Institute of Oceanology, Chinese Academy of Sciences
Article Information
- YAN Xu, CAO Min, FU Qiang, YANG Ning, WANG Ningning, SONG Lin, LI Chao
- Expression profile of long non-coding RNAs in the intestine of black rockfish Sebastes schlegelii in response to Edwardsiella tarda infection
- Journal of Oceanology and Limnology, 41(1): 376-391
- http://dx.doi.org/10.1007/s00343-021-1230-7
Article History
- Received Jul. 15, 2021
- accepted in principle Sep. 17, 2021
- accepted for publication Dec. 22, 2021
2 School of Marine Science and Engineering, Qingdao Agricultural University, Qingdao 266109, China
Large numbers of non-coding RNAs (ncRNAs) have been reported in mammalian and other eukaryotes genomes. Majority of these ncRNAs are by-products of RNA processing, performing various regulatory functions in cells (Mercer et al., 2009). NcRNAs were initially thought to be spurious transcriptional noise resulting from low RNA polymerase fidelity because their low protein-coding capacity compared with other RNAs (Struhl, 2007). However, previous research showed ncRNAs could take part in a variety of biological processes (McHugh et al., 2015). As for ncRNA types, they can be divided into sncRNA (length between 20–50 nt), mncRNA (length between 50–200 nt) and long non-coding RNAs (lncRNAs) (length over 200 nt) by different lengths. Numerous functional studies on ncRNAs have been conducted in recent years especially for lncRNA. For instance, tens of thousands lncRNAs have been identified in species including human (Homo sapiens), mouse (Mus musculus), zebrafish (Danio rerio), and Caenorhabditis elegans (Zhao et al., 2016). Depending on their relative positions to coding genes, lncRNAs can be classified into antisense lncRNAs, intronic lncRNAs, divergent lncRNAs, and intergenic lncRNAs (Ransohoff et al., 2018). lncRNAs play important roles in higher-order chromosome dynamics, chromatin modification, telomere biology, subcellular structural organization, transcriptional regulation, and post-transcriptional regulation. Mercer et al. (2009) found the down-regulation of a lncRNA called TROJAN impaired the proliferative potential of breast cancer cell lines in vitro, while over-expression of TROJAN promoted this ability (Jin et al., 2019). In addition, many lncRNA molecules related to human immune response were explored in early studies, such as Morrbid (Kotzin et al., 2016), lnc-Lsm3b (Jiang et al., 2018), NKILA (Huang et al., 2018). Moreover, innate immune responses of lncRNAs were widely investigated in other species. For example, mammals like sheep (Zhang et al., 2021a), arthropods like Drosophila (Zhang et al., 2020), several mollusks (Zhang et al., 2021b), even for plants (Gómez and Pallás, 2013). Therefore, the function of lncRNAs in the process of animal immune regulation is worth further exploration.
In teleost, the potential roles of lncRNAs have also been increasingly explored in recent years. For example, in common carp (Cyprinus carpio) undifferentiated gonads, 124 lncRNAs showed differential expression between experimental and control groups, which associated with gonadal development (Song et al., 2019). In the process of muscle growth, rainbow trout (Oncorhynchus mykiss) harbored 1 160 differentially expressed lncRNAs among different groups in the back muscle, involved in regulation of muscle growth (Paneru et al., 2018). Moreover, the lncRNAs in teleost involved in immune response against pathogenic infections was widely reported in previous studies. For example, 556 lncRNA showed differential expression when the rainbow trout infected by Flavobacterium psychrophilus. Several of them exhibited associations with immune related genes and pathways (Paneru et al., 2016). Furthermore, such immune related lncRNAs have also been identified in zebrafish (D. rerio) (Chen et al., 2018), olive flounder (Paralichthys olivaceus) (Xiu et al., 2021), and Nile tilapia (Oreochromis niloticus) (Li et al., 2018). However, knowledge of detailed immune regulation roles of lncRNAs in teleost is still limited.
Black rockfish (Sebastes schlegelii) is one of the common economic fish species that widely distributed in the east coast of China, Korea, and Japan (Wang et al., 2020). The gram-negative pathogen Edwardsiella tarda in seawater that seriously endangers the aquaculture environment. The infection caused by E. tarda can result in systematic disease to fish, which can eventually lead to mass death (Xu and Zhang, 2013). To study the mechanism of black rockfish in response to pathogen invasion, several immune molecules were identified in recent studies including MyD88 (Shanaka et al., 2019), CTL (Du et al., 2018), CCL25 (Wang et al., 2020), TLRs (Cao et al., 2020), and NLRs (Cao et al., 2021a). Moreover, transcriptome analysis of non-coding RNAs in black rockfish, such as miRNAs (Im et al., 2020) and cricRNAs (Cao et al., 2021b) were conducted to explain their regulatory functions in black rockfish. However, the function of lncRNAs in black rockfish has not been studied so far. Accordingly, the differentially expressed lncRNAs and mRNAs between different infection groups and the control group were analyzed in the intestine of black rockfish. Potential lncRNA-mRNA interactions were thereafter analyzed based on the co-expression analysis of differentially expressed lncRNAs and mRNAs. Meanwhile, Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses were performed with the targeted mRNAs of lncRNAs. The results will provide novel knowledge about lncRNAs in the intestinal immune responses in black rockfish, and serve as important resources for further investigating roles of lncRNAs during pathogen infections in teleost.
2 MATERIAL AND METHOD 2.1 Bacterial challenge and samplingHealthy black rockfish were purchased from a fish farm in Qingdao, Shandong Province, China with length of about 15±2 cm. The experimental protocols were approved by the Committee on the Ethics of Animal Experiments of Qingdao Agricultural University Institutional Animal Care and Use Committee (IACUC). E. tarda was isolated from diseased black rockfish. Subsequently, the purified E. tarda was inoculated in Luria-Bertani (LB) medium at 28 ℃ with 180 r/min. The concentration of E. tarda was 1×107 CFU/mL in the experimental group tank. The survival rate for 1×107 colony forming unit (CFU)/mL 4 h is about 62%, which was detected in our previous study (Cao et al., 2021a). In particular, individuals in the experimental group were cultured in tank with E. tarda for 4 h and then returned back to seawater. Meanwhile, the individuals in control group (CON) were cultured with seawater. The bath-challenge include four infection time points of 2 h, 6 h, 12 h, and 24 h. Therefore, the intestinal tissues from experimental groups were designated as EI2H, EI6H, EI12H, and EI24H, respectively. Furthermore, each time point had three replicates, and each replicate included 6 random individuals. All samples were flash-frozen in liquid nitrogen and then stored in a -80-℃ ultra-low freezer until preparation of RNA.
2.2 RNA isolation, cDNA library construction, and sequencingRNAs were extracted and purified using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) and treated with RNase-Free DNase I reagent (TIANGEN, Beijing, China). Subsequently, 1% agarose gels were used to check the contamination and degradation. And the RNA concentration was examined in NanoPhotometer® spectrophotometer. Meanwhile, the RNA Nano 6000 Assay Kit was used for measuring the integrity and quantity of RNA in Bioanalyzer 2100 system. According to manufacturer's suggestion, 5-μg total RNA per sample was used to construct sequence libraries with Illumina TruSeq RNA Sample Preparation Kit (Illumina, San Diego, USA). Then, the prepared libraries were sequenced on an Illumina Hiseq 4000 platform. When the raw data obtained, five sections of invalid data were removed including the reads with 5′ adapter, the reads without 3′ adapter, the reads with more than 10% N, the reads with poly DNA bases and the reads with low sequencing quality (bases with low quality values represented more than 50% of the entire reads). After above steps, the clean reads were obtained. And the parameters of clean data including Q20, Q30, and GC content were calculated.
