Institute of Oceanology, Chinese Academy of Sciences
Article Information
- LI Yuhang, SUN Zhongmin, NAGUMO Tamotsu, XU Kuidong
- Two new marine benthic diatom species of Hippodonta (Bacillariophyceae) from the coast of China: H. nanjiensis sp. nov. and H. qingdaoensis sp. nov.
- Journal of Oceanology and Limnology, 39(3): 1033-1041
- http://dx.doi.org/10.1007/s00343-020-0104-8
Article History
- Received Feb. 27, 2020
- accepted in principle Apr. 6, 2020
- accepted for publication Jul. 22, 2020
2 Echigo Natural History Laboratory, Kowada 719, Ojiya, Niigata 947-0041, Japan;
3 Laboratory for Marine Biology and Biotechnology, Pilot National Laboratory for Marine Science and Technology(Qingdao), Qingdao 266237, China;
4 University of Chinese Academy of Sciences, Beijing 100049, China;
5 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
The genus Hippodonta Lange-Bertalot, Metzeltin et Witkowski in Lange-Bertalot et al. (1996) was established and separated from Navicula Bory sensu lato based on its characters of a simple raphe system without an accessory rib, transapically elongated and cap-like terminal areas, and external raphe distal endings not or weakly deflected without extending to the valve mantle (Witkowski et al., 2000; Pavlov et al., 2013). Lange-Bertalot et al. (1996) described ten new Hippodotna species and transferred 11 species into this genus, which included eight species from coastal areas. Later, three new species from coastal areas were added to the genus (Witkowski et al., 2000; Potapova, 2013; Zhao et al., 2017). In contrast to these coastal species, most species of Hippodonta have been described from (ancient) lakes and rivers in various continents (Metzeltin et al., 2005; Blanco et al., 2012; Kulikovskiy et al., 2012; Van de Vijver et al., 2012; Pavlov et al., 2013; Peng et al., 2014). Presently, 95 species are included in the genus Hippodonta (Guiry 2020), of which only 11 have been described from coastal areas. Pavlov et al. (2013) separated Hippodonta into eight morphological groups. Most Hippodonta species from coastal areas were assigned into Groups 7 and 8, both of which have a linear valve outline.
In the past decade, marine benthic diatoms from the Bohai Sea, Yellow Sea and East China Sea have received increased attention. New genera, viz., Moreneis Park, Koh et Witkowski and Sternimirus Witkowski et Li, and many new species have been described in this region (Park et al., 2012, 2013; Li et al., 2015; Liu et al., 2015a, b, c; Witkowski et al., 2016; Chen et al., 2017; Zhao et al., 2017; Li et al., 2018). Molecular data indicate additional potential new taxa in this region (Witkowski et al., 2016; An et al., 2017). However, within the genus Hippodonta, only two species have been reported in this area, along the China Fujian coast of the East China Sea (Zhao et al., 2017). In the present study, we describe two new Hippodonta species sampled from intertidal sediments of the East China Sea and the Yellow Sea, providing insight into the diversity of Hippodonta from the coast of China.
2 MATERIAL AND METHODSampling was conducted at the Dasha'ao (27°27′45.6"N, 121°03′ 28.80"E) sandy beach in the Nanji Islands National Marine Natural Reserve, the East China Sea, on 13 November 2016 and at the No. 1 Bathing Beach, Qingdao City, the Yellow Sea (36°03′16.97"N, 121°20′19.27"E) on 2 September 2016 and 1 May 2019. At each site, three stations were selected for sample collection, one in each of the high, middle and low tidal zones. The upper 1–2 cm of sediment at each station was collected using a glass tube (2 cm in diameter), and glutaraldehyde was added a final concentration of 2.5% as fixative (Round et al., 1990).
