Institute of Oceanology, Chinese Academy of Sciences
Article Information
- PANG Bijian, LI Hongjun, LI Mingmin, LUO Xin, CHEN Ying, LI Tianshen, LAN Wenlu
- Effects of shellfish culture on the community and mortality of zooplankton in a subtropical Bay
- Journal of Oceanology and Limnology, 41(2): 458-468
- http://dx.doi.org/10.1007/s00343-022-2073-6
Article History
- Received Feb. 16, 2022
- accepted in principle Apr. 28, 2022
- accepted for publication May 25, 2022
2 National Marine Environmental Monitoring Center, Dalian 116023, China
China is the world's leading aquaculture producer (Jiang et al., 2012). According to statistics, in 2020, the production of mariculture in China was 21.4×106 t, among which the shellfish accounted for 69.3% of the total production (China Fishery Statistics Yearbook, 2021). Shellfish aquaculture in China is mainly dominated by filter-feeding bivalves such as oyster, scallop and mussel. Filter-feeding bivalves ingests large amounts of phytoplankton and organic particles and has high biodeposition rate, so they can aggregate organic matter from water column to surface sediment, which may affects the ecosystem of the aquaculture farms and the surrounding environment (Fenchel, 1991). Excessive exploitation and expansion of shellfish culture may impose a negative impact on marine and estuarine ecosystem (Jiang et al., 2012), such as the attenuation of suspended particulate, accelerated deposition of particulate organics, and uncontrolled plankton dynamics (Zhang et al., 2009).
Zooplankton acts as a crucial route in the marine food web by ingesting phytoplankton and transferring energy to the higher trophic levels through fishes and other marine organisms (Abdullah Al et al., 2020). Its dynamic changes in abundance and community affect the biomass of producer and consumer in the marine (Jin et al., 2010; Viñas et al., 2013). Characterized by its short lifespan and sensitivity to environmental disturbances perturbations, zooplankton has been widely served as bioindicator species to marine ecosystem (Hitchcock et al., 2016; Beaugrand and Kirby, 2018). Studies reveal that top-down effects caused by the shellfish's excessive prey on phytoplankton had an indirect impact on zooplankton in the culture area (Liu et al., 2015, 2019; Su et al., 2018, 2019). High-density shellfish culture could affect or even alter the production pattern of phytoplankton and zooplankton, resulting in the miniaturization of zooplankton community structure (Guo et al., 2015). Filtering effect of shellfish culture has indirect impact on the spatial and temporal changes of zooplankton biomass and diversity (Liu et al., 2015; Su et al., 2018).
To understand the relationship between the distribution pattern of the zooplankton community and environmental parameters in shellfish aquaculture, this research was done based on monthly sampling in the Qinzhou Bay from January 2018 to December 2018, one of the major oyster culture farms in the northern Beibu Gulf, South China Sea. We hope to reveal the characteristics and ecological variations of zooplankton in shellfish culture area and the impact of shellfish culture on zooplankton through environmental adaptation and food webbing.
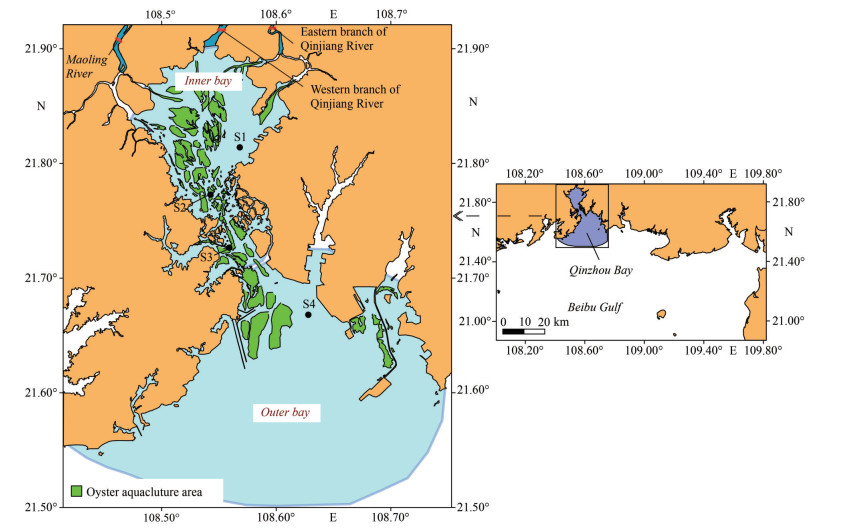
2 MATERIAL AND METHOD 2.1 Study areaSamples were collected at both near-farm sites at S1 and S4 and in-farm sites at S2 and S3 from January to December in 2018 (Fig. 1), where average water depth was 4.6, 7.5, 11.8, and 6.8 m, respectively. The parameters for the survey include the zooplankton, phytoplankton, and chlorophyll a (chl a), as well as environmental parameters.
