Institute of Oceanology, Chinese Academy of Sciences
Article Information
- YIN Sicheng, HUANG Ruohan, JEPPESEN Erik, CHEN Lijing, WANG Liqing, FANG Xin, ZHANG Wei
- The effect of cylindrospermopsin on the bacterioplankton community: a microcosm experiment on water from Dishui Lake, Shanghai, China
- Journal of Oceanology and Limnology, 40(5): 1829-1839
- http://dx.doi.org/10.1007/s00343-022-2081-6
Article History
- Received Feb. 27, 2022
- accepted in principle Apr. 2, 2022
- accepted for publication Jun. 6, 2022
2 Department of Ecoscience, Aarhus University, Silkeborg 8600, Denmark;
3 Sino-Danish Centre for Education and Research (SDC), University of Chinese Academy of Sciences, Beijing 100049, China;
4 Limnology Laboratory and EKOSAM, Department of Biological Sciences, Middle East Technical University, Ankara 06800, Turkey;
5 Institute of Marine Sciences, Middle East Technical University, Mersin 33731, Turkey;
6 School of Business, Macau University of Science and Technology, Taipa, Macao 999078, China
With global warming and the intensification of eutrophication of water bodies, the problem of cyanobacteria blooms has become increasingly serious (Yan et al., 2017). With these blooms, cyanobacteria metabolites enter the water column, affecting severely the aquatic ecosystem. Among the metabolites, cyanotoxins have become a central issue due to their strong toxicity and the environmental hazards that they pose (Briland et al., 2020), potentially inhibiting the growth of dominant aquatic organisms, such as Microcystis sp., Lemna minor, Daphnia magna, and Wolffia arrhizal (Jámbrik et al., 2010; Bednarska et al., 2014; Rzymski et al., 2014), and shifting the food web in lakes and oceans (Pohnert et al., 2007). The cyanotoxins accumulate in consumers up through the food chain, thereby affecting survival and reproduction (Wilson et al., 2006). Microcystis is the most common blooming cyanobacteria, and impacts of microcystins (MC), produced by these algae, on the aquatic ecosystem have been widely reported. Studies have shown that MC can affect the underwater organs of macrophytes and their growth (Chen et al., 2020), cause abnormal development of the freshwater snail Lymnaea stagnalis (Lance et al., 2007, 2011) and inhibit the photosynthesis of microalgae (e.g., Chlorella sp.), which ultimately inhibits the proliferation of algae (Singh et al., 2001; Campos et al., 2013).
In recent years, other cyanobacteria with a strong dispersal ability and invasive potential have been observed to develop blooms, e.g. in Chinese rivers, lakes, and reservoirs (Jiang et al., 2014; Lei et al., 2014). Among these, CYN-producing Cylindrospermopsis and Chrysosporium have attracted special attention (Fadel et al., 2014; Burford et al., 2016; Zhang et al., 2017) as they have shown strong competitiveness and may replace Microcystis under certain conditions (Lei et al., 2014; Zhang et al., 2017), in part as result of CYN production (Zhang et al., 2017). Several studies have focused on the impact of CYN on various aquatic organisms. Beyer et al. (2009) found that CYN inhibited the growth of reed roots and caused necrosis of root epidermal cells. Zhu and Chen (2012) showed that CYN induced oxidative damage to grass carp lymphocytes, leading to cell apoptosis. Another investigation found that CYN stimulated sympatric phytoplankton to produce extracellular alkaline phosphatase (APA), thereby increasing the available phosphate content in the water column (Bar-Yosef et al., 2010). These results are helpful to understand the potential damage of CYN to aquatic ecosystems and how the CYN-producing species may dominate water bodies; however, knowledge about the response of microbial assemblages to CYN in aquatic ecosystems is limited.
As decomposers and consumers, bacteria play an important role in the material circulation and energy flow of the surface water ecosystem (Zhou et al., 2009). Moreover, when the lake water is exposed to contaminants, specific bacterial groups will be activated and degrade the toxic substances, thereby reducing or eliminating their harm (Hyenstrand et al., 2003). Because microorganisms are more sensitive to environmental changes and have a shorter growth cycle, they typically respond to environmental pollutants before other species (Bloem and Breure, 2003). Studies have shown that cyanobacteria blooms and cyanotoxins affect the community structure and function of bacterioplankton in different ways (Wilhelm et al., 2011). Non-toxic soluble organic carbon (DOC) released by cyanobacteria can be used as a carbon source to stimulate the growth of bacteria (Wang and Priscu, 1994), but cyanotoxins may also have a negative impact on bacterioplankton, for instance by reducing bacteria diversity or slowing down the process of the nitrogen cycle by inhibiting the growth of denitrifying bacteria (Yang et al., 2008; Li et al., 2016).
Dishui Lake, a coastal man-made lake with a total area of 5.56 km2, is located in the southeast of Shanghai, China (Lin et al., 2014). In recent years, the CYN-producing cyanobacteria Chrysosporum ovalisporum has been observed in the lake, with effects on the phytoplankton and zooplankton communities (Zhang et al., 2018). In this study, we aimed to explore the effect of CYN on the structure and function of the bacterioplankton community in Dishui Lake in a microcosmic experiment involving addition of pure CYN and high-throughput sequencing analysis. We hypothesised that 1) CYN can inhibit the growth of bacterioplankton and thus change their community structure and 2) the high concentrations of CYN can shift the functional taxa in the aquatic ecosystem. Our study provides new insight into the relationship between heterotrophic bacteria and cyanobacterial toxins and, thus, the carbon and nitrogen cycles in shallow lakes.
