Institute of Oceanology, Chinese Academy of Sciences
Article Information
- CAO Jingya, YANG Shengxiong, TANG Danling, FENG Junxi, LIANG Jinqiang
- Mg/Ca, Ba/Ca, and S/Ca ratios as environmental and growth proxies for bivalve shells from the Haima cold seep, South China Sea
- Journal of Oceanology and Limnology, 41(2): 660-672
- http://dx.doi.org/10.1007/s00343-022-2010-8
Article History
- Received Jan. 11, 2022
- accepted in principle Mar. 28, 2022
- accepted for publication Jun. 6, 2022
2 Department of Ocean Science, Hong Kong University of Science & Technology, Hong Kong 999077, China;
3 MLR Key Laboratory of Marine Mineral Resources, Guangzhou Marine Geological Survey, China Geological Survey, Ministry of Natural Resources, Guangzhou 511458, China
Cold seeps are seafloor manifestations of methane-rich fluids which are mainly composed of methane and hydrogen sulfide and are globally distributed on the seafloor of continental margins, transporting these fluids from sediments into the ocean and even the atmosphere (Campbell, 2006; Boetius and Wenzhöfer, 2013; Suess, 2014; Ceramicola et al., 2018). Now, cold seeps, one of the important sinks for carbon and sulfur, are recognized as a significant part of the global carbon and sulfur cycles (Peckmann and Thiel, 2004; Yoshinaga et al., 2015; Zhang et al., 2017; Wang et al., 2018; Bowles et al., 2019; Sun et al., 2019; Dong et al., 2020). In cold seeps, seepage of the methane-rich fluids results in the formation of the authigenic carbonates and chemoautotrophic biocoenoses which mainly depend on a specific mechanism of anaerobic oxidation of methane (AOM) (Boetius et al., 2000). Meanwhile, these authigenic carbonates and chemoautotrophic biocoenoses also become faithful recorders of the methane-rich fluids seepage (Callender and Powell, 1999; Feng et al., 2016). Peckmann et al. (2009) first stated that the cement content in authigenic carbonates could be used as an approximation of seepage intensity since more intense seepage appears to favor cement precipitation. After that, a series of indicators, including the Mg/Ca and Sr/Ca ratios and Mo content of submarine sediment (Chen et al., 2016; Wang et al., 2018), S isotopes of sulfur compound (Zhang et al., 2018), and biomarker contents in authigenic carbonates (Guan et al., 2016), have been proposed to trace the seepage history and even the seepage intensity of the methane-rich fluids in cold seeps. These pioneer studies have provided important efforts to assess the seepage history of cold seeps.
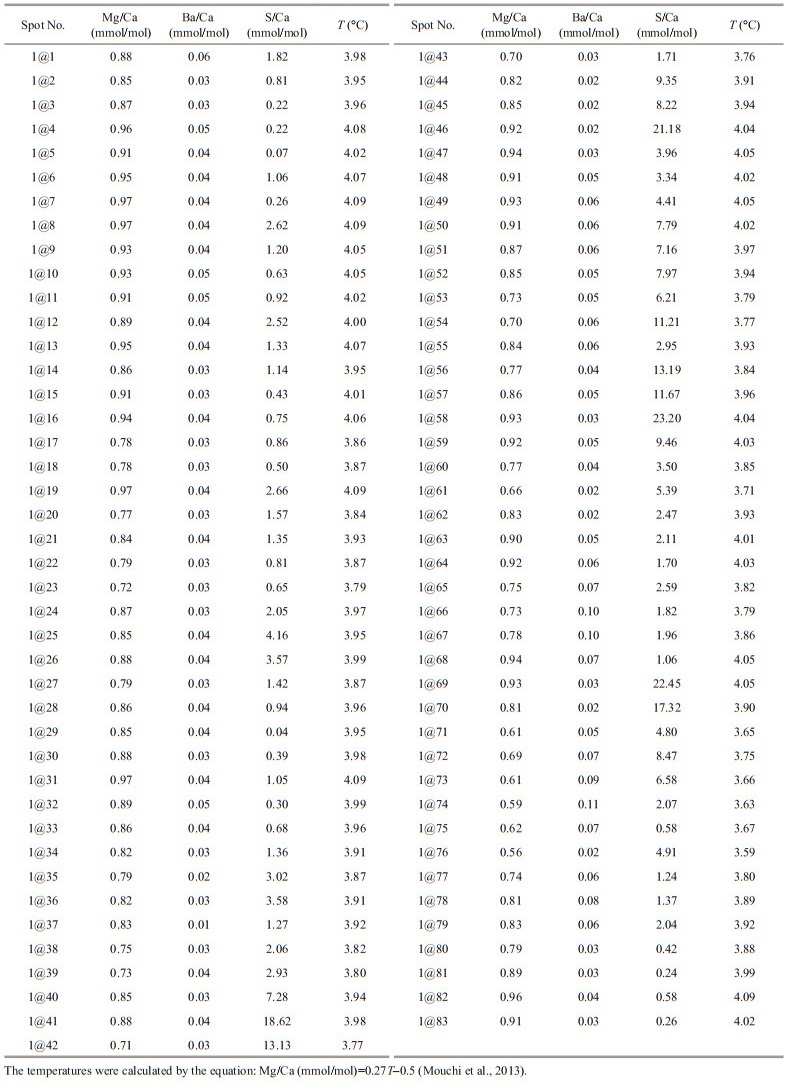
As the important parts of these chemoautotrophic biocoenoses, bivalve species, e.g., Gigantidas platifrons, Akebiconcha marissinica, and Archivesica marissinica, are common in cold seeps. The growth processes of these bivalves are influenced by the methane-rich fluids and the surrounding seawater, which might be geochemically and isotopically recorded by bivalve shells (e.g., Lietard and Pierre, 2008, 2009; Yang et al., 2014; Feng et al., 2018a; Riekenberg et al., 2018). Previous studies have shown that some specific elements in bivalve shells in marine and freshwaters, e.g., Mg, Ba, S, and Sr, are greatly influenced by variations in temperature and salinity of waters, making they were widely used in reflecting the palaeoenvironment (Putten et al., 2000; Gillikin et al., 2006; Schöne, 2008; Poulain et al., 2015). However, rare studies were involved in the bivalves in cold seeps. Hence, a series of geochemical analyses, including LA-ICP-MS elemental and mapping, in-situ XRD, and SEM analyses, were conducted on a bivalve shell fossil of the G. platifrons, collected in the ROV2 site of Haima cold seep, South China Sea (SCS) (Fig. 1). We examine the Mg/Ca, Ba/Ca, and S/Ca ratios of the profile, aiming to assess the physical environment variation and provide insight into the relationship between the S/Ca ratios and growth rate and/or seepage intensity variation of the cold seep activity history.