|
| Fig.1 Sampling sites in Qinzhou Bay, Beibu Gulf, the South China Sea |
Water temperature and salinity were measured using a multiparameter water quality monitor (Germany WTW, Multi350i) at each site. Following the specifications guide for marine monitoring (General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China and Standardization Administration of the People's Republic of China, 2008), seawater was taken near the surface (at a depth of about 0.5 m) for measurement of nutrients concentration. Dissolved inorganic ammonium (NH4+), nitrate (NO3-), nitrite (NO2-), phosphate (PO43-), and chl a were measured using a UV-Vis spectrophotometer (UV-2450, Shimadzu, Japan). Dissolved inorganic nitrogen (DIN) was calculated as the sum of the concentrations of NH4+, NO3-, and NO2-. Zooplankton was sampled by a plankton net with a mesh size of 0.505 mm (mouth diameter of 0.50 m). The net mouth was equipped with a flowmeter to measure the volume of filtered water in cubic meters during each tow when the net was towed vertically from the bottom to the surface. One liter of subsurface water was collected using a water sampler for the analysis of phytoplankton, and the plankton samples were preserved immediately in 5% solution of formaldehyde in seawater. The composition and abundance of plankton taxa were measured by a stereomicroscope and optical microscope in laboratory. Additionally, samples for the mortality analysis of zooplankton were collected at S2 and S4; neutral red staining was used to distinguish carcass and living zooplankton (Elliott and Tang, 2009). Filled 500 mL of filtered seawater from the sampling site into a 2-L brown jar, directly transferred the zooplankton samples collected into the same jar and continued filling the jar using the seawater from the sampling site until reaching volume of 1 L. Samples were stained for 15 min using 1.5-mL neutral red solution (10 g/L), filtered, sealed in Petri dishes, and stored at -20 ℃ until enumeration. The samples were then acidified with HCl to a pH of less than seven to develop the neutral red stain color and viewed under a dissecting microscope with dark field illumination. The samples with bright red staining were living bodies, while those without staining or with pink staining were dead bodies.
2.3 Data analysisThe distribution map of sampling stations was plotted by Surfer software (version 11.0). CANOCO software was utilized to calculate the Canonical Correspondence Analysis (CCA) as a measure of the correlation between the abundance of zooplankton and the major environmental factors.
The dominance (Y) of each species is expressed in:
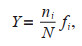
where ni is the abundance of the ith species, fi represents the occurrence frequency of the ith species, and N stands for the total abundance. A Y value equal to or greater than 0.02 indicates a dominant species (Xu et al., 1995).
3 RESULT 3.1 Environmental variableSeasonal variation of water temperature ranged from 11.7 to 31.1 ℃ (Fig. 2), which was higher in summer and lower in winter. Salinity ranged from 5.1 to 27.9, and the seasonal change of salinity was lower in summer and higher in winter. The salinity gradient increased from estuary in the inner bay to the outer bay, with lowest at S1 and highest at S4 (Fig. 2).
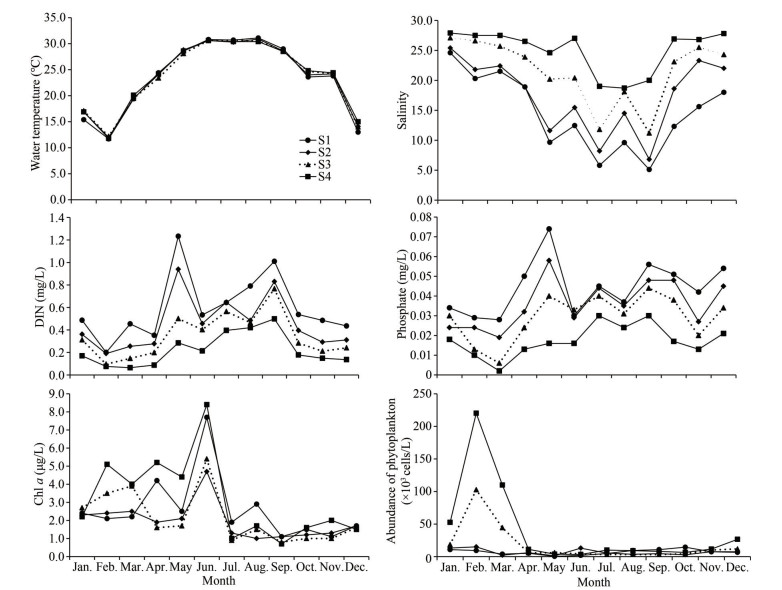
|
| Fig.2 Distribution of environmental parameters of the Qinzhou Bay |
The spatial and temporal distributions of the DIN and phosphate were largely the same, and higher in the wet season and lower in the dry season. The average concentration of DIN and phosphate gradient decreased from inner bay to outer bay. The spatial and temporal distributions of chl-a concentration were significantly different (Fig. 2). The average concentration of chl a was lower in the farming areas than that in the near-farm areas. The abundance of phytoplankton ranged from 3.2×103 to 220.2×103 cells/L, and the average abundance of phytoplankton was lower in the farming areas than that in the near-farm areas.
3.2 Variation and composition of zooplankton speciesA total of 76 species belonging to 11 classes of zooplankton were identified, including 27 species of Copepoda, 12 species of Medusa, 11 species of Chaetognatha, and 11 species of the pelagic larvae. The number of the species of other groups such as Tunicata, Decapod, Cladocera, Ostracoda, Cumacea, and Protozoa was negligible. The number of zooplankton species increased gradually from S1 to S4, and the dominant zooplankton species at the four sites were mainly composed of Copepoda and pelagic larvae. From S1 to S4, the number of the species of other groups such as Medusa and Chaetognatha gradually increased. Zooplankton species at each site varied greatly from month to month, but there was no obvious seasonal variation (Fig. 3).
