Institute of Oceanology, Chinese Academy of Sciences
Article Information
- YANG Yeyin, HUANG Bozhu, TANG Yingzhong, XU Ning
- Allelopathic effects of mixotrophic dinoflagellate Akashiwo sanguinea on co-occurring phytoplankton: the significance of nutritional ecology
- Journal of Oceanology and Limnology, 39(3): 903-917
- http://dx.doi.org/10.1007/s00343-020-0132-4
Article History
- Received Mar. 25, 2020
- accepted in principle May. 5, 2020
- accepted for publication May. 21, 2020
2 Guangdong Environmental Monitoring Center, Guangzhou 510308, China;
3 Key Laboratory of Marine Ecology and Environmental Sciences, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China
Over recent decades, harmful algal blooms (HABs) have increased in coastal regions all over the world, and have become a threat to fisheries, environments, public health and economies worldwide (Fleming et al., 2006; Hallegraeff, 2010; Gobler and Sunda, 2012; Chakraborty and Feudel, 2014; Castro et al., 2016). The formation mechanisms of HABs are very complex and related to various aspects of physical chemistry and biology. Studies have shown that degraded water quality from increased nutrient pollution, the imbalance of nitrogen (N) to phosphorus (P) and interactions between marine microalgae (e.g. allelopathy), high irradiance and hydrological factors (water column stability instead of turbulence) may play important roles for the formation and maintenance of HABs (Anderson et al., 2008; Heisler et al., 2008; Tang and Gobler, 2010; Phlips et al., 2011; Driscoll et al., 2013; Accoroni et al., 2015; Ou et al., 2017).
Allelopathic effects of harmful algae on the composition of phytoplankton communities and the dynamics of HABs have attracted much attention (Granéli and Hansen, 2006; Tang and Gobler, 2010; Hattenrath-Lehmann and Gobler, 2011). Some harmful algae can produce and release allelochemicals (secondary metabolites) into the ambient environments, which inhibiting or killing target species (competitors or predators), thus causing massive algal proliferation (Driscoll et al., 2013). Allelopathy is considered to be a relevant part of the ecology giving harmful algae growth advantages over other algae (Fistarol et al., 2004). Algal allelochemicals can be released into the environment during growth, and they have a wide range of effectiveness and various modes of action on co-occurring algal cells (Legrand et al., 2003; Hakanen et al., 2014). The typical mode of action includes cell lysis, blistering, inhibiting the photosynthetic system, paralyzing cells and growth inhibition. For example, Karenia brevis secretes lipophilic allelochemicals to fuse with the cell membrane of target algae, damaging membrane integrity and leading to cell dissolution and death (Prince et al., 2008). Further research showed that K. brevis allelochemicals can reduce photosynthesis of target algae (Poulin et al., 2018). So far, the potential ecological significance of allelopathic effects of harmful algae, and the role in development and formation of HABs remains unclear.
The species Akashiwo sanguinea is widespread all over the world, and has been recorded in North America (Horner et al., 1997; Du et al., 2011; Badylak et al., 2014), South America (Kahru et al., 2004), Europe (Gómez and Boicenco, 2004), Asia (Katano et al., 2011) and Australia (Hallegraeff, 1992). Akashiwo sanguinea is eurythermal and euryhaline (Matsubara et al., 2007) and is famous for forming large, noticeable blooms (Horner et al., 1997) especially in spring or summer. In South Korea, A. sanguinea blooms have occurred almost every year for the past 30 years, causing huge economic losses (Park et al., 2013). In China, A. sanguinea is also a common harmful alga, reported in the coastal waters of Hong Kong (Hodgkiss and Lu, 2004), Xiamen (Yang et al., 2012) and Yantai (Chen et al., 2015). The outbreak of A. sanguinea blooms is directly or indirectly related to large numbers of deaths of fish and seabirds (Harper and Guillen, 1989; Jessup et al., 2009). Laboratory experiments have shown that A. sanguinea is harmful to abalone larvae (Botes et al., 2003). Our previous study demonstrated that strains of A. sanguinea isolated from Daya Bay in the South China Sea exhibited toxic effects on various marine animals, including fish, prawns and clams, and even caused acute damage to fish epidermis (Xu et al., 2017). However, the influence of A. sanguinea on cooccurring phytoplankton is not clear.
In this study, the allelopathic effects and the action mode of A. sanguinea on different marine microalgae were investigated. The regulation mechanism of nutrient factors on allelopathy of A. sanguinea was further explored, to reveal the ecological significance of allelopathy in the formation of the mixotrophic dinoflagellate A. sanguinea and provide a theoretical basis for the prevention and control of A. sanguinea blooms.
2 MATERIAL AND METHOD 2.1 Algal species and culture conditionsStains of A. sanguinea JX13 and JX14 were isolated from Daya Bay, South China Sea. Stain A. sanguinea CCMA256, provided by Dr. GU Haifeng of the Third Institute of Oceanography State Oceanic Administration, was isolated from Xiamen, Fujian, China. Stains of A. sanguinea ASNP6 and AS2 were provided by Dr. TANG Yingzhong: ASNP6 was isolated from Northport Bay (New York, USA) (Tang and Gobler, 2015), and AS2 was isolated from Chesapeake Bay (Virginia, USA) (Tang and Gobler, 2010).
Four target phytoplankton species were used in the study: two species of dinoflagellates (Scrippsiella trochoidea JX20 and Prorocentrum micans JX8), a haptophyte (Phaeocystis globosa JX4) and a cryptophyte (Rhodomonas salina CCMP1319). Strains JX20, JX4 and JX8 were isolated from the South China Sea and CCMP1319 was donated by Dr. Christopher J. Gobler of Stony Brook University.
All cultures were grown in sterile silicate-free f/2 (f/2-Si) culture medium (Guillard, 1975) which made with 0.22-μm filtered and autoclaved seawater. The salinity range of sterile f/2-Si culture medium was 30±1. Cultures were placed in an incubator (GXZ intelligent light incubator, China) (23±1 ℃) with a 12-h꞉12-h light꞉dark cycle. The light source in an incubator was provided by fluorescent lamps and the light intensity reached ~100 μmol quanta/(m2·s). In mid-exponential phase growth, all cultures were used for experiments.
2.2 Co-culture experiments of A. sanguinea and other phytoplankton 2.2.1 Allelopathic effects of A. sanguinea on coculturing phytoplanktonAkashiwo sanguinea JX14 in mid-exponential growth phase (4 days after inoculation) was corespectively cultured with three species of microalgae from different categories (S. trochoidea JX20, Pr. micans JX8 and Ph. globosa JX4), in six-well culture plates for 48 h. In order to provide adequate nutrition during the experiment, the f/2-Si medium was added at the beginning. The initial cell density of A. sanguinea JX14 was 500 or 2 000 cells/mL and the initial cell density of each target alga was 500 cells/mL. Monocultures of A. sanguinea and target algae under the same conditions were used as controls. All treatments and controls had three replicates.
At 0–6, 12, 24, and 48 h, observing and photographing morphological changes and behaviors of cells were under an inverted microscope (Olympus BX53, Japan) with a mounted digital insight camera (DP27). At 48 h, 1 mL of culture was fixed with Lugol's solution (final concentration of 2%) for counting under a microscope (Olympus CX41, Japan). Before and after the experiments, pH in cultures was determined by pH meter (PHB-3, China). After the experiments, culture filtrate in all treatments was obtained by filtration through a 0.22-μm polycarbonate filter and then N and P concentrations were determined by AutoAnalyzer 3 (Bran+Luebbe, Germany).
2.2.2 Allelopathic effects of multiple strains of A. sanguinea on R. salinaTo determine whether the allelopathic effects displayed by strain JX14 were a strain-specific feature, experiments were conducted with five strains of A. sanguinea: JX13, JX14, CCMA256, ASNP6 and AS2. In the following experiments, R. salina CCMP1319 was used as a model target alga.