Sediments (0.5 g) were cleaned using domestic bleach (Pip Unish, Uni. Charm, Tokyo) for 20 min and repeatedly washed with distilled water (via centrifugation three to five times at 1 000×g for 7 min; Nagumo and Kobayasi, 1990; Nagumo, 1995). Cleaned samples were mounted on glass slides with Mountmedia (Wako Pure Chemical Industries, Ltd. Osaka, Japan) for light microscopy (LM) observation. A Zeiss Imager Z2 and Nikon Eclipse 80i LM equipped with differential interference contrast (DIC) were used for LM observation. For scanning electron microscopy (SEM) observation, cleaned samples were placed on coverslips, air-dried and coated with osmium. A Hitachi S-3400 was used for SEM observation. For transmission electron microscopy (TEM) observation, cleaned samples were placed on Formvar-coated copper mesh grids and air-dried. A JEOL-2000EX was used for TEM observations.
Environmental factors were measured at Dasha'ao sandy beach, Nanji Islands. The temperature in situ was measured using a thermometer with the bulb immersed in the sediment to a depth of 2 cm on each sampling occasion. Pore water from the upper sediment was collected for salinity measurement using a refractometer (Model: RHS-10ATC). Grain size analysis was performed using a Laser Diffraction Particle Size Analyzer (Cilas 940L). The water content of the sediment layers was measured as a percentage of weight loss by drying the sediment at 60 ℃ for 48 h.
Terminology follows Anonymous (1975), Ross et al. (1979) and Round et al. (1990).
3 RESULTSpecies description
Class Bacillariophyceae Haeckel
Order Naviculales Bessey
Family Naviculaceae Kützing
Genus Hippodonta Lange-Bertalot, Metzeltin et Witkowsk
Hippodonta nanjiensis Yuhang Li, Nagumo et Kuidong Xu, sp. nov. (Figs. 1 & 2)

|
| Fig.1 LM and SEM micrographs of Hippodonta nanjiensis a–g. LM micrographs of Hippodonta nanjiensis, showing the morphological variation among individual cells; h, i, k. SEM micrographs of the external view of valve, showing the large round central area and longitudinal elongate areolae around the apices (arrow); j. SEM micrographs of the internal view of valve, showing the simple raphe without an accessory rib. Scale bars=10 μm (a–g), 2 μm (h–k). |

|
| Fig.2 SEM micrographs of Hippodonta nanjiensis a–b. external raphe terminal slits are slightly curved (arrows); c–d. external central raphe endings are slightly curved and expanded. Large and round central area with one or two areolae (arrows) near the valve margin. Areolae adjacent raphe slits near the central area are radiate (arrowheads). Scale bars=2 μm (a–b), 1 μm (c–d). |
Description: Frustule rectangular with rounded corners. Valves lanceolate with protracted acutely round apices, 13.0–31.0 μm (20.9±5.9 μm, mean±SD, n=15) in length and 2.5–5.2 μm (3.7±0.7 μm, n=15) in width. Raphe straight. Axial area narrow. Central area very large. Striae radiate near center and convergent near apices, 12–16/10 μm (Fig. 1a–g).
In SEM, valves are convex and depressed at the center (Fig. 1h–i). The raphe external distal endings are weakly curved to the same direction at apices, not extending to the valve mantle (Figs. 1h, i, 2a arrow, & b arrow). Internally distal raphe ends terminate in small helictoglossae (Fig. 1j). The external proximal raphe endings are close and slightly expanded externally (Fig. 2c & d). The internal proximal raphe endings are close and straight (Fig. 1j). The transapical striae are radiate near center and become convergent near apices (Fig. 1a–g). The striae forming areolae are apically elongate and parallel, except those adjacent to the central area which are oblique, (38–51)/10 μm (Fig. 2c & d arrowheads). The areolae are occluded internally by hymenes. The central area is large and round (Figs. 1h, i, 2c, & d), with one or two areolae near the valve margin (Fig. 2c & d arrows). A uniseriate rows of areolae surround the apices (Fig. 1h). The two central ones are longer than the others (Fig. 1h arrow).
Holotype: Holotype MBMCAS286531, illustrated in Fig. 1a, deposited in the Marine Biological Museum, Chinese Academy of Sciences (MBMCAS), Qingdao, China.
Isotype: MBMCAS286533, deposited in the MBMCAS, Qingdao, China.
Type locality: The Dasha'ao beach in the Nanji Islands, East China Sea (27°27′45.6"N, 121°03′28.80"E).
Etymology: Species name is derived from the type locality Nanji Islands.