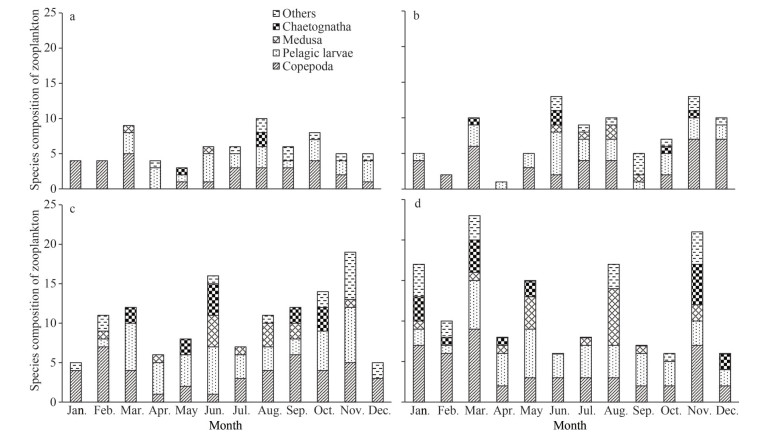
|
| Fig.3 Variation of the composition of zooplankton species a. S1; b. S2; c. S3; d. S4. |
The abundance of zooplankton ranged from 1.40 to 312.0 inds./m3. The abundance of zooplankton in the farming areas was significantly lower than that outside, demonstrated by S4 having the highest average abundance of zooplankton (109.85 inds./m3), followed by S1, whereas S2 having the lowest average abundance of zooplankton (30.85 inds./m3). Observation of monthly changed abundance showed that the abundance of zooplankton at both S1 and S2 was much higher in wet season than other seasons with a unimodal peak whereas multimodal peak at S3 (in January, June, and November) and S4 (in May and October) (Fig. 4).
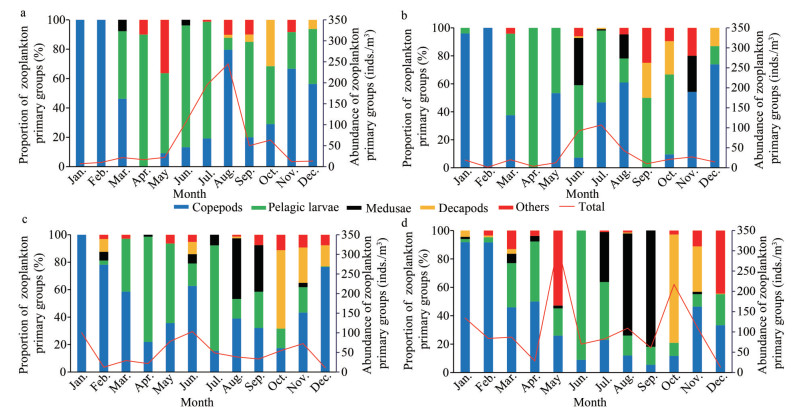
|
| Fig.4 Distributions of the abundance of zooplankton major groups a. S1; b. S2; c. S3; d. S4. |
Furthermore, the 4 sites significantly differed in community structure and monthly succession of zooplankton (Fig. 4). Planktic larvae, mainly zoeae (Bradyura), was the dominant group from April to July at S1, substituted in the remaining observation period by copepod, mainly estuarine brackish-water groups such as Misophria sinensis and Pseudodiaptomus incisus. Similar result was found at S2. The copepod was the dominant group of zooplankton at S3 and S4, where the offshore warm-water species such as Centropages tenuiremis and Temora turbinata were more noticeable in winter and spring, and Acartia pacifica was the primary species in summer and autumn. Compared with S1 and S2, S3 and S4 showed a significant increase in the appearance and abundance of medusa and decapod from July to November, and the dominant species were mainly Pleurobrachia globosa and Lucifer hanseni.
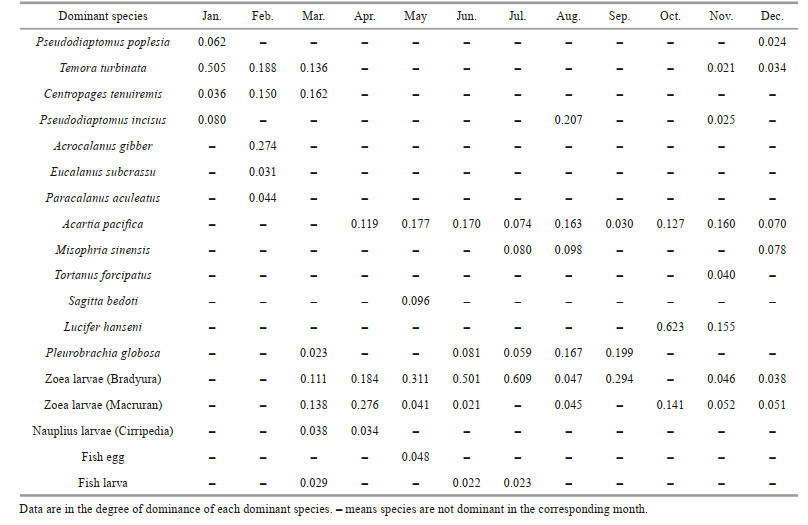
3.4 Variation of the dominant zooplankton speciesEighteen mainly dominant zooplankton species were identified, including 10 species of Copepod, 5 species of planktonic larvae, 1 species each of Chaetognatha, Decapoda, and Medusa. The most dominant zooplankton species appeared in November, while the least in September and October. Acartia pacifica continued to be one of the main species from April to December. The number and species of planktonic larvae also began to increase in March, and zoeae (Bradyura and Macruran) were the primary and important groups in the community of zooplankton at four sites from March to December. Pleurobrachia globosa became the dominant group from June to September, and the number of Lucifer hanseni increased dramatically from October to November, making them the main species (Table 1).