|
| Fig.1 Location of ROV2 site in the Qiongdongnan Basin, South China Sea Modified after Feng et al. (2018b). |
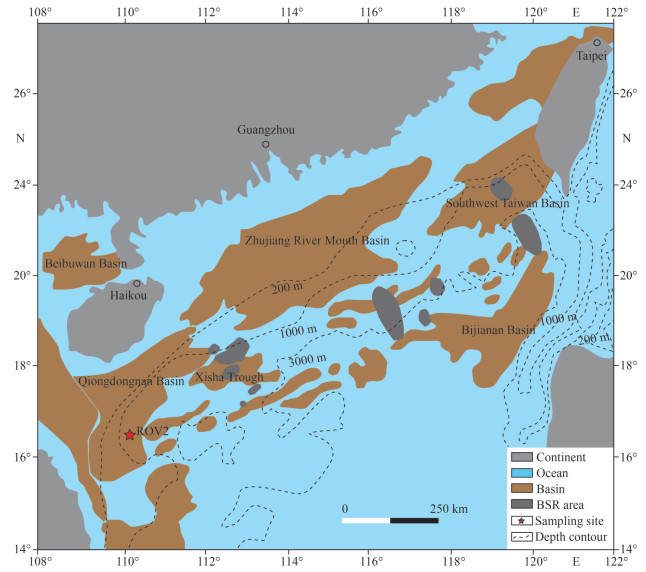
The active Haima cold seeps with an area of 350 km2, first discovered in 2015, are located at approximately 1 370–1 390-m water depth on the lower continental slope of the northwestern SCS. The Haima cold seeps host abundant chemosynthetic communities, methane-derived authigenic carbonates, and massive gas hydrates (Liang et al., 2017). The bivalve shell fossils were collected from the Haima cold seep of the southern Qiongdongnan Basin, SCS, at a water depth of 1 381 m by the Remote Operated Vehicle (ROV) "Haima" during the Haiyang-6 cruise conducted by Guangzhou Marine Geological Survey in 2016. These bivalve shell fossils are cemented by the authigenic carbonates (Fig. 2a). The 14C ages of these shell fossils were 5.1–6.1 ka. BP, reported by Liang et al. (2017). These authigenic carbonates, mainly white to grey in color, are composed of aragonite, foraminifer fossils, and microcrystalline carbonate matrix with minor Fe-sulfides (Fig. 2a–d).
|
| Fig.2 Hand specimens of the bivalve shell fossil (a–b), rock slice (c), and micrographs of the authigenic carbonates (d) |
The shell piece, with a length of 1 cm and a width of 2 mm, was cut by a handheld cutting machine (Fig. 2b). Firstly, the sample was soaked in the sodium hydroxide solution with a mass fraction of 20% for 1 h in a water bath of 65 ℃ to remove the organic materials (Zhao et al., 2015). Secondly, it was both cleaned with deionized water and then dried for 24 h. Thirdly, this shell piece was mounted into an epoxy resin block. Finally, this cross-section was polished to obtain a flat surface. But before the analysis, the surface of the sample was etched by using the acetic acid with a mass fraction of 10%.
2.2 Scanning electron microscope (SEM) imaging for the bivalve shell fossilThe SEM imaging for this sample was carried out on the TESCAN MIRA 3 SEM instrument at the Guangzhou TuoYan Analytical Technology Co., Ltd., Guangzhou, China. This instrument equips a high-intensity Schottky field emission gun with electron energy of 200 eV–30 keV, beam current of 2 pA–100 nA, and resolution ratio of 1.2 nm@30 keV. Before the analyses, the samples were first cleaned in an ultrasonic cleaner which is filled with deionized water. Then, it was plated with gold to enhance its electrical conductivity. The location of these SEM images was marked in Fig. 3a.

|
| Fig.3 Photo of the shell section (a) and SEM images (b–g) of the bivalve shell from the Haima cold seep |
The XRD analyses for the shell section were carried out on a Rigaku Rapid IIR micro-diffractometer at the Key Laboratory of Metallogenic Prediction of Nonferrous Metals and Geological Environment Monitoring, Ministry of Education, Central South University, Changsha. The X-ray was emitted by a copper target. The measuring process was set as the following parameters, i.e., a voltage of 40 kV and electricity of 250 mA. In addition, the measuring duration was 20 min for each analysis. The raw data was conducted by Jade 6.5.
2.4 In-situ LA-ICP-MS trace element analysis and mapping of the bivalve shellTrace element analysis and map scanning of bivalve shells were conducted by LA-ICP-MS at the Guangzhou TuoYan Analytical Technology Co., Ltd., Guangzhou, China. Laser sampling was performed using an NWR 193 laser ablation system. An iCAP RQ ICP-MS instrument was used to acquire ion-signal intensities. The trace element composition analysis was conducted with helium as a carrier gas. Argon was used as the make-up gas and mixed with the carrier gas via a Y-connector before entering the ICP. The spot size, frequency, and energy of the laser were set to 50 µm, 5 J/cm2, and 6 Hz, respectively. In this study, analyses were carried out in the middle of the calcite layer of the bivalve shell fossil with a spot spacing of 100 μm along with the growth profile. Glass standards reference NIST 610 and NIST 612 and carbonate standard MACS-1 were used as external standards for trace element calibration. Each analysis incorporated a background acquisition of approximately 30 s followed by 40 s of data acquisition from the sample. Raw data were calibrated against multiple silicate reference materials without using an internal standard (MRM-NoIS) by using an Excel-based software ICPMSDataCal (Liu et al., 2008). Analytical accuracy was recommended values within 10% for trace elements and better than 5% for major elements by using the MRM-NoIS calibration strategy, which was described in detail by Chen et al. (2011). The LA-ICP-MS trace element mapping was carried out, using the laser size, frequency, energy, and scanning speed of 30 µm, 3.5 J/cm2, 6 Hz, and 150 µm/s, respectively. A detailed measuring procedure was described by Paton et al. (2011).
