Institute of Oceanology, Chinese Academy of Sciences
Article Information
- SONG Minjie, YAN Tian, KONG Fanzhou, WANG Yunfeng, ZHOU Mingjiang
- Increased diversity and environmental threat of harmful algal blooms in the Southern Yellow Sea, China
- Journal of Oceanology and Limnology, 40(6): 2107-2119
- http://dx.doi.org/10.1007/s00343-021-1209-4
Article History
- Received Jun. 28, 2021
- accepted in principle Oct. 28, 2021
- accepted for publication Dec. 16, 2022
2 Laboratory for Marine Ecology and Environmental Science, Pilot National Laboratory for Marine Science and Technology(Qingdao), Qingdao 266237, China;
3 University of Chinese Academy of Sciences, Beijing 100049, China;
4 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
Harmful Algal Blooms (HABs) constitute exceptional ecological events caused by algae, which can attain high-biomass and/or result in proliferation of toxic cells (Hallegraeff, 1993; Kudela et al., 2017). Generally, HABs may include so-called 'red, brown, green, and golden tides', which can endanger ecosystem health and marine organisms survival through toxin production or by causing gill damage and water quality deterioration, although they are not always evidenced by water discoloration (Anderson et al., 2012; Kudela et al., 2017; Li et al., 2021). Moreover, they can contaminate seafood products, such as shellfish, with algal toxins that can be transferred through the food web, thereby harming human health (Reich et al., 2008). The frequency of HABs and the diversity of their causative species are on the rise in coastal China, and the modes of action of harmful algae that affecting the marine ecological environment and coastal human populations are becoming increasingly complex (Lu et al., 2014; Anderson et al., 2015).
In China, compared with the Bohai Sea, and the East and South China Seas, the Yellow Sea provides a relatively healthy ecological environment in which fewer HABs have been documented before the 21st century (Zhou et al., 2001). However, this changed in 2007 when large-scale blooms of the green macroalga Ulva prolifera (green tides) appeared and recurred annually thereafter (MNR, 2009–2020; Zhang et al., 2019). Furthermore, an unusual co-occurrence of green, golden (Sargassum horneri), and harmful red tides (Kerenia mikimotoi and Heterosigma akashiwo) was observed in the Southern Yellow Sea (SYS) in 2017 (Kong et al., 2018), while these HABs appeared individually in other sea areas. In addition to marine toxin concerns, HABs in the SYS have a greater diversity and ecological impact than those in adjacent seas. Over the past 30 years, algal toxins occasionally detected in shellfish and shellfish poisoning outbreaks, and sometimes were even associated with human fatalities, have been reported along the coast of the SYS (Liang et al., 2019). Manila clams, Ruditapes philippinarum, were contaminated with paralytic shellfish poisoning (PSP) toxins accumulated from Alexandrium minutum cells, resulting in six people poisoned and one person died in Lianyungang City, Jiangsu in 2008 (Yu and Liu, 2016; Yu and Luo, 2016). Furthermore, HABs formed by non-toxic species may also threaten the health and safety of coastal populations. For example, U. prolifera, which is non-toxic, can produce toxic gases such as hydrogen sulfide (H2S) and ammonia (NH3) during bulk deposition and decay along the coast (Yu and Liu, 2016), which can cause sea and atmospheric contamination, and endanger the health of coastal residents and tourists.
Harmful algal blooms in the SYS have also caused serious economic losses. A massive U. prolifera green tide occurred in the coastal area of Qingdao between May and July 2008 prior to the Qingdao Olympic Regatta. Motivated by the bloom, local government made a heroic effort to remove the green algae, costing an estimated 2 billion RMB (Ye et al., 2011), and caused economic losses of 640 million RMB in 2009 (Yu and Liu, 2016). Invasion of the brown macroalga S. horneri in the Subei Shoal resulted in a loss of more than 500 million RMB to Neopyropia farming in the winter of 2016 (Xing et al., 2017). Blooms of the dinoflagellates Karlodinium veneficum and Takayama acrotrocha occurred in Haizhou Bay off Lianyungang in North Jiangsu during summer 2020, and also harmed Neopyropia farming (Zhang et al., 2022).
In the SYS, different types of algal blooms co-occur; therefore, more attention should be paid to this unique sea area. This appears to be the result of the combined effects of economic development and global climate change in recent decades (Anderson et al., 2012; Trainer et al., 2020).
This paper comprehensively reviews the current status and trends of HABs appearance along the coast of China, with particular focus on the SYS, to document the history of HAB events that have occurred in the water mass over the years. It describes the pattern of increasing HAB diversity in the SYS, and analyzes their formation characteristics to better understand the associated threats to the environment and human health.
2 BACKGROUND AND HAB DIFFERENTIATIONAlgal blooms, often referred to as red tides, are exceptional ecological events during which some marine microalgae or even protists proliferate resulting in discoloration at high concentrations (Zhou et al., 2001). However, this description is inadequate for toxic or harmful algal blooms that are not red, or that are toxic despite their occurrence at relatively low concentrations. The definition of HAB is often a societal concept rather than a scientific definition. Generally, HABs in the sea may include red, brown, green, and golden tides.
2.1 Harmful red tides and algal toxinsAs a natural phenomenon, red tides have existed since ancient times. In this study, the term "harmful red tides" is used to distinguish them from green and golden tides that are all herein referred to as harmful algal blooms. The production of toxins is frequently responsible for the harmful effects of marine microalgae. As harmful red tide causative species, most Alexandrium species produce a variety of saxitoxin derivatives that are responsible for PSP syndrome (Caruana and Amzil, 2018). Shellfish are the main vectors for the transfer of algal toxins to humans. Of the global poisoning incidents caused by the consumption of contaminated seafood, 35% have been attributed to PSP and 30% to diarrhetic shellfish poisoning (DSP) (Hallegraeff et al., 2021).
