Institute of Oceanology, Chinese Academy of Sciences
Article Information
- TANG Jia, CAI Wenqi, YAN Zhicong, WU Zhongjie, YANG Qianxi, ZHOU Zhi
- Involvement of the ammonium assimilation mediated by glutamate dehydrogenase in response to heat stress in the scleractinian coral Pocillopora damicornis
- Journal of Oceanology and Limnology, 40(5): 2001-2011
- http://dx.doi.org/10.1007/s00343-021-1266-8
Article History
- Received Aug. 19, 2021
- accepted in principle Oct. 11, 2021
- accepted for publication Oct. 24, 2021
2 Hainan Academy of Ocean and Fisheries Sciences, Haikou 570228, China
Scleractinian corals flourish in the oligotrophic tropical and subtropical marine environment due to the subtle coral-Symbiodiniaceae symbiosis (Tong et al., 2017). In the symbiotic association, the coral hosts absorb and assimilate inorganic nutrients from seawater environment, and further transfer these nutrients to symbiotic Symbiodiniaceae. In exchange, the symbionts provide its photosynthetic products as organic nutrients to the coral hosts (Wang andDouglas, 1999; Rosic et al., 2014). However, the symbiosis has been threatened by the rise of sea surface temperature owing to global warming(Douglas, 2003; Claar et al., 2020), and the threatening process was affected by the assimilation and exchange of nitrogen nutrients in the symbiotic association (Rädecker et al., 2015; Morris et al., 2019).
The coral-Symbiodiniaceae symbiotic association assimilates ammonium as preferred nitrogen resource, and the resulting nitrogen cycling plays an important role in the maintenance of the symbiosis. It has been proposed that ammonium can be assimilated mostly by the coral hosts through glutamine synthetase and/ or glutamate dehydrogenase (GS/GDH) pathway, and slightly by symbiotic Symbiodiniaceae through glutamine synthetase/glutamine: 2-oxoglutarate aminotransferase (GS/GOGAT) pathway (Rädecker et al., 2015; Su et al., 2018). The symbiotic Symbiodiniaceae need more nitrogen resource than the coral hosts, and therefore the symbionts are under nitrogen limitation, which can regulate symbiont density to maintain their symbiosis (Muscatine et al., 1989; Yellowlees et al., 2008; Rädecker et al., 2015). It has been also reported that elevated nitrogen availability disrupts the nitrogen limitation of symbiotic Symbiodiniaceae, and ultimately causes coral bleaching (Wiedenmann et al., 2013; Wooldridge, 2013). Furthermore, high temperature has been considered to be able to disrupt the symbiosis via increasing the ammonium assimilation rates of coral hosts and regulating the nitrogen limitation of symbiotic Symbiodiniaceae (Rädecker et al., 2015). However, the molecular mechanism underlying the process is still not explored well in scleractinian corals.
GDH widely exists in most organisms and catalyzes the reversible oxidative deamination of L-glutamate to α-ketoglutarate and ammonia using NAD(P)H as coenzyme, which is the primary pathway of ammonia assimilation (Dudler et al., 1987; Hudson and Daniel, 1993). GDH is classified into three types according to their coenzyme specificity, including NADPH-specific, NADH-specific, and nonspecific enzymes, and different types of GDH show distinct regulatory and kinetic properties (Catmull et al., 1987; Dudler et al., 1987). Several GDHs have been also identified in cnidaria, such as coral Acropora formosa and sea anemone Anthopleura xanthogrammica, and play an important role in the nitrogen metabolism in cnidarian-dinoflagellate symbiosis (Male and Storey 1983; Catmull et al., 1987; Dudler and Miller 1988). Furthermore, our previous study showed that the catalytic activity of NADH-GDH was induced by acute heat stress in the scleractinian coral P. damicornis (Tang et al., 2020). The detailed activity characteristics and heat stress response mode of GDH in scleractinian corals is still unclear, in spite of its important role in the nitrogen metabolism in the symbiotic association.
Pocillopora damicornis is a scleractinian coral belonging to the family Pocilloporidae, and native to the tropical and subtropical regions of the Indian and Pacific Oceans. It is a bleaching-sensitive coral and commonly used as a model species for studying experimental biology and physiology of scleractinian corals (Hill et al., 2014; Cunning et al., 2018). This study focuses on the following critical questions: (1) identify the homologue of GDH presented in the scleractinian coral P. damicornis (designated as PdGDH); (2) explore the effects of temperature, pH, green tea catechin, and epigallocatechin-3-gallate (EGCG) on PdGDH activity; (3) investigate the expression and activity changes of PdGDH in response to heat and ammonium stress. These results pave a new way to further understand the ammonium assimilation and nitrogen cycling mechanisms under stress response and environmental acclimatization of scleractinian corals.
2 MATERIAL AND METHOD 2.1 CoralPocillopora damicornis colonies were collected from a fringing reef in Wenchang (Hainan Province, China), and then cultured in 500-L flow-through tanks with natural seawater (temperature: 26; salinity: 35). Cultured corals were illuminated with two white and two blue fluorescent bulbs (Philips T5HO Activiva Active 54 W) in a 12-h꞉12-h light-dark cycling to acclimatize for one month in the laboratory condition.
