Institute of Oceanology, Chinese Academy of Sciences
Article Information
- HE Xiuping, CHEN Junhui, WANG Jiuming, SUN Xia, XIN Ming, WANG Baodong, LIANG Shengkang
- Spatial variation of lipophilic marine algal toxins and its relationship with physicochemical parameters in spring in Laizhou Bay, China
- Journal of Oceanology and Limnology, 40(6): 2242-2255
- http://dx.doi.org/10.1007/s00343-022-1426-5
Article History
- Received Feb. 21, 2022
- accepted in principle Mar. 31, 2022
- accepted for publication Mar. 3, 2022
2 Laboratory for Marine Ecology and Environmental Science, Pilot National Laboratory for Marine Science and Technology(Qingdao), Qingdao 266237, China;
3 Qingdao Key Laboratory of Analytical Technology Development and Standardization of Chinese Medicines, Qingdao 266590, China;
4 Shandong University of Science and Technology, Qingdao 266590, China;
5 Key Laboratory of Marine Chemistry Theory and Technology, Ministry of Education, Ocean University of China, Qingdao 266100, China
Marine algal toxins are a type of secondary metabolites produced by some marine microalgae. In solubility, these toxins can be divided into hydrophilic marine algal toxins and lipophilic marine algal toxins (LMATs). Among the 300 types of marine algal toxins identified to date, LMATs account for more than 90% of the total toxins and are widely distributed in the coastal areas worldwide (Wang et al., 2015). LMATs include mainly okadaic acid (OA) and its derivatives, yessotoxins (YTXs), azaspiracids (AZAs), pectenotoxins (PTXs), cyclic imines (CIs), and brevetoxins (BTXs) (Gerssen et al., 2011; Chen et al., 2018). LMATs have strong hydrophobicity and excellent stability, and are easily absorbed by fishery organisms, such as shellfish through filter feeding and then accumulate in the body. Therefore, the threat and impact of LMATs on fishery production and human health cannot be ignored (Reguera et al., 2014; Li et al., 2018).
In the past decades, the widespread distribution of LMATs affect global ecosystem considerably. Europe is the most seriously affected by LMATs (Nicolas et al., 2017). For example, a serious diarrhetic shellfish poisoning (DSP) event occurred in Greece in 2000, in which more than 200 people poisoned, causing huge economic losses in the shellfish industry (Koukaras and Nikolaidis, 2004). In 2002, a large-scale DSP incident caused the poisoning of 403 people (De Schrijver et al., 2002). In 2009, 11 DSP-related poisoning events occurred in France, resulting in the poisoning of 45 people (Hossen et al., 2011). In 2010, more than 300 people in Italy were poisoned by shellfish contaminated with high levels of OA (Visciano et al., 2016). North and South America also suffered from shellfish poisoning. For examples, the United States (Trainer et al., 2013), Canada (Taylor et al., 2013), Chile (Reguera et al., 2014; Nicolas et al., 2017), and Brazil (Mafra et al., 2019) had algal toxin poisoning cases in recent years. In Asia, the impact of LMATs, especially OAs and PTXs, is highly serious (Reguera et al., 2014). A number of poisoning incidents caused by LMATs have been recorded in China. The most serious incidents occurred in Zhejiang and Fujian Province, China in May 2011, in which more than 200 people were poisoned after eating blue mussel contaminated by OA and DTX1 in high concentration of 215 and 195 μg/100 g, respectively (Jiang et al., 2017). LMAT-related contamination events have also been reported in Korea (Kim et al., 2010), Singapore (Holmes and Teo, 2002), Indonesia (Likumahua et al., 2020), Australia (MacKenzie et al., 2002; Madigan et al., 2006), New Zealand (Grattan et al., 2016; Mackenzie, 2019), Morocco, and Tunisia (Elgarch et al., 2008; Armi et al., 2012). Hence, fishery organisms of many coastal countries in the world have been contaminated by LMATs, indicating that LMATs have become a global concern that affects mariculture industries and public health. However, the comprehensive monitoring of LMATs in the coastal aquatic environment remains limited, and the detection of algal toxins is focused on mariculture organisms, such as scallops and clams (Suzuki et al., 2005; Farabegoli et al., 2018). Such method is complicated and time-consuming, and cannot provide an early warning of the contamination risk from algal toxins in the shellfish and aquatic environment. During survival and competition, toxin-producing algae often secrete toxins out of the cell, and monitoring of extracellular toxins in seawater is more efficient and faster (Lefebvre et al., 2008; Pan et al., 2017). In addition, the seawater is an important medium for the survival of marine organisms. To promote the healthy development of aquaculture, it is important to secure the safety of coastal aquatic environment.
In recent years, with the intensification of eutrophication and the impact of global climate change, the frequency of harmful algal blooms has gradually increased (Hallegraeff, 2010), and the number of toxin-producing algae has been involved to a certain extent, resulting in variation in the content of algal toxins in marine environment, especially in spring (Yu and Liu, 2016; Jiang et al., 2017; Wu et al., 2019). Studies on the environmental factors affecting the toxin-producing algae have been increasing gradually. Ajani et al. (2016) constructed a model and analyzed the abundance and environmental factors of Dinophysis in South Australia for 12 years. They found that the abundance of D. acuminata was closely related to seasonal change, thermal stratification, and nutrients supply, while the abundance of D. caudata was associated with nutrients, salinity, and dissolved oxygen. Current studies on the effect of temperature on the growth and toxin production of toxin-producing algae generally believe that the increase in temperature can promote the growth of toxigenic alga and then increase the total toxin production. However, the temperature was not positively correlated with the amount of cellular toxin production of individual toxigenic alga (Kamiyama et al., 2010; Basti et al., 2015, 2018). Hattenrath-Lehmann and Gobler (2015) found that organic and inorganic nitrogen could promote the growth rate of D. acuminata, rather than the nutrient stimulation of the growth of its prey (Mesodinium rubrum), which in turn stimulated the growth of D. acuminata. The above investigations show that the study on environmental factors affecting the toxin-producing algae have mainly focused on laboratory cultivation. Studies on actual aquatic environment, especially in mariculture zones, are lacking. In fact, a significant difference exists between the actual environment and the laboratory cultivation. Therefore, a reliable conclusion based only on the results of laboratory cultivation is difficult to achieve.
To date, no systematic studies have been conducted on the occurrence of LMATs and its relationship to physicochemical parameters in the coastal mariculture bay in spring. In spring season, algal toxins often contaminate mariculture shellfish seriously. To better understand this problem, LMATs and various physiochemical parameters in a typical mariculture bay (Laizhou Bay) were investigated to: (1) clarify the composition, concentration level, and distribution characteristics of LMATs in the aquatic environment (seawater and phytoplankton) of Laizhou Bay and (2) probe into the relationship between the distribution of LMATs and environmental factors during spring.
2 MATERIAL AND METHOD 2.1 Reagent and materialGlass fiber filters (GF/F, 0.7 μm, 47 mm; GF/A, 1.6 μm, 47 mm) were purchased from Whatman (Maidstone, England). The nylon membrane filter (0.22 μm) was supplied by Jinteng (Tianjin, China). SPE cartridges (Oasis HLB: 6 mL, 200 mg, 30 μm) were purchased from Waters (Milford, MA, USA). HPLC-grade acetonitrile and methanol were purchased from Merck (Darmstadt, Germany). LC/MS-grade ammonium hydroxide (≥25%) was obtained from Fluka (St. Louis, MO, USA). The certified reference standards of OA, dinophysistoxin1 (DTX1), YTX, AZA1, AZA2, gymnodinium (GYM), 13-desmethyl spirolide C (SPX1), and PTX2 were obtained from the National Research Council of Canada (Halifax, Nova Scotia, Canada). Ultrapure water was prepared using an in-house Milli-Q water purification system (Millipore Ltd, USA). Methanol was used as the dilution solvent to dilute OA, PTX2, DTX1, YTX, AZA1, AZA2, SPX1, and GYM to concentrations of 200.00, 220.35, 21.29, 245.83, 64.84, 61.21, 352.87, and 125.00 μg/L, respectively. The working solution was prepared by mixing all eight LMAT solutions together using methanol. All solutions were stored at -20 ℃ before use.
