Institute of Oceanology, Chinese Academy of Sciences
Article Information
- DONG Dong, LI Xinzheng
- Two new species of the genus Munidopsis Whiteaves, 1874 (Crustacea: Anomura: Munidopsidae) from the Caroline Ridge, South of the Mariana Trench
- Journal of Oceanology and Limnology, 39(5): 1841-1853
- http://dx.doi.org/10.1007/s00343-021-0385-6
Article History
- Received Oct. 9, 2020
- accepted in principle Nov. 6, 2020
- accepted for publication Jan. 27, 2021
2 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China;
3 University of Chinese Academy of Sciences, Beijing 100049, China;
4 Laboratory for Marine Biology and Biotechnology, Pilot National Laboratory for Marine Science and Technology (Qingdao), Qingdao 266237, China
The genus Munidopsis Whiteaves, 1874 is a group of macrobenthic crustaceans that typically dwell in abyssal waters and noticeably have high biodiversity in the seamount environment (Ahyong and Poore, 2004; Schnabel and Bruce, 2006; Ahyong, 2007; Baba et al., 2008; Rowden et al., 2010). The tropical western Pacific Ocean contains numerous seamounts around the Yap Trench and the Mariana Trench. The seamounts that are close to the Coral Triangle (Briggs, 1999; Hoeksema, 2007; Veron et al., 2009) provide complex and diverse habitats for deep-sea macrobenthos, thereby being potential of regional biodiversity significance for harboring large numbers of new species. In recent decades, several seamounts have been surveyed in this area, and some new Munidopsis species had been reported (Zarenkov and Khodkina, 1981; Williams and Baba, 1989; Cubelio et al., 2008; Komai and Tsuchida, 2014; Dong et al., 2017, 2019).
To study the biodiversity of the tropical western Pacific seamounts, a series of expeditions were organized by the Institute of Oceanology, Chinese Academy of Sciences (IOCAS) in recent years. The latest expedition was undertaken in 2019 to survey the seamounts in the Caroline Ridge, which is located near the south of the Mariana Trench. Two Munidopsis species were collected during this survey. After morphological examination, they were determined new to science. In addition to the morphological analysis, DNA barcodes and phylogenetic tree were deciphered in this study, with which the systematic status of the new species was revealed.
The present article reports these two new species, making a contribution to the knowledge concerning squat lobster biodiversity in the seamounts beneath the tropical western Pacific Ocean.
2 MATERIAL AND METHOD 2.1 Sample collection and morphological examinationThe material for this study was collected during the seamount expedition on the Caroline Ridge, south of Mariana Trench, in May 2019. The investigated location was at the M5 (temporarily named) seamount. Specimens were collected using the remotely operated vehicle (ROV) Faxian (Discovery in Chinese) deployed onboard the R/V Kexue (Science in Chinese). The specimens collected were fixed in 95% ethanol immediately after being brought on deck and were later brought to the laboratory for further study.
The size of the specimen is given as the postorbital carapace length (PCL), which refers to the carapace length excluding the rostrum. The terminology used mainly follows Baba (2005). All specimens collected from the seamount survey expeditions were deposited in the Marine Biological Museum of the Chinese Academy of Sciences in Qingdao, China. The abbreviations used in the text are as follows: ovig., ovigerous; P1, pereopod 1 (chelipeds); P2–4, pereopods 2 to 4 (first to third walking legs).
2.2 DNA extraction, amplification, and sequencingTotal genomic DNA was extracted from abdominal muscle tissue using the EasyPureⓇ Marine Animal Genomic DNA Kit (TransGen, Beijing, China) following the manufacturer's instructions. Extracted DNA was eluted in double-distilled H2O (ddH2O). Partial sequences of a mitochondrial gene, cytochrome oxidase subunit I (COI), were amplified via polymerase chain reaction (PCR). Reactions were carried out in a 30-mL volume containing: 15-mL Premix Taq (2×T5 Super PCR Mix plus dye; Tsingke, Beijing, China), 1.2-mL each of forward and reverse primers (10 mmol/L), respectively, 1.2-mL DNA template, and 11.4-mL ddH2O. A new pair of primers was employed for the amplification: HCO2198 (Folmer et al., 1994) and LCOgalaA (5'-TTTCTACAAACCACAAAGACATTGG-3'). This new primer pair was utilized to obtain a fragment (ca. 660 bp) of the COI gene with the annealing temperature of 49 ℃.
After being purified, the PCR products were bidirectionally sequenced using the same primers. Sequences were checked by the sequence peak height and then assembled based on the contigs using the DNASTAR Lasergene software package (DNASTAR, Inc., Madison, WI, USA).
Four new sequences were generated in the present study (Aside from the new species, we also sequenced COI gene of M. verrilli Benedict, 1902 from Site F cold seep, northern South China Sea). Additional COI gene sequences of Munidopsis species were downloaded from NCBI GenBank for phylogenetic analysis (Table 1). These species include two species with trifid rostrum, M. trifida Henderson, 1885 and M. comarge Taylor, Ahyong and Andreakis, 2010, and other species are all distributed in the Indo-West Pacific.
|
The sequences were aligned and trimmed using the software package MEGA6.06 (Tamura et al., 2013). After trimming, each sequence was 501-bp long and was employed for phylogenetic analysis.
The genetic distances based on COI barcoding were estimated according to the Kimura 2-parameter (Kimura, 1980) model in MEGA 6.06.