Eighteen dominant species of zooplankton were selected for Canonical Correspondence Analysis (CCA) with 10 environmental factors (dominance degree ≥ 0.020, frequency ≥5). The Monte Carlo test results show that the first axis (P=0.002) and the whole axis (P=0.002) are significantly different (P≤0.010), indicating that the sorting result is reliable.
The results show that water temperature (the correlation coefficient of correlated weight (CW) on axis 1; CW=-0.857 2), dissolved oxygen (CW=-0.777 8), phytoplankton abundance (CW=-0.686 9), and salinity (CW=-0.623 4) were the main environmental factors affecting the distribution of the 18 zooplankton species. The species-environment correlation coefficients of the first axis and the second axis were 0.919 and 0.776, respectively, and explained 41.7% and 18.9% of species variables, respectively, indicating that the 18 major zooplankton species were well correlated with 10 environmental factors.
The graph was obtained by sorting CCA analysis results. The phytoplankton species Group Ⅰ (Zoea larva (Brachyura), Misophria sinensis, Pseudodiaptomus incises, and Pleurobrachia globosa were positively correlated with nutrients. The species of Group Ⅱ, including nauplius larva (Cirripdia), Sagitta bedoti, Eucalanus subcrassus, and Tortanus forcipatus were negatively correlated with salinity, chl a, and pH. The zooplankton in Group Ⅲ, including Paracalanus aculeatus, Temora turbinata, Acrocalanus gibber, and Centropages tenuiremis were positively correlated with dissolved oxygen and phytoplankton abundance, and negatively correlated with water temperature (Fig. 5).
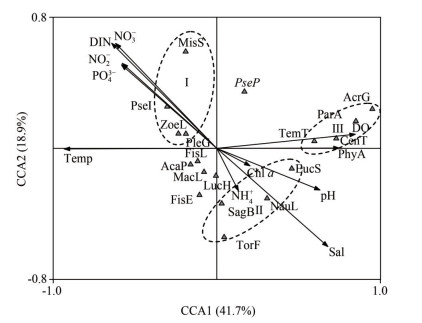
|
| Fig.5 CCA ordination of main zooplankton species with environmental variables Temp: temperature of water; Sal: salinity; DIN: dissolved inorganic nitrogen; DO: dissolved oxygen; PhyA: phytoplankton abundance; MisS: Misophria sinensis; ZoeL: Zoea larva (Brachyura); PseI: Pseudodiaptomus incises; PleG: Pleurobrachia globose; NauL: nauplius larva (Cirripdia); SagB: Sagitta bedoti; EucS: Eucalanus subcrassus; TorF: Tortanus forcipatus; ParA: Paracalanus aculeatus; TemT: Temora turbinata; AcrG: Acrocalanus gibber; CenT: Centropages tenuiremis; FisE: fish eggs; AcaP: Acartia pacifica. |
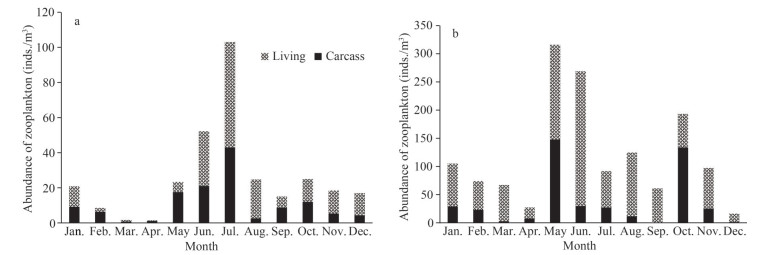
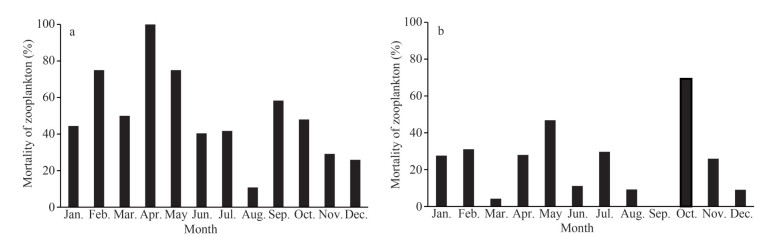
The abundance of carcass zooplankton at S2 peaked in July (43.00 inds./m3) and dropped to the lowest value in March (0.83 inds./m3) (Fig. 6). The mortality of zooplankton peaked in April (100%) and fell to the lowest value in August (18.1%) on average of 49.9% (Fig. 7).
|
| Fig.6 Variation of the abundance of carcass zooplankton at S2 and S4 a. S2; b. S4. |
|
| Fig.7 Variation of the mortality of zooplankton at S2 and S4 a. S2; b. S4. |
The abundance of carcass zooplankton at S4 peaked in May (148.00 inds./m3) and dropped to the lowest value in September (0.00 inds./m3) (Fig. 6). The mortality of zooplankton peaked in October (69.3%) and dropped to the lowest value in September (0%) (Fig. 7), on average of 24.87%.
