Institute of Oceanology, Chinese Academy of Sciences
Article Information
- LIU Qi, LUO Xi, XIE Shulian, KOCIOLEK John Patrick
- New and interesting diatoms from the Shimen Wetwalls, Yunnan Province, China. Ⅱ. The diatom genus Nupela Vyverman & Compère (Bacillariophyceae)
- Journal of Oceanology and Limnology, 41(1): 327-341
- http://dx.doi.org/10.1007/s00343-021-1281-9
Article History
- Received Jul. 16, 2021
- accepted in principle Sep. 1, 2021
- accepted for publication Dec. 4, 2021
2 Museum of Natural History, University of Colorado, Boulder, CO 80309, USA;
3 Department of Ecology and Evolutionary Biology, University of Colorado, Boulder, CO 80309, USA
The diatom genus Nupela was described by Vyverman and Compère (1991: 175) for a biraphid diatom (N. gilwuensis Vyverman and Compère 1991: 178) from Papua New Guinea with external areolae having a thin layer occluding the openings. Since the original description with a single species, the genus has grown to over 85 taxa (Kociolek et al., 2022). The feature thought to unite these taxa is the external hymenate covering over the striae and areolae (Potapova, 2003). Some members of the genus have been transferred to Nupela from genera as diverse as Brachysira Kützing (1836: 3), Achnanthidium Kützing (1844: 75), Navicula Bory (1822: 128), and Neidium Pfitzer (1871: 39), and new species have been described from several continents (e.g., Kulikovskiy et al., 2009, 2020; Potapova, 2011; Buczkó et al., 2013; Tremarin et al., 2015; Yu et al., 2017; Guiry and Guiry, 2021; Kociolek et al., 2022). The genus appears to be unknown from Antarctica and Australia. The first report of the genus in the fossil record is from 40 million years ago during the Eocene Epoch (Siver et al., 2010).
The breadth of morphological forms assigned to the genus has grown dramatically to include biraphid and monoraphid diatoms (Vyverman and Compère, 1991; Lange-Bertalot, 1993; Potapova, 2011), species with symmetrical and asymmetrical central areas (e.g., Siver et al., 2007; Tremarin et al., 2015), with smooth or highly mottled axial areas (Potapova, 2011), being asymmetrical about the transapical axis (Kulikovskiy et al., 2015), the apical axis (Lange-Bertalot, 1993; Metzeltin and Lange-Bertalot, 1998) or symmetrical to both axes (most species), and valves smooth and planar to having deep depressions (Potapova, 2011; Tremarin et al., 2015). The genus has been characterized as occurring in water of low mineral content and pH (Vyverman and Compère, 1991; Sala et al., 2014), but is also known from alkaline environments with high conductivity (Yu et al., 2017).
A single species of Nupela has been previously described from China. Nupela major Yu, Q. M. You & Kociolek (in Yu et al., 2017: 246) was described from a wet wall habitat in the Maolan Nature Reserve, Guizhou Province. This species is the largest described so far (length 20.0–41.5 μm, breadth 6.5–9.5 μm) for the entire genus and has a flat raphe valve and rapheless valve with a deep central trough.
New diatoms of Yunnan Province have been described initially by Skuja (1937), then in a series of papers by Chen (1980) and Li et al.(2010a, b), and more recently by Levkov et al. (2013), You et al. (2015), Liao and Li (2018), Cheng et al. (2018), and Jiang et al. (2018). Yunnan is a province with many microhabitats, and one that harbors significant biodiversity of terrestrial and aquatic environments (e.g., Li, 1993, 1995; Guo and Long, 1998; Zhou and Chen, 2006; Naruse et al., 2018). While the province has a number of new taxa described in the historical and more recent literature, the diverse geography, large lakes, three parallel rivers, hot springs, waterfalls, and other important aquatic environments make it home to most of the endemic freshwater fishes in China (Chen, 2013), and we suppose, potentially the diatoms as well. Our first report on the diatoms of the Shimen wet walls was focused on the valve ultrastucture and transfer of Melosira sinuata-radiata var. yunnanica Chen to the genus Orthoseira Thwaites (Kociolek et al., 2021).
It is the purpose of the present report to present light and scanning electron microscopic observations on five Nupela species collected from wet walls in Yunnan Province, and to formally describe these species as new.
2 MATERIAL AND METHODThe diatom samples investigated in this study were obtained from Shimen Valley of Ailao Mountain, Yunnan Province, China. Kociolek et al. (2021) describes the ecological setting of this region.
Epilithic samples were collected from a variety of areas of wet wall in Shimen Valley. The individual samples considered herein include: 201904033, 201904055.
Samples were digested with hydrochloric acid to remove organic matter. After repeated washing of the treated material with distilled water, aliquots were dried on cover glasses and mounted in Naphrax (Brunel Microscopes Ltd., Chippenham, UK). All LM observations were performed using Olympus BX-51 light microscopes with a 100× oil immersion objective (numerical aperture 1.40). Specimen images were taken using Olympus DP72 digital cameras at Shanxi University, China, or at the University of Colorado, Boulder, USA.
Measurements of each species were based on 50 specimens. For scanning electron microscope (SEM) observations, the cleaned specimens were air dried onto glass cover slips and attached to copper stubs and coated with approximately 15 nm of gold-palladium. Scanning electron microscope observations were made using a Hitachi SU8010 SEM at a working distance less than 6 mm and accelerating voltage set at 1–2 kV at Shanghai Normal University.
Holotype slides and material are stored at the Freshwater Algal Herbarium of Shanxi University (SXU), Taiyuan, China. Isotype slides are deposited in the Kociolek Collection at the University of Colorado (COLO).