Phylogenetic relationships were inferred using maximum likelihood (ML) and Bayesian inference (BI) methods. The best nucleotide base substitution model selected in this study, which is HKY+I+G, was determined by MrModeltest v2 (Nylander, 2004) under the Akaike Information Criterion (AIC). The ML analyses were assembled in PhyML 3.1 (Guindon and Gascuel, 2003) with 1 000 replicates, based on which the bootstrap support (BS) was estimated. A Bayesian inference (BI) tree was constructed using MrBayes 3.2.7 (Huelsenbeck and Ronquist, 2001). Markov chains were run for 1 000 000 generations, sampled every 100 generations; the first 25% trees were discarded as burn-in, after which remaining trees were used to construct the 50% majority-rule consensus tree and to estimate posterior probabilities (PP). Given the uncertainty of systematic relationships within the family Munidopsidae, Munida militaris Henderson, 1885 was selected as the outgroup. The phylogenetic trees reconstructed from both the ML and BI analyses were generally congruent; the combined tree is presented based on the topology structure derived from the ML analysis.
3 RESULTSystematics
Munidopsidae Ortmann, 1898
Munidopsis Whiteaves, 1874
Munidopsis ahyongi sp. nov. (Figs. 1–3)
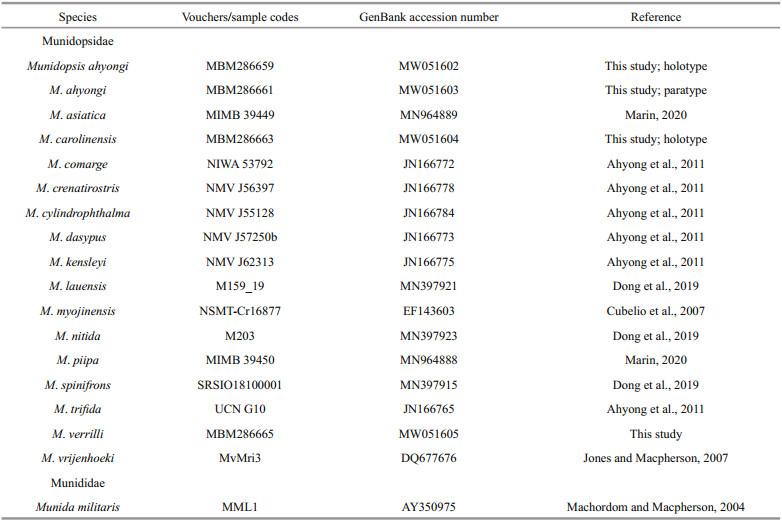
|
| Fig.1 Munidopsis ahyongi sp. nov. a, c–h. MBM286659, holotype, ovig. female; b. MBM286661, paratype, female (a. carapace and abdominal segments 1-4, dorsal view; b. carapace, dorsal view; c. left pterygostomian, lateral view; d. sternal plastron, ventral view; e. telson, extensor view; f. left antennular and antennal peduncles, ventral view; g. left third maxilliped, ventral view; h. left crista dentata, ventral view). Scale bars equal 1.0 mm. |
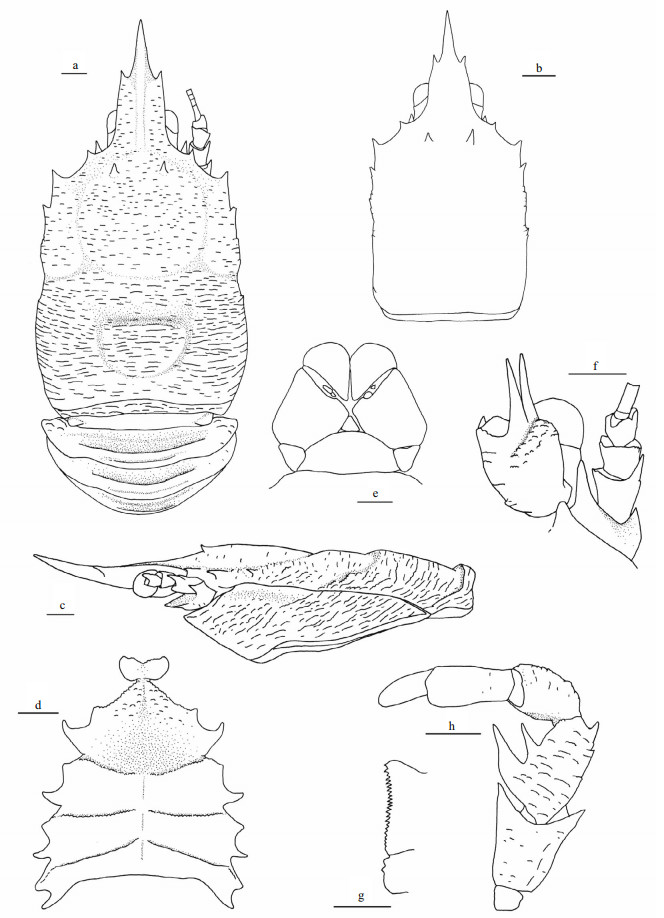
|
| Fig.2 Munidopsis ahyongi sp. nov., MBM286659, holotype, ovig. female a. right P1 (setae only shown on merus), dorsal view; b. right P1, ventral view; c. left P2 (with setae), lateral view; d. left P3, lateral view; e. left P4, lateral view; f. dactylus of left P2, lateral view. Scale bars equal 1.0 mm. |

|
| Fig.3 Munidopsis ahyongi sp. nov., MBM286659, holotype, ovig. female, whole body, conserved in ethnol, dorsal view |
Material examined
Holotype, MBM286659, 1 ovig. female (PCL 10.8 mm), stn. FX-Dive213, M5 seamount on the Caroline Ridge, West Pacific, 140°11'E, 10°04'N, 817–1 017 m, coll. Faxian ROV on R/V Kexue, 31 May 2019. Paratype, MBM286661, 1 female (PCL 6.1 mm), stn. FX-Dive213, M5 seamount on the Caroline Ridge, West Pacific, 140°11'E, 10°04'N, 817–1 017 m, coll. Faxian ROV on R/V Kexue, 31 May 2019.
