Institute of Oceanology, Chinese Academy of Sciences
Article Information
- CHAO Jinyu, FENG Song, HAO Yingdong, LIN Jianing, ZHANG Bin
- Pollution characteristics and risk assessment of organophosphate esters in aquaculture farms and natural water bodies adjacent to the Huanghe River delta
- Journal of Oceanology and Limnology, 41(1): 251-266
- http://dx.doi.org/10.1007/s00343-021-1233-4
Article History
- Received Aug. 12, 2021
- accepted in principle Sep. 28, 2021
- accepted for publication Dec. 10, 2021
2 Institute of Eco Environmental Forensics, Shandong University, Qingdao 266237, China;
3 School of Environmental Science and Engineering, Shandong University, Qingdao 266237, China;
4 School of Food and Biotechnology, Xihua University, Chengdu 610039, China;
5 CAS Key Laboratory of Marine Ecology and Environmental Sciences, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China;
6 Laboratory for Marine Ecology and Environmental Science, Pilot National Laboratory for Marine Science and Technology(Qingdao), Qingdao 266237, China;
7 Shandong Yellow River Delta National Nature Reserve Administration Committee, Dongying 257091, China
In 2018, the global use of flame retardants reached about 3 million tons (Lai et al., 2019). Brominated flame retardants, especially polybrominated diphenyl ethers (PBDEs), have been widely used in the past few decades. However, due to their persistence, bioaccumulation potential, and toxicity, PBDEs were added to the list of persistent organic pollutants in a meeting held by the United Nations Environment Programme in 2009, and the use of PBDEs was gradually banned (Campone et al., 2010; Hu et al., 2014; Wang et al., 2019). In recent years, as a substitute for PBDEs, the production and usage of organophosphate esters (OPEs) have increased rapidly worldwide. The global use of OPEs increased from 209 000 tons in 2004 to 680 000 tons in 2015, and the global production of OPEs flame retardants grew with an annual rate of 5.2% from 2016 to 2021 (Ou, 2011; van der Veen and de Boer, 2012; Wang et al., 2017c, 2018; Sala et al., 2019). In China, the usage of OPEs increased from 70 000 tons in 2007 to 179 000 tons in 2013 (Li et al., 2018).
OPEs are widely used as flame retardants and plasticizers in the fields of plastics, electronics, furniture, textiles, construction, and transportation. Because OPEs are synthetic substances added to artificial materials through physical addition rather than chemical bonding, they can easily enter the environment through volatilization, leaching, and wearing (Marklund et al., 2003; Möller et al., 2012; Hu et al., 2014; Wang et al., 2017b; Yadav et al., 2018; Zeng et al., 2018a, 2020; Chen et al., 2019a; Pantelaki and Voutsa, 2019; Xing et al., 2020). Numerous studies have reported the occurrence of OPEs in surface water, groundwater, sewage, indoor dust, atmosphere, sediments, and organisms all over the world (Woudneh et al., 2015; Sühring et al., 2016; Giulivo et al., 2017; Guo et al., 2017; Wang et al., 2017a, 2018; Li et al., 2018, 2019a, b; Shi et al., 2018; Chen et al., 2019a; Ji et al., 2019; Liang et al., 2021).
Studies have shown that OPEs in the water environment can induce carcinogenicity, teratogenicity, neurotoxicity, and embryonic developmental toxicity, and have endocrine disrupting effects by direct exposure or dietary exposure (Dishaw et al., 2014; Wei et al., 2015; Wang et al., 2017a; Arukwe et al., 2018; Shi et al., 2018; Zhang et al., 2018; Liu et al., 2019a; Sala et al., 2019; Castro et al., 2020). Moreover, OPEs could come into the human body through atmospheric particulate matter inhalation, dietary intake, and skin contact. OPEs have been detected in human placenta, breast milk, and blood, which have been reported to suppress human hormones, affect male reproductive ability, reduce the concentration of serum thyroid hormone in males and affect adolescent glucose metabolism and pregnant women's pregnancy (Meeker and Stapleton, 2010; Kim et al., 2014; Ding et al., 2015; Chen et al., 2019b; Ya et al., 2019; Luo et al., 2020).
To date, the researches on OPEs in different countries have mainly been focused on natural environment such as water, atmosphere, and soil, however there was very little attention paid on aquacultural environment and aquatic product safety. According to statistics, since 2019, China's total aquatic output has reached 64.803 6 million tons, of which aquaculture products accounted for more than 79.8% (Huang et al., 2021). At present, China's farmed aquatic products account for more than 60% of the world's total aquatic product farming output. Marine aquaculture farms are the main source of people's consumption of seafood. Due to the detection frequency in the natural environments and the toxicity of OPEs to organisms and even human, the pollution of OPEs in the aquacultural environment and aquatic product, and the health risks caused by them cannot be ignored. In the study conducted in aquaculture farms in the Beibu Gulf in China, Zhang et al. (2020) found that OPEs concentrations were 21.3–138.0 ng/g dry weight (dw), with an average concentration of 55.5 ng/g dw. The concentration of OPEs in mussels from European farms ranged from 0.5 to 102.0 ng/g dw (Aznar-Alemany et al., 2018). Huanghe (Yellow) River delta area is one of the largest aquaculture centers in China, where farmed a wide variety of species (e.g., prawns, hairy crabs, swimming crabs) with an aquaculture output of more than 500 000 tons.
