Institute of Oceanology, Chinese Academy of Sciences
Article Information
- YANG Shun, MKINGULE Idefonce, LIU Long, CHEN Wenqi, YUAN Xiangyu, MA Zixuan, LIANG Liang, QIAN Shichao, HUANG Mengmeng, FEI Hui
- Protective efficacy evaluation of immunogenic protein AHA_3793 of Aeromonas hydrophila as vaccine candidate for largemouth bass Micropterus salmoides
- Journal of Oceanology and Limnology, 41(1): 392-400
- http://dx.doi.org/10.1007/s00343-022-1326-8
Article History
- Received Oct. 12, 2021
- accepted in principle Dec. 13, 2021
- accepted for publication Jan. 13, 2022
2 Zhejiang Development&Planning Institute, Hangzhou 310012, China;
3 Huzhou Baijiayu Biotech Co., Ltd., Huzhou 313000, China
Aeromonas hydrophila is a gram-negative pathogen with broad distribution in the aquatic environment, which can not only infect terrestrial animals, but also a variety of fishes, such as channel catfish (Ictalurus Punetaus), European eel (Anguilla anguilla), and Chinese breams (Parker and Shaw, 2011; Wang et al., 2013b; Feng et al., 2017; Abdelhamed et al., 2019). The outbreaks of disease caused by A. hydrophila in fish farming have resulted in huge economic losses in aquaculture. Therefore, the development of vaccines is essential to reduce fish mortality in farming caused by A. hydrophila and use of antibiotics in aquaculture. So far, many studies have focus on the development of vaccines against A. hydrophila, including various vaccination strategies (Abdelhamed et al., 2017; Wang et al., 2017; Yun et al., 2017; Guo et al., 2018; Han et al., 2019; Zhang et al., 2019). However, the major problem in developing vaccines against A. hydrophila is the antigenic diversity of strains, which requires us to develop targeted vaccines against different strains.
The subunit vaccine has unique advantages by using recombinant antigens that can induce protective of the body as effectors. Currently, the studies on subunit vaccines of A. hydrophila mainly focus on its outer membrane proteins (Omps). Many studies have shown that the Omps of gram-negative bacteria play an important role in bacterial adhesion and infection (Lopez et al., 2020; Guan et al., 2021). The function of Omps in the interaction between bacteria and host has been a research hotspot (Li et al., 2020; Passalia et al., 2020). In addition, Omps are the epitope exposed on the cell surface, resulting in it easier to interact with the host immune system. Therefore, many Omps with high immunogenicity are good candidates for vaccine development (Liu et al., 2016; Xing et al., 2017a, b). In Labeo rohita, the rOmp F can induce fish immune response and increased the relative percentage survival (RPS) by about 44% on day 140 post-immunization against A. hydrophila (Yadav et al., 2018). In European eel, previous study has reported that Omp can induce the protective immunity of fish against A. hydrophila as well as Aeromonas sobria (Guan et al., 2011). Moreover, a conserved 46-kD Omp of maltoporin also exhibited high protection against three strains of A. hydrophila in European eel (Feng et al., 2017). In Chinese breams, high immunogenicity Omp 38 in A. hydrophila identified by immunoproteomic analysis can effectively induce both specific and non-specific immune responses, protecting fish against A. hydrophila infection (Wang et al., 2013b).
Largemouth bass (Micropterus salmoides) is a freshwater fish species originating in North America, which has become an important economic fish in many areas because of favored by consumers (Zhong et al., 2020). Our previous study has reported that A. hydrophila infection caused metabolic disorder, high inflammation, reduced adaptive immune response, and destruction of microbial community homeostasis of largemouth bass, resulting in high mortality (Yuan et al., 2021). The outbreaks of frequent diseases caused by A. hydrophila have severely restricted the development of largemouth bass farming. However, few studies have been conducted to develop vaccine against A. hydrophila for largemouth bass. In present study, we identified a high immunogenicity Omp, AHA_3793, with combined western blotting and mass spectrometry using largemouth bass anti-A. hydrophila antibodies. The recombinant AHA_3793 (rAHA_3793) was obtained by prokaryotic expression, then evaluated for protective efficacy in largemouth bass. The specific antibody production and relative percentage survival were monitored after immunization with rAHA_3793. Our data demonstrated that AHA_3793 can serve as a vaccine candidate for protection against A. hydrophila infection in largemouth bass farming.