Description
Carapace (excluding rostrum) approximately 1.3 times longer than broad, bearing very sparse fine setae on lateral margin and dorsal surface. Frontal margins oblique, antennal spines well developed. Lateral margins parallel; posterior orbital margin (posterior to outer orbital angle) oblique; anterior branchial margin with short, elevated and transverse rugae, armed with strong anterolateral spine followed by another relatively small spine and deep constriction on lateral end of posterior cervical groove; posterior branchial margin slight convex or parallel, unarmed, with numerous transverse or oblique rugae extending onto dorsal surface. Posterior margin straight, with strongly elevated and unarmed submarginal ridge. Dorsal surface with numerous transverse rugae; cervical grooves distinct; regions well delimitated, forming distinct depression anterior to cardic region; gastric region elevated, armed with pair of epigastric spines. Rostrum elongate, half of remaining carapace length, 3.7 times longer than broad (measured between bases of eyestalks), distally trifid; median spine elongate, weakly upturned, 0.4 to half of rostrum length; dorsal surface carinate along midline; lateral proximal margins parallel (holotype) or slightly convex (paratype). Pterygostomial flap with numerous oblique rugae on lateral surface, anterior end blunt.
Sternal plastron approximately longer than broad, widening posteriorly. Sternite 3 approximately 2.1 times broader than long, anterior margin with shallow median notch. Sternite 4 having anterolateral margin denticulate, straight and oblique; ventral surface with short rugae and median groove on anterior region and distinctly depressed on posterior region. Sternites 5–7 smooth on ventral surface, each with transverse ridge interrupted by shallow median groove.
Abdominal tergites with dorsal surfaces glabrous and unarmed; tergites 2–4 each with elevated transverse ridge; tergites 2 and 3 with additional short, low posterior ridge; tergite 6 with straight posterior margin.
Telson composed of 8 main plates.
Eyestalk movable, partially concealed by rostrum. Peduncle unarmed, slightly longer than cornea length. Cornea hemispheric, globular. Epistomial spine well-developed between eyestalk and antennal peduncle.
Antennular peduncle with basal article longer than broad; distal margin armed with slender ventrolateral and dorsolateral spines; lateral surface inflate, bearing short rugae.
Antennal peduncle reaching proximal 0.3 of rostrum. Article 1 short and immovable, distal margin with strong ventromesial tooth and small lateral tooth. Article 2 armed with strong distolateral spine. Article 3 and article 4 short, unarmed.
Third maxilliped stout. Ischium slightly shorter than merus, disto-extensor and distoflexor corners produced into strong spines; crista dentata well-developed; ventral surface with short rugae. Merus with slightly convex extensor margin, armed with distinct disto-extensor spine followed by pointed tubercle; flexor margin with blunt distal corner, bearing 2 strong spines on proximal part, proximal spine much produced and perpendicular to flexor margin; ventral surface with short transverse rugae. Carpus with extensor margin slightly rugose. Propodus subrectangular, unarmed. Dactylus short and unarmed.
P1 unequal (right P1 larger than left P1 in holotype) or subequal (paratype), approximately 3.0 times as long as PCL (measurement based on right P1 of holotype, similarly hereinafter); surfaces and margins covered with numerous scale-like rugae and bearing sparse setae (slightly plumose). Ischium short, approximately 0.3 times merus length, armed with distinct dorsodistal spine; ventrodistal margin produced, laterally denticulate and mesially with stout subterminal spine. Merus 1.2 times PCL, distal margin with strong dorsal, dorsomesial, ventromesial and ventrolateral spines; dorsodistal spine somewhat posterior to distal margin, followed by row of spines along midline of dorsal surface, distal dorsomesial spine followed by 2 strong spines, distal ventromesial spine followed by 2 or 3 spines on proximal part. Carpus subrectangular, 0.4 times merus length; dorsodistal margin with strong spine followed by 1–3 small spines or pointed tubercles; distal dorsomesial corner denticulate, followed by strong subterminal spine; ventrodistal margin with small lateral spine. Palm (excluding fingers) 0.6 times merus length, lateral and mesial margins straight, with elevated rugae, left palm of holotype with small median spine on mesial margin. Fingers 0.8 times palm length, tips spooned; occlusal margins crenulated, nearly straight, with indistinct median tooth on fixed finger.
P2–4 covered with numerous scale-like rugae and bearing sparse setae (slightly plumose) on surfaces and margins of each segment; P2 approximately 1.7 times PCL, reaching at most proximal part of P1 palm. Meri slender, subequal in breadth and decreasing in length posteriorly, P2 merus 0.6 times PCL and 4.2 times longer than broad, P3 and P4 merus approximately 0.8 times P2 merus length; extensor margin armed with row of slender spines; flexor margin rugose or with elevated transverse ridges, armed with strong distal spine. Carpi short, subequal in length from P2–4, approximately 0.4 times P2 merus length; extensor margin with 2 longitudinal ridges, mesial ridge armed with strong distal spine followed by additional small spines (P2) or tubercles (P3 and P4). Propodi slender, subequal in breadth and slightly increasing in length from P2–4, P2 and P3 propodi 0.9 times P2 merus length, and 5.6 times longer than broad, P4 propodus approximately 1.1 times P2 propodus length; flexor margin with 3 corneous spines on distal part including distal pair. Dactyli approximately 0.6 times propodus length, ending in slightly curving claw; extensor margin nearly straight on proximal three-fourth; flexor margin straight, with 11 movable corneous spines on entire length, each spine based on triangular tooth, distalmost spine small, closely appressed to claw.
