Institute of Oceanology, Chinese Academy of Sciences
Article Information
- FENG Jianlong, LIU Lulu, LIU Qiulin, ZHAO Liang
- The potential distribution of adult Antarctic krill in the Amundsen Sea
- Journal of Oceanology and Limnology, 40(4): 1566-1577
- http://dx.doi.org/10.1007/s00343-021-1181-z
Article History
- Received Jun. 15, 2021
- accepted in principle Aug. 20, 2021
- accepted for publication Sep. 23, 2021
2 National Marine Data & Information Service, Tianjin 300457, China;
3 College of Marine and Environmental Science, Tianjin University of Science and Technology, Tianjin 300457, China
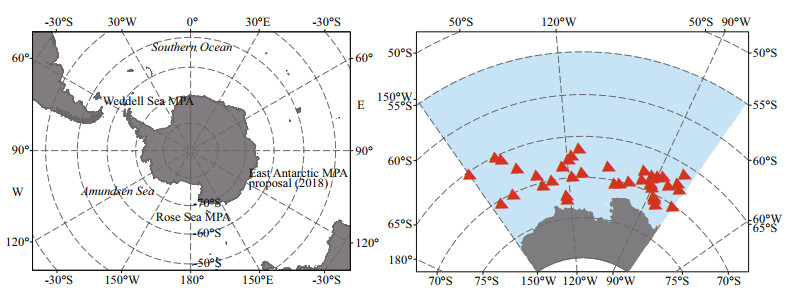
In 1982, the Commission for the Conservation of Antarctic Marine Living Resources (CCAMLR) was established with the objective of conserving living marine resources in the Southern Ocean. At present, the Southern Ocean has contributed a considerable proportion of the marine protected areas (MPAs) network at a global scale due to the uniqueness of the Antarctic environment (Brooks et al., 2020; Teschke et al., 2020). The Amundsen Sea, lying between Cape Flying Fish to the east and Cape Dart on Slip Island to the west (Fig. 1), has a relatively narrow continental shelf and a large amount of perennial sea ice, and a number of coastal polynyas located adjacent to large ice shelves (Arrigo and Van Dijken, 2003). Results from satellite show that the coastal ecosystems of the polynyas in the Amundsen Sea have the largest phytoplankton productivity in the Antarctic (Arrigo and Van Dijken, 2003; Arrigo et al., 2012). At present, the CCAMLR has listed the Amundsen Sea as one of the nine MPAs reserves to be planned in the Antarctic region.
|
| Fig.1 Locations of the Southern Ocean, Weddell Sea MPA, Rose Sea MPA, and Amundsen Sea (left), locations of the Antarctic krill in the Amundsen Sea (right) |
Antarctic krill is a keystone species of the neritic ecosystem in the Southern Ocean, as well as a key dietary resource for several predators, such as Antarctic fur seals, whales, Adélie, chinstrap, and several species of fish, squid, albatross and other invertebrates (Croxall and Prince, 1980; Siegel and Piatkowski, 1990; Forcada et al., 2012). Research showed that variability in the abundance and distribution of krill have substantial effects on reproductive performance of krill-dependent predators (Croxall et al., 1999; Reid et al., 2005; Forcada et al., 2012). In addition to their ecological role, krill is also the dominant fished species in the Southern Ocean, which has a potential sustainable yield equivalent to 11% of global fishery landings (Grant et al., 2013). But the habitat of Antarctic krill in the Southern Ocean is subject to on-going climate changes, such as sea ice decline, temperature rise, and ocean acidification (Jacquet et al., 2010; Flores et al., 2012a, b; Sylvester et al., 2021). Recently more and more concerns were forced on the future sustainability of krill harvesting under the cumulative pressure of climate and fisheries (Schiermeier, 2010; Meyer et al., 2020; Watters et al., 2020). Resource and conservation management in the Southern Ocean will become more adaptive as Antarctic krill populations and marine ecosystems are responding to climate change (Flores et al., 2012a). The CCAMLR is responsible for managing marine living resources in the Antarctic Ocean using an ecosystem-based approach to management (Constable et al., 2000), in which krill play a central role in the marine food web of the wider ecosystem (Constable et al., 2000; Ballerini et al., 2014; Dahood et al., 2020). The effectiveness of management is highly dependent on detailed knowledge of their distribution, and how the distributions are affected by a complex range of environmental variables (Silk et al., 2016). Thus, understanding the spatial distribution of krill and how the distribution are effected by the environment conditions is very important for the sustainable fishery management and conservation policy making (such as identifying suitable Marine Protected Areas). At present, knowledge about krill spatial distribution mostly comes from scientific surveys using acoustics or nets or data from the fishery (Reiss et al., 2008; Atkinson et al., 2012; Kinzey et al., 2015; La et al., 2015). However, these data only cover a tiny fraction of the South Ocean, especially in the Amundsen Sea, which fail to meet the need for spatial distribution.
Habitat suitability models, which can significantly improve the understanding on species niche requirements, have been widely used to predict the potential distributions of species by using distribution points and environmental variables (Peterson et al., 2002; Hirzel et al., 2006; Xavier et al., 2016). Implementation of habitat suitability started with terrestrial species, with increasing numbers of publications each year (Phillips et al., 2006; Saatchi et al., 2008; Robinson et al., 2011; Melo-Merino et al., 2020). In the Southern Ocean, Bombosch et al. (2014) modelled habitat suitability of humpback and Antarctic minke whales in the Southern Ocean, they produced daily basinwide/circumpolar prediction maps of habitat suitability. Do Amaral et al. (2015) used ecological niche modeling to estimate the potential distribution of Stenella dolphins in the southwestern Atlantic Ocean, they found that different species of Stenella have distinct environmental requirements. Xavier et al. (2016) used habitat suitability models to assess favorable areas from 15 cephalopods in the Southern Ocean. Nachtsheim et al. (2017) detected the suitable habitats for crabeater seals within the Weddell Sea using maximum entropy model (Maxent). Wege et al. (2020) modeled habitat suitability for crabeater seals in the Weddell Sea using satellite images.
In this work, a presence-only habitat modelling approach named Maxent was used to estimate the possible distribution and to investigate the influence of certain environmental variables on the distribution of Antarctic krill in the Amundsen Sea. In this way, favored conditions for Antarctic krill were detected within the Amundsen Sea, which is critically important for the planning of marine protected areas in the Amundsen Sea.