2.2 Study area and sample collectionLaizhou Bay is located in the south of the Bohai Sea and north of the Shandong Peninsula. It starts from the Huanghe (Yellow) River mouth in the west and ends at the Qimu Cape in Longkou in the east. The total area of Laizhou Bay is 6 966.93 km2, making it one of the three major bays in the Bohai Sea. The average water depth of Laizhou Bay is below 10 m. The Huanghe River, Xiaoqing River, Weihe River, Jiaolai River, Zhimai River, and Bailang River discharge into the bay with abundant nutrients and fresh water (Zhang and Gao, 2015; Guo et al., 2017).
Surface seawater and phytoplankton samples were collected from Laizhou Bay in May 2019. (Fig. 1). Forty-three seawater samples and 18 phytoplankton samples were collected (Supplementary Table S1). All seawater samples were collected from a depth of approximately 0.5 m from the surface by using a stainless steel water sampler. The samples were stored in polyethylene terephthalate bottles at 4 ℃ in dark. Phytoplankton samples were collected using a double-layer net (diameter, 25 cm; upper layer: 200-μm mesh size and 0.5 m long; lower layer: 20-μm mesh size and 1.4 m long) by vertical net hauls from 15 m to the surface. If the water depth was less than 15 m, the trawling from the bottom to the surface was repeated until the total depth was 15 m. All samples were transported to laboratory for processing and analysis within 24 h after collection.
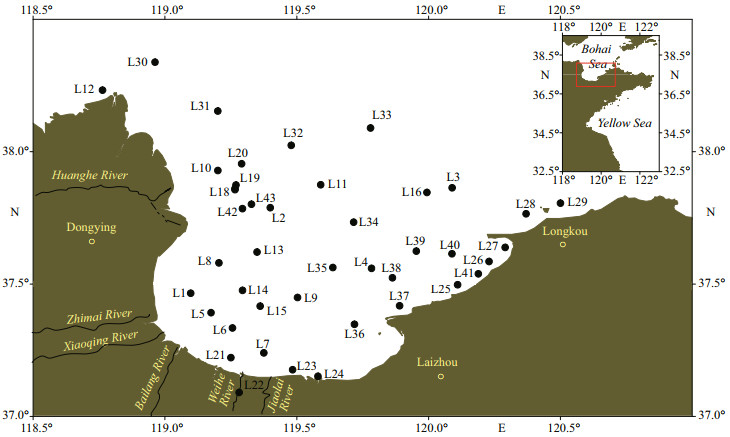
|
| Fig.1 Sampling stations in Laizhou Bay of the Bohai Sea, China |
Seawater samples were filtered using 0.7-μm GF/F filters to remove the particles for LMATs, chlorophyll a (chl a), and nutrients analysis. Filtered seawater samples for the analysis of LMATs were extracted using Oasis HLB SPE column as described in our previous study (Chen et al., 2018). Phytoplankton samples were filtered on the GF/A filter, and the filter was freeze-dried and weighed. The LMATs on the filter were extracted using 10 mL of methanol and ultrasonicated for 10 min. This step was repeated thrice. The extracts were merged and rotary-evaporated at 40 ℃ to dry. The obtained extracts were re-dissolved in 1 mL of methanol and then filtered with 0.22-μm nylon filter for detection by liquid chromatography-tandem mass spectrometry (LC-MS/MS).
Seawater physiochemical parameters, including temperature, salinity, and pH, were determined in situ using a Seabird 911 conductivity-temperature-depth sensor (Seabird Electronics, Bellevue, WA, USA). DO was determined using the Winkler titration method. Nutrients, including PO4-P, SiO3-Si, NO3-N, NO2-N, and NH4-N were manually analyzed by spectrophotometry method after filtration. The suspended particulate matter (SPM) was calculated using a gravimetric method. The content of chl a was filtered on the GF/F filters and extracted with 90% (v꞉v) acetone aqueous solution. A Turner Designs TD-700 fluorometer was used for chl-a determination. All these methods were in accordance with that developed by Parsons et al. (1984). Dissolved inorganic N (DIN) was the total of NO3-N, NO2-N, and NH4-N.
A 1200 series HPLC system coupled with a 6320 series ion-trap mass spectrometer (Agilent, USA) consisting of an electrospray ionization (ESI) interface was employed to analyze the LMATs. A ZORBAX Extend-C18 column (3 mm×150 mm, 3.5 μm, Agilent, USA) was used for the chromatographic separation at 20±2 ℃. Water (A) and 90% aqueous acetonitrile solution (B) which both contained 6.6-mmol/L ammonium hydroxide, were used for the mobile phase with a flow rate of 0.4 mL/min. The elution process was as follows: 0-15-20-45-50 min, 20%-30%-47.5%-100%-100%B. The LMATs were detected in multiple reaction monitoring mode by using five retention time windows (Supplementary Table S2). The MS conditions were as follows: electrospray ion source, capillary voltage, 4.5 kV; nebulizer pressure, 40 psi; drying gas flow rate, 11 L/min; drying gas temperature, 350 ℃; and scan range, 100–1 300 m/z. LMATs with standards (OA, DTX1, PTX2, YTX, AZA1, AZA2, GYM, and SPX1) were determined using the external standard method and those without standards (DTX2, PTX2 SA, 7- epi-PTX2 SA, and PTX11) were semi-quantitatively. Specifically, the concentrations of PTX2 SA, 7-epi-PTX2 SA, and PTX11 were quantified according to the standard curve of PTX2, while that of DTX2 was quantified according to the standard curve of DTX1.
2.4 Quality assurance and quality controlDuring the whole experiment, ultrapure water and methanol were used to rinse all containers for 2–3 times to prevent exogenous contamination. For every 10 samples, the solvent blank and calibration check standards were used to ensure reliable results. Three times signal to noise ratio (S/N) and ten times S/N were defined as the limit of detection (LOD) and the limit of quantification (LOQ), respectively. The LODs of the LMATs were 0.012 ng/L (OA), 0.056 ng/L (DTX1), 0.017 ng/L (PTX2), 0.025 ng/L (GYM), 0.053 ng/L (AZA1), 0.017 ng/L (AZA2), 0.064 ng/L (YTX), and 0.023 ng/L (SPX1). The corresponding LOQs were 0.04, 0.187, 0.057, 0.083, 0.177, 0.057, 0.213, and 0.077 ng/L. Recovery of the target LMATs in the seawater and phytoplankton samples ranged from 75.5% to 103.8% and from 85.3% to 105.2%, respectively.
2.5 Statistical analysisThe sampling station map and the contour map of the distribution of LMATs were drawn using Ocean Data View (ODV 4). The Statistical Package for the Social Sciences version 25 (IBM Corporation, Armonk, NY, USA) was used to analyze the correlations between the LMATs and seawater physiochemical parameters by using the Pearson correlation coefficient. The quality of fit was determined by the correlation coefficients (R) and probabilities (P). Differences were considered to be statistically significant when P < 0.05 and extremely significant when P < 0.01. Origin 8.5 (OriginLab Corporation, USA) was employed to explain the variations in the composition and concentrations of individual LMATs at each station. The total concentrations of all LMATs were expressed as the sum of the concentration levels of all detected LMATs (ΣLMATs). To calculate the ΣLMATs, concentrations below the LOD and the not-found data were all assigned as zero. Concentrations below the LOQ were calculated as half of the LOQ for the ΣLMATs and individual analysis. For LMATs with standard substances, external standard method was used for quantification.