Pereopods without epipod.
Habitat: Hard and sandy substrate.
Distribution: Only known from the type locality, Caroline Ridge near Mariana Trench, tropical western Pacific; depth 817–1 017 m.
Etymology: The specific name is in honor of Shane T. Ahyong, for his outstanding contributions to the galatheoid systematics.
Remarks: The new species is morphologically similar to species of the M. serricornis (Lovén, 1852) complex, but according to Ahyong's (2014) definition, it should be excluded from this complex because it has the posterior orbital margins and frontal margins of the carapace in-line and sloping posteriorly at the same angle. The new species, however, can be sorted into a group with some species from the M. serricornis complex in having a combination of the following characters: the carapace has a pair of epigastric spines and at least 2 spines on the lateral margin, the abdominal segments are unarmed, the P2–4 meri have a row of slender spines on the extensor margin, and the P1 palm is unarmed (at least without row of spines). This group of species contains M.macphersoni Ahyong, 2014, M. mina Benedict, 1902, M. pyrochela Ahyong, 2014, M. spiridonovi Ahyong, 2014, M. transtridens Pequegnat and Pequegnat, 1971, and M. tridens (A. Milne Edwards, 1880). In addition to the oblique posterior orbital margins, the new species is different from these related congeners in having 2 spines on the lateral margin of the carapace and the rostral median spine approximately half of the rostrum length. In the new species, the lateral branchial margin posterior to the second spine has elevated ridges and tubercles but never bears spines. In contrast, those related species have 4 spines on the lateral margin of the carapace and a considerably shorter rostral median spine. The new species further differs from M. macphersoni, M. mina, M. pyrochela, M. spiridonovi, and M. transtridens in having only 2 strong spines instead of having additional denticles or spines on the distoflexor margin of the third maxilliped merus. The new species further differs from M. tridens in having 2 rows of spines instead of only one spine on the mesial side of the P1 merus. In addition, M. macphersoni, M. pyrochela, M. spiridonovi, and M. transtridens have a small but distinct spine on the distomesial corner of the P1 carpus, whereas the new species lacks such spine.
The new species also resembles M. trifida, but can be distinguished from the latter in having 2 spines on the lateral carapace margin and unarmed P1 palm, whereas M. trifida has 4 spines on the lateral carapace margin and a row of spines on the mesial margin of the P1 palm (Henderson, 1885).