2.2 Brown tidesBrown tides are caused by picoplanktonic (2–3 μm) microalgae that can attain extremely high cell densities (up to 2–3 million cells/mL) (Sieburth et al., 1988), and can last up to 8 weeks (Probyn et al., 2001); they exhibit a brown color at high cell densities, differentiating them from other HABs (Cosper et al., 1990). The main causative species of brown tides worldwide are the pelagophytes Aureococcus anophagefferens and Aureoumbra lagunensis (DeYoe et al., 1997). When concentrations exceed ~2×105 cells/mL, brown tides can result in mass mortalities of wild and cultured shellfish populations; alter the structure and function of marine ecosystems; and affect the reproduction, recruitment, and survival of bivalve species (Gastrich and Wazniak, 2002). Due to their broad ecological effects, they have been referred to as ecosystem disruptive algal blooms (Sunda et al., 2006).
2.3 Green tidesGenerally, green tides are caused by the rapid and large-scale proliferation or aggregation of macroalgal chlorophytes after they break away from the attachment base to form aggregations of their thalli (Yu et al., 2018). They usually occur in semi-enclosed sea areas such as estuaries or inner bays, and are mostly caused by the genera Ulva, Cladophora, and Chaetomorpha (Ye et al., 2011). Green tides can harm coastal ecosystems by virtue of their sheer physical mass. They can block the photosynthesis of local primary producers via shading and lead to negative effects such as degradation of seagrass habitat (Valiela et al., 1997; Barnes, 2019). Additionally, if not removed in time, they may result in H2S production from their anoxic interior (Smetacek and Zingone, 2013), which will have further detrimental effects on coastal ecology and tourism economy (Fletcher, 1996).
2.4 Golden tidesGolden tides are formed by the floating macroalga Sargassum, when it detaches from the sea bottom (Yoshida, 1963). They grow rapidly and accumulate on the sea surface over large areal scales, and have previously been mainly restricted to the beaches between the Gulf of Mexico and Bermuda (Smetacek and Zingone, 2013). However, due to the continuous expansion of Atlantic Sargassum in recent years, multiple blooms have occurred along the Brazilian coast in the South Atlantic, particularly from 2011 to 2015 (Sissini et al., 2017). Additionally, Sargassum is widely distributed along the coast of China, Japan, and Korea (Komatsu et al., 2014; Xu et al., 2018). Blooms of Sargassum in the Atlantic, extending from the West Indies across to West Africa, have been found to contain high concentration of toxic arsenic (Devault et al., 2021).
In the SYS, Sargassum attains high biomass, but toxin production has not been recorded. Before 2012, floating Sargassum in the East China Sea was mainly distributed in the open sea causing no major harm, and therefore did not attract much attention. However, the frequency and distribution area of floating and drifting Sargassum in the Yellow Sea and the East China Sea have significantly increased since 2015, thus, critically affecting the coastal social economy of Dalian Beach, Rongcheng aquaculture facilities, the Haiyang Nuclear Power Plant, and Neopyropia farming in Jiangsu Province. The impact on coastal social and economic development has greatly increased over the last decade.
3 HAB DIVERSITY IN THE SOUTHERN YELLOW SEAHarmful algal blooms in China are characterized by their species diversity, and have different sources and modes of action (Gu et al., 2021; Liu et al., 2021a; Xiao et al., 2021). In the spring and summer of 2017, a 35°N transect in the SYS documented a hitherto rare co-occurrence of green, golden, and harmful red tides (Kong et al., 2018), indicating degradation of the marine environment and severe ecosystem changes in this region. The interaction among HABs, and their succession and evolution, have thus become increasingly important and worthy of attention.
Previously, the SYS has attracted a great deal of attention due to the prevalence of green tides. In recent years, however, golden tides and harmful red tides have also raised considerable alarm, without diminishing the importance of green tides that have continued to occur in the region.
3.1 Characteristics of green tide formation in the SYSGreen tides in the SYS are mainly formed by Ulva, more specifically, a newly identified floating ecotype of Ulva (Zhao et al., 2015), that persists for many years. During early ontogeny, the living environment of the floating Ulva is very similar to that of the intertidal stationary Ulva. However, during the former's later growth stage, it always floats at the sea surface. These algae occur both floating on the surface and in suspension below the surface, and therefore have different light adaptation strategies depending on where in the water column they occur (Zhao et al., 2016).
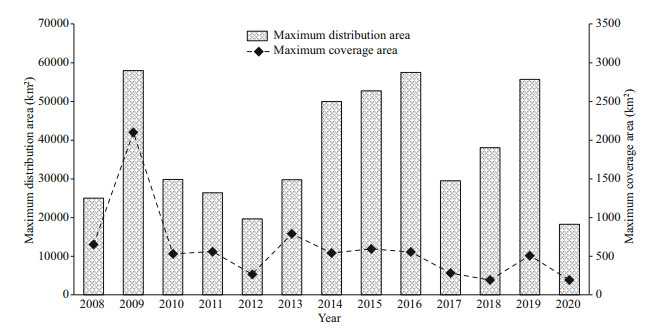
Green tides of the SYS originate every year from the sandbanks of the Subei Shoal, in April through May. Neopyropia culture rafts play a role in 'amplifying' the initial biomass of green tides by providing a substrate near the surface. After Neopyropia harvest, the attached green algae are removed from the rafts and fragments of U. prolifera fronds become floating (Zhou et al., 2015). Then, under the action of wind and currents at the surface, the floating chlorophytes drift northward during which rapid proliferation. The green tide area has progressively expanded, eventually affecting the south coast of the Shandong Peninsula. The distribution area, equal to the sum of the coverage area and the gap between the floating patches, reaches its maximum in June–July. Additionally, during June-July, green tides reach the mainland and successively affected a larger coastal area. Finally, in late July and August, they mostly disappear in the coastal waters off the Shandong Peninsula. The occurrence of green tides over the years is shown in Fig. 1.
|
| Fig.1 Variation on the maximum daily distribution and coverage area of the U.prolifera green tides in the SYS since 2008 Data source: the Bulletin of China Marine Disaster: 2009-2020 (MNR, 2009–2020). |
Ulva is a non-toxic green macroalgae that can be used for food, animal feed, fertilizer, and bio-energy production (Zhang et al., 2019). However, if green tides attain a large biomass of floating Ulva and cannot be effectively removed or harvested within a short time, they will also produce toxic gases from their anoxic interior, which pollute the sea and air, and negatively affect the health of coastal residents and tourists. During late green tide stages, the decomposition of this chlorophyte also leads to the release of high concentrations of nutrients that stimulate the growth of microalgae, and can thus cause secondary impacts such as harmful red tides (Kong et al., 2018).