2 MATERIAL AND METHOD 2.1 Exposure experiment of planktonic microorganisms to CYNIn September 2019, surface water samples were collected at 0.5-m depth in a nearshore zone of Dishui Lake, an area without cyanobacteria blooms. In-situ water temperature (WT), pH, dissolved oxygen (DO), and salinity (SAL) were measured using an YSI Pro-plus multisensor sonde. Other water quality parameters, total phosphorus (TP), and chlorophyll a (chl a) were measured simultaneously, and the results of these measurements can be found in our previous study (Zhang et al., 2018). Average values of physical and chemical parameters of Dishui Lake are given in Table 1.

|
The water samples were kept cold at 4 ℃ and transported to the laboratory where they were mixed evenly and transferred into 1-L beakers. The samples were tested for CYN before our experiments, and no CYN was detected. Three concentration levels of pure CYN (Enzo life science): low group (6 μg/L), medium group (20 μg/L), and high group (40 μg/L) were set up in the exposure experiment. The initial lake water without CYN was used as control. The final culture volume was 500 mL. All the beakers were kept at 25±1 ℃ under a light intensity of 2 500 lx (12-h dark:12-h light cycle). Each treatment group had three replicates, and the beakers were manually shaken three times a day during the experimental cycle.
After 48 hours of incubation, 300 mL of the mixed water sample was removed and filtered through 5-μm and 0.2-μm filters. The filter membrane was placed in a sterile centrifuge tube, quickly frozen with liquid nitrogen and then stored in a refrigerator at -20 ℃. The samples were then forwarded to Metabio Science & Technology Co., Ltd. (Wuxi, China) for Miseq (Illumina) high-throughput sequencing.
2.2 Sample DNA extractionMetagenomic DNA was extracted by PowerSoil® DNA Isolation Kit from MOBIO Laboratories, Inc., according to the manufacturer's instructions. UV spectrophotometry was used to detect the concentration and purity. The specific method was applied following the instructions of the kit.
2.3 PCR amplification and sequencing analysisIn this study, the variable region of bacterial 16S rDNA V3–V4 was selected as the target amplification fragment for the subsequent high-throughput sequencing, and 16S rDNA V3–V4 universal primer 341F/805R was chosen as extract. The primer sequences were 341F: 5'-CCCTACACGACGCTCTTCCGATCTG-3', 805R: 5'-GACTGGAGTTGGCACCCGAGAATTCCA-3', and Barcode discrimination sequence samples were added at the end of 3' (Caporaso et al., 2011). PCR reaction conditions were: pre-denaturation at 98 ℃ for 30 s, denaturation at 98 ℃ for 15 s, annealing at 50 ℃ for 30 s, extension at 72 ℃ for 30 s, 25–27 cycles, incubation at 72 ℃ for 5 min; and storage at 4 ℃ (Biorad C1000 Touch PCR, Shanghai).
The products were detected by electrophoresis 2% agarose gel, and the Axygen gel recovery kit recovered the target fragments. Quant-i T PicoGreen dsDNA Assay Kit was used to quantify the PCR products on the Microplate reader (BioTek, FLx800), and then the samples were mixed according to the amount of data required for each sample.
The original image data file obtained from Illumina Miseq sequencing was transformed into raw reads by base calling analysis. Each sample was distinguished with a barcode, and the sequence quality was controlled and filtered. The sequence was spliced according to the overlap relationship, and the spliced sequence was subjected to quality control and was filtered again. Finally, the optimized sequence (total: 2 309 479, average: 192 457 per sample, max: 300 694, min: 43 461) was obtained for downstream analysis. The optimized sequences were analyzed by Operational Taxonomic Units (OTUs) cluster analysis and species taxonomy. All sequences were divided into OTUs relative to different similarity levels. In this study, the biological information of OTU at 97% similarity level was statistically analyzed, and OTUs with an abundance below 2 were removed. The sequences and related date were deposited into the National Center for Biotechnology Information (NCBI) database with the Sequence Read Archive (SRA) accession number of SRR18810468.
2.4 Statistical analysisThe results were handled in Past V3.22 and R 4.0.2 software. We calculated the α diversity index in the Past V3.22 program. The results of Bray-Curtis distances were visualized by Principal Coordinate Analysis (PCoA) plots in the R 4.0.2 environment using the "ape" package, and significant differences in bacterioplankton community structure among different treatment groups were tested using one-way analysis of similarities (ANOSIM). The species with an absolute abundance greater than 10 in each group were selected for analysis of the co-occurrence network (Carey et al., 2017). For the observed and simulated networks, we calculated structural properties that assessed network complexity. These characteristics included the number of nodes and edges (i.e., taxa richness), connectance (the proportion of potential connections in a network that are actually connected), average degree (average number of connected edges per node), average path length (the mean shortest path length between all nodes), betweenness centralization (the ratio of the number of paths passing through this node among all the shortest paths to the total number of shortest paths), degree centralization (measuring node centrality), clustering coefficient (a metric of how connected nodes are in the network), and diameter (maximum value among shortest distances) (Newman, 2003; Kara et al., 2013; Carey et al., 2017; Zhu et al., 2021). We used the "Psych" package in R 4.0.2 to calculate inter-species correlations. Networks were visualized with the software programs Cytoscape v.2.8.3 and Gephi 0.9.2. Venn charts and heat maps were made using the software Origin 2016. Bar charts were mapped using Excel.
