Institute of Oceanology, Chinese Academy of Sciences
Article Information
- ZHAO Jin, KONG Fanzhou, LIU Qianchun, LI Fengjie, WEI Xiu, YAN Tian, JIANG Peng
- Tempo-spatial distribution of Ulva spp. micro-propagules in the Yellow Sea during and after green tide in 2019
- Journal of Oceanology and Limnology, 40(6): 2462-2472
- http://dx.doi.org/10.1007/s00343-022-1365-1
Article History
- Received Nov. 1, 2021
- accepted in principle Dec. 28, 2021
- accepted for publication Feb. 14, 2022
2 Laboratory for Marine Biology and Biotechnology, Pilot National Laboratory for Marine Science and Technology(Qingdao), Qingdao 266237, China;
3 CAS Key Laboratory of Marine Ecology and Environmental Sciences, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China;
4 Laboratory of Marine Ecology and Environmental Science, Pilot National Laboratory for Marine Science and Technology(Qingdao), Qingdao 266237, China;
5 University of Chinese Academy of Sciences, Beijing 100049, China;
6 College of Life Science, Qingdao University, Qingdao 266071, China
The Yellow Sea green tide re-occurred frequently in spring and summer in the recent decade since 2007, which brought serious troubles to both the ecology and the economy. The floating ecotype of Ulva prolifera has been proved to be the dominant algae (Zhao et al., 2013, 2015). It was proved that Subei radial sand ridges are the geographical origin for the green tide as seen from satellite remote sensing data and cruise surveys (Keesing et al., 2011; Zhang et al., 2014; Wang et al., 2015; Xing et al., 2019). It is suggested that the micro-propagules of Ulva spp. in the environment, including gametes, spores, zygotes, micro-germlings, and vegetative fragments, can attach to the nori aquaculture facilities, grow up and accumulate their initial biomass (Huo et al., 2014; Song et al., 2018). During the nori harvest, green algae were cleaned up from the facilities and became floating at seawater surface, which is thought to be the direct source of the bloom-forming algae (Liu et al., 2009, 2010a, 2021a, b, 2022; Zhang et al., 2019). Micro-propagules, which is considered as the "seed bank" of green algae, play an important role to the annual occurrence of green tide (Liu et al., 2012, 2013).
Based on the studies carried out between 2009 to 2016, green algal micro-propagules were persistently detected in the environment in Subei radial sand ridges in the year (Liu et al., 2012, 2013, 2017; Li et al., 2014; Song et al., 2015; Huo et al., 2016; Miao et al., 2020). U. prolifera was found to be the dominant species, accounting more than 50% of the micro-propagules in April (Liu et al., 2013; Huo et al., 2016; Song et al., 2018). However, most of previous studies paid little attention to the genetic diversity at the intra-species level of U. prolifera. It has been proved that the Yellow Sea green tide is dominated by a unique ecotype of U. prolifera, which is genetically different from the attached populations widely distributing along the coastline of Jiangsu Province (Zhang et al., 2011; Zhao et al., 2015). The proportion of the floating ecotype of U. prolifera micro-propagules in Subei radial sand ridges was reported in May 2016 only, accounting for approximately 88% (Miao et al., 2020). It is necessary to do further study at intra-species level to compare the inter-annual variation in quantity of the bloom-forming algae. Moreover, most of the reports on the tempo-spatial distribution of the micro-propagules in Subei radial sand ridges were before 2017. It was reported that the macroalgal blooms in the Yellow Sea varied obviously in recent years, and the golden tide caused by Sargassum horneri co-occurred with the green tide every summer since 2017 (Zhang et al., 2019; Xiao et al., 2020a). A great amount of stranded S. horneri were found from nori aquaculture facilities and might affect the epiphytic green algae on the rafts, which were considered to be the contributors to the micro-propagules to the water column (Li et al., 2014; Miao et al., 2020; Xiao et al., 2020b). Therefore, the community structure of green algal micro-propagules in Subei radial sand ridges might be altered, which deserves more indepth studies.
During the northward drifting of the floating seaweeds, micro-propagules were detected in the open sea area of the Yellow Sea (Li et al., 2014; Huo et al., 2016). The floating matured thalli are considered as the donor of micro-propagules, and U. prolifera was the dominant species in this stage (Li et al., 2014; Miao et al., 2020). These studies covered the blooming period of the Yellow Sea green tide. However, the tempo-spatial distribution pattern of U. prolifera micro-propagules after green tide in the Yellow Sea was poorly studied. At present, only a few studies paid attention to the existence time of micro-propagules along Qingdao coastline (Liu et al., 2010b; Miao et al., 2018; Zhao et al., 2018), and the condition in the open sea area is unknown.
In this study, the tempo-spatial distribution and genetic composition at both the inter- and intra-species level of green algae micro-propagules in the Yellow Sea during and after green tide in 2019 were studied. In addition, previous studies on the micro-propagules in the Yellow Sea before 2019 were reviewed and compared with the new data. The aims are (1) to investigate the potential variation of the micro-propagule composition in Subei radial sand ridges in response to the alternation of macroalgal tides in the Yellow Sea in the latest years; (2) to specify the duration of micro-propagules of the floating ecotype after green tide in the open sea area of the Yellow Sea, and (3) to speculate the final destination of the micro-propagules left from floating thalli.