Distribution and ecology: Hippodonta nanjiensis is currently known only from the type locality, where it occurs mainly in the middle intertidal zone of the Dasha'ao sandy beach at salinities ranging from 26 to 29. The sediment temperature at collection was approximately 26℃. The water content of the sediment at collection was 31.6%. The median grain size of the sediment is 254 μm. Amphora spp., Carinasigma minuta (Donkin) Reid, Donkinia spp. and Hantzschia marina (Donkin) Grunow were dominant diatoms at the type locality.
Hippodonta qingdaoensis Yuhang Li, Nagumo et Kuidong Xu, sp. nov. (Figs. 3 & 4)

|
| Fig.3 LM and SEM micrographs of Hippodonta qingdaoensis a–g. LM micrographs of Hippodonta qingdaoensis showing morphological variation among individual cells, note the two longitudinal lines of areolae on the secondary side of the valves; h–k. SEM micrographs of the external and internal valve face; note the deflected external raphe endings and the less developed areolae of the valve secondary side. Scale bars=5 μm (a–g), 2 μm (h–k). |
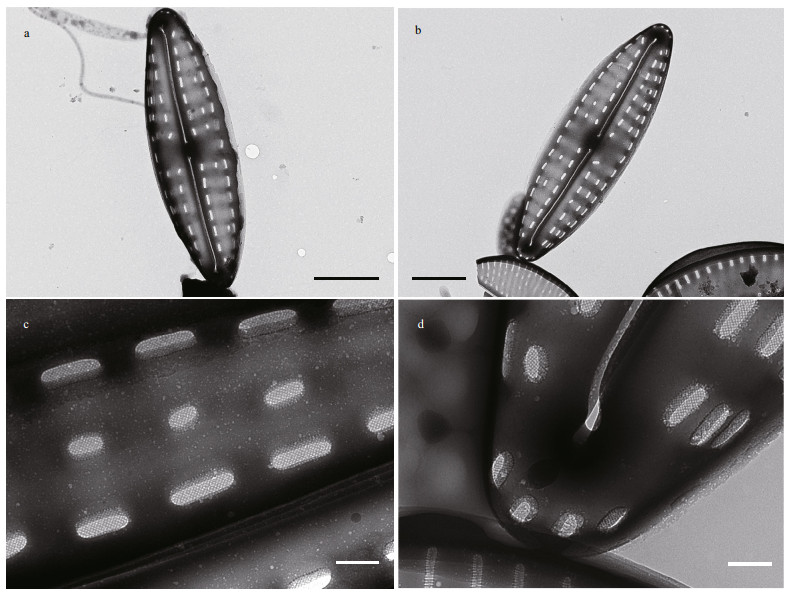
|
| Fig.4 TEM micrographs of Hippodonta qingdaoensis a–b. the whole valves, showing the less developed areolae on the valve secondary side; c. the shorter middle areolae; d. terminal area of the valve, showing that the external raphe terminal does not extend to the valve mantle and the four areolae on the apical mantle. Scale bars=2 μm (a–b), 200 nm (c–d). |
Description: Valves lanceolate with obtusely round apices, 6.8–11.0 μm (8.5±1.2 μm, mean±SD, n=15) in length and 2.2–3.9 μm (3.2±0.6 μm, n=15) in width. Raphe filiform, slightly curved. Axial area narrow. Central area rectangular or bow-tie shaped. Transapical striae slightly radiate, (11–14)/10 μm. Striae appear composed of two areolae on the primary side of the valve (Fig. 3a–g).
In SEM, valves are convex. The raphe external distal ends deflect to the same direction at apices, not extending to the valve mantle (Figs. 3h–k, 4a, b, & d). Internally distal raphe ends terminate in small helictoglossae (Fig. 3j). External proximal raphe endings are straight and slightly expanded (Fig. 3h, i, & k), whereas internally raphe endings are simple and straight (Fig. 3j). In TEM, the areolae in the middle transapical striae are less developed or absent on the primary side of the valve (Fig. 4a–c), presenting two areolae per stria under LM (Fig. 3a–g). The central area is rectangular or bow-tie-shaped, with one or two areolae near the valve margin (Figs. 3a–g & 4a–b). A uniseriate row of areolae surround the apices (Fig. 4d). The areolae internally occluded by hymenes, (26–33)/10 μm (Fig. 4c & d).
