Institute of Oceanology, Chinese Academy of Sciences
Article Information
- ZHAO Xinyu, ZHONG Yi, ZHANG Huanxin, QU Tongfei, HOU Chengzong, GUAN Chen, LIU Feng, TANG Xuexi, WANG Ying
- Comparison of environmental responding strategies between Ulva prolifera and Sargassum horneri: an in-situ study during the co-occurrence of green tides and golden tides in the Yellow Sea, China in 2017
- Journal of Oceanology and Limnology, 39(6): 2252-2266
- http://dx.doi.org/10.1007/s00343-021-0397-2
Article History
- Received Oct. 19, 2020
- accepted in principle Nov. 24, 2020
- accepted for publication Jan. 5, 2021
2 Qingdao National Laboratory for Marine Science and Technology, Qingdao 266237, China;
3 College of Geography and Environment, Shandong Normal University, Jinan 250000, China;
4 CAS Key Laboratory of Marine Ecology and Environmental Sciences, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China
In the 1970s, the biomass of macroalgae along the coast of industrialized countries began to increase. By the 1990s, macroalgae had become a public hazard in many coastal areas. Harmful algal blooms caused by large seaweeds around the world increased during the 2000 (Ye et al., 2011). Although macroalgae does not produce toxic substances by themselves, they can cause many serious effects. The macroalgae can block the channel. Large-scale expansion of macroalgae can cause damage to the ecological balance of estuarine communities, and may cause economic losses to fisheries and tourism (Keesing et al., 2016).
Harmful algal blooms caused by macroalgae show a significant impact on the Yellow Sea. Large-scale green tides occurred for thirteen consecutive years since 2007. The occurrence of green tides in the Yellow Sea has typical characteristics. The origin of green tides is in the southern Yellow Sea, while the location of the bloom is in the northern Yellow Sea (Liu et al., 2010). Long-duration of floating drift is the typical characteristic of green tides. The causative species of green tides in the Yellow Sea is Ulva prolifera (Ye et al., 2008). Large-scale floating Sargassum horneri were observed at the site during the Yellow Sea green tides outbreak in 2017 (Liu et al., 2018). The emergence of a large-scale floating S. horneri caused a new kind of harmful algal bloom (golden tides). The green tides and golden tides always occur separately. The unusual co-occurrence of the green tides and golden tides could be observed in the Yellow Sea in 2017, which increased the difficulty of monitoring and controlling of harmful algal blooms.
Different species of macroalgae show different environmental responding strategies. Cyclic electron flow (CEF), non-photochemical quenching (NPQ), and antioxidant system are important environmental responding strategies in U. prolifera. CEF can enhance the desiccation tolerance of Ulva sp. (Gao et al., 2011). Photosystem Ⅰ (PSI) shows a higher tolerance to osmotic stress than photosystem Ⅱ (PSII) in U. prolifera (Gao et al., 2014). U. prolifera can utilize CO2 in the atmosphere directly for rapid growth (Huan et al., 2016). NPQ plays an important role in protecting U. prolifera under high light stress (Mou et al., 2013). Antioxidant system also plays an important role in protecting U. prolifera under the environmental stress (Wang et al., 2012). These characteristics enhance the photosynthetic plasticity and environmental adaptability of U. prolifera. Many studies have also studied the response characteristics of S. horneri. Moderately elevated temperatures, especially under elevated CO2 levels at the same time, could significantly enhance the growth of S. horneri (Li et al., 2020). The demand of S. horneri for nitrogen increased in spring, and the high temperature and nitrogen concentration in spring leaded to the high biomass accumulation of drifting thalli (Yu et al., 2019). The existing researches were finished in the laboratory, and there was no in-situ comparative study on the physiological response characteristics of U. prolifera and S. horneri. The in-situ comparative study about U. prolifera and S. horneri is meaningful.
In this study, the in-situ environmental indicator parameters were monitored. We also measured the in-situ chlorophyll fluorescence and pigment changes in U. prolifera and S. horneri. Furthermore, we compared the antioxidant capacity of the two kinds of macroalgae, and the relationship among the development of green tides & golden tides and the environmental factors were analyzed.
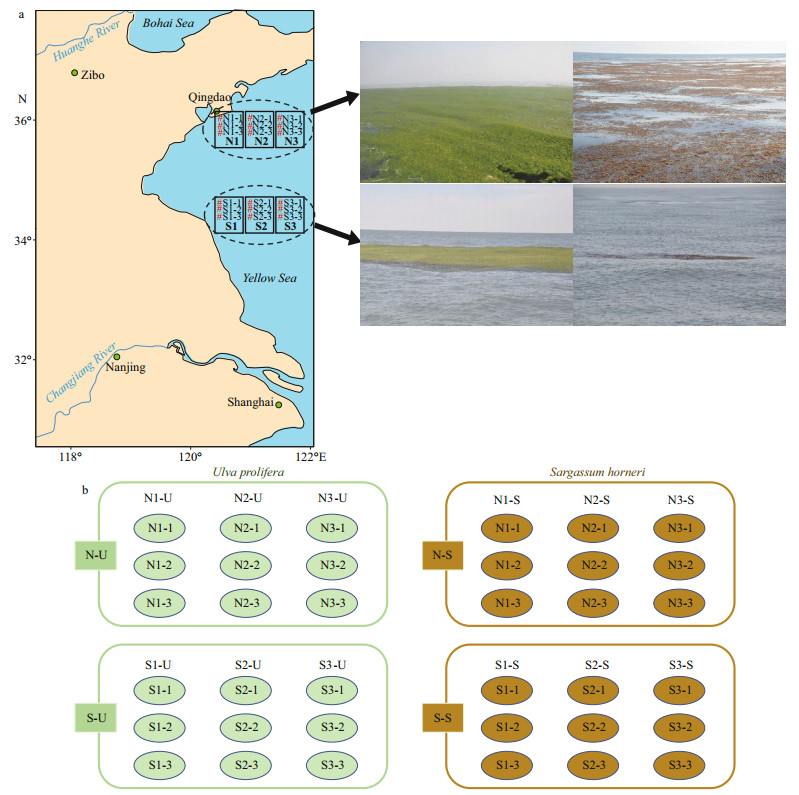
2 MATERIAL AND METHOD 2.1 Study area and field samplingThree sampling locations were distributed along each of the two transects (34°25′N–36°00′N) in the Southern Yellow Sea between 120°30′E–121°30′E. Three replicates of both U. prolifera (N-U and S-U) and S. horneri (N-S and S-S) were sampled at each site, which resulted in eighteen samples (N1-1, N1-2, N1-3, N2-1, N2-2, N2-3, N3-1, N3-2, N3-3, S1-1, S1-2, S1-3, S2-1, S2-2, S2-3, S3-1, S3-2, and S3-3) in total for each species (Fig. 1; Table 1).