2 MATERIAL AND METHOD 2.1 Cruise investigation and sampling sitesFour survey cruises, including a nearshore cruise in the Subei Shoal and three major cruises covering a large open sea area of the Yellow Sea, were conducted to investigate the distribution and species diversity of green algae micro-propagules during and after the Yellow Sea green tide in 2019. The nearshore cruise was conducted in mid-April, 2019, when green tide just started. Two of the three major cruises were conducted during green tide in the mid-June and late July, respectively. And the last one was carried out in the early September, when the green tide ended, to investigate the duration of the micro-propagules in the open sea environment of the Yellow Sea. Seawater and surface sediment samples were collected during the cruises (Fig. 1; Supplementary Table S1), and stored in cooling boxes before being transported to the laboratory.
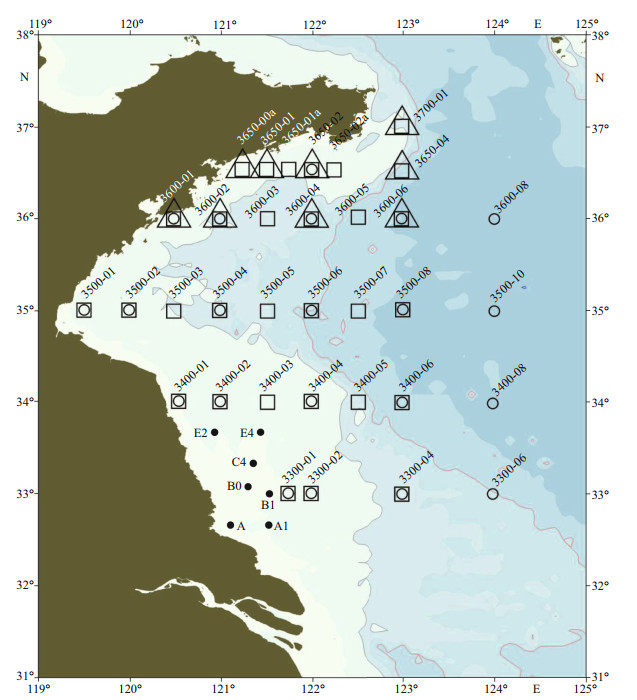
|
| Fig.1 Deployment of sampling sites of the four research cruises in this study Black dots show the sites of the nearshore cruise in the Subei Shoal in mid-April. Sites of the major cruises in the Yellow Sea in mid-June are indicated by cycles, those in late July by squares, and those in early September by triangles. |
Green algae micro-propagules in the seawater and sediment samples were cultivated using the method reported by Liu et al. (2013) with some modifications as described by Zhao et al. (2018). For seawater, 500 mL of each sample was enriched with 500 μL of 1 000× VSE (von Stosch's enriched) solution in 500-mL glass beaker. For sediments, a wet weight of 100 g of each sample was put into a 1 000-mL glass beaker filled with 500-mL sterile VSE medium. The sediment suspension was stirred vigorously and then the liquid was filtered out with the Nylon mesh (with the bore size of 75 μm) into a 500-mL glass beaker. Saturated GeO2 solution of 250 μL was added in each beaker to inhibit the growth of diatoms (Shea and Chopin, 2007). Triplicated treatments were set up for each seawater or sediment sample in an incubator (Jiangnan, Ningbo, China) at 20 ℃ with 100 μmol photons/(m2·s) and 12 h: 12 h light: dark light cycle. After three-week culture, the number of green algae germlings was counted. The abundance of micro-propagules (A, inds./L) was calculated as A=N/V or A=N/m (N: total number of germlings; V: volume of seawater sample cultured; m: weight of sediment sample cultured). The individual thallus was randomly selected from each sample for further DNA extraction and species identification.
2.3 Molecular identificationGenomic DNA was extracted from individual thallus using a Plant Genomic DNA Kit (TIANGEN, Beijing, China) following the manufacture's protocols. All of the specimens were identified by their internal transcribed spacer (ITS) sequences (Leskinen and Pamilo, 1997). Then, for the LPP (U. linza-proceraprolifera) clade, morphological features and 5S rDNA spacer sequences were analyzed to separate U. linza and U. prolifrea (Shimada et al., 2008; Tseng, 2009). The most prominent morphological feature of U. prolifera is the dense branching of the tubular thallus, while U. linza has a distromatic thallus with bilateral thin tubular margins. The sequence characterized amplified region (SCAR) marker developed by Zhao et al. (2015) was used to trace the unique floating ecotype of U. prolifera dominating in the Yellow Sea green tide. A specific 830-bp band can be amplified from the floating ecotype, while is absent from the attached populations. PCR products were checked and purified on 1.5% Tris Acetate-EDTA (TAE) agarose gels stained with DuRed (US Everbright Inc.) and sequenced by Sangon Biotech (Shanghai) Co., Ltd. Sequences were aligned using ClustalX (1.83) (Larkin et al., 2007). Eighteen ITS sequences from Ulva, Blidingia, and Urospora species and ten 5S rDNA space sequences from LPP clade are downloaded from GenBank as references to elucidate the relationship among the algal samples. Phylogenetic trees are built using Maximum Likelihood (ML) and Neighbor-Joining (NJ) methods with Mega (4.0) (Tamura et al., 2007). Robustness of the phylogenetic tree was tested by boot strapping with 1 000 replicates of the data.