The outline of this paper are as follows: a brief description of the datasets and methods were provided in Section 2, Section 3 listed the results, discussions were provided in Section 4, and Section 5 presented the main conclusions draw from this study.
2 DATA SET AND METHODOLOGY 2.1 Data setThe presence data of the Antarctic krill is from KRILLBASE, which is an open access database of net-based juvenile and adult Antarctic krill (Atkinson et al., 2016). In total, we get 40 effective locations for Antarctic krill (Fig. 1). Nearly all selected locations were concentrated in January, February, March, November, and December. These are too little data to get the temporal variation (There are only 8 points on monthly average), so we put these locations together to get the spatial distribution in this work. In order to avoid the spatial auto-correlation, which affects the accuracy of the model, we randomly removed a point with distance less than 0.1° between two points (Milchev, 2009; Zhang et al., 2019).
In this work, 8 physical and 9 ecological variable of the ocean were used to analyze the habitat preferences of Antarctic krill (Table 1). These variables are derived from the Global Ocean Reanalysis Simulation (GLORYS2v4) (http://marine.copernicus.eu/service-portfolio/) as monthly mean value of January, February, March, November, and December from 1993 to 2015 with a resolution of 0.25°×0.25°. All the data used in this work was the first layer of the variables. The parameters used in the Maxent contained the average states of the variables (January, February, March, November, and December), their variability (maximum mean, minimum mean, and long-term change rate). The sea ice persistence index (ICE) was calculated as the proportion of the overall time during which the grid was covered by sea ice (the sea ice concentration larger than 60%). The index was calculated as ICE=M1/M, where M1 is the number of months which monthly sea ice concentration is less than 60%, and M is the number of months used in a year (in this work it is 5).The extent of all variables was clipped to match the study area, ranging from 80°W to 150°W and 55°S to 80°S. Furthermore, correlation analysis was performed on the attribute values of 17 variables, as too many variables would increase the complexity and random error of the model, which would reduce the accuracy of the results (Jiang, 2018; Zhang et al., 2019). The factors with Pearson's correlation coefficient larger than 0.7 was removed (Nachtsheim et al., 2017). In addition, the variables that contribute less than 0.01 to the Maxent model were removed (Nachstheim et al., 2017). Finally, 10 parameters, ICE (sea ice persistence index), PHYC (total phytoplankton), Fe_min (minimum dissolved iron), SPCO2_min (minimum surface CO2), U_max (maximum eastward velocity), Fe (dissolved iron), NPPV_min (minimum total primary production of phytoplankton), MLP_max (maximum density ocean mixed layer thickness), PO4_min (minimum phosphate), and V_max (maximum northward velocity) for the Antarctic krill were selected in this work.
In this work, the maximum entropy model (Maxent) was used to calculate the constraints and estimates the possible distribution of the Antarctic krill using the environmental variables and krill presence points. Maxent is quite prevalent in habitat modeling as only presence points was needed and works well with small sample sizes (Phillips and Dudik, 2008; Merow et al., 2013; Saupe et al., 2015). The program Maxent (version 3.4.1, https://biodiversityinformatics.amnh.org/open_source/maxent/, Phillips et al., 2006; Phillips and Dudik, 2008) was used in this work, 75% of the specie locations were selected to build the model, and the remaining 25% of the locations to test the model. Within the setting window, a bootstrap replicate run type was selected for 10 replicates with a random test percentage of 25% used. We used the bootstrap to sample the presence data for multiple runs. The cloglog was chosen as the output format, which gives a rough estimate of a probability of presence. The maximum test sensitivity plus specificity was selected as the threshold. Jackknife test was used to get the contribution rate and importance of variables. Model performance was assessed using receiver operating characteristic (ROC) curves using both the training and test data (Fielding and Bell, 1997; Nachtsheim et al., 2017). The area under the curve (AUC) can range between 0 and 1, the model can be judged as excellent if AUC is higher than 0.9 and good if AUC is between 0.8 and 0.9 (Fielding and Bell, 1997; Phillips et al., 2006; Nachtsheim et al., 2017).
Habitat suitability was defined based on the particular value in each cell and ranked into four different categories (Boitani et al., 2002; Elith et al., 2011).
"Unsuitable": where the ecological requirement of the species are not fulfilled (with the probability less than 0.2);
"Low Suitability": where habitat features cannot support a permanent species presence (with the probability between 0.2 and 0.4);
"Moderate": where habitat features support species presence at a sub-optimal level (with the probability between 0.4 and 0.6);
"High Suitability": where habitat features support species presence at an optimal level (with the probability larger than 0.6).
Mann-Kendall test, first introduced by Mann (1945), is a non-parametric statistical method to test the significant of change trend of time series. It has been widely used to assess the long-term trend and abrupt change point of climate factors. The statistic UFk is an order normalization parameter of time series X calculated in order. If UFk is larger (smaller) than 0, it indicates that the sequence is increasing (decreasing). For a given significant level α, if the absolute value of UFk is greater than Uα (for α=0.05, Uα=±1.96), the sequence has a significant trend. UBk is an order normalized parameter calculated in reverse order of time series X. If there is an intersection point between the two curves of UFk and UBk, and the point is between the Uα, the point corresponding to the abrupt change point.
3 RESULT 3.1 Habitat suitability maps for Antarctic krillIn this work, Maxent performed well in terms of generating species distribution models for Antarctic krill in the Amundsen Sea (Fig. 2), with the AUC values equal to 0.91 (0.92 for training data and 0.90 for test data). Results show that the high suitable habitat for Antarctic krill located between 65°S and 72°S, which account for 8.1% of the total area of the Amundsen Sea. The moderate suitability habitat mostly located at the border area of the high suitable habitat and there was also a small area in the central west of Amundsen Sea, and account for 6.7% of the total area. The low suitability habitat account for 11.2% of the total area, and mostly located at south of 65°S. The unsuitable habitat occupied the largest percentage in area (74.0% of the total area), which located around the coastline (south of 73°S) and between 55°S and 63°S.