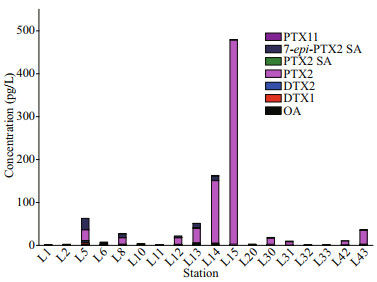
3 RESULT AND DISCUSSION 3.1 Composition and concentration of LMATs in the seawater of Laizhou BayThe composition and concentration of LMATs in seawater are shown in Fig. 2. Eight out of twelve LMATs, namely, OA, PTX2, DTX1, DTX2, PTX2 SA, 7-epi-PTX2 SA, SPX1, and PTX11 were detected from the surface seawater of Laizhou Bay during spring. The composition of LMATs in the seawater of Laizhou Bay during spring was more abundant than that during summer (He et al., 2020). Among the eight detected LMATs, OA, DTX1, and PTX2 SA were detected in all collected seawater samples. PTX2 could be detected at all stations, except at the L14 station. The detection frequencies of DTX2, 7-epi-PTX2 SA, PTX11, and SPX1 were 53.33%, 25.58%, 4.65%, and 2.33%, respectively. SPX1 was detected at the L31 station only, and PTX11 was detected at the L14 and L15 stations only. Besides, the concentrations of SPX1 and PTX11 just reached the LOQ. Therefore, SPX1 and PTX11 were only used as reference values here. The results indicate that OA, DTX1, PTX2, and PTX2 SA were common toxins in Laizhou Bay during spring. It is found that PTX group and OA group toxins are the most commonly found toxins in the coastal water of China, which is consistent with many other reports (Li et al., 2014, 2016, 2017; Chen et al., 2017, 2018; He et al., 2019, 2020; Wu et al., 2019; Wang et al., 2021).
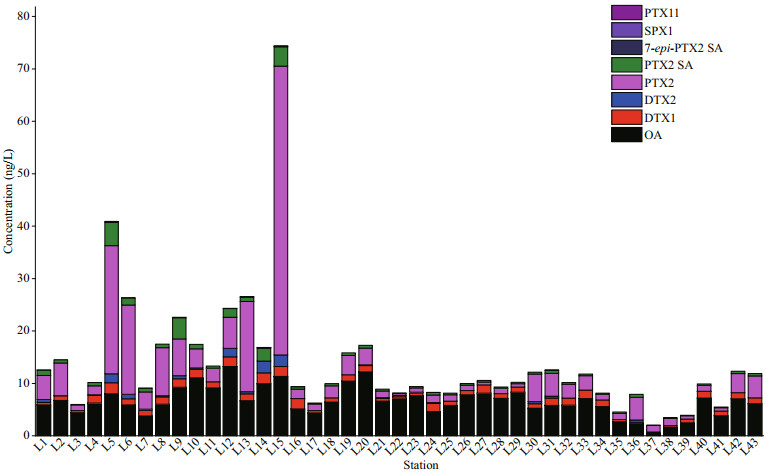
|
| Fig.2 The composition and concentration of individual lipophilic marine algal toxins in seawater at each station |
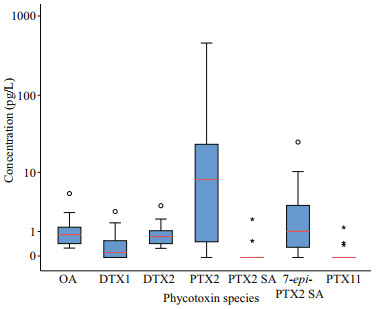
The concentration of OA ranged 0.53–13.23 ng/L on average of 6.58 ng/L. It is indicated that the surface seawater of Laizhou Bay was dominated by OA during spring. The concentration of PTX2 in the surface seawater of Laizhou Bay ranged from not detected (ND) to 55.14 ng/L on average of 4.98 ng/L, which was the second to that of OA. As shown from Fig. 3, the interquartile range of PTX2 was larger than that of OA, indicating that the fluctuation range of PTX2 content was larger than that of OA and was also the highest value. The concentrations of DTX1, DTX2, and PTX2 SA were in the ranges of 0.18–2.11, 0–2.23, and 0.07–4.48 ng/L, on average of 1.10, 0.30, and 0.75 ng/L, respectively. These values are all within the interquartile range, except for higher values in some stations (Fig. 3). The median value of DTX1 was 1.16 ng/L, above the average value. However, the median value of DTX2 was only 0.03 ng/L, indicating that DTX2 was not detected at most stations. The content of PTX2 SA in the Laizhou Bay was relatively stable. The contents of 7-epi-PTX2 SA, SPX1, and PTX11 at each station were all less than 1.00 ng/L. Thus, these contents were not considered in this study.
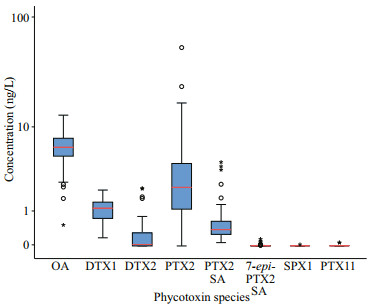
|
| Fig.3 Individual lipophilic marine algal toxins concentrations in surface seawater of Laizhou Bay Red lines represent the median concentration; the box height represents the interquartile range (IQR, 25%–75%), the circles and small stars are outliers, and the black vertical line represents the range of 1.5 IQR within the maximum and minimum interquartile values. |
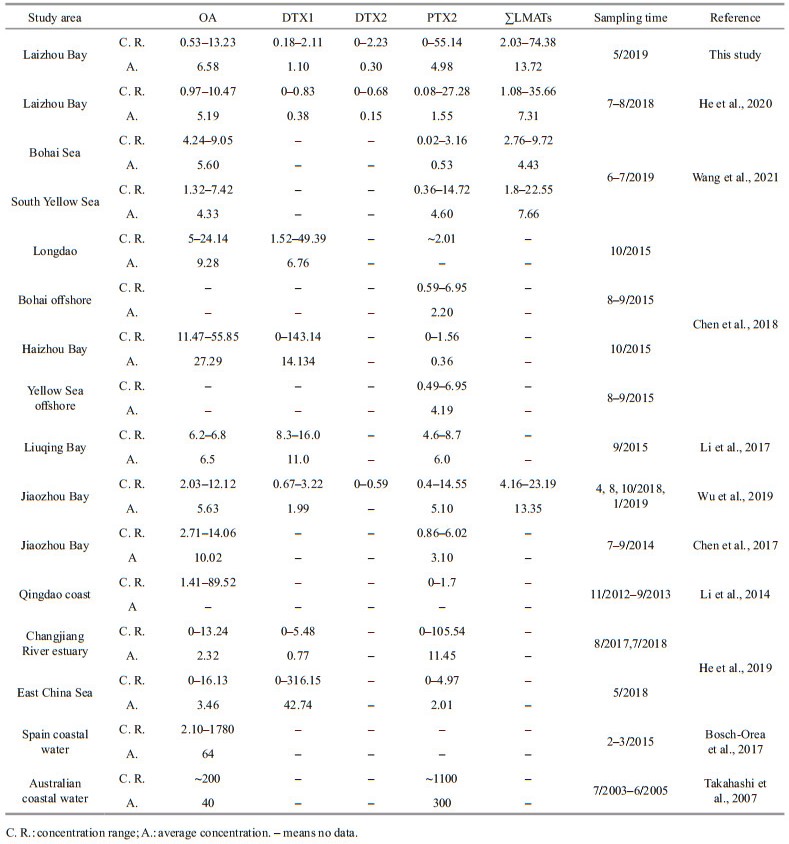
The concentrations of LMATs worldwide are listed in Table 1 (only samples prepared by the SPE method are listed). As seen in Table 1, the average and maximum contents of all LMATs detected in the seawater during spring were all higher than those during summer in the Laizhou Bay (He et al., 2020). The result was contrary to those found in Jiaozhou Bay and Yellow Sea (Li et al., 2014; Wu et al., 2019), where the highest average contents of LMATs were found during summer. The difference may be due to the distinct research areas with different environmental factors and toxin-producing algae. The average concentrations of OA and PTX2 during spring in Laizhou Bay were both higher than those in the offshore of Bohai Sea and Yellow Sea (Chen et al., 2018; Wang et al., 2021) and the coastal area of East China Sea (He et al., 2019). However, the average concentration of OA was lower than those in Longdao on the west coast of Bohai Sea and Haizhou Bay of the Yellow Sea (Chen et al., 2018), but equivalent to the average value in Liuqing River of the Yellow Sea (Li et al., 2017). Moreover, the OA content in the Laizhou Bay during spring was markedly lower than those in the coastal waters of Spain (Bosch-Orea et al., 2017) and Australia (Takahashi et al., 2007). The average content of PTX2 in Laizhou bay was lower than those in Jiaozhou Bay (Wu et al., 2019), Liuqing Bay (Li et