Institute of Oceanology, Chinese Academy of Sciences
Article Information
- CHEN Zishuo, LI Tao, YANG Bingjie, JIN Xuejie, WU Hualian, WU Jiayi, LU Yandu, XIANG Wenzhou
- Isolation of a novel strain of Cyanobacterium sp. with good adaptation to extreme alkalinity and high polysaccharide yield
- Journal of Oceanology and Limnology, 39(3): 1131-1142
- http://dx.doi.org/10.1007/s00343-020-0113-7
Article History
- Received Mar. 5, 2020
- accepted in principle Apr. 22, 2020
- accepted for publication Jun. 8, 2020
2 State Key Laboratory of Marine Resource Utilization in South China Sea, College of Oceanology, Hainan University, Haikou 570228, China;
3 Southern Marine Science and Engineering Guangdong Laboratory(Guangzhou), Guangzhou 511458, China;
4 University of Chinese Academy of Sciences, Beijing 100049, China
Cyanobacteria, existing in diverse morphology, are a large group of prokaryotic organisms with promising biotechnological and commercial applications. These oldest photoautotrophic microbes on the earth colonize a diversity of habitats, from fresh or seawater to terrestrial environments (Lau et al., 2015). They can adapt to wide environmental stresses, such as high alkalinity, high salinity, and low irradiance. Some species can grow rapidly with excellent carbon capture and yield high-value metabolites (Khan et al., 2018). Thus, cyanobacteria are considered as suitable candidates to produce biologically active compounds, biodiesel, bioplastics, and so forth (Grossmann et al., 2019).
Polysaccharides produced by cyanobacteria have received increasing attention due to various biological activities, such as antiviral, antitumor, antioxidant, antidiabetic, and immunoregulation effects (Demay et al., 2019). The calcium sulfate polysaccharide isolated from hot water extract of Spirulina showed a pronounced effect on human immunodeficiency virus type 1, making itself a potential candidate for the treatment of Acquired Immune Deficiency Syndrome (Hayashi et al., 1996). Exopolysaccharides (EPSs) which can be easily collected from liquid cultures also have drawn extensive attention in recent years. Oral administration of EPS released by Aphanothece halophytica significantly inhibited influenza virus hemagglutinin type 1 and neuraminidase type 1-induced pneumonia in mice (Zheng et al., 2006). EPS from Nostoc commune showed marked hydroxyl radical and superoxide anion scavenging activity and moisture absorption and retention capacity, which may promote its commercial application in skin-care products (Morone et al., 2019).
Apart from polysaccharides, other cyanobacteriaderived molecules have applied to many fields. For example, phycobiliproteins, the light-harvesting soluble proteins with associated pigments, are well known for anti-oxidation, anti-tumor, and antidiabetes; phycocyanin has been approved by the Food and Drug Administration as a natural dye and commercialized as a raw food material in Europe (Pagels et al., 2019). Besides that, bioactive carotenoids and polyunsaturated fatty acids have been widely utilized in pharmaceutical, nutraceutical, and cosmeceutical industries (Meléndez-Martínez, 2019).
Therefore, the mass culture of economical algal species is of great significance. Nowadays, only several species such as Spirulina platensis, Chlorella vulgaris, and Dunaliela salina have been successfully cultivated on a large scale (Schipper et al., 2019), largely due to their extraordinary adaptation to outdoor open pond systems. The open pond system is exceedingly common because it is cost-effective, energy-efficient, and environmentally-friendly (Grossmann et al., 2019). However, considering the almost inevitable biological contamination in open ponds, the isolation of promising algal species with good adaptation to environmental stresses such as high alkalinity is important. For example, high concentrations of NaHCO3 were used in open pond cultures of Spirulina to inhibit the contamination with other algae, and thus maintained an outdoor algal monoculture (Volkmann et al., 2008).
Alternative cyanobacterial species aside from Spirulina that have the potential to produce valueadded molecules and tolerate different environmental stresses also need investigation, such as Cyanobacterium aponinum, which was isolated from different thermal springs (Moro et al., 2007; Meng et al., 2018; Strunecký et al., 2019). Gris et al. (2017) reported that C. aponinum was able to accumulate released polysaccharides, phycocyanin, β-carotene, and zeaxanthin. The crude lipid content and C16 and C18 methyl ester yield of C. aponinum were also notably high compared with other previously reported cyanobacterial strains (Karatay and Dönmez, 2011). Also, the immunomodulatory calciferous exopolysaccharide (EPS-Ca) released from C. aponinum was beneficial for the psoriasis patients bathing in the Blue Lagoon (Gudmundsdottir et al., 2015, 2019). In virtue of skin conditioning functions, C. aponinum Ferment was put on the list of International Nomenclature of Cosmetic Ingredients (Mourelle et al., 2017). Additionally, C. aponinum had a broad range of tolerance to environmental stresses such as high temperature, high concentrations of carbon dioxide (CO2), and low pH (Meng et al., 2018). Despite all this, at present, the cultivation of C. aponinum still limits to a laboratory scale, except one pilot-scale culture in open ponds with detectable contaminations with green algae and diatoms (Winckelmann et al., 2016).