3 RESULT 3.1 Bacterial taxa diversityThe OTUs of the four groups were 20 748, 20 834, 20 956, and 11 386, respectively, and 5 272 taxa were common for all four groups. The control group had the highest (n=125) taxa richness of particular taxa (occurring only in one group), but shared 11 092, 11 158, and 5 639 OTUs with the low, medium, and high CYN groups, constituting 53%, 53%, and 50% of the total taxon number, respectively (Fig. 1a). By contrast, the three treatment groups had higher numbers of shared taxa, the highest (n=11 338) being shared between the low and medium CYN groups (Fig. 1a). With increasing CYN concentrations, the number of unique OTUs in each treatment group gradually decreased.

|
| Fig.1 OTUs overlapping Venn charts and α diversity analysis a. the Venn charts showing the distribution of OTUs; b. comparison of the number of OTUs; c. Shannon index of each group; d. Margalef index of each group; e. Chao-1 index of each group. *: P < 0.05, significant differences between the experimental group and the control group; **: P < 0.01, highly significant differences between the experimental group and the control group, the same below. |
Compared with the control group, the number of OTUs in the low and medium CYN groups tended to be higher, though not significantly so (P > 0.05), while the number of OTUs in the high CYN group was significantly lower (P < 0.01). The Shannon index, the Margalef index, and the Chao-1 index of the high CYN groups were also significantly lower than those of the control group (P < 0.01) (Fig. 1b–e).
3.2 Abundance and composition of bacterioplanktonThe total bacteria abundances in the low and medium CYN groups were 1.17 and 1.29 times that of the control group, but the difference was not significant (P > 0.05). Contrarily, the bacteria abundance in the high CYN group was significantly lower (68.3%) than in the control group (P < 0.05). A different response pattern to CYN was found for the clades, phyla. The relative abundances of Proteobacteria and Comamonadaceae in the high CYN group were significantly lower than those of the other three groups (P < 0.05 and P < 0.01). Unlike Proteobacteria, the difference in absolute abundance of actinobacteria between the four groups was not significant (P > 0.05) (Fig. 2). Compared with the other groups, the relative abundance of Methylophilaceae was significantly higher in the medium CYN group (P < 0.01) (Fig. 3).
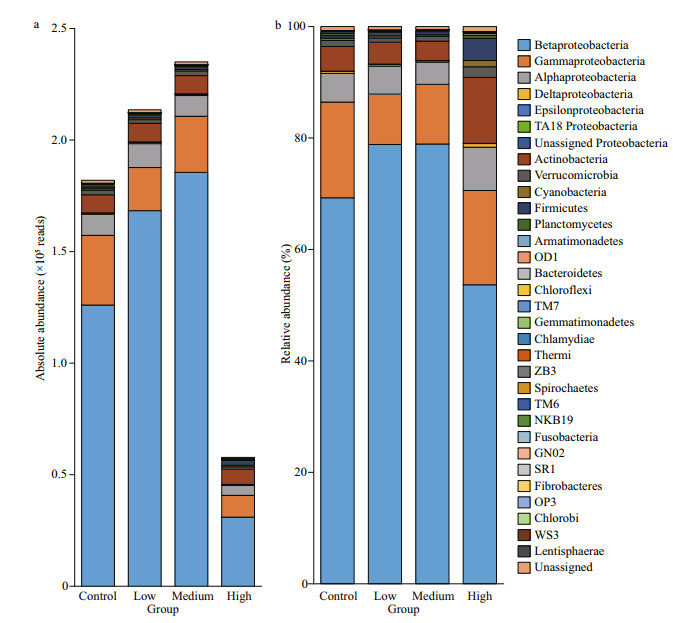
|
| Fig.2 The abundance and relative abundance of the bacterioplankton community at phylum or class level |
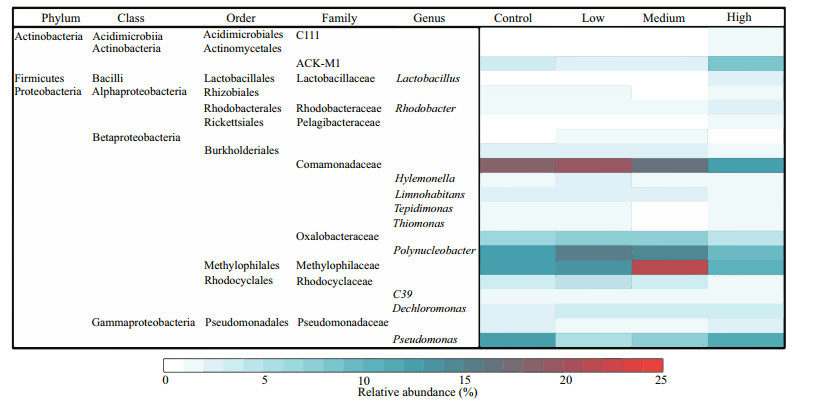
|
| Fig.3 The relative abundance of the dominant genera Relative abundances below 1% were not considered. |
Compared with the control group, the abundances of the most dominant genera increased in the low and medium concentration groups and decreased in the high concentration group. The abundances of Pseudomonas, unclassified Comamonadaceae, and unclassified R4-41B were significantly lower in the high CYN group than in the control group (P < 0.05) (Fig. 4).