2.3 Identification of lncRNAsFirstly, the software HISAT2 was used to process the clean reads and mapped them onto the reference genome. For selecting lncRNAs in transcript libraries, filter conditions were set for all the clean-reads as follows: (1) selection of transcripts with exon ≥2, length > 200 bp; (2) filtering out the transcripts which overlapped with database annotated exon regions; (3) removal of the transcripts with protein-coding capability based on the information in CNCI, Pfam, and CPC2 database. The names of novel lncRNAs were identified according to the rules of HUGO Gene Nomenclature Committee (HGNC). Novel lncRNA sequences were also inspected and identified based on known lncRNA and mRNA sequences (Supplementary Dataset 1).
2.4 Differential expression analysis of lncRNAsA direct embodiment of a gene's expression level was the abundance profile of its transcripts. And higher transcript abundance reflected higher levels of gene expression. The expression levels of genes were estimated by the count of sequencing sequences (reads) that mapped to a genomic regions or exonic regions. For quantification, we employed the StringTie's network stream algorithm to calculate FPKM values for each gene. Differential expression analysis was performed using EdgeR software. And the hierarchical clustering method was used to cluster the expression values of the samples. The padj-value were set less than 0.05 to filter differentially expressed genes (DEGs).
2.5 Co-expression analysis between lncRNA and their target mRNAsThe cis-acting target gene prediction and trans-acting target gene prediction were used for finding the target genes of lncRNAs. The specific method of cis-acting target gene prediction was used to select the protein-coding genes which located within 10–100 kb of lncRNAs. As for trans-acting target prediction, Pearson' correlations coefficients were used to calculate trans-acting regulatory elements between the coding genes and lncRNAs.
To better summarize the regulatory functions of lncRNAs in black rockfish, the interaction of lncRNA-mRNA was identified between the differentially expressed lncRNAs and mRNAs. When the number of samples was ≥5, the co-expression acting target genes were selected and merged with the differentially expressed mRNAs for analysis. The differentially expressed lncRNAs and mRNAs were connected by analyzing the association between the target genes of differentially expressed lncRNAs and the differentially expressed mRNAs through co-expression information.
2.6 The gene ontology and KEGG analysisThe gene ontology (GO) is a major bioinformatics database which defined one gene's function into three domains: cellular component (CC), molecular function (MF), and biological process (BP) (Young et al., 2010). Meanwhile, the KEGG (Kyoto Encyclopedia) database focuses on the pooling of pathway maps of genes, RNAs, chemical reactions, and the others (Kanehisa et al., 2008). In order to perform GO and KEGG enrichment analysis in black rockfish, the target genes of differentially expressed lncRNAs with length corrected were processed by the ClusterProfiler R package. And the enrichment results with corrected P-values less than 0.05 were considered significant.
2.7 Real-time quantitative PCR (qRT-PCR) validation of lncRNA and mRNA expression analysisFor validation of the expression analysis of lncRNAs and mRNAs in sequencing, total RNA was extracted from black rockfish. PrimeScriptTM RT reagent Kit (TaKaRa, Japan) was used to reversely transcribe RNA into cDNA according to the manufacturer's instructions. Elongation factor-1 alpha (EF1-α) was chosen as internal control. The validated qRT-PCR primers were designed by PrimerQuest Tool (https://www.idtdna.com/pages/tools/primerquest). Subsequently, CFX96 real-time PCR detection system (Bio-Rad Laboratories, Hercules, CA) was used to verify the expression levels of lncRNA and mRNA. The qRT-PCR reaction system is as follows: 10 μL of SYBR® Premix Ex TaqTM II (TliRNaseH Plus), 0.6 μL of each primer, 2 μL of the 10-fold dilution cDNA, 6.8-μL nucleotide-free water. The qRT-PCR running program is set as follows: 5 min at 95 ℃, followed by 35 cycles of 95 ℃ for 5 s, 56 ℃ for 30 s and 72 ℃ for 30 s, then up to 95 ℃ with a rate of 0.1 ℃/s increment. After qRT-PCR, 2-ΔΔCt method was used to calculate the expression fold change of lncRNA and mRNA (Livak and Schmittgen, 2001).
3 RESULT 3.1 Sequencing dataThe sequencing libraries were named by their infection time points as: CON, EI2H, EI6H, EI12H, and EI24H. The sequenced reads were determined and classified into four data types include Raw_reads, Clean_reads, Raw_bases, and Clean_bases. A total of 2 935 704 384 raw reads were produced in 15 groups. Meanwhile, the 2 872 246 718 clean reads were generated after removing the low-quality, adapter-containing reads, and N-containing reads from the raw reads. All libraries had available sequencing quality with more than 15.06 Gb clean bases for each library. Meanwhile, in 15 sequencing libraries, the sequencing quality related parameters with the values of Q20 ≥97.93, Q30≥94.07, and error rate ≤0.03 (Supplementary Table S1).
A total of 9 311 lncRNAs were obtained from clean reads after quality control. The length of lncRNAs ranged from 201 to 71 349 bp, and the number of exons ranged from 2 to 37. Overall, the exon numbers of novel lncRNA were mostly in the range of 0–15, much smaller than that of mRNAs (Fig. 1c). The distribution tendency of open reading frames (ORF) is similar to that of exons (Fig. 1b), and the ORF lengths of lncRNA were all located within 1 000 bp, while those of mRNAs can reach up to 3 000 bp. In terms of length, novel lncRNAs had similar trends to annotated mRNAs, with the majority of the full length residing between 2 000–2 500 bp, and novel RNAs had a broader range and general longer full length (Fig. 1b). The number of included lncRNAs showed a decreasing trend as the length range increased stepwise (Fig. 1d). The proportion of exon, intergenic, and intronic in all samples were presented in Fig. 1e. Among the different types of lncRNAs, 65.7% of the discovered lncRNAs belong to long intergenic non-coding RNA (lincRNA), 16.2% of them belong to antisense, and 18.1% of them were overlapping. In addition, there were no sense intronic lncRNAs type existed (Fig. 1f).
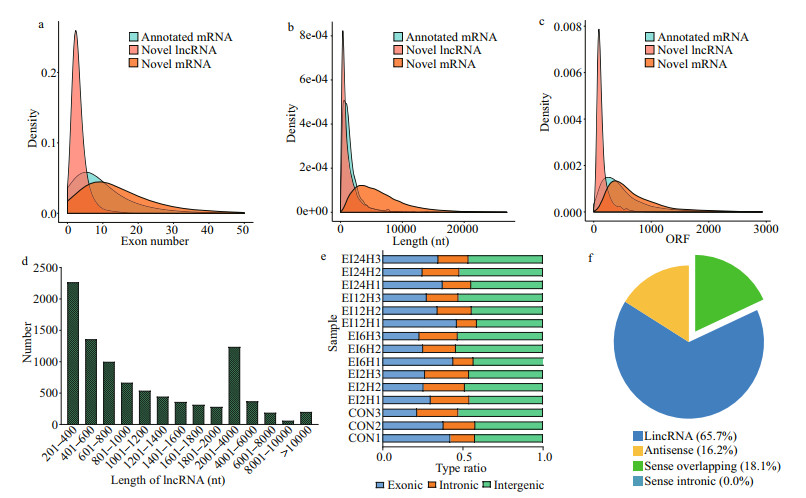
|
| Fig.1 Overview of the sequencing data a–c. the magnitude distribution of exon, length, and ORF in novel lncRNAs, novel mRNAs, and annotated mRNAs; d. the length distribution of lncRNAs; e. proportion information of reads mapping to variant locations across 15 samples; f. classification of lncRNAs at four time points. |
A total of 102 lncRNAs underwent changes in expression trends, with 10 up-regulated and 12 down-regulated at 2 h, 34 up-regulated and 16 down-regulated at 6 h, 8 up-regulated and 16 down-regulated at 12 h, and 16 up-regulated and 12 down-regulated at 24 h (Fig. 2a). The most up-regulated lncRNAs were observed at 6 h with 34. Subsequently, 66 lncRNAs with significant expression changes at each time point were selected and subjected to expression heatmaps (Fig. 2b). Obviously, evm.TU.Chr8.478 was down-regulated at EI2H. Meanwhile, evm.TU.Chr5.1155 showed significant up-regulation at EI6H. Particularly, the lncRNA evm.TU.Chr22.794 showed obvious up-regulation trend at four infection time points, whereas XLOC_012887 showed obviously down-regulation trends at four infection time points. Overall, the number of up-regulated lncRNAs was slightly higher than down-regulated lncRNAs.