3 RESULT 3.1 Phylogenetic analysis of individual specimens cultivated from micro-propagulesA total number of 238 green algae seedlings cultured from the seawater and sediment samples were selected for molecular identification. ML analysis of the ITS sequences showed that all the individual samples fell into three distinct clades: the LPP clade (160 samples), U. aragoënsis clade (75 samples), and U. compressa clade (3 samples, Fig. 2). Phylogenetic tree built by NJ method shows similar topological structures to the ML tree (Supplementary Fig.S1). It should be noted that the species, which has long been identified as U. flexuosa according to the ITS sequence, was just corrected to U. aragoënsis recently (Hiraoka et al., 2017; Krupnik et al., 2018), and the sequences from the micro-propagule samples belonging to this complex were previously named after U. flexuosa (Liu et al., 2012, 2013; Huo et al., 2016; Song et al., 2018). Morphological and 5S rDNA spacer identification of the samples in the LPP clade show that 38 out of the 160 LPP samples were U. linza, and the remaining 122 samples were U. prolifera (Supplementary Fig. S2). Based on the PCR results of the specific SCAR marker, the floating ecotype accounted for 81% of the U. prolifera individual samples.
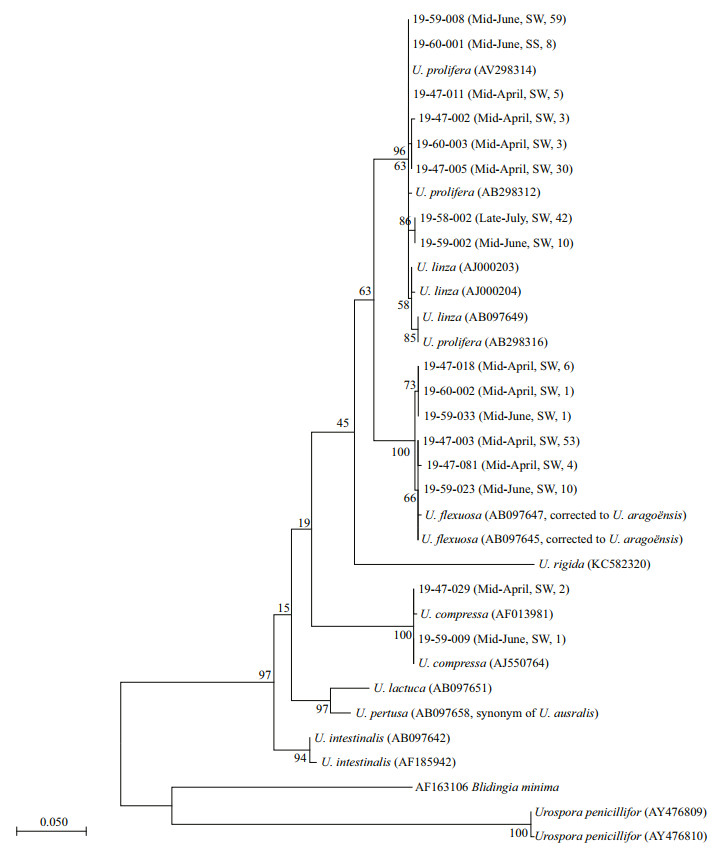
|
| Fig.2 Phylogenetic tree of the ML analysis inferred from the ITS sequences of the green algae seedlings cultured form the seawater and sediment samples The sample IDs are followed in bracket by the sampling time, sample type (SW represents seawater and SS represents surface sediment), and the number of individuals that have the same ITS sequences. |
A nearshore cruise was conducted on April 18–22, 2019, to investigate the density and species composition of the green algae micro-propagules in the Subei Shoal at the initially formatting stage of the green tide. Seawater samples were collected from seven sites. The density of the micro-propagules ranged 178–2 104 inds./L on average of 908 inds./L. The highest micro-propagule density was found in two sites around the 33°N transect (Fig. 3a; Supplementary Table S1). Four species were detected from the green algae micro-propagules, including U. aragoënsis (61%), U. linza (33%), U. prolifera (4%), and U. compressa (2%). U. aragoënsis and U. linza were the major species of green algae micro-propagules in the Subei Shoal during survey in this study. U. prolifera micro-propagules were detected at a low percentage and 25% of the U. prolifera individuals were SCAR-positive (Fig. 3b).
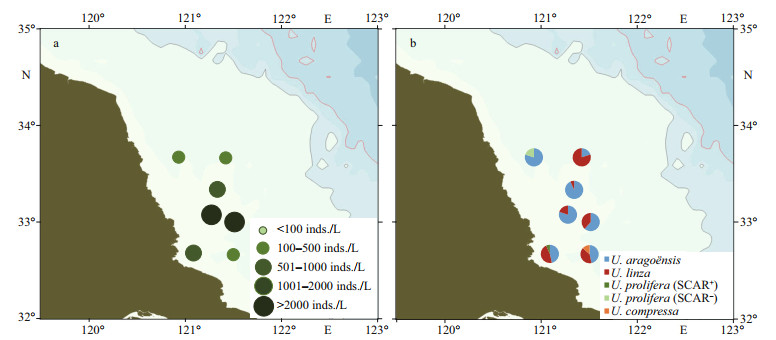
|
| Fig.3 Density (a) and community composition (b) of green algae micro-propagules in seawater of the Subei Shoal in mid-April, 2019 |
During June 11–19, 2019, seawater and surface sediment were sampled in 20 and 21 sites respectively along five transects (33°00'N, 34°00'N, 35°00'N, 36°00'N, and 36°30'N). In the seawater samples, after three-week cultivation, Ulva sprouts grew out in five samples and the micro-propagule density ranged from 0 to 60 inds./L (Fig. 4a; Supplementary Table S1). Three species were detected, i.e., U. prolifera (85%), U. aragoënsis (14%), and U. compressa (1%) (Fig. 4b). In the sediment samples, Ulva micro-propagules were detected from three samples, in which the micro-propagule density ranged from 0 to 40 inds./kg (Fig. 4c; Supplementary Table S1). Three species were detected, i.e., U. prolifera (59%), U. linza (33%), and U. aragoënsis (8%) (Fig. 4d). U. prolifera was the dominant Ulva species in both seawater and sediment samples. Eighty-four percentages of U. prolifera individuals were floating ecotype as revealed by the SCAR marker detection.