3.2 Characteristics of golden tide formation in the SYSSargassum has appeared in the Yellow Sea in China since 2000 (Komatsu et al., 2008). It occurred in the coastal waters of Zhejiang Province in 2012, and has reappeared almost every year in February to March since then (Qi et al., 2017). In recent years, Sargassum, accompanied by Ulva, has ravaged the southern coast of Shandong Peninsula and Dalian waters in the summer. After invading the Neopyropia farming area of the Subei Shoal, a large biomass has also damaged a large number of raft facilities. Taking 2016 as an example, the massive invasion of S. horneri resulted in a total direct loss of 500 million RMB (77 million US$) to the Neopyropia farming industry in Yancheng and Nantong, Jiangsu Province.
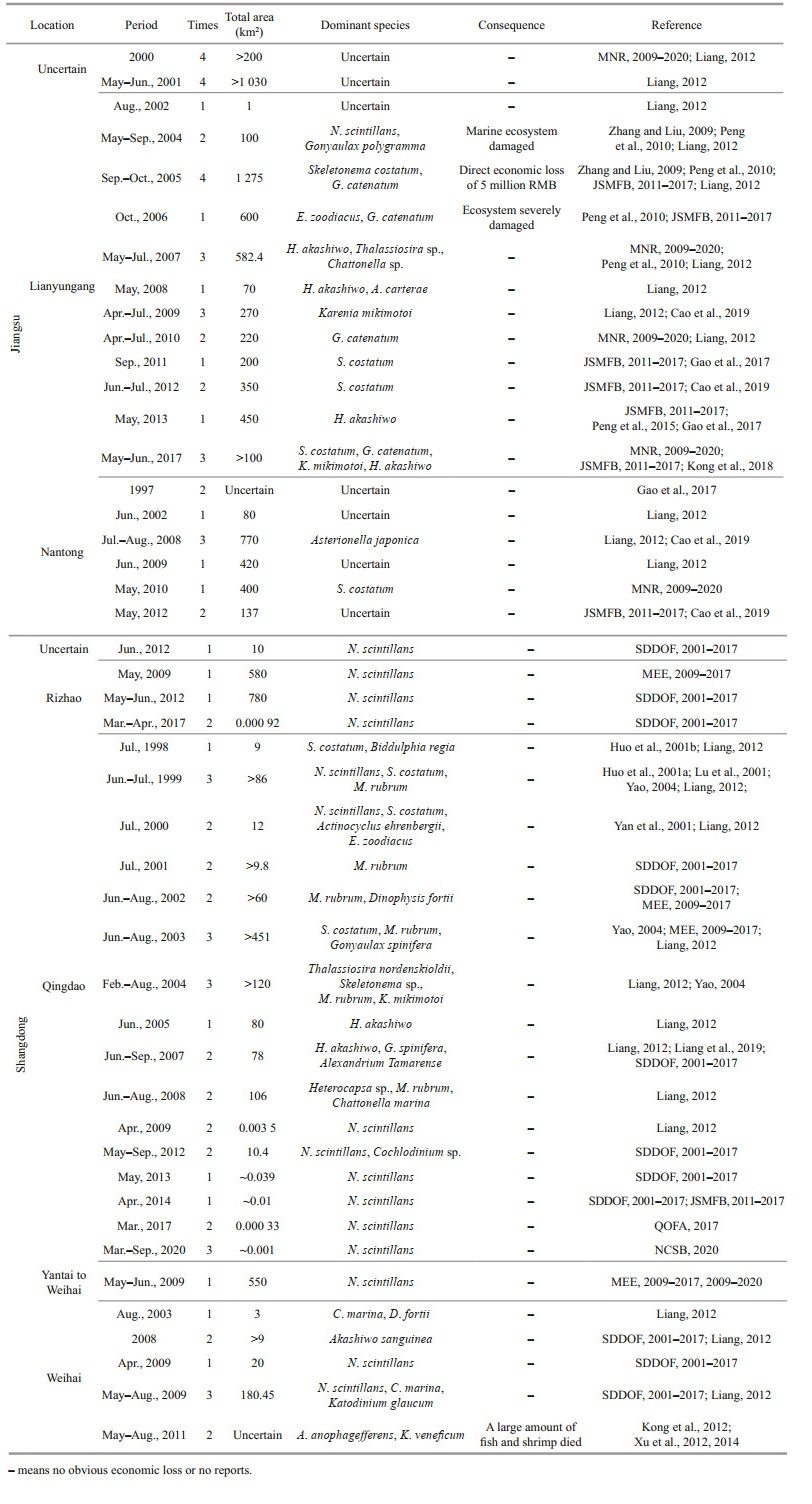
Habitats along the Jiangsu coast are unsuitable for the growth of benthic S. horneri populations, but adjacent regions may all provide a source (Huang et al., 2018). Using satellite imagery, Xing et al. (2017) found in 2016 that the floating path of S. horneri golden tides could be traced from coastal waters at the eastern end of the Shandong Peninsula to the Subei Shoal. Qi et al. (2017) focused on the drift path of the golden tide in 2017 and speculated that it originated in the coastal waters of Zhejiang Province. Ding et al.(2019) believed that sessile S. horneri in the mussel, Mytilus galloprovincialis, farming area along the coast of Zhejiang may be one of the sources of golden tides. Thus, golden tides in the SYS may have a dual origin, although this remains unclear. Additionally, the Sargassum golden tide is known to influence the occurrence of Ulva green tides, indicating that the interaction between multiple HABs requires further attention. The occurrence of golden tides over the years is shown in Table 1.
