Institute of Oceanology, Chinese Academy of Sciences
Article Information
- WU Qiong, LI Qiuhua, LUO Huan, CHEN Qian, CHEN Huaxiang, DONG Yanjun, LI Shenghua
- Comparison in phytoplankton diversity-productivity-community stability between river-type reservoir and lake-type reservoir
- Journal of Oceanology and Limnology, 40(4): 1485-1507
- http://dx.doi.org/10.1007/s00343-021-1175-x
Article History
- Received Jun. 7, 2021
- accepted in principle Jul. 2, 2021
- accepted for publication Sep. 30, 2021
2 Pearl River Water Resources Research Institute, Guangzhou 510611, China;
3 Key Laboratory of the Pearl River Estuary Regulation and Protection of Ministry of Water Resources, Guangzhou 510611, China;
4 Guangdong Provincial Engineering Technology Research Center for Life and Health of River & Lake, Guangzhou 510611, China;
5 Key Laboratory for Information System of Mountainous Area and Protection of Ecological Environment of Guizhou Province, Guizhou Normal University, Guiyang 550001, China;
6 Bureau of Ecological Environment of Xunwu County of Jiangxi Province, Xunwu 342200, China
At present, biodiversity is being seriously lost due to the global climate change and frequent human activities (Cardinale et al., 2012). Biodiversity refers to the characteristics of diversity and variability of genes, species, and functional groups, of which the species diversity is the most important. Species diversity not only reflects the rich species resources of the ecosystem, but also complex interrelationship among species and between species and environment (Zhang et al., 2006a). Community stability refers to the self-renewal and maintenance of the community after reaching the climax of succession in evolution, so that the structure and function of the community can be maintained at a higher but less fluctuating level for a long time; it also refers to the restoration of the original structure, functional state, and antiinterference ability of the community after being disturbed (Holling, 1973; Pimm, 1984; Sennhauser, 1991). At present, impact of biodiversity on community stability and productivity has become one of the key ecological issues (Giller et al., 2004). The greater the biodiversity, the higher the stability (Hodapp et al., 2014). Phytoplankton, as the main primary producer in aquatic ecosystem, has special physiological and ecological characteristics. Variations in community composition and population reflect directly and quickly the dynamic changes of water environment (Reynolds, 1984). However, a functional group that is defined according to the physiological, morphological, and ecological attributes of species has similar responses to environmental changes. They can replace each other and have a little impact on the ecosystem, and can well reflect the relationship between species and habitats (Liu, 2008). Phytoplankton functional group is an important theory proposed by Reynolds et al. (2002) and further modified by Padisák et al. (2009). Therefore, it is of great significance to study the stability of phytoplankton community and phytoplankton functional groups for better understanding species change in phytoplankton community and ecosystem function.
The river-type reservoir has dual characteristics of river and reservoir. Different from common reservoirs, the river-type reservoir has a shallow and narrow but long water surface. In dry season, the upstream water is insufficient, and the reservoir would be in high water level operation, showing the characteristics of reservoir and lake. In wet season, due to the need of flood control, the reservoir operate at low water level, and the inflow is equal to the discharge, showing characteristics of river channel (Li et al., 2013). River-type reservoirs have significant spatial heterogeneity (Zhang et al., 2006b). Although the nutrient concentration is relatively high, the water body can still maintain a strong exchange capacity, and the water flow velocity is relatively fast, which is not conducive to the growth of phytoplankton and the maintenance of community structure organization. The water flow velocity in the reservoir area slows down, and the water retention time increases. The exchange capacity of water body is weakened and a large amount of suspended solids deposited, resulting in a significant increase of transparency in the transitional type area, which improves the growth conditions of phytoplankton, thus the biomass and growth rate of phytoplankton are higher than those in the river areas. In the head area of the reservoir and the corresponding tributary backwater bay, the flow velocity is very slow, water retention time is long, nutrients are easy to be retained; therefore, water exchange capacity is relatively weak, vertical stratification is easy to occur, and the retained nutrients are easy to cause the rapid growth of phytoplankton. On the other hand, a lake-type reservoir is generally built in a plain, a plateau platform, or a low-lying area. Its shape and ecological environment are similar to those of a shallow lake, with open water surface, and flat bottom. However, the water flow is not easy to mix and the distribution of water quality is uneven, especially in deep-water reservoirs.
After a reservoir is commissioned by damming and impoundment, a series of changes would take place in phytoplankton community due to drastic changes in the hydrodynamic conditions and water retention time in the habitat. Generally, it takes 4–5 years to stabilize the community structure (Ioriya et al., 1998). On one hand, the response of phytoplankton is different due to the difference in hydraulic retention time before and after impoundment (Sun et al., 2019). On the other hand, after the impoundment, with the decomposition of the covered vegetation and the release of organic matter and nutrients in the soil, the reservoir would have a "nutrient rising period" (Straškraba et al., 1993). At present, studies on river-type reservoir and lake-type reservoir focus on the water quality (Bai et al., 2017; Zhang et al., 2018). Most of the studies are based on a biological community in laboratory, and those on a natural ecosystem are scarce. At present, a comparative analysis on stability of phytoplankton community in river-type reservoir and lake-type reservoir has not been reported to the authors' best knowledge.
Both Jiuquwan Reservoir and Taihu Reservoir are located in the upstream of Xunwu River, which is an important drinking water source for Xunwu County. Jiuquwan Reservoir has been in operation for many years. It is a canyon-shaped river-type reservoir with an average water depth of less than 10 m, and has experienced a course from algal bloom outbreak, cyanobacteria control, to water ecological restoration. Taihu Reservoir is a newly built lake-type reservoir with an average water depth of about 35 m. In September 2018, it began to store water, and worked for less than five years so far. The two reservoirs have similar geographical conditions, the same meteorological conditions, and both are drinking water sources. However, they also have significant differences: many years of impoundment vs. new water storage, river-type vs. lake-type, and shallow water vs. deep water. Therefore, relationships between phytoplankton diversity, productivity, and community stability in the two reservoirs were compared and analyzed, and coefficient of variation (CV) was used to measure the community stability of phytoplankton. In addition, the stability of phytoplankton functional groups was measured in the Bray Curtis dissimilarity index; the relationships among phytoplankton diversity, productivity, and community stability were explored; and the differences among them in the two reservoirs were revealed, aiming to provide a theoretical basis for water environment management and water ecological restoration for reservoir.
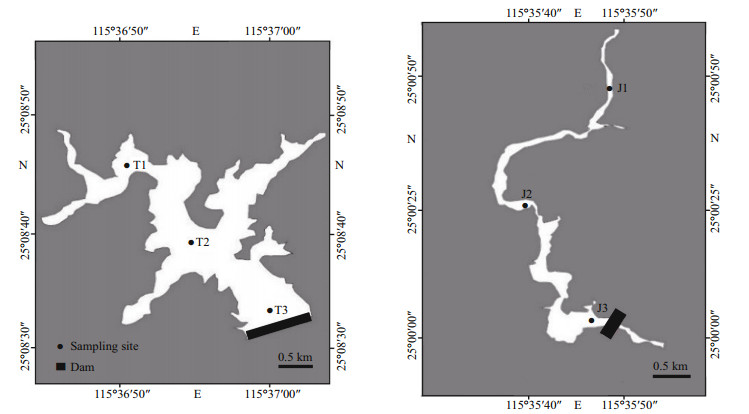
2 MATERIAL AND METHOD 2.1 The study regionJiuquwan Reservoir (24°59'57.22"N– 25°00'57.62"N, 115°35'36.63"E–115°35'53.25"E) is located in the middle Mati River, a branch of Xunwu River, and further tributary to the upper stream of Dongjiang River, 9 km north of Xunwu County, Jiangxi Province. The reservoir runs through a river valley with a watershed area of 71 km2, has a water surface area of 250 000 m2, total storage capacity of 4.15 million m3, and normal water level of 352 m. The length and width of submerged area below normal water level of reservoir is 2 315 and 108 m, respectively, and the average water depth is 9.52 m. Additional to water supply, the reservoir has comprehensive utilization benefits such as flood control and power generation. Taihu Reservoir (25°08'32.25"N–25°08'48.12"N, 115°36'46.03"E–115°37'04.36"E) is located in the upstream of Xunwu River tributary to Dongjiang River of Zhujiang (Pearl) River Basin. It is situated in Shuiyuan Town, 47 km northwest of Xunwu County, Jiangxi Province. Taihu Reservoir is a newly built reservoir and was impounded in September 2018. The basin area is 42.8 km2; the water area of the reservoir is about 340 000 m2; the normal water level is 443 m; the total storage capacity is 23.82 million m3; and the average water depth is 35 m. The reservoir mainly supplies water, and has comprehensive benefits such as irrigation and flood control. In this study, Jiuquwan Reservoir and Taihu Reservoir were sampled in dry season (December 2019), normal season (March 2020), and wet season (August 2020). The sampling sites were selected from upstream to downstream according to specific sampling protocols and ecological characteristics of the reservoirs. Jiuquwan Reservoir was set with three sampling sites: J1 (25°00'45.28"N, 115°35'48.15"E), J2(25°00'25.36"N, 115°35'38.55"E), J3 (25°00'03.12"N, 115°35'45.16"E). The sampling sites are divided into three layers: surface layer (0.5 m), middle layer (6 m), and bottom layer (12 m). Taihu Reservoir was set with three sampling sites, i.e., T1 (25°08'45.16"N, 115°36'50.53"E), T2 (25°08'38.32"N, 115°36'54.23"E), T3(25°08'33.26"N, 115°36'59.36"E), which were divided into three layers: surface layer (0.5 m), middle layer (15 m), and bottom layer (30 m) (Fig. 1; Table 1).
|
| Fig.1 Distribution of sampling sites in Taihu Reservoir (left) and Jiuquwan Reservoir (right) |
A total of 99 samples were collected in this study, among them there were 54 samples for physical and chemical index analysis, and 99 samples for biological index analysis.
Qualitative samples of phytoplankton: a #25 plankton net was slowly trawled in ∞-shaped route for 3–5 min in the reservoir, and 3%–5% Lugo solution was immediately added for fixation. Quantitative samples of phytoplankton: 1.5 L of water was taken from each layer with a water sampler, and was immediately fixed by adding Lugo solution, then brought back to the laboratory for static sedimentation for 24–48 h, and concentrated to 30 mL by siphon method. Algae identification and counting were carried out under the microscope for identification and classification of algae species as per Hu and Wei (2006). Phytoplankton productivity was determined based on the primary productivity that was measured by black-and-white bottle: once the transparency at measuring point is about 1 m, the sampling sites were divided into five layers: 0.0, 0.5, 1.0, 2.0, and 3.0 m. One black bottle and two white bottles per each layer were used; the initial dissolved oxygen was fixed during sampling. After 24-h exposure, dissolved oxygen was determined by the Winkler method (Iodic Titration).