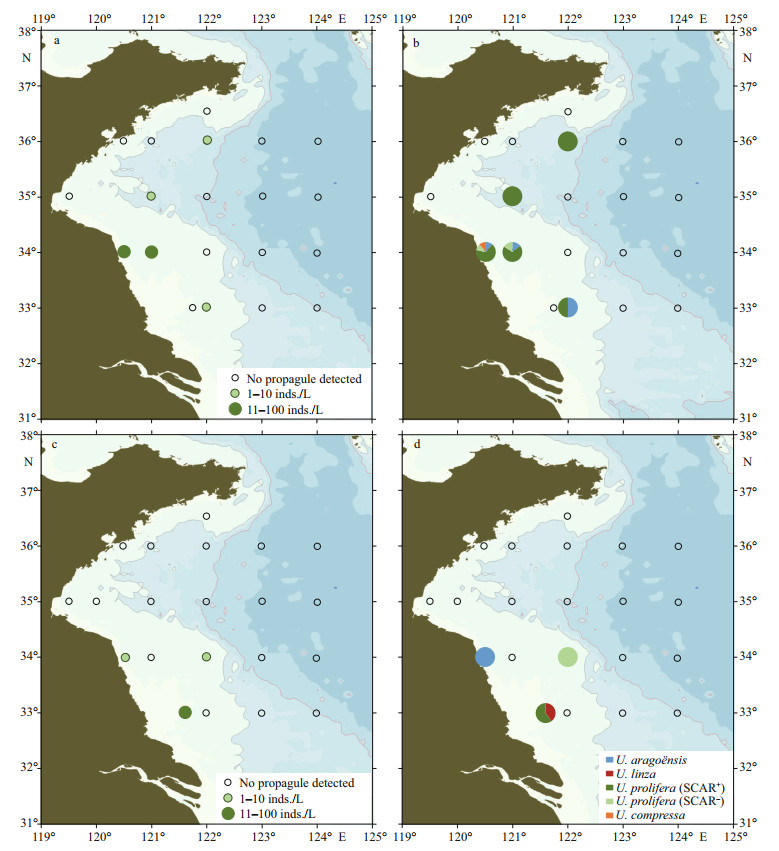
|
| Fig.4 Distribution and community composition of green algae micro-propagules in the Yellow Sea in mid-June, 2019 a. density of the micro-propagules in the seawater samples; b. community composition of the micro-propagules in the seawater samples; c. density of the micro-propagules in the sediment samples; d. community composition of the micro-propagules in the sediment samples. |
Twenty-two seawater samples and 26 surface sediment samples were collected from six transects (33°00'N, 34°00'N, 35°00'N, 36°00'N, 36°30'N, and 37°00'N) on July 21–28, 2019. Ulva germlings grew out from five seawater samples. The micro-propagule density ranged from 0 to 62 inds./L (Fig. 5a; Supplementary Table S1). Only one species, U. prolifera was detected from the germlings, and 81% of the U. prolifera individuals were SCAR-positive (Fig. 5b). No Ulva micro-propagule was detected from all the sediment samples (Supplementary Table S1).
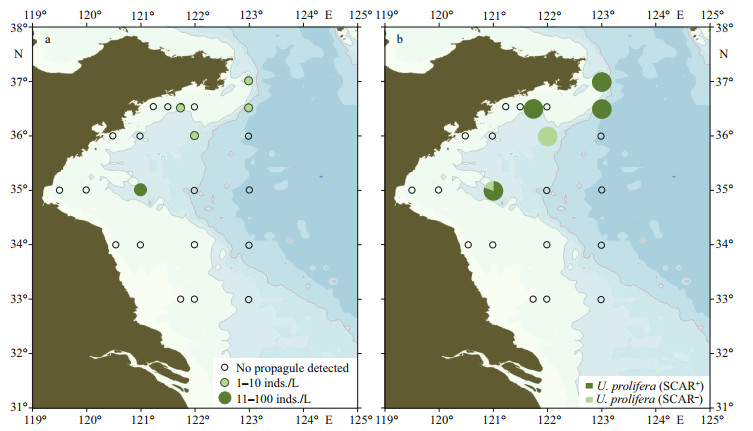
|
| Fig.5 The density (a) and community composition (b) of green algae micro-propagules in water samples of the Yellow Sea in late July, 2019 |
To investigate the duration of the micro-propagules existence in the open sea environment of the Yellow Sea, a cruise was carried out on September 3–6, 2019 when green tide ended. Seawater and surface sediment samples were collected from nine sites along three transects (36°00'N, 36°30'N, and 37°00'N). However, Ulva micro-propagule was not detected from all of the samples (Supplementary Table S1).
4 DISCUSSION 4.1 Significant variation in community composition of micro-propagules in Subei radial sand ridges under the background of the alternation of macroalgal tidesIn the present study, a great number of green algae micro-propagules were detected in all the investigation sites in the Subei Shoal in April, 2019, with the average density of 908 inds./L. The high density of micro-propagules was in accordance with the results of many former studies in this sea area in the season. In the Aprils of 2012 and 2013, Ulva micro-propagule densities were reported to be 4 800 inds./L and 1 189 inds./L, respectively (Huo et al., 2014, 2016). On the other hand, it was found that the composition percentages of different Ulva spp. micro-propagules in April 2019 in this area varied dramatically from that in the same season of previous years. In this study, U. aragoënsis and U. linza were found the major species of green algae micro-propagules in the Subei Shoal, and U. prolifera micro-propagules were detected at a low percentage (4%) during the investigation period in April 2019. From 2009 to 2014, studies on the species composition of Ulva micro-propagules in this region found repeatedly that the proportion of U. prolifera was more than 50%, and Ulva species was dominant in April (Song et al., 2015; Huo et al., 2016). In May, 2016, U. prolifera was the second dominant species, composing 35% of the total green algae micro-propagules in the Subei Shoal (Miao et al., 2020). The quantity of U. prolifera micro-propagules in the Subei Shoal sharply decreased in April 2019 from that in the same season of earlier years from 2009 to 2016.