al., 2017), and Changjiang (Yangtze) River estuary (He et al., 2019). This toxin was also significantly lower than that in the offshore waters in Australia (Takahashi et al., 2007). As shown in Table 1, the concentration range and average value of DTX1 during spring were higher than those during summer in Laizhou Bay (He et al., 2020), the offshore of Bohai Sea and the Yellow Sea (Chen et al., 2018; Wang et al., 2021), and the Changjiang River estuary (He et al., 2019). However, the content of DTX1 in the surface seawater of Laizhou Bay during spring was lower compared with those in the nearshore of Bohai and Yellow Sea (Li et al., 2017; Chen et al., 2018; Wu et al., 2019) and the coast of East China Sea (He et al., 2019). The contents of ∑LMATs ranged 2.03–74.38 ng/L on average of 13.72 ng/L. The content level of ∑LMATs during spring was higher than those of summer in Laizhou Bay (He et al., 2020), Bohai Sea, South Yellow Sea (Wang et al., 2021), and Jiaozhou Bay (Wu et al., 2019). Therefore, OA, PTX2, and DTX1 were the dominant LMATs in the Laizhou Bay in spring. Compared with other coastal waters of China, the composition of LMATs in the Laizhou Bay was more abundant, but the content level was lower than those in most coastal waters in China, and considerably lower than those in the coastal waters of Europe and Australia.
|
The spatial distribution of LMATs in the seawater of Laizhou Bay during spring is shown in Fig. 4. As shown in Fig. 4a, the highest concentration of OA was found in the northern Huanghe River estuary (L12), followed by those in the southern Huanghe River estuary (L20) and the inner bay (L15). The high-concentration area of PTX2 (Fig. 4b) was located near the L15 and L5 stations at the mouth of Xiaoqing River, which was the same as the area with the second highest value of OA. The concentration of DTX1 in the whole bay fluctuated slightly, and only the L5 and L14 stations in the southwest had a slightly higher DTX1 content (Fig. 4c), which is similar to the distribution of PTX2. The distribution of DTX2 (Fig. 4d) was approximately the same as that of OA. The high values were detected at the mouth of the Huanghe River and the inner bay near L14 and L15 stations. The high value of PTX2 SA (Fig. 4e) was distributed mainly near the Huanghe River estuary. Given the low detection rates and contents of 7-epi-PTX2 SA, SPX1, and PTX11, their distributions were not analyzed. The maximum value of ∑LMATs (Fig. 4f) appeared near station L15 near the Xiaoqing River estuary, which is in the southwest of the Laizhou Bay. The second highest value area of ∑LMATs was detected near station L12 in the northern Huanghe River estuary, and the low value area of ∑LMATs was near station L37 located east of the bay. Overall, the content of LMATs in Laizhou Bay during spring was higher in the Huanghe River estuary and southwest of the inner bay.
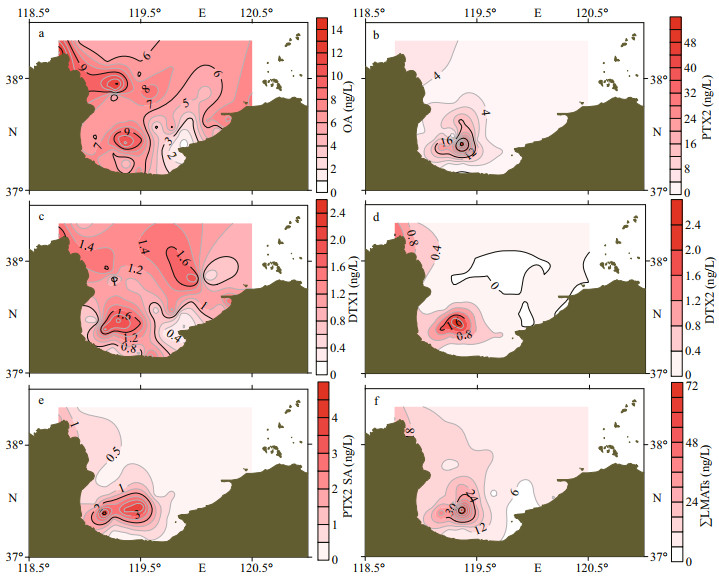
|
| Fig.4 The spatial distribution of lipophilic marine algal toxins in seawater during spring |
The unique distribution characteristics of LMATs in seawater of Laizhou Bay is mainly affected by the joint effect of terrestrial input (Huanghe River, Xiaoqing River, and Weihe River) and clockwise circulation in the bay. The low-nutrient tongue outside the bay intruded into the bay from the mouth, and the high-nutrient tongue from the Huanghe River, Xiaoqing River, and Weihe River at the top and southwest formed a clockwise extension trend in the southwest direction of the bay (Zhao et al., 1995; Huang et al., 1998; Sun, 2006). The strong clockwise circulation in the Laizhou Bay carried nutrients from the Huanghe River, Xiaoqing River, and Weihe River to the bay, which increased the feeding source of the toxin-producing algae. The distribution characteristics of the ∑LMATs in the Laizhou Bay of spring were significantly different from those of summer (He et al., 2020). On the one hand, the clockwise circulation in the Laizhou Bay during summer was obviously weaker than that during spring (Guo et al., 2017). On the other hand, temperature, light, nutrients, and other factors could also affect the growth and toxin production of toxin-producing algae. Thus, the growth and toxin production of toxin-producing algae were obviously affected by environmental factors. Hence, the spatial distribution of LMATs was higher at southwest of the inner Laizhou Bay and the Huanghe River estuary, but lower at east of the inner Laizhou Bay due to the joint influence of terrigenous input and internal circulation. The content and distribution of LMATs in the Laizhou Bay varied obviously in season.
