Institute of Oceanology, Chinese Academy of Sciences
Article Information
- WU Zhongxing, YANG Songqi, SHI Junqiong
- Overview of the distribution and adaptation of a bloom-forming cyanobacterium Raphidiopsis raciborskii: integrating genomics, toxicity, and ecophysiology
- Journal of Oceanology and Limnology, 40(5): 1774-1791
- http://dx.doi.org/10.1007/s00343-022-2003-7
Article History
- Received Sep. 27, 2021
- accepted in principle Dec. 4, 2021
- accepted for publication Mar. 28, 2022
Raphidiopsis raciborskii (basonym Cylindrospermopsis raciborskii; Aguilera et al., 2018) is a solitary, planktonic, and filamentous diazotrophic cyanobacterium belonging to the order Nostocales. Because it has two terminal heterocysts, R. raciborskii was originally described as Anabaena raciborskii Wołoszyńska from the samples collected in Java, Indonesia, in 1912 (Wołoszyńska, 1912). Original observations were limited to the Indo-Malayan realm, so R. raciborskii was considered to be a tropical species. However, an increasing number of reports have been made from every continent except Antarctica (Padisák, 1997; Sinha et al., 2012) that R. raciborskii forms bloom in approximately 18% of freshwater lakes, reservoirs, and rivers (Xiao et al., 2020a). These results make it reasonable to assume that R. raciborskii is an invasive species (Padisák, 1997; Briand et al., 2004; Antunes et al., 2015).
Apart from its dispersal potential, R. raciborskii has attracted scientific interest due to its association with toxic effects. The cyanotoxin alkaloid cylindrospermopsin (CYN) was first identified by Ohtani et al. (1992) isolated from R. raciborskii, after which more CYN variants were found (Li et al., 2001; Rzymski and Poniedziałek, 2014; Wimmer et al., 2014). Recently, some strains of R. raciborskii have also been found that can produce other toxins, including alkaloid saxitoxin (STX; Lagos et al., 1999; Zingone and Enevoldsen, 2000; Neilan et al., 2003; Miotto et al., 2017) and lipophilic congeners of phorbol 12-myristate 13-acetate (PMA; Rzymski et al., 2017).
Several studies have highlighted the distinctive features of this species that aid its succession and dominance. For example, laboratory studies have observed that R. raciborskii can utilize all kinds of different nitrogen sources (Harris and Baxter, 1996; Moisander et al., 2012; Ammar et al., 2014) and a high uptake affinity and storage capacity for phosphorus (Isvánovics et al., 2000; Posselt et al., 2009; Wu et al., 2009; Xiao et al., 2020a), as well as can thrive in a wide range of light intensities (Padisák and Istvánovics, 1997; Padisák and Reynolds, 1998; Briand et al., 2002; Pierangelini et al., 2014). Therefore, information on the ecology, phylogeography, and toxicology of this species has been reviewed (Padisák and Istvánovics, 1997; Griffiths and Saker, 2003; Antunes et al., 2015; Burford et al., 2016, 2018). However, as interest in R. raciborskii has increased, some issues regarding its phylogeography, molecular selection, and ecophysiological adaptation have been questioned. Here, the latest proposals on its distribution and adaptation through the integration of genomics, toxicity, and ecophysiology are discussed. As well, managing implications for R. raciborskii are also presented according to new insights into the success of this species in different environments.
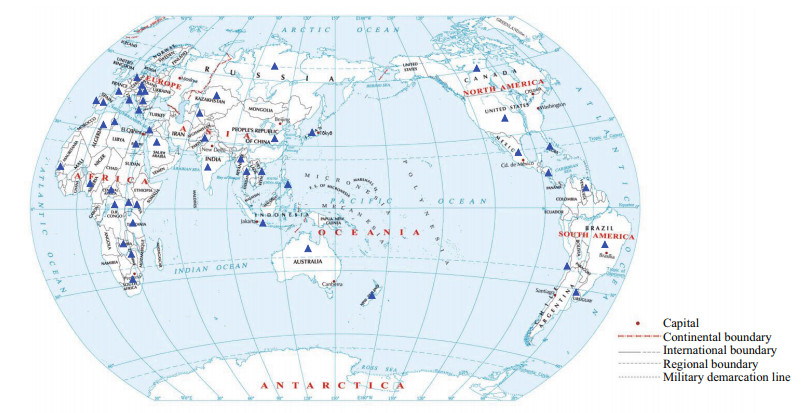
2 DISTRIBUTIONRaphidiopsis raciborskii was first identified by Wołoszyńska (1912) in 1899–1900 from samples taken from the lakes in Java, Indonesia. In Europe, R. raciborskii was first observed in Lake Kastoria, Greece (Skuja, 1937), and later in Hungary (Padisák, 1997). In Africa, R. raciborskii was recorded in detail in Lake Victoria by Komárek and Kling (1991) and was probably first detected in 1938 by Huber-Pestalozzi (1938). Additionally, this species was first reported in America in 1955 (Prescott and Andrews, 1955), in Australia in 1979 (Hawkins et al., 1985), and in the Middle East in 1998 (Zohary, 2004). To date, an increasing number of observations have localized this species in rivers, lakes, reservoirs, and shallow waters in the northern and southern hemispheres (Fig. 1). This species has been found, for example, in Spain (Romo and Miracle, 1994), Thailand (Li et al., 2001), New Zealand (Stirling and Quilliam, 2001), Germany (Fastner et al., 2003), Japan (Chonudomkul et al., 2004; Zarenezhad et al., 2012), Brazil (Soto-Liebe et al., 2010; Stucken et al., 2010), Poland (Kokociński et al., 2010), Italy (Messineo et al., 2010), Russia (Vinogradska, 1974; Babanazarova et al., 2015; Sidelev et al., 2020), Vietnam (Dao et al., 2010; Nguyen et al., 2017), USA (Yilmaz and Phlips, 2011), and Myanmar (Ballot et al., 2020; Swe et al., 2021). In China, R. raciborskii was first reported in a fish pond in Kunming, Yunnan, in 2006 (Wu et al., 2011); thereafter, this species was found to be present in freshwater bodies in Guangdong (Lei et al., 2014; Yu et al., 2014), Hubei (Wu et al., 2011, Jiang et al., 2014), Shanghai (Wu et al., 2011), Guizhou (Chen et al., 2011), Jiangsu (Wu et al., 2012a), Taiwan (Yamamoto and Shiah, 2012), Fujian (Lv et al., 2013; Jiang et al., 2014; Tan et al., 2021), Beijing (Xie et al., 2018), Sichuang (Tao et al., 2016), Shangdong (Wang, 2019), Chongqing (Zhang, 2019), and Zhejiang (Chao et al., 2021).
|
| Fig.1 The global geographic distribution of Raphidiopsis raciborski Data are obtained from Padisák, 1997; Sinha et al., 2012; Antunes et al., 2015; Panou et al., 2018; Sidelev et al., 2020; Yang et al., 2021. Map review No. GS(2016)2958. The blue triangles stand for the countries and regions where R. raciborskii was observed. |
Several hypotheses have been proposed to explain the origin and dispersal routes of R. raciborskii from tropical/subtropical zones to northern latitudes. The "radiation center" hypothesis was proposed by Padisák (1997) only based on the high diversity and salinity tolerance characteristics of R. raciborskii. Padisák and Istvánovics (1997) suggested that two radiation centers, Africa as the primary center and Australia as the secondary center, were responsible for expansion in Central America and Asia, respectively. Two possible routes, such as an oceanic route to the America by migratory birds or by unintentional human activities and a continental route to Central Asia and then to European by river course or by birds, are thought to explain the expansion of R. raciborskii from Australia to temperate regions (Padisák and Istvánovics, 1997; Moreira et al., 2011).
Another "refuge" hypothesis for the current geographic distribution was proffered by Gugger et al. (2005) based on a phylogeographic study. They found that three clusters of R. raciborskii strains were grouped: (ⅰ) America, (ⅱ) Europe, and (ⅲ) Africa and Australia using the 16S–23S internally transcribed spacer (ITS1) sequences. Therefore, they suggested that recent spread of R. raciborskii across the Americas and Europe occurred from restricted warm refuge areas rather than through intercontinental exchanges. Wood et al. (2014) indicated that cryptic akinetes of R. raciborskii were already present in lake sediment layers in New Zealand long before they were discovered as phytoplankton in 2003. A similar finding was found in the Blanca subtropical lagoon (De La Escalera et al., 2014). However, the refuge hypothesis has been repeatedly challenged as significant genetic differences were found in strains of R. raciborskii from southern Europe (Spain, Greece, and Italy) and northern Europe (Germany, Hungary, and Russia) (Cirés et al., 2014; Panou et al., 2018; Sidelev et al., 2020).
Later, a new hypothesis was raised by Haande et al. (2008) and Moreira et al. (2015) through the phylogeographic analysis of strains from all five continents based on three genetic markers, 16S rRNA gene, 16S–23S rRNA larger fragment (ITS-L), and RNA polymerase (rpoC1). They postulated that the primary evolutionary center of R. raciborskii was the tropical area of America, from where this species spread to the African continent, followed by Australia, Asia, and Europe. The hypothesis was supported by recent studies which indicated that strains from Spain, Greece, Italy, Tunisia, Russia, and New Zealand are more genetically similar to strains from the Americas (Cirés et al., 2014; Wood et al., 2014; Panou et al., 2018).
Although various hypotheses have been raised to explain the spread of R. raciborskii, there is a lack of high-quality paleontological evidence to support each hypothesis (Padisák et al., 2016; Kokociński et al., 2017). Sidelev et al. (2020) suggested that close genetic relatedness between the southern European, Tunisian, and American strains, as well as between the African and Australian strains, may be the result of the ancient origin of the species inhabiting the continents, rather than new transport in some cases through birds, insects, humans, or rivers in some cases. Recently, however, Vico et al. (2020) showed Central Africa as the primary center of distribution based on the analysis of 354 orthologous genes from all available genomes and ITS sequences. A nested clade analysis (NCA; Posada et al., 2006) was performed to test the phylogeography of 96 strains of R. raciborskii from different continents in our laboratory (Fig. 2). Our results revealed that these strains isolated from Uganda, Senegal, and Australia formed a tight cluster, confirming the result of Padisák and Istvánovics (1997) and Vico et al. (2020). It suggests that R. raciborskii can spread from the tropical zone to temperate and northern regions (Padisák and Istvánovics, 1997). However, closer relationships between some Chinese strains and other strains (e.g. European and American strains) were also noted in our results. It suggests that the biogeography of R. raciborskii has become even more confused with the increasing studies of more strains being isolated from around the world.