The water temperature (WT), dissolved oxygen (DO), and pH values were measured in situ by YSI multi-function water quality parameter instrument, and the transparency was measured with Secchi disk (SD). Chemical indexes, including total nitrogen (TN), total phosphorus (TP), inorganic nitrogen (DIN) (including nitrate nitrogen (NO3--N), nitrite nitrogen (NO2--N), and ammonium nitrogen (NH4+-N)), and orthophosphate (PO43--P), were determined according to the analytic method of environmental quality standard for surface water (China's National Standard GB 3838-2002). Chlorophyll-a (Chl-a) concentration was measured using 500-mL water sample that was filtered through 0.45-μm cellulose acetate membrane, extracted by repeated freeze-thaw, and finally determined in acetone extraction method (Lin et al., 2005).
2.3 Data process and analysisThe thickness of euphotic layer (Zeu) was calculated as 2.7 times the transparency (Cole, 1994), and that of mixed layer (Zmix) was estimated according to water temperature variation (≥1 ℃). The ratio of euphotic layer to mixed layer in thickness (Zeu/Zmix) can be used to indicate the light availability in mixed layer (Jensen et al., 1994).
The relative water column stability (RWCS) (Padisák et al., 2003) was calculated using Eq.1:
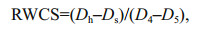 (1)
(1)where, Dh is the density of bottom water body, Ds is the density of surface water body, D4 and D5 represent the density of water body at 4 ℃ and 5 ℃, respectively.
Water density can be calculated by the Krambeck equation (Rodríguez-Rodríguez et al., 2004):
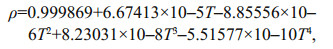 (2)
(2)where ρ is the water density and T is the water temperature (℃).
Based on Redfield ratio (Redfield et al., 1963), when the ratio of nitrogen to phosphorus is greater than 16:1, phosphorus is the limiting factor, and when the ratio of nitrogen to phosphorus is less than 10:1, nitrogen is the limiting factor. During our investigation period, the ratio of nitrogen to phosphorus in Jiuquwan Reservoir is 56:1 and that of Taihu Reservoir is 22:1. According to the Redfield ratio, phosphorus is the limiting factor in both reservoirs. Therefore, we can use the ratio of phytoplankton biomass to total phosphorus (TP) to measure the resource use efficiency of phytoplankton (RUEpp) (Ptacnik et al., 2008) according to the following formula:
 (3)
(3)where Bio(Phyto) is phytoplankton biomass (mg/L), and TP is total phosphorus concentration (mg/L). The biomass of phytoplankton was calculated via the density, the cell volume, and the abundance of phytoplankton. The cell volume of phytoplankton was calculated based on the volume of its similar geometric shape (Sun et al., 1999), and the density of phytoplankton was assumed to be 1 g/m3 to calculate the biomass of phytoplankton (Algal cell wet weight).
The Shannon-Wiener index (H') was used to determine species diversity (Luding and Reynolds, 1988):
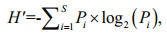 (4)
(4)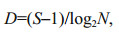 (5)
(5)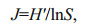 (6)
(6)where D is the Margalef's richness index, J is Pielou's species evenness index. The community stability is described by the coefficient of variation (CV) calculated according to the following formula (Tilman et al., 2006; Thibaut and Connolly, 2013; Venail et al., 2013):
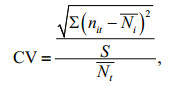 (7)
(7)where Pi=n/N,
n is the number of single species, N is the number of all species, and S is the number of all species in the community; 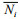
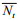
The stability of functional groups was measured by the Bray Curtis dissimilarity (Filstrup et al., 2014; Xu et al., 2016)
 (8)
(8)where BC is the Bray Curtis dissimilarity indicating the community replacement; yi1 is the biomass (mg/L) of species i measured for the first time in the community; yi2 is the biomass (mg/L) of species i measured for the second time in the community; n is the total number of species identified by two measurements in the community. The value range of Bray Curtis dissimilarity index (BC) is [0, 1]. The closer the BC value is to 1; the stronger the species replacement is, the weaker the stability is.
All the data process and analysis in this study were completed using MS Excel 2010, Origin 8.5, and ArcGIS10.6. Using the Pearson correlation method, the Poisson coefficient (P) was used to determine the correlation between different factors, and P < 0.05 was set as a significant correlation, while P < 0.01 is a very significant correlation. Meanwhile, the corresponding regression equation was established. Independent sample t-test was adopted for significant difference test to compare the two reservoirs for the same seasons and to compare any two seasons in the same reservoir. Meanwhile, One-way ANOVA was executed to analyze each sampling site in the same reservoir or the same season. The major concepts, symbols, factors, and statistical analyses methods involved in this study are shown in Table 2.
|
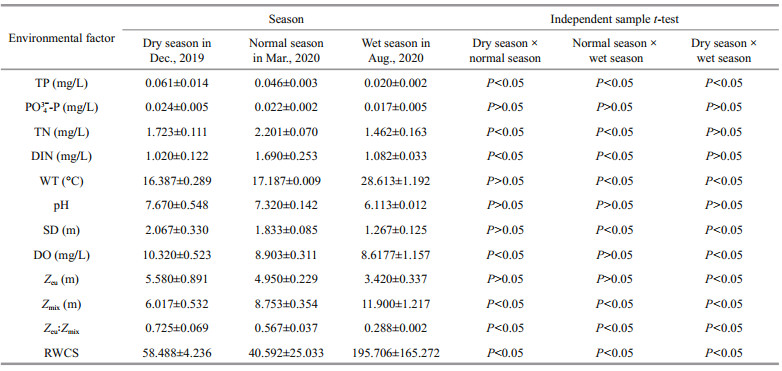
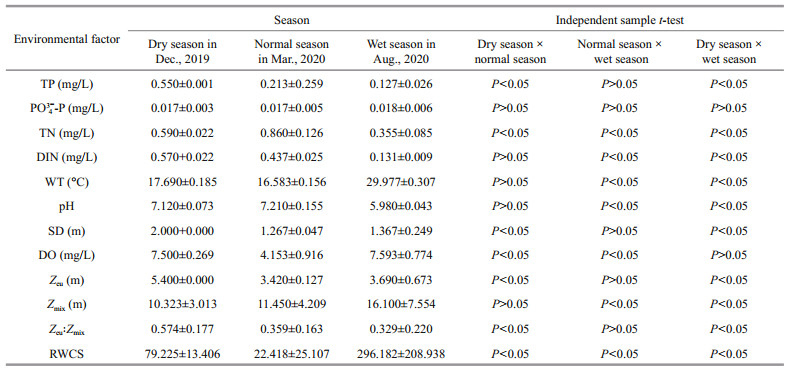
Except for PO43--P, environmental factors showed significant differences between the two reservoirs (P < 0.05). Generally speaking, the indices were greater in Jiuquwan Reservoir than those in Taihu Reservoir (Tables 3 & 4). TN, TP, and DIN varied largely in different seasons, and phosphorus decreased from dry season to wet season in both reservoirs. The average TP concentration of Jiuquwan Reservoir decreased from 0.061 to 0.002 mg/L, and that of Taihu Reservoir from 0.550 to 0.127 mg/L from dry season to wet season. Nitrogen in both reservoirs reached peak value in normal season. There was no significant difference in WT and SD between the two reservoirs (P>0.05). WT stayed at about 17 ℃ in dry season and normal season, and at about 29 ℃ in wet season, and SD reached the maximum in dry season. There was no significant difference in pH value between the two reservoirs (P > 0.05), but pH decreased in the wet season. DO concentration of Jiuquwan Reservoir was higher than that of Taihu Reservoir, and Taihu Reservoir was in anoxic state during normal season. In addition, there was no significant difference (P > 0.05) in Zeu and Zeu: Zmix between the two reservoirs, but Zeu: Zmix was higher in dry season than in normal and wet seasons in the two reservoirs. Significant difference was observed in RWCS between the two reservoirs (P < 0.05), being significantly higher in Taihu Reservoir than in Jiuquwan Reservoir (P < 0.05). RWCS was lower in normal season than in dry season, and much lower in dry season than in wet season, which indicates that the water body was stable and stratified in wet season. Independent sample t-test result shows that the water environmental factors of the two reservoirs changed significantly in different water regime periods or seasons. Except for PO43--P and pH, other environmental factors of Jiuquwan Reservoir showed certain variation rules in different seasons (P < 0.05), while in Taihu Reservoir, environmental factors exclusive of PO43--P presented certain variation rules in different seasons (P < 0.05).
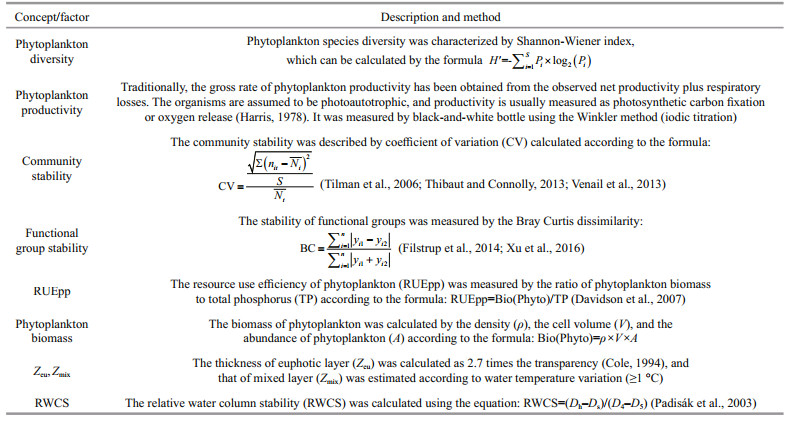
During the investigation period, 67 species (genera) of 7 phyla were identified in Jiuquwan Reservoir, including 8 species (genera) of Cyanophyta, 36 species (genera) of Chlorophyta, 16 species (genera) of Bacillariophyta, 1 species (genera) of gymnophyta, 2 species (genera) of Cryptophyta, 3 species (genera) of dinoflagellate, and 1 species (genera) of Xanthophyta. Chlorophyta was the most, accounting for 53.73% of the total species, followed by Bacillariophyta and Cyanophyta, accounting for 23.88% and 11.94% of the total species, and the rest account for a relatively small proportion. In Jiuquwan Reservoir, 23 species (genera) of 6 phyla were detected in dry season, 29 species (genera) of 5 phyla were detected in normal season, and 67 species (genera) of 7 phyla were detected in wet season. A total of 61 phytoplankton species (genera) belonging to 7 phyla were identified in Taihu Reservoir. Among them, 6 species (genera) belong to Cyanophyta, 33 species (genera) belong to Chlorophyta, 13 species (genera) belong to Bacillariophyta, 2 species (genera) belong to Nudophyta, 2 species (genera) belong to Cryptophyla, 4 species (genera) belong to dinoflagellate, and 1 species (genera) belongs to Chrysophyta. Chlorophyta was the most, accounting for 54.10% of the total species, followed by Bacillariophyta and Cyanophyta, accounting for 21.31% and 9.84% of the total species, respectively. There were 32 species (genera) in 5 phyla in dry season, 23 species (genera) in 7 phyla in normal season and 44 species (genera) in 5 phyla in wet season. There was no significant difference in phytoplankton species composition between the two reservoirs. In Jiuquwan Reservoir, 23, 29, and 67 species were collected in dry season, normal season, and wet season (Fig. 2a), while in Taihu Reservoir, 32, 23, and 44 species were collected, respectively (Fig. 2b). Chlorophyta, Bacillariophyta, and Cyanophyta were the main phytoplankton species in the two reservoirs, and the number of phytoplankton species was the largest in wet season. Species inventory and relative abundance of the two reservoirs were shown in the supplementary material (Appendix 1).