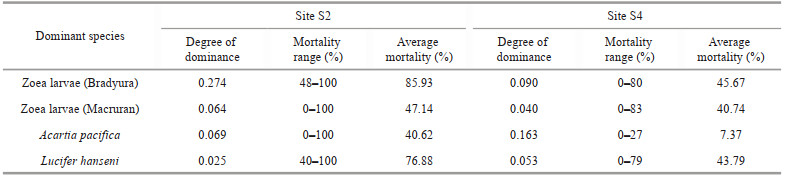
3.7 Mortality of the dominant species of zooplanktonAmong the 4 dominant species of zooplankton at S2, the zoeae (Bradyura) had the highest mortality and the mortality ranged from 48% to 100%, on average of 85.93%, the Acartia pacifica had the lowest mortality ranged from 0 to 100%, on average of 40.62%. At S4, the mortality of zoeae (Bradyura) was 45.67%, and Acartia pacifica had the lowest mortality, only 7.37%. Among the four common dominant species, the mortality at S2 was significantly higher than S4 (Table 2).
The state of estuary is the principal driver to the seasonal variation of zooplankton in the Qinzhou Bay. Data analysis showed no similarity in temporal distributions (Fig. 4) among samples collected in the oyster farm area (S2 and S3), which is also true for samples out of the farming areas (S1 and S4) (Fig. 1). On the contrary, similar temporal distribution of the structure and succession of zooplankton was found in samples collected from station closed to estuary at S1 and S2 (Fig. 3). Similar conclusion applies to the samples away from the estuary at S3 and S4. Freshwater from the river caused significant seasonal change in salinity (Fig. 2), resulting in peak during wet season in zooplankton abundance from June to August when salinity was low. However, seawater salinity kept higher and more stable at S3 and S4 (Fig. 2). Moreover, the number of species of zooplankton remarkably increased from the estuary to the outer sea, implying that the succession of the community structure of zooplankton and the variation of the abundance of zooplankton were driven by the characteristics of the hydrological environment (Fig. 3). Studies showed that aquaculture have significantly changed the temporal distributions of zooplankton (Papa et al., 2011; Dias et al., 2012), but results of our observation show that oyster culture did not significantly change the original temporal characteristics of zooplankton in the oyster farms in the Qinzhou Bay.
In contrast, oyster culture imposed a significant impact on the spatial distributions of the abundance of zooplankton of the Qinzhou Bay, suggested by this study. Spatial abundance was significantly lower in the culture farms at S2 and S3 than that outside the farms at S1 and S4 (Fig. 4). The dramatic changing in hydrological environment in the estuary was the driven factor causing reduction in the abundance of zooplankton (Wei et al., 2001). The higher average abundance of zooplankton in the estuary at S1 and outside the estuary at S4 than those at S2 and S3 in the oyster farms, indicate that oyster culture was the principal factor determining the spatial distribution of the abundance of zooplankton in the Qinzhou Bay.
Oyster culture caused reduction in phytoplankton biomass in Qinzhou Bay, resulting in the reduction of abundance of zooplankton in spatial scale. Correlation analysis suggested positive relationship between the abundance of zooplankton and phytoplankton (Fig. 5). Chl-a concentration representing the biomass of phytoplankton (Luo et al., 2016) was lower in the oyster farms at S2 and S3 than those at S1 and S4, which proved that phytoplankton as the food organisms for zooplankton is involved directly in the regulation of zooplankton communities. Surveys conducted on shellfish culture in other estuaries reported similar findings (Cadée and Hegeman, 1974; Asmus and Asmus, 1991; Yan et al., 1999). Additionally, some small zooplankton and planktonic larvae were filtered by the shellfish (Jiang et al., 2002; Deng and Yang, 2009), which further affected the zooplankton communities.
4.2 Effects of oyster culture on the mortality of zooplanktonCarcass zooplankton always took up a significant proportion of zooplankton collected at S2 and S4, a salient phenomenon of the Qinzhou Bay. The higher mortality of the crustacean may indicate that zooplankton was more easily affected by environmental stress such as hunger, salinity stress, and ultraviolet radiation (Elliott and Tang, 2011) at the larvae stage (Table 2). The average mortality of zooplankton outside the farms at S4 was 24.87%, consistent with the results reported for other waters (Elliott et al., 2010; Tang and Elliott, 2014), whereas the average mortality of zooplankton in the farms at S2 was almost doubled to be 49.90% (Figs. 6–7). Specifically, the mortality of Acartia pacifica and Lucifer hanseni inside the oyster farms was much higher than that outside (Table 2). Some studies reported that the complex and changeable water environment in estuary was not conducive to the growth of zooplankton (Wei et al., 2001; He et al., 2009). We discovered the mortality of Acartia pacifica, the main dominant species that suits low salinity at estuary (Lan et al., 2015) at S4, was only 7.37% versus, up to 40.62% at S2. Similarly, Lucifer hanseni, a common dominant specie in the Qinzhou Bay (Pang et al., 2018), its mortality in the oyster farms was up to 76.88%, followed by zoeae (Bradyura) at 85.93%. Additional to the state of estuary where other common species are well adapted, with the significant variation in mortality of zooplankton inside and outside of the oyster farms, the oyster culture may be the main reason for the high mortality of zooplankton in the oyster farms.