2 MATERIAL AND METHOD 2.1 FishLargemouth bass were purchased from a fish farm in Huzhou, Zhejiang Province, China, for this study. Then largemouth bass reared for two weeks in the tanks containing aerated filtered fresh water at 28±0.5 ℃ with commercial feed prior to experiments. The rearing conditions of fish have been remained as above throughout the experiment. Fish was anesthetized with ethyl 3-amino-benzoate-methanesulfonic acid (MS-222) before sacrificing and sampling. The treatment of fish in this study complied with provisions for the welfare ethics of experimental animals in the state and Zhejiang Province.
2.2 Preparation of largemouth bass anti-A. hydrophila antibodiesFor A. hydrophila vaccination, the A. hydrophila isolated from diseased largemouth bass was inactivated with 0.5% formaldehyde solution, and adjusted to 1×108 CFU/mL. Then 40 healthy largemouth bass (50±4.0 g) were immunized with 200 μL of inactivated A. hydrophila emulsified with equal volume of complete Freund's adjuvant (Sigma) by intraperitoneal injection. Two weeks later, largemouth bass were intraperitoneal injected with 200 μL of inactivated A. hydrophila emulsified with equal volume of incomplete Freund's adjuvant (Sigma) for first booster immunization. Subsequently, two booster injections were performed by intraperitoneal injection with 100 μL of inactivated A. hydrophila at one-week intervals. Finally, largemouth bass serum was collected one week after last injection, and largemouth bass anti-A. hydrophila antibodies were characterized by ELISA.
2.3 Expression and purification of recombinant AHA_3793The sequence of AHA_3793 was obtained from GenBank (No. A0KPP0), and then specific primers (F: 5'-CGCGGATCCATGAATAAAACACTGATTACCTTGC-3', BamH I; R: 5'-CCCAAGCTTCTGCTGAACTTCCGAGATCC-3', Hind III) were designed to amplify the sequences encoding AHA_3793 by PCR. The PCR products were purified and ligated into pET-28 vector after digestion with specific restriction enzymes to construct recombinant plasmid pET-28a-AHA_3793. Then pET-28a-AHA_3793 was transformed into Escherichia coli (DE3), and the positive transformant was screened by colony PCR and sequencing. Subsequently, positive transformant was cultured in LB medium, then induced with isopropyl β-D-1-thiogalactopyranoside (IPTG). Recombinant AHA_3793 (rAHA_3793) was purified using HisTrapTM HP Ni-Agarose (GE Healthcare) referring to manufacturer's instructions. Moreover, purified rAHA_3793 was subjected to stepwise dialysis for refolding as previously described, and then treated with Triton X-114 for removing endotoxin. Finally, purified rAHA_3793 was analysed with sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE), and the concentration was determined with bicinchoninic acid (BCA) method.
2.4 Analysis of the immunogenicity of AHA_3793The A.hydrophilawas analyzed by SDS-PAGE, then electrophoretically transferred onto polyvinylidene fluoride (PVDF) membranes (Merck Millipore, Germany). The PVDF membranes were blocked using phosphate buffered saline (PBS) containing 5% bovine serum albumin (BSA) for 2 h at 37 ℃, then washed with PBS containing 0.05% tween-20 (PBST) three times. Then, PVDF membranes were incubated with largemouth bass anti-A. hydrophila antibodies (1 100) for 3 h at 28 ℃, and then washed with PBST. Subsequently, PVDF membranes were incubated with polyclonal antibodies against largemouth bass IgM at 37 ℃ for 1 h as previously described (Yang et al., 2020). After washing with PBST, PVDF membranes were incubated with goat anti-rabbit Ig-horseradish peroxidase conjugate (GAM-HRP, 1 5 000) at 37 ℃ for 1 h. Next, membranes were stained with 3, 3N-diaminobenzidine tertrahydrochloride (DAB) substrate solution (Solarbio, China) for 10 min, and stopped by washing with distilled water. Healthy largemouth bass serum instead of largemouth bass anti-A. hydrophila antibodies were used as control. According to the results of western blotting, the corresponding bands on SDS-PAGE gel were cut, then send Shanghai Sangon Biotech for liquid chromatography-mass spectrometry (LC-MS). Finally, appropriate outer membrane protein of A. hydrophila was selected from the results of LS-MS.