|
| Fig.2 A nested clade analysis (NCA) of nif gene for 96 strains of R. raciborskii isolated from different continents Some genes data are from NCBI: https://www.ncbi.nlm.nih.gov. |
The cyanotoxin cylindrospermopsin (CYN) first became known in scientific documents as the "Palm Island Mystery Disease, " which occurred in 1979 on Palm Island, Australia. One hundred forty-eight persons were hospitalized with severe symptoms of anorexia, vomiting, and tender livers after consumption of cyanobacterial bloom water treated with copper sulfate (Byth, 1980; Ohtani et al., 1992). Another implication of R. raciborskii in the poisoning was in northern Queensland, Australia, 13 cattle died in 1992 after drinking from a water source with a heavy cyanobacterial bloom (Thomas et al., 1998).
A novel structure for CYN was proposed by Ohtani et al. (1992). To date, four different CYN variants, 7-epicylindrospermopsin (7-epi-CYN), 7-deoxy-cylindrospermopsin (7-deoxy-CYN), 7-deoxy-desulfo-cylindrospermopsin, and 7-deoxy-desulfo-12-acetyl-cylindrospermopsin, have been described (Norris et al., 1999; Banker et al., 2000; Rzymski and Poniedziałek, 2014; Wimmer et al., 2014). Their novel structures, chemical properties, and toxicological effects and occurrences have been extensively reviewed (see reviews by De La Cruz et al., 2013; Burford et al., 2016; Adamski et al., 2020; Yang et al., 2021). Moreover, R. raciborskii can also produce neurotoxic STX and its analogs (i.e., neo-STX, gonyautoxins 2 and 3 [GTX-2 and GTX-3], decarbamoyl STX [dc-STX], and decarbamoyl-neo-saxitoxin [dc-neo-STX]), collectively known as paralytic shellfish toxins (PST) (Lagos et al., 1999; Li et al., 2001; Griffiths and Saker, 2003; Molica et al., 2005; Soto-Liebe et al., 2010). A 43-kb cyr gene cluster (Stucken et al., 2014) and a 35-kb stx gene cluster (Kellmann et al., 2008) were responsible for the production of CYN and STX, respectively. Recently, another toxic compound, polymethoxy-1-alkene (PMA) was reported from strains isolated from North America (Rzymski et al., 2017).
Most studies on the dispersal route of R. raciborskii are based on molecular genetic markers (i.e., 16S rRNA, ITS, PC-IGS, nifH, and rpoC1), and did not consider the phenotypes and genotypes of their toxicity (Vico et al., 2020). Studies have shown that R. raciborskii's ability to produce toxins appears to show a geographic pattern. For example, CYNs isolated from Australia, New Zealand, and Asia can be produced (Hawkins et al., 1997; Saker et al., 2003; Wood and Stirling, 2003; Chonudomkul et al., 2004; Jiang et al., 2014; Lu et al., 2021), while the South American strains are associated with STX producers (Lagos et al., 1999; Antunes et al., 2015). In contrast, strains from Africa, Europe, and North America are neither PST nor CYN- producers (Fastner et al., 2003; Neilan et al., 2003; Kellmann et al., 2006; Yılmaz et al., 2008; Mowe et al., 2015). Vico et al. (2020) found that the strains analyzed were divided into two clades, one with the South American strains (mostly PSP-producers) and another with the non-toxic strains isolated from Europe and Sun-Saharan Africa and CYN-producers isolated from Oceania. A similar result was also reported by Jiang et al. (2020), who indicated that Clade Ⅳ included all PST-producing strains, while CYN-producing strains were divided into two clusters, Clade Ⅱ and Clade Ⅴ.
Nevertheless, partial sequences of cyr genes are determined in American non-CYN producing strains (Piccini et al., 2011) and in PST-producing strains from Brazil (Hoff-Risseti et al., 2013). Recently, Vico et al. (2020) found that the partial genes of cyrA, cyrB, and cyrC are present again in the strains isolated from South America. Meanwhile, Yilmaz and Phlips (2011) found that cyr genes exhibit more exchange changes within North American strains of CYN-producing Aphanizomenon (Chrysosporum) ovalisporum than between species. A hypothesis raised by Vico et al. (2020), is therefore that (ⅰ) non-toxic R. raciborskii spread early from tropical Africa as the primary evolutionary center to North Africa, North America, and Mediterranean Europe; (ⅱ) a secondary evolutionary event was involved to acquire the cluster for CYN synthesis. These CYN-producing species spread warm climates across sub-Saharan Africa, Oceania, and South America. Later, the populations in South America somehow lost the cyr cluster and acquired the stx cluster through horizontal gene transfer, then the STX-producing species migrated to North America. In fact, the secondary evolutionary event mentioned by Vico et al. (2020) was the result of a phylogenetic analysis based on the ribosomal ITS of the species. Moreover, the estimated divergence time calculated for Raphidioposis may coincide with the time when Gondwana was split into Oceania and the South American continent. However, strains isolated from North America, Europe, Africa, and the Middle East have not been reported to produce CYN (Neilan et al., 2003; Yılmaz et al., 2008; Alster et al., 2010), which does not support this secondary evolutionary evidence that African strains are a source of the CYN gene cluster.
Recently, Jiang et al. (2020) found that strains of R. raciborskii isolated from China (i.e., CHAB3409, CHAB3422, and CHAB3426) produce STX, neo-STX, and dc-STX, and these strains and American strains have been clustered into different clades, further supporting the recent intercontinental spread events of toxic R. raciborskii (Antunes et al., 2015), but not the geographic origin of the strains. In fact, other cyanobacterial genera, including Chrysosporum, Aphanizomenon, Anabaena, Umezakia, Microseira, and Oscillatoria, have been reported to produce CYN (Rzymski and Poniedziałek, 2014). Hence, a complex history of acquisition and loss in the cyr gene cluster may be associated with its intra- and inter-genomic transfers (Jiang et al., 2014, 2020; Burford et al., 2016). Similarly, Moustafa et al. (2009) also suggested that STX is a common ancestral trait of R. raciborskii strains. Therefore, to answer these questions, additional genomic data from these diverse lineages, including closely related toxic and non-toxic strains, are needed.
Based on the literature and our NCA analysis (Fig. 2), we partially agree with Padisák's early hypothesis of the tropical region as the evolutionary center of R. raciborskii, but do not support the finding of Africa and Australia as the primary and secondary centers, and the high genetic and toxic diversity of Chinese strains indicates a high heterogeneity of the R. raciborskii population (Cirés et al., 2014; Moreira et al., 2015; Panou et al., 2018). Willis et al. (2018) also suggested that R. raciborskii exhibits high plasticity due to frequent gain or loss of genes. These reflect that the ability of R. raciborskii to produce toxins may not be a geographical pattern but the result of environmental responses and adaptations (Willis et al., 2019; Jiang et al., 2020). Therefore, a larger number of strains with different toxicity are required to test the comprehensive biogeography of this species, as previously suggested (Cirés et al., 2014).
3 ADAPTATION AND ACCLIMATION 3.1 Genome variations and adaptationBased on ecological and genomic studies, new insights into the genomic adaptation of marine picocyanobacteria to the local environment have been provided (Kashtan et al., 2014; Larsson et al., 2014; Biller et al., 2015). However, due to the lack of balanced genomic samples, the genomic adaptation of cyanobacteria to a wide variety of environments is still poorly understood (Chen et al., 2021). The first R. raciborskii genome was sequenced from the toxigenic strain CS-505 (Stucken et al., 2010), followed shortly thereafter by those of CS-506 and CS-509 (Sinha et al., 2014). To our knowledge, only one R. raciborskii genome has been closed as late as 2021. However, several draft genomes isolated from different locations were subsequently sequenced. To date, 23 drafts and 1 closed genomes sequenced from isolated strains from Australia, Brazil, United States, Uruguay, China, and Korea were available from NCBI (see Table 1 for detailed information). The average nucleotide identity (ANI) between strains was 0.997 6 (range 0.995 0–0.998 7, between genome pairs; Willis and Woodhouse, 2020).
Compared to other cyanobacteria (e.g., Microcystis, Nostoc, Dolichospermum, and Aphanizomenon), a smaller genome was found in R. raciborskii with a genome size of 3.74±0.24 Mb, 40.24%±0.15% G+C content, and 3 144±292.35 coding sequences (Table 1). Stucken et al. (2010) has suggested that a small genome found in R. raciborskii may be in the process of reducing superfluous functions. Typically, a downsizing of a genome is seen as an indication of an evolutionary adaptation strategy to different environments (Rocap et al., 2003; Shi and Falkowski, 2008; Larsson et al., 2011; Willis et al., 2018). It suggests that genome variants in R. raciborskii are responsible for the global expansion into new habitats.
Willis et al. (2018) stated that the R. raciborskii pan-genome contains about 16% of R. raciborskii genome with 847 variables and 433 strain-specific orthologous groups, suggesting that there is greater genetic diversity in R. raciborskii strains. In addition, variation, arrangement, or shifting of genes are always found in R. raciborskii when seven strains of R. raciborskii are compared with the strain Raphidiopsis brookii D9 strain. These genes are involved in natural product biosynthesis, heterocyst glycolipid formation, nitrogen fixation, and toxin production. Similar results are reported by Abreu et al. (2018), who found variable genes involved in amino sugar metabolism, DNA modification, and carbohydrate biosynthesis. Shi and Falkowski (2008) suggested that selective pressures and evolution can affect the core and variable genes, resulting in strain variability in different environments (Kashtan et al., 2014).
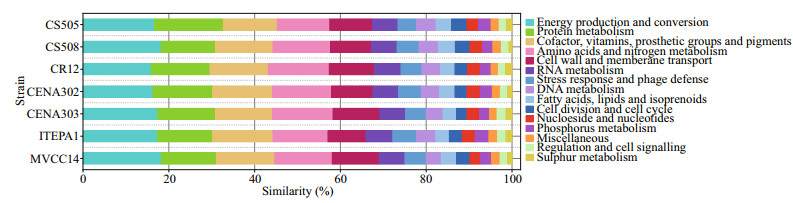
A comparative genome analysis showed that strains of R. raciborskii contained a variety of strain-specific (or non-homologous) genes (Stucken et al., 2010; Sinha et al., 2014; Abreu et al., 2018). These genes are involved in energy production and conversion, stress response and phage defense, DNA repair and recombination, cell cycle control, and the nutrients transport and uptake (Fig. 3), all of which are largely related to environmental response and adaptation. Moreover, the gene clusters associated with toxin production and heterocyst differentiation (i.e., hassallidin [hass], cylindrospermopsin [cyr], saxitoxin [sxt], heterocyte glycolipid [hgl], and nitrogen fixation [nif, fdxN, hesA and B, and feoaA]) also indicate phenotypic plasticity (Sinha et al., 2014; Abreu et al., 2018). Stucken et al. (2010) suggested that the absence or loss of the cyr cluster, rather than indicating mutations or partial deletions, was associated with the absence of toxicity in some strains of R. raciborskii. However, several reports have found that some R. raciborskii strains retained the partial cyr cluster, i.e., cyrA, cyrB, or/and cyrC are still unable to produce CYN (Kellmann et al., 2006; Rasmussen et al., 2008; Hoff-Risseti et al., 2013). This supports that horizontal gene transfer or subsequent loss of the cyr gene is responsible for the acquisition of the cyr genes (Christiansen et al., 2008; Moustafa et al., 2009). Willis et al. (2018) has observed that 21 proteins, particularly those involved in sugar transport, phosphonate substrate binding, and CRISPR/Cas phage-defense systems, yield a greater copy number in the coiled compared to the straight morphotypes of R. raciborskii. Larsson et al. (2011) proposed that gene duplication can expand phenotype and adaptive behavior in cyanobacteria.