Holotype: Holotype MBMCAS286532, illustrated in Fig. 3f, deposited in the Marine Biological Museum, Chinese Academy of Sciences, Qingdao, China.
Isotype: MBMCAS286534, deposited in the MBMCAS, Qingdao, China.
Type locality: No. 1 Bathing Beach in Qingdao City, the Yellow Sea (36°03′16.97"N, 121°20′19.27"E).
Etymology: Species name is derived from the type locality Qingdao City.
Distribution and ecology: Hippodonta qingdaoensis is currently known only from the type locality, where it occurs mainly in the middle intertidal zone at salinities from 28 to 30. Small celled Anorthonies excentrica (Donkin) Grunow, Cocconeiopsis kantsiensis (Giffen) Witkowski, Lange-Bertalot et Metzeltin, Fallacia spp. and Navicula spp. were dominant diatoms at the type locality.
4 DISCUSSIONBoth Hippodonta nanjiensis and H. qingdaoensis have two features that differ from those of Navicula s.s., viz., a simple raphe without an accessory rib on the internal valve face (vs. raphe slit opening laterally in a markedly rib-like raphe sternum for most of its length) and raphe slits that are weakly curved at external valve apices without extending to the valve mantle (Fig. 4d; vs. usually curved or hooked terminal fissures extending to the valve mantle). Both features justify the assignment of the two species to the genus Hippodonta.
Hippodonta nanjiensis has a unique large round central area, whereas in all other congeners the central area is rhombic or lanceolate or bow-tie-shaped or rectangular fascia (Pavlov et al., 2013). Hippodonta nanjiensis mostly closely resembles H. subcostulata (Hustedt) Lange-Bertalot, Metzeltin et Witkowski in valve length, width, stria density, and stria orientation (Table 1). However, in addition to having a unique central area, H. nanjiensis differs from H. subcostulata in having a more acute narrow valve and a more elevated valve face. Hippodonta nanjiensis resembles H. grunowii Lange-Bertalot, Metzeltin et Witkowski in valve outline, length, width and stria density but differs from the latter in having striae on the subapical area (which are absent in H. grunowii). In addition, H. nanjiensis has a uniseriate row of areolae around the apices, with the two middle areolae being longer than the others. This feature is also exhibited by H. fujianensis, a species recently described from the southeast coast of China. However, the two species can be distinguished by the valve outline (narrowlinear with protracted apices in H. nanjiensis vs. elliptic-lanceolate to rhombic-lanceolate with nonprotracted apices in H. fujianensis), stria orientation (slightly radiate near center becoming convergent towards apices vs. radiate on the whole valve face) and the unique large round central area in H. nanjiensis.

|
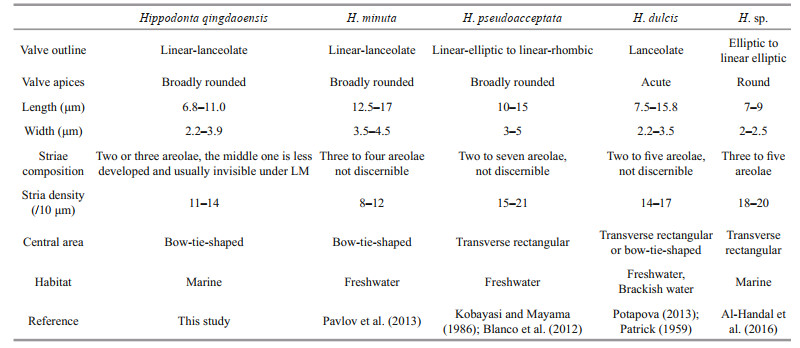
Hippodonta qingdaoensis differs from congeners in having a unique stria pattern: the areolae in the middle transapical striae are less developed or absent on the primary side of the valve, appearing two areolae per stria under LM (Table 2). One of the areolae is adjacent to the raphe and the other one is along the valve margin. This kind of stria pattern also appears in Navicula wasmundii Witkowski, Metzeltin et Lange-Bertalot. However, Navicula wasmundii lacks the two diagnostic features of Hippodonta mentioned above. Hippodonta qingdaoensis most closely resembles Hippodonta minuta Pavlov, Levkov, D.M. Williams et Edlund; both species have linear-lanceolate valves with broadly rounded apices and bow-tie-shaped central areas. However, in addition to exhibiting the unique feature mentioned above, H. qingdaoensis can be distinguished from