2.2 Heat and ammonium stress experimentThirty-five coral nubbins were employed in the heat and ammonium stress experiments. Specially, five untreated coral nubbins were sampled at 0 h as the blank group, and the remaining thirty nubbins were randomly divided into three groups: control, heat, and heat_NH4 (n=10 per group). The coral nubbins in the control and heat groups were placed in seawater at 26 ℃ and 32 ℃, respectively, whereas the coral nubbins in the heat_NH4 group were placed in ammonium seawater at 32 ℃. The ammonium seawater was prepared by adding ammonium chloride (NH4Cl) to filtered seawater at a final concentration of 10 μmol/L. Five nubbins were sampled from three groups (control, heat, and heat_NH4) at 12 and 24 h after treatment. The obtained samples were used for subsequent RNA extraction and NADH-GDH activity assay.
2.3 RNA extraction and cDNA synthesisThe sampled coral nubbins were grinded in liquid nitrogen, followed by mixing with 1-mL Trizol reagent (Invitrogen), and then the harvested homogenates were centrifuged at 5 000 g, 4 ℃ for 5 min to remove the calcium carbonate skeleton. Total RNA was extracted according to the protocol described by Stefanik (Stefanik et al., 2013), and reversed into cDNA using M-MLV kit (Promega, USA) with oligo (dT)-adaptor as primer (Table 1) (Jiang et al., 2013). The synthesized cDNA libraries were diluted to 1 100 and stored at -80 ℃ for subsequent gene cloning and quantitative real-time PCR.
Transcriptome libraries of the scleractinian coral P. damicornis after ammonium stress were previously sequenced and assembled (Yuan et al., 2017), and one transcript (No. TR73996_c2_g2_i1) was revealed through BLAST search to be homologous to GDHs identified previously in other animals. The transcript contained a completed open reading frame (ORF), which represented the whole-length coding region of PdGDH. Two specific primers (Table 1) were designed to clone the PdGDH ORF. After PCR amplification, the product fragment (1 611 bp) was purified using the MiniBEST Agarose Gel DNA Extraction Kit (TaKaRa, Japan) and cloned into pMD19-T simple vector (TaKaRa, Japan). After being transformed into the competent cells of Escherichia coli DH5α (Transgen, China), three positive clones were identified through PCR screening and sequenced on an ABI 3730 XL Automated Sequencer (Applied Biosystems) to verify the sequence.
The theoretical isoelectric point and molecular weight of the deduced PdGDH protein were calculated with ExPASy (https://web.expasy.org/compute_pi/). The possible signal peptide and domain of PdGDH protein were predicted by SignalP 4.1 Server (http://www.cbs.dtu.dk/services/SignalP/) and Conserved Domain Database at NCBI (http://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml/), respectively. Homologous molecules for PdGDH were searched using BLAST algorithms in NCBI GenBank (http://www.ncbi.nlm.gov/blast/). Multiple alignment of amino acid sequences of PdGDH and other GDHs was analyzed by ClustalX program, and secondary structure of PdGDH was predicted using Protein Data Bank (https://www.rcsb.org/), following by the visualization using ESPript (http://espript.ibcp.fr/ESPript/ESPript/) (Robert and Gouet, 2014). Phylogenetic analyses of PdGDH and other GDHs were performed using the neighbor-joining (NJ) method in the MEGA X software, and a bootstrap analysis was performed based on 1 000 bootstrap replications.
2.5 Quantitative real-time PCR and gene expression analysisQuantitative real-time PCR was carried out to examine the relative expression level of PdGDH in coral nubbins at 12 h after heat and ammonium stress in accordance with a previous protocol (Zhou et al., 2012). A PdGDH fragment was amplified using two specific primers, and the fragment of elongation factor (PdEF) was used as endogenous control. All primers used in the present study were shown in Table 1. Three replicates were conducted for each coral nubbin sample. The 2-ΔΔCt method was used to calculate the relative mRNA expression levels of PdGDH in corals (Livak and Schmittgen, 2001).
2.6 Recombinant expression and purification of PdGDHThe sequence encoding PdGDH mature peptide was amplified using specific primers (Table 1) with the vector pMD19-T-PdGDH as templates, and then inserted into pEASY-E1 expression vector (TransGen, China). Next, the pEASY-E1-PdGDH construct was transformed into E. coli BL21 (DE3)-Transetta (TransGen, China), and the positive transformants were cultured in 100 mL of lysogeny broth (LB) medium with 100-mg/mL ampicillin at 37 ℃ with shaking at 200 r/min. When the culture media reached OD600 of 0.6, isopropyl β-D-thiogalactopyranoside (IPTG) was added at the final concentration of 1.0 mmol/L to induce the expression of PdGDH recombinant protein (rPdGDH) for 4 h. The bacteria were collected, sonicated, and centrifuged to remove the insoluble debris. The rPdGDH with His-tag was purified through a Ni2+ chelating Sepharose column (Sangon Biotech, China), and the purified protein sample was analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The purified rPdGDH was refolded in gradient urea-TBS buffer (50-mmol/L Tris, 50-mmol/L NaCl, 1-mmol/L EDTA.2Na, 1-mmol/L reduced glutathione, 2-mmol/L oxidized glutathione, 133-mmol/L glycine, a gradient urea concentration of 8-, 6-, 4-, 2-, 1-, and 0-mol/L urea in each gradient, pH 7.4, each gradient at 4 ℃ for 12 h). The concentration of purified rPdGDH was quantified using bicinchoninic acid (BCA) Protein Assay kit (Sangon Biotech, China).