In mid-exponential growth phase, CCMP1319 and strains of A. sanguinea inoculated into six-well culture plates for 72 h. The f/2-Si medium was added as described in Section 2.2.1. The initial cell densities in the experiment were 500 and 2 000 cells/mL for A. sanguinea and 500 cells/mL for CCMP1319. Monocultures of five strains of A. sanguinea and target alga were used as controls under the same conditions. All treatments and controls had three replicates.
Observing and photographing morphological changes and behaviors of cells were under an inverted microscope (Olympus BX53) every 24 h. At 72 h, 1 mL of culture was fixed with Lugol's solution (final concentration of 2%) for counting under a microscope (Olympus CX41). The pH, N, and P concentrations were determined as described in Section 2.2.1.
2.2.3 Effects of R. salina on A. sanguinea growthTo understand the effects of R. salina on the growth of A. sanguinea, experiments were conducted with 3 strains of A. sanguinea: JX13, JX14 and AS2.
In mid-exponential growth phase, strains of A. sanguinea were added into 6-well culture plates for co-culture with R. salina for 72 h under the same conditions used for maintaining cultures. f/2-Si medium was added at the beginning of the experiment to ensure sufficient nutrition in the experiment. The initial cell densities of R. salina in the experiment were 1 000, 2 000, and 5 000 cells/mL, respectively, 3 strains of A. sanguinea was 1 000 cells/mL. Monocultures of 3 strains of A. sanguinea and tested alga under the same conditions were used as controls. All treatments and controls were in triplicate.
One milliliter culture was fixed with Lugol's solution (final concentration of 2%) for counting under the microscope (Olympus CX41) at each 24 h. The pH, N, and P concentrations were determined as described in Section 2.2.1.
2.3 Mechanisms of allelopathic effects in A. sanguinea 2.3.1 Allelopathic effects of different fractions of A. sanguinea culture on R. salinaTo compare whether different fractions of A. sanguinea JX14 culture displayed different inhibition on target alga growth, experiments were conducted with four components of JX14 culture: sonicated culture, filtrates of sonicated culture, wholecell culture and culture filtrate.
Four components of JX14 culture obtained in mid- exponential growth phase. We obtained culture filtrate after filtrating 50-mL cultures of A. sanguinea JX14 with a 0.22-μm polycarbonate filter. We obtained a sonicated culture by treating 100-mL cultures of A. sanguinea JX14 with a sonicator (10 min, 5 s/5 s, 60%) (JYD-900L, China), and ensured that all cells were broken by examination under a microscope. The filtrates of sonicated culture were obtained afterfi ltrating 50 mL of the sonicated cultures with a 0.22-μm polycarbonate filter.
The R. salina was inoculated into four components of A. sanguinea JX14 culture in 10-mL culture tubes for 72 h. The f/2-Si medium was added as described in Section 2.2.1. The initial cell densities for JX14 and R. salina were both 500 cells/mL. Monoculture of R. salina with the same cell density was used as the control. All treatments and control had three triplicates. Every 24 h, 1 mL of culture was fixed with Lugol's solution (final concentration of 2%) for counting under the microscope (OLYMPUS CX41).
2.3.2 Effects of crude extract of A. sanguinea JX14 culture on R. salina growthThe crude extract of JX14 was prepared as follows: in mid-exponential growth phase, 500-mL cultures of JX14 (cell density: 3 000 cells/mL) were collected and centrifuged at 1 000 r/min at 4 ℃ (10 min) and then JX14 cells were re-suspended in 25 mL of chloroform꞉methanol꞉water (13꞉7꞉5) solution. The suspension was treated with a sonicator (10 min, 5 s/5 s, 60%) (JYD-900L) and checked under a microscope to ensure all algal cells were broken. The broken-cell culture was transferred to a separating funnel until complete stratified. The lower solution obtained from the separating funnel was transferred to a rotary evaporator (temperature 60 ℃) and evaporated under vacuum to dryness. Crude extract was re-dissolved in 10 mL of methanol (i.e. 1/50 of culture of JX14) and stored at -18 ℃ for later use.
To explore the effects of crude extract of JX14 on the growth of AS2 and CCMP1319, experiments were conducted with A. sanguinea AS2 and R. salina CCMP1319. The AS2 and CCMP1319 in midexponential growth phase were inoculated into sixwell culture plates and 2, 6, or 10 μL of crude extract respectively were added to the co-culture system with a final volume of 10 mL, which was maintained for 48 h. The f/2-Si medium was added as described in Section 2.2.1. The initial cell densities of AS2 and CCMP1319 were both 500 cells/mL. The controls were set as follows: monoculture of CCMP1319, coculture of AS2 and CCMP1319 without methanol and extract, co-culture of AS2 and CCMP1319 respectively with 2, 6, and 10 μL of methanol. All treatments and controls had three triplicates.
During the experiment, morphological changes and behaviors of cells were observed and photographed at 0–6, 24, and 48 h under a microscope (OLYMPUS BX53). At 48 h, 1 mL of cultures was taken out and fixed with Lugol's solution (final concentration of 2%) for counting under a microscope (OLYMPUS CX41).
2.4 Effects of nutrients on allelopathic activity of A. sanguineaTo explore the effects of nutrients on allelopathic activities of A. sanguinea, strains JX13 and JX14 of A. sanguinea were cultured under different conditions. (1) The JX13 and JX14 were grown in cultures of three different nutrient levels (all μg/L): middle (180 N and 20 P), high (448 N and 50 P) and low (12 N and 1.3 P); (2) the JX13 and JX14 were grown at three N꞉P ratios (all μg/L): 10꞉1 (226 N and 50 P), 20꞉1 (448 N and 50 P), and 30꞉1 (672 N and 50 P). Nitrate and phosphate were used as N and P sources, respectively.
Before the experiment, JX13 and JX14 were precultured for three cycles under the above six nutrient conditions. Cultures of JX13 and JX14 were treated with a sonicator (10 min, 5 s/5 s, 60%) (JYD-900L) and we ensured that all cells were broken under a microscope. The sonicated culture was filtered to obtain filtrate using a 0.22-μm polycarbonate membrane.
The R. salina CCMP1319 was inoculated into sonicated filtrates of JX13 and JX14, in 10-mL culture tubes for 48 h. The f/2-Si medium was added as described in Section 2.2.1. The initial cell densities of JX13 and JX14 were 500 and 2 000 cells/mL, and that for CCMP1319 was 500 cells/mL. Monoculture of CCMP1319 with the same cell density was used as the control. All treatments and control had three replicates. At 48 h, 1 mL of samples was fixed with Lugol's solution (final concentration of 2%) for counting under the microscope (Olympus CX41).
2.5 StatisticsExperimental data were analyzed by one-way analysis of variance (ANOVA). This statistical analysis was performed by SPSS version 17.0. Treatments differences were considered to be statistically significant at P < 0.05 and extremely significant at P < 0.01.
Inhibition rate=(1−Ntreatment/Ncontrol)×100%
(Williamson and Richardson, 1988),
where Ntreatment and Ncontrol are numbers of target algal cells in treatments and controls, respectively.
Specific growth rate (μ)=ln(Nt/N0)/T,
where Nt is the number of algal cells at time t, N0 is the number of algal cells at the beginning of the experiment and T is the time of co-culturing.
3 RESULT 3.1 Co-culture experiments of A. sanguinea and other phytoplankton 3.1.1 Allelopathic effects of A. sanguinea on coculturing phytoplanktonThe results showed that A. sanguinea JX14 significantly reduced the cell densities of two target species, the dinoflagellate S. trochoidea and the prymnesiophyte P. globosa, compared to their respective controls during co-culturing experiments (P < 0.05, Fig. 1). For JX14 treatments of 2 000 cells/mL, the cell densities of S. trochoidea and Ph. globosa were significantly lower than controls (P < 0.05; Fig. 1), and the inhibition rates on both species were around 40%. It is noteworthy that the JX14 growth rate increased when co-cultured with S. trochoidea and Ph. globosa in all treatments compared with its monoculture. However, JX14 had no significant negative effect on growth of Pr. micans (P>0.05). At the end of experiments, the final nitrate concentration in culture medium was 4.096–7.158 mg/L, final phosphate concentration was 0.430 3–1.073 mg/L, and pH was 7.6–8.5.