The scarcity of food caused by the excessive oyster culture may be another reason for the higher mortality of zooplankton in oyster farms. Large consumption of phytoplankton by the filter feeder, oysters, in the Qinzhou Bay brought not just direct mortality of the small zooplankton but also competition for the zooplankton over the food chain. Higher mortality of zooplankton occurred from February to May at S2, which coincided with the rapid decrease in the concentration of chl a in parallel. On the other hand, the rich nutrients brought by the river rapidly promote the growth of phytoplankton in estuary from June to August in the wet season with matching increase of concentration of chl a by almost three times (Fig. 2), coincided with decrease of the mortality of zooplankton at S2 (Fig. 7).
4.3 Effects of culture on the community structure of zooplanktonHigh-density oyster culture in Qinzhou Bay affected the community structure of phytoplankton and further reshaped the community structure of zooplankton. Oysters selectively feed on larger size of phytoplankton (Trottet et al., 2008). Study on the phytoplankton of Qinzhou Bay revealed that the dominant species of phytoplankton in the oyster farms were composed of nano- and pico-phytoplankton such as cyanobacteria (Lan et al., 2011). Influenced by the large-scale oyster culture, the phytoplankton in the oyster farms was mainly composed of the small-sized. Our studies revealed the community structure of zooplankton in the Qinzhou Bay was dominated by meso- and microzooplankton, with relatively small amount of macrozooplankton species. Among the dominant species of copepods, except for the Eucalanus subcrassu, which belongs to the herbivorous filter feeding species, the species of Centropagidae, Temoridae, Pseudodiaptomidae, and Acartiidae are all omnivorous filter feeding species (Li et al., 2012). When phytoplankton are lacking, these omnivorous copepods can maintain their metabolic consumption for survival by feeding on animal food such as copepods larvae and debris (Gong et al., 2015). The lack of phytoplankton for feeding herbivorous copepods led to a community structure of zooplankton dominated by meso- and microzooplankton species and omnivorous copepods.
Oyster culture facilities such as rafting frame and longline can significantly reduce the flow velocity and exchange rate (O'Donncha et al., 2013). The water exchange capacity of Qinzhou Bay is relatively weak (Chen et al., 2017), coupled with the influence of floating rafts, resulted in a low water exchange rate between the inside and outside of the oyster farms which increased the spatial heterogeneity of the community structure of zooplankton in the Qinzhou Bay. With weak swimming capability, zooplankton can drift only along with the tides, making hydrological factors such as runoff and ocean current keys to its distribution (Marques et al., 2007). Tens of thousands of rafts floating on the surface of the water just like a huge wave elimination device, and hampered zooplankton movement alongside the tide. Our study revealed that the species of offshore warm-temperature group outside of the oyster farms could not reach the inside with the tides even in the normal and dry seasons when the outer sea water has a great influence on the tides. The dominant species and groups of large-sized zooplankton such as the Chaetognatha, Medusae, and Decapoda were mainly presented at S4. There was a high abundance of Sagitta bedoti of Chaetognatha at S4, as opposed to the oyster farms. Lucifer hanseni was abundant at S4, but it was barely found in the estuary area (Fig. 4) due to its high mortality in the oyster farms (Table 2). Similarly, the estuarine species rarely reach the outer sea area with the current of runoff even in the wet season. Consequently, the species composition and the abundance of zooplankton at S4 outside of the oyster farms are significantly different from those at S2 and S1 in the estuary area (Figs. 3–4).
5 CONCLUSIONThis study shows that the oyster culture was the principal driver to the zooplankton abundance in spatial scale and to the high spatial heterogeneity of the zooplankton community structure, whereas the hydrological state of estuary imposed the impact on zooplankton abundance in temporal scale. The scarcity of nutrient caused by the excessive oyster culture might be the main reason for the higher mortality of zooplankton in the oyster farms. Our survey provided a new perspective on the effects of shellfish aquaculture on zooplankton and had some interesting findings and conclusions. With the rapid development of shellfish aquaculture in estuarine coastal waters of the world, more and more estuarine ecosystems will be formed for shellfish aquaculture. Therefore, our research has practical significance for promoting ecological environment protection and sustainable utilization of resources. In the future, we will focus on the effect and mechanism of shellfish farming on the changes of zooplankton community structure from the perspective of zooplankton-oyster feeding.
6 DATA AVAILABILITY STATEMENTAll data generated and/or analyzed during this study are available from the corresponding author on reasonable request.
Abdullah Al M, Akhtar A, Rahman M F, et al. 2020. Temporal distribution of zooplankton communities in coastal waters of the northern Bay of Bengal, Bangladesh. Regional Studies in Marine Science, 34: 100993.
DOI:10.1016/j.rsma.2019.100993 |
Asmus R M, Asmus H. 1991. Mussel beds: limiting or promoting phytoplankton?. Journal of Experimental Marine Biology and Ecology, 148(2): 215-232.