2.7 Activity assay of rPdGDH at different temperature, pH conditions, and EGCG concentrationsThe activity of rPdGDH was measured at different temperature and pH conditions according to the description of Yang et al. (2005). Briefly, 10 μL of rPdGDH was added to 800 μL of reaction mixture (100-mmol/L Tris-HCl, 2-mmol/L α-ketoglutarate, 0.2-mmol/L NAD(P)H, and 10-mmol/L NH4Cl), and then absorbance at 340 nm was measured at 0 min and 5 min. To evaluate the effects of temperature and pH on the rPdGDH activity, it was measured at temperatures ranging from 20 to 35 ℃ at 5-℃ intervals (pH 7.5), and pH ranging from 6.5 to 9.5 at 0.5 pH intervals (at 35 ℃). Temperature and pH were changed by water bath and adding hydrochloric acid respectively, and the pH value was detected by pH meter (METTLER TOLEDO, FiveEasy Plus, FE28-Micro). One unit of enzyme was defined as that the amount which catalyzes the consumption of 1 nmol/L of NAD(P)H per minute, and the specific activity was standardized to per mg protein and expressed as U/mg.
The inhibitory effect of different concentrations of EGCG on rPdGDH activity was also evaluated according to the above-mentioned methods. To avoid the influence of EGCG color on the experimental results, each concentration of EGCG required a control tube. Specially, EGCG was added into the reaction mixture (35 ℃, pH 7.5) at final concentrations of 0, 10-4, 10-5, and 10-6 mol/L, and then 10 μL of rPdGDH was added to the reaction mixture. Similar process was performed in the control tube, expect that rPdGDH samples were replaced with equal volumes of phosphate buffered saline (PBS, 377-mmol/L NaCl, 2.7-mmol/L KCl, 8.09-mmol/L Na2HPO4, 1.47-mmol/L KH2PO4, pH 7.4). The relative activities of rPdGDH at different concentrations of EGCG were determined as the ratio of OD340 to that at 0-mol/L EGCG.
2.8 Activity assay of NADH-GDH after heat and ammonium treatmentNADH-GDH activity was measured in the coral nubbins at 0, 12, and 24 h after heat and ammonium stress. Coral tissue homogenates were prepared with the Waterpik water jet and pre-cooled PBS (4 ℃), and the homogenates were centrifuged at 5 000×g, 4 ℃ for 15 min to obtain the supernatants for the determination of NADH-GDH activity. Coral NADH-GDH activity was also determined according to the above-mentioned methods, except for replacing 10-μL rPdGDH with 200-μL coral supernatant. Moreover, the concentration of total protein in the supernatant was quantified using BCA method, and NADH-GDH activity was normalize to per mg protein and expressed as U/mg.
2.9 Statistical analysisAll data were expressed as mean±standard deviation (SD). Statistical differences were assessed through one-way analysis of variance (ANOVA) followed by multiple comparison (S-N-K). The above operations were all performed using software IBM SPSS 20.0 (IBM Inc.), and significant differences were considered at P < 0.05.
3 RESULT 3.1 Molecular characteristics of PdGDHThe ORF of PdGDH with the length of 1 611 bp was cloned and identified, and it was the same with the sequence of XM_027194020 (GenBank ID) which was from the genome annotation of P. damicornis (Cunning et al., 2018). PdGDH encoded a polypeptide of 536 amino acid residues, and its theoretical molecular weight and isoelectric point were 59.27 kDa and 7.57, respectively. No signal peptide was predicted by SignalP software. The deduced PdGDH protein contained a GdhA domain (from Val95 to Tyr525) and a NAD(P)H binding site (Gly290, Asn291, Val292, Glu312, Arg313, Arg364, Asn365, Gly385, Ala386, and Asn387).
The results of amino acid sequence alignment showed that PdGDH shared 96% identity with GDH from Stylophora pistillata (XP_022804879.1), 87% with that from Acroporamillepora(XP_029200822.1), and 90% with that from Orbicella faveolata (XP_020616550.1). The alignment also revealed eight conserved cysteine residues (Cys46, 92, 126, 152, 234, 297, 307, and 357). Furthermore, the predicted secondary structure of PdGDH protein was composed of 17 α-helix, 10 β-sheet, 7 3/10-helix, 1 α-turns, and 6 β-turns (Fig. 1).