2 MATERIAL AND METHOD 2.1 Isolation of Cyanobacterium sp. SCSIO-45682The cyanobacterial strain was isolated by streak plate method from water samples collected from the open pond of a marine oleaginous green alga (Picochlorum sp. SCSIO-45015) (109°19′38″E, 18°18′31″N, Hainan, China) and was numbered as SCSIO-45682. The medium for the isolation of the algal strain was f/2 medium (with a salinity of 25) composed of: NaHCO3 (0.50 g/L); NaNO3 (0.40 g/L); NaH2PO4·2H2O (20 mg/L); Na2EDTA·2H2O (4.4 mg/L); FeCl3·6H2O (3.2 mg/L); MnCl2·4H2O (0.18 mg/L); ZnSO4·7H2O (22 μg/L); CoCl2·6H2O (10 μg/L); CuSO4·5H2O (9.8 μg/L); Na2MoO4·2H2O (6.3 μg/L). The water samples were streaked on the solid medium containing 1.5% agar and cultured at 25±1 ℃ and illuminated with fluorescent lamps at 60±5 μmol photons/(m2·s) on a 24 h꞉0 h (light: dark) photoperiod (Philips T8, Koninklijke Philips Electronics N.V., China). After cultivation for two weeks, cyanobacterial colonies growing on the plates were partly picked out, and were observed under a light microscope (BX53, Olympus Co., Ltd., Japan, magnification up to 1 000×). Monoclonal colonies of cyanobacterial cells characterized by morphology were obtained after plate streaking for three times. Monoclonal colonies were picked and transferred to a 250-mL Erlenmeyer flask containing 150-mL f/2 medium. The culture was maintained under the same temperature and light conditions as mentioned above.
2.2 Identification of Cyanobacterium sp. SCSIO-45682The light microscope (LM) observation was made with an optical microscope (BX53, Olympus Co., Ltd., Japan) equipped with digital image acquisition (Leica Application Suite X, Leica Camera AG, Germany). Genomic DNA was extracted using a DNA kit (E.Z.N.A.TM HP Plant DNA Kit, OMEGA Bio-Tech Co., Ltd., Georgia). The 16S rRNA gene was amplified by polymerase chain reaction (PCR, 2720, Thermo Fisher Scientific, China) using primers LZF (5′-AGAGTTTGATCCTGGCTCAG-3′) and LZR (5′-AAGGAGGTGATCCAGCCGCA-3′). The PCR reaction used in the following conditions: predenaturation at 94 ℃ for 6 min, followed by 36 cycles of denaturation at 94 ℃ for 45 s, annealing at 50 ℃ for 45 s, and extension at 72 ℃ for 90 s with a final extension at 72 ℃ for 10 min. The internal transcribed spacer (ITS) rRNA gene was amplified using the primers 322F (5′-TGTACACACCGCCCGTC-3′) and 340R (5′-CTCTGTGTGCCTAGGTATCC-3′). The PCR conditions were pre-denaturation at 94 ℃ for 3 min, followed by 10 cycles of denaturation at 94 ℃ for 45 s, annealing at 53 ℃ for 40 s, extension at 68 ℃ for 75 s, and 25 cycles of denaturation at 90 ℃ for 45 s, annealing at 53 ℃ for 40 s, extension at 68 ℃ for 75 s with a final extension at 68 ℃ for 7 min. Both the PCR products were purified and sequenced by Guangzhou Tianyi Huiyuan Gene Technology Co., Ltd., China. The identities of the sequences were checked using the BLAST program at the NCBI web server. Phylogenetic trees were built with MEGA 6.0 Software.
2.3 NaHCO3 treatment experimentThe inoculum of Cyanobacterium sp. SCSIO-45682 was grown in a 2 000-mL Erlenmeyer flask containing 1 200-mL f/2 medium under 150±5 μmol photons/ (m2·s) with a 24 h꞉0 h (light꞉dark) photoperiod (Philips T8, Koninklijke Philips Electronics N.V., China) at 25±1 ℃. For the NaHCO3 treatment experiment, f/2 medium supplemented with the initial NaHCO3 concentrations of 0, 1.0, 2.1, 4.2, 8.4, and 16.8 g/L, respectively, were prepared. The bicarbonate alkalinity of each medium was examined by Nordmann titration (Rieger and Weiland, 2006) and reached 111.00, 804.75, 1 387.50, 2 608.50, 4 939.49, and 10 489.48 mg/L (ormalized as CaCO3), respectively. The inoculum during exponential phase was centrifuged (3 000 r/min for 10 min). The pellets were gently washed with fresh NaHCO3-free f/2 medium and then transferred into f/2 medium with the different initial NaHCO3 concentrations for 14 days cultivation. The starting optical density at 750 nm (OD750) was 0.10 (the initial inoculum concentration was 0.030 g/L). The NaHCO3 treatments were maintained at the same temperature and light conditions as that of the inoculum. The growth was measured on Days 0, 2, 4, 6, 8, 10, 12, and 14. The pH was concurrently determined by a pH meter (FE20, Mettler-Toledo Instruments Co., Ltd., China). After 14 days of cultivation, the cyanobacterial biomass was harvested by centrifugation (8 500 r/min for 10 min). The pellets were subsequently washed triple with deionized water, freeze-dried at -50 ℃ for 48 h (FD-1-50, Beijing Boyikang Laboratory Instrument Co., Ltd., China), and stored at -20 ℃ for biochemical composition determination. Each treatment had three biological replicates (n=3), and all the measurements were conducted in triplicate (n=3).
2.4 Growth measurement 2.4.1 Optical density at the wavelength of 750 nmFor each culture, optical density at the wavelength of 750 nm (OD750) was evaluated using a visible spectrophotometer (722S; Shanghai Shun Yu Heng Ping Scientific Instrument Co., Ltd., China).
2.4.2 Dry weightA 5–100-mL (according to OD750) culture sample was filtered through pre-weighed 0.45-μm filters. After rinsing with deionized water for three times, the filters were dried at 80 ℃ until the weights were constant.
Specific growth rate (μ, /d) was calculated according to the following equation:
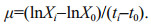 (1)
(1)Biomass productivity (P, g/(L·d)) was calculated according to the following equation:
 (2)
(2)where Xi and X0 are the dry weight (DW, g/L) at culture days ti and t0, respectively.
2.5 Biochemical composition determination 2.5.1 Intracellular total saccharide productionA 0.5-mol/L sulfuric acid solution was used to hydrolyze a 10-mg freeze-dried specimen at 80 ℃ for 1 h. The process was repeated four times. The intracellular total saccharide content was measured by phenol-sulfuric acid method (Dubois et al., 1956), utilizing D-glucose as standard. The results were normalized on DW of the specimens.