3 RESULTNupela shimenensis, Q. Liu, S. L. Xie & Kociolek, sp. nov. Figures 1 (LM) & 2–3 (SEM)
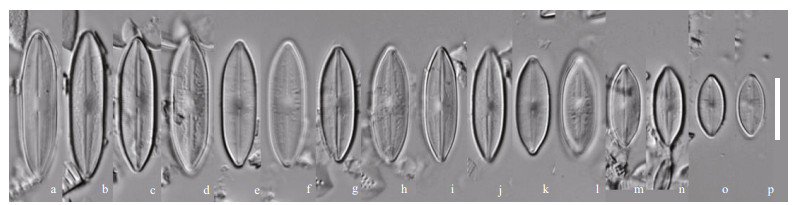
|
| Fig.1 Nupela shimenensis Q. Liu, S. L. Xie, & Kociolek, sp. nov. under LM diminution series, showing both valves of eight frustules. Holotype is illustrated in (a & b). Scale bar=10 μm for all figures. |
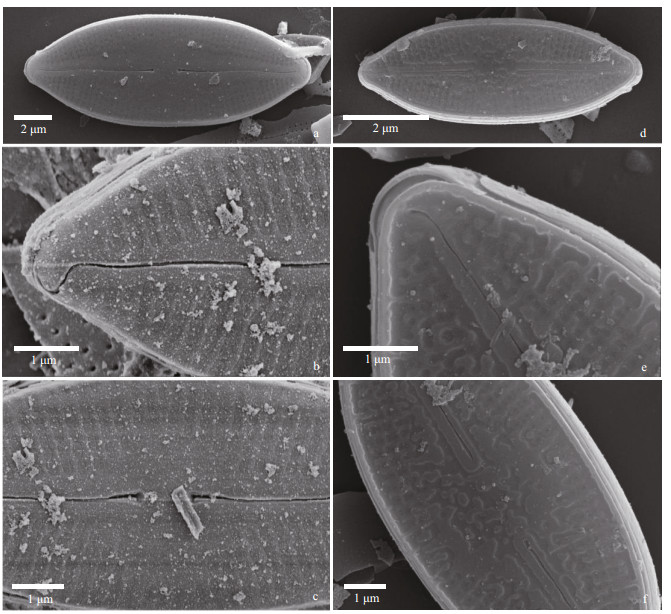
|
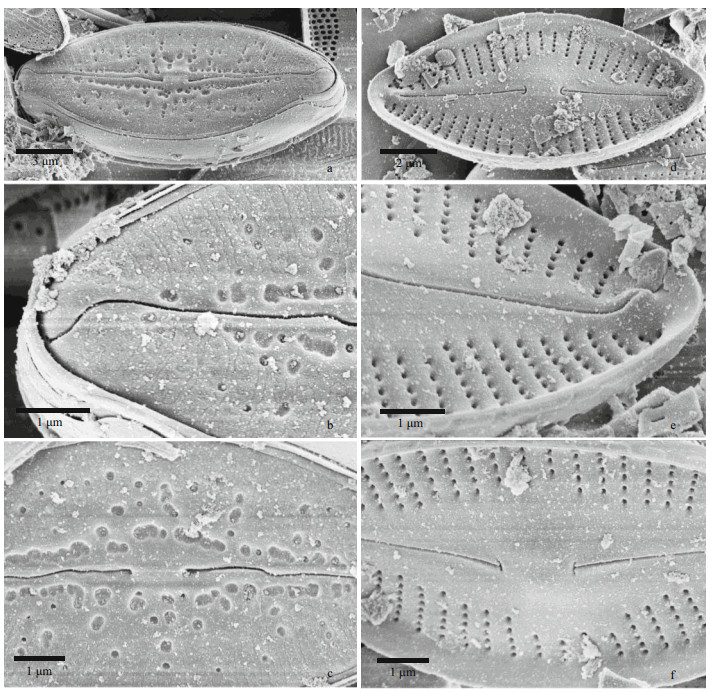
| Fig.2 Nupela shimenensis Q. Liu, S. L. Xie, & Kociolek, sp. nov. under SEM (valve exterior) a & d: entire valve showing slightly undulate raphe and radiate striae; b & e. valve apices, with hooked distal raphe end. In (b), the exterior covering of the areolae is evident; c & f. central area of the valve. Proximal raphe ends are dilated. In (d–f), the valve has a thickened covering of silica on it. |
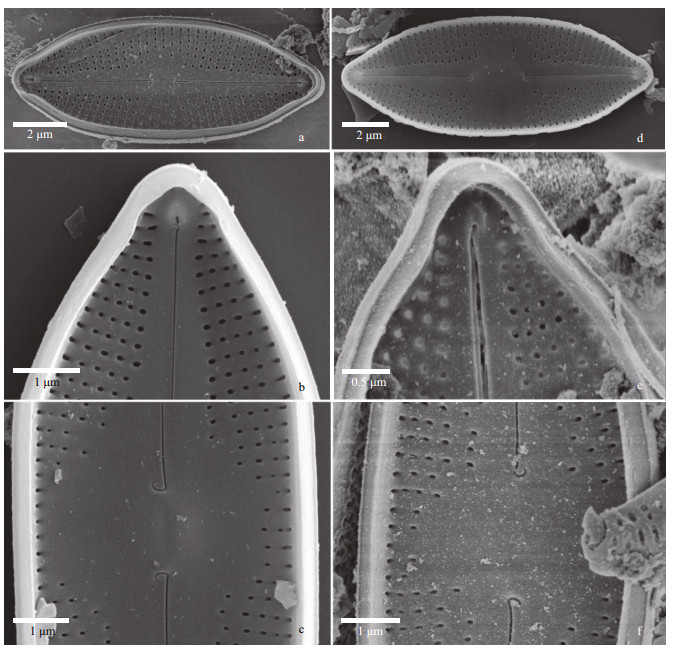
|
| Fig.3 Nupela shimenensis Q. Liu, S. L. Xie, & Kociolek, sp. nov. under SEM (valve interior) a & d. entire valves. In (a), a girdle band is attached and bears a small septum. Axial area is narrowly lanceolate and the helictoglossa at each pole is evident; b & e. valve apices, with prominent septum and helictoglossae evident; c & f. central portion of the valve, with small central nodule evident. Unilaterally hooked proximal raphe ends are shown at the base on either side of the central nodule. |
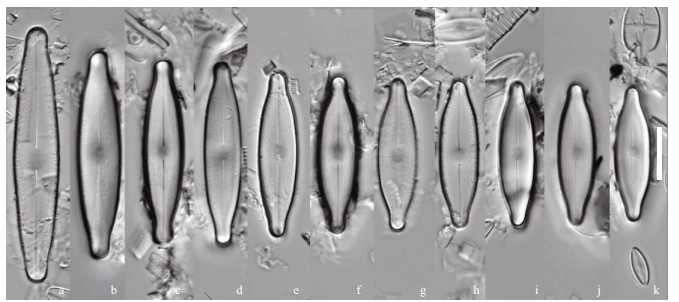
Description: Frustules biraphid, valves elliptical, tapering quickly to the apex, poles barely protracted, rounded. Length 14–24 μm, breadth 5.0–6.5 μm. Axial area narrow at the poles, lanceolate in shape, expanded laterally to form an asymmetrical central area, with one side expanded to the margin, the other side with few striae of irregular length present. Raphe appears filiform, and more or less straight. The proximal raphe ends are dilated. Striae fine, radiate, some of differing lengths, 28–32/10 μm. A small round central nodule is visible.
In the SEM, the valve exterior (Fig. 2) can have varying degrees of silicification. In cells with the smallest amount of silica seen (Fig. 2a–c), individual areolae have coverings over the opening, and there are very small, round openings around the periphery of each areola. The raphe appears straight to slightly undulate, the distal ends of the raphe curve onto the mantle, and there is no siliceous rim around the margin of the valve. In valves with more silica (Fig. 2d–f), the coverings over the striae are less organized, and are thicker, there is a rim of silica at the margin and the raphe is less undulate and the distal ends do not curve onto the mantle.
Internally in the SEM (Fig. 3) the raphe has hooked proximal ends laying at the base of the small, round central nodule (Fig. 3a, c, d, f). The small helictoglossae are positioned at tip of the valve apex (Fig. 3b & e). Areolae are unoccluded internally (Fig. 3b, c, e, f). The valvocopulae appear to have a small septum associated with them (Fig. 3b & e).
Holotype: Slide No. 201904033-1 in collection of SXU, Freshwater Algal Herbarium of Shanxi University, Taiyuan, China, holotype illustrated in Fig. 1a & b.
Type locality: Yunnan Province, Shimen Valley, wet wall, periphyton, China (23°58ʹ22ʺN, 101°31ʹ25ʺE, above sea level (ASL): 1 987.7 m).