|
| Fig.2 Species composition of phytoplankton in Jiuquwan Reservoir (a) and Taihu Reservoir (b) in different sites and seasons of sampling |
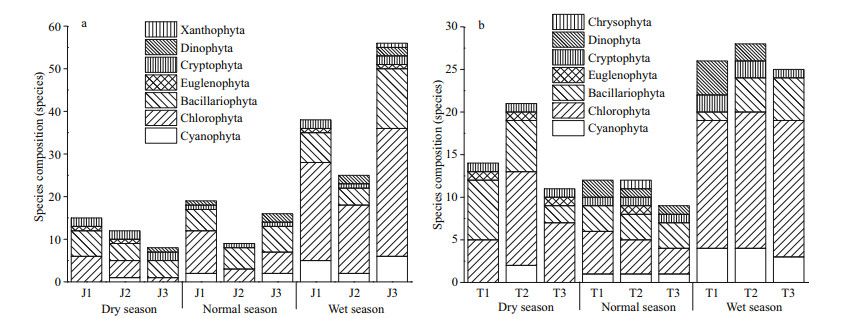
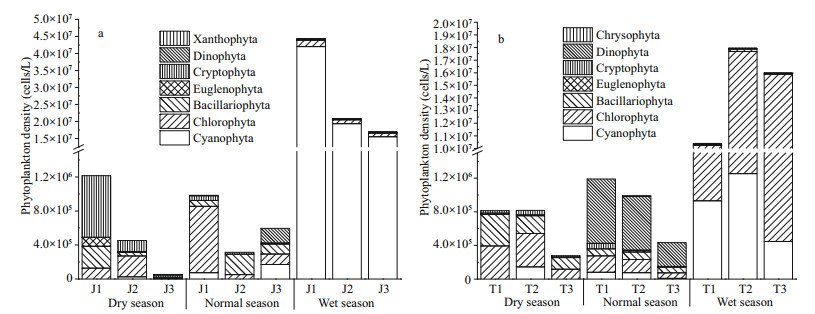
The phytoplankton density of Jiuquwan Reservoir was (5.39–4 427.97)×104 cells/L (Fig. 3a), and that of Taihu Reservoir was (27.88–1 794.56)×104 cells/L (Fig. 3b). The average phytoplankton density was the highest in wet season on a time scale. There was significant difference in phytoplankton density between the two reservoirs (P < 0.05). In Jiuquwan Reservoir, Cryptophyta, Chlorophyta, and Bacillariophyta were dominant in dry season, of which the relative abundance are 51.26%, 21.80%, and 18.39%. Among them, Cryptomonas sp., Pandorina morum, and Aulacoseria granulata account for a relatively large proportion. Chlorophyta, Bacillariophyta, and Cyanophyta were dominant in normal season with the relative abundance of 50.52%, 22.56%, and 12.97%. Among them, Gomphosphaeria sp., Discostella stelligera, and Pseudanabaena sp. have relatively high abundance. Cyanophyta is absolutely dominant in wet season with the relative abundance of 93.81%, in which Chroococcus minutus has the highest abundance. In Taihu Reservoir, Chlorophyta and Bacillariophyta were dominant in dry season with the relative abundance of 47.50% and 37.91%. Among them, Coelastrum microporum and Discostella stelligera have relatively high abundance. Dinophyta and Chlorophyta were dominant in normal season with the relative abundance of 64.52% and 16.05%. Among them, Peridiniopsis borgei has relatively high abundance. Chlorophyta is absolutely dominant in wet season with the relative abundance of 92.90%, in which Cosmarium sp. has the highest abundance. The relationship between phytoplankton species and biomass is shown in Fig. 4. There was a significant positive correlation between phytoplankton species and biomass in the two reservoirs (P < 0.05), indicating that phytoplankton biomass increased with the increase of phytoplankton species. The linear regression relationship of Jiuquwan Reservoir was y=-0.241+0.122x, the Pearson correlation coefficient R=0.686, P < 0.01. The regression coefficient was 0.122, meaning that each additional species of phytoplankton community increased in Jiuquwan Reservoir, the biomass would increase by 0.122 mg/L on average. The linear regression relationship of Taihu Reservoir was y=0.127+0.307x, the Pearson correlation coefficient R=0.317, P < 0.05, which indicates that for every additional species increased in the phytoplankton community in Jiuquwan Reservoir, its biomass would increase by 0.307 mg/L on average.
|
| Fig.3 Phytoplankton density of Jiuquwan Reservoir (a) and Taihu Reservoir (b) in different sites and seasons of sampling |
|
| Fig.4 Effect of phytoplankton species on biomass in Jiuquwan Reservoir (a) and Taihu Reservoir (b) |
As shown in Appendix 2, there is no significant difference in Shannon diversity index between the two reservoirs (P > 0.05, t-test), and the maximum diversity index of Taihu Reservoir is higher than that of Jiuquwan Reservoir. The trend of seasonal variation in phytoplankton diversity of the two reservoirs is consistent, which was dry season > normal season > wet season (Fig. 5). Meanwhile, no significant difference was observed among sampling sites in the two reservoirs in different seasons (P > 0.05, ANOVA). Based on the time series stability analysis of the two reservoirs, it was found that the stability of phytoplankton community fluctuated in the three hydrological seasons, but it did not show obvious seasonality (Fig. 6). In addition, no significant difference was found in the stability between the two reservoirs (P > 0.05, t-test), and the CV value increased with time, indicating that the stability of phytoplankton community was low in both the reservoirs. Moreover, no significant difference in the stability among sampling sites was noticed in the two reservoirs in different seasons (P > 0.05, AVONA). It can be seen from Appendix 2 that there are significant differences in phytoplankton productivity between the two reservoirs (P < 0.05, t-test). The productivity of Taihu Reservoir was higher than that of Jiuquwan Reservoir, and the trends of seasonal variation in phytoplankton productivity of the two reservoirs were the same, i.e., wet season > normal season > dry season (Fig. 7). There was no significant difference among sampling sites in Taihu Reservoir in all seasons (P > 0.05). While there was no significant difference among the sampling sites in Jiuquwan Reservoir in normal and wet seasons (P > 0.05), but significant difference in dry season (P < 0.05).
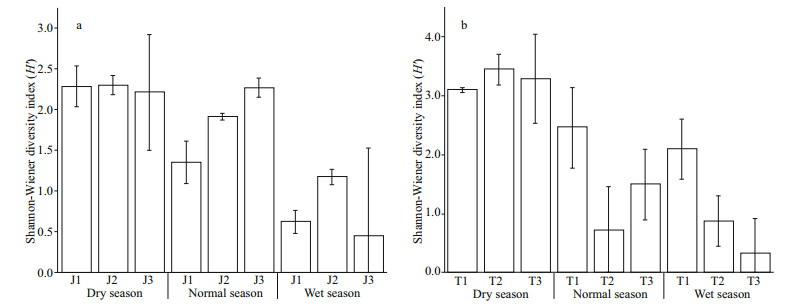
|
| Fig.5 Shannon diversity index (H') of Jiuquwan Reservoir (a) and Taihu Reservoir (b) in different sites and seasons of sampling |
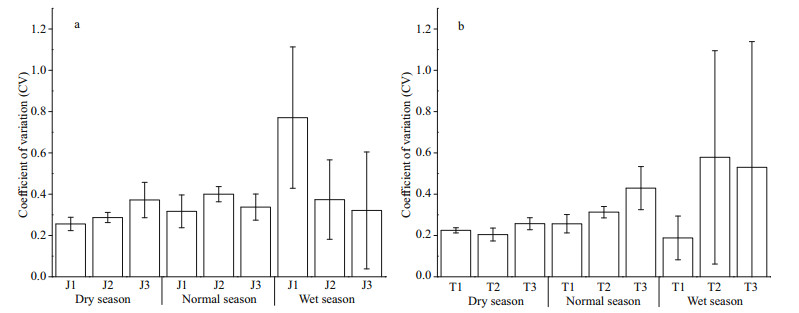
|
| Fig.6 The stability of Jiuquwan Reservoir (a) and Taihu Reservoir (b) in different sites and seasons of sampling |
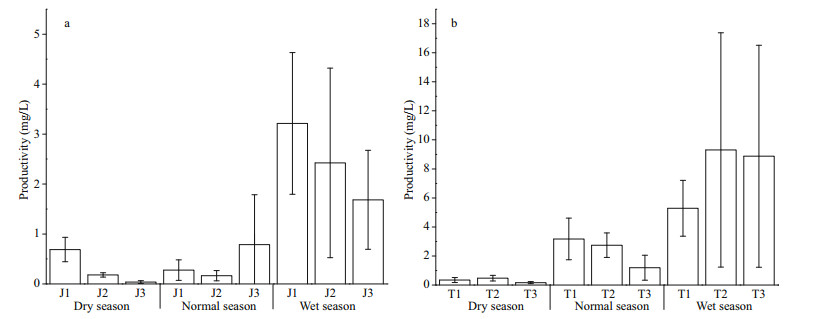
|
| Fig.7 Bioproductivity in Jiuquwan Reservoir (a) and Taihu Reservoir (b) in different sites and seasons of sampling |
The phytoplankton coefficient of variation (CV) and diversity of the two reservoirs were significantly negatively correlated (P < 0.05) (Fig. 8). The linear regression relationship of Jiuquwan Reservoir was y=2.326–1.704x, Pearson correlation coefficient R= -0.555, P < 0.01 (Fig. 8a); while the linear regression relationship of Taihu Reservoir was y=2.524–1.624x, Pearson correlation coefficient R=-0.527, P < 0.01 (Fig. 8b). The phytoplankton coefficient of variation (CV) value of Jiuquwan Reservoir had no significant correlation with richness (P > 0.05) (Fig. 8c), while the Taihu Reservoir showed a weak negative correlation (P < 0.05). The linear regression relationship was y=1.728–0.705x, Pearson correlation coefficient R=-0.358, P < 0.05 (Fig. 8d). The phytoplankton coefficient of variation (CV) and evenness of the two reservoirs showed a significant negative correlation (P < 0.05). The linear regression relationship of Jiuquwan Reservoir was y=1.095–0.691x, Pearson correlation coefficient R=-0.381, P < 0.05 (Fig. 8e), the linear regression relationship of Taihu Reservoir was y=1.047–0.563x, Pearson correlation coefficient R=-0.458, P < 0.05 (Fig. 8f). In summary, the phytoplankton coefficient of variation (CV) of the two reservoirs showed a certain negative correlation with diversity, richness, and evenness, indicating that the coefficient of variation (CV) decreased and the community stability increased with the increases of diversity, richness, and evenness. The correlation coefficients between phytoplankton stability and diversity, richness, and evenness in Taihu Reservoir were higher than those in Jiuquwan Reservoir.