3 RESULT 3.1 SEM imaging and XRD analysesBased on the observation, the shell can be subdivided into two major parts: unaltered zones and altered zones. The former mainly contains two microstructures, i.e., prismatic structure and stratified structure (Fig. 3b–e). The prismatic structure is mainly distributed in clusters with a series of uneven prismatic materials (Fig. 3b–e). The stratified structure material, the major part of the nacreous layer, is composed of the laminated lamella, with each lamella of 1–2 μm (Fig. 3d–e). In addition, the altered zones are mainly characterized by amorphous features (Fig. 3f–g), indicating that these zones might be influenced by the authigenic carbonates and/or the methane-derived fluids.
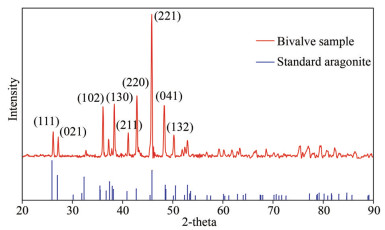
XRD analyses on the prismatic layer of the bivalve shell were carried out in this study. As shown in Fig. 4, the legible spectral peaks of the fibrous prismatic layers indicate a high degree of crystallinity. In addition, the prismatic layers of the bivalve shell show similar diffraction peaks of each lattice plane to the standard aragonite, i.e., (111), (021), (102), (130), (220), (221), and (041). The result illustrates that the mineralogical composition of the prismatic layer of the bivalve shell is composed of aragonite.
|
| Fig.4 XRD spectrum of the bivalve shell from the Haima cold seep |
LA-ICP-MS mapping results for the shell profile were represented in Fig. 5. Generally, the prismatic layer has lower Ba/Ca, S/Ca, and Mg/Ca ratios than the nacreous layer (Fig. 5), which is confirmed by the detailed studies from Takesue et al. (2008). However, some altered zones of this profile, marked in Fig. 5, contain abnormal high Ba/Ca, Mg/Ca, and S/Ca ratios which are ten times more than these unaltered zones.
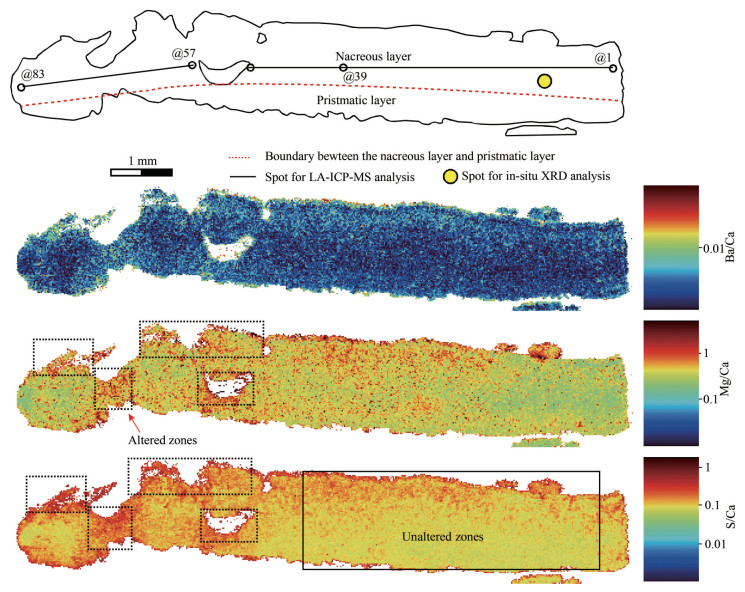
|
| Fig.5 LA-ICP-MS mapping for the section of the bivalve shell from the Haima cold seep |
Eighty-three analyses were carried out on the prismatic layer along with the growth profile (Fig. 5). The results of Mg/Ca, Ba/Ca, and S/Ca ratios were represented in Table 1. Mg/Ca, Ba/Ca, and S/Ca ratios range 0.56–0.97 mmol/mol (mean value of 0.84 mmol/mol), 0.01–0.11 mmol/mol (mean value of 0.04 mmol/mol) and 0.04–23.2 mmol/mol (mean value of 4.11 mmol/mol), respectively. However, in order to avoid the influences from the altered zones, a series of data (spots Nos. 1–39) were selected, with Mg/Ca, Ba/Ca and S/Ca ratios of 0.72–0.97 mmol/mol (mean=0.87 mmol/mol), 0.01–0.06 mmol/mol (mean= 0.04 mmol/mol) and 0.04–4.16 mmol/mol (mean=1.32), respectively. Generally, the selected Mg/Ca, Ba/Ca, and S/Ca ratios show wave-type variability (Fig. 6). However, due to the relatively low Mg/Ca and Ba/Ca ratios, Mg/Ca and Ba/Ca ratios did not show obvious variations along the section, implying that Mg/Ca and Ba/Ca ratios remain constant (Fig. 6). In addition, Mg/Ca ratios generally correspond to the S/Ca ratios, whereas the Ba/Ca did show a positive relationship with the Mg/Ca and S/Ca ratios (Fig. 6).