The delta received the discharge of treated and untreated domestic and industrial wastewater from the Huanghe River and its tributaries. As a "sink" of pollutants, new pollutants such as microplastics and polycyclic aromatic hydrocarbons (PAHs) have been detected in the water samples of the Huanghe River estuary, however, there are few reports on OPEs (Li et al., 2017; Han et al., 2020). However, the information about the pollution of OPEs in the Huanghe River delta is still lacking. As an emerging pollutant, studies of the distribution, main sources, and ecological health risks of OPEs in the aquatic environment in the Huanghe River delta and its adjacent waters are needed.
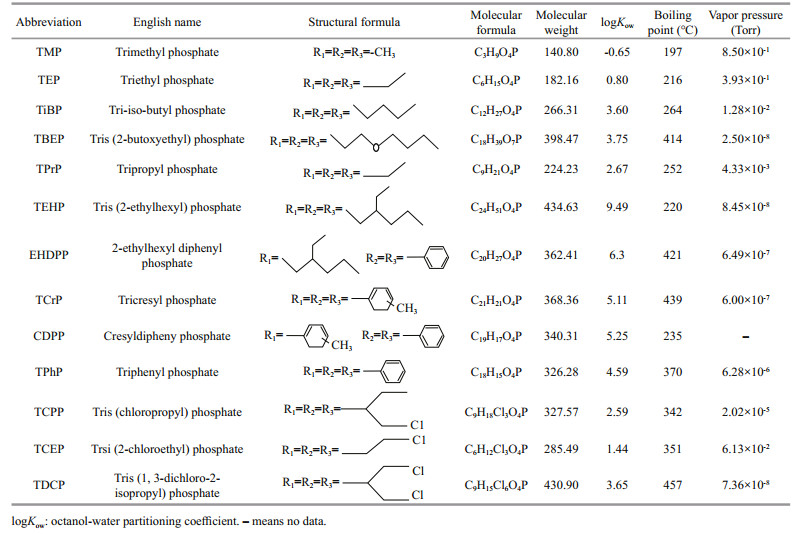
OPEs in the environment are mainly divided into alkyl OPEs, aryl OPEs and chlorinated OPEs. The use of chlorinated OPEs and alkyl OPEs in China are more extensive, especially tris (2-chloroethyl) phosphate (TCEP), tris (chloroisopropyl) phosphate (TCPP) and tris (1, 3-dichloro-2-isopropyl)phosphate (TDCP) in polyurethane foam, plastics, furniture, and other industries, tris (2-ethylhexyl) phosphate (TEHP), tris-iso-butyl phosphate (TiBP), tris (2-butoxyethyl) phosphate (TBEP) in rubber, lubricants, concrete, and other industries, which were also frequently detected in the water environment (Liao et al., 2020; Zhang et al., 2020; Pantelaki and Voutsa, 2021). Therefore, we selected these typical contaminants and some homogenous congers in this research, including three chlorinated OPEs, six alkyl OPEs, and four aryl OPEs (Table 1).
The purpose of this study was to clarify the pollution characteristics of 13 typical OPEs in water samples, sediments in the aquatic environment, and farmed organisms from the Huanghe River delta. We analyzed the distribution of OPEs in water, sediment, and organisms, evaluated the biological enrichment between sediments and organisms, and revealed the health risks of OPEs in seafood (i.e., hazard quotient, HQ). This study could provide basic information for the pollution feature of OPEs in the delta farms, as well as the health risk assessment by aquatic products consumption in the coastal aquaculture. The Huanghe River Basin plays a very important role in the economic and social development and ecological security of China, for which this research also could provide basic data for the management and prevention of the emerging pollutants.
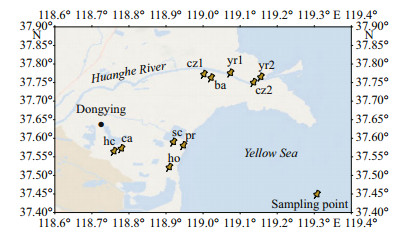
2 MATERIAL AND METHOD 2.1 Sample collectionIn September 2020, we selected five different types of aquaculture farms in Dongying City, Shandong Province, where aquaculture organisms (carp, prawn, hairy crab, swimming crab, and holothurian) and the corresponding five sediment samples and five surface water samples were collected. As a contrast, four surface water samples and five sediments samples in natural water bodies were also obtained (Fig. 1). Biological samples collected from aquaculture farms consisted of five carp, six prawns, four hairy crabs, four swimming crabs, and six holothurians, preserved in polyethylene bags. Sediment samples were collected using stainless steel grabs from the five aquaculture farms and five natural water sites, and stored in polyethylene bags at -20 ℃. Nine 1-L water samples were collected using stainless steel buckets from the five aquaculture farms and four natural water sites. All the samples were immediately transported back to the laboratory and stored at -20 ℃.
|
| Fig.1 Distribution of sampling sites cz1 (core zone1), cz2 (core zone2), ba (buffer area), yr1 (yellow river1), and yr2 (yellow river2) indicates the five sampling sites in natural waters; sc (swimming crab), hc (hairy crab), ho (holothurian), ca (carp), and pr (prawn) indicates the five sampling sites in aquaculture areas. |
In this study, we measured the concentrations of 13 types of OPEs: trimethyl phosphate (TMP), triethyl phosphate (TEP), tripropyl phosphate (TPrP), TCEP, TiBP, trisphenyl phosphate (TPhP), TCPP, cresyldiphenyl phosphate (CDPP), 2-ethylhexyl diphenyl phosphate (EHDPP), tricresyl phosphate (TCrP), TBEP, TDCP and TEHP. Standards (purity ≥98%) for TiBP, tributyl phosphate (TnBP)-d27 and TCPP-d18 were purchased from Toronto Research Chemicals (Toronto, Canada). TPrP-d21 was provided by Chiron AS (Trondheim, Norway). Isopropanol, ammonia, and acetonitrile (all pure) were purchased from Sinopharmaceutical Chemical Reagents Co., Ltd. (Shanghai, China). Formic acid was bought from Sigma-Aldrich Company (St. Louis, MO, USA).