|
| Fig.3 Comparison of strain-specific (or non-homologous) genes in Raphidiopsis raciborskii strains Each layer represents a genome and each color of the layer indicates a subsystem. The values (%) show a similar percentage of the subsystem in the genome with R. raciborskii C04 strain (Genes data from Stucken et al., 2010; Abreu et al., 2018; Willis et al., 2018; Vico et al., 2020, and NCBI: https://www.ncbi.nlm.nih.gov/). |
In short, comparative genomics provides new insights into the genotypic and phenotypic plasticity of the species R. raciborskii. A high proportion of variable strain-specific genes associated with environmental responses and adaptation, particularly in some key cellular processes (e.g., cell regulation, biosynthesis, and transport), are found in this species, reflecting that successful adaptation to specific habitat in R. raciborskii may allow the exploration of a wide range of environmental conditions. Furthermore, the co-existence of multiple strains within a R. raciborskii population in a single water sample can confer fitness advantages to this species in variable environments by eliciting their niche adaptation (Piccini et al., 2011; Willis et al., 2018). In addition, Abreu et al. (2018) found that the comparative genome analysis showed that the five South American genomes CENA302, CENA303, ITEP-A1, MVCC14, and D9 (Brazil and Uruguay) are slightly smaller and more conserved than the non-South American CS-505, CS-508, and CR12 (Australia and Singapore) genomes, suggesting that genomes from South America underwent gene loss events. However, due to the lack of genome sequences in European and African strains, no more precise conclusions can be drawn about the influence of the geographic environment on their genomic plasticity. Therefore, in order to explain the very different strategies for genomic organization and adaptation mechanisms in R. raciborskii, more strains from a range of habitats or regions needed to be sequenced and compared in the future.
3.2 Ecophysiology and adaptation 3.2.1 PhosphorusPhosphorus is considered a key factor in the ecophysiology and dominance of R. raciborskii. A positive or negative correlation between R. raciborskii cell densities and phosphorus concentrations has been reported in field studies (Bonilla et al., 2012; Muhid et al., 2013; Soares et al., 2013a; Zhao et al., 2017). Several studies have illustrated that R. raciborskii has a high uptake affinity for dissolved inorganic phosphorus (Isvánovics et al., 2000; Wu et al., 2009) and a high phosphorus storage capacity (Posselt et al., 2009; Willis et al., 2017), as well as a superior scavenger for dissolved organic phosphorus (Bai et al., 2014). Furthermore, both uptake and conversion of phosphorus were more effective in R. raciborskii than in Microcystis aeruginosa and Aphanizomenon flos-aquae (Wu et al., 2009). Therefore, these traits are favorable for the dominance of R. raciborskii populations (Isvánovics et al., 2000), which is supported by the results of Chislock et al. (2014), who indicated that R. raciborskii can dominate at different phosphorus concentrations.
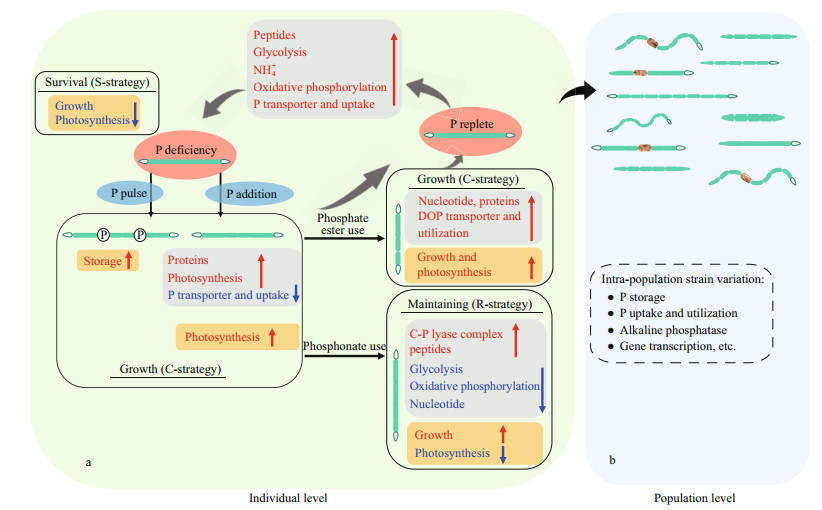
Physiological and molecular studies have suggested that R. raciborskii can evolve a variety strategies in response to environmental phosphorus (Fig. 4a). Under phosphorus deficient conditions, strains show little metabolic activity to keep sustain themselves (i.e., "S-adapted strains"). In this environment, the growth and photosynthesis of these strains are significantly inhibited and the genes encoding photosynthesis and protein synthesis are markedly downregulated, while alkaline phosphatase and the genes encoding phosphate uptake and transport, ATP-consumption, and energy metabolism are markedly upregulated (Wu et al., 2012a; Bai et al., 2014; Willis et al., 2018; Shi et al., 2022). Under organic phosphate conditions, the strains showed a rapid growth (i.e., "C-adapted strains or K-adapted strains"). In this state, rapid growth is noted, which is caused by a significant increase in genes encoding phosphate-specific transporters, alkaline phosphatase, and ribosomes (Bai et al., 2014; Willis et al., 2018; Shi et al., 2022). However, under phosphonate conditions (i.e., "R-adapted strains or r-adapted strains"), a slight inhibition of growth and photosynthesis is observed because alkaline phosphatase and the genes encoding carbon-phosphorus lyase, genetic information, and environmental information are dramatically upregulated (Willis et al., 2018; Shi et al., 2022). Additionally, since R. raciborskii has a high phosphorus storage capacity, pulsed additions of dissolved inorganic phosphorus are more favorable for the growth of this species compared to constant feeds of dissolved inorganic phosphorus (Posselt et al., 2009; Marinho et al., 2013; Amaral et al., 2014), referred to as "C-adapted strains or K-adapted strains" (Xiao et al., 2020a).
|
| Fig.4 Diagram for the response to changing ambient phosphorus in Raphidiopsis raciborskii Red texts: upregulated genes; blue texts: downregulated genes; gray and yellow squares: gene and physiological parameters, respectively (from Wu et al., 2012a; Bai et al., 2014; Willis et al., 2015, 2017; Burford et al., 2016; Xiao et al., 2020a; Shi et al., 2022). |
Recent studies have provided evidence that different strains in a R. raciborskii population exhibit significant differences in growth, storage, and molecular response to phosphorus concentrations and pulses (Fig. 4b, Amaral et al., 2014; Willis et al., 2015, 2017, 2019; Guedes et al., 2019; Xiao et al., 2020a). For example, Xiao et al. (2020a) showed that phosphorus storage capacity can vary four-fold in six toxic strains of R. raciborskii. Willis et al. (2019) have also pointed out that gene copy number and expression patterns for phosphorus metabolism show differences between the coiled and straight R. raciborskii strains under phosphorus replete and deficiency conditions. This finding suggests that the intraspecific variability of R. raciborskii can lead to changes in the proportion of strains within a population (Burford et al., 2018). In addition, it has been suggested that R. raciborskii dominance can be promoted under both high and low nitrogen-to-phosphorus ratios (Posselt et al., 2009; Chislock et al., 2014).
Moreover, previous studies have confirmed that P availability can affect intracellular CYN concentration (QCYNS) or a shift in the proportion of toxic and non-toxic R. raciborskii. For example, Mohamed and Al-Shehri (2013) found that QCYNS of R. raciborskii cells was increased when P concentrations were higher in a Saudi lake. Burford et al. (2014) also indicated that the proportion of toxic R. raciborskii strains increases with increasing phosphorus availability, regardless of whether N was supplied using a mesocosm study. Lu et al. (2021) showed that phosphorus deficiency stimulates R. raciborskii dominance by facilitating CYN-induced alkaline phosphatase secretion. A similar finding was reported by Bar-Yosef et al. (2010) in CYN-producing cyanobacteria, Chrysosporum ovalisporum. The results may indicate that CYN may be facilitated by the dominance of R. raciborskii under P deficiency.
3.2.2 NitrogenRaphidiopsis (Cylindrospermopsis) raciborskii was originally described as a Nostocales species with heterocytes, distinguished from other Raphidiopsis by its lack of heterocytes and nitrogen-fixing ability (Padisák, 1997). However, Abreu et al. (2018) found that the C. raciborskii strain CENA303 isolated from Brazil does not differentiate heterocytes due to the absence of nif and hgl gene clusters involved in nitrogen fixation and thick heterocyte glycolipid envelope formation, respectively. Therefore, Cylindrospermopsis and Raphidiopsis are considered to be a unifying genus, with Raphidiopsis using a morphological, ultrastructural, physiological, and molecular approach (Aguilera et al., 2018). In general, nitrogen-fixing ability is often associated with an ecological advantage of R. raciborskii over non-nitrogen-fixing species (Harris and Baxter, 1996; Hadas et al., 2012). Studies have indicated that the terminal heterocyst cells of R. raciborskii can fix nitrogen, allowing this species to survive in low dissolved nitrogen environments (Harris and Baxter, 1996; Présing et al., 1996; Padisák and Istvánovice, 1997; McGregor and Fabbro, 2000; Spröber et al., 2003; Plominsky et al., 2013; Willis et al., 2016). However, a preference for different forms of dissolved nitrogen (i.e., ammonia, nitrate, and urea) has now been demonstrated in R. raciborskii (Hawkins et al., 2001; Saker and Neilan, 2001; Burford et al., 2006; Ammar et al., 2014; Figueredo et al., 2014; Yu et al., 2014). Ammar et al. (2014) showed that R. raciborskii can grow faster than the species Planktothix agardhii, a perennial biomass and phytoplankton community dominant in a Tunisian reservoir, at high ammonia concentrations. Dai et al. (2015) also found that the growth of R. raciborskii was significantly inhibited under conditions of low nitrogen (< 0.5 mg/L).
The relationship between CYNs concentrations and nitrogen has led to conflicting conclusions. For example, Saker and Neilan (2001) found that the highest and lowest CYNs concentrations were determined in R. raciborskii grown in the absence of a fixed N source and ammonium, respectively. Rigamonti et al. (2018) also indicated that a positive association between CYN production and nitrogen fixation was observed in R. raciborskii. In contrast, Vico et al. (2016) showed that nitrate availability is not related to the biosynthesis of saxitoxin and analogs in R. raciborskii. However, compared to nitrate uptake, nitrogen fixation is an inefficient and energetically expensive process (Shafik et al., 2001; Burford et al., 2006). Abreu et al. (2018) found that the non-nitrogen-fixing strain R. raciborskii CENA303 lacks the nitrogen fixation (nif) and heterocyte glycolipid (hgl) gene clusters. Therefore, nitrogen availability can shift the proportion of toxic and non-toxic R. raciborskii and regulate the formation of R. raciborskii bloom. Switching between dissolved nitrogen assimilation and nitrogen fixation in R. raciborskii is an adaptive strategy to respond to fluctuations in environmental nitrogen (Moisander et al., 2012).