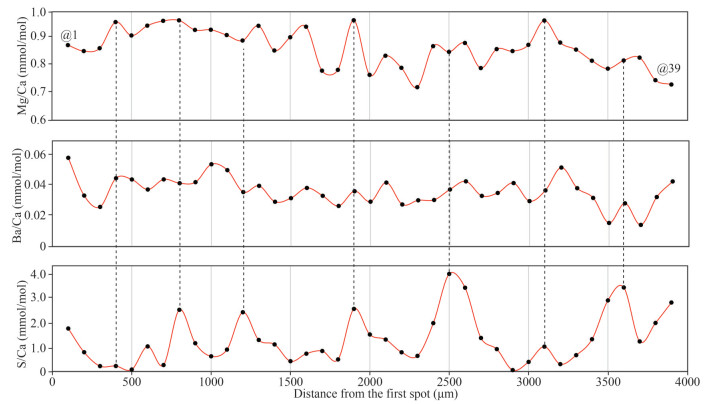
|
| Fig.6 Mg/Ca, Ba/Ca, and S/Ca ratios along with the shell profile of unaltered zones from the Haima cold seep |
Bivalves can incorporate some specific elements into their shells during the growth process which are sensitive to the physical and biological properties of the surrounding seawater (Poulain et al., 2015). These elements will be enriched and/or depleted in response to the physical and biological variations of the surrounding seawater and organisms, therefore, they can be used as efficient tracers to reflect these variations (Schöne et al., 2002, 2010, 2011; Schöne, 2008; Gillikin and Dehairs, 2013). Previous studies proposed that the Mg/Ca ratios of carbonate minerals in solids and liquids show a close relationship with the temperatures (Mucci, 1987). In addition, it is well accepted that Mg/Ca ratios in seawater remain invariable if the seawater salinity is over 10% (Dodd and Crisp, 1982). Therefore, the Mg/Ca ratios of shells mainly reflect the temperature of the surrounding seawater (e.g., Mucci, 1987; Putten et al., 2000). In this study, Haima bivalve shell fossils show low Mg/Ca ratios of the 0.56–0.97 mmol/mol (mean= 0.84 mmol/mol), remarkably lower than the Mg/Ca ratios of Mytilus edulis reported by Putten et al. (2000), Wanamaker et al. (2008), and Freitas et al. (2008). In addition, the low Mg/Ca variations along with the section further imply that the surrounding seawater temperatures keep constant. Based on this positive correlation between the Mg/Ca ratios and temperature, a series of Mg/Ca-temperature calibration curves have been successfully developed to estimate the water temperature (e.g., Putten et al., 2000; Freitas et al., 2012; Mouchi et al., 2013; Tynan et al., 2017). The temperatures for these selected data, calculated by the model from Mouchi et al. (2013), range from 3.8 to 4.1 ℃ (mean=4.0 ℃), consistent with the measured data of 3.9 ℃ which was conducted by a conductivity-temperature-depth system (CTD) during the same cruise. In addition, the LA-ICP-MS mapping results also show that the Mg/Ca ratios of the section show a rising trend in these altered zones, but the detailed Mg/Ca ratios vary in a limited range which has a minor impact on these calculated temperatures (Fig. 5). Therefore, these calculated temperatures show minor variations, indicating that the surrounding seawater temperatures likely remain constant, which is consistent with the low temperatures of the deep-sea environment.
4.2 Ba/Ca constraints on the salinity of the cold-seep provinceA previous study proposed that the Ba concentration profile of aragonite biominerals was regarded as a forceful tool for productivity which is mainly governed by the Ba concentration of ambient water (e.g., Elliot et al., 2009). A further study reported that the shell Ba/Ca ratios were controlled by Ba/Ca ratios of the surrounding waters (Poulain et al., 2015). This property makes the shell Ba/Ca ratio an efficient tool to track the Ba/Ca ratio of waters and then rebuild the salinity of waters (Gillikin et al., 2006, 2008). However, Rosenheim et al. (2005) hypothesized that Ba incorporation into shell may be restricted by ambient water with high Ba concentration, implying that Ba/Ca be not a reliable paleoenvironmental proxy where the Ba concentration is high. Other factors might also influence the Ba/Ca ratios of shells, e.g., type of bivalve species, shell mineral compositions, and growth rate (Rosenberg et al., 2001; Gillikin et al., 2008; Izumida et al., 2011; Hatch et al., 2013; Marali et al., 2017). However, Ba/Ca in carbonate skeletons, as a palaeoenvironment proxy, plays a significant role in the reconstruction of the paleosalinity (Gillikin et al., 2006; Sarimin and Mohamed, 2014; Poulain et al., 2015; de Nooijer et al., 2017). In this study, the section of bivalve shell is featured by relatively low Ba/Ca ratios of 0.01–0.11 mmol/mol (mean=0.04 mmol/mol) which is consistent with the unaltered zones of 0.01–0.06 mmol/mol (mean=0.04 mmol/mol). In addition, the LA-ICP-MS mapping also reveals that this section hosts invariable Ba/Ca ratios (Fig. 5). Consequently, these minor variations of Ba/Ca ratios indicate that the surrounding seawater salinity remains constant during the bivalve growth.
4.3 S/Ca: a potential tracer for recording the growth rate?Previous studies indicated that sulfur (S) might be served as organic S and inorganic S in bivalve species (Dauphin et al., 2005; Yoshimura et al., 2013, 2014; Tamenori and Yoshimura, 2018). The former exists in the organic tissues as amino acids and sulfated polysaccharides (Dauphin et al., 2005; Tamenori and Yoshimura, 2018), whereas the latter is incorporated in the shell CaCO3 as inorganic sulfate (Yoshimura et al., 2013, 2014). In addition, Yoshimura et al. (2014) further illustrated that inorganic sulfate is the primary species in coral calcite skeletons, with a minor distribution of organic sulfurs. Some researchers argued that those higher shell organic contents might be associated with high sulfur concentrations in biogenic mollusk carbonates (Rosenberg et al., 2001; Dauphin et al., 2005; Lazareth et al., 2007). One conceivable reason for his debate is the difference in the nature of the bivalve species (Tamenori and Yoshimura, 2018). Previous studies have proposed that S in bivalve shell cross-sections is correlated with the annual lines, which could be used as a tracer to record the growth process of shells (Dauphin and Cuif, 1999; Cuif et al., 2008; Takesue et al., 2008). In general, element substitution in CaCO3 is governed by equilibrium partitioning or kinetically controlled inorganic partitioning, therefore, the S content tends to increase with the CaCO3 precipitation rate (Busenberg and Plummer, 1985). Commonly, shallow sea bivalves will have a higher growth rate in summer than in other seasons since the high temperature will promote CaCO3 precipitation. Inversely, the higher growth rate of these bivalves will result in the elevation of the CaCO3 precipitation and the assimilation of some specific elements, e.g., Mg and S (Lorens and Bender, 1980; Poulain et al., 2015). As aforementioned, growth rate have a powerful impact on the Mg/Ca ratios of the bivalve shells, Mg in shell carbonate is highly regulated by the organism during shell precipitation, which means that a high growth rate likely promotes the compatibility for Mg in the shells (Lorens and Bender, 1980). In addition, Shirai et al. (2008) reported a positive relationship between Mg and S contents in shells of the Bathymodiolus platifrons. In addition, detailed fields with Mg and S contents did not conform to the locations of growth rims (Shirai et al., 2008), indicating that locations of growth rims, representing a low growth rate, are negatively related to the high Mg and S contents. Therefore, the growth rate might affect the variations of not only the Mg/Ca ratios but also the S/Ca ratios. In order to avoid the negative influence on the S/Ca ratios by the authigenic carbonate rocks and/or methane-derived fluids. A series of data for the unaltered zones of the shell section was intercepted for further discussion. As shown in Fig. 6, the selected S/Ca ratios have regular variations which generally correspond to the variations of the Mg/Ca ratios, confirming that the S/ Ca ratios of bivalve shells can also reflect the growth rate of the G. platifrons. Although the sample was firstly conducted by removal procedures of organic materials in this study, it is not certain whether the organic S was removed thoroughly. Therefore, the shell aragonite mainly contains inorganic S in the shell of the G. platifrons, whereas the residual organic S might also be a potential S source for the data in this study. However, the influences from the residual organic sulfurs might be negligible for the following reasons: 1) the sample was firstly conducted by removal procedures of organic materials in this study; and 2) the shell mainly contain inorganic sulfurs with a few organic sulfurs as proposed by Tamenori et al. (2014).