2.5.2 Exopolysaccharide productionA 5–100-mL aliquot of the cultures was centrifuged at 8 500 r/min for 10 min. The supernatant was collected and dialyzed against deionized water for 72 h, cutting off polysaccharide with a molecular weight over 500 Da to avoid a possible disturbance of monosaccharide, oligosaccharide, and salts (MD77MM membrane, Viskase Co., Ltd., USA). EPS concentration was determined according to the phenol-sulfuric acid method (Dubois et al., 1956), using D-glucose as standard.
2.5.3 Crude protein productionThe crude protein content of a 0.10-g specimen was measured using the Kieldahl method (Ma and Zuazaga, 1942), with a value of 6.25 as a conversion factor. The results were normalized on DW of the specimens.
2.5.4 Total lipid productionThe total lipid content of an 80-mg specimen was measured using the method according to KhozinGoldberg et al. (2005). The results were normalized on DW of the specimens.
2.5.5 Pigment and phycobiliprotein productionA 10-mg specimen was used to extract pigments by 100% acetone, stirring at 4 ℃ for 48 h (avoid light). Chlorophyll a and total carotenoids contents were measured by a spectrophotometer (TU-1810, Persee Instrument Co., Ltd., China) and calculated according to the equations proposed by EhlingSchulz et al. (1997). Carotenoids profiles were determined by analyzing the same specimens using high performance lipid chromatography (HPLC, 1525, Waters Corporation, USA), with a photodiode detector (2996, Waters Corporation, USA). An aliquot of 20 μL of the extract was injected, and the mobile phase comprised 90% acetonitrile as solvent A and 100% ethyl acetate as solvent B. The retention time and absorption spectrum were used to identify single pigments. Phycobiliproteins were determined according to Bennett and Bogorad (1973). The results were normalized on DW of the specimens.
2.6 Statistical analysisThe data were presented as mean±standard deviation of three independent biological replicates and three technical replicates. Differences between treatments were statistically analyzed by one-way analysis of variance (ANOVA) using SPSS 18.0 (SPSS Inc., USA) (P value less than 0.05 was considered to indicate significance). Graphing was performed using Origin Pro 8.5 software (OriginLab Corporation, USA).
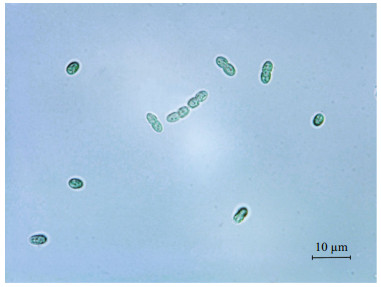
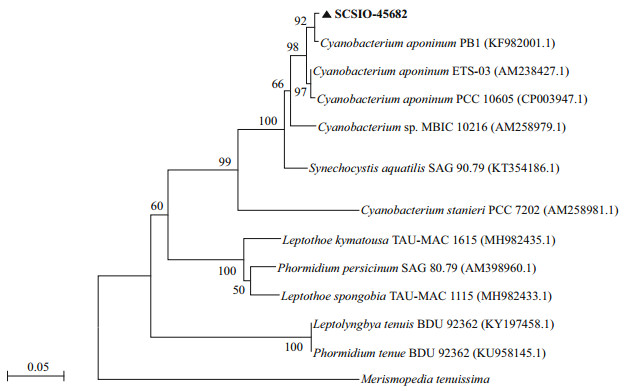
3 RESULT 3.1 Identification of Cyanobacterium sp. SCSIO-45682The cells of isolated strain numbered SCSIO-45682 were solitary or showed in pairs during binary fission under the optical microscope. They appeared bluegreen, oval, 2–3 μm in length, and 1–2 μm in diameter (Fig. 1). The 16S rDNA sequence was 1 421 bp in length, and the ITS rDNA sequence was 479 bp long. BLAST analysis showed that SCSIO-45682 was closely related to C. aponinum PCC 10605 (with a high identity of 99.6% in 16S rDNA sequence and 98.7% in ITS rDNA sequence). The 16S rDNA phylogenetic tree revealed that SCSIO-45682 clustered with C. aponinum PCC 10605 (with a bootstrap value of 98%) (Fig. 2). The ITS rDNA phylogenetic tree indicated that SCSIO-45682 clustered with C. aponinum PB1 (with a bootstrap value of 92%) (Fig. 3). Thus, SCSIO-45682 was closely related to C. aponinum and was named as Cyanobacterium sp. SCSIO-45682.
|
| Fig.1 Optical microscopy graphs of Cyanobacterium sp. SCSIO-45682 grown in f/2 medium Scale bar=10 μm. |

|
| Fig.2 The Maximum-likelihood phylogenetic tree based on nearly complete 16S rDNA sequences of Cyanobacterium sp. SCSIO-45682 Bootstrap values (expressed as percentages of 1 000 replicates) >50% are shown at branch points (scale bar=0.02 substitutes per nucleotide position). |
|
| Fig.3 The Maximum-likelihood phylogenetic tree based on ITS rDNA sequences of Cyanobacterium sp. SCSIO-45682 Bootstrap values (expressed as percentages of 1 000 replicates) >50% are shown at branch points (scale bar=0.05 substitutes per nucleotide position). |
Cyanobacterium sp. SCSIO-45682 showed significant differences in growth under different initial NaHCO3 concentrations ranging from zero to 16.8 g/L. According to OD750 (Fig. 4a), the growth of Cyanobacterium sp. SCSIO-45682 improved under high NaHCO3 concentrations, and the group with no NaHCO3 added revealed noticeably slow growth (OD750=0.3 on the final day of culture). After day 8, OD750 of the 8.4-g/L NaHCO3 group exceeded that of the 16.8-g/L NaHCO3 group, and reached the highest OD750 of 2.5 on Day 14.