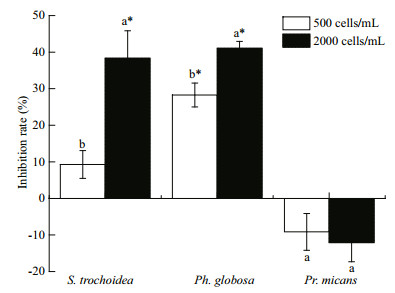
|
| Fig.1 Inhibition rates of A. sanguinea on three marine microalgae A. sanguinea JX14 (initial cell densities of 500 and 2 000 cells/mL) co-cultured for 48 h with three microalgae: S. trochoidea JX20, Pr. micans JX8 and Ph. globosa JX4 (initial cell densities 500 cells/mL). Results are mean±standard deviation (SD) (n=3). Different lowercase letters indicate that the inhibition rate of A. sanguinea significantly differs between treatments within the same tested alga (P < 0.05); *: cell densities of target microalgae significantly differ to the monoculture (P < 0.05). |
We did observe a series of morphological changes of the target algae when co-cultured with JX14. For example, 30 min after co-culturing with JX14, cells of S. trochoidea and Ph. globosa gradually lost motility, and cell morphology showed a series of significant changes, losing typical surface characteristics, swelling into spheres and finally cell membrane breakage occurred (i.e. cell contents released) (Fig. 2a–e). However, Pr. micans morphology did not change and the cells remained intact (Fig. 2f & g).
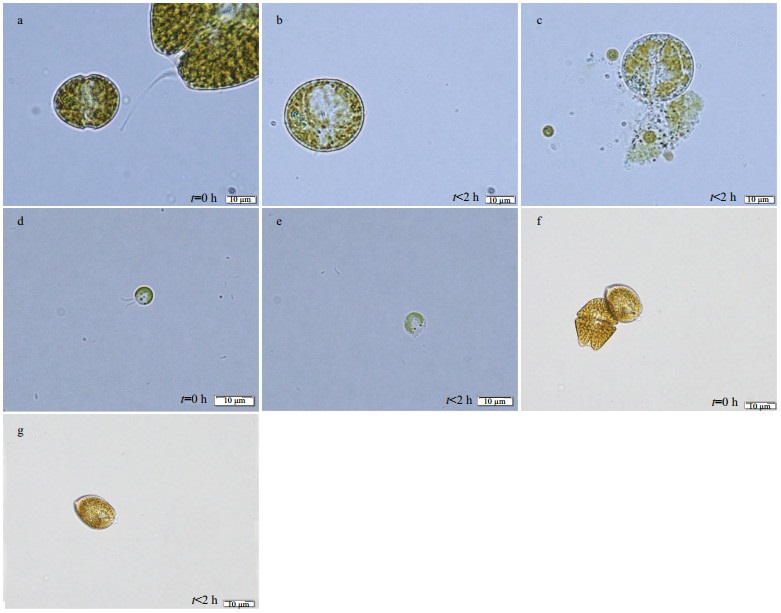
|
| Fig.2 Light microscope photos of three microalgae co-cultured with A. sanguinea JX14 a–c. morphologic change of normal cells of S. trochoidea (initial cell density 500 cells/mL) to abnormal cells when co-cultured with A. sanguinea JX14 (initial density 500 and 2 000 cells/ mL); d, e. morphologic change of Ph. globosa (initial density 500 cells/mL) to abnormal cells when co-cultured with JX14 (initial density 500 and 2 000 cells/mL); f, g. Pr. micans cells remained intact. Exposure time (t) is as shown. |
F our strains of A. sanguinea significantly inhibited the growth of R. salina, but not for strain AS2 (Fig. 3). In the treatments of 2 000 cells/mL for four A. sanguinea strains (JX13, JX14, CCMA256, and ASNP6), the R. salina cell densities significantly differed to those of the monoculture (P < 0.01); the inhibition rates on R. salina significantly differed among A. sanguinea strains (P < 0.05; Fig. 3). The growth-inhibiting effects of strains JX13, JX14, CCMA256, and ASNP6 on R. salina were densitydependent. Specifically, the inhibition rates of JX13, JX14, and CCMA256 for 2 000 cells/mL treatments on R. salina significantly differed from those at 500 cells/mLin 72 h (P < 0.05; Fig. 3). In all treatments, CCMA256 (initial cell density 2 000 cells/mL) showed the highest inhibition rate of around 90%. At the end of experiments, nitrate concentration in culture medium was 1.008–4.824 mg/L, phosphate concentration was 0.092–0.647 mg/L, and pH was 7.8–8.8.
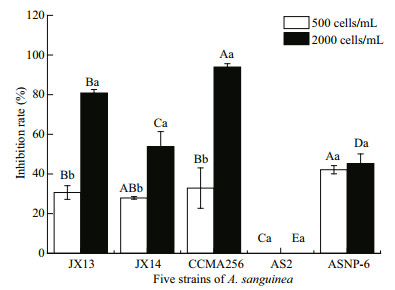
|
| Fig.3 Inhibitory effects of five strains of A. sanguinea on R. salina R. salina (initial cell density 500 cells/mL) was co-cultured with five strains of A. sanguinea (initial density 500 or 2 000 cells/ mL) respectively in six-well culture plates for 72 h. Results are means±SD (n=3). Different capital letters indicate significant differences in inhibition rate on R. salina among strains of A. sanguinea at the same cell density (P < 0.05). Different lowercase letters indicate significant differences in inhibition rate on R. salina between different densities of the same A. sanguinea strain (P < 0.05). |
The presence of R. salina had significant implications for growth of A. sanguinea. When JX13 or JX14 were co-cultured with R. salina, their growth rates significantly increased compared with monocultures, but AS2 treatment did not show the same trend (Fig. 4). That is, the presence of R. salina may have promoted growth of A. sanguinea.
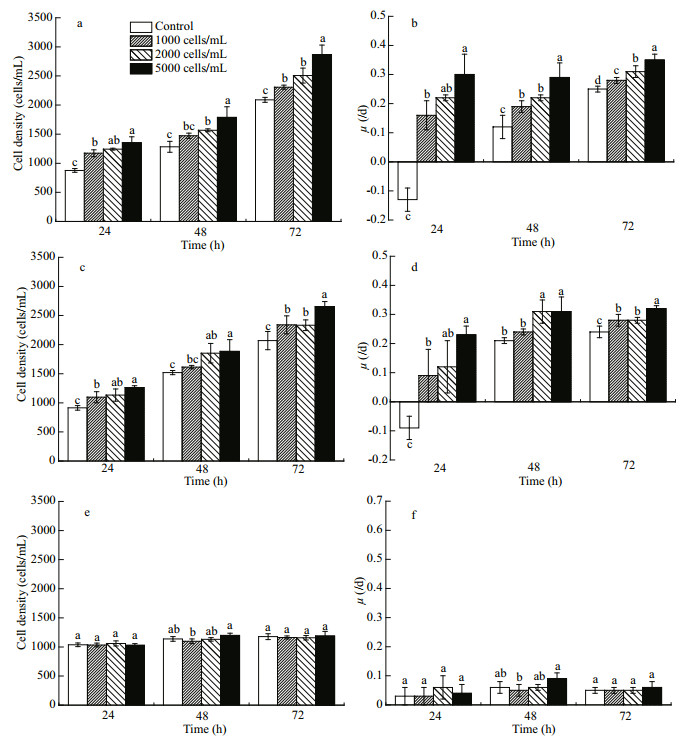
|
| Fig.4 Cell density and growth rate of different strains of A. sanguinea co-cultured with R. salina Three strains of A. sanguinea (JX13, JX14, and AS2, initial cell density 1 000 cells/mL) co-cultured with R. salina (initial cell density 1 000, 2 000, and 5 000 cells/mL) respectively for 72 h. Cell density (a) and growth rate (b) of A. sanguinea JX13; cell density (c) and growth rate (d) of A. sanguinea JX14; cell density (e) and growth rate (f) of A. sanguinea AS2. Results are means±SD (n=3). Different lowercase letters indicate significant differences among different R. salina cell densities within the same time (P < 0.05). |
The cell densities of A. sanguinea JX13 in all treatments were significantly higher compared with the monoculture at 24 and 72 h (P < 0.05; Fig. 4a), with a similar trend at 48 h. The specific growth rates of JX13 co-cultured with R. salina were significantly higher than for monoculture at 24, 48, and 72 h (P < 0.05; Fig. 4b). When R. salina cell density was higher, the cell densities and the specific growth rate of JX13 were higher at 24, 48, and 72 h. The cell densities and specific growth rate of JX13 increased by around 40% compared with control when R. salina cell density was 5 000 cells/mLat 72 h.