Compared with other bodies of water off the coast of China, harmful red tides recorded in the SYS were previously much less important in terms of the toxic outbreak frequency and the geographic range of toxin influence (Yu et al., 2012); however, more recently an increasing trend has been documented. Several studies in 2020 showed that the coast of Lianyungang City experienced harmful red tides of the dinoflagellates K. veneficum and T. acrotrocha, Heterocapsa sp. (unpublished data). Based on the data in Table 2, we can draw the following conclusions. Harmful red tides in the Jiangsu sea area of the SYS have occurred mainly since 2000. The timing of the blooms varies from April to November, although they are most common in May, and are mainly located along the coast of Lianyungang and Nantong, Jiangsu Province. The area of harmful red tide outbreaks has ranged from hundreds to thousands of km2, and the main causative species of these blooms have been the diatoms Skeletonema sp., Thalassiosira sp., and Eucampia zoodiacus, and the dinoflagellates Gymnodinium catenatum, Heterosigma akashiwo, Amphidinium carterae, and Noctiluca scintillans, and more rarely, raphidophytes. All these species have damaged the marine ecosystem due to their toxicity and/or high biomass. In the Shandong area of the SYS, there were eight records of harmful red tides in 2009, making it the year with the most frequent red tides in the history of this region. The causative species of harmful red tides since 2009 are mainly N. scintillans, while red tides of the ciliate Mesodinium rubrum were more prevalent before 2009. The locations of harmful red tide occurrence are concentrated in the water adjacent to Qingdao, Rushan, Rizhao, and Rongcheng. The initiation of red tides has shown a trend of earlier appearance over the years. The data by area is shown in Table 2.
There are approximately 5 000 species of phytoplankton in the ocean, of which around 200 are toxic (Sournia, 1995; Du and Lu, 2008; Sarkar, 2018; Hallegraeff et al., 2021). Marine microalgae can produce a variety of toxins, most commonly including paralytic shellfish toxins (PSTs), diarrhetic shellfish toxins (DSTs), amnesic shellfish toxins (ASTs) or domoic acid, neurotoxic shellfish toxins (NSTs) or brevetoxins, and ciguatoxins (CTXs) (Egmond et al, 2004). The status of algal toxins in the SYS over the years, in terms of samples at various locations and their toxicities, is shown in Table 3.
The algal toxins in the adjacent sea area of Shandong in the SYS are dominantly DSTs, while both PSTs and DSTs have been detected in the seas adjacent to Jiangsu. The poisoning incidents caused by algal toxins along the SYS coast can mostly be attributed to PSTs. The toxic outbreaks caused by red tides in Lianyungang warrant further attention due to their high frequency. Real-time monitoring of shellfish poisoning, and parallel tracking of the occurrence of toxic HABs, will help provide a better scientific basis for developing suitable biotoxin management strategies.
4 CAUSES AND TRENDS IN HAB DIVERSITY IN THE SYSWhile the evidence for increase in HABs worldwide is not clear (Hallegraeff et al., 2021), the diversity, frequency, and intensity of HABs in the SYS have clearly increased from 2000 to 2020. The ecological environment of the SYS has changed, as evidenced by the co-occurrence of diverse HABs, which is rare worldwide. Harmful algal blooms are becoming increasingly severe, resulting in intensification of the impacts to the ecological environment, mariculture and the coastal society and economy. As indicated earlier, they have also become more diverse and complex.
The diverse HABs in the SYS occur in the context of global warming and climate change, human activities, marine environmental conditions, and ocean dynamic processes. First, with regard to global warming, the water temperature in coastal China has increased significantly (Pei et al., 2017; Yu et al., 2019; Li et al., 2020), such that warm water HAB species now tend to extend northward. Secondly, the development of China's coastal industries and agriculture, sewage and waste discharge activities have led to eutrophication of coastal waters, and exerts great pressure on the local marine ecology as well as providing a basis for the occurrence of HABs. Furthermore, China has a well-developed marine aquaculture industry; the scale of Neopyropia farming in coastal cities, such as Nantong, Yancheng, and Lianyungang in Jiangsu, has also been expanding (Zhang et al., 2014) and this massive increase in coastal aquaculture facilities has greatly changed the marine ecological environment. Finally, the appearance and transport of HABs are closely related to the physical environment and dynamic processes in the sea area, especially wind-driven currents caused by the southward winter monsoon and northward summer monsoon that can drive HABs northward and southward, respectively.
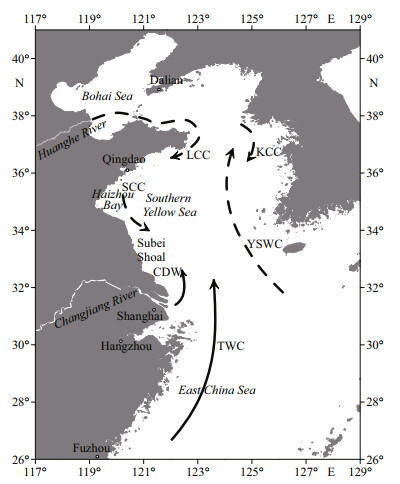
The offshore waters of the SYS in China mainly include the seas south of the Shandong Peninsula and those east of Jiangsu Province (Fig. 2). As a part of a semi-enclosed continental shelf marginal sea in the Western Pacific, the SYS not only lies in the East Asian monsoon area, but is also affected by the Kuroshio Current and El Niño phenomenon (Wu et al., 2018; Liu et al., 2021b). The ecology of the region is affected by both climate change and intensive human activities, resulting in the increasing prominence of ecological problems such as HABs (Gu et al., 2021). Currents in various directions in different seasons have also contributed to the particular co-occurrence of diverse HABs in the SYS (Fig. 2).
|
| Fig.2 The location and currents of the SYS in China CDW: Changjiang River diluted water; TWC: Taiwan Warm Current; YSWC: Yellow Sea Warm Current; SCC: Subei Coastal Current; LCC: Lubei Coastal Current; KCC: Korea Coastal Current. The arrowed solid lines and dashed lines mark the currents that predominated in summer and winter, respectively (modified after Guo et al. (2020)). |
The diverse HABs in the SYS are also interconnected. Firstly, there is competition for nutrients and space among algal species, as well as mutual inhibition or promotion via allelopathy. Laboratory studies have found that the relationship between different HAB species is largely one of mutual inhibition (Liu, 2015; Cai et al., 2019), but the dominant species tend to change under different conditions. For example, Ulva plays a dominant role in its interactions with microalgae at different development stages (Liu, 2015). Both S. horneri decomposition medium and culture medium can inhibit the attachment and germination of microscopic propagules of U. prolifera at high concentrations but can promote them at low concentrations (Cai et al., 2019), and can also inhibit the growth of some causative species of harmful red tides (Cai et al., 2019). Field observations have indicated that there is also connection among HABs. As shown in Table 1 and Fig. 1, the maximum distribution area of the golden tide in 2017 reached its peak (about 160 000 km2), while the maximum distribution area of the green tide in the same year was relatively small (29 522 km2). At the same time, the transport of nutrients and metabolites released during the decomposition of an organism may contribute to the occurrence of harmful red tides elsewhere. These interactions and their underlying mechanisms remain unclear and merit further investigation.