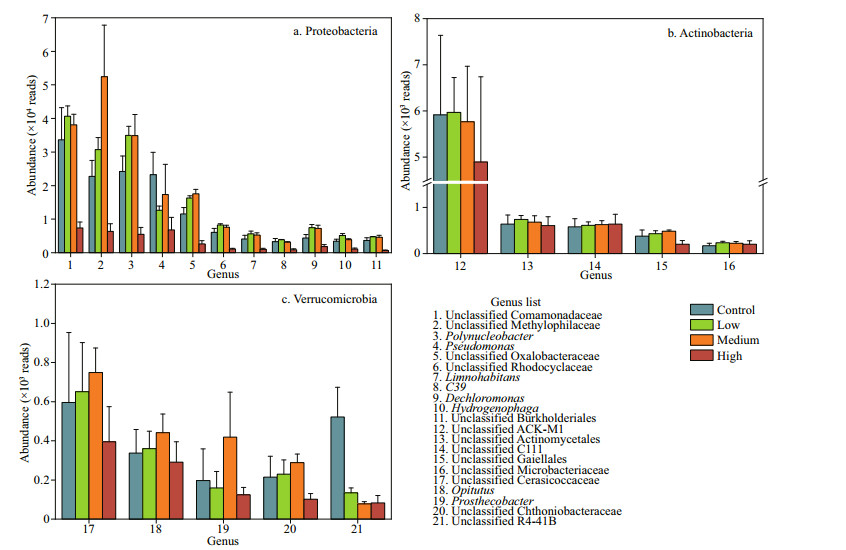
|
| Fig.4 The abundances of the dominant genera of Proteobacteria, Actinobacteria, and Verrucomicrobia |
The PCoA results showed that CYN exposure, especially at the high concentration level, changed the community of the main bacteria clades. Bacteria, Proteobacteria, and Planctomycetes in the high CYN group were significantly different from the other groups (P < 0.05). Actimobacteria, Verrucomicrobia, Firmicutes, and Cyanobacteria in the three treatment groups were not significantly different from those of the control group (P > 0.05). Armatimonadetes were significantly different from the control group and here also differences between the high CYN group and the other treatment groups were found (P < 0.05) (Fig. 5).
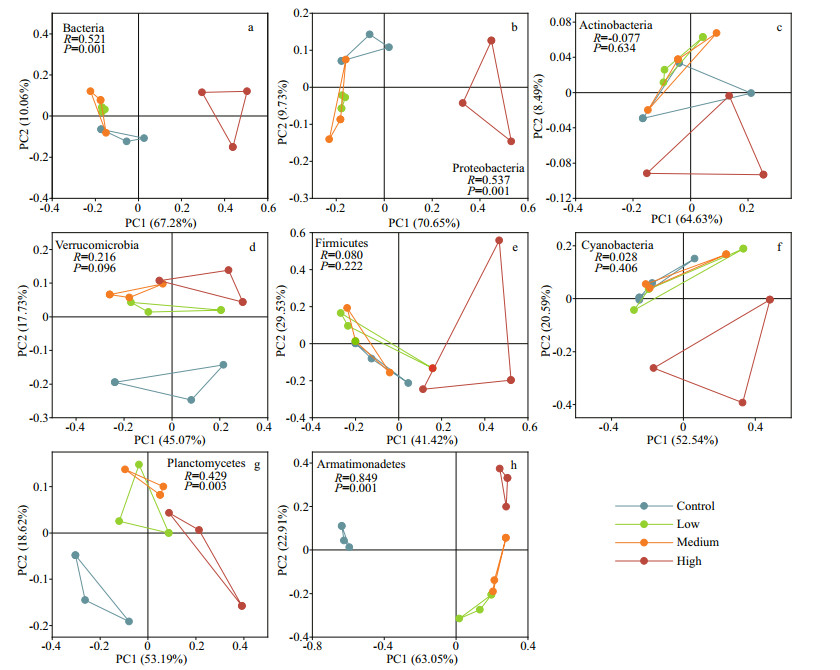
|
| Fig.5 PCoA analysis diagram of different dominant phyla based on Bray-Curtis distance a. Bacteria; b. Proteobacteria; c. Actinobacteria; d. Verrucomicrobia; e. Firmicutes; f. Cyanobacteria; g. Planctomycetes; h. Armatimonadetes. |
The taxa from Proteobacteria and Actinobacteria were closely related to other phyla, while OD1 and Armatimonadetes were less related to other phyla (Fig. 6). The bacteria communities in the control group exhibited significantly higher network complexity than the CYN treatment groups as indicated by more nodes (i.e., greater richness) and edges, higher network connectance and average degrees (Fig. 7a–d). The transitivity among taxa was poorer in the high CYN group than in the other groups as suggested by lower clustering coefficients and larger diameters (Fig. 7h–i). However, community stability tended to increase with rising CYN concentrations, reaching relatively stable conditions in the high CYN group as indicated by connectance, betweenness centralization, and degree centralization (Fig. 7c, f, & g).