Etymology: Named for the region where it was found.
Comments: Nupela shimenensis is similar in outline with N. decipiens (Reimer) Potapova, and the two species also have widened central areas (Reimer and Reimer, 1966; Potapova, 2013). The two species differ in that the specimens from South Carolina, USA, described originally by Reimer and Reimer (1966) and subsequently documented by Potapova (2011) are distinctly heterovalvate, possessing raphe and rapheless valves that are ornamented differently (Potapova, 2013). In N. shimenensis, frustules are not heterovalvate, but rather all valves possess a raphe system and have similar ornamentation.
Nupela yunnanica, Q. Liu, S. L. Xie & Kociolek, sp. nov. Figures 4 (LM) & 5–6 (SEM)
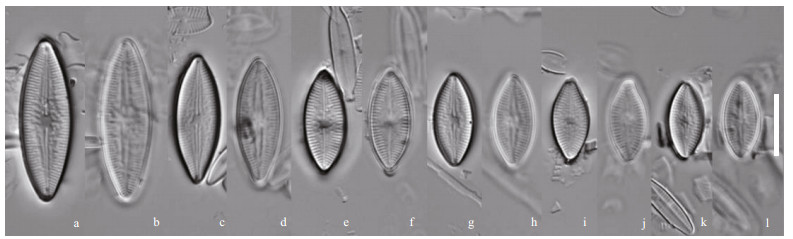
|
| Fig.4 Nupela yunnanica Q. Liu, S. L. Xie, & Kociolek, sp. nov. under LM Size diminution series, showing both valves of six frustules. Holotype is illustrated in (a) & (b). Scale bar=10 μm for all figures. |
|
| Fig.5 Nupela yunnanica Q. Liu, S. L. Xie, & Kociolek, sp. nov. under SEM (raphe valves) a–c. external views. In (a): entire valve showing high degree of flexure about the transapical axis and mottled/pitted appearance of the valve face. In (b): towards the terminus of the valve showing somewhat undulate raphe with deflected distal end that extends onto the valve mantle. Areolae have an external covering. In (c): central part of the valve with raphe slit evident and many pits around the center of the valve; d–f: internal views. Entire valve view sowing the highly flexed valve, and prominent, anchor-shaped proximal raphe ends. In (e): view towards the end of a valve, showing prominent helictoglossa that is connected to the apex of the valve. Striae with narrow vimines are separated by relatively thick virgae. In (f): valve center with anchor-shaped proximal raphe ends and a small central nodule. |
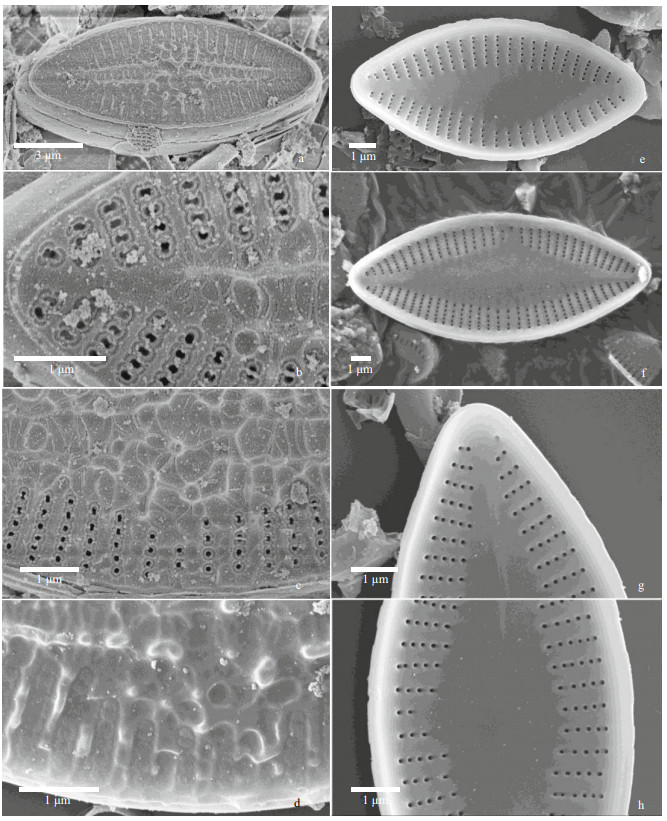
|
| Fig.6 Nupela yunnanica Q. Liu, S. L. Xie, & Kociolek, sp. nov. under SEM (rapheless valves) a–d. external views. In (a): entire valve with prominent siliceous ribs and nodules on the valve face. A rim or border can be seen around the margin. In (b): view near the valve apex showing borders around the areolae. In (c): central part of the valve showing a network of siliceous outgrowths. Areolae are bordered. In (d): view showing siliceous nodules. e–h: internal views. In (e–h): entire valves showing lanceolate axial area. In (g–h): apex and central area showing 'ghost' raphe in the axial area. |
Description: Frustules monoraphid, valves ovoid-elliptical, without protracted apices. Length 11–28 μm, breadth 5.5–8.0 μm. Axial area narrow, narrowly-lanceolate in shape, no distinct central area formed. Axial area mottled with siliceous nodules and depressions. Raphe valve with straight, filiform raphe with dilated proximal ends. Striae radiate, with some striae of differing lengths. Striae 19–23/10 μm.
In the SEM, the exterior of the raphe valve (Fig. 5a–c) has many round to irregular, variously-sized pits or depressions mostly along the axial area but scattered across the valve face. There is a covering over the entirety of each striae that occludes the areolae. The raphe is undulate, with the proximal ends dilated slightly and distal ends curved in the same direction and extending onto the mantle. Internally, the raphe valve (Fig. 5d–f) has distinctly radiate striae and a small, round central nodule. Distal raphe ends terminate as helictoglossae. The proximal ends are anchor-shaped. Areolae are unoccluded internally.
The exterior of the rapheless valves (Fig. 6a–d) show a network of thickened siliceous outgrowths mostly away from the ends and concentrated down the center of the valves. Areolae have thickenings around them. The margin of the valve has a thickened rim. Internally (Fig. 6e–f), the broadly-lanceolate axial area dominates the valve interior of the rapheless valve. Areolae are small, round and without occlusions. A "ghost raphe" can be seen on some valves (Fig. 6f & g).
Holotype: Slide No. 201904055-1 in collection of SXU, Freshwater Algal Herbarium of Shanxi University, Taiyuan, China, holotype illustrated in Fig. 4a & b.
Type locality: Yunnan Province, Shimen Valley, wet wall, periphyton, China (23°58ʹ22ʺN, 101°31ʹ21ʺE, ASL: 2 065.7 m).
Etymology: Named for the province in which it was found.