3.3 Composition, concentration, and distribution of LMATs in the phytoplankton of Laizhou BayGiven the limitation of sampling, only 18 stations of phytoplankton samples in the north-western part of Laizhou Bay were collected during spring. The composition and concentration of the LMATs in the phytoplankton samples at each station are shown in Fig. 5. Seven LMATs, including PTX2, OA, 7-epi-PTX2 SA, DTX2, DTX1, PTX2 SA, and PTX11, were detected in the phytoplankton samples, and this result was consistent with that of the phytoplankton samples during summer in Laizhou Bay (He et al., 2020). However, the abundance of LMATs in the phytoplankton samples was lesser than that in the seawater samples. OA and DTX2 could be detected in all phytoplankton samples. The detection rates of 7-epi-PTX2 SA and PTX2 were 88.89% and 83.33%, respectively. The detection rate of DTX1 was approximately 50%. PTX11 was only detected at the L8, L13, and L14 stations. PTX 2SA was detected only at the L12 and L13 stations. In addition, the concentrations of PTX11 and PTX 2SA were low and only reached the LOQ. Therefore, the concentrations of PTX11 and PTX 2SA were only used as reference values here. OA, DTX1, and PTX2 were the commonly found toxins in Daya Bay of South China Sea (Jiang et al., 2017), Changjiang River estuary, East China Sea (He et al., 2019), and the Bohai Sea (Liu et al., 2017). Compared with the other coastal areas of China, LMATs in the phytoplankton detected in this study area were more abundant.
|
| Fig.5 The composition and concentration of individual lipophilic marine algal toxins in phytoplankton samples at each station |
PTX2 was the dominant toxin in the phytoplankton samples during spring in Laizhou Bay. The concentration of PTX2 ranged from ND to 476.05 pg/L, with the average concentration of 43.32 pg/L, which was consistent with that during summer in Laizhou Bay (He et al., 2020). As shown in Fig. 6, the concentrations of PTX2 were all within the interquartile range, without outliers. However, the interquartile range was large, indicating that the concentration of PTX2 considerably varied. Although the detection rate of PTX2 was less than 100%, its concentration at most detected stations were high. The results showed that the toxin-producing algae could gather at some locations in the study area. The maximum value of PTX2 obtained in this study was lower than that in Hangu (the west of Bohai Sea) but higher than those in Laishan (southeast of Bohai Sea), southeast of Laizhou Bay, Qinhuangdao and Huludao (northwest of Bohai Sea) (Liu et al., 2017). Contrary to the results detected in the phytoplankton samples from the Bohai Sea (Liu et al., 2017) and Yellow Sea (Li et al., 2017), the concentration of 7-epi-PTX2 SA was considerably high, which was secondary to PTX2. The concentration of 7-epi-PTX2 SA ranged from ND to 26.64 pg/L, with an average of 3.84 pg/L. The high concentration of 7-epi-PTX2 SA was also different from that detected during summer in Laizhou Bay (He et al., 2020). This result indicated that the composition and concentration of LMATs were remarkably affected by the change in the external environment. This incident is the first time that 7-epi-PTX2 SA with high frequency and content has been detected in the coastal area of China. The concentration of OA ranged from 0.31 pg/L to 5.29 pg/L, with an average content of 1.27 pg/L. The maximum concentration of OA was lower than those detected at four stations around the coastal area of Bohai Sea in 2013–2014 (Liu et al., 2017), indicating that phytotoxin species in this area are gradually transforming from OA to PTX2. The contents of DTX2 and DTX1 were relatively low, ranging 0.30– 3.43 pg/L and 0–2.75 pg/L, on average of 1.01 pg/L and 0.48 pg/L, respectively. The outliers of DTX2 and DTX1 were few, and the fluctuation of content level was not obvious (Fig. 6). The detection rates and concentration levels of PTX11 and PTX2 SA at each station were low. Therefore, the pollution status in the whole Laizhou Bay was relatively light, which could be ignored for the moment. The content of ∑LMATs ranged 0.98– 479.27 pg/L on average of 50.20 pg/L.
|
| Fig.6 Individual lipophilic marine algal toxin concentrations in phytoplankton samples of Laizhou Bay The red line represents the median, the box height represents the interquartile range (IQR, 25%–75%), the circles and small stars are outliers, and the black vertical line represents the range of 1.5 IQR within the maximum and minimum interquartile values. |
The distribution of all detected LMATs are shown in Fig. 7. The highest concentrations of OA (Fig. 7a), PTX2 (Fig. 7b), DTX1 (Fig. 7c), DTX2 (Fig. 7d), 7-epi-PTX2 SA (Fig. 7e), and ∑LMATs (Fig. 7f) were found at the southwest area of Laizhou Bay near the Xiaoqing River estuary (near L15 and L5 stations). The concentration of LMATs decreased from this area to all directions. The distribution of LMATs in the phytoplankton was consistent with that in seawater, indicating that the source of LMATs in the seawater and phytoplankton was consistent. Meanwhile, the reason for the distribution trend of LMATs in the phytoplankton may be similar to that in seawater. However, the distribution of LMATs in the phytoplankton samples during spring was significantly different from that during summer in Laizhou Bay (He et al., 2020). The high values during spring were located at the southwest part of the bay, while those during summer were located at the eastern part of the bay mouth. This phenomenon may be caused by the strong circulation during spring and weak circulation during summer (Guo et al., 2017). Meanwhile, environmental factors may also play an important role in the concentration and distribution of LMATs. Therefore, PTX2, 7-epi-PTX2 SA, and OA were the dominant LMATs in the phytoplankton samples during spring in the Laizhou Bay. The detection rate and concentration of 7-epi-PTX2 SA were extremely high in the phytoplankton samples during spring in Laizhou Bay. The composition of LMATs during spring was the same as that during summer, but the dominant LMATs were different. The distribution of LMATs in the phytoplankton was consistent with that in seawater and showed obvious seasonal variation.
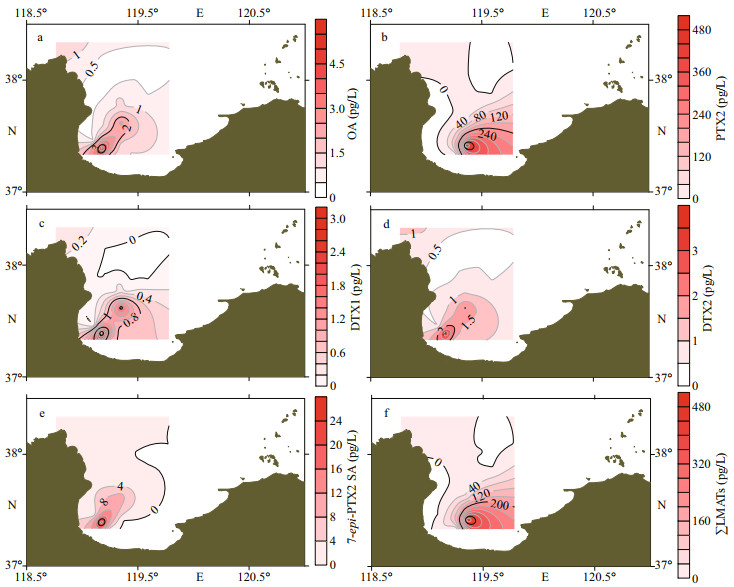
|
| Fig.7 The spatial distribution of lipophilic marine algal toxins in phytoplankton during spring |
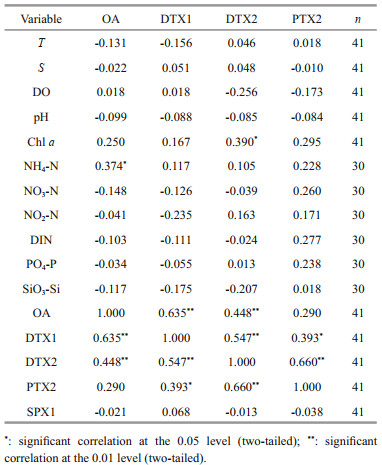
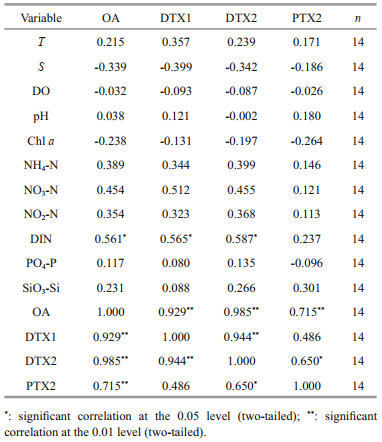
Algal toxins in seawater originated from toxin-producing algae. The abundance and toxin production of toxin-producing algae may be influenced by various environment factors, and the toxin composition and level even in the same toxigenic species may vary significantly under different environmental conditions. For example, some studies have proven that D. acuminata can produce OA, DTX1, and PTX2 (Hackett et al., 2009; Reguera et al., 2014), but D. acuminata collected from Spain, Portugal (Jrgensen and Andersen, 2007), and France (Reguera and Pizarro, 2008) produced only OA. D. acuminata collected from Chile and Denmark produced only PTX2 (Miles et al., 2004; Nielsen et al., 2012), while those from Japan could produce PTX2 with a small amount of DTX1 (Suzuki et al., 2009). Hence, environmental factors play an important influence on the production of toxins. To explore the influence factors that affect the content and distribution of LMATs, we conducted a correlation analysis between the distribution of LMATs and the environmental physicochemical factors in the Laizhou Bay during spring. The results are shown in Tables 2–3.