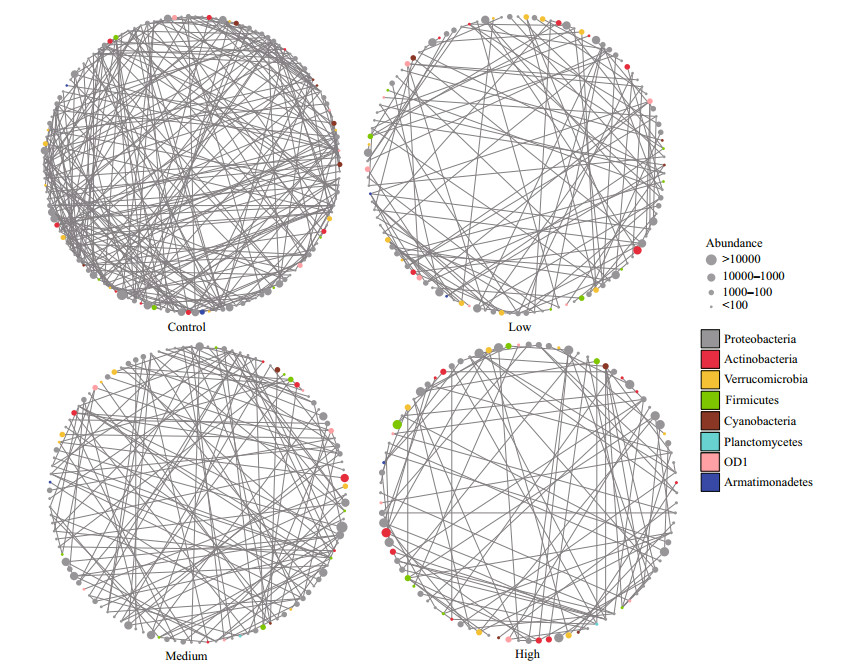
|
| Fig.6 Observed networks of bacterioplankton co-occurrences in the four CYN concentration treatments (0, 6, 20, and 40 μg/L) Each colored circle represents a node or taxon in the bacterioplankton community, with the line (or edge) connecting taxa representing an observed co-occurrence. The color of the node denotes the division of the bacterioplankton taxon, and the size of the nodes represents different levels of OTU reads. |
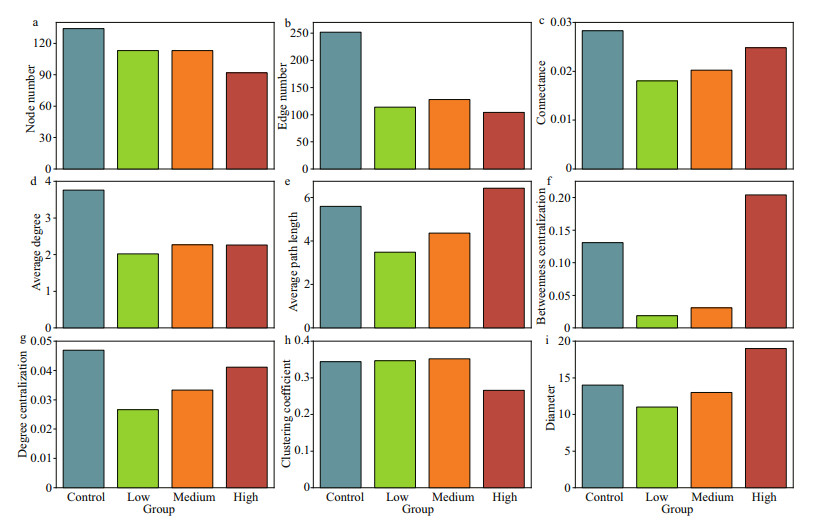
|
| Fig.7 Parameters of the networks of bacterioplankton co-occurrences in Fig. 6 a. node number; b. edge number; c. connectance; d. average degree; e. average path length; f. betweenness centralization; g. degree centralization; h. clustering coefficient; i. diameter. |
In aquatic ecosystems, cyanotoxins can inhibit the growth of other aquatic organisms and promote their own growth (Valdor and Aboal, 2007). Our results showed that most bacterioplankton taxa in Dishui Lake exhibit a certain tolerance to CYN, but a high CYN concentration (40 μg/L) led to significant decrease in the abundance of the dominant microbes, such as Comamonadaceae (Fig. 4). CYN can inhibit protein and glutathione synthesis and cause gene damage (Campos et al., 2013). Glutathione is the most important non-protein sulfhydryl compound in bacteria cells and plays an anti-oxidant stress role (Copley and Dhillon, 2002). Strong oxidative stress produced by high concentrations of CYN may be one of the reasons for the significant decrease in bacteria abundance. We found that Actinomycetes were most resistant to CYN (Figs. 4–5). A variety of actinobacteria (e.g., taxa belonging to Streptomyces) have the ability to dissolve Microcystis cells and degrade microcystins (Choi et al., 2005). The high tolerance of actinobacteria to CYN indicates that they also have a potential for degrading CYN. Although a review showed that CYN concentrations may exceed 100 μg/L (Rzymski et al., 2014), most of the field survey results from lakes or reservoirs have shown CYN concentrations under Cylindrospermopsis blooming events < 10 μg/L (Fadel et al., 2014; Lei et al., 2014; Lu et al., 2021). We found that CYN increased the abundance of bacterioplankton at 6- and 20-μg/L CYN, thus showing "hormesis", as also found in toxicological experiments elucidating the impact of antibiotics, microcystins and other substances on microorganisms (Chen and Jiang, 2011). Another investigation showed that CYN had allelopathic effects and stimulated other cyanobacteria to produce alkaline phosphatase (APA) (Rzymski et al., 2014). We speculate that the low and medium dosages of CYN in our experiment may have promoted the release of APA by bacterioplankton and that the increase of soluble inorganic phosphorus in the water column promoted the growth of these organisms.