|
| Fig.1 Multiple sequence alignment of PdGDH with other glutamate dehydrogenases from Stylophora pistillata (SpGDH, XP_022804879.1), Acropora millepora (AmGDH, XP_029200822.1), and Orbicella faveolata (OfGDH, XP_020616550.1) in the NCBI GenBank The red background in all four proteins indicates conserved residues, and secondary structure of PdGDH was indicated at the top of each panel. α: α-helix; β: β-sheet; η: 3/10-helix; TT: β-turns; TTT: α-turns. |
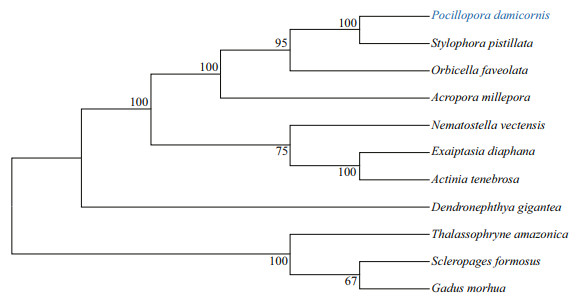
A phylogenetic tree was constructed based on the polypeptide sequences of PdGDH and GDHs from other animals. Two distinct clades were observed in the tree. The PdGDH was firstly clustered with GDHs from cnidaria S. pistillata (XP_022804879.1), O. faveolata (XP_020616550.1), A. millepora (XP_029200822.1), Nematostella vectensis (XP_032237771.1), Exaiptasia diaphana (KXJ18637.1), Actinia tenebrosa (XP_031562890.1), and Dendronephthya gigantea (XP_028408260.1) to form the first clade. All the GDHs from vertebrates were clustered into the second clade, including Thalassophryne amazonica (XP_034040172.1), Scleropages formosus (XP_029110146.1), and Gadus morhua (XP_030234570.1) (Fig. 2).
|
| Fig.2 A phylogenetic neighbor-joining tree based on sequence alignment of glutamate dehydrogenases from different animals The numbers at the branches indicate the bootstrap. The protein sequences used for phylogenetic analysis include: Stylophora pistillata (XP_022804879.1), Orbicella faveolata (XP_020616550.1), Acropora millepora (XP_029200822.1), Nematostella vectensis (XP_032237771.1), Exaiptasia diaphana (KXJ18637.1), Actinia tenebrosa (XP_031562890.1), Dendronephthya gigantea (XP_028408260.1), Thalassophryne amazonica (XP_034040172.1), Scleropages formosus (XP_029110146.1), and Gadus morhua (XP_030234570.1). The scleractinian coral Pocillopora damicornis was marked in blue. |
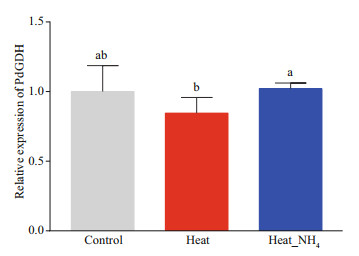
The quantitative real-time PCR was employed to determine the expression level of PdGDH mRNA in coral nubbins at 12 h after heat and ammonium stress. The expression level of PdGDH mRNA did not change significantly in both heat and heat_NH4 groups, in comparison with that in the control group. However, the expression level of PdGDH mRNA in the heat_NH4 group (1.21-fold, P<0.05) was significantly higher than that in the heat group (Fig. 3).
|
| Fig.3 Temporal variability in the expression of NADH-GDH in the coral nubbins at 12 h of heat and ammonium stress Data are presented as the mean±SD (n=5), and bars with different letters are significantly different (P < 0.05). |
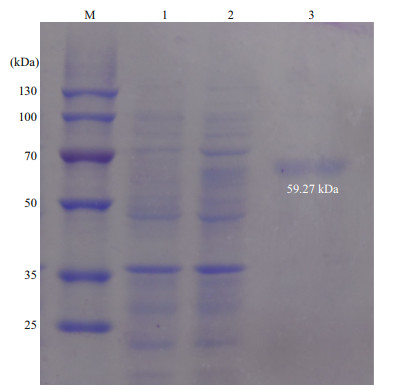
To further reveal the possible function of PdGDH, its recombinant protein rPdGDH was expressed in the E. coli BL21 (DE3) which received an IPTG induction. A specific band with a molecular weight of ~60 kDa was exhibited through Ni2+ chelating Sepharose column purification and 12% SDS-PAGE analysis, which was consistent with the predicted molecular weight of rPdGDH (Fig. 4). The concentration of purified rPdGDH was measured to be 0.3 mg/mL.
