Institute of Oceanology, Chinese Academy of Sciences
Article Information
- ZHANG Anqi, LIU Honghan, LI Chenhong, CHEN Changping, LIANG Junrong, SUN Lin, GAO Yahui
- Relationship between toxic and harmful microalgae and environmental factors in typical mariculture areas of East China Sea
- Journal of Oceanology and Limnology, 40(6): 2401-2415
- http://dx.doi.org/10.1007/s00343-022-2125-y
Article History
- Received Mar. 14, 2022
- accepted in principle Jun. 1, 2022
- accepted for publication Jul. 18, 2022
2 State Key Laboratory of Marine Environmental Science, Xiamen University, Xiamen 361102, China;
3 Key Laboratory of Ministry of Education for Coastal and Wetland Ecosystems, Xiamen University, Xiamen 361102, China
Harmful algal blooms (HABs) are usually caused by the rapid proliferation and accumulation of toxic and harmful microalgae in the ocean. They can influence the structure and function of the ecosystem and have a negative impact on the ecological environment and human society (Berdalet et al., 2017; Chen et al., 2021b). Microalgae are an important part of the marine ecosystem, however, some toxic and harmful microalgae form algal blooms that can have a serious impact on fisheries and aquaculture (Berdalet et al., 2016). Some HAB species can produce powerful toxins that contaminate seafood and can accumulate in the food chain, leading to mass mortality in wild and farmed fish, shellfish, and even poisoning humans (Anderson et al., 2002; Wang, 2008). Similarly, nontoxic HABs can also be harmful to the environment, as they can lead to significant reductions in oxygen concentrations in coastal sheltered areas (Brandenburg et al., 2019), which can have consequential human or ecological health impacts and damage to local aquaculture and economic development. In recent years, HAB events have occurred more frequently and lasted longer in more locations than in past decades (Furuya et al., 2018). Due to the recent widespread and stable events of HAB that have adversely affected human health and aquaculture, there is a much greater need for society to recognize these phenomena than in the past (Zohdi and Abbaspour, 2019).
Mariculture is well developed in China, ranking first in the world in terms of area and production (Li et al., 2014). As a traditional industry, marine aquaculture plays an important role in promoting the economic development of coastal areas (Liao and Yang, 2019), but the existing problems associated with HABs are becoming increasingly prominent. The coastal areas of the East China Sea are well-known "hot spots" for HABs in China (Chen et al., 2021c), and the East China Sea has become an area of frequent HAB occurrence. Fujian and Zhejiang are major marine provinces in China, with vast coastal areas, rich marine resources, and important mariculture areas, and are also the areas with frequent incidences of red tide (Huang et al., 2020). Gouqi Island is located in the eastern part of Shengsi Island, which is located in the subtropical marginal sea area (Zhang et al., 2007), and mussel aquaculture is the main economic industry of Gouqi Island (Chen et al., 2012; Bao et al., 2020). Sandu Bay is located in the northeastern part of Fujian Province, it is a typical semi-enclosed harbor and the only inlet spawning ground of Pseudosciaena crocea in China (Zheng, 2007), mainly aquaculture of Pseudosciaena crocea and abalone (Lin, 2013). Dongshan Bay is the largest bay in southern Fujian Province (Zheng, 2009), and it is also an important mariculture area in Fujian Province, where the main products are abalone and scallops (Zhang et al., 2010). However, in recent years, eutrophication and HABs have been frequent in the mariculture areas, and these phenomena have a significant impact on the ecosystem health (Li, 2000; Chai et al., 2013) and affect the marine culture industry.
HAB events are usually associated with rapid abnormal proliferation or high biomass accumulation of toxic or harmful microalgae on the sea surface or in water bodies (Anderson et al., 2012), and many HABs are associated with increasing eutrophication (Glibert and Burkholder, 2011). Understanding the key characteristics of HAB species diversity and population dynamics is essential (Zhou and Zhu, 2006), and this understanding will serve as the basis for improved monitoring (Zingone and Enevoldsen, 2000). Therefore, the investigation of the diversity of toxic and harmful microalgae in mariculture areas and their relationship with environmental factors is of great significance for the prediction and prevention of harmful algal blooms. There have been various studies on the phytoplankton community structure in the East China Sea (Tang et al., 2013; Zhou et al., 2018), but there are few studies focusing on the distribution of toxic and harmful microalgal species and their relationship with environmental factors in the aquaculture areas. Hence the aim of this essay is to investigate the distribution of toxic and harmful microalgae diversity in typical mariculture areas of the East China Sea (Gouqi Island, Sandu Bay, and Dongshan Bay) and to reveal the relationship between toxic and harmful microalgae and environmental factors in the mariculture areas. Understanding the baseline species composition could give insight to better anticipate and prevent HABs in mariculture areas of this region.
2 MATERIAL AND METHOD 2.1 Sample collectionAccording to the distribution of mariculture areas, a total of 12 sampling stations were set up in the typical mariculture areas of Gouqi Island (122°44′57″E–122°49′27″E, 30°42′34″N– 30°43′51″N), Sandu Bay (119°44′58″E–119°54′19″E, 26°22′13″N–26°37′17″N), and Dongshan Bay (117°20′6″E–117°42′8″E, 23°36′18″N–23°56′31″N), and 4 stations were set up in each mariculture area (Fig. 1).
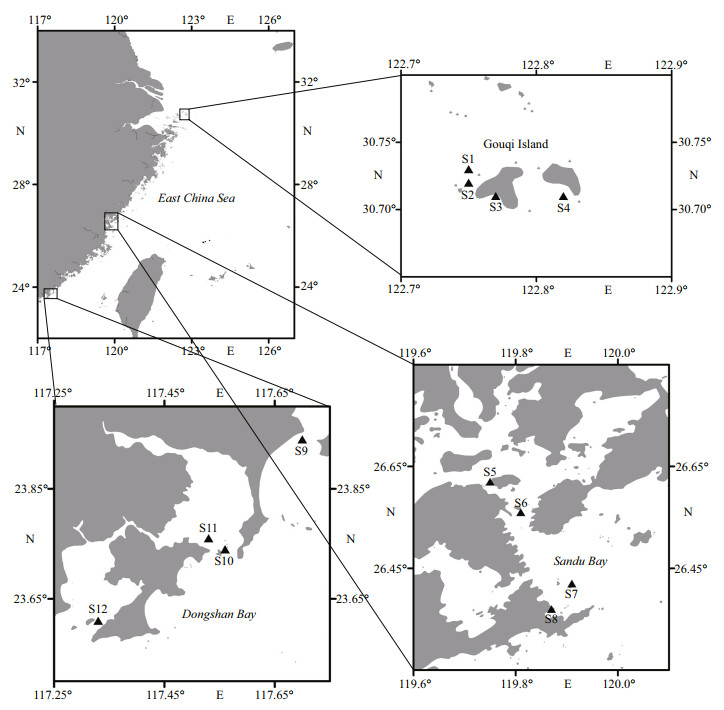
|
| Fig.1 The sampling stations in typical mariculture areas of the East China Sea |
Four field sampling surveys were conducted in May 2020, August 2020, November 2020, and March 2021. At each sampling site, water samples were collected from the surface layer at a depth of 2 m with a Plexiglas water collector, and 1 L of water was fixed with 15-mL Lugol's solution. To determine of the species composition and cell density of phytoplankton and toxic and harmful microalgae, we filtered 15 L of water through a 20-μm pore size phytoplankton net and collected 100 mL. Then, this subsample was fixed in 1.5 mL of formaldehyde.