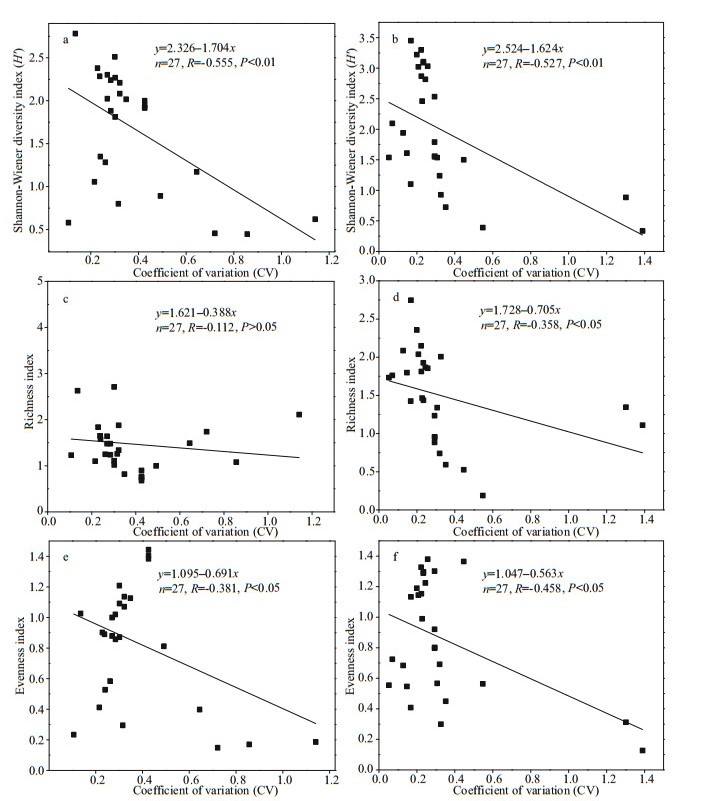
|
| Fig.8 Relationships of the CV value to the diversity, richness, and evenness in Jiuquwan Reservoir (left panel) and Taihu Reservoir (right panel) |
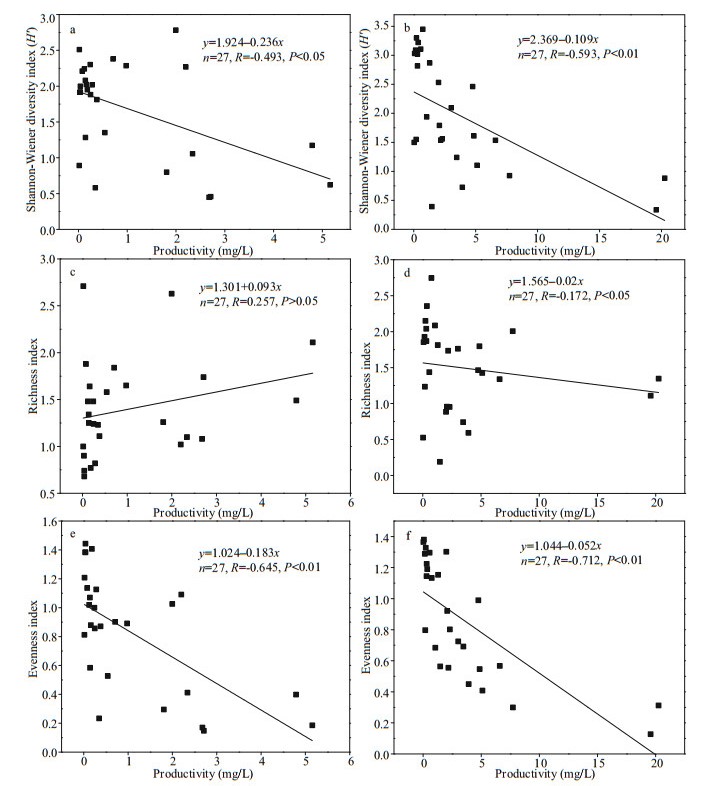
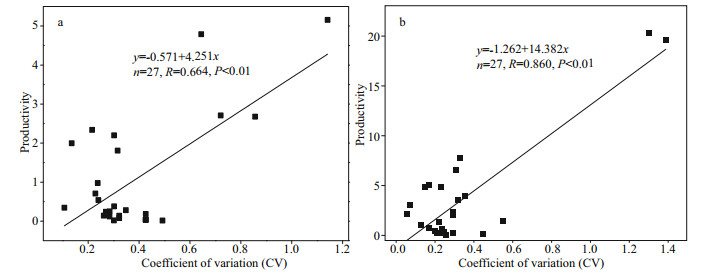
The productivity of phytoplankton community was negatively correlated with diversity and evenness (P < 0.05), but not with richness (P > 0.05) (Fig. 9). The Pearson correlation coefficients of productivity and diversity, richness and evenness in Jiuquwan Reservoir were -0.493 (Fig. 9a), 0.257 (Fig. 9c), and -0.645 (Fig. 9e), while those of Taihu Reservoir were -0.593 (Fig. 9b), -0.172 (Fig. 9d), and -0.712 (Fig. 9f), respectively, indicating that the productivity of the two reservoirs decreased with the increase of phytoplankton diversity index (H') and evenness. With the increase of productivity, the richness index did not increase nor decrease obviously. The relationship between phytoplankton productivity and the CV values are shown in Fig. 10. There is a significant positive correlation between phytoplankton productivity and the CV value in the two reservoirs (P < 0.01). The linear regression relationship of Jiuquwan Reservoir was y=-0.571+4.251x, Pearson correlation coefficient R=0.664, P < 0.01 (Fig. 10a); and those of Taihu Reservoir were y=-1.262–14.382x, Pearson correlation coefficient R=0.860, P < 0.01 (Fig. 10b). With the increase of phytoplankton productivity, the CV value increased and community stability weakened in the two reservoirs.
|
| Fig.9 Relationship of the productivity to the diversity, richness, and evenness of phytoplankton in Jiuquwan Reservoir (left panel) and Taihu Reservoir (right panel) |
|
| Fig.10 Relationship between phytoplankton productivity and CV value in Jiuquwan Reservoir (a) and Taihu Reservoir (b) |
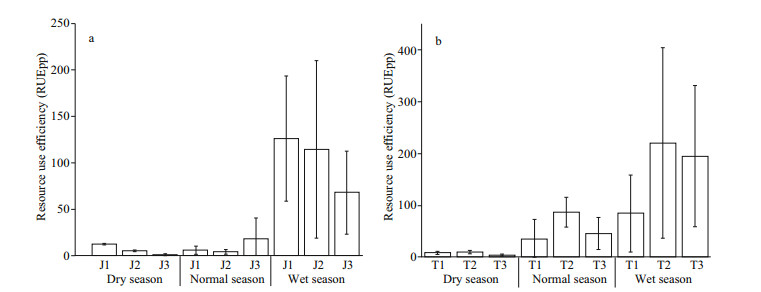
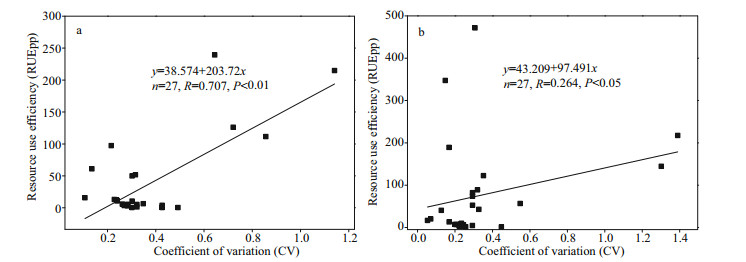
As shown in Appendix 2, there was no significant difference in RUEpp between the two reservoirs (P > 0.05, t-test), and the phytoplankton RUEpp was greater in Taihu Reservoir than in Jiuquwan Reservoir. The phytoplankton RUEpp in both two reservoirs had obvious seasonal fluctuation (Fig. 11). There were significant differences in the phytoplankton RUEpp between wet season and dry season (P < 0.05, t-test). There was no significant difference in Jiuquwan Reservoir between normal season and dry season (P > 0.05, t-test), while the RUEpp of phytoplankton in wet season was significantly higher than that in dry-and-normal seasons. The RUEpp was in peak in wet season, and the standard deviation of mean RUEpp value of the two reservoirs in wet season was large, which indicates that phytoplankton resources utilization ability in different water layers was also different. There was a significant positive correlation between RUEpp and the CV value (P < 0.01). The linear regression relationship of Jiuquwan Reservoir was y=38.574+203.72x, Pearson correlation coefficient R=0.707, P < 0.01 (Fig. 12a), and for Taihu Reservoir, they were y=43.209+97.491x, R=0.264, P < 0.05 (Fig. 12b). The results show that the CV value increased while the community stability decreased with the improvement of resource use efficiency of phytoplankton.