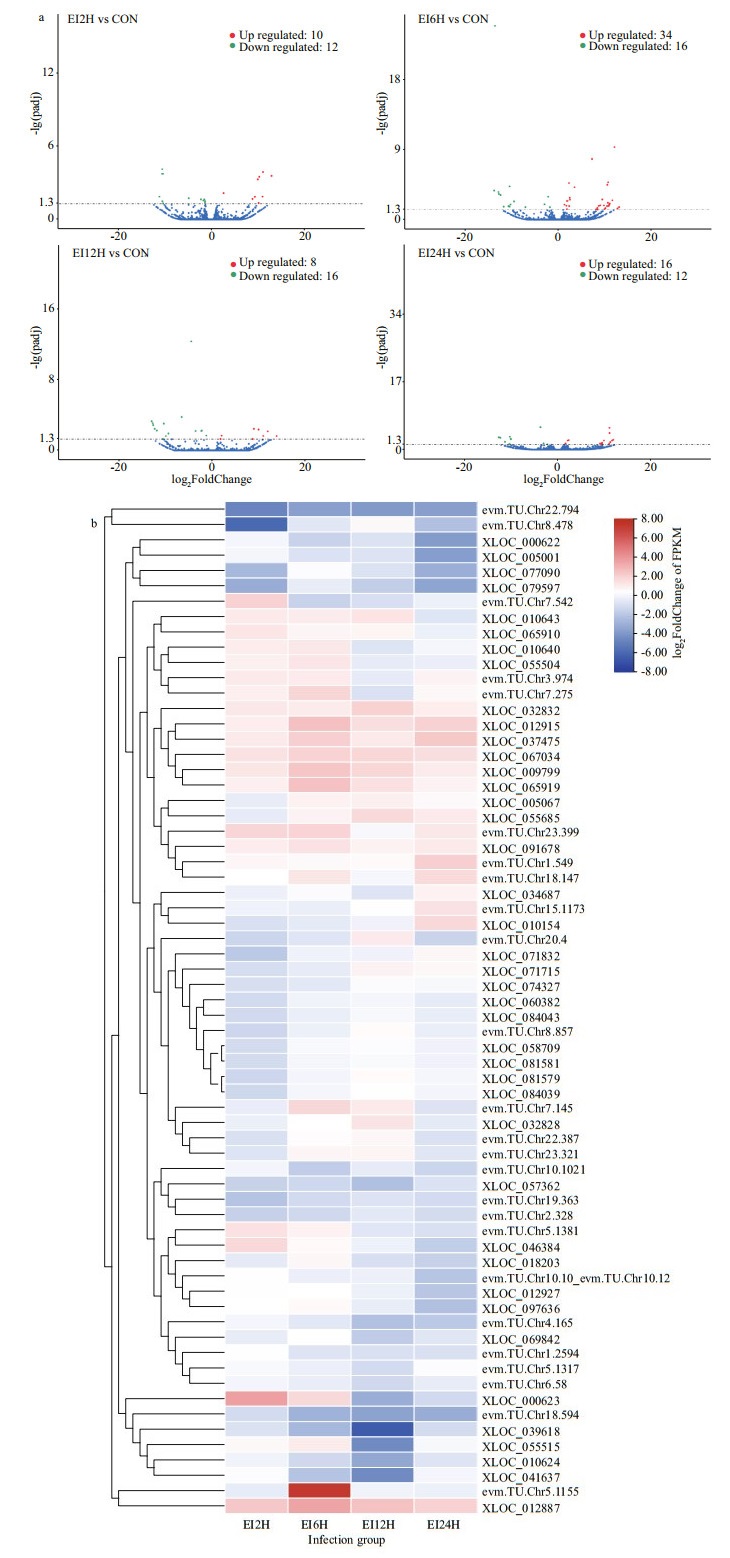
|
| Fig.2 Differential expression analysis of lncRNAs after E. tarda infection a. volcano plot of differential lncRNAs expression compared with control at different infection time points; b. hierarchical clustering plot (Heatmap) of differential lncRNAs expression. |
A total of 3 348 target mRNA were confirmed between groups to groups for further research. And the GO enrichment analysis was performed on all the target mRNAs (Fig. 3). It mainly contained three categories: cellular component, biological process and molecular function. The results of GO enrichment at 2 h, 12 h, and 24 h after infection with E. tarda showed higher number of genes involved in molecular function than the other two functions. Differently, the genes of the process function accounted for a higher proportion in EI6H group. Specifically, in EI2H group, the number of genes possessing catalytic activity was the greatest, followed by hydrolase activity. In EI6H group, the highest number of genes had the defense response function. Meanwhile, peptidase activity emerged as the function performed by the largest number of genes in EI12H group. Finally, in EI24H group, the number of genes with catalytic activity was significantly higher than the other enriched functions.