H. minuta by its smaller valve size (6.8–11.0 μm vs. 12.5–17 μm) and higher stria density ((11–14)/10 μm vs. (8–12)/10 μm). Hippodonta qingdaoensis also resembles H. pseudoacceptata (Kobayasi) LangeBertalot and H. dulcis Potapova but differs from them in valve outline (linear-elliptic to linear rhombic in H. pseudoacceptata and lanceolate with acute apices in H. dulcis) and stria density, which is lower in H. qingdaoensis ((11–14)/10 μm in H. qingdaoensis vs. (15–21)/10 μm in H. pseudoacceptata and (14–17)/10 μm in H. dulcis). Moreover, H. minuta, H. pseudoacceptata and H. dulcis are all originally reported from freshwater or brackish water rather than marine environments (Kobayasi and Mayama, 1986; Pavlov et al., 2013; Potapova, 2013). Hippodonta qingdaoensis also resembles H. sp. sensu Al-Handal, Compére et Riaux-Gobin (2016). Both have a similar valve outline. However, H. qingdaoensis differs from the latter species in having its unique stria pattern and a much lower stria density ((11–14) /10 μm vs. (18–20)/10 μm).
|
During our survey of marine benthic diatoms in Dasha'ao, Nanji Islands and Qingdao No.1 Bathing Beach, we observed five Hippodonta species: H. nanjiensis sp. nov., H. qingdaoensis sp. nov., H. linearis (Østrup) Lange-Bertalot, Metzeltin, et Witkowski, H. cf. dulcis and H. fujianensis. These species represent all the Hippodonta species recorded from the China coast of the Pacific. In contrast, among the 13 Hippodonta species reported from coastal areas, nine were reported from the coastal areas of the Atlantic, viz., H. caotica Witkowski, Lange-Bertalot et Metzeltin; H. germainii Lange-Bertalot, Metzeltin et Witkowski; H. kaiseri Lange-Bertalot, Metzeltin et Witkowski; H. kociolekii Lange-Bertalot, Metzeltin et Witkowski; H. lesmonenis Lange-Bertalot, Metzeltin et Witkowski; H. linearis, H. ruthnielseniae Lange-Bertalot, Metzeltin et Witkowski; and H. umbilicatissima (Reichardt) Reichardt (Lange-Bertalot et al., 1996; Witkowski et al., 2000). AlHandal et al. (2016) also mentioned seven Hippodonta species in the West Indian Ocean; unfortunately, they could not identify these species due to insufficient specimens. The data indicate that Hippodonta species commonly occur in coastal areas worldwide. However, Hippodonta species are usually low in abundance and easily confused with Navicula species in routine LM examination because the diagnostic features cannot be revealed under light microscope. Thus, the diversity of Hippodonta in coastal areas is presumably still underestimated. Extensive taxonomic works remain needed to reveal the diversity and distribution of Hippodonta.
5 CONCLUSIONTwo new species of marine benthic diatoms, Hippodonta nanjiensis sp. nov. and H. qingdaoensis sp. nov., are described from the intertidal sandy sediments of Nanji islands in the East China Sea and Qingdao No. 1 Bathing Beach in the Yellow Sea, respectively. H. nanjiensis can be easily distinguished by its unique large round central area. H. qingdaoensis has a unique stria pattern from that of its congeners. This study expands our knowledge of the diversity and distribution of the genus Hippodonta on the coast of China.
6 DATA AVAILABILITY STATEMENTThe authors declare that the data supporting the findings of this study are available within the article. The data will be available on request from the corresponding author.
7 ACKNOWLEDGMENTThe authors are grateful to three anonymous reviewers for providing constructive suggestions, and to Mrs. Houcai CAI, Wandong CHEN, and the staff of the Nanji Islands National Marine Natural Reserve Research Institute for their help in sample collection.