Comments: Several species of Nupela have been described with the mottled look about the axial area, including N. lapidosa (Krasske) Lange-Bertalot and Genkal (1999) and N. major Yu, You & Kociolek (in Yu et al., 2017). Nupela yunnanica differs from these species by being significantly smaller than N. major and having a valve shape that is more elliptical than what is seen in N. lapidosa (Potapova, 2011)
Nupela magna, Q. Liu, S. L. Xie & Kociolek, sp. nov. Figures 7 (LM) & 8–9 (SEM)
|
| Fig.7 Nupela magna Q. Liu, S. L. Xie, & Kociolek, sp. nov. under LM Size diminution series. Holotype is illustrated in (c). Scale bar=10 μm for all figures. |
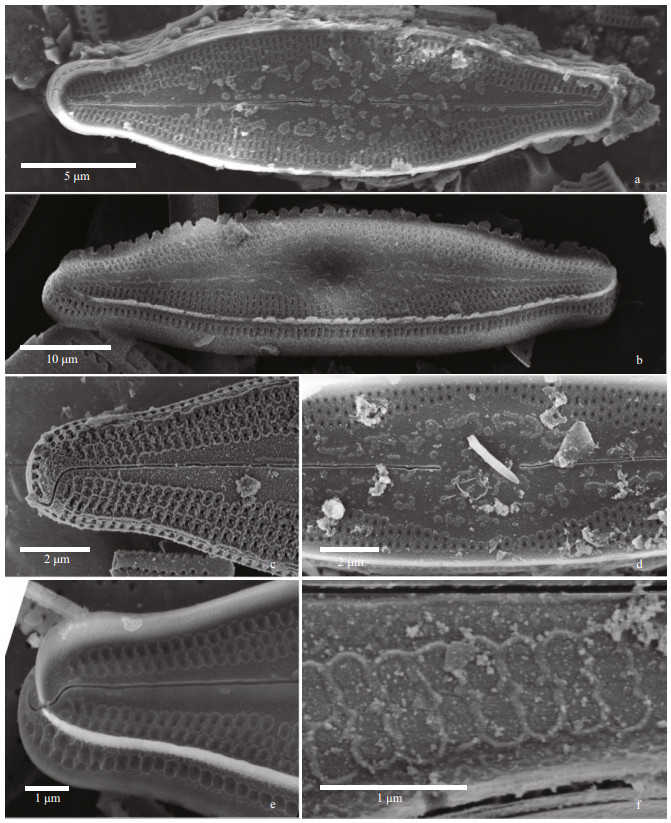
|
| Fig.8 Nupela magna Q. Liu, S. L. Xie, & Kociolek, sp. nov. under SEM (external views) a. entire valve view, with robust marginal rim evident. The raphe is slightly undulate near the center; b. entire valve view with fimbriate marginal rim. Striae extend onto the mantle. c & e. valve terminus, showing no marginal rim (c) or substantial marginal rim (e). Areolae are bordered by thick rims. Distal raphe ends bent and extend onto valve mantle; d. central part of the valve with wide central area evident. Proximal raphe ends are dilated slightly; f. detail of external occlusions on areolae. |
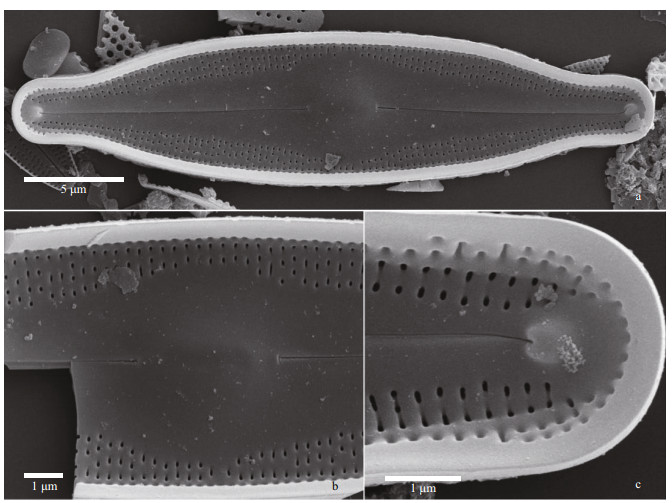
|
| Fig.9 Nupela magna Q. Liu, S. L. Xie, & Kociolek, sp. nov. under SEM (internal views) a. entire valve with lanceolate axial area evident. Small central nodule is round; b. central nodule showing proximal raphe ends are anchor-shaped, but with one side larger than the other. Areolae of the striae appear offset from one another; c. valve terminus with large helictoglossa present at raphe terminus. |
Description: Valves linear-elliptical with produced ends that are broadly-rounded. Length 25–38 μm, breadth 6.0–7.5 μm. Axial area wide, lanceolate, forming a circular central area in most specimens. The central nodule is round. Raphe filiform, with external proximal ends deflected slightly in the same direction. Striae short, fine, composed of small dash-like areolae, radiate, ca. 28–32/10 μm, slightly offset from one another.
In the SEM, the valve exterior (Fig. 8) has areolae covered with occlusions. The raphe is straight to slightly undulate, the external proximal raphe ends are not dilated and the distal ends are hooked in the same direction and extend onto the valve mantle. Areolae are bordered by oblong silica thickenings. Specimens may lack a marginal siliceous border, or the marginal border may be fimbriate or entire (e.g., Fig. 8b & e). Internally (Fig. 9), there is a distinct central nodule at the base of which sit the proximal raphe ends. Internal proximal raphe ends are anchor-shaped, with one side branch larger than the other. Areolae are unoccluded. Distally, the raphe terminates as distinct helictoglossae.
Holotype: Slide No. 201904055-1 in collection of SXU, Freshwater Algal Herbarium of Shanxi University, Taiyuan, China, holotype illustrated in Fig. 7c.
Type locality: Yunnan Province, Shimen Valley, wet wall, periphyton, China (23°58ʹ22ʺN, 101°31ʹ21ʺE, ASL: 2 065.7m).
Etymology: Named for being a large-sized Nupela species.
Comments: Structure of the striae as seen with LM, the areolae in adjoining striae are slightly offset from one another, a feature that is reminiscent of Anomoeoneis or Brachysira (Round et al., 1990). Valves appear as similar to large specimens of Humidophila, but the striae are comprised of more than a single areola.