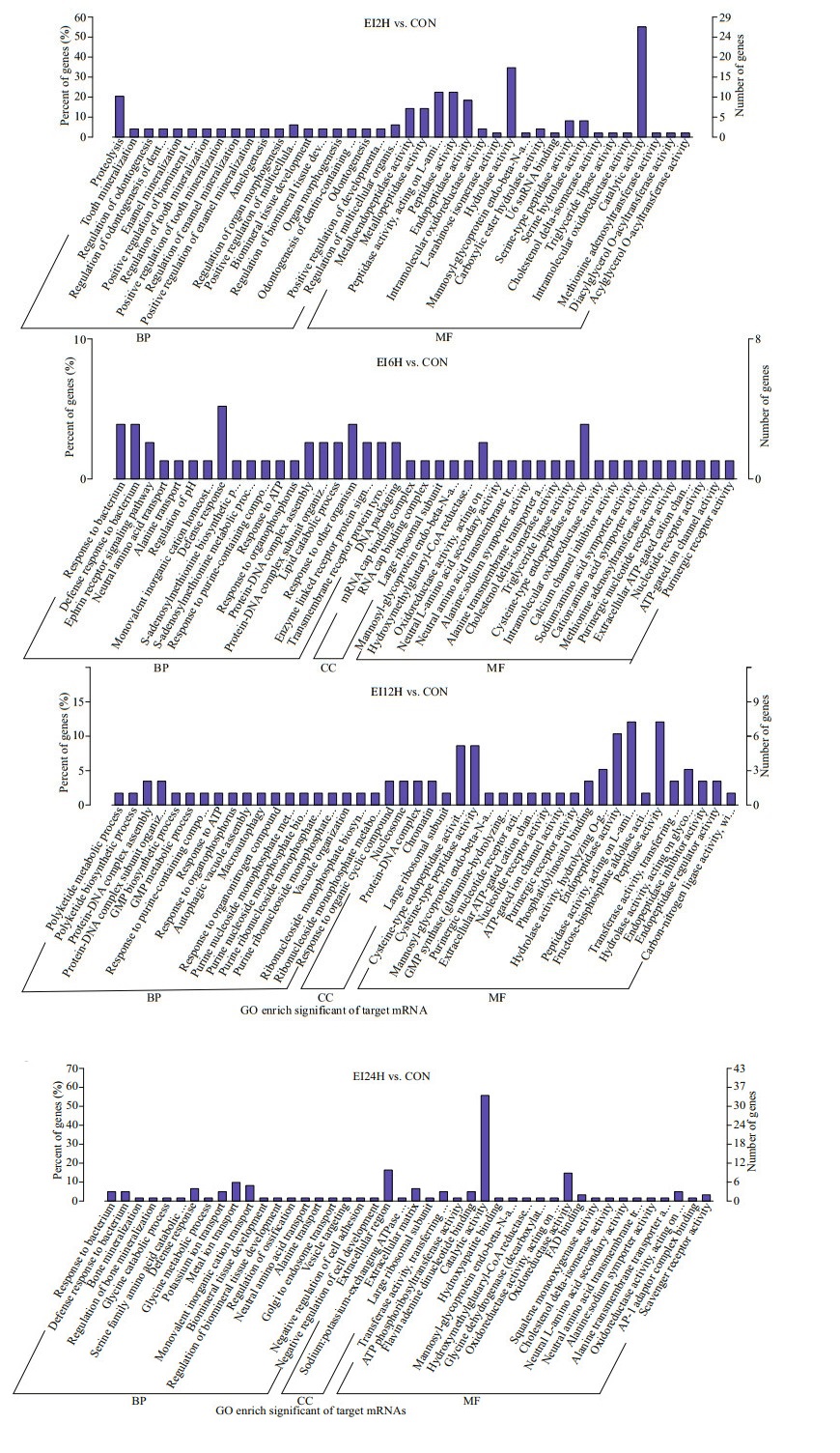
|
| Fig.3 Gene ontology (GO) analysis of mRNA genes in EIH2, EIH6, EIH12, and EIH24 BP: biological process; CC: cellular component; MF: molecular function. |
KEGG enrichment of target gene also represents different functional enrichment at 2-h, 6-h, 12-h, and 24-h infection time points (Fig. 4). The EI2H group showed most genes played a role in metabolic pathways, especially for the inositol phosphate metabolism, glycerophospholipid metabolism, and carbon metabolism. There were also a number of immune-related pathways such as nucleotide oligomerization domain (NOD)-like receptor signaling pathway in enrichment analysis results. Meanwhile, the EI6H and EI24H groups showed similar results to the previous group. The difference was that the classic immune pathway, RIG-I-like receptor signaling pathway was appeared at these infection time points. And for EI12H group, in addition to metabolic and immunity pathways such as phagosome, calcium signaling pathway and focal adhesion, neuroactive ligand-receptor interaction and lysosome were also functionally clustered with a larger number of genes. Besides, few genes with high rich factor and low q-value displayed in steoid biosynthesis from EI2H, EI6H, and EI24H groups. Especially in EI24H group, the enrichment was significant in selenocompound metabolism.

|
| Fig.4 Results of KEGG enrichment of mRNA genes in EIH2, EIH6, EIH12, and EIH24 The q-values from high to low are shown in a gradient from blue to red. The size of the circles represents the number of genes. |
Among the results of the GO and KEGG enrichment analysis, 38 differentially expressed mRNAs (DEmRNAs) in 4 groups (EI2H, EI6H, EI12H, and EI24H) which had functions in important pathway were further selected, such as metabolic or immune pathway. Finally, 8 DEmRNA (evm.TU.Chr9.463_ MMP13, evm.TU.Chr13.188_CARNMT2, and evm. TU.Chr16.796_BET1, evm.TU.Chr2.1072_GATM, evm.TU.Chr8.51_TNFSF13B, evm.TU.Chr16.518_ FCGBP, evm.TU.Chr2.1283_MUC5B, evm. TU.Chr7.943_DHCR3), which showed consistent trends of regulation with differentially expressed lncRNAs at two or more time points were selected. Meanwhile, 47 lncRNAs with strong co-expression for these DEmRNA were also selected (Fig. 5). In this analysis, most lncRNAs were co-expressed with one mRNA. Meanwhile, there was also a member of lncRNAs co-expressed with two and more DEmRNAs. Among them, the number of lncRNA co-expressed with two DEmRNA was thirteen, while the number of lncRNA co-expressed with three DEmRNAs was four (XLOC_067928, evm.TU.Chr1.1983, XLOC_030904, and XLOC_032649). In particular, only four DEmRNAs (Chr 13.188_CARNMT2, Chr 21.072_GATM, Chr 16.518_FCGBP, and Chr 2.1283_MUC5B) were the focal objects when one lncRNA was co-expressed with multiple DEmRNAs. The remaining DEmRNAs were all co-expressed with only a single lncRNA.
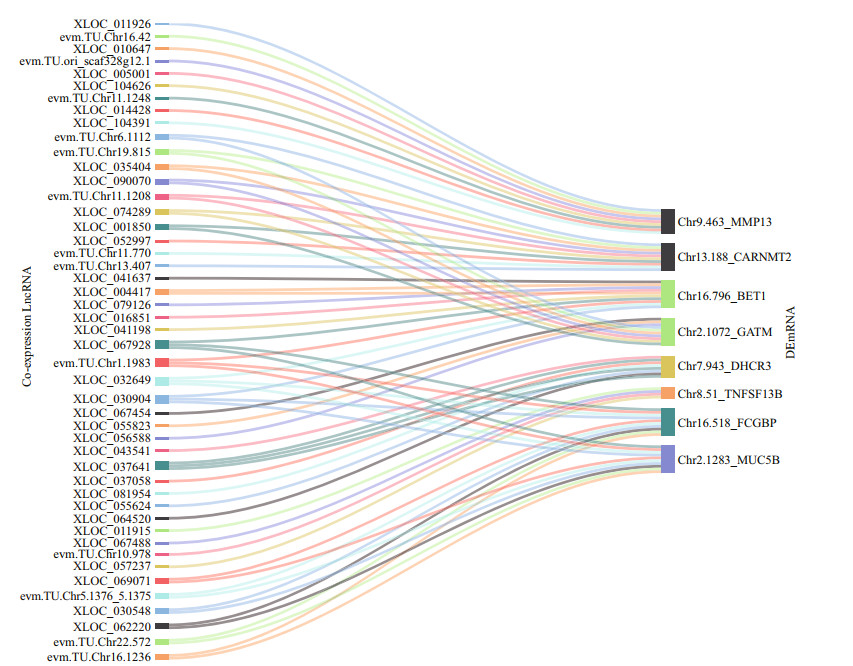
|
| Fig.5 Co-expression relationship between the 8 DEmRNAs and the 47 lncRNAs in Sankey diagram |
The identified eight DEmRNAs obtained from black rockfish were listed in Table 1. The function prediction of DEmRNAs were obtained according to the NR database. Among them, DEmRNA evm.TU.Chr8.51_TNFSF13B was identified as tumor necrosis factor like superfamily member 13b (TNF13B), a gene involved in the positive regulation of T-cell proliferation and associated with B-cell maintenance homeostasis in human and an important immune factor. Evm.TU.Chr9.463_ MMP13, evm.TU.Chr13.188_CARNMT2, and evm. TU.Chr7.943_DHCR3 were annotated as Collagenase 3 (MMP-13), Carnosine N-methyltransferase 2 and 3-beta-hydroxysteroid-Delta (8). Moreover, other DEmRNAs had different biological activities like mediating protein transport (evm.TU.Chr16.796_ BET1), involving in creatine biosynthesis (evm. TU. Chr2.1072_GATM), maintaining the mucosal architecture (evm.TU.Chr16.518_FCGBP), binding collagen and Ca2+ (evm.TU.Chr9.463_MMP13), and inhibited endopeptidases activity (evm.TU.Chr2.1283_ MUC5B) (Fig. 6).