2.3 Sample pretreatment and instrument analysisThe sample pretreatment and instrumental analysis methods used in this study were optimized based on previously established methods (Guo et al., 2017; Wang et al., 2019; Ding et al., 2020). The water samples were filtered through GF/F (0.22 μm) glass fiber membrane (Whatman International Ltd., Maidstone, England) and placed in brown glass bottles with an appropriate amount of deuterium internal standard added as the recovery standard. Before intubation, acetonitrile (6 mL) and ultrapure (6 mL) water were added successively to activate the solid phase extraction (SPE) columella. Then started the solid-phase extraction (6 cc, 500 mg, Oasis® HLB, Waters, MA, USA) of the OPEs of the water sample, controlled the speed at about one drop per second, drained the sample for 10 min after the sample was dripped, and then performed post-elution with 6 mL of 5% ammonia acetonitrile. The collected eluent was concentrated by blowing nitrogen via a gentle stream of nitrogen (Detelogy, MFV-24), then the volume was made up to 1 mL with acetonitrile. The sample was placed in a brown vial and stored at -20 ℃ until instrumental analysis.
The freeze-dried (48 h, -40 ℃) sediments were crushed with a pestle in a mortar and sifted through a 75-mesh (0.2 mm) steel sieve. Weighed 1 g of sediment, added an appropriate amount of deuterium internal standard, and mixed. A mixture of 10-mL isopropanol/acetone (V꞉V=1꞉1) was added and the solution was swirled (2 min). After ultrasonic extraction (30 min, 20 ℃) followed by centrifugation (10 min, 8 000 r/min), the supernatant was transferred to a brown glass bottle. These steps were repeated three times to obtain the three supernatants, which were pooled, and then ultra-pure water was added to reach a constant volume of 400 mL. Solid phase extraction was carried out, and the subsequent steps were the same as those used in the water samples. Weighed 1 g of the sediment sample after grinding and sieving, and measured the total organic carbon (TOC) content of the sediment with a TOC analyzer (TOC-L CPH).
In this study, all individuals of each organism were dissected and homogenized before pre-processing. The biological samples were defrosted and rinsed with ultrapure water. The muscles of the organisms were extracted using a clean scalpel, then freeze-dried and screened through a steel sieve. One gram biological sample were weighed, and appropriate amount of deuterium internal standard were added and mixed. The samples then were processed following the same steps as those used for the sediment samples. The prepared sample was transferred to a centrifuge tube, refrigerated for centrifugation (10 min, 2 400 r/min), and then the supernatant was transferred to a brown loading vial.
All samples were quantitatively analyzed by an ultraperformance liquid chromatography-electrospray ion source-tandem triple quadrupole mass spectrometer (Xevo T-QS, Waters, MA, USA) on an ACQUITY UPLC® BEH C18 column (Waters, MA, USA). The injection volume was 5 μL, the flow rate was 0.4 mL/min, the column temperature was 4 ℃, and the mobile phase consisted of 0.1% formic acid water (A) and acetonitrile (B). The gradient elution procedures were as follows: 0–1 min 2% B, 1.1–2.4 min 30% B, 2.5–6.4 min 55% B, 6.5–7.1 min 65% B, 7.2–8.9 min 98% B, and 9–10 min 2% B. Nitrogen was the desolvation and atomization gas, the desolvation temperature and the ion source temperature were 150 ℃ and 400 ℃, respectively, the collision gas was helium, and the capillary voltage was 0.5 kV.
2.4 Quality assurance and quality control (QA/QC)Strict quality control was adopted in the process of sample collection and laboratory processing and analysis. To avoid contamination, all glassware was soaked in acid barrels for more than 24 h, and washed for three times each with tap water, pure water, and ultrapure water, and dried after sealing with aluminum foil. The matrix blank and laboratory blank were set. The isotope internal standard was used to correct the loss and matrix effect in the pre-processing and detection process. The linear range of the internal standard quantitative method was 0.1–200 μg/L, and the standard curve of each substance was R2 > 0.99. The recoveries of the 13 types of OPES were 89.7%–107.9%. The limit of detection (LOD, S/N=3) of OPEs was 0.1–0.7 ng/L, and the limit of quantification (LOQ, S/N=10) of the method was 0.3–2.5 ng/L.
2.5 Water-sediment distribution coefficient, biological enrichment, and ecological health riskThe distribution of OPEs between water and sediment can be reflected by the distribution coefficient between water and sediment (Kd, L/kg) and the standardized distribution coefficient of organic carbon (Koc, L/kg). The calculation formulas are as follows:
 (1)
(1)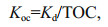 (2)
(2)where Csediment (ng/g dw) is the concentration of OPEs in sediments, Cwater (ng/L) is the concentration of OPEs in water, and TOC is the total organic carbon in sediments.