|
| Fig.11 The resource use efficiency of phytoplankton in Jiuquwan Reservoir (a) and Taihu Reservoir (b) in different sites and seasons of sampling |
|
| Fig.12 Relationship between resource use efficiency of phytoplankton and the CV value in Jiuquwan Reservoir (a) and Taihu Reservoir (b) |
According to the classification of phytoplankton functional group proposed by Reynolds et al. (2002) and Padisák et al. (2009), the identified algae species were classified. For the Jiuquwan Reservoir, they were classified into 18 functional groups, namely A, B, D, F, G, J, LM, LO, MP, N, P, S1, T, TC, W1, X1, X2, and Y; for Taihu Reservoir, 19 functional groups were classified, namely, B, D, E, F, G, H1, J, LM, LO, MP, N, P, S1, T, W1, W2, X1, X2, and Y (Table 5). The functional groups with relative biomass greater than 5% were defined as advantageous functional groups, and those with relative biomass greater than 50% as dominant functional groups. The advantageous function groups of Jiuquwan Reservoir during dry season were W1, Y, N, and P, that during normal water period were LO, B, and P, that during wet season were LO, N, and J. The advantageous function group of Taihu Reservoir during dry season included B, N, W1, W2, and P, the dominant function group in normal water season was LO, and that in wet season was N. The results of the analysis of phytoplankton functional groups in the two reservoirs in the three sampling seasons show that the phytoplankton community replacement of the two reservoirs showed alternation and fluctuation, and the overall stability was low (Fig. 13). But the stability of Jiuquwan Reservoir was higher than that of Taihu Reservoir. The functional group N of Jiuquwan Reservoir had a large fluctuation range, and the stability was high during dry and normal seasons. The functional groups LM, TC, X1, T, J, G, and A had a small fluctuation range, and their stability was low. The functional groups S1, W1, and F of Taihu Reservoir had a large fluctuation range. S1 had higher stability during normal and wet seasons. W1 had higher stability during dry and normal seasons, and F had higher stability during wet and dry seasons. The functional groups LM, LO, H1, T, N, W2, and E had a small fluctuation range, and their stability was low. Comparing the dominant function groups and non-dominant function groups of the two reservoirs, we found no significant difference in community replacement value (P > 0.05, t-test) between the two reservoirs (Appendix 2). In Jiuquwan Reservoir, dominant functional groups and non-dominant functional groups had the same trend of stability changes. The law of stability changes was: dry season-normal season > normal-wet season > wet-dry season (Fig. 14a). In Taihu Reservoir, the variation trends of dominant function group and non-dominant function group were inconsistent. The variation trend of the stability of dominant function group was consistent with that of Jiuquwan Reservoir, and that of non-dominant function group was: wet-dry season > dry-normal season > normal-wet season (Fig. 14b). There was no signifi cant correlation between the number of phytoplankton functional groups and the community stability in the two reservoirs (P > 0.05) (Fig. 15a & b). The number of phytoplankton functional groups in Jiuquwan Reservoir was significantly positively correlated with community productivity (P < 0.01) (Fig. 15c), while in Taihu Reservoir, no significant correlation was observed (P > 0.05) (Fig. 15d).
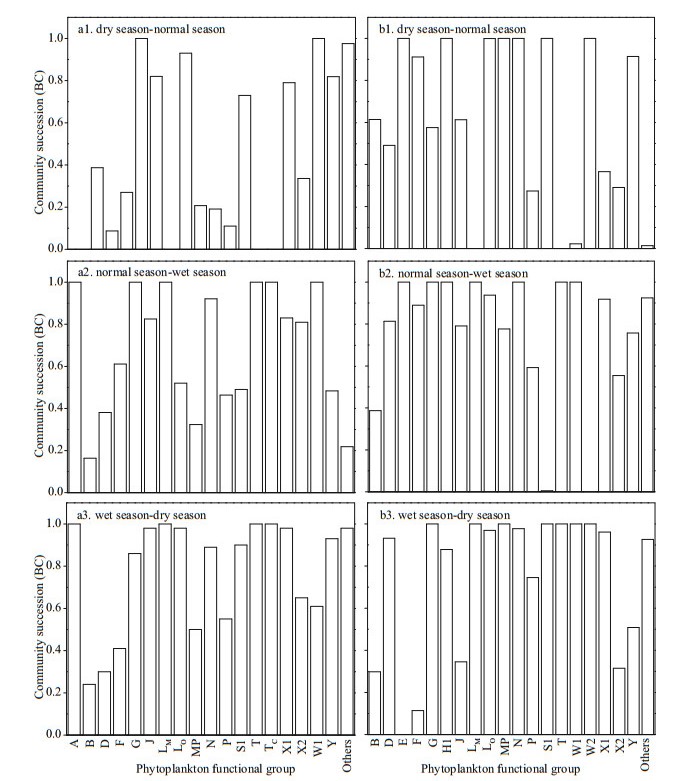
|
| Fig.13 The community replacement index BC (see Eq.8) of Jiuquwan Reservoir (a1–a3, left panel) and Taihu Reservoir (b1–b3, right panel) |
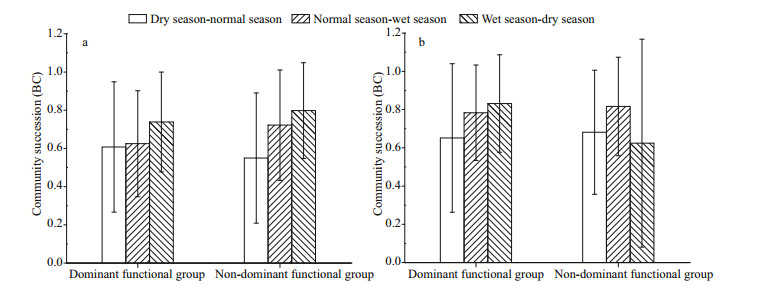
|
| Fig.14 The community replacement index BC (see Eq.8) of dominant and non-dominant functional groups in Jiuquwan Reservoir (a) and Taihu Reservoir (b) |
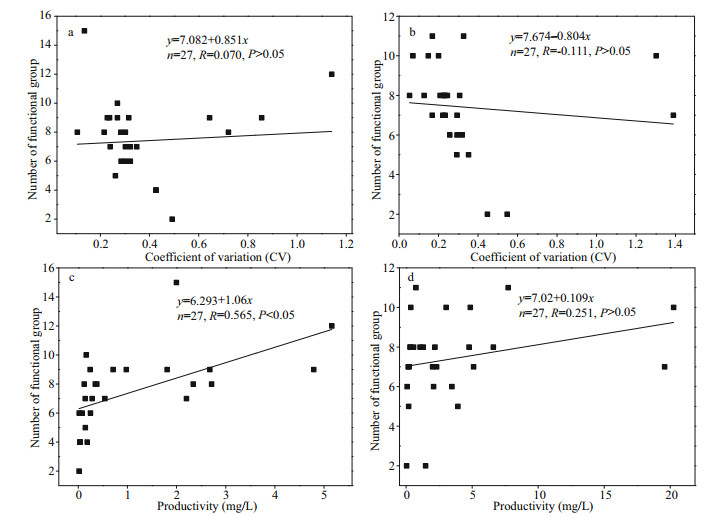
|
| Fig.15 Relationship of the number of functional groups to the CV value and productivity in Jiuquwan Reservoir (a & c) and Taihu Reservoir (b & d) |
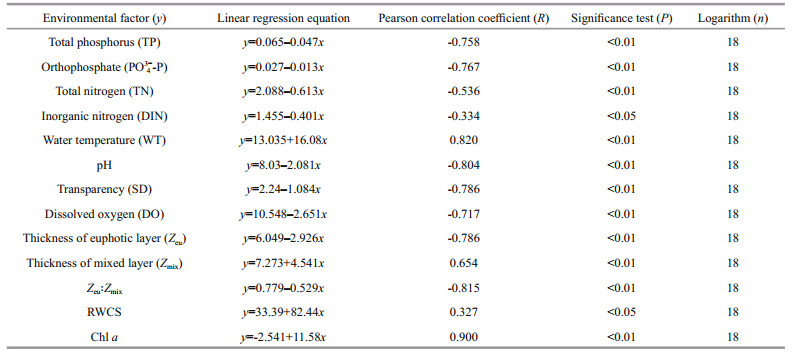
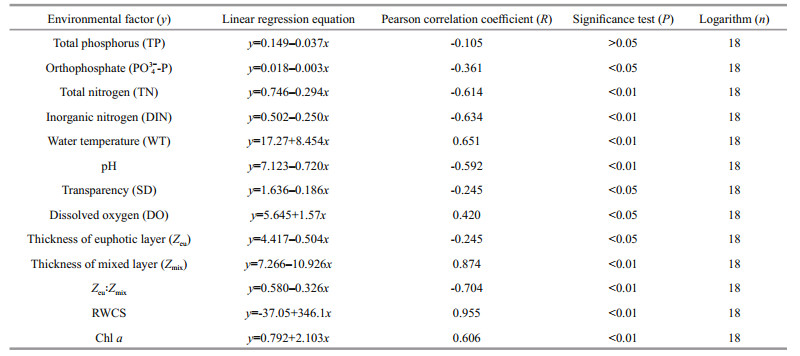
The environmental factors and community stability in the two reservoirs were analyzed, from which linear regression was performed (Tables 6 & 7). In Jiuquwan Reservoir, the CV value had significant linear relationship with 13 environmental factors (P < 0.05), and had negative linear relationship with TP, PO43ˉ-P, TN, DIN, pH, SD, DO, Zeu, and Zeu: Zmix, showing that these factors could promote the stability of the community; and positively correlated with WT, Zmix, RWCS, and Chl a, indicating that they inhibited the stability of the community. In Taihu Reservoir, the CV value had significant linear relationship with all the environmental factors (P < 0.05) except for of TP which had no significant linear relationship (P > 0.05); PO43ˉ-P, TN, DIN, pH, SD, Zeu, and Zeu: Zmix showed a negative correlation, indicating that they could promote the stability of the community, while WT, DO, Zmix, RWCS, and Chl a showed a significant positive correlation, showing that they could inhibit the stability of the community.
|
|
Jiuquwan Reservoir has experienced a course from cyanobacteria bloom outbreak to the treatment and recovery. In the outbreak event in 2014, 83 species from 7 phyla of phytoplankton were detected (Chen et al., 2015). In the treatment period in 2014–2015 and in the recovery period in 2016–2018, 81 species from 6 phyla of phytoplankton were detected (Yan et al., 2020). In this study, 67 species from 7 phyla of phytoplankton from 2019 to 2020 were detected. Compared with the previous result, the number of phytoplankton species decreased, indicating that the species diversity of Jiuquwan Reservoir has been decreased and the ecosystem degenerated. Twenty-two functional groups were classified during the investigation period 2017–2018, namely B, C, D, F, G, H1, J, K, LO, M, MP, N, P, S1, SN, TC, W1, W2, X1, X2, X3, and Y (Yan et al., 2020), while in this study there were 18 functional groups detected, namely A, B, D, F, G, J, LM, LO, MP, N, P, S1, T, T C, W1, X1, X2, and Y.