In addition, the rAHA_3793 was also electrophoretically transferred onto PVDF membranes. Then, the binding of largemouth bass anti-A. hydrophila antibodies with rAHA_3793 was analyzed by western blotting as above. Healthy largemouth bass serum instead of largemouth bass anti-A. hydrophila antibodies were used as control.
2.5 Vaccination and samplingTwo hundred and forty healthy largemouth bass (4±0.4 g) were equally divided into two groups. For rAHA_3793 vaccination, the concentration of rAHA_3793 was adjusted to 1 mg/mL, then largemouth bass of one group was immunized with 50 μL of rAHA_3793 emulsified with equal volume of complete Freund's adjuvant by intraperitoneal injection. The other group was intraperitoneal injected with 50-μL PBS as control.
For quantitative real-time PCR (qRT-PCR) analysis, the spleen and head kidney were isolated from nine largemouth bass for three individual samples at 0, 1, 2, 3, 5, 7, and 14 d post vaccination, then frozen in liquid nitrogen and stored at -80 ℃ until usage. In addition, the blood was sampled from caudal vein of nine largemouth bass for three individual samples in weeks 4 and 5 post vaccination, then clotted overnight at 4 ℃ to prepare serum by centrifugation at 3 000×g at 4 ℃ for 15 min. The serum was stored at -20 ℃ until used for ELISA analysis.
2.6 ELISAThe inactivated A. hydrophila was resuspended in carbonate-bicarbonate buffer (50 mmol/L, pH=9.6) and adjusted to 1×108 CFU/mL, then 100 μL of inactivated A. hydrophila and purified rAHA_3793 (50 μg/mL) were coated in wells of microplates (96-wells, Costar) at 4 ℃ overnight, respectively. After washing with PBST, 200 μL of 5% BSA per well was added and blocked at 37 ℃ for 1 h. Then, 100 μL of serum from vaccinated largemouth bass (1 200) per well was added and incubated at 28 ℃ for 3 h after washing with PBST again. The serum from control group instead of serum from vaccinated largemouth bass was used as control. Next, the wells were washed with PBST, and 100 μL of polyclonal antibodies against IgM per well was added and incubated at 37 ℃ for 1 h (Yang et al., 2020). After washing again, 100 μL of goat anti-rabbit Ig-horseradish peroxidase conjugate (GAM-HRP, 1 5 000) per well was added and incubated at 37 ℃ for 1 h. Following the last wash, 3, 3′, 5, 5′-tetramethylbenzidine (TMB) substrate solution (Beyotime, China, 100 μL/well) was added and incubated in the dark at room temperature for 15 min, then stopped by 1-mol/L HCl (100 μL/well). Finally, the microplate was added into an ELISA reader to measure the absorbance at 450 nm.
2.7 Quantitative real-time PCRTotal RNA was extracted from spleen and head kidney of largemouth bass using RNA extraction kit (Tiangen, China) referring to manufacturer's instructions, and then first-strand cDNA was synthesized using FastKing RT kit (Tiangen, China) following quality inspection of RNA by Bioanalyzer 2100 (Agilent Technologies, USA). The concentrations of cDNAs were analyzed and adjusted to 100 ng/μL, then quantitative real-time PCR (qRT-PCR) was performed in a LightCycler® 480 II real time system (Roche, Switzerland) using SYBR Green I Master Mix (Tiangen, China) as described previously (Yuan et al., 2021). The relative abundance of MHC IIα and CD4-2 were determined with the 2-ΔΔCt method using β-actin gene as internal reference. The primers for MHC IIα and CD4-2 were designed and shown in Table 1.
Forty vaccinated largemouth bass were randomly selected from rAHA_3793 vaccinated group and control group in week 5 after immunization for challenge experiment. The A. hydrophila was cultured in LB media at 30 ℃ for 24 h, then adjusted to 1×106 CFU/mL. Then all fish used for challenge experiment was intraperitoneally injected with 50 μL of A. hydrophila per fish. Mortalities of largemouth bass were monitored over a period of 14 days following the challenge, and then relative percentage of survival rate (RPS) was calculated as previously described.
2.9 Statistical analysisThe data was analyzed using statistical product and service solution (SPSS) software (Version 20.0; SPSS, Inc). Statistical significance was analyzed by independent-samples t-test, and differences were considered significant when P < 0.05. All data was shown as the mean±S.D.