5 CONCLUSIONFrom a global perspective, once they appear, many HABs become recurrent in a given sea area, which gradually deteriorates the marine environment and negatively affects human health. The set of co-occurring complex and diverse HABs in the SYS is unique worldwide. It strongly indicates a deteriorating environment, and a potential threat to ecology and human health. Based on field observations and remote sensing, more data should be collected for further research on the early warning and prediction of diverse HABs to help develop comprehensive prevention and control measures. Furthermore, diverse HABs and aquaculture activities are interrelated. To some extents, human activities have intensified HABs in the SYS by affecting the marine environment. Moreover, interrelationships among diverse HABs under field conditions remain unclear. Therefore, there is an urgent need for the early warning and control of HABs based on the characteristics of diverse HABs, their environmental adaptability, the mechanisms of inter-specific competition, and their main controlling factors.
6 DATA AVAILABILITY STATEMENTAll data generated and/or analyzed during this study are included in this published article.
Anderson C R, Moore S K, Tomlinson M C et al. 2015. Living with harmful algal blooms in a changing world: strategies for modeling and mitigating their effects in coastal marine ecosystems. In: Shroder J F, Ellis J T, Sherman D J eds. Coastal and Marine Hazards, Risks, and Disasters. Elsevier, Boston. p. 495-561, https://doi.org/10.1016/B978-0-12-396483-0.00017-0.
|
Anderson D M, Cembella A D, Hallegraeff G M. 2012. Progress in understanding harmful algal blooms: paradigm shifts and new technologies for research, monitoring, and management. Annual Review of Marine Science, 4: 143-176.
DOI:10.1146/annurev-marine-120308-081121 |
Barnes R S K. 2019. Context dependency in the effect of Ulva-induced loss of seagrass cover on estuarine macrobenthic abundance and biodiversity. Aquatic Conservation: Marine and Freshwater Ecosystems, 29(2): 163-174.
DOI:10.1002/aqc.2977 |
Cai J C, Geng H X, Kong F Z, et al. 2019. Simulation study on the effect of Sargassum Horneri on other harmful bloom causative species. Oceanologia et Limnologia Sinica, 50(5): 1050-1058.
(in Chinese with English abstract) DOI:10.11693/hyhz20190100028 |
Cao B, Gao Q Q, He P D, et al. 2019. Study on the relationship between occurrence and extinction of red tide and the hydrological and meteorological factors in Jiangsu sea area. Transactions of Oceanology and Limnology, (3): 36-42.
(in Chinese with English abstract) |
Caruana A M N, Amzil Z. 2018. Microalgae and toxins. In: Levine I A, Fleurence J eds. Microalgae in Health and Disease Prevention. Elsevier, Academic. p. 263-305, https://doi.org/10.1016/B978-0-12-811405-6.00013-X.
|
Cosper E M, Lee C, Carpenter E J. 1990. Novel "Brown Tide" blooms in Long Island embayments: a search for the causes. In: Granéli E, Sundström B, Edler L et al eds. Toxic Marine Phytoplankton: Proceedings of the Fourth International Conference on Toxic Marine Phytoplankton. New York, Elsevier. p. 17-28.
|
Devault D A, Modestin E, Cottereau V, et al. 2021. The silent spring of Sargassum. Environmental Science and Pollution Research, 28(13): 15580-15583.
DOI:10.1007/s11356-020-12216-7 |
DeYoe H R, Stockwell D A, Biolagare R R, et al. 1997. Description and characterization of the algal species Aureoumbra lagunensis gen.et sp. Nov. And referral of Aureoumbra and Aureococcus to the pelagophyceae. Journal of Phycology, 33(6): 1042-1048.
DOI:10.1111/j.0022-3646.1997.01042.x |
Ding X W, Zhang J H, Zhuang M M, et al. 2019. Growth of Sargassum horneri distribution properties of golden tides in the Yangtze Estuary and adjacent waters. Marine Fisheries, 41(2): 188-196.
(in Chinese with English abstract) |
Du K M, Jiang T J, Wu N. 2013. The pattern of paralytic shellfish poisoning in shellfish cultured in the coast of Yellow Sea, China. Marine Environmental Science, 32(2): 182-184.
(in Chinese with English abstract) |
Du W, Lu D D. 2008. Harmful effects and detection of toxic algae and their algal toxins. Journal of Marine Sciences, 26(2): 89-97.
(in Chinese with English abstract) DOI:10.3969/j.issn.1001-909X.2008.02.013 |
Egmond H P, Van Apeldoorn M E, FAO. 2004. FAO Food and nutrition paper 80. Marine biotoxins. Food and Agriculture Organization of the United Nations, Rome. p. 185-218.
|
Fletcher R L. 1996. The occurrence of "Green Tides"—a review. In: Schramm W, Nienhuis P H eds. Marine Benthic Vegetation: Recent Changes and the Effects of Eutrophication. Springer, Berlin. p. 7-43, https://doi.org/10.1007/978-3-642-61398-2_2.
|
Gao Q Q, Cao B, Yang B, et al. 2017. Characteristics of the red tide in the sea area of Jiangsu. Marine Science Bulletin, 36(2): 217-221, 229.