DOI:10.1016/0022-0981(91)90083-9 |
Beaugrand G, Kirby R R. 2018. How do marine pelagic species respond to climate change? Theories and observations. Annual Review of Marine Science, 10: 169-197.
DOI:10.1146/annurev-marine-121916-063304 |
Cadée G C, Hegeman J. 1974. Primary production of the benthic microflora living on tidal flats in the Dutch Wadden Sea. Netherlands Journal of Sea Research, 8(2-3): 260-291.
DOI:10.1016/0077-7579(74)90020-9 |
Chen Z H, Xia C S, Qiao F L. 2017. Numerical simulation of water exchange in the Qinzhou Bay of China. Haiyang Xuebao, 39(3): 14-23.
(in Chinese with English abstract) DOI:10.3969/j.issn.0253-4193.2017.03.002 |
Deng B P, Yang Y F. 2009. Comparative studies on water quality and community structure of zooplankton between the sea surface microlayer and the subsurface microlayer in mariculture areas in Dapeng Cove. Journal of Jinan University (Natural Science), 30(1): 101-105.
(in Chinese with English abstract) DOI:10.3969/j.issn.1000-9965.2009.01.024 |
Dias J D, Simões N R, Bonecker C C. 2012. Zooplankton community resilience and aquatic environmental stability on aquaculture practices: a study using net cages. Brazilian Journal of Biology, 72(1): 1-11.
DOI:10.1590/S1519-69842012000100001 |
Elliott D T, Harris C K., Tang K W. 2010. Dead in the water: the fate of copepod carcasses in the York River estuary, Virginia. Limnology and Oceanography, 55(5): 1821-1834.
DOI:10.4319/lo.2010.55.5.1821 |
Elliott D T, Tang K W. 2009. Simple staining method for differentiating live and dead marine zooplankton in field samples. Limnology and Oceanography Methods, 7(8): 585-594.
DOI:10.4319/lom.2009.7.585 |
Elliott D T, Tang K W. 2011. Spatial and temporal distributions of live and dead Copepods in the Lower Chesapeake Bay (Virginia, USA). Estuaries and Coasts, 34(5): 1039-1048.
DOI:10.1007/s12237-011-9380-z |
Fenchel T. 1991. Bivalve filter feeding: hydrodynamics, bioenergetics, Physiology and Ecology. C. Barker Jorgensen. The Quarterly Review of Biology, 66(3): 353.
DOI:10.1086/417296 |
General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China, Standardization Administration of the People's Republic of China. 2008. The specification for marine monitoring-Part 4: Seawater Analysis: GB 17378.4-2007. Beijing: China Standards Press. (in Chinese)
|
Gong Y Y, Zhang C X, Chen Z Z, et al. 2015. Structural characteristics of zooplankton populations and their annual changes in Zhanjiang Bay. Marine Sciences, 39(12): 46-55.
(in Chinese with English abstract) |
Guo Y J, Li J W, Luo Z L, et al. 2015. Characteristics of zooplankton community in aquaculture areas of Liusha bay. Progress in Fishery Sciences, 36(5): 8-17.
(in Chinese with English abstract) DOI:10.11758/yykxjz.20150502 |
He Q, Sun J, Luan Q S, et al. 2009. Phytoplankton in Changjiang estuary and adjacent waters in winter. Marine Environmental Science, 28(4): 360-365.
(in Chinese with English abstract) DOI:10.3969/j.issn.1007-6336.2009.04.004 |
Hitchcock J N, Mitrovic S M, Hadwen W L, et al. 2016. Zooplankton responses to freshwater inflows and organic-matter pulses in a wave-dominated estuary. Marine and Freshwater Research, 67(9): 1374-1386.
DOI:10.1071/MF15297 |
Jiang S, Huang C J, Chen S W, et al. 2002. Community structure and temporal and spatial distribution of zooplankton in Zhelin Bay, China (2000~2001). Acta Ecologica Sinica, 22(6): 828-840.
(in Chinese with English abstract) DOI:10.3321/j.issn:1000-0933.2002.06.006 |
Jiang Z B, Chen Q Z, Zeng J N, et al. 2012. Phytoplankton community distribution in relation to environmental parameters in three aquaculture systems in a Chinese subtropical eutrophic bay. Marine Ecology Progress Series, 446: 73-89.
DOI:10.3354/meps09499 |
Jin X S, Zhang B, Xue Y. 2010. The response of the diets of four carnivorous fishes to variations in the Yellow Sea ecosystem. Deep Sea Research Part II, 57(11-12): 996-1000.
DOI:10.1016/j.dsr2.2010.02.001 |
Lan W L, Li T S, Li M L, et al. 2015. Characteristics of zooplankton community in the Qinzhou Bay during flood and dry seasons. Haiyang Xuebao, 37(4): 124-132.
(in Chinese with English abstract) DOI:10.3969/j.issn.0253-4193.2015.04.012 |
Lan W L, Wang X H, Li M M. 2011. Phytoplankton community structure in Qinzhou Bay during flood season by analysis of HPLC photosynthetic pigment signatures. Acta Ecologica Sinica, 31(13): 3601-3608.