|
| Fig.4 SDS-PAGE analysis of rPdGDH Lane M: protein molecular weight marker; lane 1: negative control for rPdGDH; lane 2: IPTG induced rPdGDH; lane 3: purified rPdGDH. |
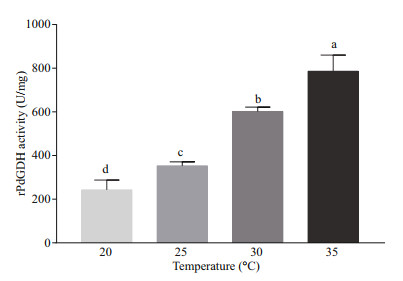
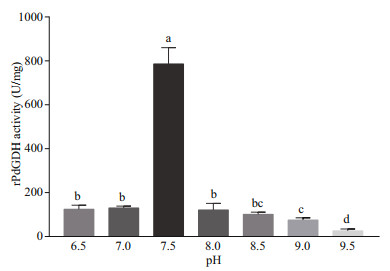
The activities of rPdGDH were determined at different temperatures (20, 25, 30, and 35 ℃) and pH (6.5, 7.0, 7.5, 8.0, 8.5, 9.0, and 9.5). The rPdGDH only uses NADH as coenzyme, and its activity increased steadily with increasing temperature, with the highest level at 35 ℃ (785.98 U/mg). Significant differences were observed in all pairwise comparisons (P < 0.05) (Fig. 5). In addition, the highest activity was observed at pH 7.5 (785.98 U/mg), which was significantly higher than those in all other pH (P < 0.05). The activities of rPdGDH at pH 6.5 (124.44 U/mg), 7.0 (130.23 U/mg), 8.0 (121.54 U/mg), 8.5 (101.29 U/mg), and 9.0 (75.24 U/mg) were also significantly higher than that at pH 9.5 (26.05 U/mg, P < 0.05) (Fig. 6).
|
| Fig.5 The relative activities of rPdGDH at temperature 20, 25, 30, and 35 ℃ (pH 7.5) Data are presented as the mean±SD (n=3), and bars with different letters are significantly different (P < 0.05). |
|
| Fig.6 The relative activities of rPdGDH at pH 6.5, 7.0, 7.5, 8.0, 8.5, 9.0, and 9.5 (35 ℃) Data are presented as the mean±SD (n=3), and bars with different letters are significantly different (P < 0.05). |
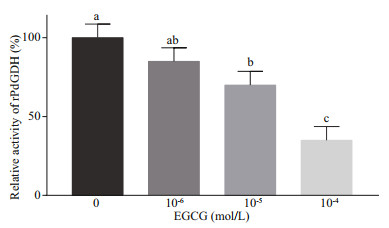
The inhibitory effect of EGCG on rPdGDH activities was investigated at the concentrations of 10-4, 10-5, and 10-6 mol/L (35 ℃, pH 7.5). The relative activity of rPdGDH decreased as EGCG concentration increased, and began to decrease significantly at 10-5-mol/L EGCG (70.0%, P < 0.05), in comparison with that without EGCG. The lowest activity of rPdGDH was detected at 10-4-mol/L EGCG (35.0%, P < 0.05), and was less than 50% of that without EGCG (Fig. 7).
|
| Fig.7 The relative activities of rPdGDH at EGCG 10-4, 10-5, and 10-6 mol/L (35 ℃, pH 7.5) Data are presented as the mean±SD (n=3), and bars with different letters are significantly different (P < 0.05). |
The NADH-GDH activity in the P. damicornis nubbins was determined at 0, 12, and 24 h after heat and ammonium stress. The NADH-GDH activities increased significantly only at 12 h in the heat and heat_NH4 groups (32.66 U/mg and 11.26 U/mg, respectively, P<0.05), in comparison with that in the control group. Besides, the NADH-GDH activity in the heat group at 12 h was significantly higher than that in the heat_NH4 group (2.90-fold, P<0.05) (Fig. 8).

|
| Fig.8 Temporal variability in the activities of NADH-GDH in the coral nubbins at 0, 12, and 24 h of heat and ammonium treatment Data are presented as the mean±SD (n=5). * Means significant difference between treatment group and control group (P < 0.05). # Means significant difference between different treatment groups (P < 0.05). |
The nitrogen cycling between coral hosts and symbiotic Symbiodiniaceae is vital to the stability of the symbiotic relationship between the two partners (Pernice et al., 2012). GDH is involved in the crucial nitrogen cycling through catalyzing the assimilation of ammonium, which is the main nitrogen resource for the symbiotic association (Yellowlees et al., 1994). In the present study, a GDH gene was identified from the scleractinian coral P. damicornis (PdGDH), and its ORF encoded a polypeptide of 536 amino acid residues. The amino acid sequence of PdGDH protein shared the highest identity (96%) with that of GDH from coral S. pistillata (XP_022804879.1). Structure analysis predicted that PdGDH had one GdhA domain (from Val95 to Tyr525), which contained 10 amino acid residues for binding NAD(P)H. Phylogenetic tree showed that PdGDH was first clustered with GDHs from other cnidarians, and then clustered with GDHs from vertebrates. Furthermore, the catalytic activity of rPdGDH was determined to rely on NADH not NADPH, and reduced gradually with EGCG concentration, with more than 50% inhibition at 10-4-mol/L EGCG. The present study demonstrated that PdGDH used only NADH as co-factor. Further research is needed on the types of GDH in P. damicornis, because the activity of GDH in coral Acropora latistella has shown both NADH- and NADPH-specific (Dudler et al., 1987). EGCG also has the ability to specifically inhibit rPdGDH activity, and this can provide an inhibitor choice for related research in the future (Li et al., 2007; Zhang et al., 2016). Together, these results suggest that PdGDH, as a homologue of glutamate dehydrogenase, is able to catalyze the reversible oxidative deamination of L-glutamate to α-ketoglutarate and mediate the ammonium assimilation in the scleractinian coral P. damicornis.