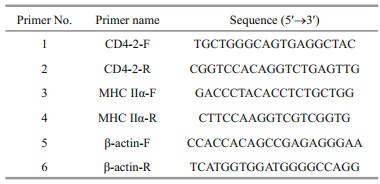
3 RESULT 3.1 Expression and purification of recombinant AHA_3793The AHA_3793 from A. hydrophila was recombinantly expressed using pET-28a vector. SDS-PAGE analysis showed that AHA_3793 was successfully expressed with a distinct band of about 44 kDa after IPTG induction, which was consistent with predicted molecular mass of rAHA_3793 plus His-tag (Fig. 1). As shown in Fig. 1, the high-purity rAHA_3793 was obtained by purification with Ni2+ affinity chromatography and stepwise dialysis refolding.

|
| Fig.1 SDS-PAGE analysis of expression and purification of rAHA_3793 protein Lane M: molecular mass marker; Lane 1: transformated E. coli without IPTG induction; Lane 2: transformated E. coli induced with IPTG; Lane 3: purified rAHA_3793. |
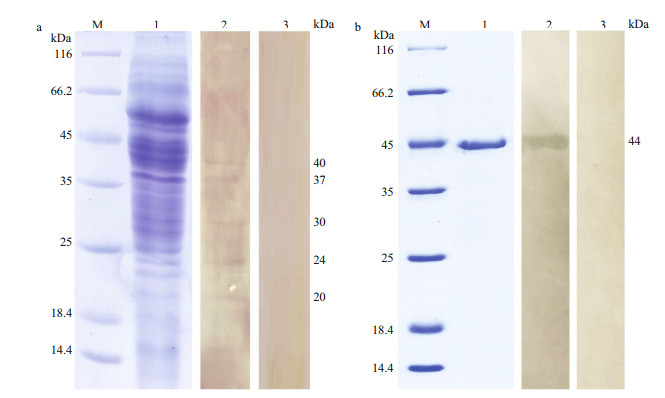
The binding of largemouth bass anti-A. hydrophila antibodies with A. hydrophila was analyzed by western blotting, and the results showed that five positive bands were observed with molecular mass of about 40, 37, 30, 24 and 20 kDa (Fig. 2a). As shown in Table 2, the mass spectrometric (MS) showed that positive bands matched with six outer membrane proteins of A. hydrophila, which were AHA_1280, AHA_1281, AHA_3953, AHA_3793, AHA_4200, and AHA_1130. Among them, only AHA_3793 and AHA_4200 were successfully expressed and purified (Fig. 1 and Supplementary Fig.S1). Western blotting also showed that rAHA_3793 could react specifically with largemouth bass anti-A. hydrophila antibodies but not rAHA_4200 (Fig. 2b and Supplementary Fig. S2), which suggested that AHA_3793 in A. hydrophila possessed high immunogenicity to largemouth bass.
|
| Fig.2 Analysis of the immunogenicity of AHA_3793 a. western blotting analysis of binding of largemouth bass anti-A. hydrophila antibodies with A. hydrophila. Lane M: molecular mass marker; Lane 1: A. hydrophila; Lane 2: A. hydrophila immuno-stained using largemouth bass anti-A. hydrophila antibodies; Lane 3: negative control; b. western blotting analysis of binding of largemouth bass anti-A. hydrophila antibodies with rAHA_3793. Lane M: molecular mass marker; Lane 1: rAHA_3793; Lane 2: rAHA_3793 immuno-stained using largemouth bass anti-A. hydrophila antibodies; Lane 3: negative control. |
|
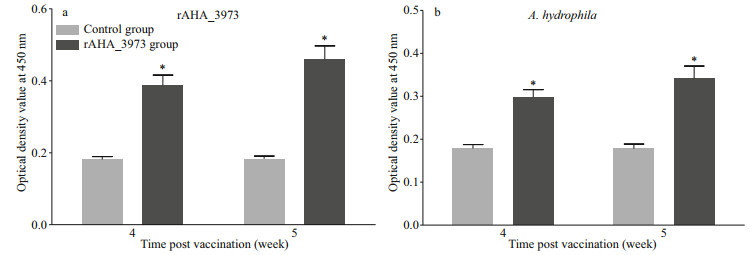
ELISA analysis showed that the specific serum antibodies of largemouth bass against rAHA_3793 in vaccinated group in weeks 4 and 5 after immunization were significantly higher than those in control group, and higher levels of specific serum antibodies were observed in week 5 after immunization (Fig. 3a). Moreover, the specific serum antibodies of largemouth bass against A. hydrophila in vaccinated group in weeks 4 and 5 after immunization were also significantly higher than those in control group, and higher levels of specific serum antibodies against A. hydrophila were also observed in week 5 after immunization (Fig. 3b). These results suggested that rAHA_3793 could induce the production of specific serum antibodies of largemouth bass against rAHA_3793 and A. hydrophila.