In addition, the bioaccumulation factor (BAF) of OPEs in each aquaculture pond and the biota-sediment accumulation factors (BSAFs) of OPEs in sediments can reflect the degree of bioaccumulation of OPEs. The calculation formulas are as follows:
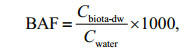 (3)
(3)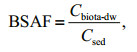 (4)
(4)where Cbiota-dw (ng/g dw) is based on the concentration of each OPEs in the dry weight of the biological sample, Cwater (ng/L) is the concentration of OPEs in water, and Csed (ng/g TOC) is the concentration of OPEs in the sediment after standardization by TOC.
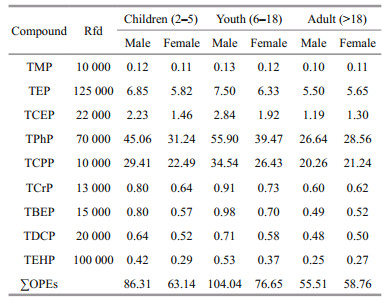
This study assessed the Hazard quotient (HQ) of individual OPEs in seafood by measuring the Daily Intake (Estimed Daily Intake (EDI) of carp, prawn, hairy crab, swimming crab, and holothurian), and the calculation formulas are Eqs.5 & 6, respectively:
 (5)
(5)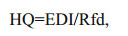 (6)
(6)where CBi (ng/g dw) is the concentration of OPEs in the biological sample, DC (g/day) is the daily intake of seafood, which refers to the Chinese dietary survey (Guo et al., 2010), BW is body weight, 65.6 kg and 56.5 kg for adult males and females (> 18 year-old); 41.7 kg and 39.0 kg for adolescent males and females (6–18-year-old); 16.8 kg and 16.0 kg for male and female children (2–5-year-old), respectively (Yang et al., 2005). Rfd (reference dose) was based on previous studies (Ali et al., 2012; Ding et al., 2015, 2018; Liu et al., 2019b). According to the standards of the United States Environmental Protection Agency (USEPA), when HQ≥1, the health risk of OPEs is considered high; an HQ between 0.1 and 1 indicates medium risk; a value between 0.01 and 0.1 means lower risk; HQ < 0.01 indicates that there is no health risk. We also calculated the hazard index (HI) of all OPEs by summing the HQ of each OPEs:
 (7)
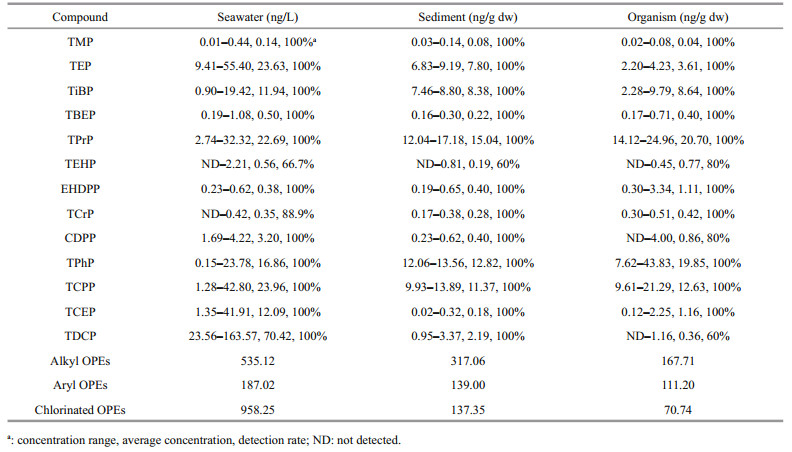
(7)The OPEs detected in surface water, sediments, and biological samples included six alkyl OPEs (TMP, TEP, TiBP, TBEP, TEHP, TPrP), four aryl OPEs (EHDPP, TCrP, TPhP, CDPP), and three chlorinated OPEs (TCEP, TCPP, TDCP). Except for TCrP, TEHP, CDPP, and TDCP, the detection rates of other OPEs were 100% (Table 2).
|
The ∑13OPEs in water samples ranged from 51.53 to 272.17 ng/L, with an average concentration of 186.71 ng/L. Except for TEHP (66.7%) and TCrP (88.9%), all the other OPEs were 100% detectable. In both aquaculture and natural water samples, the concentration of chlorinated OPEs was the highest (57.03%), followed by alkyl OPEs (31.85%). In detail, TDCP, TCPP, and TEP were the main contributor of OPEs, which accounted for 37.72%, 12.83%, 12.65%, respectively (Fig. 2a).