2.2 Phytoplankton and environmental factorsThe water and net samples were brought to the laboratory and concentrated to 10 mL and 50 mL. After sedimentation for 24 h, the supernatant was discarded with a siphon tube. Phytoplankton were identified and counted using a 0.1-mL phytoplankton counting chamber under a light microscope (Olympus BX51, Olympus Corporation, Tokyo, Japan) at a magnification of 200–1 000× (Jiang et al., 2019).
For nutrient analysis, water samples for nutrient measurements were collected using a 0.45-μm pore size cellulose acetate membrane filter for orderly filtration of seawater samples. Water temperature (WT), salinity (Sal), pH, and dissolved oxygen (DO) were measured with a U-50 multi-parameter water quality monitor (HORIBA, Japan) on site. Concentrations of phosphate (PO43-), nitrate (NO3-), nitrite (NO2-), and silicate (SiO32-) were measured and analyzed with an AA3 Nutrient Salt Automatic Analyzer (SEAL, Germany).
2.3 Data analysisThe phytoplankton and environmental data were analyzed using SPSS 28.0 software. One-way ANOVA was conducted to evaluate the differences in the phytoplankton or toxic and harmful microalgal cell densities. Difference in seasonal variation of environmental factors at 12 sampling sites was analyzed by two-way ANOVA (Cui et al., 2018). The statistical significance was set at P < 0.05. Dominance was calculated by the species cell density/total cell density to define dominant species (Chen et al., 2016).
The analysis of species data and environmental factors was performed using the "ggplot2" package (Wickham et al., 2020) and the "vegan" package (Oksanen et al., 2020) in RStudio 1.4.1717. Redundancy analysis (RDA) was used to characterize the correlation between environmental factors and toxic and harmful microalgae. Since our toxic and harmful algae data were zero-inflated, we employed the Helliger transformation to our species and environmental variables (Legendre and Gallagher, 2001). The significance of environmental factors in relation to species community composition was assessed using Monte Carlo permutation tests (Yu et al., 2016). All other graphing and analyses were performed in RStudio 1.4.1717 and Microsoft Office Excel 2019.
3 RESULT 3.1 Phytoplankton assemblageAcross the 12 stations, we identified 199 phytoplankton species belonging to 70 genera, of which 145 species were Bacillariophyceae (72.9%), 49 species from Dinophyceae (24.6%), 2 from Cyanophyceae (1%), 2 from Chrysophyceae (1%), and 1 from Raphidophyceae (0.5%). Of the 17 dominant species, we found eight harmful and toxic microalgal species (Table 1).
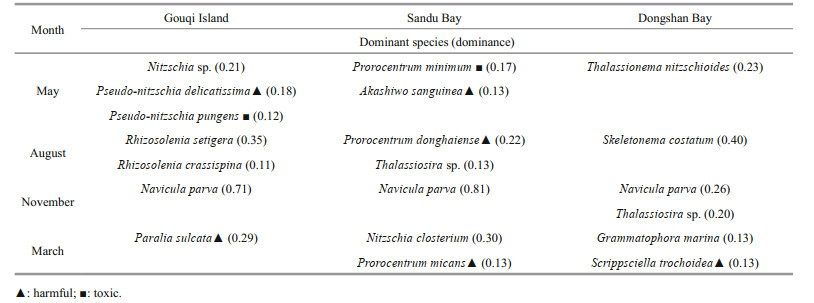
|
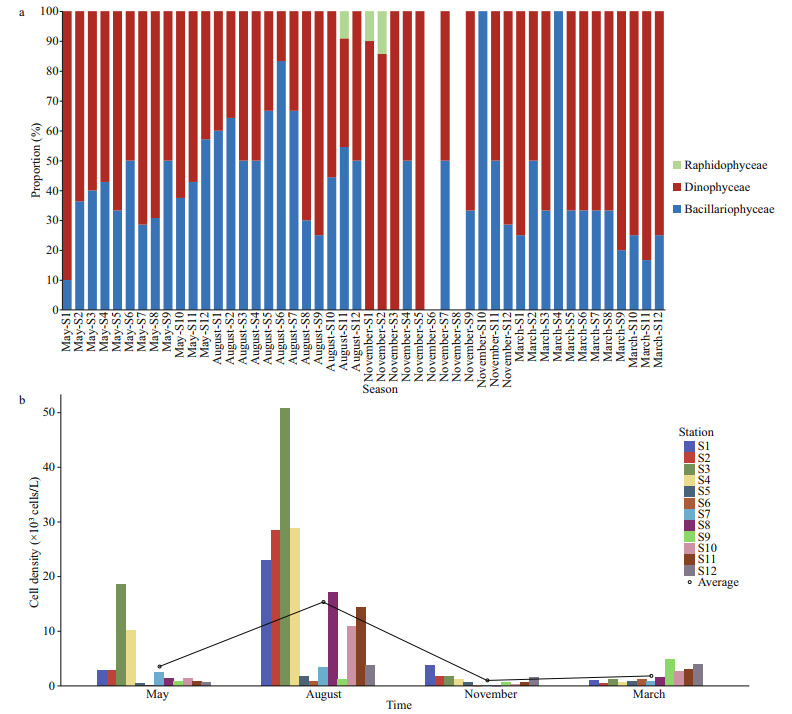
Phytoplankton cell densities were highest during the summer and relatively lower during the autumn, spring, and winter (Fig. 2). The phytoplankton cell density was significantly higher during the summer than during the other seasons (ANOVA, df=3, F=9.437, P < 0.001), with an average of (80.03±77.81)×103 cells/L in summer (August), (13.87±11.49)×103 cells/L in autumn (November), (8.90±10.08)×103 cells/L in spring (May), and (6.53±6.13)×103 cells/L in winter (March).
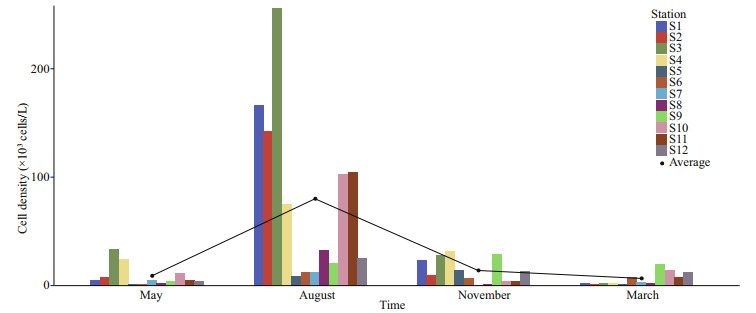
|
| Fig.2 Temporal variation of phytoplankton cell density in typical mariculture areas of the East China Sea Different colors represent sampling stations. The black line represents the connecting line of the average phytoplankton cell density in each season |
A total of 38 toxic and harmful microalgal species belonged to 21 genera were found among the identified phytoplankton species (Table 2). With 24 species belonging to Dinophyceae and 13 species belonged to Bacillariophyceae, and 1 species belonged to Raphidophyceae (Yuan et al., 2009).