The cell densities and the specific growth rates of JX14 in all treatments were significantly higher compared with monoculture at 24 and 72 h (P < 0.05; Fig. 4c & d). When R. salina cell density was 5 000 cells/mL at 72 h, the cell densities and specific growth rate of JX14 were increased by around 30% compared with control.
Although the cell density and growth rate of JX13 and JX14 were significantly promoted in coculturing treatments with R. salina, there were no significant differences between AS2 co-cultured with R. salina and AS2 monoculture (P>0.05; Fig. 4e & f).
3.2 Mechanisms of allelopathy in A. sanguinea 3.2.1 Allelopathic effects of four components of A. sanguinea cultures on R. salinaFour culture fractions of A. sanguinea JX14 (i.e. whole-cell culture, culture filtrate, sonicated culture, and filtrates of sonicated culture) all had significant inhibitory effects on R. salina growth (P < 0.05; Fig. 5); however, the effects of four culture fractions on R. salina were different. After incubation for 72 h, sonicated JX14 culture showed the highest inhibitory rate on R. salina (32%), followed by whole-cell culture (28%), filtrates of sonicated culture (17%), with the lowest inhibition rate for culture filtrate (8%). The inhibition rate of sonicated culture significantly differed to that for other components at 24, 48, and 72 h (P < 0.05; Fig. 5). Sonicated culture and filtrates of sonicated culture show the highest inhibitory rate at 48 h, and then decreased with time. Whole-cell culture exhibited stronger inhibition of R. salina with prolonged incubation time. Filtrates of sonicated culture show stronger inhibitory effects than wholecell culture at 24 and 48 h. However, the inhibition rate of culture filtrate on R. salina was significantly lower at 24, 48, and 72 h than for all other treatments (P < 0.05; Fig. 5).
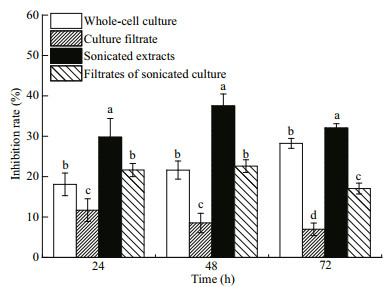
|
| Fig.5 Inhibitory effects of four different components of A. sanguinea JX14 on R. salina R. salina (initial cell density 500/mL) exposed to four components of JX14 (initial density 500/mL) in 10-mL culture tubes for 72 h. Results are means±SD (n=3). Different lowercase letters indicate significant differences among the treatments at the same time (P < 0.05). |
The results revealed that R. salina growth was significantly inhibited by all A. sanguine a JX14 crude extract treatments compared with all monoculture and co-culture controls after 48 h (P < 0.01; Fig. 6a). There was no significant difference between co-culture control and methanol controls, with both showing inhibition rates of around 0%. The inhibition rates significantly differed among the three dosages (2, 6, and 10 μL) of crude extract treatments (P < 0.05; Fig. 6a). When adding 10 μL of crude extract of JX14 (equivalent to 1 500 cells) into co-culture of AS2 and R. salina, the highest inhibition rate was 23% at 48 h.
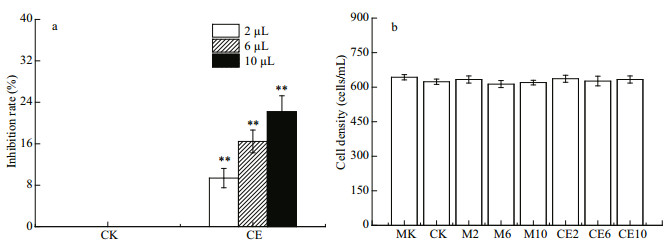
|
| Fig.6 Effects of crude extract of A. sanguinea JX14 on growth of R. salina and A. sanguinea AS2 a. inhibitory effects of crude extract of JX14 on R. salina; b. cell density of AS2. MK: monoculture of AS2. CK: AS2 and R. salina co-culture with no methanol and crude extract. M2, M6, M10: AS2 and R. salina co-culture with 2, 6, or 10 μL of methanol. CE: AS2 and R. salina co-culture with 2, 6, or 10 μL of crude extract of JX14. Initial cell densities for AS2 and R. salina were 500 cells/mL. Results are means±SD (n=3). **: significant differences in CK (P < 0.01). |
The growth rate of A. sanguinea AS2 was around 0.1/d and did not significantly differ among all treatments (Fig. 6b).
3.3 Effects of nutrients on allelopathic activity in A. sanguineaNutrient level could change the allelopathic ability of A. sanguinea. The inhibition rates on R. salina were higher if JX13 and JX14 were cultured under high nutrient conditions (Fig. 7a & b). In all treatments, the inhibition rates of JX13 or JX14 on R. salina significantly differed among the low, medium, and high nutrient conditions (P < 0.05; Fig. 7a & b). The highest inhibition rate on R. salina of 70% was for A. sanguinea JX13 treatment with 2 000 cells/mL under high nutrient conditions at 48 h.

|
| Fig.7 Inhibitory effect of A. sanguinea JX13 and JX14 on R. salina at different nutrient concentrations and nutrient ratios R. salina (initial cell density 500 cells/mL) was inoculated into sonicated filtrates of JX13 and JX14 respectively, in 10-mL culture tubes for 48 h, initial cell densities of JX13 and JX14 were 500 or 2 000 cells/mL. Inhibitory effects of JX13 (a) and JX14 (b) on R. salina at different nutrient levels. Inhibitory effects of JX13 (c) and JX14 (d) on R. salina for different N꞉P ratios. Results are means±SD (n=3). Different lowercase letters indicate significant differences among the treatments with the same density (P < 0.05). |
Different N꞉P ratios also exhibited remarkable influence on A. sanguinea allelopathic ability (Fig. 7c & d). When A. sanguinea JX13 and JX14 were 2 000 cells/mL, treatments with N꞉P of 10꞉1 and 30꞉1 had significantly higher inhibition rates than for N꞉P of 20꞉1 (P < 0.05; Fig. 7c & d). The A. sanguinea JX13 and JX14 at 2 000 cells/mL treatments had higher inhibition rates on R. salina than treatments with 500 cells/mL. In JX14 treatment at 2 000 cells/mL for N꞉P of 10꞉1, the inhibition rate increased by 1.7 times compared with those for N꞉P of 20꞉1.
4 DISCUSSION 4.1 Characteristics of allelopathy in A. sanguineaIt was demonstrated that strains of A. sanguinea had allelopathic effects on multiple co-occurring phytoplankton species. In the co-culture experiment, A. sanguinea JX14 showed significant inhibitory effects on three target species: Ph. globosa, S. trochoidea and R. salina (Figs. 1 & 3). For example, the highest inhibition rate was 94% when A. sanguinea CCMA256 was co-cultured with R. salina at 72 h (Fig. 3). In Section 3.2.1, cell-free treatments (culture filtrate, sonicated culture, and filtrates of sonicated culture) of A. sanguinea JX14 had inhibitory effects on R. salina, suggesting that the inhibitory effect did not require direct cell contact. The inhibitory effect decreased with time, indicating that the concentration of allelopathic substances decreased (i.e. by consumption or degradation). However, the inhibitory rate of whole-cell culture treatment gradually increased with time, indicating that the complete living cells could continuously release allelopathic substances. The results in Section 3.2.2 showed that R. salina growth was significantly inhibited when allelopathic extracts of A. sanguinea JX14 were added, and the inhibition rate was positively correlated with the dose of allelopathic extracts. Thus, the allelochemicals could stabilize in the environment and inhibit competitors. These results showed that A. sanguinea produced and released allelochemicals—secondary metabolites that inhibit the growth of target species.