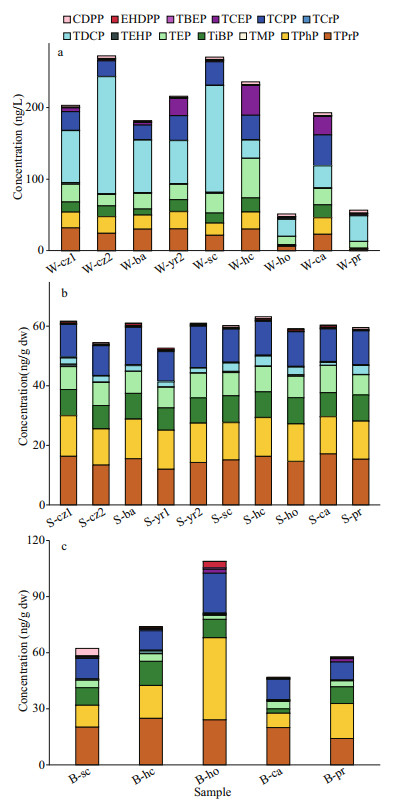
|
| Fig.2 The concentration and composition of individual OPEs in samples a, b, and c represented water (W), sediment (S), and organism (B) samples, respectively; cz1, cz2, ba, yr1, and yr2 were samples from natural waters; sc, hc, ho, ca, and pr were samples from aquaculture water; sc, hc, ho, ca, and pr represent the swimming crab, hairy crab, holothurian, carp, and prawn water samples, respectively. |
The concentration of ∑13OPEs in natural water ranged from 181.79 to 272.17 ng/L (average: 218.22 ng/L); for the aquaculture water, ∑13OPEs were detected in the range of 51.53–270.44 ng/L (average: 161.50 ng/L). There was no significant difference in the concentration of OPEs between the two water bodies (P > 0.05). There were significant differences in the different aquacultural ponds (P < 0.05). The concentrations of OPEs in the swimming crab and hairy crab ponds were similar, followed by that in carp ponds, but the concentration of OPEs in holothurian and prawn ponds was relatively lower.
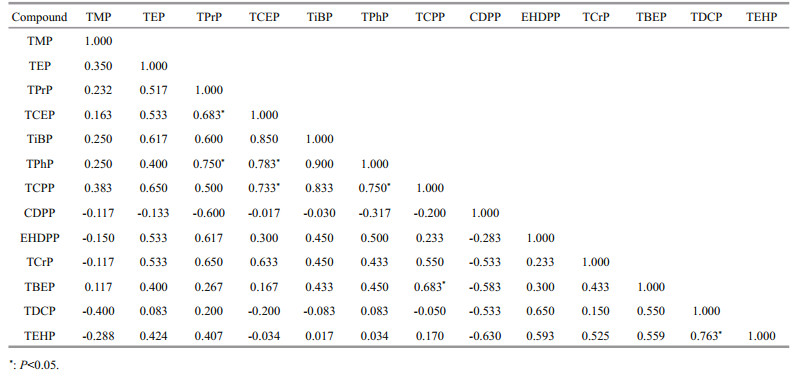
The PCA analysis show that there were two main components affecting the OPEs distribution in the water, together explaining 68.4% of the variance, with PC1 representing 47.3%, PC2 representing 21.1% (Fig. 3). W-yr2, W-ca, and W-hc had higher scores on PC1, and W-cz2, W-cz, W-sc, W-ba showed similar higher scores on PC2. Similar higher loadings of TPhP, TPrP, TCPP occurred in PC1, and TDCP, TEHP occurred in PC2. Significant positive correlation appeared between TPrP and TPhP, TCEP and TPhP, TCEP and TCPP, TPhP and TCPP, and TDCP and TEHP (Table 3), implying that these OPEs congeners might origin from the similar source and release behaviors, which was nearly consistent with the results of PCA analysis (Liu et al., 2018). By PCA analysis, the load value of PC1 was high in the water samples from the natural water bodies and the swimming crab, prawn, and holothurian culture waters, indicating that the comprehensive indicators of the water samples represented by PC1 had similar OPEs concentration characteristics. However, the water samples from the hairy crab and carp ponds had high loading values in PC2, which depicted that the comprehensive indicators of water samples in these culture areas had similar OPEs characteristics.
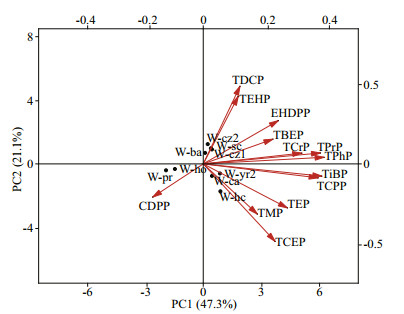
|
| Fig.3 Principal component analysis (PCA) of water samples W-cz1, W-cz2, W-ba, and W-yr2 represent water samples from natural waters; W-sc, W-hc, W-ho, W-ca, and W-pr represent swimming crab, hairy crab, holothurian, carp, and prawn culture water samples, respectively. |
The total concentration of OPEs in sediments ranged from 52.63 to 63.17 ng/g dw, with an average concentration of 59.34 ng/g dw. The detection of OPEs was 100% except for TEHP (60%). In contrast with the OPEs profiles in water, the concentration of alkyl OPEs was the highest (53.43%), with aryl OPEs and chlorinated OPEs followed (23.42% and 23.15%). TPrP was the most abundant OPEs (25.34%), followed by TPhP (21.60%) and TCPP (19.15%) (Fig. 2b). There was no significant difference between the concentration OPEs in aquaculture and natural areas (P > 0.05). In addition, there was no difference in the concentration of OPEs in the sediments of the five different farms (P > 0.05).