(in Chinese with English abstract) DOI:10.11840/j.issn.1001-6392.2017.02.013 |
Gastrich M D, Wazniak C E. 2002. A Brown Tide Bloom Index based on the potential harmful effects of the brown tide alga, Aureococcus anophagefferens. Aquatic Ecosystem Health & Management, 5(4): 435-441.
DOI:10.1080/14634980290002011 |
Gu H F, Wu Y R, Lȹ S H, et al. 2021. Emerging harmful algal bloom species over the last four decades in China. Harmful Algae, 111: 102059.
DOI:10.1016/j.hal.2021.102059 |
Guan C J, Feng Z Q, Ma M H, et al. 1999. Monitor of paralytic shellfish poison in the commercial shellfishes in the coast from Yalujiang to Changjiang of China. Marine Environmental Science, 18(2): 49-52.
DOI:10.3969/j.issn.1007-6336.1999.02.010 |
Guo C C, Zhang G C, Sun J, et al. 2020. Seasonal responses of nutrient to hydrology and biology in the southern Yellow Sea. Continental Shelf Research, 206: 104207.
DOI:10.1016/j.csr.2020.104207 |
Hallegraeff G M, Anderson D M, Belin C, et al. 2021. Perceived global increase in algal blooms is attributable to intensified monitoring and emerging bloom impacts. Communications Earth & Environment, 2(1): 117.
DOI:10.1038/s43247-021-00178-8 |
Hallegraeff G M. 1993. A review of harmful algal blooms and their apparent global increase. Phycologia, 32(2): 79-99.
DOI:10.2216/i0031-8884-32-2-79.1 |
Huang B X, Ding L P, Qin S, et al. 2018. The taxonomical status and biogeographical distribution of Sargassum horneri with the origin analysis of its drifting population in the end of 2016 at the western Yellow Sea. Oceanologia et Limnologia Sinica, 49(1): 214-223.
|
Huang X, Jiang T J, Wu N. 2013. The pattern of diarrhetic shellfish poisoning in shellfish cultured in the coast of Yellow Sea, China. Marine Environmental Science, 32(2): 178-181.
(in Chinese with English abstract) |
Huo W Y, Yu Z M, Zou J Z, et al. 2001a. Analysis of dynamic process and the causes of Eucampia Zoodiacus red tide in Jiaozhou Bay. Journal of Fisheries of China, 25(3): 222-226.
(in Chinese with English abstract) |
Huo W Y, Yu Z M, Zou J Z, et al. 2001b. Outbreak of Skeletonema Costatum red tide and its relations toenvironmental factors in Jiaozhou Bay. Oceanologia et Limnologia Sinica, 32(3): 311-318.
(in Chinese with English abstract) |
Jiangsu Marine and Fisheries Bureau (JSMFB). 2011-2017. The bulletin of marine environment quality of Jiangsu. Jiangsu Marine and Fisheries Bureau, Nanjing. (in Chinese)
|
Komatsu T, Matsunaga D, Mikami A, et al. 2008. Abundance of drifting seaweeds in eastern East China Sea. Journal of Applied Phycology, 20(5): 801-809.
DOI:10.1007/s10811-007-9302-4 |
Komatsu T, Mizuno S, Natheer A, et al. 2014. Unusual distribution of floating seaweeds in the East China Sea in the early spring of 2012. Journal of Applied Phycology, 26(2): 1169-1179.
DOI:10.1007/s10811-013-0152-y |
Kong F Z, Jiang P, Wei C J, et al. 2018. Co-occurence of green tide, golden tide and red tides along the 35£N transect in the Yellow Sea during Spring and Summer in 2017. Oceanologia et Limnologia Sinica, 49(5): 1021-1030.
|
Kong F Z, Yu R C, Zhang Q C, et al. 2012. Primary analyses on the causative species of a bloom in the Sanggou Bay. Marine Environmental Science, 31(6): 824-829.
|
Kudela R M, Berdalet E, Enevoldsen H, et al. 2017. GEOHABjthe global ecology and oceanography of harmful algal blooms program: motivation, goals, and legacy. Oceanography, 30(1): 12-21.
DOI:10.5670/oceanog.2017.106 |
Li J. 2005. Research on Biotoxins in Shellfish in Coastal Areas of China. The Institute of Oceanology, Chinese Academy of Sciences, Qingdao. (in Chinese with English abstract)
|
Li W C, Luan G, Li L, et al. 2000. Investigation of shellfish toxins in some sea areas of China. Marine Sciences, 24(9): 19-22, 18.
(in Chinese) DOI:10.3969/j.issn.1000-3096.2000.09.008 |
Li X Y, Yu R C, Geng H X, et al. 2021. Increasing dominance of dinoflagellate red tides in the coastal waters of Yellow Sea, China. Marine Pollution Bulletin, 168: 112439.
DOI:10.1016/j.marpolbul.2021.112439 |
Li Y, Mu L, Wang Q Y, et al. 2020. High-quality sea surface temperature measurements along coast of the Bohai and Yellow Seas in China and their long-term trends during 1960-2012. International Journal of Climatology, 40(1): 63-76.
DOI:10.1002/joc.6194 |
Li Z X, Guo M M, Yang S G, et al. 2010. Investigation of pectenotoxin profiles in the Yellow Sea (China) using a passive sampling technique. Marine Drugs, 8(4): 1263-1272.
DOI:10.3390/md8041263 |
Liang Y B, Li D M, Yao J Y, et al. 2019. Progresses in investigation and research on phycotoxins and toxic microalgaes in the coastal waters of China. Oceanologia et Limnologia Sinica, 50(3): 511-524.
|
Liang Y B. 2012. Zhongguo Chichao Zaihai Diaocha Yu Pingjia (1933-2009). Ocean Press, Beijing.
(in Chinese)
|
Liu J L, Xia J, Zhuang M M, et al. 2021a. Golden seaweed tides accumulated in Pyropia aquaculture areas are becoming a normal phenomenon in the Yellow Sea of China. Science of the Total Environment, 774: 145726.