The two type specimens of the new species have intraspecific genetic distances of 0.4% based on COI. The genetic distances between the new species and M. trifida from Chile (Ahyong et al., 2011) are 15.3%–15.6%. The genetic distances between the new species and M. comarge (another species with trifid rostrum) from New Zealand (Ahyong et al., 2011) are 14.9%–15.2%.
In the phylogenetic tree (Fig. 4), M. ahyongi sp. nov., M. comarge, and M. trifida form a well-supported clade (BS=78, PP=1.00), which indicates a close relationship. This also suggests that species within (and resembling) the M. serricornis complex may have a monophyletic origin, but more molecular research is needed to verify this evolutionary pattern.
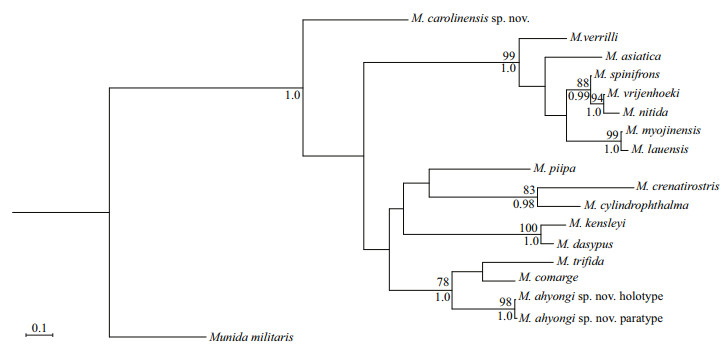
|
| Fig.4 Phylogenetic tree obtained by the maximum likelihood analysis based on the COI gene sequences BS (above, based on maximum likelihood analysis) and PP (below, based on Bayesian inference) are indicated adjacent to each node. Only values of BS≥75 and PP≥0.95 are shown. |
Munidopsis carolinensis sp. nov. (Figs. 5-7)
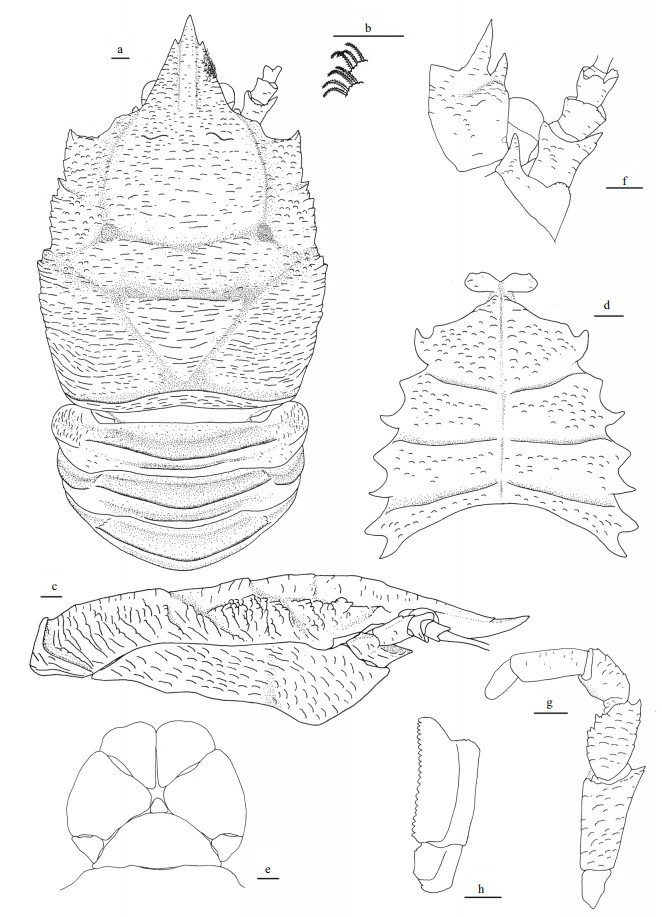
|
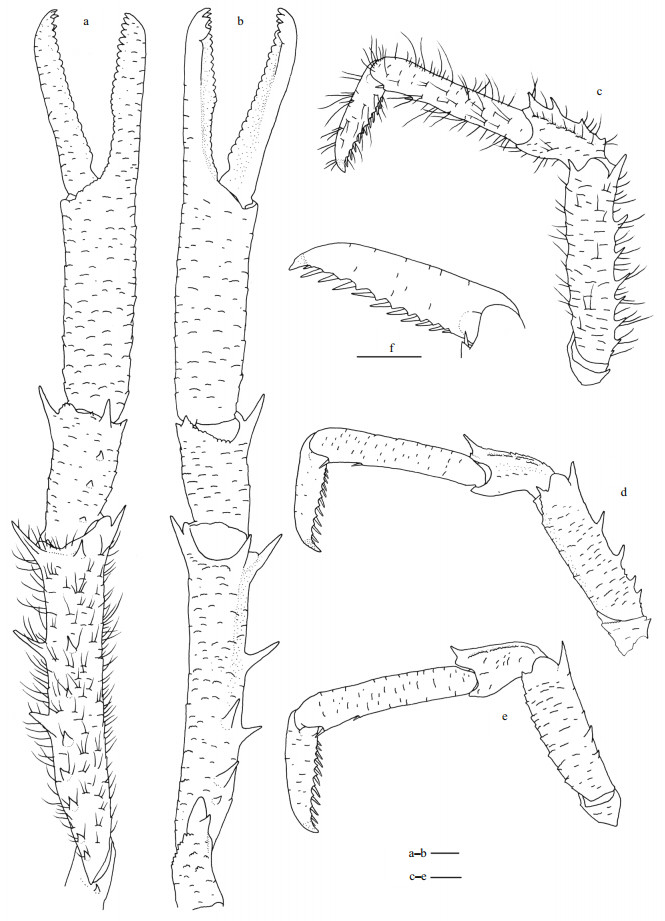
| Fig.5 Munidopsis carolinensis sp. nov., holotype, MBM286663, female a. carapace and abdominal segments 1-4 (setae only show on right lateral margin of rostrum), dorsal view; b. setae on carapace and rostrum, dorsal view; c. right pterygostomian, lateral view; d. sternal plastron, ventral view; e. telson, extensor view; f. left antennular and antennal peduncles, ventral view; g. left third maxilliped, ventral view; h. left crista dentata, ventral view. Scale bars equal 1.0 mm. |
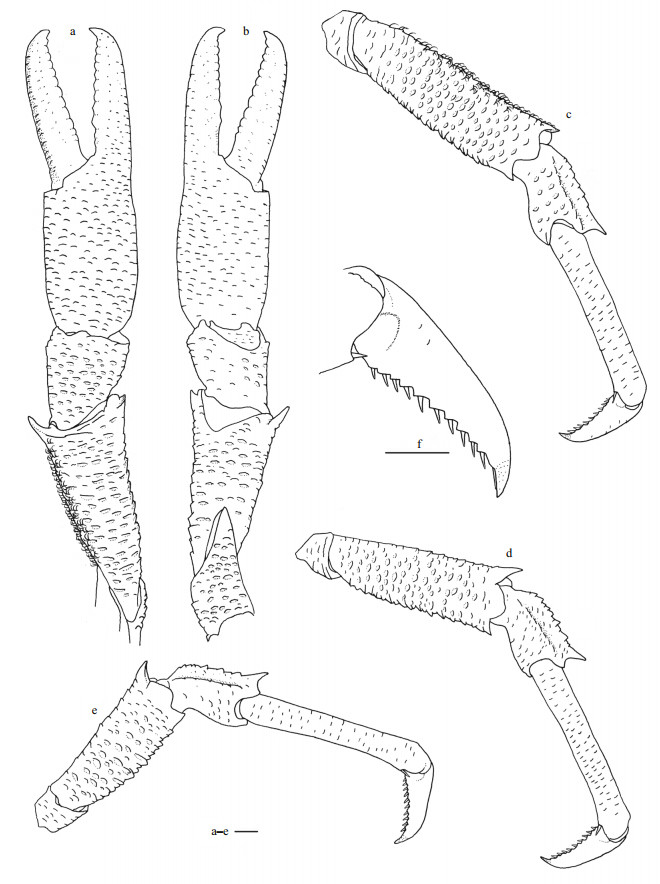
|
| Fig.6 Munidopsis carolinensis sp. nov., holotype, MBM286663, female a. right P1 (setae only shown on mesial margin of merus), dorsal view; b. right P1, ventral view; c. right P2 (setae only shown on extensor margin of merus), lateral view; d. right P3, lateral view; e. right P4, lateral view; f. dactylus of right P2, lateral view. Scale bars equal 1.0 mm. |

|
| Fig.7 Munidopsis carolinensis sp. nov., holotype, MBM286663, female, whole body a. conserved in ethnol, dorsal view; b. fresh color, dorsal view; c. fresh color, ventral view. |
Material examined
Holotype, MBM286663, 1 female (PCL 17.7 mm), stn. FX-Dive212, M5 seamount on the Caroline Ridge, western Pacific, 140°11.57'E, 10°04.10'N, 1 022 m, coll. Faxian ROV on R/V Kexue, 30 May 2019.