The diversity and community structure of bacterioplankton are closely related to their function. Our network analysis indicated that CYN caused shifts in the community structure of bacterioplankton, reducing their structural complexity, which subsequently affected their functions. We found that both the absolute and relative abundance of Comamonadaceae and Rhodocyclaceae decreased significantly in the high-CYN group (Fig. 4), and many of their taxa (e.g., Dechloromonas) play an important role in the denitrification process (Takahashi et al., 2011; Tian et al., 2015; Dong et al., 2019). The denitrification process is the key approach to transform nitrogen from reactive nitrogen to inert nitrogen with implications for the nitrogen cycle and global nitrogen balance (Murray and Knowles, 2003; Huang et al., 2017). A previous study by Yang et al. (2008) revealed that high concentrations of MC can inhibit denitrifying bacteria. However, in their study, the MC concentration had to reach mg/L level to produce the inhibitory effect, rarely occurring in natural waters. In our experiment, CYN had a significant inhibitory effect on denitrifying microorganisms (e.g., Methylophilaceae) at the μg/L level, suggesting a more potent effect on denitrification. In addition, among the several genera significantly inhibited by CYN, Pedosphaerales, mainly found in the sediments, plays an important role in the carbon cycle (Shi et al., 2018). Studies have also found that CYN can affect the predation of microorganisms by protozoa, thereby impacting the carbon transfer in the microbial food web (Bagatini et al., 2014).
We found that the high concentration of CYN could reduce the transmissibility of the bacterioplankton community (Fig. 7), indicating a lessening of the inter-species interaction among bacterioplankton (Feng et al., 2017), and thus a weakening of the "small world" nature of the bacterioplankton community (Carey et al., 2017). Generally, the stability of a weaker small world network is less affected by the hub taxa (Carey et al., 2017). This may explain the recovery of the stability of the bacterioplankton community with increasing CYN concentrations (Fig. 7).
5 CONCLUSIONOur results indicate that the high concentrations of CYN (40 μg/L) affected the abundance and the complexity and stability of the community structure of bacterioplankton in the water column, and that Proteobacteria and Verrucomicrobia were more sensitive to CYN exposure than Actinobacteria. Our results also reveal the great potential of CYN in shifting the microbial loop function in aquatic ecosystems under blooms of CYN-producing cyanobacteria.
6 DATA AVAILABILITY STATEMENTThe datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENTWe would like to express our deep thanks to Anne Mette Poulsen from Aarhus University for English assistance.
Bagatini I L, Eiler A, Bertilsson S, et al. 2014. Host-specificity and dynamics in bacterial communities associated with Bloom-forming freshwater phytoplankton. PLoS One, 9(1): e85950.
DOI:10.1371/journal.pone.0085950 |
Bar-Yosef Y, Sukenik A, Hadas O, et al. 2010. Enslavement in the water body by toxic Aphanizomenon ovalisporum, inducing alkaline phosphatase in phytoplanktons. Current Biology, 20(17): 1557-1561.
DOI:10.1016/j.cub.2010.07.032 |
Bednarska A, Pietrzak B, Pijanowska J. 2014. Effect of poor manageability and low nutritional value of cyanobacteria on Daphnia magna life history performance. Journal of Plankton Research, 36(3): 838-847.
DOI:10.1093/plankt/fbu009 |
Beyer D, Surányi G, Vasas G, et al. 2009. Cylindrospermopsin induces alterations of root histology and microtubule organization in common reed (Phragmites australis) plantlets cultured in vitro. Toxicon, 54(4): 440-449.
DOI:10.1016/j.toxicon.2009.05.008 |
Bloem J, Breure A M. 2003. Bioindicators/biomonitors-principles, assessment, concepts. In: Markert B A, Breure A M, Zechmeister H G eds. Microbial Indicators: Trace Metals and Other Contaminants in the Environment. Elsevier, Amsterdam. p. 259–282.
|
Briland R D, Stone J P, Manubolu M, et al. 2020. Cyanobacterial blooms modify food web structure and interactions in western Lake Erie. Harmful Algae, 92: 101586.
DOI:10.1016/j.hal.2019.03.004 |
Burford M A, Beardall J, Willis A, et al. 2016. Understanding the winning strategies used by the bloom-forming cyanobacterium Cylindrospermopsis raciborskii. Harmful Algae, 54(4): 44-53.
DOI:10.1016/j.hal.2015.10.012 |
Campos A, Araújo P, Pinheiro C, et al. 2013. Effects on growth, antioxidant enzyme activity and levels of extracellular proteins in the green alga Chlorella vulgaris exposed to crude cyanobacterial extracts and pure microcystin and cylindrospermopsin. Ecotoxicology and Environmental Safety, 94: 45-53.
DOI:10.1016/j.ecoenv.2013.04.019 |
Caporaso J G, Lauber C L, Walters W A, et al. 2011. Global patterns of 16s rRNA diversity at a depth of millions of sequences per sample. Proceedings of the National Academy of Sciences of the United States of America, 108(S11): 4516-4522.
DOI:10.1073/pnas.1000080107 |
Carey C C, Brown B L, Cottingham K L. 2017. The cyanobacterium Gloeotrichia echinulata increases the stability and network complexity of phytoplankton communities. Ecosphere, 8(7): e01830.