To our knowledge, G. platifrons, living in the cold-seep province, are parts of these chemosynthesis-based species and they get energy from the co-existing chemoautotrophic bacteria which may use a variety of vent or seep gases e.g., H2S and CH4 (Petersen and Dubilier, 2009; Ponnudurai et al., 2017; Wang et al., 2020; Ip et al., 2021). In addition, the extraordinary gene makes that these bivalves use energy, provided by these chemoautotrophic bacteria, rather than phytoplankton (Ip et al., 2021). Nix et al. (1995) found that the G. platifrons would have a faster growth rate in the methane-rich site. This perspective was confirmed by the study that the number of chemoautotrophic bacteria in G. platifrons is significantly influenced by the concentrations of methane (Riou et al., 2008). In this way, concentrations of H2S and CH4 might have a significant impact on the growth condition for bivalves in the cold-seep province, rather than physical conditions of the surrounding seawater, e.g., temperature and salinity, since temperature and salinity in deep-sea will remain constant within the transitory life-circle of these species which was supported by the low Mg/Ca and Ba/Ca ratios in this study. In addition, it was proposed that the S in carbonate associated sulfate (CAS) and shell organic matter (SOM) were likely sourced from the seawater and methane fluids modified by the chemosynthetic bacteria, respectively (Newton et al., 2018), which confirms that the ocean- and methane-derived S are both involved in the growth process of these bivalves in the cold-seep province. Since the SOM and CAS share a symplastic growth relationship, the SOM growth will promote the CAS growth, which will affect the S compatibility with the CAS. However, we do not know if any other factors also affect the growth rate, e.g., oxygen fugacity, pressure, current, and so on, since rare studies were carried out on these bivalve shells in the cold-seep province, constraining the further understanding of the intrinsic links between growth rate and element contents. In conclusion, the current data highlight that the S/Ca of the shell can reflect the growth rate of the G. platifrons, however, more works need to be done to confirm the direct correlation between the S/Ca ratios and seepage intensity of cold-seep fluids.
5 CONCLUSIONWe investigated Mg/Ca, Ba/Ca, and S/Ca profiles of a bivalve shell fossil by using an LA-ICP-MS technique and evaluated them as environmental and growth proxies for bivalve shells in Haima cold seep of Qiongdongnan Basin, SCS. This study strengthens the idea that Mg/Ca and Ba/Ca ratios in reflecting the environmental temperature and salinity of the surrounding water not only in the deep sea but also in the shallow sea. In addition, the invariable Mg/Ca and Ba/Ca ratios indicate that the surrounding seawater temperatures and salinity remain constant. The S/Ca ratios of the bivalve shell can be an efficient tracer to reflect the growth rate, however, further researches need to be done to evaluate if the S/Ca ratios could be the potential tracer to record the seepage intensity of cold-seep fluids.
6 DATA AVAILABILITY STATEMENTThe data that support the findings of this study are available from the corresponding author upon reasonable request.
7 ACKNOWLEDGMENTWe appreciate the constructive suggestions and comments from the editor Dr. Yang CHEN and two anonymous reviewers.
Boetius A, Ravenschlag K, Schubert C J, et al. 2000. A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature, 407(6804): 623-626.
DOI:10.1038/35036572 |
Boetius A, Wenzhöfer F. 2013. Seafloor oxygen consumption fuelled by methane from cold seeps. Nature Geoscience, 6(9): 725-734.
DOI:10.1016/10.1038/NGEO1926 |
Bowles M W, Samarkin V A, Hunter K S, et al. 2019. Remarkable capacity for anaerobic oxidation of methane at high methane concentration. Geophysical Research Letters, 46(21): 12192-12201.
DOI:10.1029/2019GL084375 |
Busenberg E, Plummer L N. 1985. Kinetic and thermodynamic factors controlling the distribution of SO32- and Na+ in calcites and selected aragonites. Geochimica et Cosmochimica Acta, 49(3): 713-725.
DOI:10.1016/0016-7037(85)90166-8 |
Callender W R, Powell E N. 1999. Why did ancient chemosynthetic seep and vent assemblages occur in shallower water than they do today?. International Journal of Earth Sciences, 88(3): 377-391.
DOI:10.1007/s005310050273 |
Campbell K A. 2006. Hydrocarbon seep and hydrothermal vent paleoenvironments and paleontology: past developments and future research directions. Palaeogeography, Palaeoclimatology, Palaeoecology, 232(2-4): 362-407.
DOI:10.1016/j.palaeo.2005.06.018 |
Ceramicola S, Dupré S, Somoza L et al. 2018. Cold seep systems. In: Micallef A, Krastel S, Savini A eds. Submarine Geomorphology. Springer, Cham. p. 367-387.
|
Chen F, Hu Y, Feng D, et al. 2016. Evidence of intense methane seepages from molybdenum enrichments in gas hydrate-bearing sediments of the northern South China Sea. Chemical Geology, 443: 173-181.
DOI:10.1016/j.chemgeo.2016.09.029 |
Chen L, Liu Y S, Hu Z C, et al. 2011. Accurate determinations of fifty-four major and trace elements in carbonate by LA-ICP-MS using normalization strategy of bulk components as 100%. Chemical Geology, 284(3-4): 283-295.