Nupela sinensis, Q. Liu, S. L. Xie & Kociolek, sp. nov. Figures 10 (LM) & 11–12 (SEM)

|
| Fig.10 Nupela sinensis Q. Liu, S. L. Xie, & Kociolek, sp. nov. under LM Size diminution series, showing both valves of eight frustules. Holotype is illustrated in (g & h). Scale bar=10 μm for all figures. |
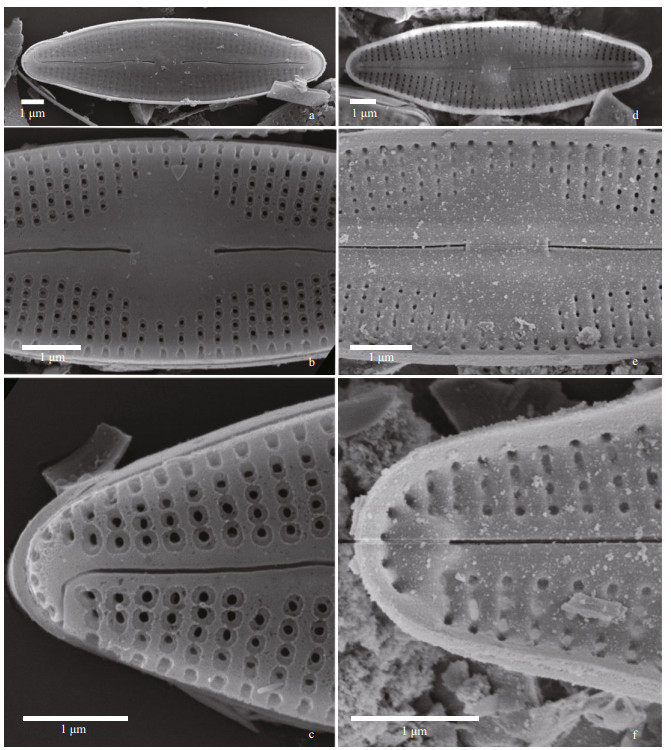
|
| Fig.11 Nupela sinensis Q. Liu, S. L. Xie, & Kociolek, sp. nov. under SEM (raphe valves) a–c. external views. In (a): entire valve view. In (b): view of the central area. Proximal raphe ends are dilated. Areolae have a narrow border around them. In (c): valve terminus. Distal raphe end is bent 90° and on to the valve mantle; d–f. internal views. In (d): entire view, with central nodule evident. In (e): central area, with central nodule bearing the anchor-shaped proximal raphe ends. In (f): terminus of valve with helictoglossa evident. |
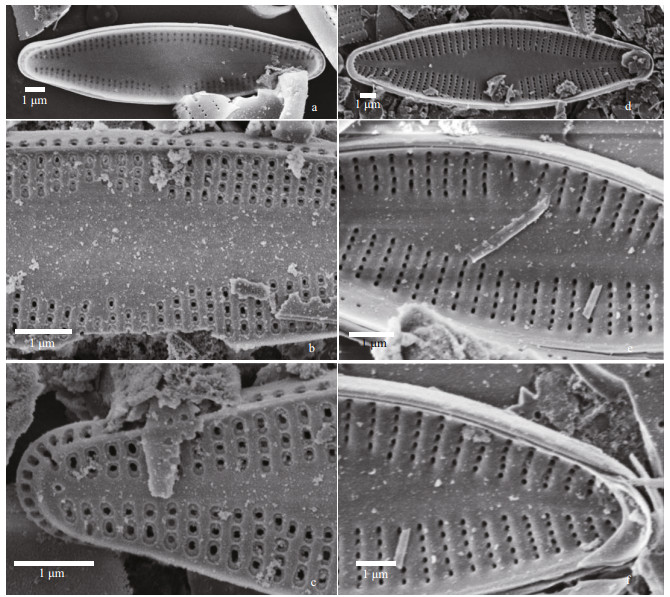
|
| Fig.12 Nupela sinensis Q. Liu, S. L. Xie, & Kociolek, sp. nov. under SEM (raphe less valves) a–c. valve exterior. In (a): entire valve view. In (b): central portion of the valve showing areolae with borders. In (c): valve terminus with bordered rectangular to elongate areolae. Note the round opening at the end of the axial area; d–f: internal views. In (d): valve view. In (e): central portion of the valve. In (f): valve terminus. Not the septum on the girdle band. |
Description: Frustules monoraphid, linear-lanceolate in shape, with slightly protracted rounded apices, length 7–25 μm, breadth 2–4 μm. Raphe valve: Axial area straight to slightly lanceolate in shape, expanded unilaterally, rectangular in shape, raphe filiform, straight, slightly swollen at the external proximal ends, distal ends indistinct. Striae radiate, individual areolae not resolved, 27–30/10 μm. Rapheless valve: central area wide, lanceolate in shape. Striae radiate throughout, 27–30/10 μm. Individual areolae not resolved.
In the SEM, the valve exterior of the raphe valve (Fig. 11a–c) has distinct areolae. No occlusions have been observed on the areolae, but there is a distinct rim around the openings. The filiform raphe is distinct, with slightly dilated proximal ends and distal ends that are hooked in the same direction, extending from the valve face onto the mantle. Internally, striae are distinct, radiate and composed of areolae that lack occlusions (Fig. 11d–f). The central nodule is small, circular and is positioned next to the small, asymmetrical, anchor-shaped proximal raphe ends. The raphe terminates at the ends as helictoglossae.
The exterior of the rapheless valve (Fig. 12a–c) has areolae bordered by siliceous rims. On the mantle, each stria opens with a single, elongated areola. In some specimens, there is an isolated opening at the terminus of the valve, at the end of the axial area (Fig. 12c). Internally (Fig. 12d–f) the areolae are unoccluded, and there is a small but distinct septum present each apex (Fig. 12d & f).
Holotype: Slide No. 201904055-1 in collection of SXU, Freshwater Algal Herbarium of Shanxi University, Taiyuan, China, holotype illustrated in Fig. 10e & f.
Type locality: Yunnan Province, Shimen Valley, wet wall, periphyton, China (23°58ʹ22ʺN, 101°31ʹ21ʺE, above sea level ASL: 2 065.7 m).
Etymology: Named for this species being found in China.
Nupela comperei, Q. Liu, S. L. Xie & Kociolek, sp. nov. Figrues 13 (LM) & 14 (SEM)
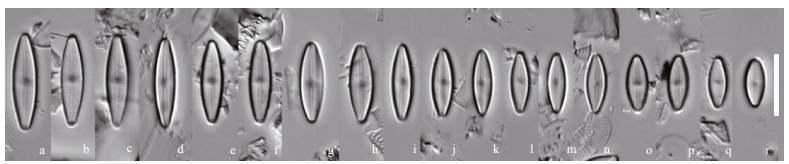
|
| Fig.13 Nupela comperei Q. Liu, S. L. Xie, & Kociolek, sp. nov. under LM Size diminution series. Holotype is illustrated in (h). Scale bar=10 μm for all figures. |
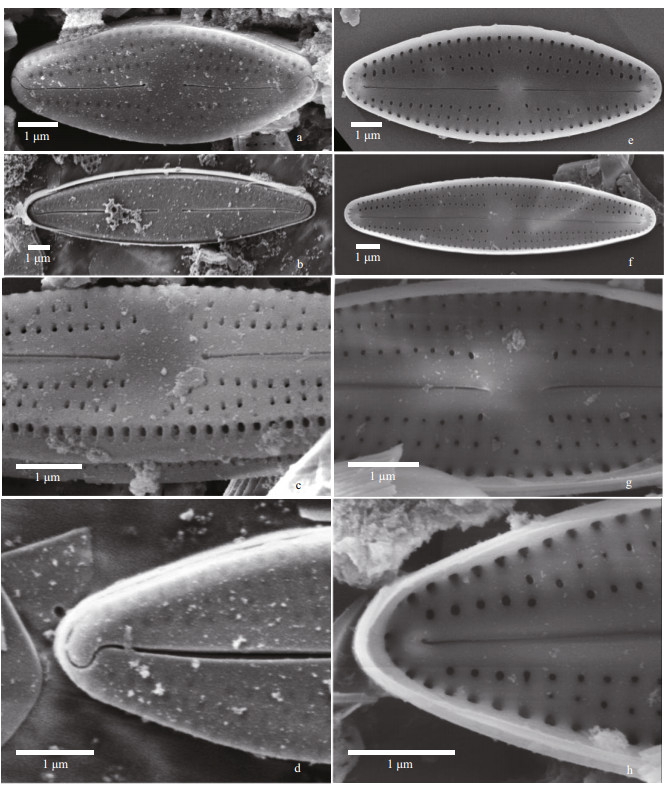
|
| Fig.14 Nupela comperei Q. Liu, S. L. Xie, & Kociolek, sp. nov. under SEM a–d. external views. In (a–b). entire valve views. The raphe is straight and striae are radiate. In (c): central portion of the valve. Proximal raphe ends are dilated and rounded. Areolae are offset from one another between striae. An undulate marginal ridge is present. In (d): valve terminus showing hooking distal raphe end, extending onto the valve margin. e–h: internal views. In (e–f): entire valve views. Central nodule is prominent. In (g): central portion of the valve, with rounded central nodule and straight proximal raphe ends. Areolae are offset from one another. In (h): valve terminus with helictoglossa evident. |
Description: Frustules biraphid. Valves linear-lanceolate to lanceolate in shape, with apices rounded. Length 8–17 μm, breadth 4–5 μm. Axial area linear, expanded laterally at the center to form a small, rectangular central area. Raphe straight to slightly undulate, filiform. Striae very fine, radiate, 35–40/10 μm, individual puncta indistinct.