|
| Fig.3 Analysis of specific serum antibodies of largemouth bass after vaccination with rAHA_3793 a. indirect ELISA analysis of specific serum antibodies against rAHA_3793; b. indirect ELISA analysis of specific serum antibodies against A. hydrophila. Asterisks on the bars indicate statistically significant differences between the immunization and control groups at the same time point (P < 0.05). |
In vaccinated group and control group, no mortality was observed before challenge experiment. Then the largemouth bass were challenged with A. hydrophila in five weeks post immunization, and the fish began to die 1 day after challenged. The cumulative mortality rate of the control group increased rapidly after challenged with A. hydrophila, but lower cumulative mortality rate was observed in rAHA_3793 vaccinated group. As shown in Fig. 4, the cumulative mortalities of rAHA_3793 vaccinated and control groups were 32.5% and 85%, respectively, which correspond to an RPS of 61.76% for rAHA_3793.
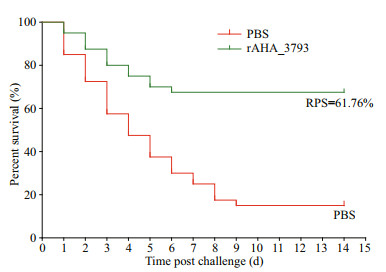
|
| Fig.4 Cumulative mortality of largemouth bass vaccinated with rAHA_3793 and PBS after challenged with live A. hydrophila |
As shown in Fig. 5, the expressions of MHC IIα and CD4-2 were significantly up-regulated after immunization with rAHA_3793. The expressions of MHC IIα reached the peaks on day 5 after immunization in both spleen and head kidney; and the peaks of CD4-2 expressions occurred on day 7 after immunization in both spleen and head kidney.
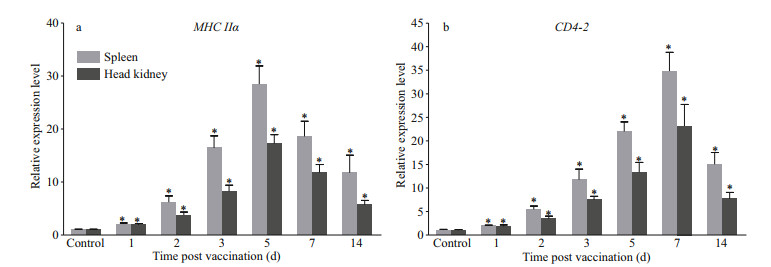
|
| Fig.5 qRT-PCR analysis of expression of largemouth bass MHC IIα (a) and CD4-2 (b) after vaccination with rAHA_3793 The mRNA levels of MHC IIα and CD4-2 were determined by normalized against β-actin gene. The asterisk on the bars indicates statistical significance of MHC IIα and CD4-2 expressions compared to control group (P < 0.05). |
Aeromonas hydrophila is a common pathogen in aquaculture that can infect various fish, which also severely restricted the development of largemouth bass farming (Blazer et al., 2010; Yuan et al., 2021; Zhou et al., 2021). Moreover, the antigenic diversity of different A. hydrophila strains required us to develop targeted vaccines, but few studies focus on developing vaccine against A. hydrophila for largemouth bass. In present study, we aimed to develop subunit vaccine against A. hydrophila for largemouth bass by immunogenic analysis using largemouth bass anti-A. hydrophila antibodies. Combined western blotting and mass spectrometric analysis showed that six outer membrane proteins, AHA_1280, AHA_1281, AHA_3953, AHA_3793, AHA_4200, and AHA_1130, could react with largemouth bass anti-A. hydrophila antibodies. However, the positive bands from western blotting contained multiple proteins, including immunogenic proteins and others, and could not be distinguished by mass spectrometry. Therefore, six outer membrane proteins were recombinantly expressed to further verify its immunogenicity, but only AHA_3793 and AHA_4200 were successfully expressed and purified. Western blotting showed that only rAHA_3793 could react specifically with largemouth bass anti-A. hydrophila antibodies, suggesting that AHA_3793 possessed high immunogenicity to largemouth bass, but AHA_4200 was the other protein contained in the positive band. The subunit vaccines play protective function by inducing protective immune response of the body, and high immunogenic proteins have higher probability of developing into subunit vaccines (Zhang et al., 2015; Kalita et al., 2019). Previous studies have also shown that immunogenic proteins as vaccines candidate can enhance specific immunity of fish, producing high immune protection against pathogenic microorganism infection (Xing et al., 2017b; Sheng et al., 2018; Zhao et al., 2020).