DOI:10.1016/j.chemgeo.2011.03.007 |
Cuif J P, Dauphin Y D, Farre B, et al. 2008. Distribution of sulphated polysaccharides within calcareous biominerals suggests a widely shared two-step crystallization process for the microstructural growth units. Mineralogical Magazine, 72(1): 233-237.
DOI:10.1180/minmag.2008.072.1.233 |
Dauphin Y, Cuif J P, Salomé M, et al. 2005. Speciation and distribution of sulfur in a mollusk shell as revealed by in situ maps using X-ray absorption near-edge structure (XANES) spectroscopy at the S K-edge. American Mineralogist, 90(11-12): 1748-1758.
DOI:10.2138/am.2005.1640 |
Dauphin Y D, Cuif J P. 1999. Relationship between mineralogy and microstructural patterns of calcareous biominerals and their sulfur contents. Annales des Sciences Naturelles-Zoologie et Biologie Animale, 20(2): 73-85.
DOI:10.1016/S0003-4339(99)80010-0 |
de Nooijer L J, Brombacher A, Mewes A, et al. 2017. Ba incorporation in benthic foraminifera. Biogeosciences, 14(14): 3387-3400.
DOI:10.5194/bg-14-3387-2017 |
Dodd J R, Crisp E L. 1982. Non-linear variation with salinity of Sr/Ca and Mg/Ca ratios in water and aragonitic bivalve shells and implications for paleosalinity studies. Palaeogeography, Palaeoclimatology, Palaeoecology, 38(1-2): 45-56.
DOI:10.1016/0031-0182(82)90063-3 |
Dong X Y, Rattray J E, Campbell D C, et al. 2020. Thermogenic hydrocarbon biodegradation by diverse depth-stratified microbial populations at a Scotian Basin cold seep. Nature Communications, 11(1): 5825.
DOI:10.1038/s41467-020-19648-2 |
Elliot M, Welsh K, Chilcott C, et al. 2009. Profiles of trace elements and stable isotopes derived from giant long-lived Tridacna gigas bivalves: potential applications in paleoclimate studies. Palaeogeography, Palaeoclimatology, Palaeoecology, 280(1-2): 132-142.
DOI:10.1016/j.palaeo.2009.06.007 |
Feng D, Peng Y B, Bao H M, et al. 2016. A carbonate-based proxy for sulfate-driven anaerobic oxidation of methane. Geology, 44(12): 999-1002.
DOI:10.1130/G38233.1 |
Feng D, Qiu J W, Hu Y, et al. 2018a. Cold seep systems in the South China Sea: an overview. Journal of Asian Earth Sciences, 168: 3-16.
DOI:10.1016/j.jseaes.2018.09.021 |
Feng J X, Yang S X, Sun X M, et al. 2018b. Geochemical tracers for methane microleakage activity in the Qiongdongnan Basin. Journal of Southwest Petroleum University (Science & Technology Edition), 40(3): 63-75.
(in Chinese with English abstract) DOI:10.11885/j.issn.1674-5086.2017.12.01.01 |
Freitas P S, Clarke L J, Kennedy H A, et al. 2008. Inter- and intra-specimen variability masks reliable temperature control on shell Mg/Ca ratios in laboratory- and field-cultured Mytilus edulis and Pecten maximus (bivalvia). Biogeosciences, 5(5): 1245-1258.
DOI:10.5194/bg-5-1245-2008 |
Freitas P S, Clarke L J, Kennedy H, et al. 2012. The potential of combined Mg/Ca and δ18O measurements within the shell of the bivalve Pecten maximus to estimate seawater δ18O composition. Chemical Geology, 291: 286-293.
DOI:10.1016/j.chemgeo.2011.10.023 |
Gillikin D P, Dehairs F. 2013. Uranium in aragonitic marine bivalve shells. Palaeogeography, Palaeoclimatology, Palaeoecology, 373: 60-65.
DOI:10.1016/j.palaeo.2012.02.028 |
Gillikin D P, Dehairs F, Lorrain A, et al. 2006. Barium uptake into the shells of the common mussel (Mytilus edulis) and the potential for estuarine paleo-chemistry reconstruction. Geochimica et Cosmochimica Acta, 70(2): 395-407.
DOI:10.1016/j.gca.2005.09.015 |
Gillikin D P, Lorrain A, Paulet Y M, et al. 2008. Synchronous barium peaks in high-resolution profiles of calcite and aragonite marine bivalve shells. Geo-Marine Letters, 28(5): 351-358.
DOI:10.1007/s00367-008-0111-9 |
Guan H X, Feng D, Wu N Y, et al. 2016. Methane seepage intensities traced by biomarker patterns in authigenic carbonates from the South China Sea. Organic Geochemistry, 91: 109-119.
DOI:10.1016/j.orggeochem.2015.11.007 |
Hatch M B A, Schellenberg S A, Carter M L. 2013. Ba/Ca variations in the modern intertidal bean clam Donax gouldii: an upwelling proxy?. Palaeogeography, Palaeoclimatology, Palaeoecology, 373: 98-107.
DOI:10.1016/j.palaeo.2012.03.006 |
Ip J C H, Xu T, Sun J, et al. 2021. Host-endosymbiont genome integration in a deep-sea chemosymbiotic clam. Molecular Biology and Evolution, 38(2): 502-518.
DOI:10.1093/molbev/msaa241 |
Izumida H, Yoshimura T, Suzuki A, et al. 2011. Biological and water chemistry controls on Sr/Ca, Ba/Ca, Mg/Ca and δ18O profiles in freshwater pearl mussel Hyriopsis sp. Palaeogeography, Palaeoclimatology, Palaeoecology, 309(3-4): 298-308.
DOI:10.1016/j.palaeo.2011.06.014 |
Lazareth C E, Guzman N, Poitrasson F, et al. 2007. Nyctemeral variations of magnesium intake in the calcitic layer of a Chilean mollusk shell (Concholepas concholepas, Gastropoda). Geochimica et Cosmochimica Acta, 71(22): 5369-5383.