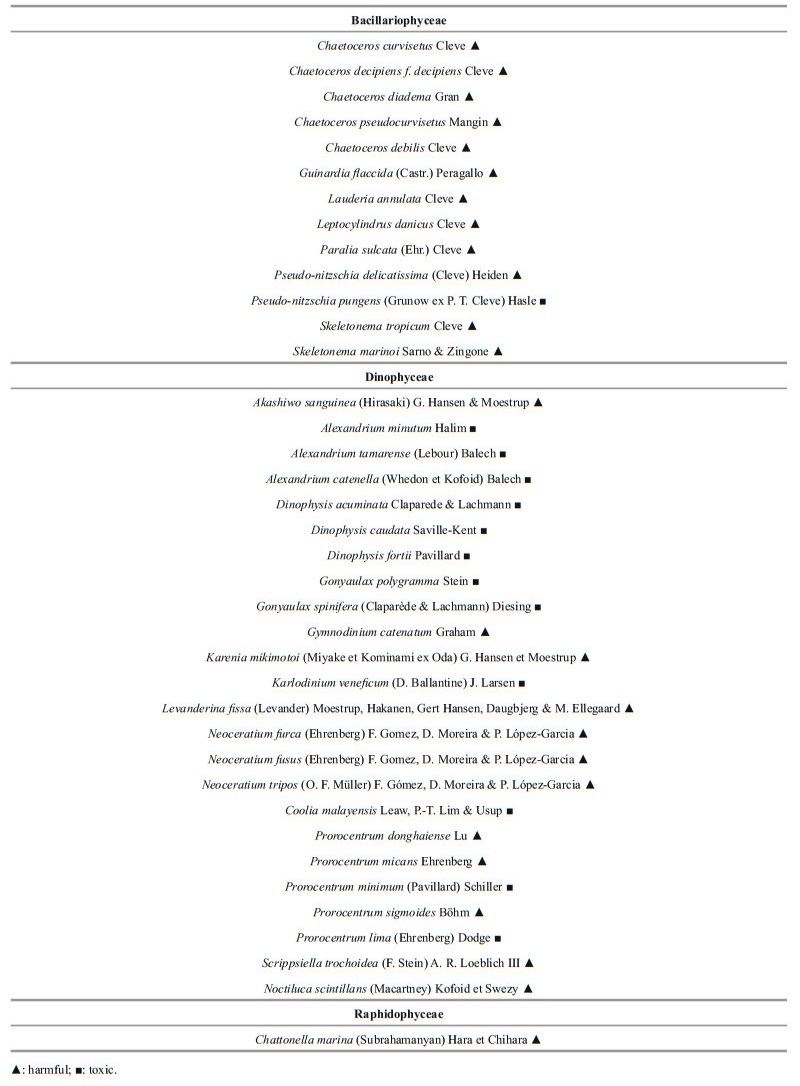
|
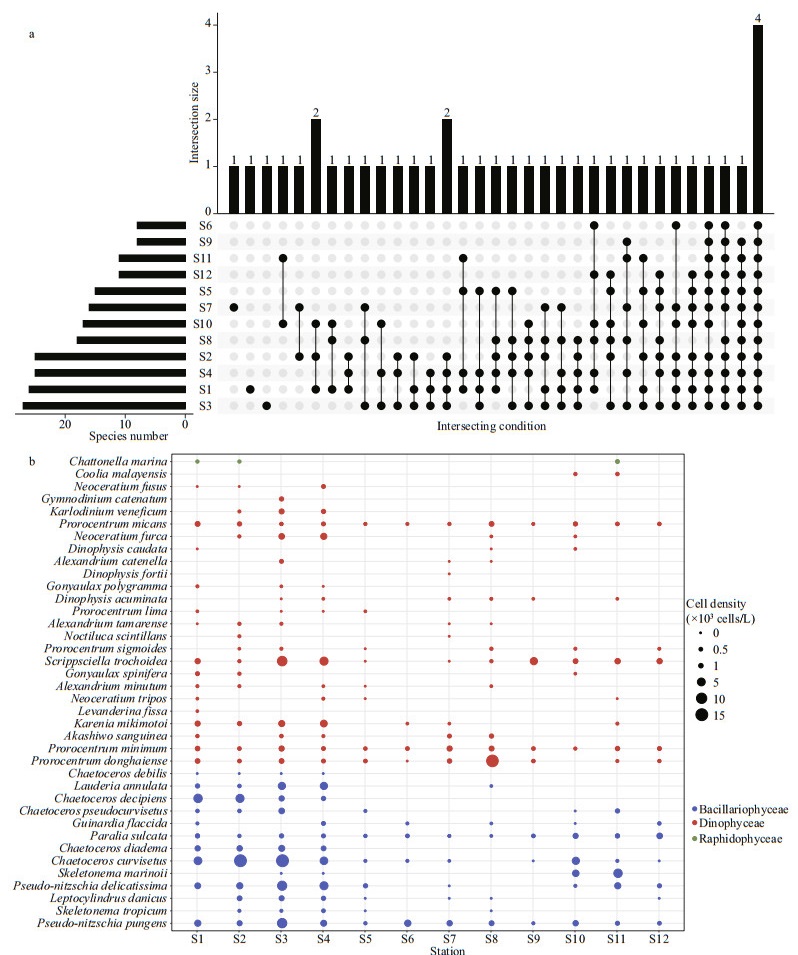
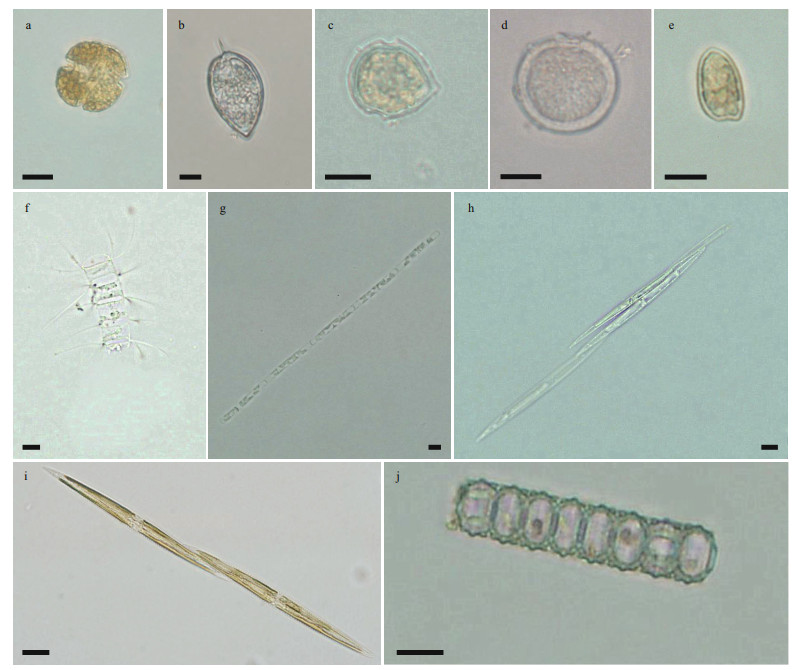
The species numbers (ANOVA, df=2, F=20.047, P < 0.001) and cell density (ANOVA, df=2, F=1.221, P < 0.05) of toxic and harmful microalgae were significantly higher at the Gouqi Island stations (Fig. 3a–b). There were 10 species of toxic and harmful microalgae with higher occurrence frequency (>6) at each station (Fig. 3a), P.pungens, P. sulcata, P. minimum, and P. micans were found at all stations during the investigation (Fig. 3b). Light microscopic photos of the high frequency toxic and harmful microalgal species are shown in Fig. 4.