Subunit vaccines are safe with negligible adverse effects, and many studies have reported that subunit vaccines formulated with certain adjuvants could induce the production of specific serum antibodies and produce highly protective effects (Wang et al., 2013a; Liu et al., 2016; Xing et al., 2017a). To evaluate the feasibility of rAHA_3793 as a candidate subunit vaccine, largemouth bass was immunized with rAHA_3793 emulsified with complete Freund's adjuvant. During a five-week observation period after immunization, no mortality was found in the rAHA_3793 group. More than that, rAHA_3793 also produced an RPS of 61.76% for largemouth bass against A. hydrophila challenge. Meanwhile, the levels of specific serum antibodies of largemouth bass induced by rAHA_3793 were also examined in present study. The results showed that rAHA_3793 induced the production of specific serum antibodies against rAHA_3793 and A. hydrophila, and higher levels of specific serum antibodies in week 5 after immunization, which indicated that rAHA_3793 could induce a strong humoral immune response of largemouth bass against A. hydrophila. In addition, it was also found that the expressions of CD4-2 and MHC IIα were up-regulated after rAHA_3793 immunization. Previous studies have shown that MHC II is responsible for processing foreign peptides and presenting them to CD4+T cells, and then activating the body's adaptive immunity (Xu et al., 2010; Chida et al., 2011; Grimholt, 2016; Dubin et al., 2019). Therefore, the up-regulation of CD4-2 and MHC IIα indicated that the recognition and presentation of largemouth bass to rAHA_3793 may involve in MHCII and CD4 pathway.
5 CONCLUSIONIn conclusion, a highly immunogenic outer membrane proteins, AHA_3793, was identified using largemouth bass anti-A. hydrophila antibodies in this study. Furthermore, rAHA_3793 could induce a strong humoral immune response of largemouth bass, and then produce high immune protective effects against A. hydrophila infection. This work suggests that identification of highly immunogenicity is an effective method for screening vaccine candidates. We will further investigate the immune protective effects of other highly immunogenic protein against A. hydrophila infection in future research.
6 DATA AVAILABILITY STATEMENTThe data that support the findings of the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Supplementary material (Supplementary Figs.S1–S2) is available in the online version of this article at https://doi.org/10.1007/s00343-022-1326-8.
Abdelhamed H, Banes M, Karsi A, et al. 2019. Recombinant ATPase of virulent Aeromonas hydrophila protects channel catfish against motile Aeromonas septicemia. Frontiers in Immunology, 10: 1641.
DOI:10.3389/fimmu.2019.01641 |
Abdelhamed H, Ibrahim I, Nho S W, et al. 2017. Evaluation of three recombinant outer membrane proteins, OmpA1, Tdr, and TbpA, as potential vaccine antigens against virulent Aeromonas hydrophila infection in channel catfish (Ictalurus punctatus). Fish & Shellfish Immunology, 66: 480-486.
DOI:10.1016/j.fsi.2017.05.043 |
Blazer V S, Iwanowicz L R, Starliper C E, et al. 2010. Mortality of centrarchid fishes in the Potomac drainage: survey results and overview of potential contributing factors. Journal of Aquatic Animal Health, 22(3): 190-218.
DOI:10.1577/H10-002.1 |
Chida A S, Goyos A, Robert J. 2011. Phylogenetic and developmental study of CD4, CD8 α and β T cell co-receptor homologs in two amphibian species, Xenopus tropicalis and Xenopus laevis. Developmental & Comparative Immunology, 35(3): 366-377.
DOI:10.1016/j.dci.2010.11.005 |
Dubin A, Jørgensen T E, Moum T, et al. 2019. Complete loss of the MHC II pathway in an anglerfish, Lophius piscatorius. Biology Letters, 15(10): 20190594.