|
| Fig.1 The sampling deployments a. sampling locations of six sampling areas (N1, N2, N3, S1, S2 and S3) and 18 corresponding sampling sites (N1-1, N1-2, N1-3, N2-1, N2-2, N2-3, N3-1, N3-2, N3-3, S1-1, S1-2, S1-3, S2-1, S2-2, S2-3, S3-1, S3-2, and S3-3) where three replicates were sampled at each site; b. sampling design of this research. |
The thalli were cleaned gently with a brush and sterile seawater to remove any attached sediment, small grazers, and epiphytes. The samples were used directly for measuring chlorophyll fluorescence. The thalli were then placed in a freezer at -80 ℃ for further study. In-situ salinity and temperature were measured by using an YSI Professional Pro Meter (YSI Inc., United States). Seawater samples were collected by using Niskin bottles, which were then filtered through precombusted (460 ℃, 6 h) Whatman GF/F filters. The filtrates were collected using polyethylene bottles at -20 ℃ for nutrient analysis. The concentrations of NO3- and NO2- were determined by using indophenol blue method, and concentration of NH4+ was measured using indophenol blue method. The concentration of PO43--P was measured by using molybdate blue method. Dissolved inorganic nitrogen (DIN) was obtained by calculating of the sum of NO3-, NO2-, and NH4+. Total dissolved nitrogen (TDN) and phosphorus (TDP) were measured through persulfate oxidation. The concentration of dissolved organic nitrogen (DON) was calculated based on the concentrations of TDN and DIN, and the concentration of dissolved organic phosphorus (DOP) was calculated based on those of TDP and PO43--P (Grasshoff et al., 1999).
2.2 Pigment extractionSamples of macroalgae were ground in liquid nitrogen, and then 90% (v/v) acetone buffer was used to extract the pigments. The mixture was centrifuged at 10 000×g at 4 ℃, and the supernatant was extracted and conserved for further analyses. To determine the chlorophyll and carotenoid contents, a TU-1800 ultraviolet-visible spectrophotometer (PERSEE, China) was used. The optical densities (OD), i.e., OD470, OD645, and OD663, were measured separately. The following equations were used to obtain the concentrations of chlorophyll a (Chl a), chlorophyll b (Chl b), total chlorophyll (total Chl), and carotenoid (Car) contents:
 (1)
(1)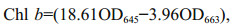 (2)
(2) (3)
(3)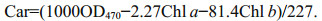 (4)
(4)All experiments were performed as three replicates; the units of the results are given as milligrams per gram (mg/g) of fresh weight (FW) (Arnon, 1949).
2.3 Measurement of chlorophyll fluorescenceA Dual-PAM-100 fluorometer (Walz, Germany) was used to measure the chlorophyll fluorescence and P700 parameters. Before the experiments, the thalli were placed in the dark for 15 min. The settings of the Dual-PAM-100 fluorometer were as described in Zhao et al. (2016). The automated induction, recovery curve routine, and repetitive application of saturation pulses were used to obtain the results of chlorophyll fluorescence. After the saturating pulse, F0 and Fm were determined. F0 is the minimal fluorescence after dark acclimation. Fm is the maximal fluorescence after saturation flashes in the dark-acclimatized sample. The parameter Fv/Fm (PSII maximum quantum yield) was calculated by the equation: Fv/Fm=(Fm−F0)/Fm, which represent the PSII maximum quantum yield in the thalli. Y(Ⅱ) is the effective quantum yield of PSII, which can also be used for evaluating the photosynthetic activity in U. prolifera. Y(NPQ) (quantum yield of regulated non-photochemical energy loss) and Y(NO) (quantum yield of non-regulated energy loss) represent the negative effects on U. prolifera from environmental stress. Y(Ⅰ) can represent the effective quantum yield of PSI. The ratio of the effective quantum yield of PSI can represent the energy distribution between PSI and PSII (Huang et al., 2013). Furthermore, 3-(30, 40-dichlorophenyl)-1, 1-dimethylurea (DCMU) was used for determining the PSI-driven CEF in the thalli. DCMU is a kind of inhibitor, which can abolish the linear electron flow (LEF) (Joët et al., 2002). ETR (Ⅰ) (photosynthetic electron transport rate of PSI) (treated with DCMU) and ETR (Ⅰ) (no inhibitor) were determined by us, and the ETR (Ⅰ) (treated with DCMU)/ETR (Ⅰ) (no inhibitor) was calculated for studying the ratios of cyclic electron flow to total electron flow.
2.4 Analysis of lipid peroxidation and H2O2 contentThalli samples of 0.1 g FW were ground in liquid nitrogen, to which 1-mL 5% (w/v) trichloroacetic acid (TCA) was added. The mixture was then centrifuged at 12 000×g for 10 min at 4 ℃. The content of H2O2 was determined using H2O2 kits according to the manufacturer's instructions (Nanjing Jiancheng, China), and the level of lipid peroxidation (MDA) was determined using thiobarbituric acid (TBA)-reacting substance contents (Buege and Aust, 1978).
2.5 Determination of antioxidant enzyme activityThallus samples of 0.1 g FW were ground in liquid nitrogen and then extracted with 1 mL of 0.05-mol/L potassium phosphate buffer (pH 7.0). The mixture was centrifuged at 12 000×g for 10 min at 4 ℃. The supernatant was used for measuring the total soluble protein (TSP) and the activities of antioxidant enzymes. The TSP content was determined using the Coomassie blue dye binding assay (Bradford, 1976). The activities of the antioxidant enzymes superoxide dismutase (SOD), catalase (CAT), and guaiacol peroxidase (GPX) were measured using commercial kits, following the manufacturer's instructions (Nanjing Jiancheng, China).