DOI:10.1016/j.scitotenv.2021.145726 |
Liu Q. 2015. The Interactions Study Between Bloom-Forming Ulva prolifera and Phytoplankton in the Yellow Sea. TheInstitute of Oceanology, Chinese Academy of Sciences, Qingdao. (in Chinese with English abstract)
|
Liu Z Q, Gan J P, Hu J Y, et al. 2021b. Progress on circulation dynamics in the East China Sea and southern Yellow Sea: origination, pathways, and destinations of shelf currents. Progress in Oceanography, 193: 102553.
DOI:10.1016/j.pocean.2021.102553 |
Lu D D, Qi Y Z, Gu H F, et al. 2014. Causative species of harmful algal blooms in Chinese coastal waters. Algological Studies, 145-146(1): 145-168.
DOI:10.1127/1864-1318/2014/0161 |
Lu M, Zhang L J, Li C, et al. 2001. Analysis of the ecological environment elements in the red tide generating and vanishing process in the eastern Jiaozhou Bay in July, 1999. Journal of Oceanography of Huanghai & Bohai Seas, 19(4): 43-50.
(in Chinese with English abstract) |
Luo X. 2011. Population Dynamics and Toxin Production of Dinophysis Species in the Coastal Waters of Qingdao. TheInstitute of Oceanology, Chinese Academy of Sciences, Qingdao. (in Chinese with English abstract)
|
Ministry of Ecology and Environment (MEE). 2009-2017. Bulletin of China Marine Ecological Environment Status. http://www.mee.gov.cn/hjzl/sthjzk/jagb/. (in Chinese)
|
Ministry of Natural Resources (MNR). 2009-2020. The Bulletin of China Marine Disaster. http://www.mnr.gov.cn/sj/sjfw/hy/gbgg/zghyzhgb/. (in Chinese)
|
North China Sea Branch of Ministry of Natural Resources (NCSB). 2020. Bulletin of Marine Disasters in the North China Sea. http://ncs.mnr.gov.cn/n1/n128/n298/210508091503128160.html. (in Chinese)
|
Ocean and Fishery Administration of Qingdao (QOFA). 2017. Report on Marine Environmental Quality of Qingdao. (in Chinese)
|
Pei Y H, Liu X H, He H L. 2017. Interpreting the sea surface temperature warming trend in the Yellow Sea and East China Sea. Science China Earth Sciences, 60(8): 1558-1568.
DOI:10.1007/s11430-017-9054-5 |
Peng M, Liang X H, Zhao A B. 2010. Analysis on occurrence of red tide and characteristics of hydrometeorological environmental factors in Haizhou Bay, Lianyungang. Ocean Development and Management, 2(9): 48-53.
(in Chinese) DOI:10.3969/j.issn.1005-9857.2010.09.014 |
Peng M, Liu S D, Zhao A B, et al. 2015. Survey on the causes and features of red tide in Lianyungang coastal waters. Marine Forecasts, 32(2): 51-56.
|
Probyn T, Pitcher G, Pienaar R, Nuzzi R. 2001. Brown tides and mariculture in Saldanha Bay, South Africa. Marine Pollution Bulletin, 42(5): 405-408.
DOI:10.1016/S0025-326X(00)00170-3 |
Qi L, Hu C M, Wang M Q, et al. 2017. Floating algae blooms in the East China Sea. Geophysical Research Letters, 44(22): 11501-11509.
DOI:10.1002/2017GL075525 |
Reich A, Blackmore C, Hopkins R, et al. 2008. Illness associated with red tide-Nassau County, Florida, 2007. Morbidity and Mortality Weekly Report, 57(26): 717-720.
|
Sarkar S K. 2018. Marine Algal Bloom: Characteristics, Causes and Climate Change Impacts. Springer, Singapore. 170p. https://doi.org/10.1007/978-981-10-8261-0.
|
Shandong Department of Ocean and Fishery (JSMFB). 2001-2017. The bulletin of marine environment status of Shandong. http://www.nmdis.org.cn/hygb/zghyhjzlgb/yhsshyhjzlgb/2008nhyhjzlgb/2008nsdshyhjzlgb/. (in Chinese)
|
Sieburth J M, Johnson P W, Hargraves P E. 1988. Ultrastructure and ecology of Aureococcus anophageferens gen.et sp. Nov. (Chrysophyceae): the dominant picoplankter during a bloom in Narragansett Bay, Rhode Island, summer 1985. Journal of Phycology, 24(3): 416-425.
DOI:10.1111/j.1529-8817.1988.tb04485.x |
Sissini M N, De Barros Barreto M B B, SzȦchy M T M, et al. 2017. The floating Sargassum (Phaeophyceae) of the South Atlantic Oceanjlikely scenarios. Phycologia, 56(3): 321-328.
DOI:10.2216/16-92.1 |
Smetacek V, Zingone A. 2013. Green and golden seaweed tides on the rise. Nature, 504(7478): 84-88.
DOI:10.1038/nature12860 |
Song X M, Xie X H, Zhao C H, et al. 2013. The study on detection of diarrhetic shellfish poisons by ELISA in Ostrea rivularis Gould. Journal of Aquaculture, 34(1): 49-52.
(in Chinese with English abstract) DOI:10.3969/j.issn.1004-2091.2013.01.012 |
Sournia A. 1995. Red tide and toxic marine phytoplankton of the world ocean: an inquiry into biodiversity. In: Lassus P, Arzul G, Gentien P et al eds. Harmful Marine Algal Blooms. Lavoisier Intercet Ltd, Paris. p. 103-112.
|
Sunda W G, Graneli E, Gobler C J. 2006. Positive feedback and the development and persistence of ecosystem disruptive algal blooms. Journal of Phycology, 42(5): 963-974.
DOI:10.1111/j.1529-8817.2006.00261.x |
Trainer V L, Moore S K, Hallegraeff G, et al. 2020. Pelagic harmful algal blooms and climate change: lessons from natureos experiments with extremes. Harmful Algae, 91: 101591.
DOI:10.1016/j.hal.2019.03.009 |
Valiela I, McClelland J, Hauxwell J, et al. 1997. Macroalgal blooms in shallow estuaries: controls and ecophysiological and ecosystem consequences. Limnology and Oceanography, 42(5part2): 1105-1118.