It was considered that the attached green macroalgae on nori aquaculture rafts were the only or substantial contributors to the micro-propagules in water column of the Subei Shoal (Huo et al., 2016; Miao et al., 2020). The community structure of raft-attached green algae was reported significantly different in 2017 from that in previous years for having a sharp decreased proportion of U. prolifera (Xiao et al., 2020b). The changed macroalgae community on raft might affect the community structure of micro-propagules in the environment of this region. On the other hand, variation in the community structure of green algae micro-propagules may further influence the species composition of attached algae on rafts, which deserves further study.
It is interesting that the Ulva species identified from micro-propagules in Subei radial sand ridges were major fouling species on nori aquaculture rafts, too (Tian et al., 2011; Shen et al., 2012; Han et al., 2013; Huo et al., 2015; Li et al., 2015). Although Subei radial sand ridges are proximal to the coastline, common green algae of Ulva species including U. simplex and U. meridionalis along the natural coast of Jiangsu Province (Xie et al., 2020) were not detected from the micro-propagules cultured in the present study. This phenomenon indicates that it is difficult for the transportation of micro-propagules along natural coastline into Subei radial sand ridges. On the other hand, abundant U. aragoënsis micro-propagules in June around the 33°N transect were rarely detected at nearshore sites in the 34°N transect, nor in the open sea area, indicating that the dispersing ability of Ulva micro-propagules from Subei radial sand ridges to the open sea area is limited.
Micro-propagules of floating ecotype of U.prolifera were proved to be present in Subei radial sand ridges. It was reported that the attached populations of U. prolifera along the natural coast of the Yellow Sea are genetically different from that of the floating ecotype that has not settled down in large scale (Zhao et al., 2015, 2018). Therefore, the natural coast could not be the source of the floating seaweeds. At present, the attached adults of floating ecotype with considering biomass are found from the nori aquaculture rafts in Subei radial sand ridges only (Zhang et al., 2018). Micro-propagules in the seawater and sediments are considered as the "seeds" of the fouling green algae on rafts (Liu et al., 2013; Huo et al., 2014; Wang et al., 2015). More studies will be conducted to understand whether the decreased quantity of U. prolifera micro-propagules in Subei radial sand ridges could affect the scale of the Yellow Sea green tide.
4.2 The green algae micro-propagules not likely exist in the open water of the Yellow Sea for a long timeThe floating green algae release micro-propagules into the environment during the northward drifting. The distribution of U. prolifera micro-propagules was found closely related to the floating algal mates in the open seawaters off the Subei Shoal in the Yellow Sea (Li et al., 2014; Miao et al., 2020). Results of the present study also support this viewpoint. In the cruise in June 2019, micro-propagules of three species, U. prolifera, U. aragoënsis, and U. compressa were detected from the seawater samples in Subei coastal area, while only U. prolifera was detected from the sites north of 35°N. In the late stage of the Yellow Sea green tide in July 2019, only U. prolifera micro-propagules were detected from the seawater samples. The distribution pattern and species composition of the green algae micro-propagules corresponded to that of the floating mats. For the sediment samples, micro-propagules were only present in the sites closed to the coastal area, but were absent from the open sea areas. Moreover, no Ulva micro-propagule was detected after the green tide in September from the seawater nor the sediment samples in any site in the open sea area of the Yellow Sea. Former studies proved that the duration of micro-propagules of the floating ecotype in the seawater and sediments around Shandong Peninsula is very limited (Miao et al., 2018; Zhao et al., 2018). These results indicate that these areas are not suitable for the foating ecotype micro-propagule preservation, and are impossible to be the "seed bank" of the green tide.
Additional to the Yellow Sea green tide, the coastal areas of Qinhuangdao in the western coast of the Bohai Sea, North China, is also an area of green tide reoccurrence from late April to late September since 2015. Cruise observations and molecular identifications of floating algae proved that the Bohai Sea green tides are originated locally, and the native attached macroalgae on seaweed beds are the major original source (Song et al., 2019a, b), which is similar to the most of green tides in relatively closed bays worldwide. Investigations on the distribution of green algae micro-propagules inshore and offshore of the Qinhuangdao coast show that the density of the micro-propagules in green-tide-affected areas is remarkable higher compared to other areas (Han et al., 2019).
Because of a huge algal biomass drifting for a long distance every year, it is generally believed that the formation and progression of the Yellow Sea green tides are quite different from those of small-scale blooms with local origin (Keesing et al., 2011; Han et al., 2019). In this study, although some U. proliera micro-propagules were found from the green-tideaffected area during the blooming period, they were not persistently detected from the investigated open sea area. There are at least two reasons to explain the disappearance of micro-propagules.