DOI:10.1002/ecs2.1830 |
Chen G Y, Zheng Z H, Bai M X, et al. 2020. Chronic effects of microcystin-LR at environmental relevant concentrations on photosynthesis of Typha angustifolia Linn. Ecotoxicology, 29(5): 514-523.
DOI:10.1007/s10646-020-02196-2 |
Chen H, Jiang J G. 2011. Toxic effects of chemical pesticides (trichlorfon and dimehypo) on Dunaliella salina. Chemosphere, 84(5): 664-670.
DOI:10.1016/j.chemosphere.2011.03.032 |
Choi H J, Kim B H, Kim J D, et al. 2005. Streptomyces neyagawaensis as a control for the hazardous biomass of Microcystis aeruginosa (cyanobacteria) in eutrophic freshwaters. Biological Control, 33(3): 335-343.
DOI:10.1016/j.biocontrol.2005.03.007 |
Copley S D, Dhillon J K. 2002. Lateral gene transfer and parallel evolution in the history of glutathione biosynthesis genes. Genome Biology, 3(5): research0025.
DOI:10.1186/gb-2002-3-5-research0025 |
Dong H H, Jiang X Y, Sun S S, et al. 2019. A cascade of a denitrification bioreactor and an aerobic biofilm reactor for heavy oil refinery wastewater treatment. RSC Advances, 9(13): 7495-7504.
DOI:10.1039/C8RA10510C |
Fadel A, Atoui A, Lemaire B J, et al. 2014. Dynamics of the toxin cylindrospermopsin and the cyanobacterium Chrysosporum (Aphanizomenon) ovalisporum in a Mediterranean eutrophic reservoir. Toxins, 6(11): 3041-3057.
DOI:10.3390/toxins6113041 |
Feng K, Zhang Z J, Cai W W, et al. 2017. Biodiversity and species competition regulate the resilience of microbial biofilm community. Molecular Ecology, 26(21): 6170-6182.
DOI:10.1111/mec.14356 |
Huang F, Pan L Q, Lv N, et al. 2017. Characterization of novel Bacillus strain N31 from mariculture water capable of halophilic heterotrophic nitrification-aerobic denitrification. Journal of Bioscience and Bioengineering, 124(5): 564-571.
DOI:10.1016/j.jbiosc.2017.06.008 |
Hyenstrand P, Rohrlack T, Beattie K A, et al. 2003. Laboratory studies of dissolved radiolabelled microcystin-LR in lake water. Water Research, 37(14): 3299-3306.
DOI:10.1016/S0043-1354(03)00180-5 |
Jámbrik K, Máthé C, Vasas G, et al. 2010. Cylindrospermopsin inhibits growth and modulates protease activity in the aquatic plants Lemna minor L. ) Horkel. Acta Biologica Hungarica, 61(S1): 77-94.
DOI:10.1556/ABiol.61.2010.Suppl.9 |
Jiang Y G, Xiao P, Yu G L, et al. 2014. Sporadic distribution and distinctive variations of Cylindrospermopsin genes in cyanobacterial strains and environmental samples from Chinese freshwater bodies. Applied and Environmental Microbiology, 80(17): 5219-5230.
DOI:10.1128/AEM.00551-14 |
Kara E L, Hanson P C, Hu Y H, et al. 2013. A decade of seasonal dynamics and co-occurrences within freshwater bacterioplankton communities from eutrophic Lake Mendota, WI, USA. The ISME Journal, 7(3): 680-684.
DOI:10.1038/ismej.2012.118 |
Lance E, Alonzo F, Tanguy M, et al. 2011. Impact of microcystin-producing cyanobacteria on reproductive success of Lymnaea stagnalis (Gastropoda, Pulmonata) and predicted consequences at the population level. Ecotoxicology, 20(4): 719-730.
DOI:10.1007/s10646-011-0613-5 |
Lance E, Paty C, Bormans M, et al. 2007. Interactions between cyanobacteria and gastropods: Ⅱ. Impact of toxic Planktothrix agardhii on the life-history traits of Lymnaea stagnalis. Aquatic Toxicology, 81(4): 389-396.
DOI:10.1016/j.aquatox.2006.12.019 |
Lei L M, Peng L, Huang X H, et al. 2014. Occurrence and dominance of Cylindrospermopsis raciborskii and dissolved cylindrospermopsin in urban reservoirs used for drinking water supply, South China. Environmental Monitoring and Assessment, 186(5): 3079-3090.
DOI:10.1007/s10661-013-3602-8 |
Li J M, Li J, Shi G, et al. 2016. Discerning biodegradation and adsorption of microcystin-LR in a shallow semi-enclosed bay and bacterial community shifts in response to associated process. Ecotoxicology and Environmental Safety, 132: 123-131.
DOI:10.1016/j.ecoenv.2016.05.033 |
Lin Q, You W H, Xu F J, et al. 2014. Zooplankton community structure and its relationship with environmental factors in Dishui Lake. Acta Ecologica Sinica, 34(23): 6918-6929.
(in Chinese with English abstract) DOI:10.5846/stxb201303060360 |
Lu Z, Lei L M, Lu Y, et al. 2021. Phosphorus deficiency stimulates dominance of Cylindrospermopsis through facilitating cylindrospermopsin-induced alkaline phosphatase secretion: Integrating field and laboratory-based evidences. Environmental Pollution, 290: 117946.