Description
Carapace slightly longer than broad (excluding rostrum), covered in dense, curved, slightly-plumose setae on dorsal surface and margins. Frontal margins distinctly oblique, with blunt outer orbital angle above antennal peduncle. Lateral margins convex; anterolateral spine stout, posterior orbital margin (posterior to outer orbital angle) distinctly transverse; hepatic and anterior branchial margin with elevated scale-like rugae, bearing low tooth on lateral end of anterior cervical groove; posterior branchial margin with relatively long and oblique rugae; lateral ends of anterior and posterior cervical grooves forming obvious notches. Posterior margin with unarmed and much elevated submarginal ridge. Dorsal surface with regions well delimitated, forming broad depression anterior to cardic region; cervical groove and branchiocardiac groove distinct; covered with numerous scale-like rugae, rugae relatively longer on posterior branchial, cardiac and intestinal regions; gastric region elevated, with pair of sinus and low epigastric rugae. Rostrum triangular, 0.3 times of remaining carapace length, as long as broad (measured between bases of eyestalks), 0.4 times as wide as distance between anterolateral spines, distally trifid, bearing rugae and curved plumose setae on surface and margins; median spine short, weakly upturned, 0.3 times of rostrum length; dorsal surface carinate along midline; lateral margins straight. Pterygostomial flap with numerous scale-like rugae on lateral surface, anterior end produced and unarmed.
Sternal plastron as long as broad, widening posteriorly, ventral surface of each sternite covered with numerous scale-like rugae bearing short setae. Sternite 3 approximately 3.5 times broader than long, anterior margin with median notch. Sternite 4 having anterolateral margin obliquely straight; ventral surface with median groove on anterior part and distinctly depressed on posterior part. Sternites 5–7 each with transverse ridge interrupted by shallow median groove.
Abdominal tergites with dorsal surfaces unarmed, bearing dense setae as on carapace; tergites 2–4 each with strongly elevated transverse ridge medially; tergite 2 and 3 with additional low and short posterior ridge; tergite 6 with straight posterior margin.
Telson composed of 8 plates, bearing faint rugae and dense setae on surface.
Eyestalk movable, partially concealed by rostrum. Peduncle unarmed, approximately as long as cornea length. Cornea hemispheric, globular. Epistomial tooth present between eyestalk and antennal peduncle.
Antennular peduncle with basal article longer than broad; distal margin armed with strong ventrolateral and dorsolateral spines; lateral surface inflate, bearing short rugae.
Antennal peduncle reaching midlength of rostrum, bearing short rugae on surfaces. Article 1 short and immovable, distal margin with produced ventromesial tooth and scale-like lateral tooth. Article 2 armed with strong distolateral spine. Article 3 and article 4 short, unarmed.
Third maxilliped slender, bearing numerous scale-like rugae on ventral surfaces. Ischium slightly longer than merus, disto-extensor corner produced into stout spine; crista dentata well-developed. Merus with straight and rugose extensor margin, armed with distinct disto-extensor spine; flexor margin denticulate, roughly with 2 strong teeth on median part. Carpus with extensor margin rugose. Propodus subrectangular and unarmed. Dactylus short and unarmed.
P1 unequal (right P1 larger than left P1 but similar in armature), approximately 3.0 times as long as PCL (measurement based on right P1, similarly hereinafter); surfaces and margins covered with numerous scale-like rugae and bearing dense setae as on carapace, rugae relatively more elevated on ischium, merus, and carpus. Ischium approximately half of merus length, dorsolateral margin strongly rugose; ventrodistal margin produced and unarmed. Merus half of PCL, distal margin with strong mesial spine and stout lateral tooth. Carpus unarmed, half of merus length, 1.2 times longer than broad. Palm (excluding fingers) unarmed, 0.8 times merus length, lateral and mesial margins slightly convex proximally. Fingers 0.9 times palm length; tips acute, hooked and crossing over each other distally; occlusal margins crenulated and straight; lateral margin of fixed finger without denticulate carina.
P2–4 covered with numerous scale-like rugae and bearing dense setae on surfaces and margins of each segment as on carapace; P2 approximately 1.6 times PCL, slightly falling short of distal end of right P1. Meri subequal in breadth and decreasing in length posteriorly, P2 merus 0.6 times PCL and 3.5 times longer than broad, P3 merus 0.8 times P2 merus length, P4 merus 0.7 times P2 merus length; extensor margin and flexor margin with elevated rugae, each bearing strong distal spine. Carpi short, subequal in length from P2–4, approximately 0.4 times P2 merus length; extensor margin with 2 longitudinal ridges, mesial ridge rugose and with strong distal spine. Propodi slender, subequal in breadth and slightly increasing in length from P2–4, P2 and P3 propodi 0.9 times P2 merus length, and 6.7 times longer than broad, P4 propodus approximately 1.1 times P2 propodus length; flexor margin with distal pair of corneous movable spines. Dactyli approximately 0.4 times propodus length, ending in strongly curving claw; extensor margin nearly straight on proximal two-third; flexor margin slightly concave, with 9–10 movable corneous spines on entire length, each spine based on triangular tooth.