3.2.3 Other factorRaphidiopsis raciborskii has shown a wide tolerance to different temperatures and light intensities. This species can grow at light intensities as low as tens to hundreds of μmol photons/(m2·s) (Saker et al., 1999; Shafik et al., 2001; Griffiths and Saker, 2003; Briand et al., 2004; Dyble et al., 2006; Mehnert et al., 2010; Yu et al., 2014). Field studies have shown that R. raciborskii can form blooms under low light intensity, which has advantages for its shade tolerance and light acclimatizaion (Padisák, 1997; Padisák and Reynolds, 1998; Briand et al., 2002; Mehnert et al., 2012). Moreover, stratified water column conditions are generally considered favorable for R. raciborskii, although it is typically dispersed throughout the water column (Bouvy et al., 1999, 2003; McGregor and Fabbro, 2000; Berger et al., 2006). This could be a factor contributing to the success of R. raciborskii (Antunes et al., 2015; Burford et al., 2016).
Raphidiopsis raciborskii also exhibits a wide tolerance to different temperatures (Briand et al., 2004; Chonudomkul et al., 2004; Everson et al., 2011; Bonilla et al., 2012). A model analysis revealed that R. raciborskii blooms can occur in the temperature range of 25 ℃ to 32 ℃ (Recknagel et al., 2014), suggesting that increasing temperature favor the bloom formation of this species (Soares et al., 2012). Studies have shown that rising temperatures are beneficial for the spread of R. raciborskii, as the akinete germination of this species is affected by early spring warming in temperate habitats (Padisák, 1997; Briand et al., 2002; Wiedner et al., 2007; Mehnert et al., 2012; Yu et al., 2014). Saker and Neilan (2001) observed that temperate strains can produce more akinetes than those in tropical strains. These results suggest that the interplay between ecophysiology and genetic evolution may have an impact on the spread of R. raciborskii. A recent study also demonstrates that temperature and light have a synergistic effect on the growth rates of R. raciborskii (Kehoe et al., 2015; Xiao et al., 2020b).
In addition, the ecological performance and selection of R. raciborskii can also be influenced by anthropogenic CO2 (Wu et al., 2012b; Pierangelini et al., 2014), pH (Bonilla et al., 2012; Holland et al., 2012), salinity (Moisander et al., 2012), allelopathy (Figueredo et al., 2007; Leão et al., 2009; Antunes et al., 2012; Mello et al., 2012), multiple disturbance (Yang et al., 2017), zooplankton (Soares et al., 2010; Bednarska et al., 2014), and other biotas (Sukenik et al., 2012; Bagatini et al., 2014; Guedes et al., 2019; Bai et al., 2020).
4 CONCLUSION AND IMPLICATIONThe abundance of studies from around the world has provided our understanding of the biogeography, toxicity, genome, and ecophysiology of R. raciborskii. Considering all the evidence, the biogeography of this species is credited with an early spread from a tropical zone to temperate regions, while the scenario of refuge and secondary radiation centers has yet to be confirmed by exploring further strains from all continents or paleontological evidence. CYN-producing strains have been identified in a limited number of country, while a geographic spread of toxic strains or a complex history of acquisition and loss in the cyr or stx gene cluster is not excluded from the intra- and inter-genomic transfers based on current studies. Studies have shown that the production and export of CYN in R. raciborskii can be a functional strategy for competition with other phytoplankton. More direct evidence is still needed to support CYN's potential biological role to facilitate its dominance or bloom.
It is obvious that this species shows flexible adaptation strategies ("C-adapted, R-adapted, and S-adapted") in nutrient dynamics based on laboratory experiments, which are very crucial for their expansion behavior. However, field studies always indicate negative or positive effects of nitrogen or phosphorus on this species or its dominance, reflecting that an interaction of nitrogen or phosphorus is likely to be underestimated. Moreover, it is clear that genome variation and ecotypes exist between co-occurring strains in a water sample. Therefore, supplementary reports on interaction effects, phenotypic differences, and population plasticity can be expected in this species. Furthermore, the impact of global climate change on the physiological resilience or existence of distinct ecotypes in this species remains unclear.
Overall, there is no doubt that rising temperatures can be associated with the spread and proliferation of this species. Moreover, flexible strategy and significant intra-population strain variation in nitrogen and phosphorus dynamics provide better resilience of a population under changing environmental nutrients. Therefore, controlling R. raciborskii bloom may not be achievable with a simple reduction in nitrogen or phosphorus loading, particularly in intermittent nutrient pulses and mixed water columns. Future efforts are essential for a comprehensive understanding of the ecophysiology of R. raciborskii in different scenarios in order to find an efficient means of the control.
5 DATA AVAILABILITY STATEMENTThe authors declare that all data supporting the findings of this study are available within the article. The raw data that support the findings of this study are available from the corresponding author upon reasonable request.
Abreu V A C, Popin R V, Alvarenga D O, et al. 2018. Genomic and genotypic characterization of Cylindrospermopsis raciborskii: toward an intraspecific phylogenetic evaluation by comparative genomics. Frontiers in Microbiology, 9: 306.
DOI:10.3389/fmicb.2018.00306 |
Adamski M, Wolowski K, Kaminski A, et al. 2020. Cyanotoxin cylindrospermopsin producers and the catalytic decomposition process: a review. Harmful Algae, 98: 101894.
DOI:10.1016/j.hal.2020.101894 |
Aguilera A, Gómez E B, Kaštovský J, et al. 2018. The polyphasic analysis of two native Raphidiopsis isolates supports the unification of the genera Raphidiopsis and Cylindrospermopsis (Nostocales, Cyanobacteria). Phycologia, 57(2): 130-146.
DOI:10.2216/17-2.1 |
Alster A, Kaplan-Levy R N, Sukenik A, et al. 2010. Morphology and phylogeny of a non-toxic invasive Cylindrospermopsis raciborskii from a Mediterranean Lake. Hydrobiologia, 639: 115-128.
DOI:10.1007/s10750-009-0044-y |
Amaral V, Bonilla S, Aubriot L. 2014. Growth optimization of the invasive cyanobacterium Cylindrospermopsis raciborskii in response to phosphate fluctuations. European Journal of Phycology, 49(1): 134-141.
DOI:10.1080/09670262.2014.897760 |
Ammar M, Comte K, Tran T D C, et al. 2014. Initial growth phases of two bloom-forming cyanobacteria (Cylindrospermopsis raciborskii and Planktothrix agardhii) in monocultures and mixed cultures depending on light and nutrient conditions. Annales de Limnologie-International Journal of Limnology, 50(3): 231-240.
DOI:10.1051/limn/2014096 |
Antunes J T, Leèo P N, Vasconcelos V M. 2012. Influence of biotic and abiotic factors on the allelopathic activity of the cyanobacterium Cylindrospermopsis raciborskii strain LEGE 99043. Microbial Ecology, 64(3): 584-592.
DOI:10.1007/s00248-012-0061-7 |
Antunes J T, Leèo P N, Vasconcelos V M. 2015. Cylindrospermopsis raciborskii: review of the distribution, phylogeography, and ecophysiology of a global invasive species. Frontiers in Microbiology, 6: 473.
DOI:10.3389/fmicb.2015.00473 |
Babanazarova O V, Sidelev S I, Fastner J. 2015. Northern expansion of Cylindrospermopsis raciborskii(Nostocales, Cyanoprokaryota) observed in shallow highly eutrophic Lake Nero (Russia). International Journal on Algae, 17(2): 131-141.
DOI:10.1615/InterJAlgae.v17.12.20 |
Bagatini I L, Eiler A, Bertilsson S, et al. 2014. Host-specificity and dynamics in bacterial communities associated with bloom-forming freshwater phytoplankton. PLoS One, 9(1): e85950.
DOI:10.1371/journal.pone.0085950 |
Bai F, Liu R, Yang Y J, et al. 2014. Dissolved organic phosphorus use by the invasive freshwater diazotroph cyanobacterium, Cylindrospermopsis raciborskii. Harmful Algae, 39: 112120.
DOI:10.1016/j.hal.2014.06.01 |
Bai F, Shi J Q, Yang S, et al. 2020. Interspecific competition between Cylindrospermopsis raciborskii and Microcystis aeruginosa on different phosphorus substrates. Environmental Science and Pollution Research, 27(34): 42264-42275.
DOI:10.1007/s11356-020-08652-0 |
Ballot A, Swe T, Mjelde M, et al. 2020. Cylindrospermopsin- and deoxycylindrospermopsin-producing Raphidiopsis raciborskii and microcystin-producing Microcystis spp. in Meiktila Lake, Myanmar. Toxins, 12(4): 232.
DOI:10.3390/toxins12040232 |
Banker R, Teltsch B, Sukenik A, et al. 2000. 7-Epicylindrospermopsin, a toxic minor metabolite of the cyanobacterium Aphanizomenon ovalisporum from Lake Kinneret, Israel. Journal of Natural Products, 63(3): 387389.
DOI:10.1021/np990498m |
Bar-Yosef Y, Sukenik A, Hadas O, et al. 2010. Enslavement in the water body by toxic Aphanizomenon ovalisporum, inducing alkaline phosphatase in phytoplanktons. Current Biology, 20(17): 1557-1561.
DOI:10.1016/).cub.2010.07.032 |
Bednarska A, Pietrzak B, Pijanowska J. 2014. Effect of poor manageability and low nutritional value of cyanobacteria on Daphnia magna life history performance. Journal of Plankton Research, 36(3): 838-847.
DOI:10.1093/plankt/fbu009 |
Berger C, Ba N, Gugger M, et al. 2006. Seasonal dynamics and toxicity of Cylindrospermopsis raciborskii in Lake Guiers (Senegal, West Africa). FEMS Microbiology Ecology, 57(3): 355-366.
DOI:10.1111/j.1574-6941.2006.00141.x |
Biller S J, Berube P M, Lindell D, et al. 2015. Prochlorococcus: the structure and function of collective diversity. Nature Reviews Microbiology, 13(1): 13-27.
DOI:10.1038/nrmicro3378 |
Bonilla S, Aubriot L, Soares M C S, et al. 2012. What drives the distribution of the bloom forming cyanobacteria Planktothrix agardhii and Cylindrospermopsis raciborskii?. FEMS Microbiology Ecology, 79(3): 594-607.
DOI:10.1111/j.1574-6941.2011.01242.x |
Bouvy M, Molica R, De Oliveira S M, et al. 1999. Dynamics of a toxic cyanobacterial bloom (Cylindrospermopsis raciborskii) in a shallow reservoir in the semi-arid region of northeast Brazil. Aquatic Microbial Ecology, 20(3): 285-297.