On the other hand, 21 species of phytoplankton in 1 phylum were detected in Taihu Reservoir in 2014 before impoundment, and 25 species from 4 phyla were detected in 2019 after impoundment (Chen et al., 2020). A total of 61 species (genera) from 7 phyla were detected from 2019 to 2020 in this study. Compared with earlier reports, the number of phytoplankton species has increased. Five functional groups were classified in 2014 before impoundment, namely D, LO, MP, P, and X3; and 8 functional groups were classified in 2019 after impoundment, namely D, J, L O, MP, P, T, X1, and X3 (Chen et al., 2020). In this study, 19 functional groups were classified, namely B, D, E, F, G, H1, J, LM, LO, MP, N, P, S1, T, W1, W2, X1, X2, and Y.
There was no significant difference in phytoplankton species composition between the two reservoirs. Chlorophyta, Bacillariophyta, and Cyanophyta were the main phytoplankton members in both reservoirs. The species number and the average phytoplankton density were the largest in wet season. There was significant difference in phytoplankton density between the two reservoirs (P < 0.05). In Jiuquwan Reservoir, Cryptophyta, Chlorophyta, and Bacillariophyta were dominant in dry season, of which the relative abundance are 51.26%, 21.80%, and 18.39%. Chlorophyta, Bacillariophyta, and Cyanophyta were dominant in normal season with the relative abundance of 50.52%, 22.56%, and 12.97%. Cyanophyta is absolutely dominant in wet season with the relative abundance of 93.81%. While in Taihu Reservoir, Chlorophyta and Bacillariophyta were dominant in dry season with the relative abundance of 47.50% and 37.91%. Dinophyta and Chlorophyta were dominant in normal season with the relative abundance of 64.52% and 16.05%. Chlorophyta is absolutely dominant in wet season with the relative abundance of 92.90%. There was a significant positive correlation between phytoplankton species and biomass in the two reservoirs (P < 0.05), indicating that phytoplankton biomass increased with the increase of phytoplankton species.
4.2 The relationship of phytoplankton diversity to the productivity and community stabilityThe higher the species richness and the more complex the nutrient structure, the higher the stability of the ecosystem when faced with environmental disturbance (MacArthur, 1955). Diversity promotes the stability of ecosystems (Loreau et al., 2001). Studies have also shown that while diversity increases the stability of the entire ecosystem, it also reduces the stability of individual species (Tilman, 1996). Pennekamp et al. (2018) pointed out that there is a U-shaped relationship between species diversity and ecosystem stability. We found that the CV value in the two reservoirs was negatively correlated in a certain degree with diversity, richness, and evenness (P < 0.05), indicating that with the increases of diversity, richness, and evenness, the CV value decreased and community became more stable, which is consistent with the results of Wittebolle et al. (2009). However, there is no significant correlation between phytoplankton community stability and abundance in Jiuquwan Reservoir, which is consistent with the results of Isbell et al. (2009) and Tian et al. (2016). Through the comparative analysis of the relationship between phytoplankton stability and diversity, abundance, and evenness in the two reservoirs, it is proved that biodiversity could promote the stability of ecosystem in the natural system to a certain extent.
The relationship between phytoplankton stability and diversity, richness, and evenness in natural system is very complex. The combination effect shall be the most important mechanism (Tian, 2017). To clarify the relationship between them, more studies are required. Ecologists have found that the relationships between phytoplankton productivity and diversity, richness, and evenness is also complex and could be recognized as the following types: stochastic type, monotonic increase type, platform type, monotonic decrease type, and unimodal (hump) type relationship according to available works (Abrams, 1995; Yang et al., 2002; Smith, 2007).
In this study, the productivity of phytoplankton community was significantly negatively correlated with diversity and evenness (P < 0.05), indicating that the phytoplankton productivity decreased with the increase of diversity index and evenness index of phytoplankton in the two reservoirs, which was a monotonic-decrease-typed relationship, but had no significant correlation with richness (P > 0.05). With the increase of productivity, the richness index did not increase nor decrease significantly, showing a stochastic-typed relationship. Waide et al. (1999) summarized the relationship between diversity and productivity of more than 200 species in published materials, and found that 30% of them were unimodal, 26% positive, 12% negative, and 32% insignificant. Therefore, the relationship between phytoplankton diversity and productivity varies with time and environment. The relationship between stability and productivity is linked by diversity. This study shows that there was a significant positive correlation between phytoplankton productivity and community variation (CV) (P < 0.01), indicating that with the increase of phytoplankton productivity, CV increased and phytoplankton community stability weakened. The stability, diversity, and productivity of the new lake-type reservoir (Taihu Reservoir) are higher than those of the river-type reservoir (Jiuquwan Reservoir).
4.3 The relationship between community stability and resource use efficiencyThe resource use efficiency refers to the ability or degree of a community to use limited resources, indicating that the current resources have not been fully utilized, which provides an available niche for the growth and reproduction of new species (Ptacnik et al., 2008). Striebel et al. (2009) found that phytoplankton species richness was positively correlated with resource use efficiency. Ptacnik et al. (2008) showed that species with higher diversity have stronger ability to utilize limited resources. In this study, there was a significant positive correlation between the utilization efficiency of phytoplankton resources and the CV value (P < 0.01) in the two reservoirs, indicating that with the improvement of the utilization efficiency of phytoplankton resources, the CV value increased and the community stability decreased.
When multiple species coexist, species complements each other for the best use of local resources (Valentine, 1971; Cardinale, 2011). Therefore, the resource utilization efficiency is also high in the community with high diversity (Ptacnik et al., 2008). However, this viewpoint seems contradictory to our findings that the increases in productivity and resource utilization efficiency weakened the stability of the community (Figs. 10 & 12). This is because those previous studies hypothesized that each species of the community had complementary effects in resource using. However, only in a stable community can the diversity persists and plays such a role. In other words, the complementary effect can play the role only when there is a relatively stable niche (Turnbull et al., 2013). In a normal (terrestrial) plant community, succession of community would take a long time to reach the top (Clewell, 2011). The same happens during phytoplankton blooms or red tides, and the duration can last 1–2 months during which a steady stage can be formed. Therefore, the utilization efficiency of community resources would be high in a steady stage by the complementary effect, while when communities are dominated by a few species, the utilization efficiency of community resources could be reduced due to the loss of diversity (Chai et al., 2020).
In this study, the environmental parameters varied considerably in the three sampling seasons (wet, normal, and dry), and the phytoplankton community structures in each season were also greatly different. Phytoplankton grows fast under ideal conditions of nutrients, light, and temperature, and the phytoplankton community can proliferate into the climax community in 1–4 weeks, and a few dominant species would occupy the most of the community's biomass (Silva et al., 2008). In the climax community, although the biodiversity is lower than that in the previous period, the dominant species can best adapt to the current environment and utilize more effectively for the nutrient resources in the water body. Researchers also found that the older phytoplankton communities could waste less energy than younger ones did (Ghedini et al., 2020). Moreover, different from the combination of attached algae used in the experiment reported by Cardinale (2011), the phytoplankton community in this study was vulnerable due to reservoir discharge and the changes of other environmental conditions, causing difficulty to form stable communities. In addition, as Cardinale et al. (2011) showed that 63% of observations suggest that diverse polycultures yielded less biomass than that of the single highest-yielding monoculture, and could not use more resources, which is consistent with our study, and indicates that under low diversity and community stability, the resource utilization efficiency of the community could be higher.
Ye et al. (2019) divided phytoplankton communities in the English Channel and Lake Kasumigaura (Japan) into different groups in functional diversity using functional diversity indexes, a more significant nonlinear positive correlation between the utilization efficiency and different type of nutrients (nitrogen, phosphorus, and silicon) was obtained. At present, our study considered only the overall efficiency of phytoplankton versus the utilization of phosphorus, and the diversity was expressed by Shannon-Wiener index. In the future study, if phytoplankton communities and the resources they use could be classified into detailed functional types, we will be able to investigate whether there are differences in efficiency of using other resources such as nitrogen, silicon, light, etc., and different results may be obtained.
The utilization efficiency of newly built lake-type reservoir (Taihu Reservoir) was higher than that of river-type reservoir (Jiuquwan Reservoir). The phytoplankton resource utilization ability was the strongest in wet season, and the standard deviation of the mean value of resource use efficiency in the two reservoirs was greater in wet season than that in other seasons, which indicated that phytoplankton resource utilization ability in different water layers was quite different. Therefore, the resource use efficiency of phytoplankton interacted with the community stability, and varied in season and water depth.
4.4 The community stability of phytoplankton functional groupsEighteen functional groups in Jiuquwan Reservoir and 19 in Taihu Reservoir were recognized, and the number of functional groups showed no significant difference. It is mainly because that the two reservoirs were shared in a common Dongjiang River basin, and the algae source was the same. Hooper et al. (2005) found that the number of functional groups and plant species richness were the main factors affecting the community productivity. The results show that the stability of phytoplankton functional groups in river-type reservoir (Jiuquwan Reservoir) is higher than that of the new lake-typed reservoir (Taihu Reservoir), the number of phytoplankton functional groups has no significant correlation with community stability (P > 0.05). The number of phytoplankton functional groups and community productivity was related positively and significantly (P < 0.01) in Jiuquwan Reservoir but no significant correlation (P > 0.05) in Taihu Reservoir, indicating that the effect of phytoplankton functional group diversity on community stability is not significant, which is not consistent with the hypothesis of "diversity-stability" (Ghedini et al., 2020), indicating that the stability of phytoplankton community has a great relationship with the change of environment.
4.5 The environmental factors and community stabilityBy performing the linear regression of 13 environmental factors (Tables 6–7) and the community stability (in terms of CV value) of the two reservoirs revealed a significant correlation between the community stability and the environmental factors in both the reservoirs. For Jiuquwan Reservoir, the correlation of linear regression was significant (P < 0.05). In specific, TP, PO43--P, TN, DIN, pH, SD, DO, Zeu, and Zeu: Zmix was significantly negative, indicating that these factors could promote the community stability; and WT, Zmix, RWCS, and Chl a was significantly positive, indicating that these factors could weaken the community stability. For Taihu Reservoir, the coefficient of variation (CV) had a significant linear relationship with 12 environmental factors (P < 0.05) exclusive of TP (P > 0.05). The CV had a significant negative correlation with PO43--P, TN, DIN, pH, SD, Zeu, and Zeu: Zmix, which indicated that these environmental factors could promote the population stability, and had a significant positive correlation with WT, DO, Zmix, RWCS, and Chl a, showing that these environmental factors could weaken the community stability.