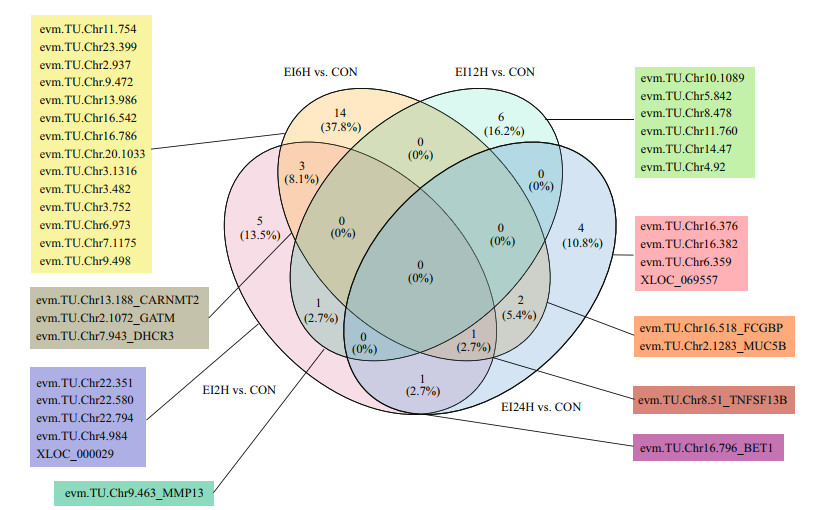
|
| Fig.6 The Venn diagram of the differentially expressed mRNAs, and genes that were targeted by differentially expressed lncRNAs at four time points The numbers in the Venn diagram represented mRNA number among different groups, the ratio represent the percentage of common and unique mRNAs of targeted differentially expressed mRNAs of lncRNAs. |
To verify the accuracy of the sequencing information, 4 DEmRNA and 8 lncRNAs were randomly selected for qRT-PCR verification. The results showed the gene expression trends of obtained by qRT-PCR were generally consistent with transcriptome data (Fig. 7). In detail, the expression patterns of most lncRNAs (XLOC_074289, XLOC_043541, XLOC_064520, XLOC_030548, XLOC_090070, evm.TU.Chr11.770, and XLOC_067928) calculated by qRT-PCR showed similar trends with those from sequencing results at two marked infection time points. For example, XLOC_074289 exhibited 6.22, 6.22, 6.14, and 6.20 fold down-regulation at 2 h, 6 h, 12 h, and 24 h infection time points based on the sequencing data. We found that the expression levels of XLOC_074289 from qRT-PCR downregulated with 3.03, 1.47, 2.00, and 2.88 fold, respectively. Similar trends were found in XLOC_043541 and XLOC_064520. As for mRNAs, the qRT-PCR fold change and transcriptome data for evm.TU.Chr8.51_TNFSF13B showed similar up-regulate trend from 2 h to 6 h. In contrast, evm. TU.Chr16.518_FCGBP showed down-regulate trend from 6 h time point to 24 h time point. In summary, the expression trends of lncRNAs and mRNAs obtained by qRT-PCR showed 71% consistency with transcriptome data. Therefore, the research on the co-expression of lncRNAs and DEmRNAs based on the transcriptome data were basically reliable.

|
| Fig.7 qRT-PCR verification of 8 selected lncRNAs and 4 DEmRNAs **: high significance at P < 0.01; *: significance at 0.01 < P < 0.05. The time points marked with red rectangle indicate the co-expression of lncRNAs and mRNAs. The histogram presents the result of qRT-PCR. |
Currently, E. tarda infection remains one of the pathogens that present a serious threat to black rockfish. In order to study the defense mechanism of black rockfish against this pathogen, we focused our research on long non-coding RNA which has been demonstrated with pivotal roles in various biological processes, especially the gene expression regulation, including transcriptional regulation, posttranscriptional control, and epigenetic processes. In recent years, the development of high-throughput sequencing technologies facilitated the discovery of lncRNAs. It has been found that lncRNAs play important roles in the regulation of immune responses in teleost (Geng and Tan, 2016). For example, the identified lncRNA-WAS and lncRNA-c8807 in grass carp (Ctenopharyngodon idellus) can interact with miRNA and activated NF-κB pathway to resist the invasion of pathogens (Fan et al., 2021). Moreover, the evolutionary conserved lncRNA were found in whitefish and could serve as biomarkers of liver injury (Florczyk et al., 2021). In the intestine of olive flounder, the identification of lncRNA from RNA-sequencing had also been carried out. And their target genes were enriched in numerous immune-related processes and exhibited a strong correlation with immune-related signaling pathways (Xiu et al., 2021). However, the relevant study of lncRNAs in black rockfish was still lacking. In this research, we obtained a total of 9 311 lncRNAs from one control group and four treatment groups infected with E. tarda. And the related sequencing quality parameters such as the value of Q20 ≥97.93, Q30 ≥94.07, and error rate ≤0.03 in all the 15 libraries can corroborate the sequencing data with high confidence.
The results of full length, ORF length, and number of exons for the novel lncRNAs presented different concentrated positions. Meanwhile, the ranges of the three parameters of lncRNAs were less than annotated mRNA and novel mRNA. It was related to the filter strategy of the transcriptome and the properties of the lncRNAs. From the identified lncRNAs classification charts, lincRNAs accounted for the highest proportion (65.7%). Based on the research recently, FANTOM5 consortium used cap analysis for the number of 27 919 human lncRNA, 13 105 (46.9%) of them were lincRNAs, which showed that lincRNAs also had a high proportion in human genome (Ransohoff et al., 2018).
GO and KEGG analyses were performed on 3 348 target mRNAs of lncRNAs, which were selected by cis-acting target gene prediction and trans-acting target gene prediction methods. Four kinds of most obvious enrichment items showed in GO analysis included catalytic activity, hydrolase activity, defense response, and peptidase activity. Actually, lncRNAs had also been identified with relevant activities in other species. For example, the catalytic activity of lncRNA was discovered in human endothelial cells which were adhered by prostate tumor cells PC-3M (Pan et al., 2021). In addition, we found that there were multiple lncRNAs co-expressed with the immune defense genes, such as lncRNA transcript-12631 and transcript-12631 in Spiroplasma eriocheiris (Ren et al., 2020). Meanwhile, a nuclear enriched lncRNA targeting ubiquitin carboxy terminal hydrolase L1 (Uchl1) could increase the transcription level of Uchl after neurodegenerative diseases in mouse (Carrieri et al., 2012). Not only in mammal, in Epinephelus coioides, lncRNAs can regulate genes related to serine hydrolase activity and mediate immune response (Tang et al., 2019). As for the peptidase activity, Liang et al. (2020) found lncRNA 2810403d21rik/ak007586/mirf can indirectly target usp15 (ubiquitin specific peptide 15) attenuated ischemic stress, which induced by cell death attenuated ischemic stress via microRNA competition pathway (Liang et al., 2020). The KEGG analysis showed the target mRNAs existed enrichment in several signaling pathways, especially in the metabolic pathway in black rockfish. For the differentially expressed lncRNAs selected from the gene panel acquired from hepatocellular carcinoma, the targeted mRNAs from them had obvious metabolic pathway enrichment (Wang et al., 2021b). And the metabolic-related pathway was further enriched by mRNA in olive flounder in previous research (Xiu et al., 2021). In addition, a partial of mRNA was also enriched in neuroactive ligand-receptor interaction and lysosome pathway with lower q-value.