In the SEM, the valve exterior (Fig. 14a–d) shows the striae comprised of 2–3 areolae, interrupted along the apical axis. Areolae are occluded externally. The valve may have a thickened margin, which appears wavy in some specimens. The thickened margin may be lacking. Raphe with proximal ends dilated slightly, with distal ends hooked and extending onto the valve mantle. Internally (Fig. 14e–h), a small, round, raised central nodule is evident. The central nodule bears the external proximal raphe ends, which are straight. The raphe terminates at the apices as helictoglossae. The areolae are unoccluded and are irregularly aligned from stria to stria.
Holotype: Slide No. 201904055-1 in collection of SXU, FreshwaterAlgal Herbarium of Shanxi University, Taiyuan, China, holotype illustrated in Fig. 13a.
Type locality: Yunnan Province, Shimen Valley, wet wall, periphyton, China (23°58ʹ22ʺN, 101°31ʹ21ʺE, ASL: 2 065.7 m).
Etymology: Named for Pierre Compère, former research scientist at the National Botanic Garden in Meise, Belgium, and one of the original describers of the genus Nupela.
4 DISCUSSIONThe concept of Nupela has broadened dramatically since its original description as a monotypic genus over 30 years ago (Vyverman and Compère, 1991). One interesting aspect of the genus as currently circumscribed is that species can be biraphid with a raphe system that extends from the central area to the apices, to biraphid taxa where the raphe may be much shorter than a "typical" raphe might be, to frustules that are heterovalvate, and there is one valve with a raphe and the other without. Potapova et al. (2003) even describe the situation in one heterovalvate species where there are degrees of differences. Work on the raphe number suggests that secondary loss of the raphe has happened 5–6 times (Kociolek et al., 2019; Kulikovskiy et al., 2020), and that even within one taxon, a species can produce biraphid, monoraphid or even araphid frustules (Liu et al., 2018). Lange-Bertalot (in Krammer and Lange-Bertalot, 1991) hinted at this possibility in species that ultimately were transferred to Nupela from Achnanthidium and Navicula (Potapova, 2011), and suggested that despite being in two different genera, the taxa might be the same taxon. However, these species, while both were transferred to Nupela, were kept separate in part due to raphe number. In the cases of the species we present here as new, three are biraphid and the other two are monoraphid. However, two species of biraphid diatom have similar structure on both valves (N. magna sp. nov. and N. comperei sp. nov), and in the third the frustule can be heterovalvate, with the length of the raphe branches differing between valves on the same frustule (N. shimenensis sp. nov.). One species (N. sinensis sp. nov.) has predominately monoraphid frustules, but in a few cases it appears to produce biraphid frustules as well, while the other monoraphid species (N. yunnanica sp. nov.) is clearly heterovalvate in terms of structure, with the raphe valve being much more rugose than the rapheless valves. Plasticity in raphe number within a species has been documented by Liu et al. (2018), where a single species can have 0, 1 or 2 raphe systems on a frustule.
In addition to the number of raphe systems per frustule, within and between species, there is also variability between Nupela taxa with regards to the structure of the raphe, in particular the internal proximal raphe ends. Some Nupela species have anchor-shaped internal proximal raphe ends, where the hooked elements are of equal proportions (e.g., N. yunnanica) while in others (such as N. magna) the anchor-shape is asymmetrical, with one side longer than the other. These anchor-shaped internal proximal raphe ends are similar to those seen in the genus Gomphosphenia Lange-Bertalot (1995; see also Kociolek et al., 1988). In some species the internal proximal raphe ends are recurved to one side only, typical of species among the freshwater gomphonemoid diatoms (e.g., Kociolek and Stoermer, 1993). Finally, N. comperei seems to have straight internal proximal raphe ends. Sala et al. (2014), Tremarin et al. (2015), and Kulikovskiy et al. (2020) showed other species with straight, not recurved, internal proximal raphe ends in Nupela species.
Nupela magna sp. nov. is quite large relative to most species assigned to the genus; it is approximately the same size as N. major Yu et al. (2017) (valve length 25–38 μm versus 20.0–41.5 μm, breadth 6.0–7.5 μm versus 6.5–9.5 μm). Taxa septa (N. sinensis and N. shiwanensis) are the first taxa in the genus Nupela reported to have these structures, further expanding the known morphological diversity of the genus.
The diagnostic feature of the genus Nupela is the presence of hymenate occlusions on the exterior of the areolae, which differentiates the genus from many naviculoid and monoraphid genera which have occlusions on the interior. Though Cox (2015) has suggested Nupela may be closely related to Brachysira (echoing the original describers of the genus (Mann in Round et al., 1990; Vyverman and Compère, 1991)); the genus has internal hymenate occlusions over the areolae (Lange-Bertalot and Moser, 1994; Anderson et al., 2013). The same is true for many other biraphid genera symmetrical about the apical and transapical axes, including others classified close to Brachysira (Round et al., 1990). In monoraphid genera such as Achnanthidium, areolae also have internal hymenes (e.g., Yu et al., 2018; You et al., 2019). Authors such as Potapova et al. (2003) have pointed to the presence of an external covering ("coalescing hymens") across the striae externally as support in their placement of species in Nupela (whether that covering was visible or not, and whether it was demonstrated in the generitype, N. gilwuensis). They discuss the placement of areolar coverings as a fundamental difference between lineages of naviculoid diatoms (Potapova et al., 2003). Kulikovskiy et al. (2020) have shown Nupela to be closely related Diploneis (Ehrenberg) P. T. Cleve (1894: 76), as part of a distinct monophyletic group with Biremis D. G. Mann & E. J. Cox in Round et al. (1990: 664) and quite separate from Brachysira. It is not clear, however, if the diversity of valve morphologies expressed in species assigned to Nupela represent a single monophyletic group and the genus is plastic in terms of raphe number and in the morphological diversity represented, or if these different morphological groups may reflect a more complex set of phylogenetic relationships. To date, only two taxa have any molecular data generated from them, and both species have straight, internal proximal raphe ends (Kulikovskiy et al., 2020). The morphological diversity seen from the wet wall in Yunnan has not yet been investigated with molecular tools.