|
|
The results from the correlation analysis between LMATs concentrations in the seawater and environmental physicochemical factors are shown in Table 2. No correlation was found between the LMATs in the seawater and other environmental physicochemical factors, except the low correlation of OA with NH 4-N (0.374) and DTX2 with chl a (0.390). Certain correlations were found among the various LMATs. Extremely significant positive correlations were found between OA and DTX1 (0.635), OA and DTX2 (0.448), DTX1 and DTX2 (0.547), and DTX2 and PTX2 (0.660). This result also showed significant positive correlation between DTX1 and PTX2 (0.393). However, no correlation was found between OA and PTX2. The differences in the correlation between LMATs may be related to their distinct sources. That is, the source of OA and parts of DTX1 and DTX2 in seawater may be the same, while the source of PTX2 and parts of DTX1 and DTX2 in seawater may be the same. However, the sources of OA and PTX2 may be different. OA, DTXs, and PTXs were reported to be produced by Dinophysis spp. (Li et al., 2015; Villalobos et al., 2015; Ajani et al., 2016; Jiang et al., 2017; Liu et al., 2017), and Procentrum spp. is also the producer of OA and DTXs (Sahraoui et al., 2013; Huang et al., 2015). However, PTX2 was reported to be produced only by Dinophysis spp. (Reguera et al., 2014). Based on the correlation results, Procentrum spp. may exist in the study area. In our previous study (He et al., 2020), both Dinophysis spp. and Procentrum spp. were found in the phytoplankton samples in Laizhou Bay during summer, which is consistent with our inferences.
Table 3 shows the correlation analysis between the LMAT contents in the phytoplankton and environmental physicochemical factors. The contents of OA, DTX1, and DTX2 in the phytoplankton samples were positively correlated with DIN; that is, the increase of DIN could promote the generation of OA, DTX1, and DTX2. Nutrients are the main components necessary for the growth of phytoplankton in the ocean and are the basis of marine primary productivity and food chain. Some studies have proved that the increase in N content could stimulate the growth of D. acuminata and increase its toxicity (Hattenrath-Lehmann and Gobler, 2015; Hattenrath-Lehmann et al., 2015), which further showed the accuracy of the correlation analysis results. Correlations were also found between some LMATs in the phytoplankton. All LMATs (i.e., : OA, DTX1, DTX2, PTX2) detected in the phytoplankton samples were positively correlated, except for PTX2 and DTX1. It indicated that parts of PTX2 and DTX1 might be derived from different toxin-producing algae. Integrated with the correlations of LMATs in seawater and phytoplankton, most of PTX2 and parts of OA and DTX1 may originate from different toxin-producing algae in Laizhou Bay.
4 CONCLUSIONIn this work, the concentration level, distribution characteristics, and influence factors of LMATs in the seawater and phytoplankton in the Laizhou Bay during spring were fully illustrated. OA, PTX2, and DTX1 were the dominant LMATs in the seawater, while PTX2, 7- epi-PTX2 SA, and OA were dominant in the phytoplankton in the study area. The concentration of LMATs in the seawater of Laizhou Bay was lower than those in most coastal areas worldwide. Affected by the joint influence of terrigenous input and internal circulation during spring, LMATs in the seawater and phytoplankton were both higher at the southwest of the inner Bay and the Huanghe River estuary but lower at the eastern part of the inner bay. Compared with our previous result during summer in Laizhou Bay, the content and distribution characteristic of LMATs in the seawater and phytoplankton in Laizhou Bay showed obvious seasonal variation characteristics. DIN played an important role in promoting the production of LMATs by toxin-producing algae, which may pose a serious threat to marine breeding industries and human health. Therefore, more attention should be paid to control the DIN discharged into the mariculture areas of the coastal bay from the land sources.
5 DATA AVAILABILITY STATEMENTAll data supporting the findings are available in this published article and the supplementary files.
Electronic supplementary material
Supplementary material (Supplementary Tables S1–S2) is available in the online version of this article at https://doi.org/10.1007/s00343-022-1426-5.
Ajani P A, Larsson M E, Rubio A, et al. 2016. Modelling bloom formation of the toxic dinoflagellates Dinophysis acuminata and Dinophysis caudata in a highly modified estuary, south eastern Australia. Estuarine, Coastal and Shelf Science, 183: 95-106.
DOI:10.1016/j.ecss.2016.10.020 |
Armi Z, Turki S, Trabelsi E, et al. 2012. Occurrence of diarrhetic shellfish poisoning (DSP) toxins in clams (Ruditapes decussatus) from Tunis north lagoon. Environmental Monitoring and Assessment, 184(8): 5085-5095.
DOI:10.1007/s10661-011-2324-z |
Basti L, Suzuki T, Uchida H, et al. 2018. Thermal acclimation affects growth and lipophilic toxin production in a strain of cosmopolitan harmful alga Dinophysis acuminata. Harmful Algae, 73: 119-128.
DOI:10.1016/j.hal.2018.02.004 |
Basti L, Uchida H, Matsushima R, et al. 2015. Influence of Temperature on growth and production of pectenotoxin-2 by a monoclonal culture of Dinophysis caudata. Marine Drugs, 13(12): 7124-7137.
DOI:10.3390/md13127061 |
Bosch-Orea C, Sanchís J, Farré M, et al. 2017. Analysis of lipophilic marine biotoxins by liquid chromatography coupled with high-resolution mass spectrometry in seawater from the Catalan Coast. Analytical and Bioanalytical Chemistry, 409(23): 5451-5462.
DOI:10.1007/s00216-017-0536-y |
Chen J H, Han T Z, Li X T, et al. 2018. Occurrence and distribution of marine natural organic pollutants: lipophilic marine algal toxins in the Yellow Sea and the Bohai Sea, China. Science of the Total Environment, 612: 931-939.
DOI:10.1016/j.scitotenv.2017.08.304 |
Chen J H, Li X, Wang S, et al. 2017. Screening of lipophilic marine toxins in marine aquaculture environment using liquid chromatography-mass spectrometry. Chemosphere, 168: 32-40.