The correlation coefficients of CV to WT, pH, and Chl a of Jiuquwan Reservoir were R > 0.8, which indicated that these environmental factors had great influence on the community stability. Studies have shown that WT has a great influence on the stability of long-term fluctuation of phytoplankton (Gonzalez and Descamps-Julien, 2004). Rothenberger et al. (2009) found that there is a strong positive correlation between the long-term fluctuation of phytoplankton community and WT, which is consistent with the results of this study. pH has an important effect on the availability of nutrients and the feeding rate of zooplankton, and it limits the growth rate of phytoplankton. The relationship between pH and the growth rate of phytoplankton is of unimodal type, with a peak value between 8.3 and 8.5 (Hansen, 2002; Hinga, 2002). During this study period, the pH of Jiuquwan Reservoir was between 6.11 and 7.67.
Chl a reflects phytoplankton productivity. In this study, phytoplankton productivity was significantly positively correlated with coefficient of variation (CV), indicating that the increase of Chl a could weaken the community stability, which agrees to the conclusion of Vörös and Padisák (1991). Therefore, WT, pH, and Chl a were the main environmental factors affecting the stability of phytoplankton in Jiuquwan Reservoir. In Taihu Reservoir, the correlation coefficient between the stability of the community and Zmix and RWCS was also significant (R > 0.8), indicating that these environmental factors had a greater impact on the stability of the community.
The vertical changes of phytoplankton functional groups are related to light, water stratification, and grazing pressure (Becker et al., 2009). After the Taihu Reservoir was built and impounded, the entire reservoir area is similar to an artificial lake, its water inflow, flow, throughput, and surface runoff are quite different from those of river-type reservoirs. In addition, the Taihu Reservoir is a sub-deep reservoir with a maximum water depth of 50 m. There are big differences in the relative stability of the water mixture layer and the water column in dry, normal, and wet seasons. In the reservoir, it was easy to form stable thermal stratification in wet season, with large temperature differences between the upper and lower layers, and the relative stability of the water body was relatively high. The water body was completely mixed during dry and normal seasons, and the relative stability of the water body was low. Therefore, Zmix and RWCS became the main environmental factors affecting the stability of phytoplankton for Taihu Reservoir. In addition, the concentrations of TN and TP in both reservoirs exceeded the internationally recognized eutrophication threshold (TN=0.2 mg/L, TP=0.02 mg/L). Therefore, nitrogen and phosphorus were no longer the limiting factors affecting the stability of the phytoplankton community. In summary, the main factors that affect the community stability of the river-type reservoirs (such as Jiuquwan Reservoir) and newly-built lake-type reservoirs (such as Taihu Reservoir) were local hydrodynamic parameters (WT, pH, Zmix, RWCS), not nutrients.
5 CONCLUSIONBased on the investigation on river-type Jiuquwan Reservoir and lake-type Taihu Reservoir, the relationship and difference in phytoplankton diversity, productivity, and community stability between the two reservoir were compared and analyzed, showing the following conclusions.
(1) There was no significant difference in functional groups and species number between the two reservoirs. The stability, diversity, productivity, and resource use efficiency of Taihu Reservoir were higher than those of Jiuquwan Reservoir, and the phytoplankton resource utilization ability was the strongest in wet season.
(2) In natural systems, biodiversity can promote productivity and ecosystem stability. The relationship between phytoplankton diversity and productivity or stability varies with time and environment. The diversity of phytoplankton functional groups had no significant effect on community stability.
(3) The stability of phytoplankton community is affected by both biotic and abiotic factors. Hydrodynamic parameters (water temperature, pH, thickness of mixed layer, and relative water column stability) are the main environmental factors affecting the stability of phytoplankton community.
6 DATA AVAILABILITY STATEMENTThe datasets generated and/or analyzed during the current study are not publicly available as the project has not been concluded but are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENTWe are grateful to Prof. Ren HU of Jinan Normal University for his tutoring on topic selection, design of field observation, and thesis formulation; to Prof. Meiying XU of Guangdong Institute of Microbiology for his inspiration and help in the research direction; to Profs. Wenlong CHEN, Fang YANG, and the Director Weijie HUANG from the Pearl River Water Resources Research Institute for a financial support and technical guidance in the field works and the manuscript preparation; to the two peer reviewers for constructive suggestions on improving the manuscript, to Dr. Roger YU for guidance in technical writing and language polishing, to Drs. Yang CHEN and Jiao LIU, the editors of this journal for review and editing of the manuscript. Thanks are extended to the research team members Mugui LI, Zubiao ZHANG, Ning LI, Rui HE, Xunan YANG, and others for the cooperation in this project and their strong support in reservoir sampling and data collection.
Abrams P A. 1995. Monotonic or unimodal diversity-productivity gradients: what does competition theory predict?. Ecology, 76(7): 2019-2027.
DOI:10.2307/1941677 |
Allen H L, Ocevski B T. 1981. Comparative primary productivity of algal epiphytes on three species of macrophyte in the lttoral zone of Lake Ohrid, Yugosiavia. Holarct. Ecol., 4: 155-160.
|
Bai M C, Ban X, Diplas P, Xiao F. 2017. Quantifying the spatio-temporal variation of flow and its ecological impacts in the middle-section of Hanjiang River following the Danjiangkou Reservoir impoundment. Resources and Environment in the Yangtze Basin, 26(9): 1476-1487.
(in Chinese with English abstract) DOI:10.11870/cjlyzyyhj201709020 |
Becker V, Huszar V L M, Crossetti L O. 2009. Responses of phytoplankton functional groups to the mixing regime in a deep subtropical reservoir. Hydrobiologia, 628(1): 137-151.
DOI:10.1007/s10750-009-9751-7 |
Cardinale B J, Duffy J E, Gonzalez A, Hooper D U, Perrings C, Venail P, Narwani A, Mace G M, Tilman D, Wardle D A, Kinzig A P, Daily G C, Loreau M, Grace J B, Larigauderie A, Srivastava D S, Naeem S. 2012. Biodiversity loss and its impact on humanity. Nature, 486(7401): 59-67.
DOI:10.1038/nature11148 |
Cardinale B J, Matulich K L, Hooper D U, Byrnes J E, Duffy E, Gamfeldt L, Balvanera P, O'Connor M I, Gonzalez A. 2011. The functional role of producer diversity in ecosystems. American Journal of Botany, 98(3): 572-592.
DOI:10.3732/ajb.1000364 |
Cardinale B J. 2011. Biodiversity improves water quality through niche partitioning. Nature, 472(7341): 86-89.
DOI:10.1038/nature09904 |
Chai Z Y, Wang H, Deng Y Y, Hu Z X, Tang Y Z. 2020. Harmful algal blooms significantly reduce the resource use efficiency in a coastal plankton community. Science of the Total Environment, 704: 135381.
DOI:10.1016/j.scitotenv.2019.135381 |
Chen Q, Wu Q, Luo H. 2020. Phytoplankton community structure characteristics before and after the impoundment in Taihu Reservoir. Ecological Science, 39(6): 75-82.
(in Chinese with English abstract) DOI:10.14108/j.cnki.1008-8873.2020.06.011 |
Chen W L, Yang F, Luo H, Cui S B, Xu L Q. 2015. Genetic mechanism of cyanobacteria blooms in drinking water reservoirs in southern mountainous areas. Pearl River, 36(1): 116-120.
(in Chinese) |
Clewell A. 2011. Forest succession after 43 years without disturbance on ex-arable land, northern Florida. Castanea, 76(4): 386-394.
DOI:10.2179/11-015.1 |
Cole G A. 1994. Textbook of Limnology. Waveland Press, Illinois, USA.
|
Davidson E A, Howarth R W. 2007. Nutrients in synergy. Nature, 449(7165): 1000-1001.
DOI:10.1038/4491000a |
Filstrup C T, Hillebrand H, Heathcote A J, Harpole W S, Downing J A. 2014. Cyanobacteria dominance influences resource use efficiency and community turnover in phytoplankton and zooplankton communities. Ecology Letters, 17(4): 464-474.
DOI:10.1111/ele.12246 |
Ghedini G, Loreau M, Marshall D J. 2020. Community efficiency during succession: a test of MacArthur's minimization principle in phytoplankton communities. Ecology, 101(6): e03015.
DOI:10.1002/ecy.3015 |
Giller P S, Hillebrand H, Berninger U G, Gessner M O, Hawkins S, Inchausti P, Inglis C, Leslie H, Malmqvist B, Monaghan M T, Morin P J, O'Mullan G. 2004. Biodiversity effects on ecosystem functioning: emerging issues and their experimental test in aquatic environments. Oikos, 104(3): 423-436.
DOI:10.1111/j.0030-1299.2004.13253.x |
Gonzalez A, Descamps-Julien B. 2004. Population and community variability in randomly fluctuating environments. Oikos, 106(1): 105-116.
DOI:10.1111/j.0030-1299.2004.12925.x |
Hansen P J. 2002. Effect of high pH on the growth and survival of marine phytoplankton: implications for species succession. Aquatic Microbial Ecology, 28(3): 279-288.
DOI:10.3354/ame028279 |
Hinga K R. 2002. Effects of pH on coastal marine phytoplankton. Marine Ecology Progress Series, 238: 281-300.
DOI:10.3354/meps238281 |
Hodapp D, Kraft D, Hillebrand H. 2014. Can monitoring data contribute to the biodiversity-ecosystem function debate?Evaluating data from a highly dynamic ecosystem. Biodiversity and Conservation, 23(2): 405-419.
DOI:10.1007/s10531-013-0609-y |
Holling C S. 1973. Resilience and stability of ecological systems. Annual Review of Ecology and Systematics, 4(1): 1-23.
DOI:10.1146/annurev.es.04.110173.000245 |
Hooper D U, Chapin F S, Ewel J J, Hector A, Inchausti P, Lavorel S, Lawton J H, Lodge D M, Loreau M, Naeem S, Schmid B, Setälä H, Symstad A J, Vandermeer J, Wardle D A. 2005. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecological Monographs, 75(1): 3-35.
DOI:10.1890/04-0922 |
Hu H J, Wei Y X. 2006. The Freshwater Algae of China: Systematics, Taxonomy and Ecology. Science Press, Beijing, China.
(in Chinese with English abstract)
|
Ioriya T, Inoue S, Haga M, Yogo N. 1998. Change of chemical and biological water environment at a newly constructed reservoir. Water Science and Technology, 37(2): 187-197.
DOI:10.1016/S0273-1223(98)00023-7 |
Isbell F I, Polley H W, Wilsey B J. 2009. Biodiversity, productivity and the temporal stability of productivity: patterns and processes. Ecology Letters, 12(5): 443-451.