The new species described herein from a wet wall in Yunnan Province provide further evidence that this province harbors a diversity of endemic diatom taxa. Skuja (1937) was the first to document unique species from Yunnan. Species described as the province, include "centric" and pennate diatoms, and in the latter group, across a wide range of lineages. And while some of Skuja's species are still present (Li et al., 2018; Liu et al., 2020), it appears they may be declining in numbers (Tao et al., 2016). In these works, only a small number of aquatic habitats have been investigated, and almost no high elevation lakes, none of the largest rivers ("3 Parallel Rivers") and habitats from the high mountainous regions have been studied in detail. Further research on the diatoms from Yunnan Province is needed to document a baseline of biodiversity present, so that the effects of human disturbance can be assessed.
5 DATA AVAILABILITY STATEMENTAll data included in this study are available upon request by contact with the corresponding author.
6 ACKNOWLEDGMENTWe are grateful to Shanxi Academy of Advanced Research and Innovation in acquiring SEM images.
Anderson K, Fate M, Hsieh C H, et al. 2013. Algal diversity in, and the description of three new diatom (Bacillariophyta) species from, Lake of the Clouds, Porcupine Mountains Wilderness State Park, Michigan USA. The Michigan Botanist, 52(1-2): 3-24.
|
Buczkó K, Wojtal A Z, Magyari E K. 2013. Late quaternary Nupela taxa of Retezat Mts (S. Carpathians), with description of Nupela pocsii sp. nov. (Bacillariophyceae). Polish Botanical Journal, 58(2): 427-436.
DOI:10.2478/pbj-2013-0059 |
Chen J Y. 1980. Notes on some new species and varieties of the genus Melosira (Bacillariophyta). Acta Hydrobiologica Sinica, 7(2): 253-256.
(in Chinese with English abstract) |
Chen X Y. 2013. Checklist of fishes of Yunnan. Zoological Research, 34(4): 281-343.
DOI:10.11813/j.issn.0254-5853.2013.4.0281 |
Cheng Y, Liu Y, Kociolek J P, et al. 2018. A new species of Gomphosinica (Bacillariophyta) from Lugu Lake, Yunnan Province, SW China. Phytotaxa, 348(2): 118-124.
DOI:10.11646/phytotaxa.348.2.6 |
Cleve P.T. 1894. Synopsis of the naviculoid diatoms. Part Ⅰ. Kongliga Svenska Vetenskapsakademiens Handlingar Series 4, 26(2): 1-194, 5 pls.
|
Cox E J. 2015. Coscinodiscophyceae, Mediophyceae, Fragilariophyceae, Bacillariophyceae (Diatoms). In: Frey Wed. Syllabus of Plant Families: Adolf Engler's Syllabus der Pflanzenfamilien. 13th edn. Borntraeger Science Publishers, Berlin, Germany. p. 64-103.
|
Guiry M D, Guiry G M. 2021. AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. Available from: http://www.alga-ebase.org (accessed on 8 November 2021).
|
Guo H J, Long C L. 1998. Biodiversity of Yunnan. Yunnan Science and Technology Press, Kunming, China. 141p. (in Chinese)
|
Jiang Z Y, Liu Y, Kociolek J P, et al. 2018. One new Gomphonema (Bacillariophyta) species from Yunnan Province, China. Phytotaxa, 349(3): 257-264.
DOI:10.11646/phytotaxa.349.3.6 |
Kociolek J P, Blanco S, Coste M et al. 2022. DiatomBase. http://www.diatombase.org. (accessed on 24 May 2022)
|
Kociolek J P, Liu Q, Danz A. 2021. New and interesting diatoms from the Shimen Wetwalls, Yunnan Province, China. I. Valve morphology of Melosira radiato-sinuata var. yunnanica Chen (Bacillariophyta: Melosirales) from Yunnan Province, China, with comments on its systematic positon. Diatom Research, 36(4): 345-351.
DOI:10.1080/0269249X.2021.2025151 |
Kociolek J P, Stoermer E F. 1993. Freshwater gomphonemoid diatom phylogeny: preliminary results. Hydrobiologia, 269(1): 31-38.
DOI:10.1007/BF00028001 |
Kociolek J P, Williams D M, Stepanek J, et al. 2019. Rampant homoplasy and adaptive radiation in pennate diatoms. Plant Ecology and Evolution, 152(2): 131-141.
DOI:10.5091/plecevo.2019.1612 |
Kociolek J P, Yang J R, Stoermer E F. 1988. Taxonomy, ultrastructure and systematic position of the Gomphonema grovei M. Schm. -species complex (Bacillariophyceae). Nova Hedwigia, 47(1-2): 145-158.
|
Krammer K, Lange-Bertalot H. 1991. Bacillariophyceae 4. Teil: achnanthaceae, Kritische Erganzungen zu Navicula (Lineolatae)und Gomphonema Gesamtliteraturverzeichnis Teil 1-4. In: Ettl H ed. Suesswasserflora von Mitteleuropa. VEB Gustav Fisher Verlag, Jena. p. 1-437.
|
Kulikovskiy M S, Lange-Bertalot H, Kuznetsova I V. 2015. Lake Baikal: Hotspot of Endemic Diatoms Ⅱ. Iconographia Diatomologica. Koeltz Scientific Books, Oberreifenberg. p. 1-657.
|
Kulikovskiy M S, Lange-Bertalot H, Witkowski A. 2009. Nupela matrioschka sp. nov., Nupela thurstonensis comb. nov. and Nupela neogracillima comb. & nom. nov. (Bacillariophyceae): critical analysis of their morphology. Polish Botanical Journal, 54(1): 13-20.
|
Kulikovskiy M S, Maltsev Y I, Glushchenko A M, et al. 2020. Preliminary molecular phylogeny of the diatom genus Nupela with the descrip-tion of a new species and consideration of the interrelationships of taxa in the suborder Neidiineae D. G. Mann sensu E. J. Cox. Fottea, 20(2): 192-204.
DOI:10.5507/fot.2020.009 |
Lange-Bertalot H, Genkal S I. 1999. Diatoms from Siberia I - Islands in the Arctic Ocean (Yugorsky-Shar Strait). In: Lange-Bertalot H ed. Iconographia Diatomologica. Koeltz Scientific Books, Königstein, Germany. p. 1-303.
|
Lange-Bertalot H, Moser G. 1994. Brachysira. Monographie der Gattung und Naviculadicta nov. gen. Bibliotheca Diatomologica, 29: 1-212.
|
Lange-Bertalot H. 1993. 85 neue taxa und über 100 weitere neu definierte Taxa ergänzend zur Süsswasserflora von Mitteleuropa, Vol. 2/1-4. In: Cramer J ed. Bibliotheca Diatomologica. Stuttgart, Berlin. p. 1-164.
|
Lange-Bertalot H. 1995. Gomphosphenia paradoxa nov. spec. et nov. gen. und Vorschlag zur Lösung taxonomischer Probleme infolge eines veränderten Gattungskonzepts von Gomphonema (Bacillariophyceae). Nova Hedwigia, 60(1-2): 241-252.
|
Levkov Z, Metzeltin D, Pavlov A. 2013. Diatoms of Europe: Diatoms of the European inland waters and comparable habitats, Luticola and Luticolopsis. Koeltz Scientific Books, Königstein. p. 1-698.
|
Li H. 1993. Flora of Dulongliang Region. Yunnan Science and Technology Press, Kunming, China. 285p. (in Chinese)
|
Li R, Chen G J, Kang W G, et al. 2018. Spatio-temporal variations of diatom community and their relationship with water environment in Fuxian Lake. Environmental Science, 39(7): 3168-3178.