|
| Fig.4 Growth of Cyanobacterium sp. SCSIO-45682 under different initial NaHCO3 concentrations The values are presented as mean±standard deviation. a. optical density at 750 nm; b. biomass concentration; c. pH. |
Biomass concentrations of Cyanobacterium sp. SCSIO-45682 under different initial NaHCO3 concentrations are shown in Fig. 4b. No remarkable differences were observed between any of the NaHCO3 concentrations in the first two days. The biomass concentrations of cultures with 4.2–16.8-g/L NaHCO3 greatly increased from Day 8 to Day 12. On the last day of cultivation, the biomass concentration of the 8.4-g/LNaHCO3 group reached 2.5 g/L, 41.7% higher than that of the 16.8-g/L NaHCO3 group (P < 0.05). Similarly, the highest average specific growth rate, maximum specific growth rate, and biomass productivity were obtained by the 8.4-g/L NaHCO3 group, which reached 0.32/d, 0.49/d, and 0.18 g/(L·d) respectively (Table 1).

|
The pH variations of the culture medium were showed in Fig. 4c. The highest pH value (10.5) among all groups was obtained by the 4.2-g/L NaHCO3 group on Day 12. The 8.4-g/L and 16.8-g/L groups reached a pH value of 10.2 and 9.7, respectively, on Day 12, and reached 9.4 and 9.3, respectively, on Day 14.
3.3 Effects of different initial NaHCO3 concentrations on intracellular total saccharide production of Cyanobacterium sp. SCSIO-45682The intracellular total saccharide content of Cyanobacterium sp. SCSIO-45682 significantly increased with the increase of initial NaHCO3 concentrations from zero to 16.8 g/L (Fig. 5a). The maximum intracellular total saccharide content reached 49.2% DW in the 16.8-g/L NaHCO3 group. However, taking the biomass concentration into account, the maximum intracellular total saccharide productivity was detected in the 8.4-g/L NaHCO3 group, which was up to 79 mg/(L·d).

|
| Fig.5 Biochemical composition of Cyanobacterium sp. SCSIO-45682 under different initial NaHCO3 concentrations The values are presented as mean±standard deviation. a. intracellular total saccharide; b. crude protein; c. total lipid. |
The crude protein content of Cyanobacterium sp. SCSIO-45682 markedly declined under initial NaHCO3 concentrations from 2.1 g/L to 16.8 g/L (Fig. 5b). The crude protein content of the 16.8-g/L NaHCO3 group decreased to 22.3% DW, which was only 45.9% of that of the 2.1-g/L NaHCO3 group (achieved the highest content compared with other groups) (P < 0.05). Whereas, due to the high biomass yield, the 8.4-g/LNaHCO3 groupexhibited the highest crude protein productivity of 44 mg/(L·d).
The total lipid content remained relatively stable ranging between 15.2% DW and 20.1% DW under different initial NaHCO3 concentrations (Fig. 5c). The 8.4-g/L NaHCO3 group showed the maximum total lipids productivity of 31 mg/(L·d) among all cultures.
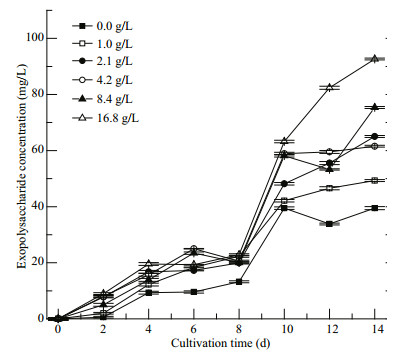
3.5 Accumulation of EPS of Cyanobacterium sp. SCSIO-45682 under different initial NaHCO3 concentrationsAs shown in Fig. 6, the EPS concentration greatly ascended with increment of initial NaHCO3 concentrations. No significant differences in EPS concentrations among different treatments were observed from Day 0 to Day 4 between the treatments. The 4.2 and 8.4-g/L NaHCO3 groups showed higher EPS concentrations than others' from Day 4 and Day 8. Notably, the EPS accumulation in all groups increased rapidly from Day 8 to Day 10. At the end of cultivation, the 16.8-g/L NaHCO3 group showed the highest EPS concentration of 93 mg/L, 22.9% higher than that of the 8.4-g/LNaHCO3 group (P < 0.05).
|
| Fig.6 Variation of exopolysaccharide concentration of Cyanobacterium sp. SCSIO-45682 under different initial NaHCO3 concentrations The values are presented as mean±standard deviation. |
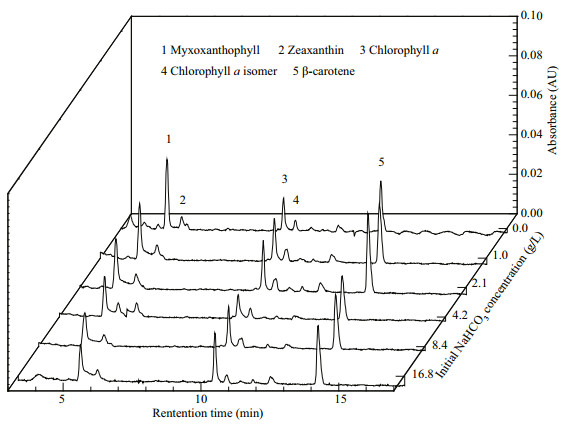
As shown in Table 2, the chl-a productivity variation was consistent with that of the biomass productivity under different initial NaHCO3 concentrations. The highest chl-a productivity and total carotenoids productivity were observed in the 8.4-g/L NaHCO3 group, up to 1.51 mg/(L·d) and 0.63 mg/(L·d), respectively. The pigments profile by HPLC (Fig. 7) suggested that Cyanobacterium sp. SCSIO-45682 possessed five major pigments which were identified as myxoxanthophyll, zeaxanthin, chl a, chl a isomer, and β-carotene, respectively. According to peak area of the zeaxanthin under different initial NaHCO3 concentrations, there was a declining trend of zeaxanthin with the increase of NaHCO3 concentrations. The highest phycocyanin productivity was found in the 16.8-g/L NaHCO3 group, reaching 6.0 mg/(L·d) (Table 2).