Pereopods without epipod.
Coloration: Whole body orange.
Habitat: Hard and sandy substrate.
Distribution: Only known from the type locality, Caroline Ridge near the Mariana Trench, tropical western Pacific; depth 1 022 m.
Etymology: The specific name refers to the type locality Caroline Ridge.
Remarks: The new species is morphologically similar to M. aurantia Lin and Chan, 2011 and M. norfanz Ahyong, 2007 in having a trifid and triangular rostrum, unarmed carapace dorsal surfaces and abdominal segments, and the P2–4 meri extensor margin rugose or dentate (but without row of spines). The new species readily differs from the two relatives in the following characters: the outer orbital angles are blunt, the frontal margins are distinctly oblique, the lateral margins of the rostrum are obliquely straight, sternites 5–7 have numerous scale-like rugae, and the P1 merus has only 2 distal spines (tooth). In contrast, the two related species have produced and rounded outer orbital angles, transverse frontal margins, slightly convex lateral margins of the rostrum, smooth sternites 5–7, and the P1 merus with 3 distal spines (Ahyong, 2007; Lin and Chan, 2011). The new species further differs from M. aurantia in having the lateral carapace margin (excluding the anterolateral spines) unarmed instead of with 2–4 spines, and the P1 carpus unarmed instead of with 4 distal spines. The new species further differs from M. norfanz in having the posterior orbital margins (posterior to the outer orbital angle) distinctly transverse instead of oblique.
Munidopsis carolinensis sp. nov. resembles species of the M. serricornis complex (Ahyong, 2014) in having the trifid rostrum but can be readily distinguished from the latter in having triangular rostrum and unarmed outer orbital angle, whereas the species in the M. serricornis complex have elongated rostrum with subparallel lateral margins and well-developed antennal spines.
No DNA information of closely related species is available for comparative analysis. In the phylogenetic tree, M. carolinensis sp. nov. is placed at the base of the Munidopsis clade (Fig. 4), suggesting that it is evolutionarily more ancient than other species in this study.
4 DATA AVAILABILITY STATEMENTAll data generated and analyzed during this study are included in this published article.
5 ACKNOWLEDGMENTWe are very grateful to Prof. Kuidong XU and Dr. Yu XU for providing us the specimens and fresh photos for this study.
Ahyong S T, Andreakis N, Taylor J. 2011. Mitochondrial phylogeny of the deep-sea squat lobsters, Munidopsidae (Galatheoidea). Zoologischer Anzeiger — A Journal of Comparative Zoology, 250(4): 367-377.
DOI:10.1016/j.jcz.2011.06.005 |
Ahyong S T, Poore G C B. 2004. Deep-water Galatheidae (Crustacea: Decapoda: Anomura) from southern and eastern Australia. Zootaxa, 472(1): 1-76.
DOI:10.11646/zootaxa.472.1.1 |
Ahyong S T. 2007. Decapod Crustacea collected by the NORFANZ Expedition: galatheidae and polychelidae. Zootaxa, 1593: 1-54.
|
Ahyong S T. 2014. Deep-sea squat lobsters of the Munidopsis serricornis complex in the Indo-West Pacific, with descriptions of six new species (Crustacea: Decapoda: Munidopsidae). Records of the Australian Museum, 66(3): 197-216.
DOI:10.3853/j.2201-4349.66.2014.1630 |
Baba K, Macpherson E, Poore G C B, Ahyong S T, Bermudez A, Cabezas P, Lin C W, Nizinski M, Rodrigues C, Schnabel K E. 2008. Catalogue of squat lobsters of the world (Crustacea: Decapoda: Anomurafamilies Chirostylidae, Galatheidae and Kiwaidae). Zootaxa, 1905(1): 1-220.
DOI:10.11646/zootaxa.1905.1.1 |
Baba K. 2005. Deep-sea chirostylid and galatheid crustaceans (Decapoda: anomura) from the Indo-Pacific, with a list of species. Galathea Reports, 20: 1-317.
|
Benedict J E. 1902. Descriptions of a new genus and forty-six new species of crustaceans of the family Galatheidae, with a list of the known marine species. Proceedings of the United States National Museum, 26(1311): 243-334.
DOI:10.5479/si.00963801.26-1311.243 |
Briggs J C. 1999. Coincident biogeographic patterns: indowest Pacific Ocean. Evolution, 53(2): 326-335.
DOI:10.1111/j.1558-5646.1999.tb03769.x |
Cubelio S S, Tsuchida S, Hendrickx M E, Kado R, Watanabe S. 2007. A new species of vent associated Munidopsis (Crustacea: decapoda: anomura: galatheidae) from the Western Pacific, with notes on its genetic identification. Zootaxa, 1435(1): 25-36.
DOI:10.11646/zootaxa.1435.1.3 |
Cubelio S S, Tsuchida S, Watanabe S. 2008. New species of Munidopsis (Decapoda: Anomura: Galatheidae) from hydrothermal vent areas of Indian and Pacific Oceans. Journal of the Marine Biological Association of the United Kingdom, 88(1): 111-117.
|
Dong D, Li X Z, Lu B, Wang C S. 2017. Three squat lobsters (Crustacea: Decapoda: Anomura) from tropical West Pacific seamounts, with description of a new species of Uroptychus Henderson, 1888. Zootaxa, 4311(3): 389-398.
DOI:10.11646/zootaxa.4311.3.4 |
Dong D, Xu P, Li X Z, Wang C S. 2019. Munidopsis species (Crustacea: Decapoda: Munidopsidae) from carcass falls in Weijia Guyot, West Pacific, with recognition of a new species based on integrative taxonomy. Peer J, 7: e8089.