DOI:10.4319/lo.1997.42.5_part_2.1105 |
Wu H, Gu J H, Zhu P. 2018. Winter counter-wind transport in the inner southwestern Yellow Sea. Journal of Geophysical Research: Oceans, 123(1): 411-436.
DOI:10.1002/2017jc013403 |
Xiao J, Wang Z L, Liu D Y, et al. 2021. Harmful macroalgal blooms (HMBs) in Chinaos coastal water: Green and golden tides. Harmful Algae, 107: 102061.
DOI:10.1016/j.hal.2021.102061 |
Xing Q G, Guo R H, Wu L L, et al. 2017. High-resolution satellite observations of a new hazard of Golden Tides caused by floating Sargassum in winter in the Yellow Sea. IEEE Geoscience and Remote Sensing Letters, 14(10): 1815-1819.
DOI:10.1109/LGRS.2017.2737079 |
Xu M, Sasa S, Komatsu T. 2018. Sargassum horneri C.Agardh space capacity estimation reveals that thallus surface area varies with wet weight. PLoS One, 13(6): e0199103.
DOI:10.1371/journal.pone.0199103 |
Xu N, Pang S J, Liu F. 2012. Molecular identification of a bloom-forming species isolated from Sanggou Bay in Shandong Province. Marine Sciences, 36(4): 13-18.
(in Chinese with English abstract) DOI:10.3969/j.issn.1001-909X.2012.04.002 |
Xu Y R, He S, Zhou C X, et al. 2014. A review of karlotoxins. Marine Sciences, 38(10): 113-118.
(in Chinese) DOI:10.11759/hykx20130819001 |
Yan T, Tan Z J, Li J, et al. 2001. A preliminary study on toxicity evaluation of HAB using bioassay-Some bioassay methods used in an HAB event in Jiaozhou Bay. Marine Environmental Science, 20(3): 5-8, 50.
DOI:10.3969/j.issn.1007-6336.2001.03.002 |
Yao Y. 2004. The Characteristics and Mechanism of Eutrophication in Jiaozhou Bay. The Institute of Oceanology, Chinese Academy of Sciences, Qingdao. (in Chinese with English abstract)
|
Ye N H, Zhang X W, Mao Y Z, et al. 2011. nGreen tideso are overwhelming the coastline of our blue planet: taking the worldos largest example. Ecological Research, 26(3): 477-485.
DOI:10.1007/s11284-011-0821-8 |
Yoshida T. 1963. Studies on the distribution and drift of the floating seaweeds. Bulletin of Tohoku Regional Fisheries Research Laboratory, 23: 141-186.
|
Yu N, Yu J S, Lv Z B, et al. 2012. Disaster characteristics of harmful algal bloom and its early warning management in Shandong coastal waters. Chinese Journal of Ecology, 31(5): 1272-1281.
(in Chinese with English abstract) |
Yu R C, Liu D Y. 2016. Harmful algal blooms in the coastal waters of China: current situation, long-term changes and prevention strategies. Bulletin of Chinese Academy of Sciences, 31(10): 1167-1174.
|
Yu R C, Luo X. 2016. Status and research perspectives on toxic algae and phycotoxins in the coastal waters of China. Studia Marina Sinica, (1): 155-166.
(in Chinese with English abstract) |
Yu R C, Sun S, Yan T, et al. 2018. Progresses and perspectives on green-tide studies in the Yellow Sea. Oceanologia et Limnologia Sinica, 49(5): 942-949.
(in Chinese with English abstract) DOI:10.11693/hyhz20180700158 |
Yu Y, Zhang H R, Jin J B, et al. 2019. Trends of sea surface temperature and sea surface temperature fronts in the South China Sea during 2003-2017. Acta Oceanologica Sinica, 38(4): 106-115.
DOI:10.1007/s13131-019-1416-4 |
Zhang C Y, Liu J T. 2009. Marine disasters and analysis of Lianyungang city. Transactions of Oceanology and Limnology, (1): 35-40.
(in Chinese with English abstract) DOI:10.13984/j.cnki.cn37-1141.2009.01.012 |
Zhang P P, Yang R, Wu X K. 2014. Laver industry in Jiangsu Province: investigation. Journal of Ningbo University(Natural Science & Engineering Edition), 27(1): 18-22.
(in Chinese with English abstract) |
Zhang Q C, Wang Y F, Song M J, et al. 2022. First record of a Takayama bloom in Haizhou Bay in response to dissolved organic nitrogen and phosphorus. Marine Pollution Bulletin, 178: 113572.
DOI:10.1016/j.marpolbul.2022.113572 |
Zhang Y Y, He P M, Li H M, et al. 2019. Ulva prolifera green-tide outbreaks and their environmental impact in the Yellow Sea, China. National Science Review, 6(4): 825-838.
DOI:10.1093/nsr/nwz026 |
Zhao J, Jiang P, Qin S, et al. 2015. Genetic analyses of floatingUlva prolifera in the Yellow Sea suggest a uniqueecotype. Estuarine, Coastal and Shelf Science, 163: 96-102.
DOI:10.1016/j.ecss.2015.05.027 |
Zhao X Y, Tang X X, Zhang H X, et al. 2016. Photosynthetic adaptation strategy of Ulva prolifera floating on the sea surface to environmental changes. Plant Physiology and Biochemistry, 107: 116-125.
DOI:10.1016/j.plaphy.2016.05.036 |
Zhou M J, Li J, Luckas B, et al. 1999. A recent shellfish toxin investigation in China. Marine Pollution Bulletin, 39(1-12): 331-334.
DOI:10.1016/S0025-326X(99)00026-0 |
Zhou M J, Liu D Y, Anderson D M, et al. 2015. Introduction to the special issue on green tides in the Yellow Sea. Estuarine, Coastal and Shelf Science, 163: 3-8.
DOI:10.1016/j.ecss.2015.06.023 |
Zhou M J, Zhu M Y, Zhang J. 2001. Status of harmful algal blooms and related research activities in China. Chinese Bulletin of Life Sciences, 13(2): 54-59, 53.
DOI:10.3969/j.issn.1004-0374.2001.02.002 |
 2022, Vol. 40
2022, Vol. 40