The allelopathic effects of A. sanguinea significantly differed among strains isolated from different areas. The results in Section 3.1.2 showed that four A. sanguinea strains, JX13, JX14, CCMA256, and ASNP6, exhibited significant inhibitory effects on R. salina. At the same density (2 000 cells/mL), the Chinese strains (JX13, JX14, and CCMA256) showed stronger toxicity and inhibitory effects on R. salina than the American strains (AS2 and ASNP6). The two American strains exhibited contrasting differences in the inhibition rates on R. salina. Even at high cell density, AS2 did not inhibit R. salina, but ASNP6 had an inhibition rate exceeding 40%. There were also some differences among Chinese strains, among which the inhibitory effects of Xiamen strain CCMA256 was the strongest and the inhibitory effect of JX13 was greater than that of JX14, although these two strains were both from samples taken in Daya Bay in the South China Sea. It was previously found that Alexandrium tamarense isolated from British waters included both virulent and non-virulent strains (Higman et al., 2001). The variation in A. sanguinea toxicity among strains may be related to their genetic diversity, but this requires more evidence.
The response of phytoplankton to A. sanguinea was species-specific. Cells of three phytoplankton species (Ph. globosa, S. trochoidea, and R. salina), when co-cultured with A. sanguinea, were lysed within 6 h, but no inhibitory effects were observed on Pr. micans. Thus, allelopathy was not always effective. Our previous study also showed that Pseudo-nitzschia spp. had significant allelopathic inhibitory effects on A. sanguinea, R. salina and Chattonella marina, but species Prorocentrum minimum and Ph. globosa were not affected by the presence of Pseudo-nitzschia pungens (Xu et al., 2015). The dinoflagellate K. brevis also can interfere with the growth of half of phytoplankton tested by allelopathic substances, which was regard as a species-specific allelopathic strategy (Kubanek et al., 2005).
4.2 Mechanisms of the allelopathy in A. sanguineaThe results in Section 3.2.1 further showed the possible allelopathy mechanisms in A. sanguinea. Cell-free filtrate of A. sanguinea JX14 significantly inhibited the growth of target alga R. salina, indicating that JX14 could secrete allelopathic substances to the ambient environment and so affect competitors. The allelopathic effects of sonicated culture of JX14 were significantly higher than other culture components, with an inhibition rate of around 40% at 48 h, indicating that the allelopathic substances were mostly stored in living cells. Our experiment showed that the allelopathic effects of cell-free culture treatments (culture filtrate, sonicated culture, and filtrate of sonicated culture) of JX14 on R. salina gradually weakened over time. However, allelopathic effects of whole-cell culture increased over time, demonstrating that A. sanguinea allelochemicals could be continuously secreted out of the cells. It is reasonable that the allelopathic effects would decrease with time without replenishment of living cells. Granéli and Johansson (2003) also found that Prymnesium parvum culture filtrate had significant inhibitory effects on Thalassiosira weissflogii, Rhodomonas cf. baltic and Pr. minimum within 36 h, but the growth of the tested algae resumed after 36 h. Our experiments supported that A. sanguinea cells can produce and secret allelochemicals and display allelopathic effects on co-existing phytoplankton. Nevertheless, we did not discuss the possible role of cell contact in the A. sanguinea allelopathy. It is clear that A. sanguinea would directly contact phytoplankton in nature, and further experimental evidence is needed to determine whether direct cellular contact affects the strength of allelopathy.
The results in Section 3.2.2 demonstrated very interesting results: in the co-culture experiment (Section 3.1.2), AS2 had no inhibitory effect on R. salina (Fig. 3); however, once the JX14 crude extract was added, AS2 showed a significant inhibitory effect with dosage response. The experimental results further confirmed that allelopathic substances stored in JX14 cells and released into the environment could inhibit the growth of co-existing phytoplankton. It is worth noting that the growth rate of AS2 did not change in the experiment with the addition of JX14 crude extract (Fig. 6).
Numerous studies have indicated that increasing HABs are related to eutrophication or excess nutrient loading (Anderson et al., 2008; Heisler et al., 2008; Glibert et al., 2010). The N and P are lost to aquatic environments by multiple pathways, such as fertilizer use for agriculture, animal excretion, industry sewage or atmospheric deposition (Galloway et al., 2002; Glibert et al., 2014, 2018). The results in Section 3.3 showed how nutrients regulated allelopathy in A. sanguinea. We found that high nutrient levels enhanced the inhibition rate on R. salina by A. sanguinea. It was showed that, when A. sanguinea JX13 was cultured at high nutrient conditions, the highest inhibition rate on R. salina (70%) was for the highest cell density treatment at 48 h (Fig. 7a), indicating that A. sanguinea cells under high nutrient conditions produced more allelochemicals. Similarly, it was reported that, as ambient nitrate concentration increased, an increase in cellular toxin occurred concurrently, and Alexandrium tamarense (ATKR- 020415) seemed to utilize excess N for toxin production (Leong et al., 2004).
Additionally, the N꞉P can also regulate the inhibition rate of A. sanguinea on R. salina. In our study, the inhibition rates of A. sanguinea were higher for lower (10꞉1) or higher (30꞉1) N꞉ P compared to those around the Redfield ratio (16꞉1). According to Granéli and Johansson (2003), nutrient restriction can activate the secondary metabolic activity of Prymnesium parvum, which raises the allelopathic effect on tested algae under nutrient restriction above that under nutrient sufficiency.
It is noteworthy that our study showed that A. sanguinea JX14 under N limitation (N꞉P=10꞉1) exhibited significantly more potent allelopathic effects on R. salina than under P limitation (30꞉1). A. sanguinea is considered a mixotrophic dinoflagellate, and when environmental nutrients are imbalanced or limited, mixotrophic dinoflagellate turn from primary producers into consumers that feed on other microorganisms to obtain more nutrients (Bockstahler and Coats, 1993). Since imbalanced nutrients favored stronger allelopathy by A. sanguinea, there may be some relationship between allelopathic effects and the nutrition strategy of A. sanguinea.
4.3 Potential ecological implications of the allelopathic effects exhibited in A. sanguineaIn regard to studies of allelopathy in microalgae, many reports have focused on inhibitory effects on growth of target species (Matsuoka et al., 2000; Adolf et al., 2006; Kim et al., 2016), but rarely mentioned the effects of target species on the growth of donor species. In this study, we not only found that the growth rates of target species, Ph. globosa, S. trochoidea and R. salina, were significantly decreased in the co-culture experiments, but also observed that the target phytoplankton were lysed and the growth of donor species A. sanguinea was promoted at the same time. For example, in Section 3.1.3, cell density and the specific growth rate of A. sanguinea JX13 increased by around 40% when R. salina cell density was 5 000 cells/mL at 72 h. The cell densities of JX13 and JX14 were positively correlated with R. salina cell density at the end of the experiment. This suggested that A. sanguinea JX13 and JX14 could absorb and utilize the organic nutrients released by R. salina and so promote their own growth. Hansen and Hjorth (2002) found that growth of Chrysochromulina ericina was affected by prey concentration under suitable illumination. The ingestion rates of Prorocentrum donghaiense and Pr. micans on cyanobacteria Synechococcus sp. increased with increasing prey concentration (Jeong et al., 2005b). It is interesting to note that strain AS2 had no inhibitory effect on R. salina; even following addition of allelopathic extracts of JX14 into the co-culture system of AS2 and R. salina, although R. salina cells were lysed, the growth of AS2 was not promoted (Fig. 6). In other words, JX13 and JX14 seemed able to promote their own growth via allelopathy, during which they produced allelochemicals lysing the cooccurring phytoplankton and exploited the resulting organic nutrients. However, strain AS2 had little (if any) allelopathic effect and also had poor heterotrophic capability. Thus, allelopathy should play an important role in the nutrition strategy of A. sanguinea, which may help it exploit organic nutrients to outcompete co-occurring phytoplankton.