|
| Fig.2 Habitat suitability maps for Antarctic krill in the Amundsen Sea |
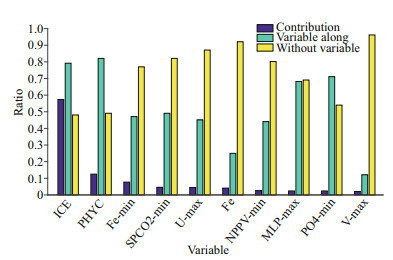
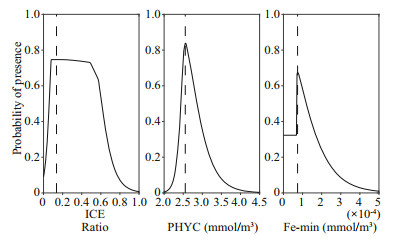
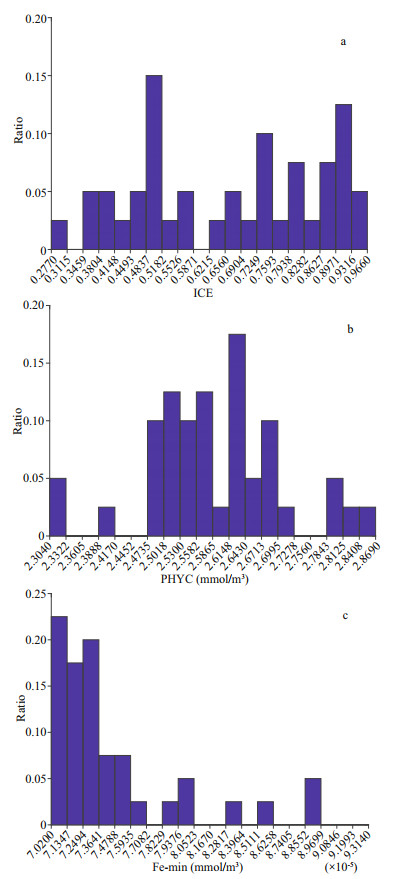
The contribution of each parameter to the modeling and the jackknife test of variable importance are shown in Fig. 3. Based on percent contribution, ICE, PHYC, and Fe_min were the top three parameters used in the prediction of the Maxent model that affected the distribution of Antarctic krill in the Amundsen Sea. The ICE was the greatest contributor (57.2%) to the model, the contributions of PHYC was larger than 10% (12.4%) and the contributions of Fe_min was smaller than 10% (7.6%). The contributions of other variables were less than 5% to the model. The jackknife test of variable importance showed the highest gain when PHYC was used in isolation containing the most information when used alone. The responses curves indicate the relationship between the probability occurrence and environmental variables (Fig. 4). Results show that the probability occurrence increased first and then decreased with the increase of the ICE. The maximum probability occurred at 0.86, and the optimum range was from 0.38 to 0.93 (with probability occurrence larger than 0.60). The probability occurrence increased first and then decreased with the increase of the PHYC. The maximum probability occurred at 2.55 mmol/m3, and the optimum range was from 2.48 to 2.77 mmol/m3. For Fe_min, the maximum probability occurred at 7.39×10-5 mmol/m3, and the optimum range was from 7.10×10-5 to 9.45×10-5 mmol/m3. The results of the responses curves are in consistent with the observations (Fig. 5). The ICE were distributed from 0.35 to 0.97, mostly were within the optimum range. The PHYC mostly ranged from 2.47 to 2.73, nearly the same as the optimum range of PHYC. The Fe_min mostly ranged from 7.02×10-5 to 7.71×10-5, also within the optimum range of Fe_min.
|
| Fig.3 The contribution and jackknife test of variable importance in Maxent |
|
| Fig.4 The responses curves between the probability occurrence and ICE, PHYC, and Fe_min The dotted line represent the value that the maximum probability occurred. |
|
| Fig.5 Distribution of ICE, PHYC and Fe_min of krill collection points |
For the Antarctic krill in the Amundsen Sea, the ICE, PHYC, and Fe_min were the main factors affecting the habitat suitability, with the contribution about 77.2% in total. Average of ICE, PHYC, and Fe_min in January, February, March, November, and December from 1993 to 2015 were calculated and shown in Fig. 6. Results show that the ICE increased as latitude increased in the Amundsen Sea. The mean value of high and moderate suitable habitat sare about 0.31 and 0.32, respectively. Results also show that the ICE might be the main restrictive factor in the northern part of the central region, where the PHYC and Fe_min were within the optimum range.
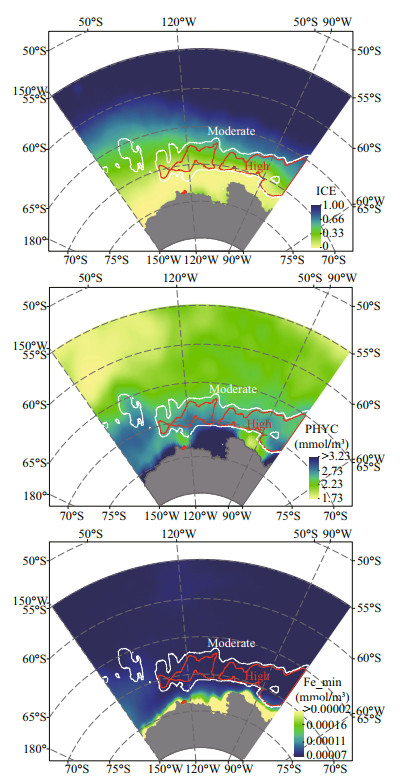
|
| Fig.6 Average of 3 selected variables in January, February, March, November, and December from 1993 to 2015 and the border of moderate (white line) and high (red line) suitable habitat |
The PHYC was largest along the coastline, especially in the Pine Island Bay (with values larger than 3.23). In general, the PHYC in the east part was higher than that in the west part. It showed that the high and moderate suitable habitat had relative higher PHYC than the outer part of the Amundsen Sea, the mean value of high and moderate suitable habitat are about 2.68 mmol/m3 and 2.30 mmol/m3, respectively. Results also show that in the northern part of the central region, where belonged to unsuitable habitat, the PHYC value was also larger than 2.48 mmol/m3 and smaller than 2.77 mmol/m3. It seemed that at these areas the food supply was not the restrictive factors for the adult Antarctic krill.
The Fe_min was largest along the coastline, and results show that the high and moderate suitable habitat had relative higher Fe_min than the outer part of the Amundsen Sea, the mean value of high and moderate suitable habitat are about 7.4×10-5 and 7.5×10-5mmol/m3, respectively.