In black rockfish, a total of 102 lncRNAs were identified after E. tarda infection. Among which, the lncRNA evm.TU.Chr5.1155 had particularly high expression at 6 h after E. tarda infection. Its target mRNA was G-protein coupled receptor activity. Recent studies had shown that lncRNA LINK-A in mouse mammary glands could facilitated crosstalk between the G-protein coupled receptor pathways and phosphatidylinositol-(3, 4, 5)-trisphosphate, finally causing metastatic mammary gland tumors (Hu et al., 2019). Thus, we can deduce that lncRNA evm. TU.Chr5.1155 can regulate the immune pathway of black rockfish with targeting mRNA. To search for functional DEmRNAs as well as lncRNAs with relevant regulatory roles in black rockfish. A total of 8 DEmRNAs with 47 related lncRNAs were selected from target mRNA and co-expression lncRNA at two or more E. tarda infection time points. As for selecting lncRNA with biological regulatory functions, the DEmRNAs involved in metabolism and immunity had been focused on. Especially, the DEmRNAs evm.TU.Chr8.51_TNFSF13B was identified as TNF13B which is also known as B-cell activating factor (BAFF) or B-lymphocyte stimulating factor (BLyS) (Ai et al., 2011). It was an important member of TNFSF family, the cytokines linked to diverse immunological and developmental pathway (Glenney and Wiens, 2007). The immune function of BAFF has also been characterized in various fish species, such as mefugu (Takifugu obscurus) (Ai et al., 2011), Japanese sea perch (Lateolabrax japonicus) (Cui et al., 2012), rainbow trout (Granja and Tafalla, 2019), and grass carp (Ctenopharyngodon idella) (Pandit et al., 2013).
Particularly, the DEmRNA evm.TU.Chr2.1283_ MUC5B and evm.TU.Chr16.518_FCGBP were previously considered to be important molecules in intestinal immunity in teleost. The evm. TU.Chr2.1283_MUC5B was annotated as Mucin-5B. In previous research, to block the invasion of pathogenic organisms, the fish bodies were always covered with a mucus layer which especially existing in the tissues like gut, epidermis, and gill (Subramanian et al., 2008). And the composition of mucus was very complex, containing mucins, immunoglobulins, complement, lectins, lysozyme, and others (Voynow and Rubin, 2009). Among them, mucin was expressed by epithelial cells and played a role in maintaining homeostasis in various organs. And the MUC2, MUC5AC, and MUC5B have received attention as three protein molecules that have been implicated in a variety of infection as cancer (Conze et al., 2010; Dong et al., 2020), gastric tumors (Mejías-Luque et al., 2010), and idiopathic interstitial pneumonia (Lou et al., 2020). In teleost, several mucin related gene segments were also explored in sequencing information of multiple fish species like Scophthalmus maximus (Gao et al., 2021), Salmo salar (Micallef et al., 2012), and Sparus aurata (Pérez-Sánchez et al., 2013). Especially for common carp, the MUC5B showed down-regulation after CyHV-3 associated disease in the skin which can cause secondary infection among other fish (Adamek et al., 2013). Moreover, the carp MUC5B was identified to have high similarity to its counterpart in mammals and birds (van der Marel et al., 2012). It means the MUC5B had a certain degree of conservation in the evolutionary process. The DEmRNA evm.TU.Chr16.518_FCGBP was identified as IgGFc-binding protein, which also can be named as FCGBP, an important intestinal mucus protein interacted with the Fc portion of IgG and with MUC2 (Johansson et al., 2009; Ehrencrona et al., 2021). Moreover, the FCGBP was ubiquitous in vertebrates, has a conserved N-terminal domain. Although its specific mechanism of action was not yet clear in teleost, its differential expression in numerous tumors made researchers interested in its immune function (Wang et al., 2021a). As for aquatic, in hepatopancreas of Sinonovacula constricta, the IgGFc-binding protein showed significant expression after Vibrio parahaemolyticus infection through the analysis of transcriptomes (Zhao et al., 2017). There is no doubt that the immune function of two DEmRNAs evm.TU.Chr2.1283_MUC5B and evm. TU.Chr16.518_FCGBP in black rockfish need further study.
For metabolism function, the DEmRNA evm. TU.Chr9.463_MMP13, evm.TU.Chr13.188_ CARNMT2, and evm.TU.Chr7.943_DHCR3 were identified as Collagenase 3, Carnosine N-methyltransferase 2, and 3-beta-hydroxysteroid-Delta(8), Delta(7)-isomerase, which performed collagen catabolism, carnosine metabolism, and sterol metabolism respectively in biological processes. Although there are limited studies on these metabolism-related molecules in teleost, their biological functions were also proved in other species. For example, Collagenase 3 played an important role in cartilage erosion independent of proteoglycan loss in mutant mice. And Carnosine N-methyltransferase 2 can transfer a methyl group to carnosine (β-alanyl-l-histidine) and closely related to the production of anserine (Cecilia Berin and Chehade, 2010), which act to retard and reverse non-enzymatic glycation (Szwergold, 2005). For 3-beta-hydroxysteroid-delta (8), delta (7)-isomerase, it is an enzyme that mainly played a role in cholesterol synthesis. The mutations in this gene can cause mosaic patchy epidermal hyperkeratinization (Man et al., 2016). These metabolic molecules and the co-expressed lncRNAs provided material for in-depth study of fish metabolic system.
5 CONCLUSIONThe expression patterns of lncRNAs in the intestine of black rockfish were investigated by high-throughput sequencing technique, and the functional enrichment analysis such as GO and KEGG were performed. The results show that DEGs are enriched in immune and metabolic-related pathways. Totally, 8 DEmRNAs and 47 lncRNAs were obtained from 3 348 target mRNAs and 1 998 associated lncRNAs. Meanwhile, the expression patterns of DEmRNAs and lncRNAs from RNA-seq were verified by qRT-PCR. Finally, the functions of immune and metabolic-related DEmRNAs and lncRNAs in black rockfish were discussed. This study laid the foundation for further investigation of the regulatory roles of lncRNAs in the intestinal immune and metabolic response of black rockfish.
6 DATA AVAILABILITY STATEMENTThe mRNA analyzed during this study is included in the published article available at https://doi.org/10.1007/s00343-021-1230-7. The lncRNA dataset analyzed during the current study is available in the Supplementary Dataset 1.
Electronic supplementary material
Supplementary material (Supplementary Dataset 1 & Table S1) is available in the online version of this article at https://doi.org/10.1016/B978-1-4377-0271-2.00044-4.
Adamek M, Syakuri H, Harris S, et al. 2013. Cyprinid herpesvirus 3 infection disrupts the skin barrier of common carp (Cyprinus carpio L.). Veterinary Microbiology, 162(2-4): 456-470.
DOI:10.1016/j.vetmic.2012.10.033 |
Ai H X, Shen Y F, Min C, et al. 2011. Molecular structure, expression and bioactivity characterization of TNF13B (BAFF) gene in mefugu, Takifugu obscurus. Fish & Shellfish Immunology, 30(6): 1265-1274.
DOI:10.1016/j.fsi.2011.03.020 |
Granja A G, Tafalla C. 2019. Different IgM+ B cell subpopulations residing within the peritoneal cavity of vaccinated rainbow trout are differently regulated by BAFF. Fish & shellfish immunology, 85: 9-17.
DOI:10.1016/j.fsi.2017.10.003 |
Cao M, Yan X, Li Q, et al. 2021a. Genome-wide identification and analysis of NOD-like receptors and their potential roles in response to Edwardsiella tarda infection in black rockfish (Sebastes schlegelii). Aquaculture, 541: 736803.
DOI:10.1016/j.aquaculture.2021.736803 |
Cao M, Yan X, Su B F, et al. 2021b. Integrated analysis of circRNA-miRNA-mRNA regulatory networks in the intestine of Sebastes schlegelii following Edwardsiella tarda challenge. Frontiers in Immunology, 11: 618687.
DOI:10.3389/fimmu.2020.618687 |
Cao M, Yan X, Yang N, et al. 2020. Genome-wide characterization of Toll-like receptors in black rockfish Sebastes schlegelii: evolution and response mechanisms following Edwardsiella tarda infection. International Journal of Biological Macromolecules, 164: 949-962.