Al-Handal A Y, Compère P, Riaux-Gobin C. 2016. Marine benthic diatoms in the coral reefs of Reunion and Rodrigues Islands, West Indian Ocean. Micronesica, 2016(3): 1-78.
|
An S M, Choi D H, Lee J H, Lee H, Noh J H. 2017. Identification of benthic diatoms isolated from the eastern tidal flats of the Yellow Sea: comparison between morphological and molecular approaches. PLoS One, 12(6): e0179422.
DOI:10.1371/journal.pone.0179422 |
Anonymous. 1975. Proposals for a standardization of diatom terminology and diagnoses. Nova Hedwigia Beiheft, 53: 323-354.
|
Blanco S, Van de Vijver B, Vinocur A, Mataloni G, Gomà J, Novais M H, Ector L. 2012. Hippodonta lange-bertalotii Van de Vijver, Mataloni & Vinocur sp. nov. and related small-celled Hippodonta taxa. Nova Hedwigia, Beiheft, 141: 39-52.
|
Chen C P, Sun J D, Zhao L, Sun L, Li X S, Liang J R, Gao Y H. 2017. Navicula amoyensis sp. nov. (Bacillariophyceae), a new benthic brackish diatom species from the Jiulong River estuary, Southern China. Phytotaxa, 291(4): 253-263.
DOI:10.11646/phytotaxa.291.4.2 |
Guiry M D. 2020. AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org/. Accessed on 2020-02-15.
|
Hustedt F. 1934. Die Diatomeenflora von Poggenpohls Moor bei Dötlingen in Oldenburg. Abhandlungen und Vorträgen der Bremer Wissenschaftlichen Gesellschaft, 8-9: 362-403.
|
Kobayasi H, Mayama S. 1986. Navicula pseudacceptata sp. nov. and validation of Stauroneis japonica H. Kob. Diatom, 2: 95-101.
|
Kulikovskiy M S, Lange-Bertalot H, Metzeltin D, Witkowski A. 2012. Lake Baikal: hotspot of endemic diatoms I. In: Lange-Bertalot H ed. Iconographia Diatomologica, vol. 23. Koeltz Scientific Books, Königstein. p. 7-607.
|
Lange-Bertalot H, Metzeltin D, Witkowski A. 1996. Hippodonta gen. nov. Umschreibung und Begründung einer neuen Gattung der Naviculaceae. In: Lange-Bertalot H ed. Iconographia Diatomologica, 4: 247-275.
|
Li Y H, Nagumo T, Xu K D. 2018. Morphology and molecular phylogeny of Pleurosira nanjiensis sp. nov., a new marine benthic diatom from the Nanji Islands, China. Acta Oceanologica Sinica, 37(10): 33-39.
DOI:10.1007/s13131-018-1298-x |
Li Y H, Suzuki H, Nagumo T, Tanaka J, Sun Z M, Xu K D. 2015. Fallacia decussata, sp. nov. : a new marine benthic diatom (Bacillariophyceae) from Northeast Asia. Phytotaxa, 224(3): 258-266.
DOI:10.11646/phytotaxa.224.3.4 |
Liu B, Blanco S, Huang B Q. 2015a. Two new Nitzschia species (Bacillariophyceae) from China, possessing a canal-raphe-conopeum system. Phytotaxa, 231(3): 260-270.
DOI:10.11646/phytotaxa.231.3.4 |
Liu B, Sterrenburg F A S, Huang B Q. 2015b. Gyrosigma xiamenense sp. nov. (Bacillariophyta) from the middle intertidal zone, Xiamen Bay, southern China. Phytotaxa, 222(4): 259-266.
DOI:10.11646/phytotaxa.222.4.3 |
Liu B, Williams D M, Huang B Q. 2015c. Gyrosigmarostratum sp. nov. (Bacillariophyta) from the low intertidal zone, Xiamen Bay, southern China. Phytotaxa, 203(3): 254-262.