Climate change has already produced spatial and temporal changes in earth's habitats and ecosystems (Parmesan and Yohe, 2003; Burrows et al., 2011; Veytia et al., 2020). Currently, ocean warming is more obvious in the Southern Ocean than global average (Vaughan et al., 2003; Meredith et al., 2019). Previous studies have shown that the climate variables of the Southern Ocean (e.g. sea temperature, ice cover, and chlorophyll) can affect the survival and growth of the Antarctic krill (Atkinson et al., 2004; Hill et al., 2013; Veytia et al., 2020). However, the effects on observed Antarctic krill distribution is a topic of debate (Atkinson et al., 2004; Loeb and Santora, 2015; Piñones and Fedorov, 2016; Cox et al., 2018). In this work, annual mean values of ICE, PHYC, and Fe_min of the high and moderate suitable habitat and Mann-Kendall (Rashid et al., 2015) test were calculated and shown in Fig. 7.

|
| Fig.7 Mean values of ICE, PHYC, and Fe_min of the high (red line) and moderate (black line) suitable regions The value where maximum probability occurred (blue dotted line) in the optimum range (black dotted lines) (a), the estimates progressive (UF) and backward (UB) series of the Mann-Kendall test of mean value of high suitable regions (b), the estimates progressive (UF) and backward (UB) series of the MannKendall test of mean value of moderate suitable regions (c). |
Results show that the ICE of the high and moderate suitable habitat were all within the optimum range, and no significant long-term trend exist in the ICE. At only three years(2006, 2008, 2013), the ICE of the high suitable habitat was smaller than 0.6, clearly decadal variations exist in ICE of the high suitable habitat. A positive trend existed in the ICE of the moderate and it was significant from 2012 to 2013.
Results of PHYC are different from that of ICE. At only 7 years the PHYC of the high suitable habitat were within the optimum range. A positive trend existed in the PHYC and it was significant after 1999. The changes of PHYC of moderate and high suitable habitat were quite similar. The PHYC of the high and the moderate suitable habitat were larger than 2.56 mmol/m3 (where the maximum probability occurred). It seems that the PHYC were becoming more beneficial to the Antarctic krill after 2000. However, it also showed that if the increase of the PHYC continues, the PHYC would be out of the optimum range in the moderate and high suitable habitat.
The Fe_min of the high suitable habitat and moderate suitable habitat were all within the optimum range. No significant long-term trend exists in the Fe_min of high suitable habitat. But a positive trend existed in the Fe_min of moderate suitable habitat and it was just significant from 2002 to 2003. At only three years(2002, 2014, 2015), the Fe_min of the moderate suitable habitat was larger than 8.4×10-5 mmol/m3, which was obviously larger than those at other years. In addition, if the increase of the Fe_min continues, the probability of presence of Antarctic krill will decrease in the moderate suitable habitat (Fig. 3).
4 DISCUSSIONDetail knowledge of species distribution is often pre-requisite to protect and utilize the species in the ecosystem. The Maxent has been widely used to predict the species abundance distributions, the suitable areas for invasive species, and the geographical distributions both on land and in the ocean (Thuiller et al., 2005; Pueyo et al., 2007; Cao et al., 2016). Antarctic krill is an ecologically and commercially important species in the Amundsen Sea. The spatial distribution and the effects of the environment conditions on the distributions are important to the development of sustainable fishery management and conservation policy. Using the distribution data and field environmental data, the potential distribution of Antarctic Krill in the Amundsen Sea was obtained based on the Maxent. It can be seen that the ICE, PHYC, and Fe_min were the main factors affecting the habitat suitability. The sea ice melt water reduced the temperature (TEM) in the high and moderate suitable habitat; a colder water was thought to be more suitable for the growth of the krill (Nicol, 2000; Atkinson et al., 2006). In addition, the sea ice can affect the phytoplankton in the Southern Ocean (Wang et al., 2014; Deppeler and Davidson, 2017). The phytoplankton in the Southern Ocean is usual limited by the iron (Fe) and light limitation due to deep vertical mixing below the critical depth (De Baar et al., 2005; Boyd et al., 2007; Moore et al., 2013).The fresh low temperature water from sea ice melt reduced the density ocean mixed layer thickness and increased light availability (Assmann et al, 2005; Holland et al., 2010). All of these made a relative high phytoplankton production (higher than deep-sea areas) in the high and moderate suitable habitat (Hofmann and Hüsrevoǧlu, 2003; Siegel and Watkins, 2016; Silk et al., 2016).
The PHYC was the food resource of the adult Antarctic krill. Food supply has the most direct effects on the animal survival, reproductive success, and population size (Newton, 2003). This result is consistent with other studies that adult krill in the open ocean are frequency associated with moderate food availability (Atkinson et al., 2008; Tarling et al., 2009; Silk et al., 2016). Atkinson et al. (2008) suggested that this relationship was a trade-off between food availability and growth potential and predation risk. The dissolved iron was an important factor limiting primary productivity. Alderkamp et al. (2015) indicated that primary productivity would be stressed by low iron concentrations during the months of December and January in the Amundsen Sea.
In this work, the presence data (40 points) of the Antarctic krill is from KRILLBASE, in which the data concentrated in January, February, March, November, and December. During these months, the adult krill swarms feed on phytoplankton in surface waters (Flores et al., 2012b). The results still agreed with what we know about the abundance and distribution of adult Antarctic krill, and the effects of environment conditions on distributions (Berglund, 1985; Atkinson et al., 2008; Krafft et al., 2010). However, the overwintering strategy of adult Antarctic krill was not taken into account. In addition, these are too little data to get the monthly variation (There are only 8 points on average). During the dark season, adult krill usually migrates to deeper water levels below 200 m or concentrates under the sea ice (Siegel, 2005; Taki et al., 2005; Flores et al., 2012b). We did not get observations during the dark season. This current method is not able to provide the habitat suitability maps of the adult Antarctic krill in the dark season. Therefore, differences may exist in the distributional behaviors and habitat preferences of Antarctic krill as the season progressed. In addition, there may be underestimate as it is difficult to get presence data in the shelf area when there is ice cover. Despite these, our results still have reference significance for the policy of the protecting potential krill habitats around the Amundsen Sea for the CCAMLR. Future improvements and more extensive-studies may be carried out when more data is available in the Amundsen Sea. Based on our results, we put forward some suggestions: First, more attention should be paid to the suitable areas of the adult krill, especially the high suitable habitat, where should be the important areas for protection. Second, the effects of climate changes on the abundance and distribution of adult Antarctic krill should be taken into account when planning protected areas. Third, more observations and studied of the adult krill are still needed in the Amundsen Sea.