DOI:10.1016/j.ijbiomac.2020.07.111 |
Carrieri C, Cimatti L, Biagioli M, et al. 2012. Long non-coding antisense RNA controls Uchl1 translation through an embedded SINEB2 repeat. Nature, 491(7424): 454-457.
DOI:10.1038/nature11508 |
Cecilia Berin M, Chehade M. 2010. Chapter 44 — mucosal immunology: an overview. In: Leung D Y M, Sampson H A, Geha R, Szefler S J eds. Pediatric Allergy: Principles and Practice. 2nd edn. Elsevier, Amsterdam. p. 471-476, https://doi.org/10.1016/B978-1-4377-0271-2.00044-4.
|
Chen W, Zhang X, Li J, et al. 2018. Comprehensive analysis of coding-lncRNA gene co-expression network uncovers conserved functional lncRNAs in zebrafish. BMC Genomics, 19(S2): 112.
DOI:10.1186/s12864-018-4458-7 |
Conze T, Carvalho A S, Landegren U, et al. 2010. MUC2 mucin is a major carrier of the cancer-associated sialyl-Tn antigen in intestinal metaplasia and gastric carcinomas. Glycobiology, 20(2): 199-206.
DOI:10.1093/glycob/cwp161 |
Cui X W, Li J F, Xiao W, et al. 2012. Molecular cloning, expression and functional analysis of TNF13b (BAFF) in Japanese sea perch, Lateolabrax japonicus. International Immunopharmacology, 12(1): 34-41.
DOI:10.1016/j.intimp.2011.10.009 |
Dong Y J, Zhou L J, Zhao D, et al. 2020. MUC5AC enhances tumor heterogeneity in lung adenocarcinoma with mucin production and is associated with poor prognosis. Japanese Journal of Clinical Oncology, 50(6): 701-711.
DOI:10.1093/jjco/hyaa016 |
Du X, Wang G H, Su Y L, et al. 2018. Black rockfish C-type lectin, SsCTL4: a pattern recognition receptor that promotes bactericidal activity and virus escape from host immune defense. Fish & Shellfish Immunology, 79: 340-350.
DOI:10.1016/j.fsi.2018.05.033 |
Ehrencrona E, van der Post S, Gallego P, et al. 2021. The IgGFc-binding protein FCGBP is secreted with all GDPH sequences cleaved but maintained by interfragment disulfide bonds. Journal of Biological Chemistry, 279(1): 100871.
DOI:10.1016/j.jbc.2021.100871 |
Fan K, Shen Y B, Xu X Y, et al. 2021. LncRNA-WAS and lncRNA-C8807 interact with miR-142a-3p to regulate the inflammatory response in grass carp. Fish & Shellfish Immunology, 111: 201-207.
DOI:10.1016/j.fsi.2021.02.003 |
Florczyk M, Brzuzan P, Woźny M. 2021. De novo profiling of long non-coding RNAs involved in MC-LR-induced liver injury in whitefish: discovery and perspectives. International Journal of Molecular Sciences, 22(2): 941.
DOI:10.3390/ijms22020941 |
Gao C B, Cai X, Cao M, et al. 2021. Comparative analysis of the miRNA-mRNA regulation networks in turbot (Scophthalmus maximus L.) following Vibrio anguillarum infection. Developmental & Comparative Immunology, 124: 104164.
DOI:10.1016/j.dci.2021.104164 |
Geng H, Tan X D. 2016. Functional diversity of long non-coding RNAs in immune regulation. Genes & Diseases, 3(1): 72-81.
DOI:10.1016/j.gendis.2016.01.004 |
Glenney G W, Wiens G D. 2007. Early diversification of the TNF superfamily in teleosts: genomic characterization and expression analysis. The Journal of Immunology, 178(12): 7955-7973.
DOI:10.4049/jimmunol.178.12.7955 |
Gómez G, Pallás V. 2013. Viroids: a light in the darkness of the lncRNA-directed regulatory networks in plants. New Phytologist, 198(1): 10-15.
DOI:10.1111/nph.12196 |
Hu Q S, Ye Y Q, Chan L C, et al. 2019. Oncogenic lncRNA downregulates cancer cell antigen presentation and intrinsic tumor suppression. Nature Immunology, 20(7): 835-851.
DOI:10.1038/s41590-019-0400-7 |
Huang D, Chen J N, Yang L B, et al. 2018. NKILA lncRNA promotes tumor immune evasion by sensitizing T cells to activation-induced cell death. Nature Immunology, 19(10): 1112-1125.
DOI:10.1038/s41590-018-0207-y |
Im J, Kim W R, Lee H E, et al. 2020. Expression analysis of LTR-derived miR-1269a and target gene, KSR2 in Sebastes schlegelii. Genes & Genomics, 42(1): 55-65.
DOI:10.1007/s13258-019-00880-0 |
Jiang M H, Zhang S K, Yang Z H, et al. 2018. Self-Recognition of an Inducible Host lncRNA by RIG-I Feedback Restricts Innate Immune Response. Cell, 173(4): 906-919.
DOI:10.1016/j.cell.2018.03.064 |
Jin X, Xu X E, Jiang Y Z, et al. 2019. The endogenous retrovirus-derived long noncoding RNA TROJAN promotes triple-negative breast cancer progression via ZMYND8 degradation. Science Advances, 5(3): eaat9820.
DOI:10.1126/sciadv.aat9820 |
Johansson M E V, Thomsson K A, Hansson G C. 2009. Proteomic analyses of the two mucus layers of the colon barrier reveal that their main component, the Muc2 mucin, is strongly bound to the Fcgbp protein. Journal of Proteome Research, 8(7): 3549-3557.
DOI:10.1021/pr9002504 |
Kanehisa M, Araki M, Goto S, et al. 2008. KEGG for linking genomes to life and the environment. Nucleic acids research, 36: D480-D484.
DOI:10.1093/nar/gkm882 |
Kotzin J J, Spencer S P, McCright S J, et al. 2016. The long non-coding RNA Morrbid regulates Bim and short-lived myeloid cell lifespan. Nature, 537(7619): 239-243.
DOI:10.1038/nature19346 |
Li B J, Jiang D L, Meng Z N, et al. 2018. Genome-wide identification and differentially expression analysis of lncRNAs in tilapia. BMC Genomics, 19(1): 729.
DOI:10.1186/s12864-018-5115-x |
Liang H H, Su X M, Wu Q X, et al. 2020. LncRNA 2810403D21Rik/Mirf promotes ischemic myocardial injury by regulating autophagy through targeting Mir26a. Autophagy, 16(6): 1077-1091.
DOI:10.1080/15548627.2019.1659610 |
Livak K J, Schmittgen T D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt Method. Methods (San Diego, Calif.), 25(4): 402-408.
DOI:10.1006/meth.2001.1262 |
Lou H Q, Huang C X, Li G Y, et al. 2020. The Association between MUC5B Rs35705950 and risks of idiopathic interstitial pneumonia, systemic sclerosis interstitial lung disease, and familial interstitial pneumonia: a meta-analysis. Iranian Journal of Public Health, 49(12): 2240-2250.
DOI:10.18502/ijph.v49i12.4801 |
Man M Q, Cheung C, Elias P M. 2016. Alterations of epidermal functions in 3 beta-hydroxysteroid-delta 8, delta 7-isomerase deficient (Conradi-Hunermann) mice. Journal of Dermatological Science, 84(1): E131.
DOI:10.1016/j.jdermsci.2016.08.392 |
McHugh C A, Chen C K, Chow A, et al. 2015. The Xist lncRNA interacts directly with SHARP to silence transcription through HDAC3. Nature, 521(7551): 232-236.