2.6 Statistical analysisAll values in this research were obtained from random collected samples. The mean values and standard deviations (mean±SD) were calculated for the replicates of each treatment condition (n=3). The results are tested in a one-way ANOVA using SPSS 22.0 statistical software (IBM Corp., United States), and Bivariate Pearson's correlation analysis was performed to assess the relationships of the parameters for photosynthetic and antioxidant systems in U. prolifera and S. horneri. Data were examined with Levene's test for homogeneity and the Shapiro-Wilk test for normality. Student-Newman-Keuls post-hoc multiple comparison test and Duncan post-hoc test were used if ANOVA indicated a significant effect. Differences were judged to be significant at P < 0.05 and P < 0.01, respectively. The figures included in this study were generated using SigmaPlot 12.5 software (Systat Software Inc., USA).
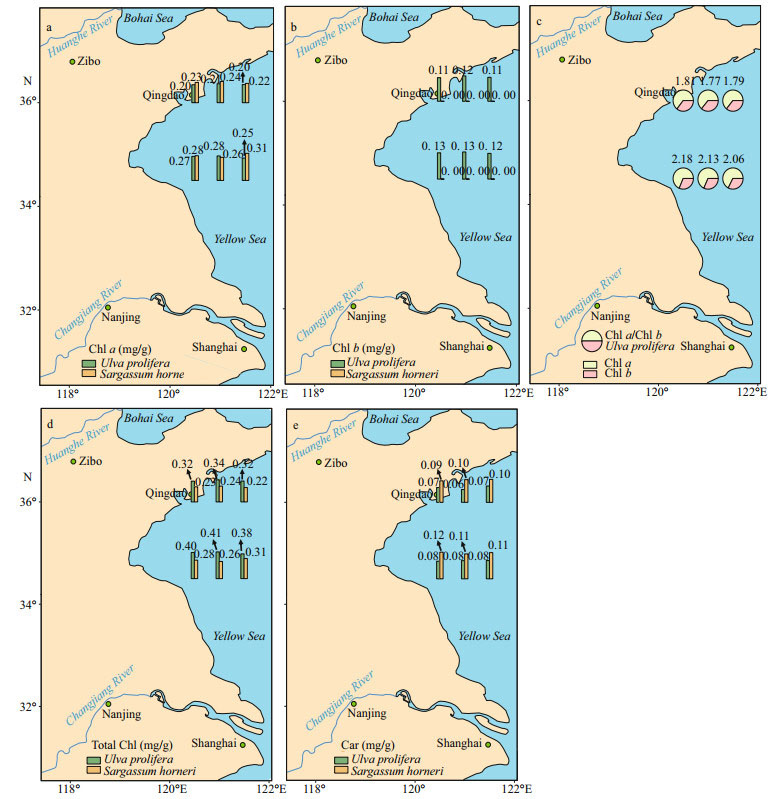
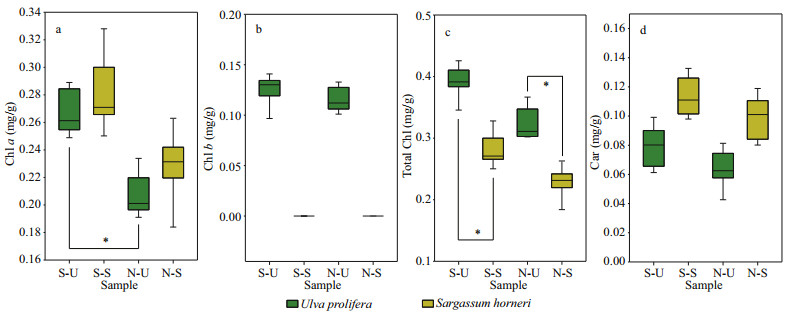
3 RESULT 3.1 Pigment analysisChlorophyll a showed significant sampling locality and latitude effects; Chl b, total Chl, and Car showed significant species effect (Table 2). Figures 2 & 3 show the contents of pigment in two kinds of macroalgae at each site. There was no significant difference in the content of Chl a in the same kind of macroalgae at the same latitude (Fig. 2a; one-way ANOVA, P > 0.05). Similar trend could also be observed in Chl b, total Chl, and Car (Fig. 2b, d, & e). For Chl a, no significant difference was seen between S-U and S-S or between N-U and N-S (Fig. 3a; one-way ANOVA, P > 0.05). No significant difference in Chl a was seen between S-S and N-S (Fig. 3a; one-way ANOVA, P > 0.05), while the content of Chl a in S-U was significantly higher than that of N-U (Fig. 3a; one-way ANOVA, P < 0.05). There was no significant difference in Chl b between S-U and N-U (one-way ANOVA, P > 0.05), and Chl b could not be measured in S-S and N-S (Fig. 3b). No significant difference in total Chl was observed between S-U and N-U or S-S and N-S (Fig. 3c; one-way ANOVA, P > 0.05). Values of total Chl in S-U and N-U were significantly higher than those of S-S and N-S, respectively (Fig. 3c; one-way ANOVA, P < 0.05). There was no significant difference in Car between S-U and N-U or S-S and N-S (Fig. 3d; one-way ANOVA, P > 0.05). No significant difference could be observed between S-U and S-S or N-U and N-S (Fig. 3d; one-way ANOVA, P > 0.05).