Although this work is not able to provide the habitat suitability maps of the adult Antarctic krill in the dark season, the results still agreed with what we know about the abundance and distribution of adult Antarctic krill. Therefore, the habitat suitability map and conclusions got from this work can provide useful information and reasonable reference for the policy of the protecting potential krill habitats around the Amundsen Sea.
5 CONCLUSIONUsing the Maxent model and sets of environmental variables, the suitable habitat distribution and how the environmental variables affect the abundance and distribution of adult Antarctic krill were carried out in this work. High suitable habitat for Antarctic krill mostly located between 65°S and 72°S in the Amundsen Sea. The high and moderate suitable habitat accounted for 14.8% of the total area. The ICE, PHYC, and Fe_min were the three largest contributors to the model, contributed 77.2% in total. Adult Antarctic krill preferred habitats with ICE of 0.42–0.93, PHYC of 2.48–2.77 mmol/m3, and Fe_min of (7.10×10-5)–(9.45×10-5) mmol/m3.
No significant long-term trend existed in the ICE of the high and moderate suitable habitat. A positive trend existed in the PHYC of the high and moderate suitable habitat and it was significant after 1999. If the increase of the PHYC continues, the PHYC will out of the optimum range in the moderate and high suitable habitat in the future. A positive trend existed in the Fe_min of moderate suitable habitat, and the probability of presence of Antarctic krill will decrease in the moderate suitable habitat if the increase of the Fe_min continues.
6 DATA AVAILABILITY STATEMENTThe presence data of the Antarctic krill can be obtained from KRILLBASE (https://www.bas.ac.uk/project/krillbase/). Environmental variables are derived from the GLORYS2v4 (http://marine.copernicus.eu/service-portfolio/).
Alderkamp A C, Van Dijken G L, Lowry K E, Connelly T L, Lagerström M, Sherrell R M, Haskins C, Rogalsky E, Schofield O, Stammerjohn S E, Yager P L, Arrigo K R. 2015. Fe availability drives phytoplankton photosynthesis rates during spring bloom in the Amundsen Sea Polynya, Antarctica. Elementa: Science of the Anthropocene, 3: 000043.
DOI:10.12952/journal.elementa.000043 |
Arrigo K R, Lowry K E, Van Dijken G L. 2012. Annual changes in sea ice and phytoplankton in polynyas of the Amundsen Sea, Antarctica. Deep Sea Research Part II: Topical Studies in Oceanography, 71-76: 5-15.
DOI:10.1016/j.dsr2.2012.03.006 |
Arrigo K R, Van Dijken G L. 2003. Phytoplankton dynamics within 37 Antarctic coastal polynya systems. Journal of Geophysical Research: Oceans, 108(C8): 3271.
DOI:10.1029/2002JC001739 |
Assmann K M, Hellmer H H, Jacobs S S. 2005. Amundsen Sea ice production and transport. Journal of Geophysical Research: Oceans, 110(C12): C12013.
DOI:10.1029/2004JC002797 |
Atkinson A, Hill S L, Pakhomov E A, Seigel V, Anadon R, Chiba S, Daly K L, Downie R, Fielding S, Fretwell P, Gerrish L, Hosie G W, Jessopp M J, Kawaguchi S, Krafft B A, Loeb V, Nishikawa J, Peat H J, Reiss C S, Ross R M, Langdon B Quetin, Schmidt K, Steinberg D K, Subramaniam R C, Tarling G A, Ward P.. 2017. KRILLBASE: A database of Antarctic krill and salp densities in the Southern Ocean, 1926 to 2016. Earth System Science Data, 9: 193-210.
DOI:10.5194/essd-9-193-2017 |
Atkinson A, Nicol S, Kawaguchi S, Pakhomov E A, Quetin L B, Ross R M, Hill S L, Reiss C, Siegel V, Tarling G. 2012. Fitting Euphausia superba into Southern Ocean food-web models: a review of data sources and their limitations. CCAMLR Science, 19: 219-245.
|
Atkinson A, Shreeve R S, Hirst A G, Rothery P, Tarling G A, Pond D W, Korb R E, Murphy E J, Watkins J L. 2006. Natural growth rates in Antarctic krill (Euphausia superba): II.Predictive models based on food, temperature, body length, sex, and maturity stage. Limnology and Oceanography, 51(2): 973-987.
DOI:10.4319/lo.2006.51.2.0973 |
Atkinson A, Siegel V, Pakhomov E A, Rothery P, Loeb V, Ross R M, Quetin L B, Schmidt K, Fretwell P, Murphy E J, Tarling G A, Fleming A H. 2008. Oceanic circumpolar habitats of Antarctic krill. Marine Ecology Progress Series, 362: 1-23.
DOI:10.3354/meps07498 |
Atkinson A, Siegel V, Pakhomov E, Rothery P. 2004. Longterm decline in krill stock and increase in salps within the Southern Ocean. Nature, 432(7013): 100-103.
DOI:10.1038/nature02996 |
Ballerini T, Hofmann E E, Ainley D G, Daly K, Marrari M, Ribic C A, Smith W O Jr, Steele J H. 2014. Productivity and linkages of the food web of the southern region of the western Antarctic Peninsula continental shelf. Progress in Oceanography, 122: 10-29.
DOI:10.1016/j.pocean.2013.11.007 |
Berglund A. 1985. Different reproductive success at low salinity determines the estuarine distribution of two Palaemon prawn species. Ecography, 8(1): 49-52.
DOI:10.1111/j.1600-0587.1985.tb01151.x |
Boitani L, Corsi F, Falcucci A, Maiorano L, Marzetti M, Masi M, Montemaggiori A, Ottaviani D, Reggiani G, Rondinini C. 2002. Rete Ecologica Nazionale. Un approccio alla conservazione dei Vertebrati Italiani. Relazione Finale [National Ecological Network. An Approach to Conservation of Italian Vertebrates. Final Report]. Università di Roma "La Sapienza", Dipartimento di Biologia Animale e dell'Uomo, Ministero dell'Ambiente, Direzione per la Conservazione della Natura, Istituto di Ecologia Applicata, Roma.
|
Bombosch A, Zitterbart D P, Van Opzeeland I, Frickenhaus S, Burkhardt E, Wisz M S, Boebel O. 2014. Predictive habitat modelling of humpback (Megaptera novaeangliae) and Antarctic minke (Balaenoptera bonaerensis) whales in the Southern Ocean as a planning tool for seismic surveys. Deep Sea Research Part Ⅰ: Oceanographic Research Papers, 91: 101-114.