Many dinoflagellate species have been shown to be of mixotrophic type (Jeong et al., 2005a; Jang et al., 2017; Ok et al., 2017), which cannot survive only by photosynthetic autotrophy, and need different kinds of organic nutrients in their growth process, i.e. a heterotrophic pattern. Mixotrophic dinoflagellates play a variety of roles in the marine food web, both as predators, preying on a variety of prey such as bacteria, algae, heterotrophic protozoa and metazoa, and as prey of a variety of predators (Jeong et al., 2016; Lim et al., 2018). Mixotrophic dinoflagellates displayed a growth advantage when feeding mixotrophically compared to strict autotrophic growth (Jeong et al., 2004; Glibert et al., 2009; Flynn et al., 2013).
For example, one study demonstrated the benefits of mixotrophy in the dinoflagellate Cochlodinium polykrikoides. When growing as a mixotroph, with cryptophytes as prey, the maximum specific growth rate of C. polykrikoides was double that when growing as a phototroph (Jeong et al., 2004). In this study, when A. sanguinea JX13 (initial cell density 1 000 cells/mL) was co-cultured with R. salina (initial cell density 5 000 cells/mL) for 72 h, the cell density of JX13 increased by 50% compared with monoculture (Fig. 4a). Obviously, mixotrophic capacity is an important nutrient strategy for dinoflagellates, making them more competitive in conditions of inorganic nutrient deficiency. Mixotrophy is also regarded as a major mode of nutrition by HABs in eutrophic waters and may contribute to the maintenance of blooms (Burkholder et al., 2008).
It has been reported that organisms with mixotrophic capacity, such as Karlodinium veneficum and Pr. parvum, could release toxins (or allelochemicals) that participate in the capture of prey (Skovgaard and Hansen, 2003; Adolf et al., 2007; Berge et al., 2012). Toxicity and allelopathic effects are different representations for different targets: toxins deter predators and allelochemicals inhibit the growth of co-existing algae (Granéli and Salomon, 2010). In our study, A. sanguinea used allelochemicals to kill or dissolve competitors (S. trochoidea, Ph. globosa, and R. salina), which inhibited their growth while also promoting its own growth. Remmel and Hambright (2012) suggested that Prymnesium toxins were used to assist micropredation, first attacking gill cells, then releasing toxins to dissolve cell membranes and finally the Prymnesium absorbed the contents of the dissolved cells. Thus, mixotrophic species may have advantages in their ability to exploit nutrients acquired from prey, such as bacterial and algal competitors and even from their predators, and toxins or allelochemicals may participate in the process of mixotrophy. Collectively, allelopathy of A. sanguinea may be related to nutrient strategy, which may play an important role in the formation and persistence of its blooms.
5 CONCLUSIONIt was demonstrated that living A. sanguinea cells could produce and release allelopathic substances continuously, lysing co-existing phytoplankton (R. salina, S. trochoidea and Ph. globosa) cells and inhibiting the growth of the target species. Different strains of A. sanguinea displayed different allelopathic effects on R. salina, and the responses of different phytoplankton to A. sanguinea were species-specific. Allelopathic substances can exist in the environment stably, but may be consumed or degraded with time in the absence of living A. sanguinea cells. Both nutrients and N꞉P regulated allelopathy in A. sanguinea. High nutrient level and lower (10꞉1) or higher (30꞉1) N꞉P enhanced the inhibition rate of A. sanguinea on R. salina.
Results show that growth of toxic A. sanguinea JX13 and JX14 was promoted by the co-occurring phytoplankton R. salina, but the growth of non-toxic A. sanguinea AS2 was not promoted, suggesting that allelopathy may play an important role in the nutrition strategy of A. sanguinea.
6 DATA AVAILABILITY STATEMENTThe datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Accoroni S, Glibert P M, Pichierri S, Romagnoli T, Marini M, Totti C. 2015. A conceptual model of annual Ostreopsis cf. ovata blooms in the northern Adriatic Sea based on the synergic effects of hydrodynamics, temperature, and the N: P ratio of water column nutrients. Harmful Algae, 45: 14-25.
DOI:10.1016/j.hal.2015.04.002 |
Adolf J E, Bachvaroff T R, Krupatkina D N, Nonogaki H, Brown P J P, Lewitus A J, Harvey H R, Place A R. 2006. Species specificity and potential roles of Karlodinium micrum toxin. African Journal of Marine Science, 28(2): 415-419.
DOI:10.2989/18142320609504189 |
Adolf J E, Krupatkina D, Bachvaroff T, Place A R. 2007. Karlotoxin mediates grazing by Oxyrrhis marina on strains of Karlodinium veneficum. Harmful Algae, 6(3): 400-412.
DOI:10.1016/j.hal.2006.12.003 |
Anderson D M, Burkholder J M, Cochlan W P, Glibert P M, Gobler C J, Heil C A, Kudela R M, Parsons M L, Rensel J E J, Townsend D W, Trainer V L, Vargo G A. 2008. Harmful algal blooms and eutrophication: examining linkages from selected coastal regions of the United States. Harmful Algae, 8(1): 39-53.
DOI:10.1016/j.hal.2008.08.017 |
Badylak S, Phlips E J, Mathews A L. 2014. Akashiwo sanguinea (Dinophyceae) blooms in a sub-tropical estuary: an alga for all seasons. Plankton and Benthos Research, 9(3): 147-155.
DOI:10.3800/pbr9.147 |
Berge T, Poulsen L K, Moldrup M, Daugbjerg N, Juel H P. 2012. Marine microalgae attack and feed on metazoans. The ISME Journal, 6(10): 1 926-1 936.
DOI:10.1038/ismej.2012.29 |
Bockstahler K R, Coats D W. 1993. Grazing of the mixotrophic dinoflagellate Gymnodinium sanguineum on ciliate populations of Chesapeake Bay. Marine Biology, 116(3): 477-487.
DOI:10.1007/BF00350065 |
Botes L, Smit A J, Cook P A. 2003. The potential threat of algal blooms to the abalone (Haliotis midae) mariculture industry situated around the South African coast. Harmful Algae, 2(4): 247-259.
DOI:10.1016/s1568-9883(03)00044-1 |
Burkholder J M, Glibert P M, Skelton H M. 2008. Mixotrophy, a major mode of nutrition for harmful algal species in eutrophic waters. Harmful Algae, 8(1): 77-93.
DOI:10.1016/j.hal.2008.08.010 |
Castro N O, Domingos P, Moser G A O. 2016. National and international public policies for the management of harmful algal bloom events. A case study on the Brazilian coastal zone. Ocean & Coastal Management, 128: 40-51.
DOI:10.1016/j.ocecoaman.2016.04.016 |
Chakraborty S, Feudel U. 2014. Harmful algal blooms: combining excitability and competition. Theoretical Ecology, 7(3): 221-237.
DOI:10.1007/s12080-014-0212-1 |
Chen T T, Liu Y, Song S Q, Li C W, Tang Y Z, Yu Z M. 2015. The effects of major environmental factors and nutrient limitation on growth and encystment of planktonic dinoflagellate Akashiwo sanguinea. Harmful Algae, 46: 62-70.
DOI:10.1016/j.hal.2015.05.006 |
Driscoll W W, Espinosa N J, Eldakar O T, Hackett J D. 2013. Allelopathy as an emergent, exploitable public good in the bloom-forming microalga Prymnesium parvum. Evolution, 67(6): 1 582-1 590.
DOI:10.1111/evo.12030 |
Du X N, Peterson W, McCulloch A, Liu G X. 2011. An unusual bloom of the dinoflagellate Akashiwo sanguinea off the central Oregon, USA, coast in autumn 2009. Harmful Algae, 10(6): 784-793.