DOI:10.1016/j.chemosphere.2016.10.052 |
De Schrijver K, Maes I, De Man L, et al. 2002. An outbreak of diarrhoeic shellfish poisoning in Antwerp, Belgium. Eurosurveillance, 7(10): 138-141.
DOI:10.2807/esm.07.10.00363-en |
Elgarch A, Vale P, Rifai S, et al. 2008. Detection of diarrheic shellfish poisoning and azaspiracids toxins in Moroccan mussels: comparison of LC-MS method with the commercial immunoassay kit. Marine Drugs, 6(4): 587-594.
DOI:10.3390/md6040587 |
Farabegoli F, Blanco L, Rodríguez L, et al. 2018. Phycotoxins in marine shellfish: origin, occurrence and effects on humans. Marine Drugs, 16(6): 188.
DOI:10.3390/md16060188 |
Gerssen A, Mulder P P J, De Boer J. 2011. Screening of lipophilic marine toxins in shellfish and algae: Development of a library using liquid chromatography coupled to orbitrap mass spectrometry. Analytica Chimica Acta, 685(2): 176-185.
DOI:10.1016/j.aca.2010.11.036 |
Grattan L M, Holobaugh S, Morris J G. 2016. Harmful algal blooms and public health. Harmful Algae, 57: 2-8.
DOI:10.1016/j.hal.2016.05.003 |
Guo F, Wang B D, Xin M, et al. 2017. Nutrient distributions in the Laizhou Bay in spring and summer of 2015. Advances in Marine Science, 35(2): 258-266.
(in Chinese with English abstract) DOI:10.3969/j.issn.1671-6647.2017.02.010 |
Hackett J D, Tong M M, Kulis D M, et al. 2009. DSP toxin production de novo in cultures of Dinophysis acuminata(Dinophyceae) from North America. Harmful Algae, 8(6): 873-879.
DOI:10.1016/j.hal.2009.04.004 |
Hallegraeff G M. 2010. Ocean climate change, phytoplankton community responses, and harmful algal blooms: a formidable predictive challenge. Limnology and Oceanography, 46(2): 220-235.
|
Hattenrath-Lehmann T, Gobler C J. 2015. The contribution of inorganic and organic nutrients to the growth of a North American isolate of the mixotrophic dinoflagellate, Dinophysis acuminata. Limnology and Oceanography, 60(5): 1588-1603.
DOI:10.1002/lno.10119 |
Hattenrath-Lehmann T K, Marcoval M A, Mittlesdorf H, et al. 2015. Nitrogenous nutrients promote the growth and toxicity of Dinophysis acuminata during estuarine bloom events. PLoS One, 10(4): e0124148.
DOI:10.1371/journal.pone.0124148 |
He X P, Chen J H, Wu D N, et al. 2019. Distribution characteristics and environmental control factors of lipophilic marine algal toxins in Changjiang estuary and the adjacent East China Sea. Toxins, 11(10): 596.
DOI:10.3390/toxins11100596 |
He X P, Chen J H, Wu D N, et al. 2020. Occurrence, distribution, source, and influencing factors of lipophilic marine algal toxins in Laizhou Bay, Bohai Sea, China. Marine Pollution Bulletin, 150: 110789.
DOI:10.1016/j.marpolbul.2019.110789 |
Holmes M J, Teo S L M. 2002. Toxic marine dinoflagellates in Singapore waters that cause seafood poisonings. Clinical and Experimental Pharmacology and Physiology, 29(9): 829-836.
DOI:10.1046/j.1440-1681.2002.03724.x |
Hossen V, Jourdan-da Silva N, Guillois-Bécel Y, et al. 2011. Food poisoning outbreaks linked to mussels contaminated with okadaic acid and ester dinophysistoxin-3 in France, June 2009. Eurosurveillance, 16(3): 1-7.
|
Huang D J, Su J L, Zhang L R. 1998. Numerical study of the winter and summer circulation in the Bohai Sea. Acta Aerodynamica Sinica, 16(1): 115-121.
(in Chinese with English abstract) |
Huang L, Zou Y, Weng H W, et al. 2015. Proteomic profile in Perna viridis after exposed to Prorocentrum lima, a dinoflagellate producing DSP toxins. Environmental Pollution, 196: 350-357.
DOI:10.1016/j.envpol.2014.10.019 |
Jiang T, Liu L, Li Y, et al. 2017. Occurrence of marine algal toxins in oyster and phytoplankton samples in Daya Bay, South China Sea. Chemosphere, 183: 80-88.
DOI:10.1016/j.chemosphere.2017.05.067 |
Jørgensen K, Andersen P. 2007. Relation between the concentration of Dinophysis acuminata and diarrheic shellfish poisoning toxins in blue mussels (Mytilus edulis)during a toxic episode in the Limfjord (Denmark), 2006. Journal of Shellfish Research, 26(4): 1081-1087.
DOI:10.2983/0730-8000(2007)26[1081:RBTCOD]2.0.CO;2 |
Kamiyama T, Nagai S, Suzuki T, et al. 2010. Effect of temperature on production of okadaic acid, dinophysistoxin-1, and pectenotoxin-2 by Dinophysis acuminata in culture experiments. Aquatic Microbial Ecology, 60(2): 193-202.
DOI:10.3354/ame01419 |
Kim J H, Lee K J, Suzuki T, et al. 2010. Seasonal variability of lipophilic shellfish toxins in bivalves and waters, and abundance of Dinophysis spp. in Jinhae Bay, Korea. Journal of Shellfish Research, 29(4): 1061-1067.
DOI:10.2983/035.029.0408 |
Koukaras K, Nikolaidis G. 2004. Dinophysis blooms in Greek coastal waters (Thermaikos Gulf, NW Aegean Sea). Journal of Plankton Research, 26(4): 445-457.
DOI:10.1093/plankt/fbh042 |
Lefebvre K A, Bill B D, Erickson A, et al. 2008. Characterization of intracellular and extracellular saxitoxin levels in both field and cultured Alexandrium spp. samples from Sequim Bay, Washington. Marine Drugs, 6(2): 103-116.
|
Li A F, Sun G, Qiu J B, et al. 2015. Lipophilic shellfish toxins in Dinophysis caudata picked cells and in shellfish from the East China Sea. Environmental Science and Pollution Research, 22(4): 3116-3126.
DOI:10.1007/s11356-014-3595-z |
Li A F, Li M H, Qiu J B, et al. 2018. Effect of suspended particulate matter on the accumulation of dissolved diarrhetic shellfish toxins by mussels (Mytilus galloprovincialis) under laboratory conditions. Toxins, 10(7): 273.
DOI:10.3390/toxins10070273 |
Li F L, Li Z X, Guo M M, et al. 2016. Investigation of diarrhetic shellfish toxins in Lingshan Bay, Yellow Sea, China, using solid-phase adsorption toxin tracking (SPATT). Food Additives & Contaminants: Part A, 33(8): 1367-1373.
|
Li M H, Sun G, Qiu J B, et al. 2017. Occurrence and variation of lipophilic shellfish toxins in phytoplankton, shellfish and seawater samples from the aquaculture zone in the Yellow Sea, China. Toxicon, 127: 1-10.
DOI:10.1016/j.toxicon.2016.12.009 |
Li X, Li Z Y, Chen J H, et al. 2014. Detection, occurrence and monthly variations of typical lipophilic marine toxins associated with diarrhetic shellfish poisoning in the coastal seawater of Qingdao City, China. Chemosphere, 111: 560-567.
DOI:10.1016/j.chemosphere.2014.05.006 |
Likumahua S, De Boer M K, Krock B, et al. 2020. Variability of dinoflagellates and their associated toxins in relation with environmental drivers in Ambon Bay, eastern Indonesia. Marine Pollution Bulletin, 150: 110778.