3.1.3 OrganismThe total concentration of the 13 types of OPEs in the organisms from the five farms ranged from 46.82 to 108.90 ng/g dw, with an average of 69.93 ng/g dw (Fig. 2c). For the three types of OPEs, the concentration of alkyl OPEs was the highest (47.97%), followed by that of aryl OPEs (31.80%) and chlorinated OPEs (20.23%). The highest concentrations in the farmed organisms were found in TPrP (29.60%), followed by TPhP (28.39%) and TCPP (18.06%). There was no diff erence in the concentration of OPEs among the fi ve farm organisms (P > 0.05). Among them, the concentration of OPEs in holothurians organisms was the highest, followed by hairy crabs, swimming crabs, and prawns, and carp had the lowest concentrations of OPEs.
3.2 Distribution and accumulation of OPEs in water, sediments, and organismsIn this study, the logKd of chlorinated OPEs (TCPP, TCEP, and TDCP) was lower than that of aryl OPEs and alkyl OPEs, thus the water solubility of chlorinated OPEs was higher. The distribution of OPEs in the water phase was consistent with previous researches (Cristale et al., 2013; Sühring et al., 2016; Ma et al., 2017). In addition, chlorinated OPEs dominated the water samples (57%) compared with sediments (23%), and the logKow of chlorinated OPEs was lower, which also proved that the distribution of OPEs between water and sediments was aff ected by logKow.
To evaluate the bioenrichment capacity of OPEs in water, we calculated the logBAF of the 13 OPEs in the culture species (Fig. 4a). Among the 13 OPEs, the logBAF of TPrP, TiBP, TPhP, TCPP, and EHDPP were greater than 3.7, indicating the "bioaccumulative eff ect" in the organism, and the logBAF of TBEP was between 3.3 and 3.7, indicating the "potential bioaccumulative eff ect". Among the fi ve organisms, the logBAF of prawns and holothurians was greater than 3.7, the logBAF of hairy crabs was between 3.3 and 3.7, and the order of bioaccumulation capacity is: prawns >holothurians >hairy crabs >swimming crabs >carp. The study found that the logBAF of alkyl OPEs and aryl OPEs was higher than that of chlorinated OPEs. TPhP had the strongest bioaccumulation ability, followed by EHDPP and TPrP. We also calculated the BSAF of OPEs in the sediments (Fig. 5). For OPEs, the diff erences in the average logBSAF values of the alkyl OPEs, aryl OPEs, and chlorinated OPEs were not signifi cant. TDCP had the lowest logBSAF value, followed by TEP and TMP. In addition, for the fi ve organisms, the logBSAF of invertebrates (prawns, hairy crabs, swimming crabs, and holothurians) was higher than that of vertebrates, and the logBSAF of holothurians was the highest.

|
| Fig.4 Bioaccumulation factor (BAF) for individual OPEs in five aquaculture species (a) and logBAF of ∑13OPEs (b) in the aquaculture area of the Huanghe River delta |
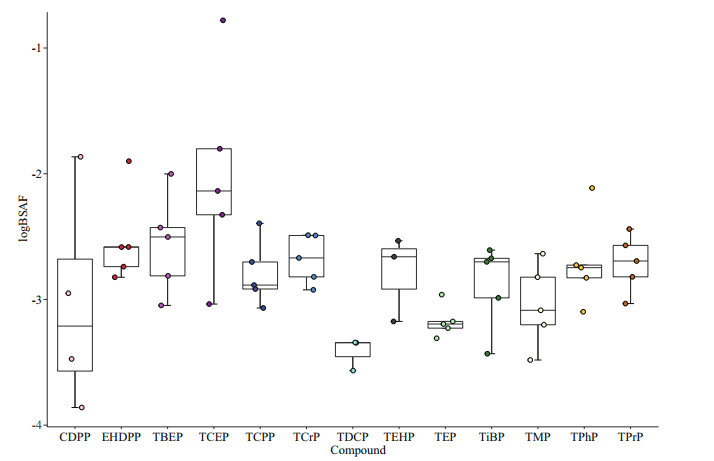
|
| Fig.5 Biota-sediment accumulation factor (BSAF) for OPEs in five aquaculture species in the aquaculture area of the Huanghe River delta |
The EDI and HQ of three age groups (2–5-year-old, 6–18-year-old, and>18-year-old) of potential male and female consumers of the fi ve type aquaculture species were calculated. The Rfd values for TPrP, EHDPP, CDPP, and TiBP were not available, so which were not included in the calculation. Table 4 shows the EDI of the nine OPEs in the three age groups. Among the fi ve species of organisms, holothurians showed the highest EDI, followed by carp and prawns. Hairy crabs and swimming crabs showed the lowest values. Among the nine detected OPEs, the EDI values of TPhP, TCPP, and TEP were highest. Among the three age groups, the adolescent group had the highest EDI, followed by children and adults. Between the sexes, males in the children group (2–5-year-old) and adolescents (6–18-year-old) were higher than females, and in the adult group (>18-yearold), females and males had no signifi cant diff erence.
|
As Fig. 6 shown, TCPP exhibited the highest HQ value, followed by TPhP. Among the three types of OPEs, chlorinated OPEs had the highest HQ, followed by aryl OPEs and alkyl OPEs. Thus, the HQ of chlorinated OPEs was higher than that of non-chlorinated OPEs, which was also reported in the Beibu Gulf (Zhang et al., 2020). Among the fi ve organisms, holothurians had the highest HQ, followed by carp, prawns, hairy crabs, and swimming crabs. Among the three age groups of consumers, the HQ of adolescents was highest, followed by children and adults. There was no significant difference in HQ between men and women. The HQ values of the nine types of OPEs in different organisms and different consumer age groups were all < 0.1, which meant that the OPEs were unlikely to cause health hazards. The HI (∑9HQ) of the nine OPEs was 0.020 4, with a range of 2.14×10-5 to 0.015, which was lower than the safety threshold of 1.