DOI:10.1016/j.dsr.2014.05.017 |
Boyd P W, Jickells T, Law C S, Blain S, Boyle E A, Buesseler K O, Coale K H, Cullen J J, Baar H J W D, Follows M, Harvey M, Lancelot C, Levasseur M, Owens N P J, Pollard R, Rivkin R B, Sarmiento J, Schoemann V, Smetacek V, Takeda S, Tsuda A, Turner S, Watson A J. 2007. Mesoscale iron enrichment experiments 1993-2005:Synthesis and future directions. Science, 315(5812): 612-617.
DOI:10.1126/science.1131669 |
Brooks C M, Crowder L B, Österblom H, Strong A L. 2020. Reaching consensus for conserving the global commons: The case of the Ross Sea, Antarctica. Conservation Letters, 13(1): e12676.
|
Burrows M T, Schoeman D S, Buckley L B, Moore P, Poloczanska E S, Brander K M, Brown C, Bruno J F, Duarte C M, Halpern B S, Holding J, Kappel C V, Kiessling W, O'connor M I, Pandolfi J M, Parmesan C, Schwing F B, Sydeman W J, Richardson A J. 2011. The pace of shifting climate in marine and terrestrial ecosystems. Science, 334(6056): 652-655.
DOI:10.1126/science.1210288 |
Cao B, Bai C K, Zhang L L, Li G S, Mao M C. 2016. Modeling habitat distribution of Cornus officinalis with Maxent modeling and fuzzy logics in China. Journal of Plant Ecology, 9(6): 742-751.
DOI:10.1093/jpe/rtw009 |
Constable A J, De La Mare W K, Agnew D J, Everson I, Miller D. 2000. Managing fisheries to conserve the Antarctic marine ecosystem: practical implementation of the Convention on the Conservation of Antarctic Marine Living Resources (CCAMLR). ICES Journal of Marine Science, 57(3): 778-791.
DOI:10.1006/jmsc.2000.0725 |
Cox M J, Candy S G, De La Mare W K, Nicol S, Kawaguchi S, Gales N. 2018. No evidence for a decline in the density of Antarctic krill Euphausia superba Dana, 1850, in the Southwest Atlantic sector between 1976 and 2016. Journal of Crustacean Biology, 38(6): 656-661.
|
Croxall J P, Prince P A. 1980. Food, feeding ecology and ecological segregation of seabirds at South Georgia. Biological Journal of the Linnean Society, 14(1): 103-131.
DOI:10.1111/j.1095-8312.1980.tb00101.X |
Croxall J P, Reid K, Prince P A. 1999. Diet, provisioning and productivity responses of marine predators to differences in availability of Antarctic krill. Marine Ecology Progress Series, 177: 115-131.
DOI:10.3354/meps177115 |
Dahood A, De Mutsert K, Watters G M. 2020. Evaluating Antarctic marine protected area scenarios using a dynamic food web model. Biological Conservation, 251: 108766.
DOI:10.1016/j.biocon.2020.108766 |
De Baar H J W, Boyd P W, Coale K H, Landry M R, Tsuda A, Assmy P, Bakker D C E, Bozec Y, Barber R T, Brzezinski MA, Buesseler K O, Boyé M, Croot P L, Gervais F, Gorbunov M Y, Harrison P J, Hiscock W T, Laan P, Lancelot C, Law C S, Levasseur M, Marchetti A, Millero F J, Nishioka J, Nojiri Y, Van Oijen T, Riebesell U, Rijkenberg M J A, Saito H, Takeda S, Timmermans K R, Veldhuis M J W, Waite A M, Wong C S. 2005. Synthesis of iron fertilization experiments: From the iron age in the age of enlightenment. Journal of Geophysical Research: Oceans, 110(C9): C09S16.
|
Deppeler S L, Davidson A T. 2017. Southern Ocean phytoplankton in a changing climate. Frontiers in Marine Science, 4: 40.
|
Do Amaral K B, Alvares D J, Heinzelmann L, Borges-Martins M, Siciliano S, Moreno I B. 2015. Ecological niche modeling of Stenella dolphins (Cetartiodactyla: Delphinidae) in the southwestern Atlantic Ocean. Journal of Experimental Marine Biology and Ecology, 472: 166-179.
DOI:10.1016/j.jembe.2015.07.013 |
Elith J, Phillips S J, Hastie T, Dudík M, Chee Y E, Yates C J. 2011. A statistical explanation of MaxEnt for ecologists. Diversity and Distributions, 17(1): 43-57.
DOI:10.1111/j.1472-4642.2010.00725.x |
Fielding A H, Bell J F. 1997. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environmental Conservation, 24(1): 38-49.
DOI:10.1017/S0376892997000088 |
Flores H, Atkinson A, Kawaguchi S, Krafft B A, Milinevsky G, Nicol S, Reiss C, Tarling G A, Werner R, Rebolledo E B, Cirelli V, Cuzin-Roudy J, Fielding S, Groeneveld J J, Haraldsson M, Lombana A, Marschoff E, Meyer B, Pakhomov E A, Rombolá E, Schmidt K, Siegel V, Teschke M, Tonkes H, Toullec J Y, Trathan P N, Tremblay N, Van De Putte A P, Van Franeker J A, Werner T. 2012a. Impact of climate change on Antarctic krill. Marine Ecology Progress Series, 458: 1-19.
DOI:10.3354/meps09831 |
Flores H, Van Franeker J A, Siegel V, Haraldsson M, Strass V, Meesters E H, Bathmann U, Jan Wolff W. 2012b. The association of Antarctic krill Euphausia superba with the under-ice habitat. PLoS One, 7(2): e31775.
DOI:10.1371/journal.pone.0031775 |
Forcada J, Trathan P N, Boveng P L, Boyd I L, Burns J M, Costa D P, Fedak M, Rogers T L, Southwell C J. 2012. Responses of Antarctic pack-ice seals to environmental change and increasing krill fishing. Biological Conservation, 149(1): 40-50.
DOI:10.1016/j.biocon.2012.02.002 |
Grant S M, Hill S L, Trathan P N, Murphy E J. 2013. Ecosystem services of the Southern Ocean: trade-offs in decisionmaking. Antarctic Science, 25(5): 603-617.
DOI:10.1017/S0954102013000308 |
Hill S L, Phillips T, Atkinson A. 2013. Potential climate change effects on the habitat of Antarctic krill in the Weddell quadrant of the Southern Ocean. PLoS One, 8(8): e72246.