DOI:10.1016/j.marpolbul.2019.110778 |
Liu Y, Yu R C, Kong F Z, et al. 2017. Lipophilic marine toxins discovered in the Bohai Sea using high performance liquid chromatography coupled with tandem mass spectrometry. Chemosphere, 183: 380-388.
DOI:10.1016/j.chemosphere.2017.05.073 |
MacKenzie L, Holland P, McNabb P, et al. 2002. Complex toxin profiles in phytoplankton and Greenshell mussels(Perna canaliculus), revealed by LC-MS/MS analysis. Toxicon, 40(9): 1321-1330.
DOI:10.1016/S0041-0101(02)00143-5 |
Mackenzie L A. 2019. A long-term time series of Dinophysis acuminata blooms and associated shellfish toxin contamination in Port Underwood, Marlborough Sounds, New Zealand. Toxins, 11(2): 74.
DOI:10.3390/toxins11020074 |
Madigan T L, Lee K G, Padula D J, et al. 2006. Diarrhetic shellfish poisoning (DSP) toxins in South Australian shellfish. Harmful Algae, 5(2): 119-123.
DOI:10.1016/j.hal.2004.12.005 |
Mafra L L, Nolli P K W, Mota L E, et al. 2019. Multi-species okadaic acid contamination and human poisoning during a massive bloom of Dinophysis acuminata complex in southern Brazil. Harmful Algae, 89: 101662.
DOI:10.1016/j.hal.2019.101662 |
Miles C O, Wilkins A L, Munday R, et al. 2004. Isolation of pectenotoxin-2 from Dinophysis acuta and its conversion to pectenotoxin-2 seco acid, and preliminary assessment of their acute toxicities. Toxicon, 43(1): 1-9.
DOI:10.1016/j.toxicon.2003.10.003 |
Nicolas J, Hoogenboom R L A P, Hendriksen P J M, et al. 2017. Marine biotoxins and associated outbreaks following seafood consumption: prevention and surveillance in the 21st century. Global Food Security, 15: 11-21.
DOI:10.1016/j.gfs.2017.03.002 |
Nielsen L T, Krock B, Hansen P J. 2012. Effects of light and food availability on toxin production, growth and photosynthesis in Dinophysis acuminata. Marine Ecology-Progress Series, 471: 37-50.
DOI:10.3354/meps10027 |
Pan L, Chen J H, Shen H H, et al. 2017. Profiling of extracellular toxins associated with diarrhetic shellfish poison in Prorocentrum lima culture medium by high-performance liquid chromatography coupled with mass spectrometry. Toxins, 9(10): 308.
DOI:10.3390/toxins9100308 |
Parsons T R, Maita Y, Lalli C M. 1984. A Manual of Chemical and Biological Methods for Seawater Analysis. Pergamon Press, New York, NY, USA.
|
Reguera B, Pizarro G. 2008. Planktonic dinoflagellates which produce polyether toxins of the Old DSP complex. In: Botana L M ed. Seafood and Freshwater Toxins: Pharmacology, Physiology and Detection. 2nd edn. Taylor & Francis, London, UK, p. 257-284.
|
Reguera B, Riobó P, Rodríguez F, et al. 2014. Dinophysis toxins: Causative organisms, distribution and fate in shellfish. Marine Drugs, 12(1): 394-461.
DOI:10.3390/md12010394 |
Sahraoui I, Bouchouicha D, Mabrouk H H, et al. 2013. Driving factors of the potentially toxic and harmful species of Prorocentrum Ehrenbergin a semi-enclosed Mediterranean lagoon (Tunisia, SW Mediterranean). Mediterranean Marine Science, 14(2): 353-362.
DOI:10.12681/mms.338 |
Sun X P. 2006. Regional Oceanography in China Seas. China Ocean Press, China, Beijing. 376p.
|
Suzuki T, Miyazono A, Baba K, et al. 2009. LC-MS/MS analysis of okadaic acid analogues and other lipophilic toxins in single-cell isolates of several Dinophysis species collected in Hokkaido, Japan. Harmful Algae, 8(2): 233-238.
DOI:10.1016/j.hal.2008.06.001 |
Suzuki T, Igarashi T, Ichimi K, et al. 2005. Kinetics of diarrhetic shellfish poisoning toxins, okadaic acid, dinophysistoxin-1, pectenotoxin-6 and yessotoxin in scallops Patinopecten yessoensis. Fisheries Science, 71(4): 948.
DOI:10.1111/j.1444-2906.2005.01049.x |
Takahashi E, Yu Q M, Eaglesham G, et al. 2007. Occurrence and seasonal variations of algal toxins in water, phytoplankton and shellfish from North Stradbroke Island, Queensland, Australia. Marine Environmental Research, 64(4): 429-442.
DOI:10.1016/j.marenvres.2007.03.005 |
Taylor M, McIntyre L, Ritson M, et al. 2013. Outbreak of diarrhetic shellfish poisoning associated with mussels, British Columbia, Canada. Marine Drugs, 11(5): 1669-1676.
DOI:10.3390/md11051669 |
Trainer V L, Moore L, Bill B D, et al. 2013. Diarrhetic shellfish toxins and other lipophilic toxins of human health concern in Washington State. Marine Drugs, 11(6): 1815-1835.
DOI:10.3390/md11061815 |
Villalobos L G, Santinelli N, Sastre V, et al. 2015. Dinophysis Species Associated with Diarrhetic Shellfish Poisoning Episodes in North Patagonian Gulfs (Chubut Argentina). Journal of Shellfish Research, 34(3): 1141-1149.
DOI:10.2983/035.034.0339 |
Visciano P, Schirone M, Berti M, et al. 2016. Marine biotoxins: occurrence, toxicity, regulatory limits and reference methods. Frontiers in Microbiology, 7: 1051.
DOI:10.3389/fmicb.2016.01051 |
Wang J M, Chen J H, He X P, et al. 2021. Simple determination of six groups of lipophilic marine algal toxins in seawater by automated on-line solid phase extraction coupled to liquid chromatography-tandem mass spectrometry. Chemosphere, 262: 128374.
DOI:10.1016/j.chemosphere.2020.128374 |
Wang Y L, Chen J H, Li Z Y, et al. 2015. Determination of typical lipophilic marine toxins in marine sediments from three coastal bays of China using liquid chromatographytandem mass spectrometry after accelerated solvent extraction. Marine Pollution Bulletin, 101(2): 954-960.
DOI:10.1016/j.marpolbul.2015.10.038 |
Wu D N, Chen J H, He X P, et al. 2019. Distribution, partitioning, and seasonal variation of lipophilic marine algal toxins in aquatic environments of a typical semi-closed mariculture bay. Environmental Pollution, 255: 113299.
DOI:10.1016/j.envpol.2019.113299 |
Yu R C, Liu D Y. 2016. Harmful algal blooms in the coastal waters of China: current situation, long-term changes and prevention strategies. Bulletin of the Chinese Academy of Sciences, 31(10): 1167-1174.
(in Chinese with English abstract) |
Zhang J F, Gao X L. 2015. Heavy metals in surface sediments of the intertidal Laizhou Bay, Bohai Sea, China: distributions, sources and contamination assessment. Marine Pollution Bulletin, 98(1-2): 320-327.
DOI:10.1016/j.marpolbul.2015.06.035 |
Zhao B R, Zhuang G W, Cao D M. 1995. Circulation, tidal residual currents and their effects on the sedimentations in the Bohai Sea. Oceanologia et Limnologia Sinica, 26(5): 466-473.
(in Chinese with English abstract) DOI:10.3321/j.issn:0029-814X.1995.05.003 |
 2022, Vol. 40
2022, Vol. 40