|
| Fig.3 Composition and distribution of toxic and harmful microalgae in typical mariculture areas of the East China Sea a. comparative analysis of Venn diagrams of toxic and harmful microalgal species, relative frequencies of toxic and harmful microalgal species, with the top bar showing species identified at one or more stations and the left bar showing the number of toxic and harmful microalgal species identified at each station; b. distribution of toxic and harmful microalgae. Different colors represent different phyla, and the size of the circles represents the cell density. |
|
| Fig.4 Light microscope photos of toxic and harmful microalgal species in typical mariculture areas of the East China Sea (frequency>6) a. Karenia mikimotoi; b. Prorocentrum micans; c. Scrippsiella trochoidea; d. Prorocentrum minimum; e. Prorocentrum donghaiense; f. Chaetoceros curvisetus; g. Leptocylindrus danicus; h. Pseudo-nitzschia delicatissima; i. Pseudo-nitzschia pungens; j. Paralia sulcata. Scale bars: 10 μm. |
During the sampling period, the distribution of toxic and harmful microalgae in the study regions was dominated by dinoflagellates in winter (March), spring (May), and autumn (November) when the temperature was relatively low. In summer (August), when the temperature was higher, diatoms were dominant (Fig. 5a). We did not observe any toxic and harmful microalgal species in autumn at Stations S6 and S8 located in Sandu Bay (Fig. 5a).
|
| Fig.5 Seasonal variations in species composition and density distribution of toxic and harmful microalgae in typical mariculture areas of the East China Sea a. seasonal variation in the proportion of toxic and harmful microalgal species. Different colors represent different phyla; b. distribution of cell density of toxic and harmful microalgae. Different colors represent sampling stations. The black line represents the connecting line of the average cell density of toxic and harmful microalgae in each season. |
Seasonal differences in the density of toxic and harmful microalgae were evident (higher in spring and summer, lower in autumn and winter). The cell densities of toxic and harmful microalgae at each station averaged of (3.53±5.42)×103 cells/L in spring, (15.34±15.28)×103 cells/L in summer, (1.00±1.08)×103 cells/L in autumn, and (1.82± 1.44)×103 cells/L in winter (Fig. 5b).
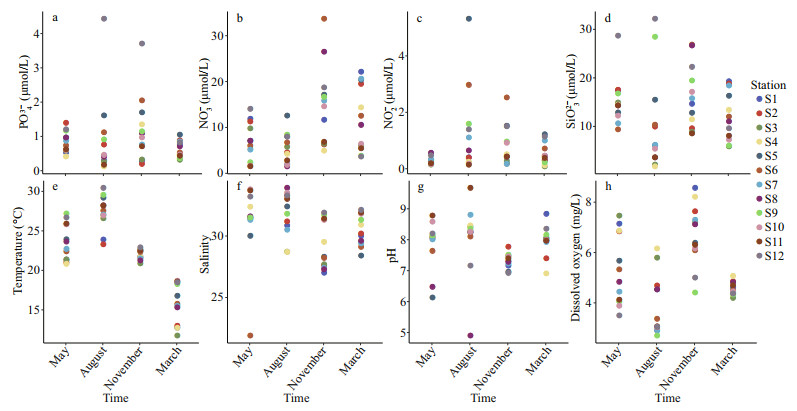
3.3 Environmental factorsAccording to the environmental factors of the 12 stations (Fig. 6), the trophic conditions in the East China Sea mariculture area during the four seasons ranged from 0.125 to 4.429 μmol/L for PO43-(Fig. 6a), 1.507 to 33.672 μmol/L for NO3-(Fig. 6b), 0.083 to 5.313 μmol/L for NO2-(Fig. 6c), and 1.768–32.160 μmol/L for SiO32-(Fig. 6d). There were no significant differences in the nutrient parameters of PO43-, NO2-, and SiO32- (two-way ANOVA, P>0.05) with seasonal variation at each station, but significant differences were found for NO3- (two-way ANOVA, P < 0.05).
|
| Fig.6 Temporal and spatial variations of the environmental factors a. PO43-; b. NO3-; c. NO2-; d. SiO32-; e. water temperature (WT); f. salinity (Sal); g. pH; h. dissolved oxygen (DO). Different stations are coded with different |
In terms of physical-chemical parameters, water temperature (WT) varied significantly with the season, ranging from 11.74 to 32.16 ℃ (Fig. 6e), and salinity (Sal) varied from 21.9 to 33.9 (Fig. 6f), pH varied over a wide range from 4.90–9.67 (Fig. 6g), and dissolved oxygen (DO) varied from 2.70–8.56 mg/L (Fig. 6h). Significant differences were found in water temperature and dissolved oxygen with season (two-way ANOVA, P < 0.001), but no significant differences were found for salinity and pH (two-way ANOVA, P>0.05).
3.4 Effects of environmental factors on toxic and harmful microalgaeWe investigated the spatial and seasonal distribution of environmental factors on toxic and harmful microalgal communities in typical mariculture areas of the East China Sea. According to the RDA results, toxic and harmful microalgae showed significant seasonal changes during the whole year of sampling (Fig. 7a). During the spring, toxic and harmful microalgae were mainly influenced by DO (Fig. 7a). During the transition period from spring to summer, the toxic and harmful microalgae were limited by nitrate NO3-. Then, in autumn, the growth of toxic and harmful microalgae was mainly influenced by pH, while in winter the main limiting environmental factor was WT. The results indicate that the succession of toxic and harmful microalgae was mainly driven by the different environmental factors during the sampling year.

|
| Fig.7 Redundancy analysis (RDA) of environmental factors and toxic and harmful microalgae data from all sampling stations during the four seasons a. ordination diagram of the distribution of all sampling stations in four seasons; b. toxic and harmful microalgae (frequency 6). Sampling stations are represented by circles, filled with yellow for spring, blue for summer, green for autumn, and red for winter. |
Among all samples, 10 species with a wide distribution (frequency>6) were selected as the species data matrix (Fig. 7b). The variation of toxic and harmful microalgal assemblages between stations and seasons was mainly affected by NO3-, WT, pH, DO, and NO2-. P. pungens, Pseudo-nitzschia delicatissima, and Chaetoceros curvisetus showed a preference for NO3-. P. sulcata and P. micans were characterized by high WT. Prorocentrum donghaiense had a particular pH. Karenia mikimotoi was positively correlated with DO. P. minimum showed particular favor to high NO2-.