DOI:10.11646/phytotaxa.203.3.4 |
Metzeltin D, Lange-Bertalot H, García-Rodríguez F. 2005. Diatoms of Uruguay. Compared with other taxa from South America and elsewhere. In: Lange-Bertalot H ed. Iconographia Diatomologica, Vol. 15. Koeltz Scientific Books, Königstein. 736p.
|
Nagumo T, Kobayasi H. 1990. The bleaching method for gently loosening and cleaning a single diatom frustule. Diatom, 5: 45-50.
|
Nagumo T. 1995. Simple and safe cleaning methods for diatom samples. Diatom, 10: 88.
|
Park J, Khim J S, Ryu J, Koh C H, Witkowski A. 2013. An emended description of the genus Fogedia (Bacillariophyceae) with reports of four species new to science from a Korean sand flat. Phycologia, 52(5): 437-446.
DOI:10.2216/12-120.1 |
Park J, Koh C H, Khim J S, Ohtsuka T, Witkowski A. 2012. Description of a new naviculoid diatom genus Moreneis gen. nov. (Bacillariophyceae) from sand flats in Korea. Journal of Phycology, 48(1): 186-195.
DOI:10.1111/j.1529-8817.2011.01087.x |
Patrick R. 1959. New species and nomenclatural changes in the genus Navicula (Bacillariophyceae). Proceedings of the Academy of Natural Sciences of Philadelphia, 111: 91-108.
|
Pavlov A, Levkov Z, Williams D M, Edlund M B. 2013. Observations on Hippodonta (Bacillariophyceae) in selected ancient lakes. Phytotaxa, 90(1): 1-53.
DOI:10.11646/phytotaxa.90.1.1 |
Peng Y M, Rioual P, Levkov Z, Williams D, Jin Z D. 2014. Morphology and ultrastructure of Hippodonta qinghainensis sp. nov. (Bacillariophyceae), a new diatom from Lake Qinghai, China. Phytotaxa, 186(2): 61-74.
DOI:10.11646/phytotaxa.186.2.1 |
Potapova M. 2013. The types of 22 Navicula (Bacillariophyta) species described by Ruth Patrick. Proceedings of the Academy of Natural Sciences of Philadelphia, 162(1): 1-23.
DOI:10.1635/053.162.0101 |
Ross R, Cox E J, Karayeva N I, Mann D G, Paddock T B B, Simonsen R, Sims P A. 1979. An amended terminology for the siliceous components of the diatom cell. Nova Hedwigia Beiheft, 64: 513-533.
|
Round F E, Crawford R M, Mann D G. 1990. The Diatoms: Biology and Morphology of the Genera. Cambridge University Press, Cambridge. 747p.
|
Van De Vijver B, Jarlman A, De Haan M, Ector L. 2012. New and interesting diatom species (Bacillariophyceae) from Swedish rivers. Nova Hedwigia Beiheft, 141: 237-254.
|
Witkowski A, Lange-Bertalot H, Metzeltin D. 2000. Diatom flora of marine coasts. In: Lange-Bertalot H ed. Iconographia Diatomologica, vol. 7. Koeltz Scientific Books, Königstein. 925pp.
|
Witkowski A, Li C L, Zgłobicka I, Yu S X, Ashworth M, Dąbek P, Qin S, Tang C, Krzywda M, Ruppel M, Theriot E C, Jansen R K, Car A, Płociński T, Wang Y C, Sabir J S M, Daniszewska-Kowalczyk G, Kierzek A, Hajrah N H. 2016. Multigene assessment of biodiversity of diatom (Bacillariophyceae) assemblages from the littoral zone of the Bohai and Yellow Seas in Yantai region of northeast China with some remarks on ubiquitous taxa. Journal of Coastal Research, 74(sp1): 166-195.
DOI:10.2112/SI74-016.1 |
Zhao L, Sun J D, Gao Y H, Liang J R, Sun L, Li X S, Chen C P. 2017. Hippodonta fujiannensis sp. nov. (Bacillariophyceae), a new epipsammic diatom from the low intertidal zone, Fujian Province, China. Phytotaxa, 295(1): 77-85.
DOI:10.11646/phytotaxa.295.1.7 |
 2021, Vol. 39
2021, Vol. 39