The rise of sea surface temperature caused by carbon dioxide emission is the main global threat to the coral-Symbiodiniaceae symbiosis, and can impair the coral growth and lower coral resilience (Anthony et al., 2011). Although ammonium assimilation plays important roles in the nitrogen cycling between coral hosts and symbiotic Symbiodiniaceae (Morris et al., 2019; Xiang et al., 2020), little is explored about the effect of environmental changes on this assimilation process. To reveal the potential effect in scleractinian coral, the catalytic activities of rPdGDH were detected firstly under different temperature and pH conditions. In the present study, the highest activity of rPdGDH was observed at 35 ℃ and pH 7.5. The rPdGDH activity increased with the elevation of temperature, and the similar rise of GDH activity in vitro at high temperature was also observed in Pycnoporus cinnabarinus (Piumi et al., 2014). Furthermore, the rPdGDH activity reached highest level at pH 7.5. Similar result was reported that GDH activity was higher at pH 7.3 than other pH conditions in coral S. pistillata (Rahav et al., 1989), and the optimal pH for GDH activity was 7.0 in sea anemone A. xanthogrammica (Male and Storey, 1983). The significant changes of PdGDH activity at different temperature and pH conditions might due to the changes in protein conformation and substrate affinity (Su et al., 2018). The higher activity of rPdGDH at high temperature and low pH would contribute to the coral host's need for more inorganic nitrogen for the repair of heat and acidification damage and acclimatization of ocean warming and acidification. These results suggested that PdGDH could keep higher catalytic activities at high temperature and lower pH, which would play an important role in the physiological acclimation of the scleractinian coral P. damicornis to ocean warming and acidification.
To further understand the potential function of PdGDH in the coral acclimatization to environmental changes such as ocean warming, the changes of NADH-GDH activity and PdGDH mRNA expression were investigated in the scleractinian coral P. damicornis after heat stress. In the present study, elevated temperature increased NADH-GDH activity significantly at 12 h, but no significant difference in the PdGDH mRNA expression level was observed after heat stress, in comparison with that in the control group. It demonstrated that heat stress induced the expression and activity of NADH-GDH encoded by PdGDH in the scleractinian coral P. damicornis. Our previous study showed that NADH-GDH activities increased significantly in P. damicornis after heat stress (Tang et al., 2020). Combined with the observation that rPdGDH activity increased at high temperature, we speculated that coral P. damicornis might increase the NADH-GDH activity and ammonium assimilation to maintain the coral-Symbiodiniaceae symbiosis and resist heat stress. It could be also supported the report that more nitrogen availability from ammonium assimilation of coral hosts contributes to the maintenance of the coral-Symbiodiniaceae symbiosis under heat stress (Yuan et al., 2017). The result collectively suggested that heat stress could induce the NADH-GDH activity at the protein level, rather than PdGDH mRNA expression level, which might increase ammonium assimilation and improve the heat acclimation of the scleractinian coral P.damicornis.
To further verify the regulation of heat acclimatization of coral by ammonium assimilation, the NADH-GDH activity and PdGDH mRNA expression were also detected in the scleractinian coral P. damicornis after heat and ammonium treatment. The NADH-GDH activity in the heat_NH4 group decreased significantly at 12 h, in comparison with that in the heat group, but was still significantly higher than that in the control group. In addition, the PdGDH mRNA expression level in the heat_NH4 group was significantly higher than that in the heat group, but showed no significantly difference in comparison with that in the control group. This was also manifested in some observation that GS activity (another ammonium assimilation-related enzyme) in coral hosts could be triggered under elevated ammonium (Su et al., 2018). The elevation of ammonium concentration could meet the elevated substrate needs of NADH-GDH in coral hosts after heat stress, and more ammonium would be assimilated to be provided to symbiotic Symbiodiniaceae, which might explain the observation that the NADH-GDH activity in the heat_NH4 group was significantly lower than that in the heat group. It also revealed that ammonium assimilation and nitrogen cycling could be optimized dynamically to maintain the symbiosis between coral hosts and symbiotic Symbiodiniaceae. To sum up, all results suggested that PdGDH-mediated ammonium assimilation could be involved in the response of the scleractinian coral P. damicornis to heat stress, which would be able to balance the coral-Symbiodiniaceae symbiosis and improve the coral acclimatization to high temperature.
5 CONCLUSIONA GDH was identified in the scleractinian coral P. damicornis, which was able to catalyze the reversible oxidative deamination of L-glutamate to α-ketoglutarate and ammonia with NADH as coenzyme. Furthermore, PdGDH-mediated ammonium assimilation was involved in response to heat stress in scleractinian coral P. damicornis. More researches are needed to unravel the mysterious symbiosis between corals and Symbiodiniaceae in the future.
6 DATA AVAILABILITY STATEMENTAll data generated and/or analyzed during this study are available from the corresponding author upon request on reasonable request.
7 ACKNOWLEDGMENTThe authors are grateful to all of the laboratory members for their continuous technical advice and helpful discussions.