4 DISCUSSION 4.1 Spatial and temporal variation of toxic and harmful microalgaeThe cell densities of toxic and harmful microalgae from the three mariculture regions displayed strong seasonal variation with the highest variation in summer (August) (Fig. 5b). The cell densities of phytoplankton and toxic and harmful microalgae in Sandu Bay and Dongshan Bay were both at low levels during the spring (Figs. 2 & 5b), which differed from past studies (Xu and Hou, 2006; Li, 2012). Previous studies showed that the high occurrence period of red tide in Fujian coastal waters was in May (Huang et al., 2020); however, in this study, the density of phytoplankton and toxic and harmful microalgae in the mariculture area of Fujian coastal waters remained at a low level in May, especially at Stations S5 and S6 (Figs. 2 & 5b). The occurrence of red tide near the study stations (including Stations S5 and S6) before the timeframe of our sampling period could be responsible for the low levels of low phytoplankton cell density observed. Phytoplankton cell densities tend to sharply decrease after red tides (Xu et al., 1992; Mu et al., 2015), which is what we observed in our study.
In recent years, there seems to be a shift in dominant taxa of algal blooms from non-toxic diatoms to toxic dinoflagellates (Glibert and Burkholder, 2011), and an increase in red tide species in the East China Sea (Zhou et al., 2001). Ouyang et al. (1993) found a total of 6 species of toxic red tide species, including 5 species of toxic dinoflagellates, in the mariculture area of Shengsi Island, Zhejiang Province, and Xu et al. (2010) identified 6 species of toxic red tide species including 5 species of toxic dinoflagellates, in Fujian coast water. In this study, 13 species of toxic microalgae were found, including 12 species of toxic dinoflagellates (Table 2), and there was a significant increase in the number of toxic dinoflagellate species. At the same time, it can be seen that the species of toxic and harmful microalgae were mostly dominated by dinoflagellates in the yearly investigation (Fig. 5a).
Four species of toxic and harmful microalgae were identified at all stations (Fig. 3a). Among them, P. pungens and P. micans are widely distributed in the East China Sea as generalist species (Wang et al., 2001; Chen et al., 2021a), and P. pungens has a strong nitrogen absorption ability (Zhang and Zhou, 1997). Glibert et al. (2008) indicated that P. minimum is a type of species that expands with the nutrient level of the marine environment, and P. sulcata, as an indicator species of coastal environments, can be a good indicator of eutrophication in seawater (McQuoid and Nordberg, 2003), so its wide distribution may reflect the eutrophication of the mariculture area.
4.2 Relationship between toxic and harmful microalgae and environmental factorsThe occurrence of harmful algal blooms caused by toxic and harmful microalgae requires a specific environment (Liu et al., 1999), such as hydrological conditions, nutrients and biological environments (Chen et al., 2001). As shown in Fig. 7a–b, the optimal environmental factors that influenced the toxic and harmful microalgae community included NO3-, WT pH, DO, and NO2-. Water temperature determines the types of harmful algal blooms that occur, which is also necessary for the germination and reproduction of some toxic and harmful microalgal cysts (Pan and Jiang, 2004). In this study, the maximum WT (Fig. 6e) occurred in August, and the peak growth of both phytoplankton and toxic microalgae occurred in this month (Figs. 2 & 5b). According to the RDA results, P. sulcata and P. micans showed a positive correlation with WT in the mariculture area (Fig. 7b), which is consistent with the results of Yang et al. (2016) and Wang et al. (2001). P. sulcata and P. micans are eurythermal species, while the East China Sea has a subtropical and temperate climate. The suitable temperature is an important reason for their wide distribution. Dissolved oxygen is a major factor in determining the growth of algae in the water column and is also the primary key factor in determining the success or failure of aquaculture (Yao and Miao, 2021). We found a positive correlation with DO and a negative correlation with Sal for K. mikimotoi (Fig. 7b), which is in clear agreement with the results of Zhao et al. (2020) and Su et al. (2020). pH not only has an important influence on the growth of phytoplankton, but also affects the toxicity effect of toxic algae producing toxins to other marine ecosystems (Schmidt and Hansen, 2001). P. donghaiense is widely distributed in the East China Sea (Chen et al., 2021a), and Yoo (1991) suggested that pH is the main factor affecting the growth of P. donghaiense, and that its population growth has obvious requirements on the pH range (Dai et al., 2011). In this study, we also investigated that pH is the growth limiting factor of P. donghaiense in the East China Sea (Fig. 7b).
Nutrients are the main limiting factor for the growth of natural phytoplankton populations in the marine environment, and some studies have suggested that eutrophication may be a key factor in the occurrence of HABs (Yang et al., 2019). Dinoflagellates can rapidly develop blooms in aquatic environments. This process requires large amounts of nutrients, especially nitrogen (Abassi and Ki, 2022). We found a positive correlation between P. minimum and NO2-, which coincides with earlier studies (Fan and Glibert, 2005; Glibert et al., 2008). The abundance of nitrogen nutrients is the material basis for red tides (Berdalet et al., 1996). Previous studies have shown that changes in nitrogen in the water column are important in influencing diatom growth (Chen et al., 2002; Fei and Jiang, 2008). P. pungens had a high correlation with NO3- in Fig. 7b, which is consistent with the results of Lü et al. (2006). Therefore, we believe that nutrients, such as NO3- and NO2-, also have major influences on the growth of toxic and harmful microalgae in the mariculture areas.
4.3 Relationship between toxic and harmful microalgae and mariculture areasThe environment of the mariculture area and the feeding effect of the mariculture products will influence the composition of toxic and harmful microalgae. Previous studies have indicated that shellfish have a greater selective tendency to feed on dinoflagellates than diatoms (Rouillon et al., 2005; Zhang et al., 2008). We found that dinoflagellate species among toxic and harmful microalgae were more abundant than diatoms in all three mariculture areas, but the density remained relatively low (Fig. 3b). Mussels are the main mariculture product, which feed selectively on phytoplankton (Kiørboe et al., 1980) and less on toxin-producing and indigestible algae (Langdon and Waldock, 1981). Interestingly, when the density of algal cells is low, mussels will feed as much as possible to ensure their growth regardless of the nature of the food (Tan and Ransangan, 2017). In this study, there was a high level of distribution of P. pungens in the Gouqi Island mariculture area (Fig. 3b). Because of the dispersion of P. pungens, mussels may selectively feed on it, and mussels in the Gouqi Island mariculture region may be exposed to amnesic shellfish poisoning (ASP) toxin.