|
| Fig.2 Horizontal distribution of the parameters a. Chl a; b. Chl b; c. Chl a/Chl b; d. total Chl; e. Car in the Yellow Sea in July 2017. |
|
| Fig.3 Box plot of all the parameters a. Chl a; b. Chl b; c. total Chl; d. Car. The mean results of three independent experiments (±SD) are shown. *: significant difference between the different groups (P < 0.05). |
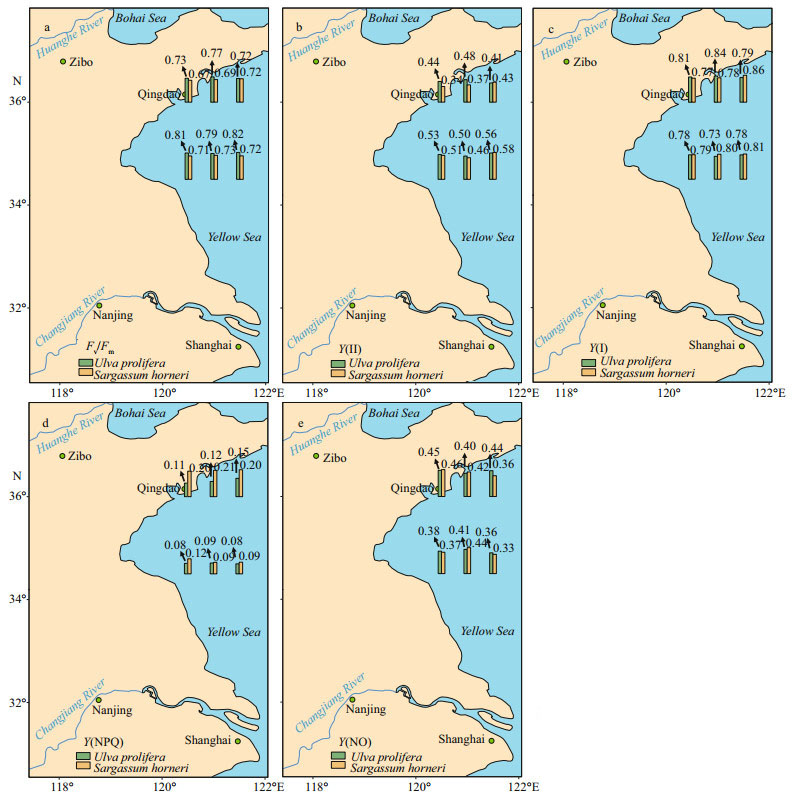
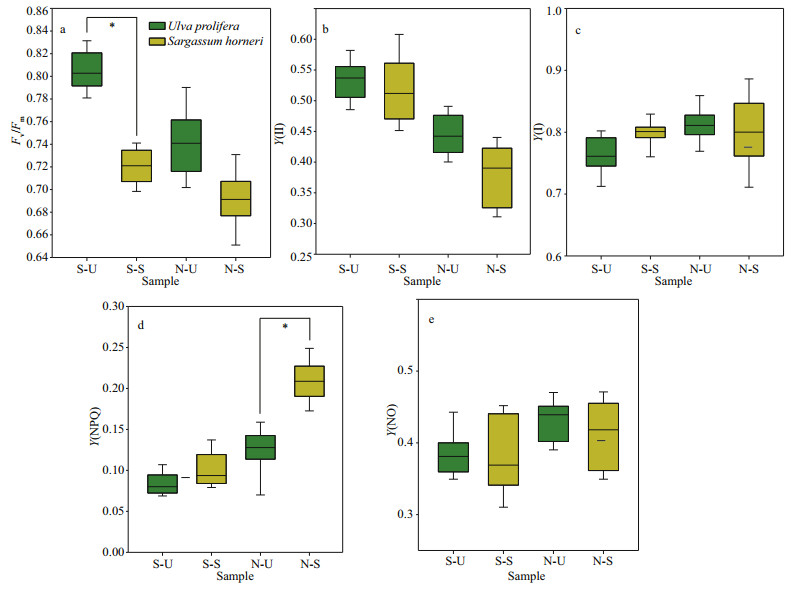
Fv/Fm and Y(NPQ) showed significant species, sampling locality, and latitude effects, and there was not significant effect of Y(Ⅱ), Y(NO), and ETR (Ⅰ) (treated with DCMU)/ETR (Ⅰ) (no inhibitor) in species (Table 2). The values of chlorophyll fluorescence could be seen in Figs. 4 & 5. Fv/Fm of S3-U and N3-S were significantly higher than those of S2-U and N1-S, respectively (Fig. 4a; one-way ANOVA, P < 0.05). The values of Y(Ⅱ) in S. horneri in the eastern part was higher than those of western part and central part, while no similar results could be observed in U. prolifera (Fig. 4b). There was no remarkable difference among the S-U, S-S, N-U, and N-S in Y(Ⅰ) (Fig. 5c; one-way ANOVA, P > 0.05). Y(NPQ) of N3-U was significantly higher than that of N1-U (Fig. 4d; one-way ANOVA, P < 0.05). Y(NO) of S2-U, S1-U, S3-U significantly decreased in turn. The ascending sequence of Y(NO) was S3-S < S1-S < S2-S. Y(NO) of N1-U and N3-U were significantly higher than that of N2-U, and Y(NO) of N1-S, N2-S, N3-S general ranked N3-S < N2-S < N1-S (Fig. 4e; one-way ANOVA, P < 0.05). There was no significant difference in Y(NO) between S-U, S-S, N-U, and N-S (Fig. 5e; one-way ANOVA, P > 0.05). During the outbreak of harmful algal bloom, the CEF activities of S. horneri were significantly higher than those of U. prolifera, and the CEF activities of the thalli located north of 35°N were higher than those of the southern thalli (Table 3).
|
| Fig.4 Horizontal distributions of the parameters in the Yellow Sea in July 2017 a. Fv/Fm; b. Y(Ⅱ); c. Y(Ⅰ); d. Y(NPQ); e. Y(NO). |
|
| Fig.5 Box plot of photosynthetic parameters and the mean (±SD) of three independent experiments a. Fv/Fm; b. Y(Ⅱ); c. Y(Ⅰ); d. Y(NPQ); e. Y (NO). *: significant difference between the different groups (P < 0.05). |
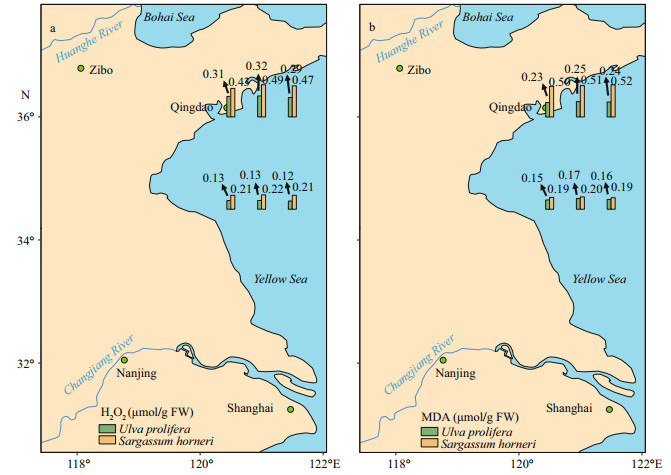
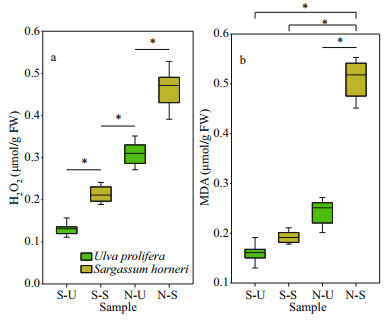
Both H2O2 and MDA showed significant species, sampling locality, and latitude effects (Table 2). There was no significant difference in the content of H2O2 and MDA in the same kind of macroalgae at the same latitude (Fig. 6; one-way ANOVA, P > 0.05). The ascending sequence of content of H2O2 was S-U < S-S < N-U < N-S (Fig. 7a; one-way ANOVA, P < 0.05). Content of MDA in the thalli of N-S was significantly higher than those of S-U, S-S and N-U (Fig. 7b; one-way ANOVA, P < 0.05).