DOI:10.1016/j.gca.2007.07.031 |
Liang Q Y, Hu Y, Feng D, et al. 2017. Authigenic carbonates from newly discovered active cold seeps on the northwestern slope of the South China Sea: constraints on fluid sources, formation environments, and seepage dynamics. Deep Sea Research Part I: Oceanographic Research Papers, 124: 31-41.
DOI:10.1016/j.dsr.2017.04.015 |
Lietard C, Pierre C. 2008. High-resolution isotopic records (δ18O and δ13C) and cathodoluminescence study of Lucinid shells from methane seeps of the eastern Mediterranean. Geo-Marine Letters, 28(4): 195-203.
DOI:10.1007/s00367-008-0100-z |
Lietard C, Pierre C. 2009. Isotopic signatures (δ18O and δ13C) of bivalve shells from cold seeps and hydrothermal vents. Geobios, 42(2): 209-219.
DOI:10.1016/j.geobios.2008.12.001 |
Liu Y S, Hu Z C, Gao S, et al. 2008. In situ analysis of major and trace elements of anhydrous minerals by LA-ICP-MS without applying an internal standard. Chemical Geology, 257(1-2): 34-43.
DOI:10.1016/j.chemgeo.2008.08.004 |
Lorens R B, Bender M L. 1980. The impact of solution chemistry on Mytilus edulis calcite and aragonite. Geochimica et Cosmochimica Acta, 44(9): 1265-1278.
DOI:10.1016/0016-7037(80)90087-3 |
Marali S, Schöne B R, Mertz-Kraus R, et al. 2017. Ba/Ca ratios in shells of Arctica islandica-potential environmental proxy and crossdating tool. Palaeogeography, Palaeoclimatology, Palaeoecology, 465: 347-361.
DOI:10.1016/j.palaeo.2015.12.018 |
Mouchi V, de Rafélis M, Lartaud F, et al. 2013. Chemical labelling of oyster shells used for time-calibrated high-resolution Mg/Ca ratios: a tool for estimation of past seasonal temperature variations. Palaeogeography, Palaeoclimatology, Palaeoecology, 373: 66-74.
DOI:10.1016/j.palaeo.2012.05.023 |
Mucci A. 1987. Influence of temperature on the composition of magnesian calcite overgrowths precipitated from seawater. Geochimica et Cosmochimica Acta, 51(7): 1977-1984.
DOI:10.1016/0016-7037(87)90186-4 |
Newton R J, Little C T S, Pape E, et al. 2018. Does carbonate-associated sulphate record nutrition in Lucinid and thyasirid bivalve shells from modern hydrocarbon seeps?. Journal of Molluscan Studies, 84(2): 170-174.
|
Nix E R, Fisher C R, Vodenichar J, et al. 1995. Physiological ecology of a mussel with methanotrophic endosymbionts at three hydrocarbon seep sites in the Gulf of Mexico. Marine Biology, 122(4): 605-617.
DOI:10.1007/BF00350682 |
Paton C, Hellstrom J, Paul B, et al. 2011. Iolite: freeware for the visualisation and processing of mass spectrometric data. Journal of Analytical Atomic Spectrometry, 26(12): 2508-2518.
DOI:10.1039/C1JA10172B |
Peckmann J, Birgel D, Kiel S. 2009. Molecular fossils reveal fluid composition and flow intensity at a Cretaceous seep. Geology, 37(9): 847-850.
DOI:10.1130/G25658A.1 |
Peckmann J, Thiel V. 2004. Carbon cycling at ancient methane-seeps. Chemical Geology, 205(3-4): 443-467.
DOI:10.1016/j.chemgeo.2003.12.025 |
Petersen J M, Dubilier N. 2009. Methanotrophic symbioses in marine invertebrates. Environmental Microbiology Reports, 1(5): 319-335.
DOI:10.1111/j.1758-2229.2009.00081.x |
Ponnudurai R, Sayavedra L, Kleiner M, et al. 2017. Genome sequence of the sulfur-oxidizing Bathymodiolus thermophilus gill endosymbiont. Standards in Genomic Sciences, 12: 50.
DOI:10.1186/s40793-017-0266-y |
Poulain C, Gillikin D P, Thébault J, et al. 2015. An evaluation of Mg/Ca, Sr/Ca, and Ba/Ca ratios as environmental proxies in aragonite bivalve shells. Chemical Geology, 396: 42-50.
DOI:10.1016/j.chemgeo.2014.12.019 |
Putten E V, Dehairs F, Keppens E, et al. 2000. High resolution distribution of trace elements in the calcite shell layer of modern Mytilus edulis: environmental and biological controls. Geochimica et Cosmochimica Acta, 64(6): 997-1011.
DOI:10.1016/S0016-7037(99)00380-4 |
Riekenberg P M, Carney R S, Fry B. 2018. Shell carbon isotope indicators of metabolic activity in the deep-sea mussel Bathymodiolus childressi. Deep Sea Research Part I: Oceanographic Research Papers, 134: 48-54.
DOI:10.1016/j.dsr.2018.02.004 |
Riou V, Halary S, Duperron S, et al. 2008. Influence of CH4 and H2S availability on symbiont distribution, carbon assimilation and transfer in the dual symbiotic vent mussel Bathymodiolus azoricus. Biogeosciences, 5(6): 1681-1691.
DOI:10.5194/bg-5-1681-2008 |
Rosenberg G D, Hughes W W, Parker D L, et al. 2001. The geometry of bivalve shell chemistry and mantle metabolism. American Malacological Bulletin, 16(1-2): 251-261.
|
Rosenheim B E, Swart P K, Thorrold S R. 2005. Minor and trace elements in sclerosponge Ceratoporella nicholsoni: biogenic aragonite near the inorganic endmember?. Palaeogeography, Palaeoclimatology, Palaeoecology, 228(1-2): 109-129.
DOI:10.1016/j.palaeo.2005.03.055 |
Sarimin A S, Mohamed C A R. 2014. Sr/Ca, Mg/Ca and Ba/ Ca ratios in the otolith of sea bass in Peninsular Malaysia as salinity influence markers. Sains Malaysiana, 43(5): 757-766.
DOI:10.1142/S0218127414500680 |
Schöne B R. 2008. The curse of physiology-challenges and opportunities in the interpretation of geochemical data from mollusk shells. Geo-Marine Letters, 28(5): 269-285.