DOI:10.1038/nature14443 |
Mejías-Luque R, Lindén S K, Garrido M, et al. 2010. Inflammation modulates the expression of the intestinal mucins MUC2 and MUC4 in gastric tumors. Oncogene, 29(12): 1753-1762.
DOI:10.1038/onc.2009.467 |
Mercer T R, Dinger M E, Mattick J S. 2009. Long non-coding RNAs: insights into functions. Nature Reviews Genetics, 10(3): 155-159.
DOI:10.1038/nrg2521 |
Micallef G, Bickerdike R, Reiff C, et al. 2012. Exploring the transcriptome of Atlantic salmon (Salmo salar) skin, a major defense organ. Marine Biotechnology, 14(5): 559-569.
DOI:10.1007/s10126-012-9447-2 |
Pan Y, Abdureyim M, Yao Q, et al. 2021. Analysis of differentially expressed genes in endothelial cells following tumor cell adhesion, and the role of PRKAA2 and miR-124-3p. Frontiers in Cell and Developmental Biology, 9: 604038.
DOI:10.3389/fcell.2021.604038 |
Pandit N P, Shen Y B, Wang W J, et al. 2013. Identification of TNF13b (BAFF) gene from grass carp (Ctenopharyngodon idella) and its immune response to bacteria and virus. Developmental & Comparative Immunology, 39(4): 460-464.
DOI:10.1016/j.dci.2013.01.004 |
Paneru B, Al-Tobasei R, Palti Y, et al. 2016. Differential expression of long non-coding RNAs in three genetic lines of rainbow trout in response to infection with Flavobacterium psychrophilum. Scientific Reports, 6(1): 36032.
DOI:10.1038/srep36032 |
Paneru B, Ali A, Al-Tobasei R, et al. 2018. Crosstalk among lncRNAs, microRNAs and mRNAs in the muscle 'degradome' of rainbow trout. Scientific Reports, 8(1): 8416.
DOI:10.1038/s41598-018-26753-2 |
Pérez-Sánchez J, Estensoro I, Redondo M J, et al. 2013. Mucins as diagnostic and prognostic biomarkers in a fish-parasite model: transcriptional and functional analysis. PLoS One, 8(6): e65457.
DOI:10.1371/journal.pone.0065457 |
Ransohoff J D, Wei Y N, Khavari P A. 2018. The functions and unique features of long intergenic non-coding RNA. Nature Reviews Molecular Cell Biology, 19(3): 143-157.
DOI:10.1038/nrm.2017.104 |
Ren Y Q, Li J Y, Guo L, et al. 2020. Full-length transcriptome and long non-coding RNA profiling of whiteleg shrimp Penaeus vannamei hemocytes in response to Spiroplasma eriocheiris infection. Fish & Shellfish Immunology, 106: 876-886.
DOI:10.1016/j.fsi.2020.06.057 |
Shanaka K A S N, Tharuka M D N, Priyathilaka T T, et al. 2019. Molecular characterization and expression analysis of rockfish (Sebastes schlegelii) viperin, and its ability to enervate RNA virus transcription and replication in vitro. Fish & Shellfish Immunology, 92: 655-666.
DOI:10.1016/j.fsi.2019.06.015 |
Song F B, Wang L M, Zhu W B, et al. 2019. Long noncoding RNA and mRNA expression profiles following igf3 knockdown in common carp, Cyprinus carpio. Scientific Data, 6(1): 190024.
DOI:10.1038/sdata.2019.24 |
Struhl K. 2007. Transcriptional noise and the fidelity of initiation by RNA polymerase II. Nature Structural & Molecular Biology, 14(2): 103-105.
DOI:10.1038/nsmb0207-103 |
Subramanian S, Ross N W, MacKinnon S L. 2008. Comparison of antimicrobial activity in the epidermal mucus extracts of fish. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 150(1): 85-92.
DOI:10.1016/j.cbpb.2008.01.011 |
Szwergold B S. 2005. Carnosine and anserine act as effective transglycating agents in decomposition of aldose-derived Schiff bases. Biochemical and Biophysical Research Communications, 336(1): 36-41.
DOI:10.1016/j.bbrc.2005.08.033 |
Tang R Q, Luo G, Zhao L M, et al. 2019. The effect of a LysR-type transcriptional regulator gene of Pseudomonas plecoglossicida on the immune responses of Epinephelus coioides. Fish & Shellfish Immunology, 89: 420-427.
DOI:10.1016/j.fsi.2019.03.051 |
van der Marel M, Adamek M, Gonzalez S F, et al. 2012. Molecular cloning and expression of two β-defensin and two mucin genes in common carp (Cyprinus carpio L.) and their up-regulation after β-glucan feeding. Fish & Shellfish Immunology, 32(3): 494-501.
DOI:10.1016/j.fsi.2011.12.008 |
Voynow J A, Rubin B K. 2009. Mucins, mucus, and sputum. Chest, 135(2): 505-512.
DOI:10.1378/chest.08-0412 |
Wang J J, Meng Z Q, Wang G H, et al. 2020. A CCL25 chemokine functions as a chemoattractant and an immunomodulator in black rockfish, Sebastes schlegelii. Fish & Shellfish Immunology, 100: 161-170.
DOI:10.1016/j.fsi.2020.02.063 |
Wang K, Guan C N, Shang X W, et al. 2021a. A bioinformatic analysis: the overexpression and clinical significance of FCGBP in ovarian cancer. Aging, 13(5): 7416-7429.
DOI:10.18632/aging.202601 |
Wang R F, Hu X B, Liu X R, et al. 2021b. Construction of liver hepatocellular carcinoma-specific lncRNA-miRNA-mRNA network based on bioinformatics analysis. PLoS One, 16(4): e0249881.
DOI:10.1371/journal.pone.0249881 |
Xiu Y J, Li Y R, Liu X F, et al. 2021. Identification and characterization of long non-coding RNAs in the intestine of olive flounder (Paralichthys olivaceus) during Edwardsiella tarda infection. Frontiers in Immunology, 12: 623764.
DOI:10.3389/fimmu.2021.623764 |
Xu T T, Zhang X H. 2014. Edwardsiella tarda: an intriguing problem in aquaculture. Aquaculture, 431: 129-135.
DOI:10.1016/j.aquaculture.2013.12.001 |
Young M D, Wakefield M J, Smyth G K, et al. 2010. Gene ontology analysis for RNA-seq: accounting for selection bias. Genome biology, 11(2): R14.
DOI:10.1186/gb-2010-11-2-r14 |
Zhang D Y, Zhang X X, Li G Z, et al. 2021a. Transcriptome analysis of long noncoding RNAs ribonucleic acids from the livers of Hu sheep with different residual feed intake. Animal, 15(2): 100098.
DOI:10.1016/j.animal.2020.100098 |
Zhang H K, Tan K, Li S K, et al. 2021b. The functional roles of the non-coding RNAs in molluscs. Gene, 768: 145300.
DOI:10.1016/j.gene.2020.145300 |
Zhang L Q, Xu W, Gao X L, et al. 2020. lncRNA sensing of a viral suppressor of RNAi activates non-canonical innate immune signaling in Drosophila. Cell Host & Microbe, 27(1): 115-128.e8.
DOI:10.1016/j.chom.2019.12.006 |
Zhao X L, Duan X M, Wang Z H, et al. 2017. Comparative transcriptome analysis of Sinonovacula constricta in gills and hepatopancreas in response to Vibrio parahaemolyticus infection. Fish & Shellfish Immunology, 67: 523-535.
DOI:10.1016/j.fsi.2017.06.040 |
Zhao Y, Li H, Fang S S, et al. 2016. NONCODE 2016: an informative and valuable data source of long non-coding RNAs. Nucleic Acids Research, 44(D1): D203-D208.
DOI:10.1093/nar/gkv1252 |
 2023, Vol. 41
2023, Vol. 41