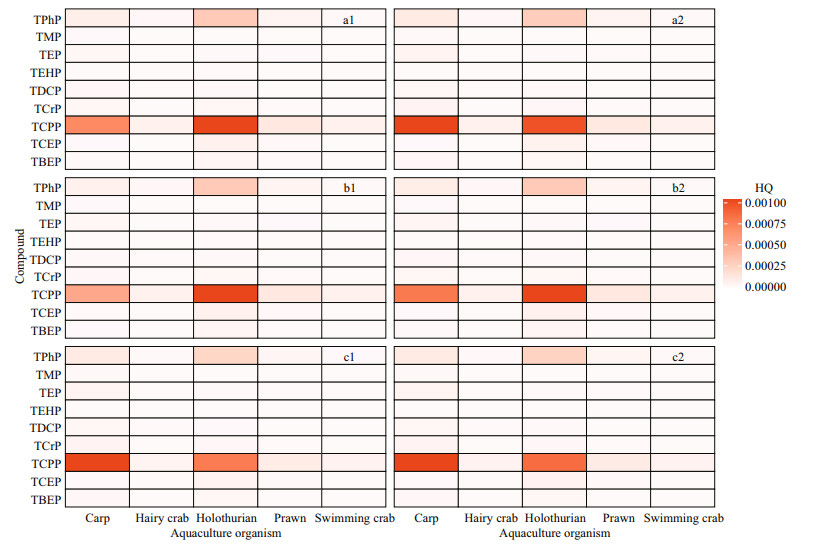
|
| Fig.6 HQ of nine OPEs in three consumer age groups and five organism types A1 and A2 represent male and female children (aged 2-5 years), respectively; B1 and B2 represent young (6-18-year-old) males and females, respectively; and C1 and C2 represent adult (> 18-year-old) men and women, respectively. |
In the past decades, chlorinated OPEs have occupied the major position in the market, and they have the characteristics of long-distance transportation, great resistance to biodegradation, and poor light-induced degradation, which may be one of the reasons why the concentration of chlorinated OPEs in water samples was higher than that of non-chlorinated OPEs (Regnery and Püttmann, 2010; Cao et al., 2017; Greaves and Letcher, 2017; Zhang et al., 2020). In addition, the concentration of OPEs in natural waters in this study was significantly lower than that of Lai et al. (2019) (253–1 720 ng/L) in the Huanghe River estuary, possibly because the location where they collected samples was relatively close to the surrounding manufacturing and production industries, but higher than the Bohai Sea (∑7OPEs was 8.12–98.04 ng/L, the average concentration was 33.42 ng/L), Laizhou Bay (0.2–28.4 ng/L, the average concentration was 5.3 ng/L), and the South China Sea (23.7–70.9 ng/L, the average concentration was 50.9 ng/L) (Zhong et al., 2017; Bekele et al., 2019; Ding et al., 2020).
In this study, the concentration of OPEs in the aquaculture water was higher than that of the Beibu Gulf farms (32.9–227 ng/L, with an average concentration of 122 ng/L) (Zhang et al., 2020), but lower than that of the European farms (0.43–867 ng/L) (Aznar-Alemany et al., 2018) reported in previous studies. In the Beibu Gulf, the concentration of OPEs in the prawn farm was also lower than that of the crab and fish (tilapia) farms (Zhang et al., 2020), which was consistent with the results of this study. These differences were likely due to the different cultivation practices and organism life habits. Although all five species evaluated in this study were cultured in artificial ponds, the prawn and holothurians were more sensitive to the cultivation environment. The water changing cycle was shorter for those two species, which may reduce the concentration of OPEs in their aquaculture water to a certain extent. In this study, TDCP, as the main pollutant in the water sample, had a significantly higher concentration than other OPEs. TDCP was mainly used as a chemical raw material and in mining transport belts, cables, electronic materials, pharmaceutical intermediates, and leather. The main industries in Dongying were petrochemical and fine chemical industries, with an annual output of tens of millions of tons (Yin and Li, 2021). Therefore, the petrochemical industry may be the main source of OPEs in water samples.
The concentration of OPEs in sediments collected by Wang et al. (2017c) nearby the Huanghe River estuary was similar to that in this study (range 11.8–102 ng/g dw, average: 36.3±23.9 ng/g dw). However, the values in sediments collected from aquaculture farms nearby the Huanghe River estuary (6.65–41.5 ng/g dw, average: 16.5±9.24 ng/g dw), the Bohai Sea (83–4 552 pg/g dw, average: 1 137 pg/g dw), southern coast of South Korea were lower than that in this study, while the OPEs in the sediments of the southern coast of South Korea were lower than this study (Zhong et al., 2018; Choi et al., 2020). The concentration of OPEs in sediments from the five ponds in this study was higher than that reported for ponds in the Beibu Gulf (22.1–35 ng/g dw, average: 10.9 ng/g dw) and comparable to that from mussel farms in Portugal (58.2 ng/g dw) (Gadelha et al., 2019; Zhang et al., 2020). In addition, by comparison, chlorinated OPEs were dominant in the sediments of Beibu Gulf farms, aryl OPEs were dominant in the sediments of European aquaculture products and Portuguese mussel farms, while the alkyl OPEs in the sediments of the five ponds in this study accounted for the main position, which may be related to the source of pollution in the region and the characteristics of OPEs in the area (Aznar-Alemany et al., 2018; Gadelha et al., 2019; Zhang et al., 2020).