The nutrient conditions in the mariculture area are also an important reason for the occurrence of toxic and harmful algal blooms (Wang et al., 2008). Previous studies found eutrophication on Gouqi Island (Bao et al., 2020), Sandu Bay (Zheng, 2007), and Dongshan Bay (Li, 2000). These regions show intensification of eutrophication, especially NO3- and PO43- (Fig. 6a & b). In the mariculture region of Gouqi Island, P. pungens and P. delicatissima were correlated with NO3- (Fig. 7b). They were found at all four locations, and were the dominant species in the spring (Fig. 3b; Table 1). NO2- was high in the Sandu Bay mariculture region (Fig. 6c). P. minimum (Sierra-Beltrán et al., 2005), with a preference for NO2-, was not only widespread but also one of the dominant species in the spring (Fig. 3b; Table 1). In Dongshan Bay, PO43- was relatively high (Fig. 6a). RDA showed a positive correlation between Scrippsiella trochoidea and PO43- (Fig. 7b). During the winter, S. trochoidea became the dominat species in Dongshan Bay and was widely distributed throughout the region (Fig. 3b; Table 1). Considering the wide distribution of these toxic and harmful microalgae and the intensification of eutrophication in the mariculture area (Bao et al., 2020; Huang et al., 2020), there is a potential risk of harmful algal blooms in typical mariculture areas in the East China Sea.
5 CONCLUSIONThis study revealed the relationship between toxic and harmful microalgae and environmental factors in three typical mariculture areas in the East China Sea. During the research period, 38 species of toxic and harmful microalgae belonging to 21 algal genera were identified. The toxic and harmful microalgae taxa were dominated by dinoflagellates. There were 8 species of toxic and harmful microalgae that appeared as dominant species: P. pungens, P. delicatissima, P. minimum, Akashiwo sanguinea, P. donghaiense, P. sulcata, P. micans, and S. trochoidea. Among them, P. pungens, P. minimum, P. sulcata and P. micans were found at all stations. The RDA results showed that interactions between toxic and harmful microalgae and environmental factors were closely related to NO3-, WT, pH, DO, and NO2-. P. pungens, P. delicatissima, P. minimum, and S. trochoidea were prevalent under high-nutrient conditions. Considering the importance of East China Sea mariculture, the monitoring of nutrient conditions in the mariculture area is necessary.
6 DATA AVAILABILITY STATEMENTThe datasets generated or analyzed during the current study are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENTThe authors would like to thank Professors Yanan LU and Chengqi FAN from the East China Sea Fisheries Research Institute for their help during sampling and data collection.
Abassi S, Ki J S. 2022. Increased nitrate concentration differentially affects cell growth and expression of nitrate transporter and other nitrogen-related genes in the harmful dinoflagellate Prorocentrum minimum. Chemosphere, 288: 132526.
DOI:10.1016/j.chemosphere.2021.132526 |
Anderson D M, Cembella A D, Hallegraeff G M. 2012. Progress in understanding harmful algal blooms: paradigm shifts and new technologies for research, monitoring, and management. Annual Review of Marine Science, 4: 143-176.
DOI:10.1146/annurev-marine-120308-081121 |
Anderson D M, Glibert P M, Burkholder J M. 2002. Harmful algal blooms and eutrophication: nutrient sources, composition, and consequences. Estuaries, 25(4): 704-726.
DOI:10.1007/BF02804901 |
Bao Y L, Duan Y L, Yang N, et al. 2020. Comparison of community structure of large seaweed in mussel culture area of Shengsi Islands and intertidal zone of Xiasanhengshan Island. Marine Fisheries, 42(5): 595-607.
(in Chinese with English abstract) DOI:10.3969/j.issn.1004-2490.2020.05.009 |
Berdalet E, Fleming L E, Gowen R, et al. 2016. Marine harmful algal blooms, human health and wellbeing: challenges and opportunities in the 21st century. Journal of the Marine Biological Association of the United Kingdom, 96(1): 61-91.
DOI:10.1017/S0025315415001733 |
Berdalet E, Kudela R, Urban E, et al. 2017. GlobalHAB: a new program to promote international research, observations, and modeling of harmful algal blooms in aquatic systems. Oceanography, 30(1): 70-81.
DOI:10.5670/oceanog.2017.111 |
Berdalet E, Marrasé C, Estrada M, et al. 1996. Microbial community responses to nitrogen- and phosphorus-deficient nutrient inputs: microplankton dynamics and biochemical characterization. Journal of Plankton Research, 18(9): 1627-1641.
DOI:10.1093/plankt/18.9.1627 |
Brandenburg K M, Velthuis M, Van de Waal D B. 2019. Meta-analysis reveals enhanced growth of marine harmful algae from temperate regions with warming and elevated CO2 levels. Global Change Biology, 25(8): 2607-2618.
DOI:10.1111/gcb.14678 |
Chai Z Y, Huo Y Z, Yu K F, et al. 2013. Assessment of Sargassum vachellianum bed ecosystem health in GouqiIsland. Marine Environmental Science, 32(3): 386-389.
(in Chinese with English abstract) |
Chen J F, Xu N, Wang C H, et al. 2002. Dynamics of Pseudo-nitzschia spp. and environmental factors in Daya Bay, the South China Sea. Acta Scientiae Circumstantiae, 22(6): 743-748.
(in Chinese with English abstract) DOI:10.3321/j.issn:0253-2468.2002.06.011 |
Chen N S, Chen Y. 2021a. Advances in the study of biodiversity of phytoplankton and red tide species in China (II): the East China Sea. Oceanologia et Limnologia Sinica, 52(2): 363-384.
(in Chinese with English abstract) DOI:10.11693/hyhz20200900264 |
Chen N S, Cui Z M, Xu Q. 2021b. Advances in the study of biodiversity of phytoplankton and red tide species in China (IV): the Changjiang Estuary. Oceanologia et Limnologia Sinica, 52(2): 402-417.
(in Chinese with English abstract) DOI:10.11693/hyhz20200800236 |
Chen Q M, Zhang S Y, Lin J, et al. 2012. Growth conditions of Mytilus edulis Linnaeus and its relationship with environmental factors in Gouqi Island. Journal of Shanghai Ocean University, 21(5): 809-815.
|
Chen X X, Deng R R, He Z J, et al. 2001. Detection of red tide on remotely sensed satellite date and an approach to red tide forecast. Acta Scientiarum Naturalium Universitatis Sunyatseni, 40(2): 112-115.
(in Chinese with English abstract) DOI:10.3321/j.issn:0529-6579.2001.02.031 |
Chen Y, Xu Q, Gibson K, et al. 2021c. Metabarcoding dissection of harmful algal bloom species in the East China Sea off southern Zhejiang Province in late spring. Marine Pollution Bulletin, 169: 112586.
DOI:10.1016/j.marpolbul.2021.112586 |
Chen Y H, Gao Y H, Chen C P, et al. 2016. Seasonal variations of phytoplankton assemblages and its relation to environmental variables in a scallop culture sea area of Bohai Bay, China. Marine Pollution Bulletin, 113(1-2): 362-370.
DOI:10.1016/j.marpolbul.2016.10.025 |
Cui L, Lu X X, Dong Y L, et al. 2018. Relationship between phytoplankton community succession and environmental parameters in Qinhuangdao coastal areas, China: a region with recurrent brown tide outbreaks. Ecotoxicology and Environmental Safety, 159: 85-93.