DOI:10.1098/rsbl.2019.0594 |
Feng J J, Lin P, Guo S L, et al. 2017. Identification and characterization of a novel conserved 46 kD maltoporin of Aeromonas hydrophila as a versatile vaccine candidate in European eel (Anguilla anguilla). Fish & Shellfish Immunology, 64: 93-103.
DOI:10.1016/j.fsi.2017.03.010 |
Grimholt U. 2016. MHC and evolution in teleosts. Biology, 5(1): 6.
DOI:10.3390/biology5010006 |
Guan Q F, Bhowmick B, Upadhyay A, et al. 2021. Structure and functions of bacterial outer membrane protein A, a potential therapeutic target for bacterial infection. Current Topics in Medicinal Chemistry, 21(13): 1129-1138.
DOI:10.2174/1568026621666210705164319 |
Guan R Z, Xiong J, Huang W S, et al. 2011. Enhancement of protective immunity in European eel (Anguilla anguilla) against Aeromonas hydrophila and Aeromonas sobria by a recombinant Aeromonas outer membrane protein. Acta Biochimica et Biophysica Sinica, 43(1): 79-88.
DOI:10.1093/abbs/gmq115 |
Guo Z, Lin Y X, Wang X Y, et al. 2018. The protective efficacy of four iron-related recombinant proteins and their single-walled carbon nanotube encapsulated counterparts against Aeromonas hydrophila infection in zebrafish. Fish & Shellfish Immunology, 82: 50-59.
DOI:10.1016/j.fsi.2018.08.009 |
Han B Q, Xu K, Liu Z T, et al. 2019. Oral yeast-based DNA vaccine confers effective protection from Aeromonas hydrophila infection on Carassius auratus. Fish & Shellfish Immunology, 84: 948-954.
DOI:10.1016/j.fsi.2018.10.065 |
Kalita P, Lyngdoh D L, Padhi A K, et al. 2019. Development of multi-epitope driven subunit vaccine against Fasciola gigantica using immunoinformatics approach. International Journal of Biological Macromolecules, 138: 224-233.
DOI:10.1016/j.ijbiomac.2019.07.024 |
Li M, Zhou H, Yang C, et al. 2020. Bacterial outer membrane vesicles as a platform for biomedical applications: an update. Journal of Controlled Release, 323: 253-268.
DOI:10.1016/j.jconrel.2020.04.031 |
Liu F G, Tang X Q, Sheng X Z, et al. 2016. Edwardsiella tarda outer membrane protein C: an immunogenic protein induces highly protective effects in flounder (Paralichthys olivaceus) against Edwardsiellosis. International Journal of Molecular Sciences, 17(7): 1117.
DOI:10.3390/ijms17071117 |
Lopez P, Guaimas F, Czibener C, et al. 2020. A genomic island in Brucella involved in the adhesion to host cells: identification of a new adhesin and a translocation factor. Cellular Microbiology, 22(11): e13245.
DOI:10.1111/cmi.13245 |
Parker J L, Shaw J G. 2011. Aeromonas spp. clinical microbiology and disease. Journal of Infection, 62(2): 109-118.
DOI:10.1016/j.jinf.2010.12.003 |
Passalia F J, Carvalho E, Heinemann M B, et al. 2020. The Leptospira interrogans LIC10774 is a multifunctional surface protein that binds calcium and interacts with host components. Microbiological Research, 235: 126470.
DOI:10.1016/j.micres.2020.126470 |
Sheng X Z, Liu M, Liu H B, et al. 2018. Identification of immunogenic proteins and evaluation of recombinant PDHA1 and GAPDH as potential vaccine candidates against Streptococcus iniae infection in flounder (Paralichthys olivaceus). PLoS One, 13(5): e0195450.
DOI:10.1371/journal.pone.0195450 |
Wang C, Hu Y H, Chi H, et al. 2013a. The major fimbrial subunit protein of Edwardsiella tarda: vaccine potential, adjuvant effect, and involvement in host infection. Fish & Shellfish Immunology, 35(3): 858-865.
DOI:10.1016/j.fsi.2013.06.021 |
Wang N, Yang Z, Zang M F, et al. 2013b. Identification of Omp38 by immunoproteomic analysis and evaluation as a potential vaccine antigen against Aeromonas hydrophila in Chinese breams. Fish & Shellfi sh Immunology, 34(1): 74-81.