DOI:10.3354/ame020285 |
Bouvy M, Nascimento S M, Molica R J R, et al. 2003. Limnological features in Tapacurá reservoir (northeast Brazil) during a severe drought. Hydrobiologia, 493: 115-130.
DOI:10.1023/A:1025405817350 |
Briand J F, Leboulanger C, Humbert J F, et al. 2004. Cylindrospermopsis raciborskii (Cyanobacteria) invasion at mid-latitudes: selection, wide physiological tolerance, or global warming?. Journal of Phycology, 40(2): 231-238.
DOI:10.1111/j.1529-8817.2004.03118.x |
Briand J F, Robillot C, Quiblier-Llobéras C, et al. 2002. Environmental context of Cylindrospermopsis raciborskii (cyanobacteria) blooms in a shallow pond in France. Water Research, 36(13): 3183-3192.
DOI:10.1016/S0043-1354(02)00016-7 |
Burford M A, Beardall J, Willis A, et al. 2016. Understanding the winning strategies used by the bloom-forming cyanobacterium Cylindrospermopsis raciborskii. Harmful Algae, 54: 44-53.
DOI:10.1016/j.hal.2015.10.012 |
Burford M A, Davis T W, Orr P T, et al. 2014. Nutrient-related changes in the toxicity of field blooms of the cyanobacterium, Cylindrospermopsis raciborskii. FEMS Microbiology Ecology, 89(1): 135-148.
DOI:10.1111/1574-6941.12341 |
Burford M A, McNeale K L, McKenzie-Smith F J. 2006. The role of nitrogen in promoting the toxic cyanophyte Cylindrospermopsis raciborskii in a subtropical water reservoir. Freshwater Biology, 51(11): 2143-2153.
DOI:10.1111/j.1365-2427.2006.01630.x |
Burford M A, Willis A, Chuang A, et al. 2018. Recent insights into physiological responses to nutrients by the cylindrospermopsin producing cyanobacterium, Cylindrospermopsis raciborskii. Journal of Oceanology and Limnology, 36(4): 1032-1039.
DOI:10.1007/s00343-018-7179-5 |
Byth S. 1980. Palm Island mystery disease. The Medical Journal of Australia, 2(1): 40-42.
DOI:10.5694/j.1326-5377.1980.tb131814.x |
Chao A M, Yu H Y, Xiao P, et al. 2021. Isolation and characterization of a Cylindrospermopsis raciborskii strain from Lake Xianghu, Hangzhou. Journal of Henan Normal University (Natural Science Edition), 49(4): 106-113.
(in Chinese with English abstract) DOI:10.16366/j.cnki.1000-2367.2021.04.015 |
Chen L L, Li Q H, Ten M D, et al. 2011. Cyanobacteria composition and microcystins distribution of Wanfeng reservoir and Baihua reservoir on Guizhou Plateau. Ecology and Environmental Sciences, 20(6–7): 1068-1074.
DOI:10.16258/j.cnki.1674-5906(2011)06-07-1068-07 |
Chen M Y, Teng W K, Zhao L, et al. 2021. Comparative genomics reveals insights into cyanobacterial evolution and habitat adaptation. The ISME Journal, 15(1): 211-227.
DOI:10.1038/s41396-020-00775-z |
Chislock M F, Sharp K L, WilsonA E. 2014. Cylindrospermopsis raciborskii dominates under very low and high nitrogen-to-phosphorus ratios. Water Research, 49: 207-214.
DOI:10.1016/j.watres.2013.11.022 |
Chonudomkul D, Yongmanitchai W, Theeragool G, et al. 2004. Morphology, genetic diversity, temperature tolerance and toxicity of Cylindrospermopsis raciborskii (Nostocales, Cyanobacteria) strains from Thailand and Japan. FEMS Microbiology Ecology, 48(3): 345-355.
DOI:10.1016/j.femsec.2004.02.014 |
Christiansen G, Yoshida W Y, Blom J F, et al. 2008. Isolation and structure determination of two microcystins and sequence comparison of the McyABC adenylation domains in Planktothrix species. Journal of Natural Products, 71(11): 1881-1886.
DOI:10.1021/np800397u |
Cirés S, Wörmer L, Ballot A, et al. 2014. Phylogeography of cylindrospermopsin and paralytic shellfish toxin-producing Nostocales Cyanobacteria from Mediterranean Europe (Spain). Applied and Environmental Microbiology, 80(4): 1359-1370.
DOI:10.1128/AEM.03002-13 |
Dai J J, Peng L, Yu T, et al. 2015. The effects of phosphorus and nitrogen on the growth of Cylindrospermopsis raciborskii N8 isolated from the Zhenhai reservoir. Acta Hydrobiologica Sinica, 39(3): 533-539.
DOI:10.7541/2015.70 |
Dao T S, Cronberg N, Lu G, et al. 2010. Toxic cyanobacteria from Tri An Reservoir, Vietnam. Nova Hedwigia, 90(3–4): 433-448.
DOI:10.1127/0029-5035/2010/0090-0433 |
De La Cruz A A, Hiskia A, Kaloudis H A, et al. 2013. A review on cylindrospermopsin: the global occurrence, detection, toxicity and degradation of a potent cyanotoxin. Environmental Science: Processes & Impacts, 15(11): 1979-2003.
DOI:10.1039/c3em00353a |
De La Escalera G M, Antoniades D, Bonilla S, et al. 2014. Application of ancient DNA to the reconstruction of past microbial assemblages and for the detection of toxic cyanobacteria in subtropical freshwater ecosystems. Molecular Ecology, 23(23): 5791-5802.
DOI:10.1111/mec.12979 |
Dyble J, Tester P A, Litaker R W. 2006. Effects of light intensity on cylindrospermopsin production in the cyanobacterial HAB species Cylindrospermopsis raciborskii. African Journal of Marine Science, 28(2): 309-312.
DOI:10.2989/18142320609504168 |
Everson S, Fabbro L, Kinnear S, et al. 2011. Extreme differences in akinete, heterocyte and cylindrospermopsin concentrations with depth in a successive bloom involving Aphanizomenon ovalisporum (Forti) and Cylindrospermopsis raciborskii (Woloszynska) Seenaya and Subba Raju. Harmful Algae, 10(3): 265-276.
DOI:10.1016/j.hal.2010.10.006 |
Fastner J, Heinze R, Humpage A R, et al. 2003. Cylindrospermopsin occurrence in two German lakes and preliminary assessment of toxicity and toxin production of Cylindrospermopsis raciborskii (Cyanobacteria) isolates. Toxicon, 42(3): 313-321.
DOI:10.1016/S0041-0101(03)00150-8 |
Figueredo C C, Giani A, Bird D F. 2007. Does allelopathy contribute to Cylindrospermopsis raciborskii (cyanobacteria) bloom occurrence and geographic expansion?. Journal of Phycology, 43(2): 256-265.
DOI:10.1111/j.1529-8817.2007.00333.x |
Figueredo C C, Von Rückert G, Cupertino A, et al. 2014. Lack of nitrogen as a causing agent of Cylindrospermopsis raciborskii intermittent blooms in a small tropical reservoir. FEMS Microbiology Ecology, 87(3): 557-567.
DOI:10.1111/1574-6941.12243 |
Fuentes-Valdés J J, Soto-Liebe K, Pérez-Pantoja D, et al. 2018. Draft genome sequences of Cylindrospermopsis raciborskii strains CS-508 and MVCC14, isolated from freshwater bloom events in Australia and Uruguay. Standards in Genomic Sciences, 13: 26.
DOI:10.1186/s40793-018-0323-1 |
Griffiths D J, Saker M L. 2003. The Palm Island mystery disease 20 years on: a review of research on the cyanotoxin cylindrospermopsin. Environmental Toxicology, 18(2): 78-93.
DOI:10.1002/tox.10103 |
Guedes I A, Pacheco A B F, Vilar M C P, et al. 2019. Intraspecific variability in response to phosphorus depleted conditions in the cyanobacteria Microcystis aeruginosa and Raphidiopsis raciborskii. Harmful Algae, 86: 96-105.
DOI:10.1016/j.hal.2019.03.006 |
Gugger M, Molica R, Le Berre B, et al. 2005. Genetic diversity of Cylindrospermopsis strains (Cyanobacteria) isolated from four continents. Applied and Environmental Microbiology, 71(2): 1097-1100.
DOI:10.1128/AEM.71.2.1097-1100.2005 |
Haande S, Rohrlack T, Ballot A, et al. 2008. Genetic characterisation of Cylindrospermopsis raciborskii (Nostocales, Cyanobacteria) isolates from Africa and Europe. Harmful Algae, 7(5): 692-701.
DOI:10.1016/j.hal.2008.02.010 |
Hadas O, Pinkas R, Malinsky-Rushansky N, et al. 2012. Appearance and establishment of diazotrophic cyanobacteria in Lake Kinneret, Israel. Freshwater Biology, 57(6): 1214-1227.
DOI:10.1111/j.1365-2427.2012.02792.x |
Harris G P, Baxter G. 1996. Interannual variability in phytoplankton biomass and species composition in a subtropical reservoir. Freshwater Biology, 35(3): 545-560.
DOI:10.1111/j.1365-2427.1996.tb01768.x |
Hawkins P R, Chandrasena N R, Jones G J, et al. 1997. Isolation and toxicity of Cylindrospermopsis raciborskii from an ornamental lake. Toxicon, 35(3): 341-346.
DOI:10.1016/S0041-0101(96)00185-7 |
Hawkins P R, Putt E, Falconer I, et al. 2001. Phenotypical variation in a toxic strain of the phytoplankter, Cylindrospermopsis raciborskii (Nostocales, Cyanophyceae) during batch culture. Environmental Toxicology, 16(6): 460-467.
DOI:10.1002/tox.10005 |
Hawkins P R, Runnegar M T, Jackson A R, et al. 1985. Severe hepatotoxicity caused by the tropical cyanobacterium (blue-green alga) Cylindrospermopsis raciborskii (Woloszynska) Seenaya and Subba Raju isolated from a domestic water supply reservoir. Applied and Environmental Microbiology, 50(5): 1292-1295.
DOI:10.1128/aem.50.5.1292-1295.1985 |
Hoff-Risseti C, Dörr F A, Schaker P D C, et al. 2013. Cylindrospermopsin and saxitoxin synthetase genes in Cylindrospermopsis raciborskii strains from Brazilian freshwater. PLoS One, 8(8): e74238.
DOI:10.1371/journal.pone.0074238 |
Holland D P, Pantorno A, Orr P T, et al. 2012. The impacts of a high CO2 environment on a bicarbonate user: the cyanobacterium Cylindrospermopsis raciborskii. Water Research, 46(5): 1430-1437.