|
|
| Fig.7 Pigment composition by HPLC of Cyanobacterium sp. SCSIO-45682 under different initial NaHCO3 concentrations |
In terms of industrialization, the screening of algal strains that can adapt to extreme environments, grow rapidly, and accumulate high-value metabolites shall be the first step towards downstream biotechnological production. In this work, one novel cyanobacterial strain was isolated and identified as Cyanobacterium sp. SCSIO-45682. The growth adaptability and biochemical composition of this strain under different initial NaHCO3 concentrations were investigated.
In this work, NaHCO3 rather than CO2 was utilized as dissolved inorganic carbon source, considering the comparatively lower solubility and the high cost of carbon capture, compression, and transportation of CO2; the use of high alkalinity can also improve the transfer of CO2 into the culture medium (Chi et al., 2014). According to previous studies, C. aponinum PCC 10605 could efficiently use both NaHCO3 and CO2 as carbon source (Gris et al., 2017), and C. aponinum OUC1 may preferentially take up bicarbonate because of a sophisticated CO2 concentrating mechanism (Badger and Price, 2003; Meng et al., 2018).
The results showed that Cyanobacterium sp. SCSIO-45682 had good adaptation to high concentrations of NaHCO3 (up to 16.8 g/L, the same NaHCO3 concentration in the Zarrouk formula) (Zarrouk, 1966) (Fig. 4a & b), which may provide a theoretical possibility for the monoculture of Cyanobacterium sp. SCSIO-45682 in outdoor open ponds. Vonshak et al. (1983) reported that a monoculture of Spirulina could be sustained both in the laboratory and outdoors under at least 16.8-g/L NaHCO3. Furthermore, high concentrations of NaHCO3 improved the growth of Cyanobacterium sp. SCSIO-45682 (Fig. 4b), which is highly significant because the accumulation of biomass is the primary index to evaluate the potential of capable algal strains. It was noteworthy that biomass yield of Cyanobacterium sp. SCSIO-45682 under high NaHCO3 concentrations was much higher than that of previously reported cyanobacterial strains, perhaps due to the good adaptation to low irradiance of Cyanobacterium sp. SCSIO-45682 observed in our laboratory (data not shown), and similar adaptation was observed in Aphanizomenon (Bradburn et al., 2012). Cyanobacterium sp. SCSIO-45682 reached a final biomass concentration of 2.5 g/L at 0.1-mol/L (8.4 g/L) NaHCO3. Gris et al. (2017) reported that C. aponinum PCC 10605 reached a final biomass of 0.513 g/L using NaHCO3 as carbon source under 150 μmol photons/(m2·s), the same light intensity as that of Cyanobacterium sp. SCSIO-45682. Chi et al. (2013) reported that an extremely alkalihalophilic cyanobacterium Euhalothece sp. reached a final biomass concentration of 4.79 g/L in medium with 1-mol/L (84.0 g/L) NaHCO3. Zhu et al. (2018) reported that Spirulina reached a biomass concentration of 1.55 g/L at 0.3-mol/L (25.2 g/L) NaHCO3 cultivated in Erlenmeyer flasks, and obtained a biomass concentration of 2.24 g/L using a 1.0-m2 floating horizontal photobioreactor with NaHCO3 as carbon source. In addition, the medium of Cyanobacterium sp. SCSIO-45682 reached the highest pH value of 10.5 at 4.2-g/L NaHCO3 rather than at higher NaHCO3 concentrations (Fig. 4c), because sufficient bicarbonate/carbonate can control high pH by acting as a strong buffer (Chi et al., 2013), which may alleviate the negative effects of extremely high pH on the growth of Cyanobacterium sp. SCSIO-45682, and similar results were also found in the cultivation of Spirulina, Synechocystis sp. and Cyanothece sp. in high NaHCO3 concentrations (Chi et al., 2014; Zhu et al., 2018).
The findings also indicated that high concentrations of NaHCO3 promoted the accumulation of polysaccharide of Cyanobacterium sp. SCSIO-45682. Cyanobacteria can absorb carbon and convert it into macromolecules such as polysaccharide, protein, and lipid, and the regulation of cultivation parameters is a feasible method to manipulate carbon allocation and to produce desirable metabolites (GonzálezFernández and Ballesteros, 2012). In the present work, the intracellular total saccharide content increased with the increment of NaHCO3 concentration, and accounted for 49.2% DW in the 16.8-g/L NaHCO3 group (Fig. 5a), reaching a considerably high carbohydrate content in comparison to cyanobacterial strains of Spirulina maxima (13%– 16% DW), Synechoccus sp. (15% DW), and Anabaena cylindrical (25%–30% DW) (Becker, 2004). Furthermore, the results showed that the crude protein content in the 16.8-g/LNaHCO3 group concomitantly declined by 51.4% (P < 0.05) compared with the group with no NaHCO3 added (Fig. 5b), which might explain the corresponding raise in the intracellular total saccharide content of Cyanobacterium sp. SCSIO-45682. This was in accordance with the findings in Porphyridium purpureum, whose carbohydrate content significantly increased with the decline of protein content with the consumption of NaNO3 (Li et al., 2019). For total lipid content, Cyanobacterium sp. SCSIO-45682 grown under different initial NaHCO3 concentrations remained relatively stable (15.2%–20.1% DW) (Fig. 5c). Karatay and Dönmez (2011) reported that crude lipids extracted from C. aponinum (45.0% DW) could act as a novel source for biodiesel production.