(in Chinese with English abstract) |
Li K Z, Tan Y H, Huang L M, et al. 2012. Feeding of planktonic copepods in the Pearl River estuary. Journal of Tropical Oceanography, 31(6): 90-96.
(in Chinese with English abstract) DOI:10.3969/j.issn.1009-5470.2012.06.014 |
Liu P, Song H J, Zhang X L, et al. 2015. Spatio-temporal distributions of zooplankton community and the impact of aquaculture activity on it in the Sanggou Bay. Advances in Marine Science, 33(4): 501-511.
(in Chinese with English abstract) DOI:10.3969/j.issn.1671-6647.2015.04.008 |
Liu P, Sun P, Song H J, et al. 2019. Spatio-temporal distribution of phytoplankton community in different aquaculture areas in the Sanggou Bay. Advances in Marine Science, 37(4): 673-680.
(in Chinese with English abstract) DOI:10.3969/10.3969/j.issn.1671-6647.2019.04.013 |
Luo X, Zeng J N, Xu X Q, et al. 2016. Distribution of zooplankton in the Zhoushan Sea and its relationship with environmental factors in summer and autumn. Acta Ecologica Sinica, 36(24): 8194-8204.
(in Chinese with English abstract) DOI:10.5846/stxb201505221032 |
Marques S C, Pardal M A, Pereira M J, et al. 2007. Zooplankton distribution and dynamics in a temperate shallow estuary. Hydrobiologia, 587(1): 213-223.
DOI:10.1007/s10750-007-0682-x |
Ministry of Agriculture and Rural Affairs Fisheries Administration. 2021. China Fishery Statistics Yearbook. The Chinese Farmer Industry Press, Beijing.
|
O'Donncha F, Hartnett M, Nash S. 2013. Physical and numerical investigation of the hydrodynamic implications of aquaculture farms. Aquacultural Engineering, 52: 14-26.
DOI:10.1016/j.aquaeng.2012.07.006 |
Pang B J, Li T S, Lan W L, et al. 2018. Distribution patterns and environmental factors of zooplankton in the Qinzhou Bay in spring and autumn. Acta Ecologica Sinica, 38(17): 6204-6216.
(in Chinese with English abstract) DOI:10.5846/stxb201708121451 |
Papa R D S, Zafaralla M T, Eckmann R. 2011. Spatio-temporal variation of the zooplankton community in a tropical caldera lake with intensive aquaculture (Lake Taal, Philippines). Hydrobiologia, 664(1): 119-133.
DOI:10.1007/s10750-010-0591-2 |
Su J Q, Zhu C B, Li J W, et al. 2019. Characteristics of zooplankton community and their correlation with fish and shellfish aquaculture in the Liusha Bay. Marine Fisheries, 41(3): 278-293.
(in Chinese with English abstract) DOI:10.3969/j.issn.1004-2490.2019.03.003 |
Su J Q, Zhu C B, Li J W, et al. 2018. Phytoplankton community characteristics in different seasons and their relationship with aquaculture in Liusha Bay. Progress in Fishery Sciences, 39(6): 11-23.
(in Chinese with English abstract) DOI:10.3969/10.19663/j.issn2095-9869.20171024002 |
Tang K W, Elliott D T. 2014. Copepod carcasses: occurrence, fate and ecological importance. In: Seuront L ed. Copepods: Diversity, Habitat and Behavior. Nova Science Publishers, New York. p. 255-278.
|
Trottet A, Roy S, Tamigneaux E, et al. 2008. Impact of suspended mussels (Mytilus edulis L.) on plankton communities in a Magdalen Islands lagoon (Québec, Canada): a me socosm approach. Journal of Experimental Marine Biology and Ecology, 365(2): 103-115.
DOI:10.1016/j.jembe.2008.08.001 |
Viñas M D, Negri R M, Cepeda G D, et al. 2013. Seasonal succession of zooplankton in coastal waters of the Argentine Sea (Southwest Atlantic Ocean): prevalence of classical or microbial food webs. Marine Biology Research, 9(4): 371-382.
DOI:10.1080/17451000.2012.745003 |
Wei M X, Tong W P, Lai T H, et al. 2001. A Preliminary study on the water environment characteristics and nutrlent status of the shellfish culture area in the inner Qinzhou Bay. Journal of Oceanography of Huanghai & Bohai Seas, 19(4): 51-55.
(in Chinese with English abstract) DOI:10.3969/j.issn.1671-6647.2001.04.007 |
Xu Z L, Wang Y L, Chen Y Q, et al. 1995. An ecological study on zooplankton in maximum turbid zone of estuarine area of Changjiang (Yangtze) River. Journal of Fishery Sciences of China, 2(1): 39-48.
(in Chinese with English abstract) |
Yan Q L, Guo H, Wang Z L, et al. 1999. A study on ecological characteristics of zooplankton inside and outside raft mariculture areas of molluscs. Journal of Oceanography of Huanghai & Bohai Seas, 17(1): 47-51.
(in Chinese with English abstract) |
Zhang J H, Fang J G, Wang W. 2009. Progress in studies on ecological carrying capacity of mariculture for filter-feeding shellfish. Journal of Fishery Sciences of China, 16(4): 626-632.
(in Chinese with English abstract) DOI:10.3321/j.issn:1005-8737.2009.04.020 |
 2023, Vol. 41
2023, Vol. 41