DOI:10.1016/j.watres.2011.11.015 |
Huber-Pestalozzi G. 1938. Das Phytoplankton des Süßwassers. Systematik und Biologie. In: die Binnengewässer, Huber-Pestalozzi G eds. Vol. 16, Stuttgart: Schweizerbartśche, 342p.
|
Isvánovics V, Shafik H M, Présing M, et al. 2000. Growth and phosphate uptake kinetics of the cyanobacterium, Cylindrospermopsis raciborskii (Cyanophyceae) in throughflow cultures. Freshwater Biology, 43(2): 257-275.
DOI:10.1046/j.1365-2427.2000.00549.x |
Jeong J Y, Lee S H, Yun M R, et al. 2020. Draft genome sequence of Raphidiopsis raciborskii strain GIHE 2018, isolated from a shallow freshwater pond in South Korea. Microbiology Resource Announcements, 9(6): e01545-19.
DOI:10.1128/MRA.01545-19 |
Jiang Y G, Chen Y X, Yang S M, et al. 2020. Phylogenetic relationships and genetic divergence of paralytic shellfish toxin- and cylindrospermopsin-producing Cylindrospermopsis and Raphidiopsis. Harmful Algae, 93: 101792.
DOI:10.1016/j.hal.2020.101792 |
Jiang Y G, Xiao P, Yu G L, et al. 2014. Sporadic distribution and distinctive variations of cylindrospermopsin genes in cyanobacterial strains and environmental samples from Chinese freshwater bodies. Applied and Environmental Microbiology, 80(17): 5219-5230.
DOI:10.1128/AEM.00551-14 |
Kashtan N, Roggensack S E, Rodrigue S, et al. 2014. Single-cell genomics reveals hundreds of coexisting subpopulations in wild Prochlorococcus. Science, 344(6182): 416-420.
DOI:10.1126/science.1248575 |
Kehoe M, O'Brien K R, Grinham A, et al. 2015. Primary production of lake phytoplankton, dominated by the cyanobacterium Cylindrospermopsis raciborskii, in response to irradiance and temperature. Inland Waters, 5(2): 93-100.
DOI:10.5268/iw-5.2.778 |
Kellmann R, Mihali T K, Jeon Y J, et al. 2008. Biosynthetic intermediate analysis and functional homology reveal a saxitoxin gene cluster in cyanobacteria. Applied and Environmental Microbiology, 74(13): 4044-4053.
DOI:10.1128/AEM.00353-08 |
Kellmann R, Mills T, Neilan B A. 2006. Functional modeling and phylogenetic distribution of putative cylindrospermopsin biosynthesis enzymes. Journal of Molecular Evolution, 62(3): 267-280.
DOI:10.1007/s00239-005-0030-6 |
Kokociński M, Akçaalan R, Salmaso N et al. 2017. Expansion of alien and invasive cyanobacteria. In: Meriluoto J, Spoof L, Codd G A eds. Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis. John Wiley & Son Ltd., Chichester. p. 28–39, https://doi.org/10.1002/9781119068761.ch4.
|
Kokociński M, Stefaniak K, Mankiewicz-Boczek J, et al. 2010. The ecology of the invasive cyanobacterium Cylindrospermopsis raciborskii (Nostocales, Cyanophyta) in two hypereutrophic lakes dominated by Planktothrix agardhii (Oscillatoriales, Cyanophyta). European Journal of Phycology, 45(4): 365-374.
DOI:10.1080/09670262.2010.492916 |
Komárek J, Kling H. 1991. Variation in six planktonic cyanophyte genera in Lake Victoria (East Africa). Algological Studies/Archiv für Hydrobiologie, Suppl 1(61): 21-45.
|
Lagos N, Onodera H, Zagatto P A, et al. 1999. The first evidence of paralytic shellfish toxins in the freshwater cyanobacterium Cylindrospermopsis raciborskii, isolated from Brazil. Toxicon, 37(10): 1359-1373.
DOI:10.1016/S0041-0101(99)00080-X |
Larsson J, Celepli N, Ininbergs K, et al. 2014. Picocyanobacteria containing a novel pigment gene cluster dominate the brackish water Baltic Sea. The ISME Journal, 8(9): 1892-1903.
DOI:10.1038/ismej.2014.35 |
Larsson J, Nylander J A A, Bergman B. 2011. Genome fluctuations in cyanobacteria reflect evolutionary, developmental and adaptive traits. BMC Evolutionary Biology, 11: 187.
DOI:10.1186/1471-2148-11-187 |
Leão P N, Vasconcelos M T S D, Vasconcelos V M. 2009. Allelopathic activity of cyanobacteria on green microalgae at low cell densities. European Journal of Phycology, 44(3): 347-355.
DOI:10.1080/09670260802652156 |
Lei L M, Peng L, Huang X H, et al. 2014. Occurrence and dominance of Cylindrospermopsis raciborskii and dissolved cylindrospermopsin in urban reservoirs used for drinking water supply, South China. Environmental Monitoring and Assessment, 186(5): 3079-3090.
DOI:10.1007/s10661-013-3602-8 |
Li R, Carmichael W W, Brittain S, et al. 2001. Isolation and identification of the cyanotoxin cylindrospermopsin and deoxy-cylindrospermopsin from a Thailand strain of Cylindrospermopsis raciborskii (Cyanobacteria). Toxicon, 39(7): 973-980.
DOI:10.1016/S0041-0101(00)00236-1 |
Lorenzi A S, Silva G G Z, Lopes F A C, et al. 2016. Draft genome sequence of Cylindrospermopsis raciborskii (Cyanobacteria) strain ITEP-A1 isolated from a Brazilian semiarid freshwater body: Evidence of saxitoxin and cylindrospermopsin synthetase genes. Genome Announc, 4(3): e00228-16.
DOI:10.1128/genomeA.00228-16 |
Lu Z, Lei L M, Lu Y, et al. 2021. Phosphorus deficiency stimulates dominance of Cylindrospermopsis through facilitating cylindrospermopsin-induced alkaline phosphatase secretion: integrating field and laboratory-based evidences. Environmental Pollution, 290: 117946.
DOI:10.1016/j.envpol.2021.117946 |
Lv H, Yang J, Liu L M. 2013. Temporal pattern prevails over spatial variability in phytoplankton communities from a subtropical water supply reservoir. Oceanological and Hydrobiological Studies, 42(4): 420-430.
DOI:10.2478/s13545-013-0098-3 |
Marinho M M, Souza M B G, Lürling M. 2013. Light and phosphate competition between Cylindrospermopsis raciborskii and Microcystis aeruginosa is strain dependent. Microbial Ecology, 66(3): 479-488.
DOI:10.1007/s00248-013-0232-1 |
McGregor G B, Fabbro L D. 2000. Dominance of Cylindrospermopsis raciborskii (Nostocales, Cyanoprokaryota) in Queensland tropical and subtropical reservoirs: implications for monitoring and management. Lakes & Reservoirs: Science, Policy and Management for Sustainable Use, 5(3): 195-205.
DOI:10.1046/j.1440-1770.2000.00115.x |
Mehnert G, Leunert F, Cirés S, et al. 2010. Competitiveness of invasive and native cyanobacteria from temperate freshwaters under various light and temperature conditions. Journal of Plankton Research, 32(7): 1009-1021.
DOI:10.1093/plankt/fbq033 |
Mehnert G, Rücker J, Nicklisch A, et al. 2012. Effects of thermal acclimation and photoacclimation on lipophilic pigments in an invasive and a native cyanobacterium of temperate regions. European Journal of Phycology, 47(2): 182-192.
DOI:10.1080/09670262.2012.683496 |
Mello M M E, Soares M C S, Roland F, et al. 2012. Growth inhibition and colony formation in the cyanobacterium Microcystis aeruginosa induced by the cyanobacterium Cylindrospermopsis raciborskii. Journal of Plankton Research, 34(11): 987-994.
DOI:10.1093/plankt/fbs056 |
Messineo V, Melchiorre S, Di Corcia A, et al. 2010. Seasonal succession of Cylindrospermopsis raciborskii and Aphanizomenon ovalisporum blooms with cylindrospermopsin occurrence in the volcanic Lake Albano, central Italy. Environmental Toxicology, 25(1): 18-27.
DOI:10.1002/tox.20469 |
Miotto M C, Costa L D F, Brentano D M, et al. 2017. Ecophysiological characterization and toxin profile of two strains of Cylindrospermopsis raciborskii isolated from a subtropical lagoon in Southern Brazil. Hydrobiologia, 802(1): 97-113.
DOI:10.1007/s10750-017-3243-y |
Mohamed Z A, Al-Shehri A M. 2013. Assessment of cylindrospermopsin toxin in an arid Saudi lake containing dense cyanobacterial bloom. Environmental Monitoring and Assessment, 185: 2157-2166.
DOI:10.1007/s10661-012-2696-8 |
Moisander P H, Cheshire L A, Braddy J, et al. 2012. Facultative diazotrophy increases Cylindrospermopsis raciborskii competitiveness under fluctuating nitrogen availability. FEMS Microbiology Ecology, 79(3): 800-811.
DOI:10.1111/j.1574-6941.2011.01264.x |
Molica R J R, Oliveira E J A, Carvalho P V C, et al. 2005. Occurrence of saxitoxins and an anatoxin-a(s)-like anticholinesterase in a Brazilian drinking water supply. Harmful Algae, 4(4): 743-753.
DOI:10.1016/j.hal.2004.11.001 |
Moreira C, Fathalli A, Vasconcelos V, et al. 2011. Genetic diversity and structure of the invasive toxic cyanobacterium Cylindrospermopsis raciborskii. Current Microbiology, 62(5): 1590-1595.
DOI:10.1007/s00284-011-9900-x |
Moreira C, Fathalli A, Vasconcelos V, et al. 2015. Phylogeny and biogeography of the invasive cyanobacterium Cylindrospermopsis raciborskii. Archives of Microbiology, 197(1): 47-52.
DOI:10.1007/s00203-014-1052-5 |
Moustafa A, Loram J E, Hackett J D, et al. 2009. Origin of saxitoxin biosynthetic genes in cyanobacteria. PLoS One, 4(6): e5758.
DOI:10.1371/journal.pone.0005758 |
Mowe M A D, Mitrovic S M, Lim R P, et al. 2015. Tropical cyanobacterial blooms: a review of prevalence, problem taxa, toxins and influencing environmental factors. Journal of Limnology, 74(2): 205-224.
DOI:10.4081/jlimnol.2014.1005 |
Muhid P, Davis T W, Bunn S E, et al. 2013. Effects of inorganic nutrients in recycled water on freshwater phytoplankton biomass and composition. Water Research, 47(1): 384-394.
DOI:10.1016/j.watres.2012.10.015 |
Neilan B A, Saker M L, Fastner J, et al. 2003. Phylogeography of the invasive cyanobacterium Cylindrospermopsis raciborskii. Molecular Ecology, 12(1): 133-140.
DOI:10.1046/j.1365-294X.2003.01709.x |
Nguyen T T L, Hoang T H, Nguyen T K, et al. 2017. The occurrence of toxic cyanobacterium Cylindrospermopsis raciborskii and its toxin cylindrospermopsin in the Huong River, Thua Thien Hue province, Vietnam. Environmental Monitoring and Assessment, 189(10): 490.