Additionally, high concentrations of NaHCO3 improved the EPS secretion of Cyanobacterium sp. SCSIO-45682. The protective response of cyanobacteria with an extracellular polymeric gel layer of EPS may inhibit the diffusion of ions through the cell surface (Kumar et al., 2007), which may explain the enhancement of EPS yield and viscosity of medium with the increase of NaHCO3concentration. It was noteworthy that EPS-Ca secreted by C. aponinum can up-regulate the interleukin-10 (Gudmundsdottir et al., 2015) as well as inhibit the expression of spleen tyrosine kinase and the encoding gene for the Dectin-1 receptor, which showed remarkable immunomodulatory activities in vitro (Gudmundsdottir et al., 2019). In the present study, the EPS yield of Cyanobacterium sp. SCSIO-45682 ascended during cultivation. By the final day of culture, the 16.8-g/L group obtained the maximum EPS yield of 93 mg/L (Fig. 6). The EPS productivity of the 16.8-g/L NaHCO3 group was 6.6 mg/(L·d) (Fig. 6), higher than that of Synechocystis sp. PCC 6803, Anabaena sp. C5, and Nostoc sp. 2S9B (Su et al., 2007). Salinity and nutrient composition of the medium for Cyanobacterium sp. SCSIO-45682 may play a significant role in the synthesis of EPS, as increasing salinity can affect the EPS secretion of cyanobacterial strains of Anabaena sp., Aphanocapsa halophyta, and Cyanothece (Sudo et al., 1995; Nicolaus et al., 1999; Su et al., 2007).
Another thing should be mentioned is that high concentrations of NaHCO3 induced the production of chl a and phycocyanin of Cyanobacterium sp. SCSIO-45682. Photosynthetic pigments are major members of light-harvesting compounds responsible for capturing light energy in cyanobacteria (Liu and Blankenship, 2019). Specifically, chlorophylls, carotenoids, and phycobiliproteins play a crucial part in the structure and photo-protection of the photosynthetic apparatus of cyanobacteria. These key pigments might help capture and assimilate light energy and promote the photosynthesis activity, which contributes to the boost of biomass production (Viola et al., 2019). This may elucidate the result that the chl-a productivity agreed with the variation of biomass productivity (Tables 1 & 2). However, there was a declining trend of zeaxanthin with the increase of initial NaHCO3 concentrations. This is probably due to the photoprotection of zeaxanthin. The stress conditions (e.g. high light intensity and carbon limitation) could obviously enhance photoinhibition in cyanobacteria (Ibelings and Maberly, 1998). The pigment zeaxanthin could protect PSII by decreasing the level of singlet oxygen (Kusama et al., 2015). In the present study, the accumulation of zeaxanthin in Cyanobacterium sp. SCSIO-45682 was found in treatments grown in low NaHCO3 concentrations. The growth was inhibited by limited inorganic carbon, and average light absorption by every cell could increase due to the lower cell density, thus zeaxanthin accumulated to protect against photoinhibition. Similar results were observed in Synechococcus sp. and Cyanobacterium aponinum PCC 10605 (Masamoto and Furukawa, 1997; Gris et al., 2017).
Therefore, Cyanobacterium sp. SCSIO-45682 can be considered as a promising cyanobacterial strain cultivated under high concentrations of NaHCO3 to obtain polysaccharide. A further attempt at a pilotscale outdoor culture with 8.4-g/L NaHCO3 was thus proposed in this work, aiming to prevent biological contamination and harvest large quantities of biomass with high-value molecules.
5 CONCLUSIONAlkalinity plays a significant role in the growth and biochemical composition of Cyanobacterium sp. SCSIO-45682 by preventing contamination and acting as an inorganic carbon source. The present work showed that Cyanobacterium sp. SCSIO-45682 had good adaptation to the NaHCO3 concentration of 16.8 g/L, which was comparable to Spirulina. Furthermore, high concentrations of NaHCO3 enhanced the biomass, intracellular total saccharide, EPS, chl a, and phycocyanin yields of Cyanobacterium sp. SCSIO-45682. Hence, it may be practicable to cultivate Cyanobacterium sp. SCSIO-45682 in alkaline systems to obtain polysaccharide and to further explore its potential of commercial applications.
6 DATA AVAILABILITY STATEMENTThe data that support the findings of the current study are available from the corresponding author on reasonable request.
7 CONFLICT OF INTERESTThe authors declare that they have no conflict of interest.
Badger M R, Price G D. 2003. CO2 concentrating mechanisms in cyanobacteria: molecular components, their diversity and evolution. Journal of Experimental Botany, 54(383): 609-622.
DOI:10.1093/jxb/erg076 |
Becker W. 2004. Microalgae in human and animal nutrition. In: Richmond A ed. Handbook of Microalgal Culture: Biotechnology and Applied Phycology. Blackwell Publishing Ltd, London, UK. p. 312-351.
|
Bennett A, Bogorad L. 1973. Complementary chromatic adaptation in a filamentous blue-green alga. The Journal of Cell Biology, 58(2): 419-435.
DOI:10.1083/jcb.58.2.419 |
Bradburn M J, Lewis Jr W M, McCutchan Jr J H. 2012. Comparative adaptations of Aphanizomenon and Anabaena for nitrogen fixation under weak irradiance. Freshwater Biology, 57(5): 1 042-1 049.
DOI:10.1111/j.1365-2427.2012.02765.x |
Chi Z Y, Elloy F, Xie Y X, Hu Y C, Chen S L. 2014. Selection of microalgae and cyanobacteria strains for bicarbonate-based integrated carbon capture and algae production system. Applied Biochemistry and Biotechnology, 172(1): 447-457.
DOI:10.1007/s12010-013-0515-5 |
Chi Z Y, Xie Y X, Elloy F, Zheng Y B, Hu Y C, Chen S L. 2013. Bicarbonate-based integrated carbon capture and algae production system with alkalihalophilic cyanobacterium. Bioresource Technology, 133: 513-521.
DOI:10.1016/j.biortech.2013.01.150 |
Demay J, Bernard C, Reinhardt A, Marie B. 2019. Natural products from cyanobacteria: focus on beneficial activities. Marine Drugs, 17(6): 320.