|
| Fig.6 Horizontal distribution of the H2O2 (a) and MDA (b) in Ulva prolifera and Sargassum horner in the Yellow Sea in July 2017 |
|
| Fig.7 Box plot of the H2O2 (a) and MDA (b) with the mean (±SD) of three independent experiments *: significant difference between the different groups (P < 0.05). |
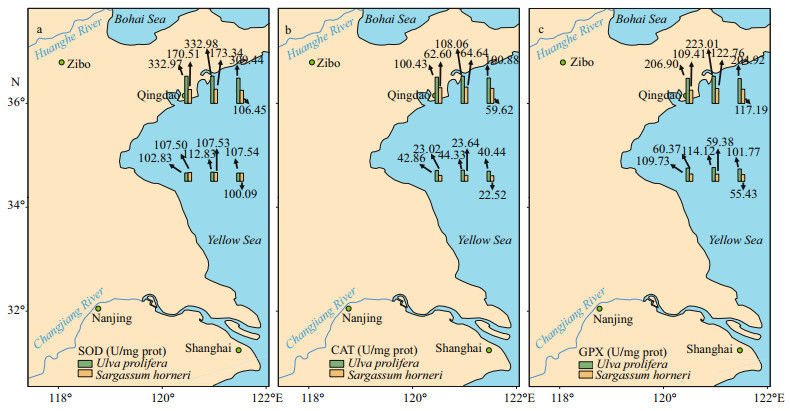
There were significant effects of SOD, CAT, and GPX in species, sampling locality, and latitude (Table 2). No significant difference in activities of SOD, CAT, and GPX in the same kind of macroalgae at the same latitude could be seen in Fig. 8 (one-way ANOVA, P > 0.05). Activity of SOD in the thalli of N-U was significantly higher than that of N-S, and that of N-S was significantly higher than those of S-U and S-S (Fig. 9a; one-way ANOVA, P < 0.05). Activity of CAT in the thalli of S-U was significantly higher than that of S-S, and activity of CAT of N-U was significantly higher than that of N-S (Fig. 9b; one-way ANOVA, P < 0.05). The trend similar to Fig. 9b could also be seen in Fig. 9c.
|
| Fig.8 Horizontal distribution of SOD (a), CAT (b), and GPX (c) in Ulva prolifera and Sargassum horner in the Yellow Sea in July 2017 |

|
| Fig.9 Box plot of SOD (a), CAT (b), and GPX (c) with the mean (±SD) of three independent experiments *: significant difference between the different groups (P < 0.05). |
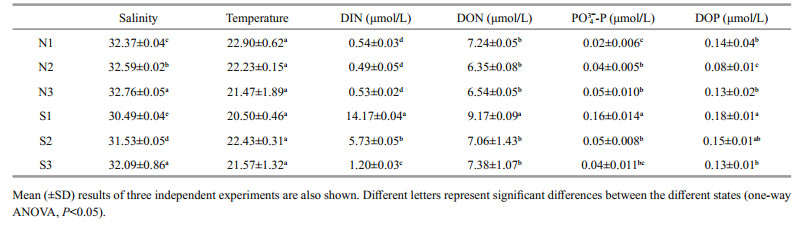
There were significant effects of salinity, DIN, DON, and DOP in species, sampling locality, and latitude but no significant difference of temperature in sampling locality and latitude (Table 2). The results of salinity, temperature, and nutrients are summarized in Table 4. Horizontal distribution of salinity during the harmful algal bloom was significantly variable among sites at the same latitude. There was no significant difference in temperature among different sites. The results of DIN at southern 3 sites were significantly higher than those of northern sites, and ascending sequence of DIN at southern sites was S1 < S2 < S3 (one-way ANOVA, P < 0.05). Furthermore, the values of DIN at southern sites were significantly higher than those of northern parts (one-way ANOVA, P < 0.05). The result of DON at site of S1 was significantly higher than the others (one-way ANOVA, P < 0.05), and no remarkable difference could be seen among the values of DON at sites of N1, N2, N3, S2, and S3 (one-way ANOVA, P > 0.05). At southern 3 sites value of PO43--P in S1 was the highest (0.16) followed by that of S2 (0.05) and S3 (0.04). The values of PO43--P at site of N2 and N3 were 0.04 and 0.05, which were significantly higher than that of N1 (one-way ANOVA, P < 0.05). The ascending sequence of DOP at southern sites was S3 < S2 < S1, and the ascending sequence of DOP at northern sites was N2 < N3 < N1.
Ulva prolifera and S. horneri are the causative species of green tides and golden tides in the Yellow Sea (Ye et al., 2008; Liu et al., 2018). Previous studies have done a lot on the environmental responding strategies of these two kinds of macroalgae, while these studies are mainly finished in the laboratories. Furthermore, green tides occurred for consecutive years in the Yellow Sea, while the golden tides only co-occurred with the green tides in the Yellow Sea since 2016. It is necessary to compare the environmental strategies of the two kinds of macroalgae.