Anthony K R N, Maynard J A, Diaz‐Pulido G, Mumby P J, Marshall P A, Cao L, Hoegh-Guldberg O. 2011. Ocean acidification and warming will lower coral reef resilience. Global Change Biology, 17(5): 1798-1808.
DOI:10.1111/j.1365-2486.2010.02364.x |
Catmull J, Yellowlees D, Miller D J. 1987. NADP+-dependent glutamate dehydrogenase from Acropora formosa: purification and properties. Marine Biology, 95(4): 559-563.
DOI:10.1007/BF00393099 |
Claar D C, Starko S, Tietjen K L, Epstein H E, Cunning R, Cobb K M, Baker A C, Gates R D, Baum J K. 2020. Dynamic symbioses reveal pathways to coral survival through prolonged heatwaves. Nature Communications, 11(1): 6097.
DOI:10.1038/s41467-020-19169-y |
Cunning R, Bay R A, Gillette P, Baker A C, Traylor-Knowles N. 2018. Comparative analysis of the Pocillopora damicornis genome highlights role of immune system in coral evolution. Scientific Reports, 8(1): 16134.
DOI:10.1038/s41598-018-34459-8 |
Douglas A E. 2003. Coral bleaching-how and why?. Marine Pollution Bulletin, 46(4): 385-392.
DOI:10.1016/S0025-326X(03)00037-7 |
Dudler N, Miller D J. 1988. Characterization of two glutamate dehydrogenases from the symbiotic microalga Symbiodinium microadriaticum isolated from the coral Acropora formosa. Marine Biology, 97(3): 427-430.
DOI:10.1007/BF00397773 |
Dudler N, Yellowlees D, Miller D J. 1987. Localization of two L-glutamate dehydrogenases in the coral Acropora latistella. Archives of Biochemistry and Biophysics, 254(1): 368-371.
DOI:10.1016/0003-9861(87)90112-3 |
Hill R, Szabó M, ur Rehman A, Vass I, Ralph P J, Larkum A W D. 2014. Inhibition of photosynthetic CO2 fixation in the coral Pocillopora damicornis and its relationship to thermal bleaching. Journal of Experimental Biology, 217(12): 2150-2162.
DOI:10.1242/jeb.100578 |
Hudson R C, Daniel R M. 1993. L-glutamate dehydrogenases: distribution, properties and mechanism. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry, 106(4): 767-792.
DOI:10.1016/0305-0491(93)90031-Y |
Jiang Q F, Zhou Z, Wang L L, Wang L L, Yue F, Wang J J, Song L S. 2013. A scallop nitric oxide synthase (NOS) with structure similar to neuronal NOS and its involvement in the immune defense. PLoS One, 8(7): e69158.
DOI:10.1371/journal.pone.0069158 |
Li M, Allen A, Smith T J. 2007. High throughput screening reveals several new classes of glutamate dehydrogenase inhibitors. Biochemistry, 46(51): 15089-15102.
DOI:10.1021/bi7018783 |
Livak K J, Schmittgen T D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt Method. Methods, 25(4): 402-408.
DOI:10.1006/meth.2001.1262 |
Male K B, Storey K B. 1983. Kinetic characterization of NADP-specific glutamate dehydrogenase from the sea anemone, Anthopleura xanthogrammica: control of amino acid biosynthesis during osmotic stress. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry, 76(4): 823-829.
DOI:10.1016/0305-0491(83)90399-1 |
Morris L A, Voolstra C R, Quigley K M, Bourne D G, Bay L K. 2019. Nutrient availability and metabolism affect the stability of coral-symbiodiniaceae symbioses. Trends in Microbiology, 27(8): 678-689.
DOI:10.1016/j.tim.2019.03.004 |
Muscatine L, Falkowski P G, Dubinsky Z, Cook P A, McCloskey L R. 1989. The effect of external nutrient resources on the population dynamics of zooxanthellae in a reef coral. Proceedings of the Royal Society B: Biological Sciences, 236(1284): 311-324.
DOI:10.1098/rspb.1989.0025 |
Pernice M, Meibom A, Van Den Heuvel A, Kopp C, Domart-Coulon I, Hoegh-Guldberg O, Dove S. 2012. A single-cell view of ammonium assimilation in coral-dinoflagellate symbiosis. The ISME Journal, 6(7): 1314-1324.
DOI:10.1038/ismej.2011.196 |
Piumi F, Levasseur A, Navarro D, Zhou S M, Mathieu Y, Ropartz D, Ludwig R, Faulds C B, Record E. 2014. A novel glucose dehydrogenase from the white-rot fungus Pycnoporus cinnabarinus: production in Aspergillus niger and physicochemical characterization of the recombinant enzyme. Applied Microbiology and Biotechnology, 98(24): 10105-10118.
DOI:10.1007/s00253-014-5891-4 |
Rädecker N, Pogoreutz C, Voolstra C R, Wiedenmann J, Wild C. 2015. Nitrogen cycling in corals: the key to understanding holobiont functioning?. Trends in Microbiology, 23(8): 490-497.