DOI:10.1371/journal.pone.0072246 |
Hirzel A H, Le Lay G, Helfer V, Randin C, Guisan A. 2006. Evaluating the ability of habitat suitability models to predict species presences. Ecological Modelling, 199(2): 142-152.
DOI:10.1016/j.ecolmodel.2006.05.017 |
Hofmann E E, Hüsrevoǧlu Y S. 2003. A circumpolar modeling study of habitat control of Antarctic krill (Euphausia superba) reproductive success. Deep Sea Research Part II: Topical Studies in Oceanography, 50(22-26): 3121-3142.
DOI:10.1016/j.dsr2.2003.07.012 |
Holland P R, Jenkins A, Holland D M. 2010. Ice and ocean processes in the Bellingshausen Sea, Antarctica. Journal of Geophysical Research: Oceans, 115(C5): C05020.
DOI:10.1029/2008JC005219 |
Jacquet J, Pauly D, Ainley D, Holt S, Dayton P, Jackson J. 2010. Seafood stewardship in crisis. Nature, 467(7311): 28-29.
DOI:10.1038/467028a |
Jiang F. 2018. Bioclimatic and altitudinal variables influence the potential distribution of canine parvovirus type 2 worldwide. Ecology and Evolution, 8(9): 4534-4543.
DOI:10.1002/ece3.3994 |
Kinzey D, Watters G M, Reiss C S. 2015. Selectivity and two biomass measures in an age-based assessment of Antarctic krill (Euphausia superba). Fisheries Research, 168: 72-84.
DOI:10.1016/j.fishres.2015.03.023 |
Krafft B A, Melle W, Knutsen T, Bagøien E, Broms C, Ellertsen B, Siegel V. 2010. Distribution and demography of Antarctic krill in the Southeast Atlantic sector of the Southern Ocean during the austral summer 2008. Polar Biology, 33(7): 957-968.
DOI:10.1007/s00300-010-0774-3 |
La H S, Lee H, Fielding S, Kang D, Ha H K, Atkinson A, Park J, Siegel V, Lee S, Shin H C. 2015. High density of ice krill (Euphausia crystallorophias) in the Amundsen Sea coastal polynya, Antarctica. Deep Sea Research Part I: Oceanographic Research Papers, 95: 75-84.
DOI:10.1016/j.dsr.2014.09.002 |
Loeb V J, Santora J A. 2015. Climate variability and spatiotemporal dynamics of five Southern Ocean krill species. Progress in Oceanography, 134: 93-122.
DOI:10.1016/j.pocean.2015.01.002 |
Mann H B. 1945. Nonparametric tests against trend. Econometrica: Journal of the Econometric Society, 13(3): 245-259.
DOI:10.2307/1907187 |
Melo-Merino S M, Reyes-Bonilla H, Lira-Noriega A. 2020. Ecological niche models and species distribution models in marine environments: A literature review and spatial analysis of evidence. Ecological Modelling, 415: 108837.
DOI:10.1016/j.ecolmodel.2019.108837 |
Meredith M, Sommerkorn M, Cassotta S, Derksen C, Ekaykin A, Hollowed A, Kofinas G, Mackintosh A, Melbourne-Thomas J, Muelbert MMC, Ottersen G, Pritchard H, Schuur EAG. 2019. Polar regions. In: Pörtner H-O, Roberts D C, Masson-Delmotte V, Zhai P, Tignor M, Poloczanska E, Mintenbeck K, Alegría A, Nicolai M, Okem A, Petzold J, Rama B, Weyer NM eds. IPCC special report on the ocean and cryosphere in a changing climate.
|
Merow C, Smith M J, Silander J A Jr. 2013. A practical guide to MaxEnt for modeling species' distributions: What it does, and why inputs and settings matter. Ecography, 36(10): 1058-1069.
DOI:10.1111/j.1600-0587.2013.07872.x |
Meyer B, Atkinson A, Bernard K S, Brierley A S, Driscoll R, Hill S L, Marschoff E, Maschette D, Perry F A, Reiss C S, Rombolá E, Tarling G A, Thorpe S E, Trathan P N, Zhu G P, Kawaguchi S. 2020. Successful ecosystem-based management of Antarctic krill should address uncertainties in krill recruitment, behaviour and ecological adaptation. Communications Earth & Environment, 1: 28.
|
Milchev B. 2009. Breeding biology of the Long-legged Buzzard Buteo rufinus in SE Bulgaria, nesting also in quarries. Avocetta, 33: 25-32.
|
Moore C M, Mills M M, Arrigo K R, Berman-Frank I, Bopp L, Boyd P W, Galbraith E D, Geider R J, Guieu C, Jaccard S L, Jickells T D, La Roche J, Lenton T M, Mahowald N M, Marañón E, Marinov I, Moore J K, Nakatsuka T, Oschlies A, Saito M A, Thingstad T F, Tsuda A, Ulloa O. 2013. Processes and patterns of oceanic nutrient limitation. Nature Geoscience, 6(9): 701-710.
DOI:10.1038/ngeo1765 |
Nachtsheim D A, Jerosch K, Hagen W, Plötz J, Bornemann H. 2017. Habitat modelling of crabeater seals (Lobodon carcinophaga) in the Weddell Sea using the multivariate approach Maxent. Polar Biology, 40(5): 961-976.
DOI:10.1007/s00300-016-2020-0 |
Newton I. 2003. The role of natural factors in the limitation of bird of prey numbers: A brief review of the evidence. In: Redpath T D B A, Fielding S M, Marquiss A H, Galbraith C A eds. Birds of Prey in a Changing Environment. Scottish Natural Heritage/The Stationary Office, Edinburgh, Scotland. p. 5-23.
|
Nicol S. 2000. Understanding krill growth and aging: the contribution of experimental studies. Canadian Journal of Fisheries and Aquatic Sciences, 57(S3): 168-177.
DOI:10.1139/f00-173 |
Parmesan C, Yohe G. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature, 421(6918): 37-42.
DOI:10.1038/nature01286 |
Peterson A T, Ball L G, Cohoon K P. 2002. Predicting distributions of Mexican birds using ecological niche modelling methods. Ibis, 144(1): E27-E32.
DOI:10.1046/j.0019-1019.2001.00031.x |
Phillips S J, Anderson R P, Schapire R E. 2006. Maximum entropy modeling of species geographic distributions. Ecological Modelling, 190(3-4): 231-259.