(1) The micro-propagules might not be able to survive long time in the open sea area. However, many studies have proved that the micro-propagules of U. prolifera could tolerate the harsh environmental conditions (Schories and Reise, 1993; Schories, 1995; Liu et al., 2012). Fang et al. (2012) reported that even after one year of low-temperature and dark treatments, about 20% of green algal micro-propagules germinated and developed into mature thalli. Therefore, it seems possible that some micro-propagules remain vital in a short period. Another possible explanation is that micro-propagules might be eaten by some predators. In an intertidal zone, predator stress is insufficient to affect the germination of living micro-propagules and to maintain the local attached Ulva spp. populations (Miao et al., 2018; Zhao et al., 2018). Although it is still unclear about the predator stress in the outer sea, the possibility that all of the micro-propagules are eaten in a short time seems very small.
(2) The micro-propagules might have been transferred to somewhere else by ocean currents. Seawater movement in the western Yellow Sea is complex, because of the intense interaction between the land and ocean. Water masses, continental shelf front, and the frontal region of the estuaries compose the unique current system in this sea area (Wei et al., 2011). The currents are considered to affect the drifting path of seaweeds (Keesing et al., 2011; Qi et al., 2017). Studies of the golden tide in the Yellow Sea show that the floating S. horneri in the sea area out of Shandong Peninsula could drift along with ocean currents to Subei radial sand ridges in autumn and winter (Xing et al., 2017). The same transportation might also occur on the U. prolifera micro-propagules in the study area. If such a case occurs, the micro-propagules, left in the open waters of the Yellow Sea post green tide in summer, might return to Subei radial sand ridges via ocean flow and overwinter to seed the green tide outbreak in the next year. Future researches are needed to demonstrate whether this hypothesis is true.
5 CONCLUSIONIn this study, a great number of Ulva micro-propagules in high density were detected in the Subei Shoal in April, 2019, which agrees with that of former studies. However, the proportion of U. prolifera was significantly lower than that in the same season from 2009 to 2016, indicating that the quantity of U. prolifera micro-propagules in 2019 was significantly decreased. During green tide outbreak in June and July, 2019, Ulva micro-propagules were detected from the sites where the floating seaweeds distributed, and U. prolifera was the dominate species. In September 2019 when the green tide ceased, no Ulva micro-propagule was detected in the open sea area of the Yellow Sea. Therefore, green algae micro-propagules were unlikely to stay in the open sea water of the Yellow Sea for a long time. The mechanism of preserving the micro-propagules in Subei radial sand ridges, and the final destination of U. prolifera micro-propagules in the open sea area of the Yellow Sea after green tide deserves further study in the future.
6 DATA AVAILABILITY STATEMENTThe datasets analyzed during the current study were available from the corresponding author on reasonable request.
Electronic supplementary material
Supplementary material (Supplementary Table S1 and Figs.S1–S2) is available in the online version of this article at https://doi.org/10.1007/s00343-022-1365-1.
Fang S, Wang Z L, Li Y, et al. 2012. The dynamics of micro-propagules before the Green tide (Ulva prolifera) outbreak in the southern Huanghai Sea and Changjiang (Yangtze) River Estuary area. Acta Oceanologica Sinica, 34(4): 147-154.
(in Chinese with English abstract) |
Han H B, Song W, Wang Z L, et al. 2019. Distribution of green algae micro-propagules and their function in the formation of the green tides in the coast of Qinhuangdao, the Bohai Sea, China. Acta Oceanologica Sinica, 38(8): 72-77.
DOI:10.1007/s13131-018-1278-1 |
Han W, Chen L P, Zhang J H, et al. 2013. Seasonal variation of dominant free-floating and attached Ulva species in Rudong coastal area, China. Harmful Algae, 28: 46-54.
DOI:10.1016/j.hal.2013.05.018 |
Hiraoka M, Ichihara K, Zhu W R, et al. 2017. Examination of species delimitation of ambiguous DNA-based Ulva (Ulvophyceae, Chlorophyta) clades by culturing and hybridisation. Phycologia, 56(5): 517-532.
DOI:10.2216/16-109.1 |
Huo Y Z, Han H B, Hua L, et al. 2016. Tracing the origin of green macroalgal blooms based on the large scale spatio-temporal distribution of Ulva microscopic propagules and settled mature Ulva vegetative thalli in coastal regions of the Yellow Sea, China. Harmful Algae, 59: 91-99.
DOI:10.1016/j.hal.2016.09.005 |
Huo Y Z, Han H B, Shi H H, et al. 2015. Changes to the biomass and species composition of Ulva sp. on Porphyra aquaculture rafts, along the coastal radial sandbank of the Southern Yellow Sea. Marine Pollution Bulletin, 93(1-2): 210-216.
DOI:10.1016/j.marpolbul.2015.01.014 |
Huo Y Z, Hua L, Wu H L, et al. 2014. Abundance and distribution of Ulva microscopic propagules associated with a green tide in the southern coast of the Yellow Sea. Harmful Algae, 39: 357-364.
DOI:10.1016/j.hal.2014.09.008 |
Keesing J K, Liu D Y, Fearns P, et al. 2011. Inter- and intra-annual patterns of Ulva prolifera green tides in the Yellow Sea during 2007-2009, their origin and relationship to the expansion of coastal seaweed aquaculture in China. Marine Pollution Bulletin, 62(6): 1169-1182.
DOI:10.1016/j.marpolbul.2011.03.040 |
Krupnik N, Paz G, Douek J, et al. 2018. Native, invasive and cryptogenic Ulva species from the Israeli Mediterranean Sea: risk and potential. Mediterranean Marine Science, 19(1): 132-146.
DOI:10.12681/mms.2104 |
Larkin M A, Blackshields G, Brown N P, et al. 2007. Clustal W and Clustal X version 2.0. Bioinformatics, 23(21): 2947-2948.