DOI:10.1007/s10661-017-6209-7 |
Norris R L, Eaglesham G K, Pierens G, et al. 1999. Deoxycylindrospermopsin, an analog of cylindrospermopsin from Cylindrospermopsis raciborskii. Environmental Toxicology, 14(1): 163-165.
DOI:10.1002/(SICI)1522-7278(199902)14:1<163::AID-TOX21>3.0.CO;2-V |
Ohtani I, Moore R E, Runnegar M T C. 1992. Cylindrospermopsin: a potent hepatotoxin from the blue-green alga Cylindrospermopsis raciborskii. Journal of the American Chemical Society, 114(20): 7941-7942.
DOI:10.1021/ja00046a067 |
Padisák J, Istvánovics V. 1997. Differential response of blue-green algal groups to phosphorus load reduction in a large shallow lake: Balaton, Hungary. Verhandlungen Internationale Vereinigung für Theoretische und Angewandte Limnologie, 26(2): 574-580.
DOI:10.1080/03680770.1995.11900783 |
Padisák J, Reynolds C S. 1998. Selection of phytoplankton associations in Lake Balaton, Hungary, in response to eutrophication and restoration measures, with special reference to the cyanoprokaryotes. Hydrobiologia, 384(1–3): 41-53.
DOI:10.1023/A:1003255529403 |
Padisák J, Vasas G, Borics G. 2016. Phycogeography of freshwater phytoplankton: traditional knowledge and new molecular tools. Hydrobiologia, 764(1): 3-27.
DOI:10.1007/s10750-015-2259-4 |
Padisák J. 1997. Cylindrospermopsis raciborskii (Woloszynska) Seenayya et Subba Raju, an expanding, highly adaptive cyanobacterium: worldwide distribution and review of its ecology. Archiv für Hydrobiologie Supplementband Monographische Beitrage, 107(4): 563-593.
|
Panou M, Zervou S K, Kaloudis T, et al. 2018. A Greek Cylindrospermopsis raciborskii strain: missing link in tropic invader's phylogeography tale. Harmful Algae, 80: 96-106.
DOI:10.1016/j.hal.2018.10.002 |
Piccini C, Aubriot L, Fabre A, et al. 2011. Genetic and eco-physiological differences of South American Cylindrospermopsis raciborskii isolates support the hypothesis of multiple ecotypes. Harmful Algae, 10(6): 644-653.
DOI:10.1016/j.hal.2011.04.016 |
Pierangelini M, Stojkovic S, Orr P T, et al. 2014. Elevated CO2 causes changes in the photosynthetic apparatus of a toxic cyanobacterium, Cylindrospermopsis raciborskii. Journal of Plant Physiology, 171(12): 1091-1098.
DOI:10.1016/j.jplph.2014.04.003 |
Plominsky Á M, Larsson J, Bergman B, et al. 2013. Dinitrogen fixation is restricted to the terminal heterocysts in the invasive cyanobacterium Cylindrospermopsis raciborskii CS-505. PLoS One, 8(2): e51682.
DOI:10.1371/journal.pone.0051682 |
Posada D, Crandall K A, Templeton A R. 2006. Nested clade analysis statistics. Molecular Ecology Notes, 6(3): 590-593.
DOI:10.1111/j.1471-8286.2006.01368.x |
Posselt A J, Burford M A, Shaw G. 2009. Pulses of phosphate promote dominance of the toxic cyanophyte Cylindrospermopsis raciborskii in a subtropical water reservoir. Journal of Phycology, 45(3): 540-546.
DOI:10.1111/j.1529-8817.2009.00675.x |
Prescott G W, Andrews T F. 1955. A new species of Anabaenopsis in a Kansas lake with notes on limnology. Journal of Aquatic Ecosystem Stress and Recovery (Formerly Journal of Aquatic Ecosystem Health), 7(1–2): 60-63.
DOI:10.1007/BF00189795 |
Présing M, Herodek S, Vörös L, et al. 1996. Nitrogen fixation, ammonium and nitrate uptake during a bloom of Cylindrospermopsis raciborskii in Lake Balaton. Archiv für Hydrobiologie, 136(4): 553-562.
DOI:10.1127/archiv-hydrobiol/136/1996/553 |
Rasmussen J P, Giglio S, Monis P T, et al. 2008. Development and field testing of a real-time PCR assay for cylindrospermopsin-producing cyanobacteria. Journal of Applied Microbiology, 104(5): 1503-1515.
DOI:10.1111/j.1365-2672.2007.03676.x |
Recknagel F, Orr P T, Cao H Q. 2014. Inductive reasoning and forecasting of population dynamics of Cylindrospermopsis raciborskii in three sub-tropical reservoirs by evolutionary computation. Harmful Algae, 31: 26-34.
DOI:10.1016/j.hal.2013.09.004 |
Rigamonti N, Aubriot L, Martigani F, et al. 2018. Effect of nutrient availability on cylindrospermopsin gene expression and toxin production in Cylindrospermopsis raciborskii. Aquatic Microbial Ecology, 82(1): 105-110.
DOI:10.3354/ame01877 |
Rocap G, Larimer F W, Lamerdin J, et al. 2003. Genome divergence in two Prochlorococcus ecotypes reflects oceanic niche differentiation. Nature, 424(6952): 1042-1047.
DOI:10.1038/nature01947 |
Romo S, Miracle M R. 1994. Population dynamics and ecology of subdominant phytoplankton species in a shallow hypertrophic lake (Albufera of Valencia, Spain). Hydrobiologia, 273(1): 37-56.
DOI:10.1007/BF00126767 |
Rzymski P, Brygider A, Kokociński M. 2017. On the occurrence and toxicity of Cylindrospermopsis raciborskii in Poland. Limnological Review, 17(1): 23-29.
DOI:10.1515/limre-2017-0003 |
Rzymski P, Poniedzialek B. 2014. In search of environmental role of cylindrospermopsin: a review on global distribution and ecology of its producers. Water Research, 66: 320-337.
DOI:10.1016/j.watres.2014.08.029 |
Saker M L, Neilan B A, Griffiths D J. 1999. Two morphological forms of Cylindrospermopsis raciborskii (Cyanobacteria) isolated from Solomon Dam, Palm Island, Queensland. Journal of Phycology, 35(3): 599-606.
DOI:10.1046/j.1529-8817.1999.3530599.x |
Saker M L, Neilan B A. 2001. Varied diazotrophies, morphologies, and toxicities of genetically similar isolates of Cylindrospermopsis raciborskii (Nostocales, Cyanophyceae) from northern Australia. Applied and Environmental Microbiology, 67(4): 1839-1845.
DOI:10.1128/AEM.67.4.1839-1845.2001 |
Saker M L, Nogueira I C G, Vasconcelos V M, et al. 2003. First report and toxicological assessment of the cyanobacterium Cylindrospermopsis raciborskii from Portuguese freshwaters. Ecotoxicology and Environmental Safety, 55(2): 243-250.
DOI:10.1016/S0147-6513(02)00043-X |
Shafik H M, Herodek S, Présing M, et al. 2001. Factors effecting growth and cell composition of cyanoprokaryote Cylindrospermopsis raciborskii (Wołoszyńska) Seenayya et Subba Raju. Algological Studies/Archiv für Hydrobiologie, Suppl 1(103): 74-103.
DOI:10.1127/algol_stud/103/2001/75 |
Shi J, Tian O Y, Yang S Q, et al. 2022. Transcriptomic responses to phosphorus in an invasive cyanobacterium, Raphidiopsis raciborskii: implications for nutrient management. Harmful Algae, 111: 102150.
DOI:10.1016/j.hal.2021.102150 |
Shi T, Falkowski P G. 2008. Genome evolution in cyanobacteria: the stable core and the variable shell. Proceedings of the National Academy of Sciences of the United States of America, 105(7): 2510-2515.
DOI:10.1073/pnas.0711165105 |
Sidelev S, Koksharova O, Babanazarova O, et al. 2020. Phylogeographic, toxicological and ecological evidence for the global distribution of Raphidiopsis raciborskii and its northernmost presence in Lake Nero, Central Western Russia. Harmful Algae, 98: 101889.
DOI:10.1016/j.hal.2020.101889 |
Sinha R, Pearson L A, Davis T W, et al. 2012. Increased incidence of Cylindrospermopsis raciborskii in temperate zones — is climate change responsible?. Water Research, 46(5): 1408-1419.
DOI:10.1016/j.watres.2011.12.019 |
Sinha R, Pearson L A, Davis T W, et al. 2014. Comparative genomics of Cylindrospermopsis raciborskii strains with differential toxicities. BMC Genomics, 15: 83.
DOI:10.1186/1471-2164-15-83 |
Skuja H. 1937. Süßwasseralgen aus Griechenland und Kleinasien. Hedwigia, 77: 15-73.
|
Soares M C S, Huszar V L M, Miranda M N, et al. 2013a. Cyanobacterial dominance in Brazil: distribution and environmental preferences. Hydrobiologia, 717: 1-12.
DOI:10.1007/s10750-013-1562-1 |
Soares M C S, Lürling M, Huszar V L M. 2010. Responses of the rotifer Brachionus calyciflorus to two tropical toxic cyanobacteria (Cylindrospermopsis raciborskii and Microcystis aeruginosa) in pure and mixed diets with green algae. Journal of Plankton Research, 32(7): 999-1008.
DOI:10.1093/plankt/fbq042 |
Soares M C S, Lürling M, Huszar V L M. 2013b. Growth and temperature-related phenotypic plasticity in the cyanobacterium Cylindrospermopsis raciborskii. Phycological Research, 61(1): 61-67.
DOI:10.1111/pre.12001 |
Soares M C S, Marinho M M, Azevedo S M O F, et al. 2011. Eutrophication and retention time affecting spatial heterogeneity in a tropical reservoir. Limnologica, 42(3): 197-203.
DOI:10.1016/j.limno.2011.11.002 |
Soto-Liebe K, Murillo A A, Krock B, et al. 2010. Reassessment of the toxin profile of Cylindrospermopsis raciborskii T3 and function of putative sulfotransferases in synthesis of sulfated and sulfonated PSP toxins. Toxicon, 56(8): 1350-1361.
DOI:10.1016/j.toxicon.2010.07.022 |
Spröber P, Shafik H M, Présing M, et al. 2003. Nitrogen uptake and fixation in the cyanobacterium Cylindrospermopsis raciborskii under different nitrogen conditions. Hydrobiologia, 506-509(1–3): 169-174.
DOI:10.1023/B:HYDR.0000008617.90245.5f |
Stirling D J, Quilliam M A. 2001. First report of the cyanobacterial toxin cylindrospermopsin in New Zealand. Toxicon, 39(8): 1219-1222.
DOI:10.1016/S0041-0101(00)00266-X |
Stucken K, John U, Cembella A, et al. 2010. The smallest known genomes of multicellular and toxic cyanobacteria: comparison, minimal gene sets for linked traits and the evolutionary implications. PLoS One, 5(2): e9235.