DOI:10.3390/md17060320 |
Dubois M, Gilles K A, Hamilton J K, Rebers P A, Smith F. 1956. Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28(3): 350-356.
DOI:10.1021/ac60111a017 |
Ehling-Schulz M, Bilger W, Scherer S. 1997. UV-B-induced synthesis of photoprotective pigments and extracellular polysaccharides in the terrestrial cyanobacterium Nostoc commune. Journal of Bacteriology, 179(6): 1 940-1 945.
DOI:10.1128/jb.179.6.1940-1945.1997 |
González-Fernández C, Ballesteros M. 2012. Linking microalgae and cyanobacteria culture conditions and key-enzymes for carbohydrate accumulation. Biotechnology Advances, 30(6): 1 655-1 661.
DOI:10.1016/).biotechadv.2012.07.003 |
Gris B, Sforza E, Morosinotto T, Bertucco A, La Rocca N. 2017. Influence of light and temperature on growth and high-value molecules productivity from Cyanobacterium aponinum. Journal of Applied Phycology, 29(4): 1 781-1 790.
DOI:10.1007/s10811-017-1133-3 |
Grossmann L, Hinrichs J, Weiss J. 2019. Cultivation and downstream processing of microalgae and cyanobacteria to generate protein-based technofunctional food ingredients. Critical Reviews in Food Science and Nutrition, published Online First, October 2019, https://doi.org/10.1080/10408398.2019.1672137.
|
Gudmundsdottir A B, Brynjolfsdottir A, Olafsdottir E S, Hardardottir I, Freysdottir J. 2019. Exopolysaccharides from Cyanobacterium aponinum induce a regulatory dendritic cell phenotype and inhibit SYK and CLEC7A expression in dendritic cells, T cells and keratinocytes. International Immunopharmacology, 69: 328-336.
DOI:10.1016/j.intimp.2019.01.044 |
Gudmundsdottir A B, Omarsdottir S, Brynjolfsdottir A, Paulsen B S, Olafsdottir E S, Freysdottir J. 2015. Exopolysaccharides from Cyanobacterium aponinum from the Blue Lagoon in Iceland increase IL-10 secretion by human dendritic cells and their ability to reduce the IL-17+RORγt+/IL-10+FoxP3+ ratio in CD4+ T cells. Immunology Letters, 163(2): 157-162.
DOI:10.1016/j.imlet.2014.11.008 |
Hayashi K, Hayashi T, Kojima I. 1996. A natural sulfated polysaccharide, calcium spirulan, isolated from Spirulina platensis: in vitro and ex vivo evaluation of anti-herpes simplex virus and anti-human immunodeficiency virus activities. AIDS Research and Human Retroviruses, 12(15): 1 463-1 471.
DOI:10.1089/aid.1996.12.1463 |
Ibelings B W, Maberly S C. 1998. Photoinhibition and the availability of inorganic carbon restrict photosynthesis by surface blooms of cyanobacteria. Limnology and Oceanography, 43(3): 408-419.
DOI:10.4319/lo.1998.43.3.0408 |
Karatay S E, Dönmez G. 2011. Microbial oil production from thermophile cyanobacteria for biodiesel production. Applied Energy, 88(11): 3 632-3 635.
DOI:10.1016/j.apenergy.2011.04.010 |
Khan M I, Shin J H, Kim J D. 2018. The promising future of microalgae: current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microbial Cell Factories, 17(1): 36.
DOI:10.1186/s12934-018-0879-x |
Khozin-Goldberg I, Shrestha P, Cohen Z. 2005. Mobilization of arachidonyl moieties from triacylglycerols into chloroplastic lipids following recovery from nitrogen starvation of the microalga Parietochloris incisa. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids, 1738(1-3): 63-71.
DOI:10.1016/j.bbalip.2005.09.005 |
Kumar A S, Mody K, Jha B. 2007. Bacterial exopolysaccharides - a perception. Journal of Basic Microbiology, 47(2): 103-117.
DOI:10.1002/jobm.200610203 |
Kusama Y, Inoue S, Jimbo H, Takaichi S, Sonoike K, Hihara Y, Nishiyama Y. 2015. Zeaxanthin and echinenone protect the repair of photosystem Ⅱ from inhibition by singlet oxygen in Synechocystis sp. PCC 6803. Plant and Cell Physiology, 56(5): 906-916.
DOI:10.1093/pcp/pcv018 |
Lau N S, Matsui M, Abdullah A A A. 2015. Cyanobacteria: photoautotrophic microbial factories for the sustainable synthesis of industrial products. BioMed Research International, 2015: 754934.
DOI:10.1155/2015/754934 |
Li T, Xu J, Wu H B, Jiang P L, Chen Z S, Xiang W Z. 2019. Growth and biochemical composition of Porphyridium purpureum SCS-02 under different nitrogen concentrations. Marine Drugs, 17(2): 124.
DOI:10.3390/md17020124 |
Liu H J, Blankenship R E. 2019. On the interface of light-harvesting antenna complexes and reaction centers in oxygenic photosynthesis. Biochimica et Biophysica Acta (BBA) - Bioenergetics, 1860(11): 148079.
DOI:10.1016/j.bbabio.2019.148079 |
Ma T, Zuazaga G. 1942. Micro-Kjeldahl determination of nitrogen. A new indicator and an improved rapid method. Industrial & Engineering Chemistry Analytical Edition, 14(3): 280-282.
DOI:10.1021/i560103a035 |
Masamoto K, Furukawa K. 1997. Accumulation of zeaxanthin in cells of the cyanobacterium, Synechococcus sp. strain PCC 7942 grown under high irradiance. Journal of Plant Physiology, 151(3): 257-261.