DOI:10.1093/bioinformatics/btm404 |
Leskinen E, Pamilo P. 1997. Evolution of the ITS sequences of ribosomal DNA in Enteromorpha (Chlorophyceae). Hereditas, 126(1): 17-23.
|
Li Y, Song W, Xiao J, et al. 2014. Tempo-spatial distribution and species diversity of green algae micro-propagules in the Yellow Sea during the large-scale green tide development. Harmful Algae, 39: 40-47.
DOI:10.1016/j.hal.2014.05.013 |
Li Y, Xiao J, Ding L P, et al. 2015. Community structure and controlled factor of attached green algae on the Porphyra yezoensis aquaculture rafts in the Subei Shoal, China. Acta Oceanologica Sinica, 34(8): 93-99.
DOI:10.1007/s13131-015-0677-9 |
Liu D Y, Keesing J K, Dong Z J, et al. 2010a. Recurrence of the world's largest green-tide in 2009 in Yellow Sea, China: Porphyra yezoensis aquaculture rafts confirmed as nursery for macroalgal blooms. Marine Pollution Bulletin, 60(9): 1423-1432.
DOI:10.1016/j.marpolbul.2010.05.015 |
Liu D Y, Keesing J K, Xing Q G, et al. 2009. World's largest macroalgal bloom caused by expansion of seaweed aquaculture in China. Marine Pollution Bulletin, 58(6): 888-895.
DOI:10.1016/j.marpolbul.2009.01.013 |
Liu F, Pang S J, Chopin T, et al. 2013. Understanding the recurrent large-scale green tide in the Yellow Sea: Temporal and spatial correlations between multiple geographical, aquacultural and biological factors. Marine Environmental Research, 83: 38-47.
DOI:10.1016/j.marenvres.2012.10.007 |
Liu F, Pang S J, Thierry C, et al. 2010b. The dominant Ulva strain of the 2008 green algal bloom in the Yellow Sea was not detected in the coastal waters of Qingdao in the following winter. Journal of Applied Phycology, 22(5): 531-530.
DOI:10.1007/s10811-009-9489-7 |
Liu F, Pang S J, Zhao X B, et al. 2012. Quantitative, molecular and growth analyses of Ulva microscopic propagules in the coastal sediment of Jiangsu province where green tides initially occurred. Marine Environmental Research, 74: 56-63.
DOI:10.1016/j.marenvres.2011.12.004 |
Liu J L, Li C X, Xia J, et al. 2021a. Epizoic Ulva attached to intertidal animals in the Subei intertidal zone are not the additional source of the famed Yellow Sea green tides. Journal of Sea Research, 174: 102065.
DOI:10.1016/j.seares.2021.102065 |
Liu J L, Tong Y C, Xia J, et al. 2022. Ulva macroalgae within local aquaculture ponds along the estuary of Dagu River, Jiaozhou Bay, Qingdao. Marine Pollution Bulletin, 174: 113243.
DOI:10.1016/j.marpolbul.2021.113243 |
Liu J L, Xia J, Zhuang M M, et al. 2021b. Controlling the source of green tides in the Yellow Sea: NaClO treatment of Ulva attached on Pyropia aquaculture rafts. Aquaculture, 535: 736378.
DOI:10.1016/j.aquaculture.2021.736378 |
Liu X Q, Wang Z L, Fan S L, et al. 2017. The distribution of green algal micro-propagules and macroalgae at the early stage of green tide in the coastal area of South Jiangsu Province in 2014. Journal of Ocean University of China, 16(1): 81-86.
DOI:10.1007/s11802-017-3008-2 |
Miao X X, Xiao J, Pang M, et al. 2018. Effect of the large-scale green tide on the species succession of green macroalgal micro-propagules in the coastal waters of Qingdao, China. Marine Pollution Bulletin, 126: 549-556.
DOI:10.1016/j.marpolbul.2017.09.060 |
Miao X X, Xiao J, Xu Q Z, et al. 2020. Distribution and species diversity of the floating green macroalgae and micro-propagules in the Subei Shoal, southwestern Yellow Sea. PeerJ, 8: e10538.
DOI:10.7717/peerj.10538 |
Qi L, Hu C M, Wang M Q, et al. 2017. Floating algae blooms in the East China Sea. Geophysical Research Letters, 44(22): 11501-11509.
DOI:10.1002/2017GL075525 |
Schories D, Reise K. 1993. Germination and anchorage of Enteromorpha spp. in sediments of the Wadden Sea. Helgol?nder Meeresuntersuchungen, 47(3): 275-285.
DOI:10.1007/BF02367169 |
Schories D. 1995. Sporulation of Enteromorpha spp. (Chlorophyta) and overwintering of spores in sediments of the Wadden Sea, Island Sylt, North Sea. Netherland Journal of Aquatic Ecology, 29(3): 341-347.
|
Shea R, Chopin T. 2007. Effects of germanium dioxide, an inhibitor of diatom growth, on the microscopic laboratory cultivation stage of the kelp, Laminaria saccharina. Journal of Applied Phycology, 19(1): 27-32.
DOI:10.1007/s10811-006-9107-x |
Shen Q, Li H Y, Li Y, et al. 2012. Molecular identification of green algae from the rafts based infrastructure of Porphyra yezoensis. Marine Pollution Bulletin, 64(10): 2077-2082.
DOI:10.1016/j.marpolbul.2012.07.021 |
Shimada S, Yokoyama N, Arai S, et al. 2008. Phylogeography of the genus Ulva (Ulvophyceae, Chlorophyta), with special reference to the Japanese freshwater and brackish taxa. Journal of Applied Phycology, 20(5): 979-989.