(in Chinese with English abstract) DOI:10.13227/j.hjkx.201710188 |
Li X W. 1995. A floristic study on the plants from the region of Yunnan Plateau. Acta Botanica Yunnanica, 17(1): 1-14.
(in Chinese with English abstract) |
Li Y L, Gong Z J, Wang C C, et al. 2010a. New species and new records of diatoms from Lake Fuxian, China. Journal of Systematics and Evolution, 48(1): 65-72.
DOI:10.1111/j.1759-6831.2009.00059.x |
Li Y L, Metzeltin D, Gong Z J. 2010b. Two new species of Sellaphora (Bacillariophyta) from a deep oligotrophic plateau lake, Lake Fuxian in subtropical China. Chinese Journal of Oceanology and Limnology, 28(6): 1160-1165.
DOI:10.1007/s00343-010-9028-z |
Liao M N, Li Y L. 2018. One new Gomphonema Ehrenberg (Bacillariophyta) species from a high mountain lake in Yunnan Province, China. Phytotaxa, 361(1): 123-130.
DOI:10.11646/phytotaxa.361.1.11 |
Liu Y, Kociolek J P, Glushchenko A, et al. 2018. A new genus of Eunotiales (Bacillariophyta, Bacillariophyceae: Peroniaceae), Sinoperonia, from Southeast Asia, exhibiting remarkable phenotypic plasticity with regard to the raphe system. Phycologia, 57(2): 147-158.
DOI:10.2216/17-21.1 |
Liu Y, Kociolek J P, Lu X X, et al. 2020. Valve ultrastructure of two species of the diatom genus Gomphonema Ehrenberg (Bacillariophyta) from Yunnan Province, China. Fottea, 20(1): 25-35.
DOI:10.5507/fot.2019.012 |
Metzeltin D, Lange-Bertalot H. 1998. Tropical diatoms of South America I: about 700 predominantly rarely known or new taxa representative of the neotropical flora. In: Lange-Bertalot H ed. Iconographia Diatomologica. Koeltz Scientific Books. Königstein, Germany. p. 1-695.
|
Naruse T, Chia J E, Zhou X M. 2018. Biodiversity surveys reveal eight new species of freshwater crabs (Decapoda: Brachyura: Potamidae) from Yunnan Province, China. PeerJ, 6: e5497.
DOI:10.7717/peerj.5497 |
Patrick R M, Reimer C W. 1966. The diatoms of the United States, exclusive of Alaska and Hawaii, volume 1. Fragilariaceae, Eunotoniaceae, Achnanthaceae, Naviculaceae. In: Monographs of the Academy of Natural Sciences of Philadelphia (Patrick R M, Reimer C W). Academy of Natural Sciences, Philadelphia. 688p.
|
Potapova M G, Ponader K C, Lowe R L, et al. 2003. Small-celled Nupela species from North America. Diatom Research, 18(2): 293-306.
DOI:10.1080/0269249X.2003.9705593 |
Potapova M. 2011. New species and combinations in the genus Nupela from the USA. Diatom Research, 26(1): 73-87.
DOI:10.1080/0269249X.2011.575111 |
Potapova M. 2013. Transfer of Achnanthes decipiens to the genus Nupela. Diatom Research, 28(2): 139-142.
DOI:10.1080/0269249X.2012.753114 |
Round F E, Crawford R M, Mann D G. 1990. The Diatoms: Biology and Morphology of the Genera. Cambridge University Press, Cambridge. 747p.
|
Sala S E, Vouilloud A A, Plata-Díaz Y, et al. 2014. Nupela species (Naviculales: Bacillariophyceae) from Colombian lowland waters including N. acaciensis nov. sp. and N. catatumbensis nov. sp. Revista de Biología Tropical, 62(1): 241-256.
DOI:10.15517/rbt.v62i1.8363 |
Siver PA, Hamilton P B, Morales E A. 2007. Notes on the genus Nupela (Bacillariophyceae) including the description of a new species, Nupela scissura sp. nov. and an expanded description of Nupela paludigena. Phycological Research, 55(2): 125-134.
DOI:10.1111/j.1440-1835.2007.00455.x |
Siver P A, Wolfe A P, Edlund M B. 2010. Taxonomic descriptions and evolutionary implications of Middle Eocene pennate diatoms representing the extant genera Oxyneis, Actinella and Nupela (Bacillariophyceae). Plant Ecology and Evolution, 143(3): 340-351.
DOI:10.5091/plecevo.2010.419 |
Skuja H. 1937. Algae. In: Handel-Mazzetti H ed. Symbolae Sinicae: Botanische Ergebnisse der Expedition der Akademie der Wissenschaften in Wien nach Sudwest-China, 1914-1918. Wien. 105p.
|
Tao J S, Chen G J, Chen X L, et al. 2016. Long-term pattern of diatom community responses to water pollution and hydrological regulation in Yangzong Lake. Geographical Research, 35(10): 1899-1911.
(in Chinese with English abstract) |
Tremarin P I, Straube A, Ludwig T A V. 2015. Nupela (Bacillariophyceae) in littoral rivers from south Brazil, and description of six new species of the genus. Fottea, 15(1): 77-93.
DOI:10.5507/fot.2015.007 |
Vyverman W, Compère P. 1991. Nupela giluwensis gen. & spec. nov. a new genus of naviculoid diatoms. Diatom Research, 6(1): 175-179.
DOI:10.1080/0269249X.1991.9705156 |
You Q M, Cao Y, Yu P, et al. 2019. Three new subaerial Achnanthidium (Bacillariophyta) species from a karst landform in the Guizhou Province, China. Fottea, 19(2): 138-150.
DOI:10.5507/fot.2019.005 |
You Q M, Kociolek J P, Wang Q X. 2015. Taxonomic studies of the diatom genus Halamphora (Bacillariophyceae) from the mountainous regions of southwest China, including the description of two new species. Phytotaxa, 205(2): 75-89.
DOI:10.11646/phytotaxa.205.2.1 |
Yu P, Kociolek J P, You Q M, et al. 2018. Achnanthidium longissimum sp. nov. (Bacillariophyta), a new diatom species from Jiuzhai Valley, southwestern China. Diatom Research, 33(3): 339-348.
DOI:10.1080/0269249X.2018.1545704 |
Yu P, You Q M, Kociolek J P, et al. 2017. Nupela major sp. nov., a new diatom species from Maolan Nature Reserve, central-south of China. Phytotaxa, 311(3): 245-254.
DOI:10.11646/phytotaxa.311.3.4 |
Zhou W, Chen B K. 2006. Biodiversity of Bitahai nature reserve in Yunnan province, China. Biodiversity and Conservation, 15(3): 839-853.
DOI:10.1007/s10531-004-1869-3 |
 2023, Vol. 41
2023, Vol. 41