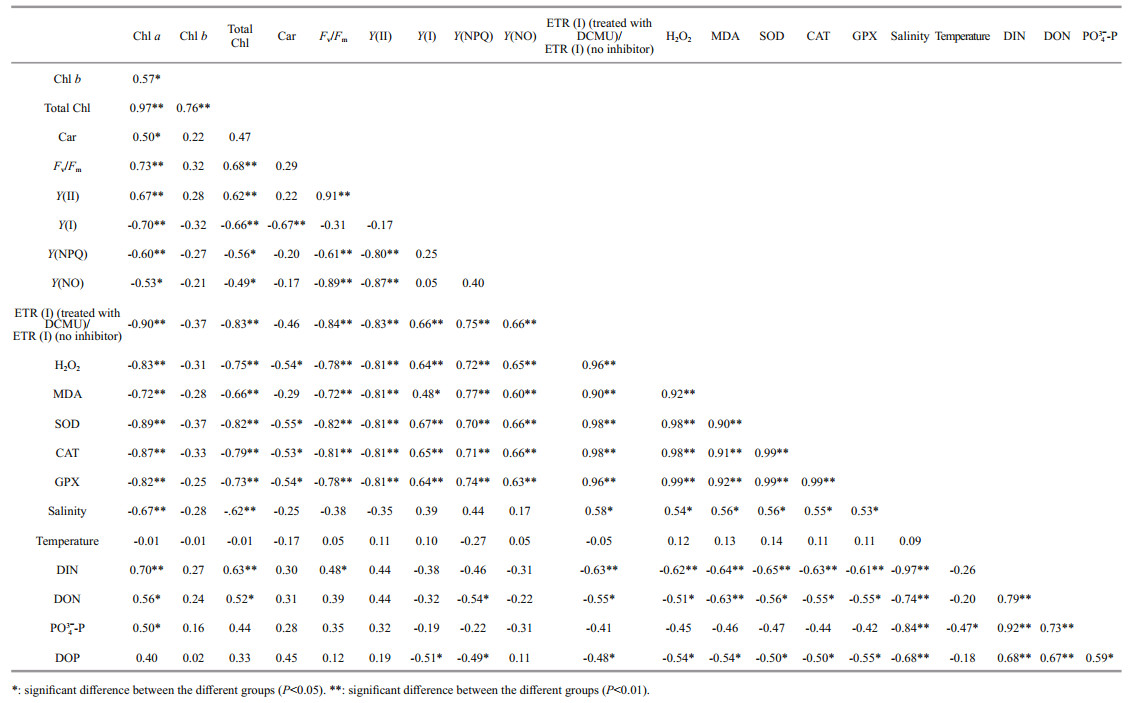
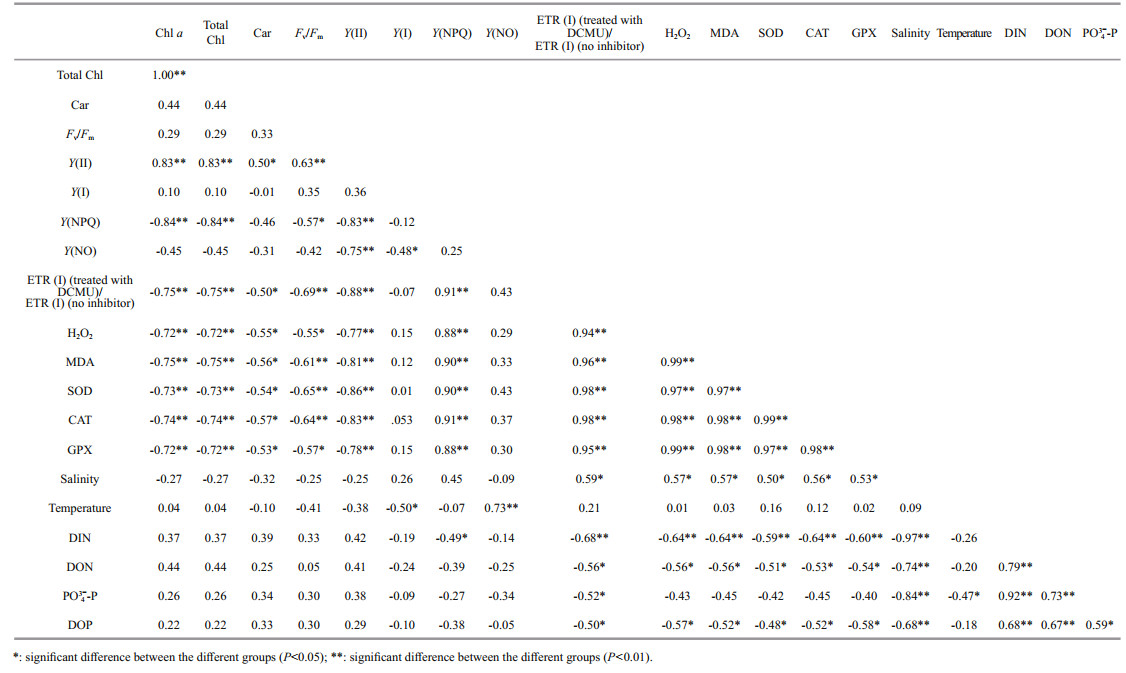
4.1 Comparison of the environmental responding strategies in U. prolifera and S. horneriDuring blooms, large-scale macroalgae gathered and rapidly expanded (Liu et al., 2015, 2018). Both the floating U. prolifera and S. horneri show strong photosynthetic adaptability for the rapidly expanding of the thalli. NPQ is a crucial photosynthetic mechanism that exists in macroalgae for adapting to the complex and changeable environment (Li et al., 2009). In the present study, the photosynthetic activity of the thalli decreased during the northward drift process, which still maintain a relatively high level (Figs. 4–5). The photosynthetic activity of the floating U. prolifera is always higher than that of floating S. horneri during its northward drift process, and the photosynthetic activity of U. prolifera and S. horneri wasn't damaged significantly during the northward drift process (Fig. 5). NPQ plays a key role in U. prolifera and S. horneri for adapting to the environment, and NPQ plays a more important role in S. horneri compared to U. prolifera, especially at the northern 3 sites (Fig. 5). There is also a remarkable correlation between Y(NO) and ETR (Ⅰ) (treated with DCMU)/ETR (Ⅰ) (no inhibitor), which means the important role of CEF under extreme environment in U. prolifera rather than in S. horneri (Tables 5–6).
|
|
Pigment composition also plays an important role in macroalgae environmental adaptation (Praba et al., 2011). The change trend of Chl-a content in U. prolifera was the same as that of S. horneri during the northward drift process (Figs. 2–3). Chl-b content in U. prolifera does not change significantly during the northward drift process, while Chl b was not detected in S. horneri (Figs. 2–3). The total Chl content in U. prolifera was always significantly higher than that in S. horneri. The total Chl content in U. prolifera does not change significantly, while total Chl content in S. horneri decreases significantly during the northward drift process (Figs. 2–3). Furthermore, a significant correlation among Fv/Fm, Chl a, and total Chl could be found in U. prolifera rather than in S. horneri (Tables 5–6). Chl b was not detected in S. horneri as it did not exist in Phaeophytes (Larkum et al., 2012). The Car content in floating S. horneri was always higher than that of U. prolifera, and the Car content in the two kinds of macroalgae does not change significantly during the northward drift process (Fig. 3).