DOI:10.1016/j.ecolmodel.2005.03.026 |
Phillips S J, Dudík M. 2008. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography, 31(2): 161-175.
DOI:10.1111/j.0906-7590.2008.5203.x |
Piñones A, Fedorov A V. 2016. Projected changes of Antarctic krill habitat by the end of the 21st century. Geophysical Research Letters, 43(16): 8580-8589.
DOI:10.1002/2016GL069656 |
Pueyo S, He F L, Zillio T. 2007. The maximum entropy formalism and the idiosyncratic theory of biodiversity. Ecology Letters, 10(11): 1017-1028.
DOI:10.1111/j.1461-0248.2007.01096.x |
Rashid M M, Beecham S, Chowdhury R K. 2015. Assessment of trends in point rainfall using continuous Wavelet Transforms. Advances in Water Resource, 82: 1-15.
DOI:10.1016/j.advwatres.2015.04.006 |
Reid K, Croxall J P, Briggs D R, Murphy E J. 2005. Antarctic ecosystem monitoring: Quantifying the response of ecosystem indicators to variability in Antarctic krill. ICES Journal of Marine Science, 62(3): 366-373.
DOI:10.1016/j.icesjms.2004.11.003 |
Reiss C S, Cossio A M, Loeb V, Demer D A. 2008. Variations in the biomass of Antarctic krill (Euphausia superba) around the South Shetland Islands, 1996-2006. ICES Journal of Marine Science, 65(4): 497-508.
DOI:10.1093/icesjms/fsn033 |
Robinson L M, Elith J, Hobday A J, Pearson R G, Kendall B E, Possingham H P, Richardson A J. 2011. Pushing the limits in marine species distribution modelling: Lessons from the land present challenges and opportunities. Global Ecology and Biogeography, 20(6): 789-802.
DOI:10.1111/j.1466-8238.2010.00636.x |
Saatchi S, Buermann W, Ter Steege H, Mori S, Smith T B. 2008. Modeling distribution of Amazonian tree species and diversity using remote sensing measurements. Remote Sensing of Environment, 112(5): 2000-2017.
DOI:10.1016/j.rse.2008.01.008 |
Saupe E E, Qiao H J, Hendricks J R, Portell R W, Hunter S J, Soberón J, Lieberman B S. 2015. Niche breadth and geographic range size as determinants of species survival on geological time scales. Global Ecology and Biogeography, 24(10): 1159-1169.
|
Schiermeier Q. 2010. Ecologists fear Antarctic krill crisis. Nature, 467(7311): 15.
DOI:10.1038/467015a |
Siegel V, Piatkowski U. 1990. Variability in the macrozooplankton community off the Antarctic Peninsula. Polar Biology, 10(5): 373-386.
|
Siegel V, Watkins J L. 2016. Distribution, biomass and demography of Antarctic krill, Euphausia superba. In: Siegel V ed. Biology and Ecology of Antarctic Krill. Cham: Springer, https://doi.org/10.1007/978-3-319-29279-3_2.
|
Siegel V. 2005. Distribution and population dynamics of Euphausia superba: summary of recent findings. Polar Biology, 29(1): 1-22.
|
Silk J R D, Thorpe S E, Fielding S, Murphy E J, Trathan P N, Watkins J L, Hill S L. 2016. Environmental correlates of Antarctic krill distribution in the Scotia Sea and southern Drake Passage. ICES Journal of Marine Science, 73(9): 2288-2301.
|
Sylvester Z T, Long M C, Brooks C M. 2021. Detecting climate signals in Southern Ocean krill growth habitat. Frontiers in Marine Science, 8: 708.
|
Taki K, Hayashi T, Naganobu M. 2005. Characteristics of seasonal variation in diurnal vertical migration and aggregation of Antarctic krill (Euphausia superba) in the Scotia Sea, using Japanese fishery data. CCAMLR Science, 12: 163-172.
|
Tarling G A, Klevjer T, Fielding S, Watkins J, Atkinson A, Murphy E, Korb R, Whitehouse M, Leaper R. 2009. Variability and predictability of Antarctic krill swarm structure. Deep Sea Research Part I: Oceanographic Research Papers, 56(11): 1994-2012.
|
Teschke K, Pehlke H, Siegel V, Bornemann H, Knust R, Brey T. 2020. An integrated compilation of data sources for the development of a marine protected area in the Weddell Sea. Earth System Science Data, 12(2): 1003-1023.
|
Thuiller W, Richardson D M, Pyšek P, Midgley G F, Hughes G O, Rought M. 2005. Niche-based modelling as a tool for predicting the risk of alien plant invasions at a global scale. Global Change Biology, 11(12): 2234-2250.
|
Vaughan D G, Marshall G J, Connolley W M, Parkinson C, Mulvaney R, Hodgson D A, King J C, Pudsey C J, Turner J. 2003. Recent Rapid regional climate warming on the Antarctic Peninsula. Climatic Change, 60(3): 243-274.
|
Veytia D, Corney S, Meiners K M, Kawaguchi S, Murphy E J, Bestley S. 2020. Circumpolar projections of Antarctic krill growth potential. Nature Climate Change, 10(6): 568-575.
DOI:10.1038/s41558-020-0758-4 |
Wang S, Bailey D, Lindsay K, Moore J K, Holland M. 2014. Impact of sea ice on the marine iron cycle and phytoplankton productivity. Biogeosciences, 11(17): 4713-4731.
|
Watters G M, Hinke J T, Reiss C S. 2020. Long-term observations from Antarctica demonstrate that mismatched scales of fisheries management and predatorprey interaction lead to erroneous conclusions about precaution. Scientific Reports, 10: 2314.
|
Wege M, Salas L, LaRue M. 2020. Citizen science and habitat modelling facilitates conservation planning for crabeater seals in the Weddell Sea. Diversity and Distributions, 26(10): 1291-1304.
|
Xavier J C, Raymond B, Jones D C, Griffiths H. 2016. Biogeography of cephalopods in the Southern Ocean using habitat suitability prediction models. Ecosystems, 19(2): 220-247.
|
Zhang J J, Jiang F, Li G Y, Qin W, Li S Q, Gao H M, Cai Z Y, Lin G H, Zhang T Z. 2019. Maxent modelling for predicting the spatial distribution of three raptors in the Sanjiangyuan National Park, China. Ecology and Evolution, 9(11): 6643-6654.
|
 2022, Vol. 40
2022, Vol. 40