DOI:10.1007/s00367-008-0114-6 |
Schöne B R, Lega J, Flessa K W, et al. 2002. Reconstructing daily temperatures from growth rates of the intertidal bivalve mollusk Chione cortezi (Northern Gulf of California, Mexico). Palaeogeography, Palaeoclimatology, Palaeoecology, 184(1-2): 131-146.
DOI:10.1016/S0031-0182(02)00252-3 |
Schöne B R, Zhang Z J, Jacob D, et al. 2010. Effect of organic matrices on the determination of the trace element chemistry (Mg, Sr, Mg/Ca, Sr/Ca) of aragonitic bivalve shells (Arctica islandica) -comparison of ICP-OES and LA-ICP-MS data. Geochemical Journal, 44(1): 23-37.
DOI:10.2343/geochemj.1.0045 |
Schöne B R, Zhang Z J, Radermacher P, et al. 2011. Sr/Ca and Mg/Ca ratios of ontogenetically old, long-lived bivalve shells (Arctica islandica) and their function as paleotemperature proxies. Palaeogeography, Palaeoclimatology, Palaeoecology, 302(1-2): 52-64.
DOI:10.1016/j.palaeo.2010.03.016 |
Shirai K, Takahata N, Yamamoto H, et al. 2008. Novel analytical approach to bivalve shell biogeochemistry: a case study of hydrothermal mussel shell. Geochemical Journal, 42(5): 413-420.
DOI:10.2343/geochemj.42.413 |
Suess E. 2014. Marine cold seeps and their manifestations: geological control, biogeochemical criteria and environmental conditions. International Journal of Earth Sciences, 103(7): 1889-1916.
DOI:10.1007/s00531-014-1010-0 |
Sun Z L, Wu N Y, Cao H, et al. 2019. Hydrothermal metal supplies enhance the benthic methane filter in oceans: an example from the Okinawa trough. Chemical Geology, 525: 190-209.
DOI:10.1016/j.chemgeo.2019.07.025 |
Takesue R K, Bacon C R, Thompson J K. 2008. Influences of organic matter and calcification rate on trace elements in aragonitic estuarine bivalve shells. Geochimica et Cosmochimica Acta, 72(22): 5431-5445.
DOI:10.1016/j.gca.2008.09.003 |
Tamenori Y, Yoshimura T. 2018. Sulfur speciation in growth layers of shell cross section of the long-lived bivalve Margaritifera laevis using synchrotron spectromicroscopy analysis. Geochimica et Cosmochimica Acta, 237: 357-369.
DOI:10.1016/j.gca.2018.07.002 |
Tamenori Y, Yoshimura T, Luan N T, et al. 2014. Identification of the chemical form of sulfur compounds in the Japanese pink coral (Corallium elatius) skeleton using μ-XRF/XAS speciation mapping. Journal of Structural Biology, 186(2): 214-223.
DOI:10.1016/j.jsb.2014.04.001 |
Tynan S, Opdyke B N, Walczak M, et al. 2017. Assessment of Mg/Ca in Saccostrea glomerata (the Sydney rock oyster) shell as a potential temperature record. Palaeogeography, Palaeoclimatology, Palaeoecology, 484: 79-88.
DOI:10.1016/j.palaeo.2016.08.009 |
Wanamaker A D Jr, Kreutz K J, Wilson T, et al. 2008. Experimentally determined Mg/Ca and Sr/Ca ratios in juvenile bivalve calcite for Mytilus edulis: implications for paleotemperature reconstructions. Geo-Marine Letters, 28(5): 359-368.
DOI:10.1007/s00367-008-0112-8 |
Wang H, Zhang H, Zhong Z S, et al. 2020. Molecular analyses of the gill symbiosis of the bathymodiolin mussel Gigantidas platifrons. iScience, 24(1): 101894.
DOI:10.1016/j.isci.2020.101894 |
Wang X D, Li N, Feng D, et al. 2018. Using chemical compositions of sediments to constrain methane seepage dynamics: a case study from Haima cold seeps of the South China Sea. Journal of Asian Earth Sciences, 168: 137-144.
DOI:10.1016/j.jseaes.2018.11.011 |
Yang K H, Chu F Y, Zhu J H, et al. 2014. Mg/Ca and Sr/Ca ratios of authigenic carbonate minerals and calcareous biogenic shells in the cold-seep carbonates, north of the South China Sea and their environmental implication. Acta Oceanologica Sinica, 36(8): 39-48.
(in Chinese with English abstract) DOI:10.3969/j.issn.0253-4193.2014.08.005 |
Yoshimura T, Tamenori Y, Kawahata H, et al. 2014. Fluctuations of sulfate, S-bearing amino acids and magnesium in a giant clam shell. Biogeosciences, 11(14): 3881-3886.
DOI:10.5194/bg-11-3881-2014 |
Yoshimura T, Tamenori Y, Suzuki A, et al. 2013. Element profile and chemical environment of sulfur in a giant clam shell: insights from μ-XRF and X-ray absorption near-edge structure. Chemical Geology, 352: 170-175.
DOI:10.1016/j.chemgeo.2013.05.035 |
Yoshinaga M Y, Lazar C S, Elvert M, et al. 2015. Possible roles of uncultured archaea in carbon cycling in methane-seep sediments. Geochimica et Cosmochimica Acta, 164: 35-52.
DOI:10.1016/j.gca.2015.05.003 |
Zhang M, Lu H F, Guan H X, et al. 2018. Methane seepage intensities traced by sulfur isotopes of pyrite and gypsum in sediment from the Shenhu area, South China Sea. Acta Oceanologica Sinica, 37(7): 20-27.
DOI:10.1007/s13131-018-1241-1 |
Zhang X, Du Z F, Zheng R E, et al. 2017. Development of a new deep-sea hybrid Raman insertion probe and its application to the geochemistry of hydrothermal vent and cold seep fluids. Deep Sea Research Part I: Oceanographic Research Papers, 123: 1-12.
DOI:10.1016/j.dsr.2017.02.005 |
Zhao L P, Xu H Z, Chen D, et al. 2015. Microstructure and spectral analysis of Mytilus coruscus shell. Journal of Zhejiang University (Science Edition), 42(3): 339-346.
(in Chinese with English abstract) DOI:10.3785/j.issn.1008-9497.2015.03.018 |
 2023, Vol. 41
2023, Vol. 41