MDA content is the parameter of lipid peroxidation, and higher H2O2 content can lead to higher lipid peroxidation (Luo and Liu, 2011). The H2O2 content in the two kinds of macroalgae increase significantly during the northward drift process, and H2O2 content in S. horneri was always significantly higher than that in U. prolifera (Fig. 7a). MDA content in the two kinds of macroalgae also increases significantly during the northward drift process (Fig. 7b). There was no significant difference in MDA content between the two kinds of macroalgae at the southern 3 sites, while the MDA content in S. horneri was significantly higher than that in U. prolifera at the northern 3 sites (Fig. 7b). Furthermore, a significant positive correlation could be observed among H2O2, MDA and Y(NO), which confirmed the close relationship of higher levels of H2O2 and MDA with irreversible damage in U. prolifera (Tables 5–6).
SOD, CAT, and GPX are all important antioxidant enzymes in plants (Imlay, 2003). The activities of CAT and GPX in U. prolifera were significantly higher than those of S. horneri at the south of 35°N, while there was no significant difference in SOD activity between the two macroalgae (Fig. 9). The activities of SOD, CAT, and GPX in the two kinds of macroalgae increase significantly during the northward drift process, and the activities of the four kinds of enzymes in U. prolifera are significantly higher than those in S. horneri (Fig. 9). The significant positive correlation among SOD, CAT, GPX, and Y(NO) could be observed in U. prolifera, which further confirmed more important roles of antioxidant system in U. prolifera than S. horneri (Tables 5–6).
4.2 Relationship between environmental factors and responding strategies in macroalgaeThe biomass and size of floating harmful algal blooms increased because of apposite temperature and nutrient richness (Shi et al., 2015; Li et al., 2017; Wu et al., 2018). Temperature is an important factor for affecting the scale, floating paths, and distribution area of harmful algal blooms (Fan et al., 2014; Song et al., 2015). Nutrient also influenced the forming of harmful algal blooms. Excess inorganic nutrients could be absorbed and utilized by U. prolifera (Li et al., 2019). Higher phosphate levels could increase the relative growth rate and the nitrate uptake rate of Sargassum muticum (Xu et al., 2017). Furthermore, Zhang et al. (2020) confirmed that DIN and DOP were main nutrients for algae. The increasingly serious eutrophication in the estuaries along the coast of Jiangsu Province is probably the key factor for the occurrence of harmful algal blooms (Zhang et al., 2020).
There was significant negative correlation among salinity, Chl a, and Car in U. prolifera, while significant positive correlation was observed between temperature and Y(NO) in S. horneri (Tables 5–6). These results may mean that salinity had a greater influence on U. prolifera, while temperature had a greater influence on S. horneri. The result of significant correlation among DIN, DOP, and Y(NPQ) may mean stronger ability to utilize DIN and DOP for U. prolifera (Tables 5–6).
5 CONCLUSIONUlva prolifera and S. horneri have different environmental responding strategies. CEF and antioxidant system play more important roles in protecting U. prolifera, while NPQ is more important for adapting to the environment in S. horneri. Meanwhile, U. prolifera has a stronger ability to utilize nutrients and rapidly increase its biomass under suitable condition compared to S. horneri.
6 DATA AVAILABILITY STATEMENTAll data generated and/or analyzed during this study are available from the corresponding author upon request on reasonable request.
Arnon D I. 1949. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiology, 24(1): 1-15.
DOI:10.1104/pp.24.1.1 |
Bradford M M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1-2): 248-254.
DOI:10.1016/0003-2697(76)90527-3 |
Buege J A, Aust S D. 1978. Microsomal lipid peroxidation. Methods in Enzymology, 52: 302-310.
DOI:10.1016/S0076-6879(78)52032-6 |
Fan X, Xu D, Wang Y T, Zhang X W, Cao S N, Mou S L, Ye N H. 2014. The effect of nutrient concentrations, nutrient ratios and temperature on photosynthesis and nutrient uptake by Ulva prolifera: implications for the explosion in green tides. Journal of Applied Phycology, 26(1): 537-544.
DOI:10.1007/s10811-013-0054-z |
Gao S, Shen S D, Wang G C, Niu J F, Lin A P, Pan G H. 2011. PSI-driven cyclic electron flow allows intertidal macro-algae Ulva sp. (Chlorophyta) to survive in desiccated conditions. Plant and Cell Physiology, 52(5): 885-893.
DOI:10.1093/pcp/pcr038 |
Gao S, Zheng Z B, Gu W H, Xie X J, Huan L, Pan G H, Wang G C. 2014. Photosystem Ⅰ shows a higher tolerance to sorbitol-induced osmotic stress than Photosystem Ⅱ in the intertidal macro-algae Ulva prolifera (Chlorophyta). Physiologia Plantarum, 152(2): 380-388.
DOI:10.1111/ppl.12188 |
Grasshoff K, Kremling K, Ehrhardt M. 1999. Methods of Seawater Analysis. 3rd edn. Wiley-VCH, New York. p. 159-228.
|
Huan L, Gu W H, Gao S, Wang G C. 2016. Photosynthetic activity and proteomic analysis highlights the utilization of atmospheric CO2 by Ulva prolifera (Chlorophyta) for rapid growth. Journal of Phycology, 52(6): 1103-1113.
DOI:10.1111/jpy.12469 |
Huang W, Fu P L, Jiang Y J, Zhang J L, Zhang S B, Hu H, Cao K F. 2013. Differences in the responses of photosystem Ⅰ and photosystem Ⅱ of three tree species Cleistanthus sumatranus, Celtis philippensis and Pistacia weinmannifolia exposed to a prolonged drought in a tropical limestone forest. Tree Physiology, 33(2): 211-220.
DOI:10.1093/treephys/tps132 |
Imlay J A. 2003. Pathways of oxidative damage. Annual Review of Microbiology, 57: 395-418.
DOI:10.1146/annurev.micro.57.030502.090938 |
Joët T, Cournac L, Peltier G, Havaux M. 2002. Cyclic electron flow around photosystem Ⅰ in C3 plants. In vivo control by the redox state of chloroplasts and involvement of the NADH-dehydrogenase complex. Plant Physiology, 128(2): 760-769.
DOI:10.1104/pp.010775 |
Keesing J K, Liu D Y, Shi Y J, Wang Y J. 2016. Abiotic factors influencing biomass accumulation of green tide causing Ulva spp. on Pyropia culture rafts in the Yellow Sea, China. Marine Pollution Bulletin, 105(1): 88-97.