DOI:10.1007/s10811-007-9296-y |
Song W, Han H B, Wang Z L, et al. 2019a. Molecular identification of the macroalgae that cause green tides in the Bohai Sea, China. Aquatic Botany, 156: 38-46.
DOI:10.1016/j.aquabot.2019.04.004 |
Song W, Jiang M J, Wang Z L, et al. 2018. Source of propagules of the fouling green macroalgae in the Subei Shoal, China. Acta Oceanologica Sinica, 37(4): 102-108.
DOI:10.1007/s13131-018-1169-5 |
Song W, Li Y, Fang S, et al. 2015. Temporal and spatial distributions of green algae micro-propagules in the coastal waters of the Subei Shoal, China. Estuarine, Coastal and Shelf Science, 163: 29-35.
DOI:10.1016/j.ecss.2014.08.006 |
Song W, Wang Z L, Li Y, et al. 2019b. Tracking the original source of the green tides in the Bohai Sea, China. Estuarine, Coastal and Shelf Science, 219: 354-362.
DOI:10.1016/j.ecss.2019.02.036 |
Tamura K, Dudley J, Nei M, et al. 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Molecular Biology and Evolution, 24(8): 1596-1599.
DOI:10.1093/molbev/msm092 |
Tian X L, Huo Y Z, Chen L P, et al. 2011. Molecular detection and analysis of green seaweeds from Rudong coasts in Jiangsu Province. Chinese Science Bulletin, 56(4-5): 309-317.
(in Chinese with English abstract) DOI:10.1360/972010-2124 |
Tseng C K. 2009. Seaweeds in Yellow Sea and Bohai Sea of China, Beijing. p. 387-388. (in Chinese)
|
Wang Z L, Xiao J, Fan S L, et al. 2015. Who made the world's largest green tide in China?—an integrated study on the initiation and early development of the green tide in Yellow Sea. Limnology and Oceanography, 60(4): 1105-1117.
DOI:10.1002/lno.10083 |
Wei Q S, Yu Z G, Ran X B, et al. 2011. Characteristics of the western coastal current of the Yellow Sea and its impacts on material transportation. Advances in Earth Science, 26(2): 145-156.
(in Chinese with English abstract) |
Xiao J, Fan S L, Wang Z L, et al. 2020a. Decadal characteristics of the floating Ulva and Sargassum in the Subei Shoal, Yellow Sea. Acta Oceanologica Sinica, 39(10): 1-10.
DOI:10.1007/s13131-020-1655-4 |
Xiao J, Wang Z L, Song H J, et al. 2020b. An anomalous bi-macroalgal bloom caused by Ulva and Sargassum seaweeds during spring to summer of 2017 in the western Yellow Sea, China. Harmful Algae, 93: 101760.
DOI:10.1016/j.hal.2020.101760 |
Xie W F, Wu C H, Zhao J, et al. 2020. New records of Ulva spp. (Ulvophyceae, Chlorophyta) in China, with special reference to an unusual morphology of U. meridionalis forming green tides. European Journal of Phycology, 55(4): 412-425.
DOI:10.1080/09670262.2020.1740946 |
Xing Q G, An D Y, Zheng X Y, et al. 2019. Monitoring seaweed aquaculture in the Yellow Sea with multiple sensors for managing the disaster of macroalgal blooms. Remote Sensing of Environment, 231: 111279.
DOI:10.1016/j.rse.2019.111279 |
Xing Q G, Guo R H, Wu L L, et al. 2017. High-Resolution satellite observations of a new hazard of golden tides caused by floating Sargassum in winter in the Yellow Sea. IEEE Geoscience and Remote Sensing Letters, 14(10): 1815-1819.
DOI:10.1109/LGRS.2017.2737079 |
Zhang J H, Huo Y Z, Wu H L, et al. 2014. The origin of the Ulva macroalgal blooms in the Yellow Sea in 2013. Marine Pollution Bulletin, 89(1-2): 276-283.
DOI:10.1016/j.marpolbul.2014.09.049 |
Zhang J H, Shi J T, Gao S, et al. 2019. Annual patterns of macroalgal blooms in the Yellow Sea during 2007-2017. PLoS One, 14(1): e0210460.
DOI:10.1371/journal.pone.0210460 |
Zhang Q C, Yu R C, Chen Z F, et al. 2018. Genetic evidence in tracking the origin of Ulva prolifera blooms in the Yellow Sea, China. Harmful Algae, 78: 86-94.
DOI:10.1016/j.hal.2018.08.002 |
Zhang X W, Xu D, Mao Y Z, et al. 2011. Settlement of vegetative fragments of Ulva prolifera confirmed as an important seed source for succession of a large-scale green tide bloom. Limnology and Oceanography, 56(1): 233-242.
DOI:10.4319/lo.2011.56.1.0233 |
Zhao J, Jiang P, Liu Z Y, et al. 2013. The yellow sea green tides were dominated by one species, Ulva (Enteromorpha) prolifera, from 2007 to 2011. Chinese Science Bulletin, 58(19): 2298-2302.
DOI:10.1007/s11434-012-5441-3 |
Zhao J, Jiang P, Qin S, et al. 2015. Genetic analyses of floating Ulva prolifera in the Yellow Sea suggest a unique ecotype. Estuarine, Coastal and Shelf Science, 163: 96-102.
DOI:10.1016/j.ecss.2015.05.027 |
Zhao J, Jiang P, Qiu R, et al. 2018. The Yellow Sea green tide: a risk of macroalgae invasion. Harmful Algae, 77: 11-17.
DOI:10.1016/j.hal.2018.05.007 |
 2022, Vol. 40
2022, Vol. 40