DOI:10.1016/j.envpol.2021.117946 |
Murray R E, Knowles R. 2003. Production of NO and N2O in the presence and absence of C2H2 by soil slurries and batch cultures of denitrifying bacteria. Soil Biology and Biochemistry, 35(8): 1115-1122.
DOI:10.1016/S0038-0717(03)00163-9 |
Newman M E J. 2003. The structure and function of complex networks. SIAM Review, 45(2): 167-256.
|
Pohnert G, Steinke M, Tollrian R. 2007. Chemical cues, defence metabolites and the shaping of pelagic interspecific interactions. Trends in Ecology & Evolution, 22(4): 198204.
DOI:10.1016/j.tree.2007.01.005 |
Rzymski P, Poniedzialek B, Kokociński M, et al. 2014. Interspecific allelopathy in cyanobacteria: cylindrospermopsin and Cylindrospermopsis raciborskii effect on the growth and metabolism of Microcystis aeruginosa. Harmful Algae, 35: 1-8.
DOI:10.1016/j.hal.2014.03.002 |
Shi W L, Jiang R D, Ma T H, et al. 2018. Molecular ecological network analysis of sedimental microbial community and its response to environmental factors in different trophic status areas of Taihu Lake. Journal of Nanjing University (Natural Science), 54(5): 1045-1056.
(in Chinese with English abstract) DOI:10.13232/j.cnki.jnju.2018.05.021 |
Singh D P, Tyagi M B, Kumar A, et al. 2001. Antialgal activity of a hepatotoxin-producing cyanobacterium, Microcystis aeruginosa. World Journal of Microbiology and Biotechnology, 17(1): 15-22.
DOI:10.1023/A:1016622414140 |
Takahashi M, Yamada T, Tanno M, et al. 2011. Nitrate removal efficiency and bacterial community dynamics in denitrification processes using poly (L-lactic acid) as the solid substrate. Microbes and Environments, 26(3): 212-219.
DOI:10.1264/jsme2.ME11107 |
Tian H L, Zhao J Y, Zhang H Y, et al. 2015. Bacterial community shift along with the changes in operational conditions in a membrane-aerated biofilm reactor. Applied Microbiology and Biotechnology, 99(7): 3279-3290.
DOI:10.1007/s00253-014-6204-7 |
Valdor R, Aboal M. 2007. Effects of living cyanobacteria, cyanobacterial extracts and pure microcystins on growth and ultrastructure of microalgae and bacteria. Toxicon, 49(6): 769-779.
DOI:10.1016/j.toxicon.2006.11.025 |
Wang L Z, Priscu J C. 1994. Stimulation of aquatic bacterial activity by cyanobacteria. Hydrobiologia, 277(3): 145-158.
|
Wilhelm S W, Farnsley S E, Lecleir G R, et al. 2011. The relationships between nutrients, cyanobacterial toxins and the microbial community in Taihu (Lake Tai), China. Harmful Algae, 10(2): 207-215.
DOI:10.1016/j.hal.2010.10.001 |
Wilson A E, Sarnelle O, Tillmanns A R. 2006. Effects of cyanobacterial toxicity and morphology on the population growth of freshwater zooplankton: meta-analyses of laboratory experiments. Limnology and Oceanography, 51(4): 1915-1924.
DOI:10.4319/lo.2006.51.4.1915 |
Yan X C, Xu X G, Wang M Y, et al. 2017. Climate warming and cyanobacteria blooms: looks at their relationships from a new perspective. Water Research, 125: 449-457.
DOI:10.1016/j.watres.2017.09.008 |
Yang C Y, Li D H, Liu Y D. 2008. The effect of microcystin on the growth and some physio-biochemical characteristics of representative microbial species. Acta Hydrobiologica Sinica, 32(6): 818-823.
DOI:10.3724/sp.j.1035.2008.00818 |
Zhang W, Jeppesen E, Wang M M, et al. 2017. Allelopathic effect boosts Chrysosporum ovalisporum dominance in summer at the expense of Microcystis panniformis in a shallow coastal water body. Environmental Science and Pollution Research, 24(5): 4666-4675.
DOI:10.1007/s11356-016-8149-0 |
Zhang W, Shang G X, Zhang J Y, et al. 2018. First report of bloom forming cyanobacterial species Chrysosporum ovalisporum in China. Plant Science Journal, 36(2): 185-190.
(in Chinese with English abstract) DOI:10.11913/PSJ.2095-0837.2018.2018 |
Zhou Q L, Li K M, Xie J, et al. 2009. Role and functions of beneficial microorganisms in sustainable aquaculture. Bioresource Technology, 100(16): 3780-3786.
DOI:10.1016/j.biortech.2008.12.037 |
Zhu G Y, Chen P P. 2012. Cytotoxicities and oxidative damage mechanisms of Ctenopharyngodon idellus lymphocytes to cylindrospermopsin. Acta Scientiae Circumstantiae, 32(5): 1199-1205.
DOI:10.13671/j.hjkxxb.2012.05.005 |
Zhu P C, Li Y C, Gao Y F, et al. 2021. Insight into the effect of nitrogen-rich substrates on the community structure and the co-occurrence network of thermophiles during lignocellulose-based composting. Bioresource Technology, 319: 124111.
DOI:10.1016/j.biortech.2020.124111 |
 2022, Vol. 40
2022, Vol. 40