DOI:10.1016/j.marpolbul.2016.02.051 |
Larkum A W D, Douglas S E, Raven J A. 2012. Photosynthesis in Algae. Springer Science & Business Media, Dordrecht.
|
Li G, Qin Z, Zhang J J, Lin Q, Ni G Y, Tan Y H, Zou D H. 2020. Algal density mediates the photosynthetic responses of a marine macroalga Ulva conglobata (Chlorophyta) to temperature and pH changes. Algal Research, 46: 101797.
DOI:10.1016/j.algal.2020.101797 |
Li H M, Zhang Y Y, Chen J, Zheng X, Liu F, Jiao N Z. 2019. Nitrogen uptake and assimilation preferences of the main green tide alga Ulva prolifera in the Yellow Sea, China. Journal of Applied Phycology, 31(1): 625-635.
DOI:10.1007/s10811-018-1575-2 |
Li H M, Zhang Y Y, Tang H J, Shi X Y, Rivkin R B, Legendre L. 2017. Spatiotemporal variations of inorganic nutrients along the Jiangsu coast, China, and the occurrence of macroalgal blooms (green tides) in the southern Yellow Sea. Harmful Algae, 63: 164-172.
DOI:10.1016/j.hal.2017.02.006 |
Li Z R, Wakao S, Fischer B B, Niyogi K K. 2009. Sensing and responding to excess light. Annual Review of Plant Biology, 60: 239-260.
DOI:10.1146/annurev.arplant.58.032806.103844 |
Liu D Y, Keesing J K, Dong Z J, Zhen Y, Di B P, Shi Y J, Fearns P, Shi P. 2010. Recurrence of the world's largest green-tide in 2009 in Yellow Sea, China: Porphyra yezoensis aquaculture rafts confirmed as nursery for macroalgal blooms. Marine Pollution Bulletin, 60(9): 1423-1432.
DOI:10.1016/j.marpolbul.2010.05.015 |
Liu F, Liu X F, Wang Y, Jin Z, Moejes F W, Sun S. 2018. Insights on the Sargassum horneri golden tides in the Yellow Sea inferred from morphological and molecular data. Limnology and Oceanography, 63(4): 1762-1773.
DOI:10.1002/lno.10806 |
Liu X Q, Li Y, Wang Z L, Zhang Q C, Cai X Q. 2015. Cruise observation of Ulva prolifera bloom in the southern Yellow Sea, China. Estuarine, Coastal and Shelf Science, 163: 17-22.
DOI:10.1016/j.ecss.2014.09.014 |
Luo M B, Liu F. 2011. Salinity-induced oxidative stress and regulation of antioxidant defense system in the marine macroalga Ulva prolifera. Journal of Experimental Marine Biology and Ecology, 409(1-2): 223-228.
DOI:10.1016/j.jembe.2011.08.023 |
Mou S, Zhang X, Dong M, Fan X, Xu J, Cao S, Xu D, Wang W, Ye N. 2013. Photoprotection in the green tidal alga Ulva prolifera: role of LHCSR and PsbS proteins in response to high light stress. Plant Biology, 15(6): 1033-1039.
DOI:10.1111/j.1438-8677.2012.00712.x |
Praba M L, Vanangamudi M, Thandapani V. 2004. Effect of low light on yield and physiological attributes of rice. International Rice Research Notes, 29: 71-73.
|
Shi X Y, Qi M Y, Tang H J, Han X R. 2015. Spatial and temporal nutrient variations in the Yellow Sea and their effects on Ulva prolifera blooms. Estuarine, Coastal and Shelf Science, 163: 36-43.
DOI:10.1016/j.ecss.2015.02.007 |
Song W, Peng K Q, Xiao J, Li Y, Wang Z L, Liu X Q, Fu M Z, Fan S L, Zhu M Y, Li R X. 2015. Effects of temperature on the germination of green algae micro-propagules in coastal waters of the Subei Shoal, China. Estuarine, Coastal and Shelf Science, 163: 63-68.
DOI:10.1016/j.ecss.2014.08.007 |
Wang Y, Wang Y, Zhu L, Zhou B, Tang X X. 2012. Comparative studies on the ecophysiological differences of two green tide macroalgae under controlled laboratory conditions. PLoS One, 7(8): e38245.
DOI:10.1371/journal.pone.0038245 |
Wu H L, Gao G, Zhong Z H, Li X S, Xu J T. 2018. Physiological acclimation of the green tidal alga Ulva prolifera to a fast-changing environment. Marine Environmental Research, 137: 1-7.
DOI:10.1016/j.marenvres.2018.02.018 |
Xu Z G, Gao G, Xu J T, Wu H Y. 2017. Physiological response of a golden tide alga (Sargassum muticum) to the interaction of ocean acidification and phosphorus enrichment. Biogeosciences, 14(3): 671-681.
DOI:10.5194/bg-14-671-2017 |
Ye N H, Zhuang Z M, Jin X S, Wang Q Y, Zhang X W, Li D M, Wang H X, Mao Y Z, Jiang Z J, Li B, Xue Z X. 2008. China is on the track tackling Enteromorpha spp forming green tide. Nature Proceedings.
DOI:10.1038/npre.2008.2352.1 |
Ye N H, Zhang X W, Mao Y Z, Liang C W, Xu D, Zou J, Zhuang Z M, Wang Q Y. 2011. 'Green tides' are overwhelming the coastline of our blue planet: taking the world's largest example. Ecological Research, 26(3): 477-485.
DOI:10.1007/s11284-011-0821-8 |
Yu J, Li J Y, Wang Q H, Liu Y, Gong Q L. 2019. Growth and resource accumulation of drifting Sargassum horneri (Fucales, Phaeophyta) in response to temperature and nitrogen supply. Journal of Ocean University of China, 18(5): 1216-1226.
DOI:10.1007/s11802-019-3835-4 |
Zhang H B, Su R G, Shi X Y, Zhang C S, Yin H, Zhou Y L, Wang G S. 2020. Role of nutrients in the development of floating green tides in the Southern Yellow Sea, China, in 2017. Marine Pollution Bulletin, 156: 111197.
DOI:10.1016/j.marpolbul.2020.111197 |
Zhao X Y, Tang X X, Zhang H X, Qu T F, Wang Y. 2016. Photosynthetic adaptation strategy of Ulva prolifera floating on the sea surface to environmental changes. Plant Physiology and Biochemistry, 107: 116-125.
DOI:10.1016/j.plaphy.2016.05.036 |
 2021, Vol. 39
2021, Vol. 39