DOI:10.1016/j.ecoenv.2018.04.043 |
Dai F F, Zhou C X, Yan X J. 2011. Effect of pH and light on population growth and activity of extracellular carbonic anhydrase in two species of dinoflagellates. Marine Environmental Science, 30(5): 694-698.
(in Chinese with English abstract) DOI:10.3969/j.issn.1007-6336.2011.05.020 |
Fan C L, Glibert P M. 2005. Effects of light on nitrogen and carbon uptake during a Prorocentrum minimum bloom. Harmful Algae, 4(3): 629-641.
DOI:10.1016/j.hal.2004.08.012 |
Fei Y J, Jiang H. 2008. Study on correlation of Chaetoceros red tide and environmental factors in Zhujiajian area. Marine Environmental Science, 27(S1): 38-41.
|
Furuya K, Iwataki M, Lim P T et al. 2018. Overview of harmful algal blooms in Asia. In: Glibert P M, Berdalet E, Burford M A et al eds. Global Ecology and Oceanography of Harmful Algal Blooms. Springer, Cham. p. 289-308.
|
Glibert P M, Burkholder J M. 2011. Harmful algal blooms and eutrophication: "strategies" for nutrient uptake and growth outside the Redfield comfort zone. Chinese Journal of Oceanology and Limnology, 29(4): 724-738.
DOI:10.1007/s00343-011-0502-z |
Glibert P M, Mayorga E, Seitzinger S. 2008. Prorocentrum minimum tracks anthropogenic nitrogen and phosphorusinputs on a global basis: application of spatially explicit nutrient export models. Harmful Algae, 8(1): 33-38.
DOI:10.1016/j.hal.2008.08.023 |
Huang C X, Chen H R, Li C. 2020. Characteristics of red tide distribution in Fujian coastal waters in 2000-2018. Journal of Applied Oceanography, 39(4): 542-550.
(in Chinese with English abstract) DOI:10.3969/J.ISSN.2095-4972.2020.04.010 |
Jiang Z B, Gao Y X, Chen Y, et al. 2019. Spatial heterogeneity of phytoplankton community shaped by a combination of anthropogenic and natural forcings in a long narrow bay in the East China Sea. Estuarine, Coastal and Shelf Science, 217: 250-261.
DOI:10.1016/j.ecss.2018.11.028 |
Kiørboe T, Mølenberg F, Nøhr O. 1980. Feeding, particle selection and carbon absorption in Mytilus edulis in different mixtures of algae and resuspended bottom material. Ophelia, 19(2): 193-205.
DOI:10.1080/00785326.1980.10425516 |
Langdon C J, Waldock M J. 1981. The effect of algal and artificial diets on the growth and fatty acid composition of Crassostrea gigas Spat. Journal of the Marine Biological Association of the United Kingdom, 61(2): 431-448.
DOI:10.1017/S0025315400047056 |
Legendre P, Gallagher E D. 2001. Ecologically meaningful transformations for ordination of species data. Oecologia, 129(2): 271-280.
DOI:10.1007/s004420100716 |
Li H Y, Chen T, Zhang H Y, et al. 2014. Evaluation of contribution of shellfish culture to ocean carbon cycle in China. Marine Sciences, 38(5): 39-45.
(in Chinese with English abstract) |
Li X D. 2012. Analysis on characteristics of red tide in Fujian coastal waters during the last 10 years. Environmental Science, 33(7): 2210-2216.
|
Li Y Z. 2000. Analysis on the water quality state and the countermeasures for the pollution control in Dongshan Bay of Fujian. Marine Environmental Science, 19(1): 64-67.
(in Chinese with English abstract) DOI:10.3969/j.issn.1007-6336.2000.01.015 |
Liao K, Yang Z Y. 2019. Analysis on the comparative advantage of mariculture in China. Chinese Fisheries Economics, 37(5): 22-29.
(in Chinese with English abstract) DOI:10.3969/j.issn.1009-590X.2019.05.005 |
Lin Y T. 2013. A preliminary study of nutrients distribution in Pseudosciaena crocea net-cage culture area of Sandu Bay. Journal of Fujian Fisheries, 35(3): 211-217.
(in Chinese with English abstract) DOI:10.3969/j.issn.1006-5601.2013.03.007 |
Liu P R, Huang X Y, Ke D. 1999. The general report of red tide mechanisms and prediction. Marine Forecasts, 16(4): 46-51.
(in Chinese with English abstract) |
Lü S H, Chen H L, He Z Q. 2006. The Effects of different c(N)/c(P) ratios on the growth of Pseudo-nitzschia pungens. Ecology and Environment, 15(4): 697-701.
(in Chinese with English abstract) DOI:10.3969/j.issn.1674-5906.2006.04.008 |
McQuoid M R, Nordberg K. 2003. The diatom Paralia sulcata as an environmental indicator species in coastalsediments. Estuarine, Coastal and Shelf Science, 56(2): 339-354.
DOI:10.1016/S0272-7714(02)00187-7 |
Mu J D, Zheng X R, Zhao Z L, et al. 2015. Ecological characteristics of phytoplankton in Qinhuangdao coastal areas during the red-tide period. Journal of Fishery Sciences of China, 22(2): 288-301.
(in Chinese with English abstract) DOI:10.3724/SP.J.1118.2015.14243 |
Oksanen J, Blanchet F G, Friendly M et al. 2020. Vegan: community ecology package. R package version 2.5-7. https://CRAN.R-project.org/package=vegan. Accessed on 2021-01-20.
|
Ouyang Y R, Chen Y H, Yu B. 1993. Studies on phytoplankton and red tide organizms in mariculture area around Shengsi Islands, China. Journal of Zhejiang College of Fisheries, 12(4): 257-264.
(in Chinese with English abstract) |
Pan K H, Jiang G X. 2004. The occurrence, ecological effects of HAB and countermeasures against it. Periodical of Ocean University of China, 34(5): 781-786.
(in Chinese with English abstract) DOI:10.3969/j.issn.1672-5174.2004.05.034 |
Rouillon G, Rivas J G, Ochoa N, et al. 2005. Phytoplankton composition of the stomach contents of the mussel Mytilus edulis L. from two populations: comparison withits food supply. Journal of Shellfish Research, 24(1): 5-14.
DOI:10.2983/0730-8000(2005)24[5:PCOTSC]2.0.CO;2 |
Schmidt L E, Hansen P J. 2001. Allelopathy in the prymnesiophyte Chrysochromulina polylepis: effect of cell concentration, growth phase and pH. Marine Ecology Progress Series, 216: 67-81.
DOI:10.3354/meps216067 |
Sierra-Beltrán A P, Cortés-Altamirano R, Cortés-Lara M C. 2005. Occurrences of Prorocentrum minimum (Pavillard) in México. Harmful Algae, 4(3): 507-517.
DOI:10.1016/j.hal.2004.08.018 |
Su J Z, Gao J, Su Y P, et al. 2020. Study on the factors affecting the proliferation of Karenia mikimotoi in Pingtan coastal area of Fujian Province. Journal of Fujian Normal University (Natural Science Edition), 36(4): 43-49, 56.