DOI:10.1016/j.tim.2015.03.008 |
Rahav O, Dubinsky Z, Achituv Y, Falkowski P G. 1989. Ammonium metabolism in the zooxanthellate coral, Stylophora pistillata. Proceedings of the Royal Society B: Biological Sciences, 236(1284): 325-337.
DOI:10.1098/rspb.1989.0026 |
Robert X, Gouet P. 2014. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Research, 42(W1): W320-W324.
DOI:10.1093/nar/gku316 |
Rosic N, Kaniewska P, Chan C K K, Ling E Y S, Edwards D, Dove S, Hoegh-Guldberg O. 2014. Early transcriptional changes in the reef-building coral Acropora aspera in response to thermal and nutrient stress. BMC Genomics, 15(1): 1052.
DOI:10.1186/1471-2164-15-1052 |
Stefanik D J, Wolenski F S, Friedman L E, Gilmore T D, Finnerty J R. 2013. Isolation of DNA, RNA and protein from the starlet sea anemone Nematostella vectensis. Nature Protocols, 8(5): 892-899.
DOI:10.1038/nprot.2012.151 |
Su Y L, Zhou Z, Yu X P. 2018. Possible roles of glutamine synthetase in responding to environmental changes in a scleractinian coral. Molecular Biology Reports, 45(6): 2115-2124.
DOI:10.1007/s11033-018-4369-3 |
Tang J, Ni X Z, Wen J Q, Wang L G, Luo J, Zhou Z. 2020. Increased ammonium assimilation activity in the scleractinian coral Pocillopora damicornis but not its symbiont after acute heat stress. Frontiers in Marine Science, 7: 565068.
DOI:10.3389/fmars.2020.565068 |
Tong H Y, Cai L, Zhou G W, Yuan T, Zhang W P, Tian R M, Huang H, Qian P Y. 2017. Temperature shapes coral-algal symbiosis in the South China Sea. Scientific Reports, 7(1): 40118.
DOI:10.1038/srep40118 |
Wang J T, Douglas A E. 1999. Essential amino acid synthesis and nitrogen recycling in an alga-invertebrate symbiosis. Marine Biology, 135(2): 219-222.
DOI:10.1007/s002270050619 |
Wiedenmann J, D'Angelo C, Smith E G, Hunt A N, Legiret F E, Postle A D, Achterberg E P. 2013. Nutrient enrichment can increase the susceptibility of reef corals to bleaching. NatureClimateChange, 3(2): 160-164.
DOI:10.1038/nclimate1661 |
Wooldridge S A. 2013. Breakdown of the coral-algae symbiosis: towards formalising a linkage between warm-water bleaching thresholds and the growth rate of the intracellular zooxanthellae. Biogeosciences, 10(3): 1647-1658.
DOI:10.5194/bg-10-1647-2013 |
Xiang T T, Lehnert E, Jinkerson R E, Clowez S, Kim R G, Denofrio J C, Pringle J R, Grossman A R. 2020. Symbiont population control by host-symbiont metabolic interaction in Symbiodiniaceae-cnidarian associations. Nature Communications, 11(1): 108.
DOI:10.1038/s41467-019-13963-z |
Yang X D, Li L F, Bi S P. 2005. Electrochemical studies of the inhibition and activation effects of Al (III) on the activity of bovine liver glutamate dehydrogenase. Sensors, 5(4): 235-244.
DOI:10.3390/s5040235 |
Yellowlees D, Rees T A V, Fitt W K. 1994. Effect of ammonium-supplemented seawater on glutamine synthetase and glutamate dehydrogenase activities in host tissue and zooxanthellae of Pocillopora damicornis and on ammonium uptake rates of the zooxanthellae. Pacific Science, 48(3): 291-295.
|
Yellowlees D, Rees T A V, Leggat W. 2008. Metabolic interactions between algal symbionts and invertebrate hosts. Plant, Cell & Environment, 31(5): 679-694.
DOI:10.1111/j.1365-3040.2008.01802.x |
Yuan C, Zhou Z, Zhang Y D, Chen G M, Yu X P, Ni X Z, Tang J, Huang B. 2017. Effects of elevated ammonium on the transcriptome of the stony coral Pocillopora damicornis. Marine Pollution Bulletin, 114(1): 46-52.
DOI:10.1016/j.marpolbul.2016.08.036 |
Zhang W, Zhu M, Wang F, Cao D H, Ruan J J, Su W K, Ruan B H. 2016. Mono-sulfonated tetrazolium salt based NAD(P)H detection reagents suitable for dehydrogenase and real-time cell viability assays. Analytical Biochemistry, 509: 33-40.
DOI:10.1016/j.ab.2016.06.026 |
Zhou Z, Wang L L, Shi X W, Yue F, Wang M Q, Zhang H, Song L S. 2012. The expression of dopa decarboxylase and dopamine beta hydroxylase and their responding to bacterial challenge during the ontogenesis of scallop Chlamys farreri. Fish & Shellfish Immunology, 33(1): 67-74.
DOI:10.1016/j.fsi.2012.04.002 |
 2022, Vol. 40
2022, Vol. 40