DOI:10.1111/j.1461-0248.2009.01299.x |
Jensen J P, Jeppesen E, Olrik K, Kristensen P. 1994. Impact of nutrients and physical factors on the shift from cyanobacterial to chlorophyte dominance in shallow Danish Lakes. Canadian Journal of Fisheries and Aquatic Sciences, 51(8): 1692-1699.
DOI:10.1139/f94-170 |
Li Y P, Hua L, Wang P F, Wang C, Tan Y F. 2013. Responses of hydrodynamical characteristics to climate conditions in a channel-type reservoir. Journal of Lake Sciences, 25(3): 317-323.
(in Chinese with English abstract) DOI:10.18307/2013.0301 |
Lin S J, He L J, Huang P S, Han B P. 2005. Comparison and improvement on the extraction method for chlorophyll a in phytoplankton. Ecologic Science, 24(1): 9-11.
(in Chinese with English abstract) DOI:10.3969/j.issn.1008-8873.2005.01.003 |
Liu S H. 2008. Effects of Plant Functional Group Diversity on Productivity in Experimental Communities. Lanzhou University, Lanzhou. (in Chinese with English abstract)
|
Loreau M, Naeem S, Inchausti P, Bengtsson J, Grime J P, Hector A, Hooper D U, Huston M A, Raffaelli D, Schmid B, Tilman D, Wardle D A. 2001. Biodiversity and ecosystem functioning: current knowledge and future challenges. Science, 294(5543): 804-808.
DOI:10.1126/science.1064088 |
Luding J A, Reynolds J F. 1988. Statistical Ecology: A Primer on Methods and Computing. John Wiley and Sons, New York.
|
MacArthur R. 1955. Fluctuations of animal populations and a measure of community stability. Ecology, 36(3): 533-536.
DOI:10.2307/1929601 |
Padisák J, Barbosa F, Koschel R, Krienitz L. 2003. Deep layer cyanoprokaryota maxima in temperate and tropical lakes. Archiv für Hydrobiologie Special Issues Advances in Limnology, 58: 175-199.
|
Padisák J, Crossetti L O, Naselli-Flores L. 2009. Use and misuse in the application of the phytoplankton functional classification: a critical review with updates. Hydrobiologia, 621(1): 1-19.
DOI:10.1007/s10750-008-9645-0 |
Pennekamp F, Pontarp M, Tabi A, Altermatt F, Alther R, Choffat Y, Fronhofer E A, Ganesanandamoorthy P, Garnier A, Griffiths J I, Greene S, Horgan K, Massie T M, Mächler E, Palamara G M, Seymour M, Petchey O L. 2018. Biodiversity increases and decreases ecosystem stability. Nature, 563(7729): 109-112.
DOI:10.1038/s41586-018-0627-8 |
Pimm S L. 1984. The complexity and stability of ecosystems. Nature, 307(5949): 321-326.
DOI:10.1038/307321a0 |
Ptacnik R, Solimini A G, Andersen T, Tamminen T, Brettum P, Lepistö L, Willén E, Rekolainen S. 2008. Diversity predicts stability and resource use efficiency in natural phytoplankton communities. Proceedings of the National Academy of Sciences of the United States of America, 105(13): 5134-5138.
DOI:10.1073/pnas.0708328105 |
Redfield A C, Ketchum B H, Richards F A. 1963. The influence of organisms on the composition of seawater. In: Hill M N ed. The Sea. Interscience, New York. p. 26-77.
|
Reynolds C S, Huszar V, Kruk C, Naselli-Flores L, Melo S. 2002. Towards a functional classification of the freshwater phytoplankton. Journal of Plankton Research, 24(5): 417-428.
DOI:10.1093/plankt/24.5.417 |
Reynolds C S. 1984. The Ecology of Freshwater Phytoplankton. Cambridge University Press, Cambridge, UK.
|
Rodríguez-Rodríguez M, Moreno-Ostos E, De Vicente I, Cruz-Pizarro L, Da Silva S L R. 2004. Thermal structure and energy budget in a small high mountain lake: La Caldera, Sierra Nevada, Spain. New Zealand Journal of Marine and Freshwater Research, 38(5): 879-894.
DOI:10.1080/00288330.2004.9517287 |
Rothenberger M B, Burkholder J M, Wentworth T R. 2009. Use of long-term data and multivariate ordination techniques to identify environmental factors governing estuarine phytoplankton species dynamics. Limnology and Oceanography, 54(6): 2107-2127.
DOI:10.4319/lo.2009.54.6.2107 |
Sennhauser E B. 1991. The concept of stability in connection with the gallery forests of the Chaco region. Vegetatio, 94(1): 1-13.
DOI:10.1007/BF00044911 |
Silva A, Mendes C R, Palma S, Brotas V. 2008. Short-time scale variation of phytoplankton succession in Lisbon Bay (Portugal) as revealed by microscopy cell counts and HPLC pigment analysis. Estuarine, Coastal and Shelf Science, 79(2): 230-238.
DOI:10.1016/j.ecss.2008.04.004 |
Smith V H. 2007. Microbial diversity-productivity relationships in aquatic ecosystems. FEMS Microbiology Ecology, 62(2): 181-186.
DOI:10.1111/j.1574-6941.2007.00381.x |
Straškraba M, Tundisi J G, Duncan A. 1993. Comparative Reservoir Limnology and Water Quality Management. Springer, Dordrecht, Netherlands.
|
State Environmental Protection Administration. 2002. China's National Standard GB 3838-2002.
|
Striebel M, Behl S, Stibor H. 2009. The coupling of biodiversity and productivity in phytoplankton communities: consequences for biomass stoichiometry. Ecology, 90(8): 2025-2031.
DOI:10.1890/08-1409.1 |
Sun J, Liu D Y, Qian S B. 1999. Study on phytoplankton biomass. Ⅰ. Phytoplankton measurement biomass from cell volume or plasma volume. Acta Oceanologica Sinica, 21(2): 75-85.
(in Chinese with English abstract) |
Sun W X, Wu D J, Pei H Y, Wei J L, Zhang S S, Wang Y T. 2019. Phytoplankton community structure and environmental factors in a newly built reservoir, Shandong Province. Journal of Lake Sciences, 31(3): 734-745.
(in Chinese with English abstract) DOI:10.18307/2019.0312 |
Thibaut L M, Connolly S R. 2013. Understanding diversitystability relationships: towards a unified model of portfolio effects. Ecology Letters, 16(2): 140-150.
DOI:10.1111/ele.12019 |
Tian W, Zhang H Y, Zhao L, Xu X, Huang H. 2016. Plankton community stability and its relationship with phytoplankton species richness in Lake Nansihu, China. Water, 8(10): 454.
DOI:10.3390/w8100454 |
Tian W. 2017. Response Mechanisms of Lake Plankton Community to Environment and Biodiversity. North China Electric Power University, Beijing, China. (in Chinese with English abstract)
|
Tilman D, Reich P B, Knops J M H. 2006. Biodiversity and ecosystem stability in a decade-long grassland experiment. Nature, 441(7093): 629-632.
DOI:10.1038/nature04742 |
Tilman D. 1996. Biodiversity: population versus ecosystem stability. Ecology, 77(2): 350-363.
DOI:10.2307/2265614 |
Turnbull L A, Levine J M, Loreau M, Hector A. 2013. Coexistence, niches and biodiversity effects on ecosystem functioning. Ecology Letters, 16: 116-127.
DOI:10.1111/ele.12056 |
Valentine J W. 1971. Resource supply and species diversity patterns. Lethaia, 4(1): 51-61.
DOI:10.1111/j.1502-3931.1971.tb01278.x |
Venail P A, Alexandrou M A, Oakley T H, Cardinale B J. 2013. Shared ancestry influences community stability by altering competitive interactions: evidence from a laboratory microcosm experiment using freshwater green algae. Proceedings of the Royal Society B: Biological Sciences, 280(1768): 20131548.
DOI:10.1098/rspb.2013.1548 |
Vörös L, Padisák J. 1991. Phytoplankton biomass and chlorophyll-a in some shallow lakes in central Europe. Hydrobiologia, 215(2): 111-119.
DOI:10.1007/BF00014715 |
Waide R B, Willig M R, Steiner C F, Mittelbach G, Gough L, Dodson S I, Juday G P, Parmenter R. 1999. The relationship between productivity and species richness. Annual Review of Ecology and Systematics, 30(1): 257-300.
DOI:10.1146/annurev.ecolsys.30.1.257 |
Wittebolle L, Marzorati M, Clement L, Balloi A, Daffonchio D, Heylen K, De Vos P, Verstraete W, Boon N. 2009. Initial community evenness favours functionality under selective stress. Nature, 458(7238): 623-626.
DOI:10.1038/nature07840 |
Xu J R, Qiao L, Han B P, Lin Q Q. 2016. Response of cladoceran assemblage to inter-basin water transfer in cascading pump reservoir systems. Chinese Journal of Applied and Environmental Biology, 22(2): 313-319.
(in Chinese with English abstract) DOI:10.3724/SP.J.1145.2015.10039 |
Yan L, Luo H, Chen H X, Wu Q, Zhang Y L. 2020. An analysis of the correlation between aquatic community structure and water quality in Jiuquwan Reservoir. China Rural Water and Hydropower, (8): 56-61.
(in Chinese with English abstract) |
Yang L M, Zhou G S, Li J D. 2002. Relationship between productivity and plant species diversity of grassland communities in Songnen Plain of northeast China. Chinese Journal of Plant Ecology, 26(5): 589-593.
(in Chinese with English abstract) |
Ye L, Chang C W, Matsuzaki S I S, Takamura N, Widdicombe C E, Hsieh C H. 2019. Functional diversity promotes phytoplankton resource use efficiency. Journal of Ecology, 107(5): 2353-2363.
DOI:10.1111/1365-2745.13192 |
Zhang B C, Li F M, Huang G B. 2006a. Effects of biodiversity on functions and stability of ecosystem. Chinese Journal of Eco-Agriculture, 14(4): 12-15.
(in Chinese with English abstract) |
Zhang Y W, Li H, Li J, Song Y, Zhang L L, Li Y, Pu X H, Huang W D. 2018. Spatiotemporal succession characteristics of algal functional groups and its impact factors for a typical Channel-type reservoir in a southwest Mountainous area. Environmental Science, 39(6): 2680-2687.
(in Chinese with English abstract) DOI:10.13227/j.hjkx.201709163 |
Zhang Y, Zheng B H, Fu G, Liu H L. 2006b. On the assessment methodology and standards for nutrition status in channel type of reservoirs based on zoning eutrophication sensitivity. Acta Scientiae Circumstantiae, 26(6): 1016-1021.
(in Chinese with English abstract) DOI:10.3321/j.issn:0253-2468.2006.06.024 |
 2022, Vol. 40
2022, Vol. 40