DOI:10.1016/j.fsi.2012.10.003 |
Wang Y Q, Chen H R, Guo Z, et al. 2017. Quantitative proteomic analysis of iron-regulated outer membrane proteins in Aeromonas hydrophila as potential vaccine candidates. Fish & Shellfish Immunology, 68: 1-9.
DOI:10.1016/j.fsi.2017.07.002 |
Xing J, Xu H S, Wang Y, et al. 2017a. Identification of immunogenic proteins and evaluation of four recombinant proteins as potential vaccine antigens from Vibrio anguillarum in flounder (Paralichthys olivaceus). Vaccine, 35(24): 3196-3203.
DOI:10.1016/j.vaccine.2017.04.071 |
Xing J, Xu H S, Wang Y, et al. 2017b. Protective efficacy of six immunogenic recombinant proteins of Vibrio anguillarum and evaluation them as vaccine candidatefor flounder (Paralichthys olivaceus). Microbial Pathogenesis, 107: 155-163.
DOI:10.1016/j.micpath.2017.03.027 |
Xu T J, Chen S L, Zhang Y X. 2010. MHC class IIα gene polymorphism and its association with resistance/susceptibility to Vibrio anguillarum in Japanese flounder (Paralichthys olivaceus). Developmental & Comparative Immunology, 34(10): 1042-1050.
DOI:10.1016/j.dci.2010.05.008 |
Yadav S K, Dash P, Sahoo P K, et al. 2018. Modulation of immune response and protective efficacy of recombinant outer-membrane protein F (rOmpF) of Aeromonas hydrophila in Labeo rohita. Fish & Shellfi sh Immunology, 80: 563-572.
DOI:10.1016/j.fsi.2018.06.041 |
Yang S, Chen W Q, He F F, et al. 2020. Comparison of the roles of IgM in systemic and mucosal immunity via tissue distribution analysis in largemouth bass (Micropterus salmoides). Aquaculture, 527: 735488.
DOI:10.1016/j.aquaculture.2020.735488 |
Yuan X Y, Zhang X T, Xia Y T, et al. 2021. Transcriptome and 16S rRNA analyses revealed differences in the responses of largemouth bass (Micropterus salmoides) to early Aeromonas hydrophila infection and immunization. Aquaculture, 541: 736759.
DOI:10.1016/j.aquaculture.2021.736759 |
Yun S, Jun J W, Giri S S, et al. 2017. Efficacy of PLGA microparticle-encapsulated formalin-killed Aeromonas hydrophila cells as a single-shot vaccine against A. hydrophila infection. Vaccine, 35(32): 3959-3965.
DOI:10.1016/j.vaccine.2017.06.005 |
Zhang D H, Xu D H, Beck B. 2019. Analysis of agglutinants elicited by antiserum of channel catfish immunized with extracellular proteins of virulent Aeromonas hydrophila. Fish & Shellfish Immunology, 86: 223-229.
DOI:10.1016/j.fsi.2018.11.033 |
Zhang H W, Qian P, Peng B, et al. 2015. A novel subunit vaccine co-expressing GM-CSF and PCV2b Cap protein enhances protective immunity against porcine circovirus type 2 in piglets. Vaccine, 33(21): 2449-2456.
DOI:10.1016/j.vaccine.2015.03.090 |
Zhao L N, Tang X Q, Sheng X Z, et al. 2020. Different immune responses of flounder (Paralichthys olivaceus) towards the full-length and N-terminal or C-terminal portion of hirame novirhabdovirus glycoprotein. Fish & Shellfish Immunology, 104: 279-288.
DOI:10.1016/j.fsi.2020.06.002 |
Zhong Y F, Shi C M, Zhou Y L, et al. 2020. Optimum dietary fiber level could improve growth, plasma biochemical indexes and liver function of largemouth bass, Micropterus salmoides. Aquaculture, 518: 734661.
DOI:10.1016/j.aquaculture.2019.734661 |
Zhou J, Zhao H, Zhang L, et al. 2021. MiRNA-seq analysis of spleen and head kidney tissue from aquacultured largemouth bass (Micropterus salmoides) in response to Aeromonas hydrophila infection. Functional & Integrative Genomics, 21(1): 101-111.
DOI:10.1007/s10142-020-00763-8 |
 2023, Vol. 41
2023, Vol. 41