DOI:10.1371/journal.pone.0009235 |
Stucken K, John U, Cembella A, et al. 2014. Impact of nitrogen sources on gene expression and toxin production in the diazotroph Cylindrospermopsis raciborskii CS-505 and non-diazotroph Raphidiopsis brookii D9. Toxins, 6(6): 1896-1915.
DOI:10.3390/toxins6061896 |
Sukenik A, Hadas O, Kaplan A, et al. 2012. Invasion of Nostocales (cyanobacteria) to subtropical and temperate freshwater lakes — physiological, regional, and global driving forces. Frontiers in Microbiology, 3: 86.
DOI:10.3389/fmicb.2012.00086 |
Swe T, Miles C O, Cerasino L, et al. 2021. Microcystis, Raphidiopsis raciborskii and Dolichospermum smithii, toxin producing and non-toxigenic cyanobacteria in Yezin Dam, Myanmar. Limnologica, 90: 125901.
DOI:10.1016/j.limno.2021.125901 |
Tan F J, Xiao P, Yang J, et al. 2021. Precision early detection of invasive and toxic cyanobacteria: a case study of Raphidiopsis raciborskii. Harmful Algae, 110: 102125.
DOI:10.1016/j.hal.2021.102125 |
Tao M, Xie B W, Qi Z M, et al. 2016. Phytoplankton community structure and water quality assessment in Tuojiang River. Oceanologia et Limnologia Sinica, 47(4): 854-861.
(in Chinese with English abstract) DOI:10.11693/hyhz20160500108 |
Thomas A D, Saker M L, Norton J H, et al. 1998. Cyanobacterium Cylindro-spermopsis raciborskii as a probable cause of death in cattle in northern Queensland. Australian Veterinary Journal, 76(9): 592-594.
DOI:10.1111/j.1751-0813.1998.tb10233.x |
Vico P, Aubriot L, Martigani F, et al. 2016. Influence of nitrogen availability on the expression of genes involved in the biosynthesis of saxitoxin and analogs in Cylindrospermopsis raciborskii. Harmful Algae, 56: 37-43.
DOI:10.1016/j.hal.2016.04.008 |
Vico P, Bonilla S, Cremella B, et al. 2020. Biogeography of the cyanobacterium Raphidiopsis (Cylindrospermopsis) raciborskii: integrating genomics, phylogenetic and toxicity data. Molecular Phylogenetics and Evolution, 148: 106824.
DOI:10.1016/j.ympev.2020.106824 |
Vinogradska T. 1974. On distribution and ecology of rare and interesting species of blue-green algae. Ukrainskiy Botanical Review, 31: 733-739.
|
Wang Y T. 2019. Variation of Cyanobacteria Community in the Main Regulating Reservoirs in Shandong Province and the Growth Characteristics of Cylindrospermopsis. Shandong University, Ji'nan. (in Chinese with English abstract)
|
Wiedner C, Rücker J, Brüggemann R, et al. 2007. Climate change affects timing and size of populations of an invasive cyanobacterium in temperate regions. Oecologia, 152(3): 473-484.
DOI:10.1007/s00442-007-0 |
Willis A, Adams M P, Chuang A W, et al. 2015. Constitutive toxin production under various nitrogen and phosphorus regimes of three ecotypes of Cylindrospermopsis raciborskii ((Wołoszyńska) Seenayya et Subba Raju). Harmful Algae, 47: 27-34.
DOI:10.1016/j.hal.2015.05.011 |
Willis A, Chuang A W, Dyhrman S, et al. 2019. Differential expression of phosphorus acquisition genes in response to phosphorus stress in two Raphidiopsis raciborskii strains. Harmful Algae, 82: 19-25.
DOI:10.1016/j.hal.2018.12.003 |
Willis A, Chuang A W, Woodhouse J N, et al. 2016. Intraspecific variation in growth, morphology and toxin quotas for the cyanobacterium, Cylindrospermopsis raciborskii. Toxicon, 119: 307-310.
DOI:10.1016/j.toxicon.2016.07.005 |
Willis A, Posselt A J, Burford M A. 2017. Variations in carbon-to-phosphorus ratios of two Australian strains of Cylindrospermopsis raciborskii. European Journal of Phycology, 52(3): 303-310.
DOI:10.1080/09670262.2017.1286524 |
Willis A, Woodhouse J N, Ongley S E, et al. 2018. Genome variation in nine co-occurring toxic Cylindrospermopsis raciborskii strains. Harmful Algae, 73: 157-166.
DOI:10.1016/j.hal.2018.03.001 |
Willis A, Woodhouse J N. 2020. Defining cyanobacterial species: diversity and description through genomics. Critical Reviews in Plant Sciences, 39(2): 101-124.
DOI:10.1080/07352689.2020.1763541 |
Wimmer K M, Strangman W K, Wright J L C. 2014. 7-Deoxy-desulfo-cylindrospermopsin and 7-deoxy-desulfo-12-acetylcylindrospermopsin: two new cylindrospermopsin analogs isolated from a Thai strain of Cylindrospermopsis raciborskii. Harmful Algae, 37: 203-206.
DOI:10.1016/j.hal.2014.06.006 |
Wołoszyńska J. 1912. Das Phytoplankton einiger Javanian Seen mit Berücksichtigung des Sawa-Planktons. Bulletin International Academiae Scientarium Cracoviae, Series. B, 6: 649-709.
|
Wood S A, Pochon X, Luttringer-Plu L, et al. 2014. Recent invader or indicator of environmental change? A phylogenetic and ecological study of Cylindrospermopsis raciborskii in New Zealand. Harmful Algae, 39: 64-74.
DOI:10.1016/j.hal.2014.06.013 |
Wood S A, Stirling D J. 2003. First identification of the cylindrospermopsin-producing cyanobacterium Cylindrospermopsis raciborskii in New Zealand. New Zealand Journal of Marine and Freshwater Research, 37(4): 821-828.
DOI:10.1080/00288330.2003.9517211 |
Wu D H, Xu Z A, Wang Y, et al. 2012. Seasonal variation of phytoplankton community structure in Hengshan reservoir. Journal of Hydroecology, 33(4): 54-57.
(in Chinese with English abstract) DOI:10.15928/j.1674-3075.2012.04.005 |
Wu Z X, Shi J Q, Li R H. 2009. Comparative studies on photosynthesis and phosphate metabolism of Cylindrospermopsis raciborskii with Microcystis aeruginosa and Aphanizomenon flos-aquae. Harmful Algae, 8(6): 910-915.
DOI:10.1016/j.hal.2009.05.002 |
Wu Z X, Zeng B, Li R H, et al. 2012a. Physiological regulation of Cylindrospermopsis raciborskii (Nostocales, Cyanobacteria) in response to inorganic phosphorus limitation. Harmful Algae, 15: 53-58.
DOI:10.1016/j.hal.2011.11.005 |
Wu Z X, Zeng B, Li R H, et al. 2012b. Combined effects of carbon and phosphorus levels on the invasive cyanobacterium, Cylindrospermopsis raciborskii. Phycologia, 51(2): 144-150.
DOI:10.2216/10.87.1 |
Xiao M, Hamilton D P, Chuang A, et al. 2020a. Intra-population strain variation in phosphorus storage strategies of the freshwater cyanobacterium Raphidiopsis raciborskii. FEMS Microbiology Ecology, 96(6): fiaa092.
DOI:10.1093/femsec/fiaa092 |
Xiao M, Hamilton D P, O'Brien K R, et al. 2020b. Are laboratory growth rate experiments relevant to explaining bloom forming cyanobacteria distributions at global scale?. Harmful Algae, 92: 101732.
DOI:10.1016/j.hal.2019.101732 |
Xie J L, Yu G L, Xu X D, et al. 2018. The morphological and molecular detection for the presence of toxic Cylindrospermopsis (Nostocales, Cyanobacteria) in Beijing city, China. Journal of Oceanology and Limnology, 36(2): 263-272.
DOI:10.1007/s00343-018-6283-x |
Yamamoto Y, Shiah F K. 2012. Factors related to the dominance of Cylindrospermopsis raciborskii (cyanobacteria) in a shallow pond in northern Taiwan. Journal of Phycology, 48(4): 984-991.
DOI:10.1111/j.1529-8817.2012.01184.x |
Yang J R, Lv H, Isabwe A, et al. 2017. Disturbance-induced phytoplankton regime shifts and recovery of cyanobacteria dominance in two subtropical reservoirs. Water Research, 120: 52-63.
DOI:10.1016/j.watres.2017.04.062 |
Yang Y M, Yu G L, Chen Y X, et al. 2021. Four decades of progress in cylindrospermopsin research: the ins and outs of a potent cyanotoxin. Journal of Hazardous Materials, 406: 124653.
DOI:10.1016/j.jhazmat.2020.124653 |
Yılmaz M, Phlips E J, Szabo N J, et al. 2008. A comparative study of Florida strains of Cylindrospermopsis and Aphanizomenon for cylindrospermopsin production. Toxicon, 51(1): 130-139.
DOI:10.1016/j.toxicon.2007.08.013 |
Yilmaz M, Phlips E J. 2011. Toxicity and genetic diversity of Cylindrospermopsis raciborskii in Florida, USA. Lake and Reservoir Management, 27(3): 235-244.
DOI:10.1080/07438141.2011.602203 |
Yu T, Dai J J, Lei L M, et al. 2014. Effects of temperature, irradiance and nitrate on the growth of Cylindrospermopsis raciborskii N8. Journal of Lake Science, 26(3): 441-446.
(in Chinese with English abstract) DOI:10.18307/2014.0315 |
Zarenezhad S, Sano T, Watanabe M M, et al. 2012. Evidence of the existence of a toxic form of Cylindrospermopsis raciborskii (Nostocales, Cyanobacteria) in Japan. Phycological Research, 60(2): 98-104.
DOI:10.1111/j.1440-1835.2012.00639.x |
Zhang H. 2019. The population dynamics of a bloom-forming cyanobacterium, Cylindrospermopsis raciborskii, and its relationship with environmental factors. Southwest University, Chongqing.
(in Chinese with English abstract) |
Zhao L, Lei L M, Peng L, et al. 2017. Seasonal dynamic and driving factors of Cylindrospermopsis raciborskii in Zhenhai Reservoir, Guangdong Province. Journal of Lake Science, 29(1): 193-197.
(in Chinese with English abstract) DOI:10.18307/2017.0121 |
Zingone A, Enevoldsen H O. 2000. The diversity of harmful algal blooms: a challenge for science and management. Ocean & Coastal Management, 43(8–9): 725-748.
DOI:10.1016/S0964-5691(00)00056-9 |
Zohary T. 2004. Changes to the phytoplankton assemblage of Lake Kinneret after decades of a predictable, repetitive pattern. Freshwater Biology, 49(10): 1355-1371.
DOI:10.1111/j.1365-2427.2004.01271.x |
 2022, Vol. 40
2022, Vol. 40