DOI:10.1016/S0176-1617(97)80250-7 |
Meléndez-Martínez A J. 2019. An overview of carotenoids, apocarotenoids, and vitamin A in agro-food, nutrition, health, and disease. Molecular Nutrition Food Research, 63(15): 1801045.
DOI:10.1002/mnfr.201801045 |
Meng F P, Cui H W, Wang Y J, Li X L. 2018. Responses of a new isolated Cyanobacterium aponinum strain to temperature, pH, CO2 and light quality. Journal of Applied Phycology, 30(3): 1 525-1 532.
DOI:10.1007/s10811-018-1411-8 |
Moro I, Rascio N, La Rocca N, Di Bella M, Andreoli C. 2007. Cyanobacterium aponinum, a new cyanoprokaryote from the microbial mat of Euganean thermal springs (Padua, Italy). Algological Studies, 123: 1-15.
DOI:10.1127/1864-1318/2007/0123-0001 |
Morone J, Alfeus A, Vasconcelos V, Martins R. 2019. Revealing the potential of cyanobacteria in cosmetics and cosmeceuticals- a new bioactive approach. Algal Research, 41: 101541.
DOI:10.1016/j.algal.2019.101541 |
Mourelle M L, Gómez C P, Legido J L. 2017. The potential use of marine microalgae and cyanobacteria in cosmetics and thalassotherapy. Cosmetics, 4(4): 46.
DOI:10.3390/cosmetics4040046 |
Nicolaus B, Panico A, Lama L, Romano I, Manca M C, De Giulio A, Gambacorta A. 1999. Chemical composition and production of exopolysaccharides from representative members of heterocystous and non-heterocystous cyanobacteria. Phytochemistry, 52(4): 639-647.
DOI:10.1016/S0031-9422(99)00202-2 |
Pagels F, Guedes A C, Amaro H M, Kijjoa A, Vasconcelos V. 2019. Phycobiliproteins from cyanobacteria: chemistry and biotechnological applications. Biotechnology Advances, 37(3): 422-443.
DOI:10.1016/j.biotechadv.2019.02.010 |
Rieger C, Weiland P. 2006. Prozessstörungen frühzeitig erkennen. Biogas Journal, 4: 18-20.
|
Schipper K, Al Muraikhi M, Alghasal G S H S, Saadaoui I, Bounnit T, Rasheed R, Dalgamouni T, Al Jabri H M S J, Wijffels R H, Barbosa M J. 2019. Potential of novel desert microalgae and cyanobacteria for commercial applications and CO2 sequestration. Journal of Applied Phycology, 31(4): 2 231-2 243.
DOI:10.1007/s10811-019-01763-3 |
Strunecký O, Kopejtka K, Goecke F, Tomasch J, Lukavský J, Neori A, Kahl S, Pieper D H, Pilarski P, Kaftan D, Koblížek M. 2019. High diversity of thermophilic cyanobacteria in Rupite hot spring identified by microscopy, cultivation, single-cell PCR and amplicon sequencing. Extremophiles, 23(1): 35-48.
DOI:10.1007/s00792-018-1058-z |
Su C D, Chi Z M, Lu W D. 2007. Optimization of medium and cultivation conditions for enhanced exopolysaccharide yield by marine Cyanothece sp. 113. Chinese Journal of Oceanology and Limnology, 25(4): 411-417.
DOI:10.1007/s00343-007-0411-3 |
Sudo H, Burgess J G, Takemasa H N, Nakamura N, Matsunaga T. 1995. Sulfated exopolysaccharide production by the halophilic cyanobacterium. Aphanocapsa halophytia. Current Microbiology, 30(4): 219-222.
DOI:10.1007/BF00293636 |
Viola S, Bailleul B, Yu J F, Nixon P, Sellés J, Joliot P, Wollman F A. 2019. Probing the electric field across thylakoid membranes in cyanobacteria. Proceedings of the National Academy of Sciences of the United States of America, 116(43): 21 900-21 906.
DOI:10.1073/pnas.1913099116 |
Volkmann H, Imianovsky U, Oliveira J L B, Sant'Anna E S. 2008. Cultivation of Arthrospira (spirulina) platensis in desalinator wastewater and salinated synthetic medium: protein content and amino-acid profile. Brazilian Journal of Microbiology, 39(1): 98-101.
DOI:10.1590/S1517-838220080001000022 |
Vonshak A, Boussiba S, Abeliovich A, Richmond A. 1983. Production of Spirulina biomass: maintenance of monoalgal culture outdoors. Biotechnology and Bioengineering, 25(2): 341-349.
DOI:10.1002/bit.260250204 |
Winckelmann D, Bleeke F, Bergmann P, Elle C, Klöck G. 2016. Detection of weed algae in open pond cultures of Cyanobacterium aponinum using PAM. International Aquatic Research, 8(1): 81-90.
DOI:10.1007/s40071-016-0126-1 |
Zarrouk C. 1966. Contribution à L'ėtude Ďune cyanophycė. Influence de Divers Facteurs physiques et Chimiques Sur la Croissance et la Photosynthėse de Spirulina maxima (Setch. Et Garndner) Geitler. Ph. D. thesis, Faculte des Sciences, Universitė de Paris, Paris.
|
Zheng W F, Chen C F, Cheng Q P, Wang Y Q, Chu C C. 2006. Oral administration of exopolysaccharide from Aphanothece halophytica (Chroococcales) significantly inhibits influenza virus (H1N1)-induced pneumonia in mice. International Immunopharmacology, 6(7): 1 093-1 099.
DOI:10.1016/j.intimp.2006.01.020 |
Zhu C B, Zhai X Q, Wang J H, Han D S, Li Y H, Xi Y M, Tang Y J, Chi Z Y. 2018. Large-scale cultivation of Spirulina in a floating horizontal photobioreactor without aeration or an agitation device. Applied Microbiology and Biotechnology, 102(20): 8 979-8 987.
DOI:10.1007/s00253-018-9258-0 |
 2021, Vol. 39
2021, Vol. 39