(in Chinese with English abstract) DOI:10.12046/j.issn.1000-5277.2020.04.007 |
Tan K S, Ransangan J. 2017. Feeding behaviour of green mussels, Perna viridis farmed in Marudu Bay, Malaysia. Aquaculture Research, 48(3): 1216-1231.
DOI:10.1111/are.12963 |
Tang F, Jiang X M, Wang T, et al. 2013. Dynamic of phytoplankton in typical sea areas of Zhoushan. Marine Environmental Science, 32(1): 67-72.
|
Wang D Z. 2008. Neurotoxins from marine dinoflagellates: a brief review. Marine Drugs, 6(2): 349-371.
DOI:10.3390/md6020349 |
Wang S F, Tang D L, He F L, et al. 2008. Occurrences of harmful algal blooms (HABs) associated with ocean environments in the South China Sea. Hydrobiologia, 596(1): 79-93.
DOI:10.1007/s10750-007-9059-4 |
Wang Z F, Zhang Q, Lu H Y. 2001. Effects of temperature, salinity, light and pH on the growth of red tide organisms Prorocentrum micans. Oceanologia et Limnologia Sinica, 32(1): 15-18.
(in Chinese with English abstract) DOI:10.3321/j.issn:0029-814X.2001.01.003 |
Wickham H, Chang W, Henry L et al. 2020. ggplot2: create elegant data visualisations using the grammar of graphics. https://CRAN.R-project.org/package=ggplot2. Accessed on 2020-12-22.
|
Xu C Y, Huang M Z, Du Q. 2010. Ecological characteristics of important red tide species in Fujian coastal waters. Journal of Oceanography in Taiwan Strait, 29(3): 434-441.
(in Chinese with English abstract) DOI:10.3969/J.ISSN.1000-8160.2010.03.020 |
Xu Z H, Hou J J. 2006. Characteristics of harmful algal bloom and its prevention in Fujian coastal waters. Journal of Oceanography in Taiwan Strait, 25(1): 143-150.
|
Xu Z L, Gu X G, Wang Y L, et al. 1992. Ecological eigenvalue analyses of plankton of red tides occurred in Xiangshan Sound, Zhejiang. Marine Science Bulletin, 11(5): 46-53.
(in Chinese with English abstract) |
Yang J L, Li X L, Yu X, et al. 2019. Changes of environmental factors and their effects on phytoplankton in scallop culture area of Laizhou Bay. Journal of Yantai University (Natural Science and Engineering Edition), 32(1): 38-46.
(in Chinese with English abstract) DOI:10.13951/j.cnki.37-1213/n.2019.01.008 |
Yang Y, Sun J, Guan X Y, et al. 2016. Seasonal variation of netz-phytoplankton community in Bohai Sea. Marine Science Bulletin, 35(2): 121-131.
(in Chinese with English abstract) DOI:10.11840/j.issn.1001-6392.2016.02.001 |
Yao Q, Miao X Y. 2021. Prediction of dissolved oxygen and ammonia nitrogen concentrations in aquaculture environment based on PCA and GA-LM. Journal of Dalian Fisheries University, 36(5): 851-858.
(in Chinese with English abstract) DOI:10.16535/j.cnki.dlhyxb.2021-082 |
Yoo K I. 1991. Population dynamics of dinoflagellate community in Masan Bay with a note on the impact of environmental parameters. Marine Pollution Bulletin, 23: 185-188.
DOI:10.1016/0025-326X(91)90672-F |
Yu J, Xu Q Q, Liu W H, et al. 2016. Response of radial growth to climate change for Larix olgensis along an altitudinal gradient on the eastern slope of Changbai Mountain, Northeast China. Chinese Journal of Plant Ecology, 40(1): 24-35.
(in Chinese with English abstract) DOI:10.17521/cjpe.2015.0216 |
Yuan M L, Wang Z H, Yang Y F. 2009. Study advances in harmful alga Chattonella biecheler (raphidophyceae). Marine Sciences, 33(11): 100-104.
(in Chinese) |
Zhang C, Zou J Z. 1997. Nutrient uptake kinetics and growth under nutrient limitation of Pseudonitzschia. Oceanologia et Limnologia Sinica, 28(6): 599-603.
|
Zhang L H, Zhang X L, Zhu M Y. 2008. Preliminary study on selective feeding of the scallop (Chlamys farreri) on diatom and dinoflagellate cells. Advances in Marine Science, 26(3): 372-376.
|
Zhang S Y, Wang Z H, Lin J, et al. 2007. Variation of fisheries resources in summer and autumn in seaweed beds of Gouqi Island. Marine Fisheries Research, 28(1): 45-52.
(in Chinese with English abstract) DOI:10.3969/j.issn.1000-7075.2007.01.008 |
Zhang Y Y, Gao Y H, Liang J R, et al. 2010. Diatom diet selectivity by early post-larval abalone Haliotis diversicolor supertexta under hatchery conditions. Chinese Journal of Oceanology and Limnology, 28(6): 1187-1194.
DOI:10.1007/s00343-010-0019-x |
Zhao C J, Liu X Z, Fu S J, et al. 2020. Variation characteristics of the evolution of Karenia mikimotoi bloom and environmental factors based on online monitoring buoy data. Journal of Tropical Oceanography, 39(2): 88-97.
(in Chinese with English abstract) DOI:10.11978/2019027 |
Zheng H D. 2009. Species composition and distribution of zooplankton in Dongshan Bay. Journal of Fujian Fisheries, (2): 11-17.
(in Chinese with English abstract) DOI:10.3969/j.issn.1006-5601.2009.02.003 |
Zheng Q H. 2007. Studies on the aquatic environment pollution and variation trends of surface layer at the aquaculture region, Sangdu Bay. Journal of Fujian Fisheries, (4): 26-30.
(in Chinese with English abstract) DOI:10.14012/j.cnki.fjsc.2007.04.002 |
Zhou L L, Ping X Y, Li L, et al. 2018. On characteristics and environmental effects of phytoplankton community structure in the coast of Pseudosciaena crocea copper seine net cage culture. Marine Fisheries, 40(4): 413-423.
(in Chinese with English abstract) DOI:10.3969/j.issn.1004-2490.2018.04.004 |
Zhou M J, Zhu M Y. 2006. Progress of the project "ecology and oceanography of harmful algal blooms in China". Advances in Earth Science, 21(7): 673-679.
|
Zhou M J, Zhu M Y, Zhang J. 2001. Status of harmful algal blooms and related research activities in China. Chinese Bulletin of Life Sciences, 13(2): 54-59, 53.
(in Chinese with English abstract) |
Zingone A, Enevoldsen H O. 2000. The diversity of harmful algal blooms: a challenge for science and management. Ocean & Coastal Management, 43(8-9): 725-748.
DOI:10.1016/S0964-5691(00)00056-9 |
Zohdi E, Abbaspour M. 2019. Harmful algal blooms (red tide): a review of causes, impacts and approaches to monitoring and prediction. International Journal of Environmental Science and Technology, 16(3): 1789-1806.
DOI:10.1007/s13762-018-2108-x |
 2022, Vol. 40
2022, Vol. 40