DOI:10.1016/j.hal.2011.06.011 |
Fistarol G O, Legrand C, Selander E, Hummert C, Stolte W, Graneli E. 2004. Allelopathy in Alexandrium spp. : effect on a natural plankton community and on algal monocultures. Aquatic Microbial Ecology, 35(1): 45-56.
DOI:10.3354/ame035045 |
Fleming L E, Broad K, Clement A, Dewailly E, Elmir S, Knap A, Pomponi S A, Smith S, Solo Gabriele H, Walsh P. 2006. Oceans and human health: emerging public health risks in the marine environment. Marine Pollution Bulletin, 53(10-12): 545-560.
DOI:10.1016/j.marpolbul.2006.08.012 |
Flynn K J, Stoecker D K, Mitra A, Raven J A, Glibert P M, Hansen P J, Granéli E, Burkholder J M. 2013. Misuse of the phytoplankton-zooplankton dichotomy: the need to assign organisms as mixotrophs within plankton functional types. Journal of Plankton Research, 35(1): 3-11.
DOI:10.1093/plankt/fbs062 |
Galloway J N, Cowling E B, Seitzinger S P, Socolow R H. 2002. Reactive nitrogen: too much of a good thing?. Ambio A Journal of the Human Environment, 31(2): 60-63.
DOI:10.1579/0044-7447-31.2.60 |
Glibert P M, Allen J I, Bouwman A F, Brown C W, Flynn K J, Lewitus A J, Madden C J. 2010. Modeling of HABs and eutrophication: status, advances, challenges. Journal of Marine Systems, 83(3-4): 262-275.
DOI:10.1016/j.jmarsys.2010.05.004 |
Glibert P M, Beusen A H W, Harrison J A, Dürr H H, Bouwman A F, Laruelle G G. 2018. Changing land-, sea-, and airscapes: sources of nutrient pollution affecting habitat suitability for harmful algae. In: Glibert P M, Berdalet E, Burford M A, Pitcher G C, Zhou M J eds. Global Ecology and Oceanography of Harmful Algal Blooms. Cham: Springer. p. 53–76.
|
Glibert P M, Burkholder J A M, Kana T M, Alexander J, Skelton H, Shilling C. 2009. Grazing by Karenia brevis on Synechococcus enhances its growth rate and may help to sustain blooms. Aquatic Microbial Ecology, 55(1): 17-30.
DOI:10.3354/ame01279 |
Glibert P M, Maranger R, Sobota D J, Bouwman L. 2014. The Haber Bosch-harmful algal bloom (HB-HAB) link. Environmental Research Letters, 9(10): 105001.
DOI:10.1088/1748-9326/9/10/105001 |
Gobler C J, Sunda W G. 2012. Ecosystem disruptive algal blooms of the brown tide species, Aureococcus anophagefferens and Aureoumbra lagunensis. Harmful Algae, 14: 36-45.
DOI:10.1016/j.hal.2011.10.013 |
Gómez F, Boicenco L. 2004. An annotated checklist of dinoflagellates in the Black Sea. Hydrobiologia, 517(1-3): 43-59.
DOI:10.1023/b:hydr.0000027336.05452.07 |
Granéli E, Hansen P J. 2006. Allelopathy in harmful algae: a mechanism to compete for resources? In: Granéli E, Turner J T eds. Ecology of Harmful Algae. Berlin, Heidelberg: Springer-Verlag. p. 189–201.
|
Granéli E, Johansson N. 2003. Increase in the production of allelopathic substances by Prymnesium parvum cells grown under N- or P-deficient conditions. Harmful Algae, 2(2): 135-145.
DOI:10.1016/s1568-9883(03)00006-4 |
Granéli E, Salomon P S. 2010. Factors influencing allelopathy and toxicity in Prymnesium parvum. JAWRA Journal of the American Water Resources Association, 46(1): 108-120.
DOI:10.1111/j.1752-1688.2009.00395.x |
Guillard R R L. 1975. Culture of phytoplankton for feeding marine invertebrates. In: Smith W L, Chanley M H eds. Culture of Marine Invertebrate Animals. Boston: Springer. p. 29–60.
|
Hakanen P, Suikkanen S, Kremp A. 2014. Allelopathic activity of the toxic dinoflagellate Alexandrium ostenfeldii: intrapopulation variability and response of co-occurring dinoflagellates. Harmful Algae, 39: 287-294.
DOI:10.1016/j.hal.2014.08.005 |
Hallegraeff G M. 1992. Harmful algal blooms in the Australian region. Marine Pollution Bulletin, 25(5-8): 186-190.
DOI:10.1016/0025-326X(92)90223-S |
Hallegraeff G M. 2010. Ocean climate change, phytoplankton community responses, and harmful algal blooms: a formidable predictive challenge. Journal of Phycology, 46(2): 220-235.
DOI:10.1111/j.1529-8817.2010.00815.x |
Hansen P J, Hjorth M. 2002. Growth and grazing responses of Chrysochromulina ericina (Prymnesiophyceae): the role of irradiance, prey concentration and pH. Marine Biology, 141(5): 975-983.
DOI:10.1007/s00227-002-0879-5 |
Harper Jr D E, Guillen G. 1989. Occurrence of a dinoflagellate bloom associated with an influx of low salinity water at Galveston, Texas, and coincident mortalities of demersal fish and benthic invertebrates. Contributions in Marine Science, 31: 147-161.
|
Hattenrath-Lehmann T K, Gobler C J. 2011. Allelopathic inhibition of competing phytoplankton by North American strains of the toxic dinoflagellate, Alexandrium fundyense: evidence from field experiments, laboratory experiments, and bloom events. Harmful Algae, 11: 106-116.
DOI:10.1016/j.hal.2011.08.005 |
Heisler J, Glibert P M, Burkholder J M, Anderson D M, Cochlan W, Dennison W C, Dortch Q, Gobler C J, Heil C A, Humphries E, Lewitus A, Magnien R, Marshall H G, Sellner K, Stockwell D A, Stoecker D K, Suddleson M. 2008. Eutrophication and harmful algal blooms: a scientific consensus. Harmful Algae, 8(1): 3-13.
DOI:10.1016/j.hal.2008.08.006 |
Higman W A, Stone D M, Lewis J M. 2001. Sequence comparisons of toxic and non-toxic Alexandrium tamarense (Dinophyceae) isolates from UK waters. Phycologia, 40(3): 256-262.
DOI:10.2216/i0031-8884-40-3-256.1 |
Hodgkiss I J, Lu S H. 2004. The effects of nutrients and their ratios on phytoplankton abundance in Junk Bay, Hong Kong. Hydrobiologia, 512(1-3): 215-229.
DOI:10.1023/B:HYDR.0000020330.37366.e5 |
Horner R A, Garrison D L, Plumley F G. 1997. Harmful algal blooms and red tide problems on the U.S. west coast. Limnology and Oceanography, 42(5part2): 1 076-1 088.
DOI:10.4319/lo.1997.42.5_part_2.1076 |
Jang S H, Jeong H J, Kwon J E, Lee K H. 2017. Mixotrophy in the newly described dinoflagellate Yihiella yeosuensis: a small, fast dinoflagellate predator that grows mixotrophically, but not autotrophically. Harmful Algae, 62: 94-103.
DOI:10.1016/j.hal.2016.12.007 |
Jeong H J, Du Yoo Y, Kim J S, Kim T H, Kim J H, Kang N S, Yih W. 2004. Mixotrophy in the phototrophic Harmful Alga Cochlodinium polykrikoides (Dinophycean): prey species, the effects of prey concentration, and grazing impact. Journal of Eukaryotic Microbiology, 51(5): 563-569.
DOI:10.1111/j.1550-7408.2004.tb00292.x |
Jeong H J, Du Yoo Y, Seong K A, Kim J H, Park J Y, Kim S, Lee S H, Ha J H. 2005a. Feeding by the mixotrophic red-tide dinoflagellate Gonyaulax polygramma: mechanisms, prey species, effects of prey concentration, and grazing impact. Aquatic Microbial Ecology, 38(3): 249-257.