DOI:10.7717/peerj.8089 |
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology, 3(5): 294-299.
|
Guindon S, Gascuel O. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology, 52(5): 696-704.
DOI:10.1080/10635150390235520 |
Henderson J R. 1885. XXXIX.—Diagnoses of the new species of Galatheidea collected during the 'Challenger' expedition. Annals and Magazine of Natural History, 16(96): 407-421.
DOI:10.1080/00222938509459908 |
Hoeksema B W. 2007. Delineation of the Indo-Malayan centre of maximum marine biodiversity: the coral triangle. In: Renema W ed. Biogeography, Time, and Place: Distributions, Barriers, and Islands. Springer, Dordrecht, Netherlands. p. 117–178.
|
Huelsenbeck J P, Ronquist F. 2001. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics, 17(8): 754-755.
DOI:10.1093/bioinformatics/17.8.754 |
Jones W J, Macpherson E. 2007. Molecular phylogeny of the east pacific squat lobsters of the genus Munidopsis (Decapoda: Galatheidae) with the descriptions of seven new species. Journal of Crustacean Biology, 27(3): 477-501.
DOI:10.1651/S-2791.1 |
Kimura M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution, 16(2): 111-120.
DOI:10.1007/BF01731581 |
Komai T, Tsuchida S. 2014. Deep-Sea decapod crustaceans (Caridea, Polychelida, Anomura and Brachyura) collected from the Nikko Seamounts, Mariana Arc, using a remotely operated vehicle "Hyper-Dolphin". Zootaxa, 3764(3): 279-316.
DOI:10.11646/zootaxa.3764.3.3 |
Lin C W, Chan T Y. 2011. Two new deep-sea squat lobsters of the genus Munidopsis Whiteaves, 1874 (Crustacea: decapoda: munidopsidae) from Taiwan. Zootaxa, 2754(1): 51-59.
DOI:10.11646/zootaxa.2754.1.4 |
Lovén S. 1852. De svenska arterna af slägtet Galathea. Ofversigt af Konglige Vetenskaps-Akademiens Förhandlingar, 9: 20-23.
|
Machordom A, Macpherson E. 2004. Rapid radiation and cryptic speciation in squat lobstersof the genus Munida (Crustacea, Decapoda) and related genera in the South West Pacific: molecular and morphological evidence. Molecular Phylogenetics and Evolution, 33(2): 259-279.
DOI:10.1016/j.ympev.2004.06.001 |
Marin I. 2020. Northern unicorns of the depths: diversity of the genus Munidopsis Whiteaves, 1874 (Decapoda: Anomura: Munidopsidae) in the northwestern Pacific Ocean, with descriptions of three new species along the Russian coast. Progress in Oceanography, 183: 102263.
DOI:10.1016/j.pocean.2020.102263 |
Nylander J A A. 2004. MrModeltest v 2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University, Uppsala. 2p.
|
Ortmann A E. 1898. Crustacea, Malacostraca. In: Gerstäcker A, Ortmann A E eds. Die Klassen und Ordnungen der. Arthropoden wissenschaftlich dargestellt in Wort und Bild, in H. G. Bronn's Die Klassen und Ordnungen der Thier-Reichs wissenschaftlich dargestellt in Wort und Bild. C.F. Winter'sche Verlagshandlung, Leipzig, 5(2): 1057–1168, pls 109–116.
|
Pequegnat W E, Pequegnat L H. 1971. New species and new records of Munidopsis (Decapoda: Galatheidae) from the Gulf of Mexico and Caribbean Sea (Supplement to Texas A & M University Oceanographic Studies, Volume 1). Gulf Publishing Co, Houston. 25p.
|
Rowden A A, Schnabel K E, Schlacher T A, Macpherson E, Ahyong S T, de Forges B R. 2010. Squat lobster assemblages on seamounts differ from some, but not all, deep-sea habitats of comparable depth. Marine Ecology, 31(s1): 63-83.
|
Schnabel K E, Bruce N L. 2006. New records of Munidopsis (Crustacea: Anomura: Galatheidae) from New Zealand with description of two new species from a seamount and underwater canyon. Zootaxa, 1172(1): 49-67.
DOI:10.11646/zootaxa.1172.1.5 |
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution, 30(12): 2725-2729.
DOI:10.1093/molbev/mst197 |
Taylor J, Ahyong S T, Andreakis N. 2010. New records and new species of the munidopsine squat lobsters (Decapoda: Anomura: Galatheidae: Munidopsinae) from Australia. Zootaxa, 2642(1): 1-18.
DOI:10.11646/zootaxa.2642.1.1 |
Veron J E N, Devantier L M, Turak E, Green A L, Kininmonth S, Stafford-Smith M, Peterson N. 2009. Delineating the coral triangle. Galaxea, Journal of Coral Reef Studies, 11(2): 91-100.
DOI:10.3755/galaxea.11.91 |
Whiteaves J F. 1874. On recent deep-sea dredging operations in the Gulf of St. Lawrence. American Journal of Science, s3-7(39): 210-218.
DOI:10.2475/ajs.s3-7.39.210 |
Williams A B, Baba K. 1989. New squat lobsters (Galatheidae) from the Pacific Ocean: mariana Back Arc Basin, East Pacific Rise, and Cascadia Basin. Fishery Bulletin, 87(4): 899-910.
|
Zarenkov N A, Khodkina I V. 1981. Decapod crustaceans. In: Benthos of the Submarine Mountains Marcus-Necker and Adjacent Pacific Regions. Academija Nauk SSSR, Moscow. p. 83–93.
|
 2021, Vol. 39
2021, Vol. 39