DOI:10.3354/ame038249 |
Jeong H J, Ok J H, Lim A S, Kwon J E, Kim S J, Lee S Y. 2016. Mixotrophy in the phototrophic dinoflagellate Takayama helix (family Kareniaceae): predator of diverse toxic and harmful dinoflagellates. Harmful Algae, 60: 92-106.
DOI:10.1016/j.hal.2016.10.008 |
Jeong H J, Park J Y, Nho J H, Park M O, Ha J H, Seong K A, Jeng C, Seong C N, Lee K Y, Yih W H. 2005b. Feeding by red-tide dinoflagellates on the cyanobacterium Synechococcus. Aquatic Microbial Ecology, 41(2): 131-143.
DOI:10.3354/ame041131 |
Jessup D A, Miller M A, Ryan J P, Nevins H M, Kerkering H A, Mekebri A, Crane D B, Johnson T A, Kudela R M. 2009. Mass stranding of marine birds caused by a surfactant-producing red tide. PLoS One, 4(2): e4550.
DOI:10.1371/journal.pone.0004550 |
Kahru M, Michell B G, Diaz A, Miura M. 2004. MODIS detects a devastating algal bloom in Paracas Bay, Peru. Eos, 85(45): 465-472.
DOI:10.1029/2004EO450002 |
Katano T, Yoshida M, Yamaguchi S, Hamada T, Yoshino K, Hayami Y. 2011. Diel vertical migration and cell division of bloom-forming dinoflagellate Akashiwo sanguinea in the Ariake Sea, Japan. Plankton and Benthos Research, 6(2): 92-100.
DOI:10.3800/pbr6.92 |
Kim J H, Jeong H J, Lim A S, Rho J R, Lee S B. 2016. Killing potential protist predators as a survival strategy of the newly described dinoflagellate Alexandrium pohangense. Harmful Algae, 55: 41-55.
DOI:10.1016/j.hal.2016.01.009 |
Kubanek J, Hicks M K, Naar J, Villareal T A. 2005. Does the red tide dinoflagellate Karenia brevis use allelopathy to outcompete other phytoplankton?. Limnology and Oceanography, 50(3): 883-895.
DOI:10.4319/lo.2005.50.3.0883 |
Legrand C, Rengefors K, Fistarol G O, Granéli E. 2003. Allelopathy in phytoplankton-biochemical, ecological and evolutionary aspects. Phycologia, 42(4): 406-419.
DOI:10.2216/i0031-8884-42-4-406.1 |
Leong S C Y, Murata A, Nagashima Y, Taguchi S. 2004. Variability in toxicity of the dinoflagellate Alexandrium tamarense in response to different nitrogen sources and concentrations. Toxicon, 43(4): 407-415.
DOI:10.1016/j.toxicon.2004.01.015 |
Lim A S, Jeong H J, Ok J H, Kim S J. 2018. Feeding by the harmful phototrophic dinoflagellate Takayama tasmanica (Family Kareniaceae). Harmful Algae, 74: 19-29.
DOI:10.1016/j.hal.2018.03.009 |
Matsubara T, Nagasoe S, Yamasaki Y, Shikata T, Shimasaki Y, Oshima Y, Honjo T. 2007. Effects of temperature, salinity, and irradiance on the growth of the dinoflagellate Akashiwo sanguinea. Journal of Experimental Marine Biology and Ecology, 342(2): 226-230.
DOI:10.1016/j.jembe.2006.09.013 |
Matsuoka K, Cho H J, Jacobson D M. 2000. Observations of the feeding behavior and growth rates of the heterotrophic dinoflagellate Polykrikos kofoidii (Polykrikaceae, Dinophyceae). Phycologia, 39(1): 82-86.
DOI:10.2216/i0031-8884-39-1-82.1 |
Ok J H, Jeong H J, Lim A S, Lee K H. 2017. Interactions between the mixotrophic dinoflagellate Takayama helix and common heterotrophic protists. Harmful Algae, 68: 178-191.
DOI:10.1016/j.hal.2017.08.006 |
Ou G Y, Wang H, Si R R, Guan W C. 2017. The dinoflagellate Akashiwo sanguinea will benefit from future climate change: the interactive effects of ocean acidification, warming and high irradiance on photophysiology and hemolytic activity. Harmful Algae, 68: 118-127.
DOI:10.1016/j.hal.2017.08.003 |
Park J, Jeong H J, Du Yoo Y, et al. 2013. Mixotrophic dinoflagellate red tides in Korean waters: distribution and ecophysiology. Harmful Algae, 30(S1): S28-S40.
DOI:10.1016/j.hal.2013.10.004 |
Phlips E J, Badylak S, Christman M, Wolny J, Brame J, Garland J, Hall L, Hart J, Landsberg J, Lasi M, Lockwood J, Paperno R, Scheidt D, Staples A, Steidinger K. 2011. Scales of temporal and spatial variability in the distribution of harmful algae species in the Indian River Lagoon, Florida, USA. Harmful Algae, 10(3): 277-290.
DOI:10.1016/j.hal.2010.11.001 |
Poulin R X, Hogan S, Poulson-Ellestad K L, Brown E, Fernández F M, Kubanek J. 2018. Karenia brevis allelopathy compromises the lipidome, membrane integrity, and photosynthesis of competitors. Scientific Reports, 8(1): 9 572.
DOI:10.1038/s41598-018-27845-9 |
Prince E K, Myers T L, Kubanek J. 2008. Effects of harmful algal blooms on competitors: allelopathic mechanisms of the red tide dinoflagellate Karenia brevis. Limnology and Oceanography, 53(2): 531-541.
DOI:10.4319/lo.2008.53.2.0531 |
Remmel E J, Hambright K D. 2012. Toxin-assisted micropredation: experimental evidence shows that contact micropredation rather than exotoxicity is the role of Prymnesium toxins. Ecology Letters, 15(2): 126-132.
DOI:10.1111/j.1461-0248.2011.01718.x |
Skovgaard A, Hansen P J. 2003. Food uptake in the harmful alga Prymnesium parvum mediated by excreted toxins. Limnology and Oceanography, 48(3): 1 161-1 166.
DOI:10.4319/lo.2003.48.3.1161 |
Tang Y Z, Gobler C J. 2010. Allelopathic effects of Cochlodinium polykrikoides isolates and blooms from the estuaries of Long Island, New York, on co-occurring phytoplankton. Marine Ecology Progress, 406: 19-31.
DOI:10.3354/meps08537 |
Tang Y Z, Gobler C J. 2015. Sexual resting cyst production by the dinoflagellate Akashiwo sanguinea: a potential mechanism contributing to the ubiquitous distribution of a harmful alga. Journal of Phycology, 51(2): 298-309.
DOI:10.1111/jpy.12274 |
Williamson G B, Richardson D. 1988. Bioassays for allelopathy: measuring treatment responses with independent controls. Journal of Chemical Ecology, 14(1): 181-187.
DOI:10.1007/BF01022540 |
Xu N, Tang Y Z, Qin J L, Duan S S, Gobler C J. 2015. Ability of the marine diatoms Pseudo-nitzschia multiseries and P. pungens to inhibit the growth of co-occurring phytoplankton via allelopathy. Aquatic Microbial Ecology, 74(1): 29-41.
DOI:10.3354/ame01724 |
Xu N, Wang M, Tang Y Z, Zhang Q, Duan S S, Gobler C J. 2017. Acute toxicity of the cosmopolitan bloom-forming dinoflagellate Akashiwo sanguinea to finfish, shellfish, and zooplankton. Aquatic Microbial Ecology, 80(3): 209-222.
DOI:10.3354/ame01846 |
Yang C Y, Li Y, Zhou Y Y, Zheng W, Tian Y, Zheng T L. 2012. Bacterial community dynamics during a bloom caused by Akashiwo sanguinea in the Xiamen sea area, China. Harmful Algae, 20: 132-141.
DOI:10.1016/j.hal.2012.09.002 |
 2021, Vol. 39
2021, Vol. 39