The concentrations of OPEs in the farmed organisms in this research were higher than those in prawn, crabs, and oysters from Beibu Gulf farms (21.3–138 ng/g dw, average: 55.5 ng/g dw) and in mussels from European farms (0.50–102 ng/g dw) (Zhang et al., 2020). However, the concentration of OPEs in the shellfish was same as that of the bivalve molluscs in the coast of South Korea (6.12–206 ng/g dw, with an average of 41.5 ng/g dw), and lower than that in the South China Sea (0.50–102 ng/g dw) (Aznar-Alemany et al., 2018; Choi et al., 2020; Ding et al., 2020). Similar to the distribution of OPEs in sediments, the concentration of alkyl OPEs in organisms was higher than that of aryl OPEs and chlorinated OPEs. Compared with chlorinated OPEs, alkyl and aryl OPEs showed stronger hydrolytic stability and accumulation in organisms. The higher accumulation in organisms may also be associated to the relatively greater ability to excrete chlorinated OPEs (Wang et al., 2017a; Shi et al., 2018). In an aerobic environment, the degradation rate of alkyl OPEs and aryl OPEs under the action of microorganisms would increase, so their concentration in water samples was generally not high (Liu et al., 2016).
The difference in the concentration of OPEs may be due to differences in the physical and chemical properties of OPEs (such as hydrophobicity and lipophilicity), bioaccumulation characteristics, degradability, toxicity, and the degree of production and use. TEP, TPrP, and TCEP have low logKow, high hydrophilicity, and water solubility. In this study, the concentration of the above-mentioned OPEs in water was higher than that in sediments (Liu et al., 2016; Li et al., 2021). Because TCEP has significant toxicity and persistence in the environment, TCEP was gradually replaced by its homologue TCPP (Liu et al., 2016; Zeng et al., 2018b). In this study, the TCPP concentration was significantly higher than TCEP, which should also be applicable. In aquaculture water bodies, except for prawns and holothurian, the concentration of OPEs in the water in several other biological aquaculture ponds was greater than that in the sediments. This may be caused by different factors such as bait, drugs, and their own excretion capacity in the breeding process of prawns and holothurians. Different hydrological conditions, human activities, maritime transportation, physical properties of OPEs, and biological differences may affect the concentration of OPEs. Therefore, we need to integrate various factors to comprehensively evaluate the environmental behavior of OPEs.
4.2 Distribution and accumulation of OPEsAccording to the standards of the European Chemicals Agency, a logBAF value > 3.7 means that the organic matter has a "bioaccumulative effect" in the organism and a value between 3.3 and 3.7 indicates a "potential bioaccumulative effect" (Zhang et al., 2020). Our results show that prawns and holothurians experienced a bioaccumulation effect and hairy crabs experienced a potential bioaccumulation effect, which was consistent with the results reported by Zhang et al. (2020). For the 13 OPEs, EHDPP and TPrP showed a potential bioaccumulative effect, whereas, TCPP, TiBP, and TPhP showed a bioaccumulative effect (Fig. 4b).
The study found that the logKow of the three OPEs with bioaccumulative capacity was not all low (TCPP logKow is 2.59, TiBP logKow is 3.60, TPhP logKow is 4.59), but EHDPP with potential bioaccumulation effect logKow (6.30) was still relatively high. In addition, except for EHDPP, the above-mentioned OPEs had higher concentrations in non-biological media (water and sediment). By comparison, we found that the logBAF and logKow in the Beibu Bay farms and Taihu Lake showed a significant positive correlation, and the positive correlation between logBAF and logKow in this study was not significant (P > 0.05) (Wang et al., 2019; Zhang et al., 2020). Therefore, the explanation is that the accumulation ability of OPEs may differ among different organisms. Studies had shown that the cumulative effect was not only related to the living environment and habitual feeding rate, nutrient level, metabolic capacity, and purification capacity of organisms, but may also be affected by various factors such as photolysis and microbial degradation in the environment (Pantelaki and Voutsa, 2020; Li et al., 2021). The cumulative effect was not only related to the living environment and habits of organisms, but also may be jointly affected by various factors such as photolysis and microbial degradation in the environment. Therefore, the accumulation capacity of OPEs in organisms was not only affected by the physical and chemical properties of OPEs and non-biological media, but also by biological factors.
The study found that logBSAF increased with the logKow of OPEs between -0.65 and 1.44, decreased from 1.44 to 3.75, then increased between 3.75 and 6.3, and decreased between 6.3 and 9.49. In the study of Wang et al. (2019), logBSAF increased with logKow between 3.75 and 5.73, and then decreased between 5.73 and 9.49, which was consistent with this study. The accumulation of organic compounds in the sediment was attributed to the combined effects of the desorption of the organic matter, the absorption from the pore water and the absorption from the ingested sediment of the combined organic pollutants (Sun et al., 2017). Among the five organisms, the logBSAF of benthic invertebrates was higher than that of fish, and the bioavailability of benthic invertebrates in sediments of OPEs was lower. Therefore, invertebrates could be more suitable for evaluating the bioavailability of OPEs in sediments, which was consistent with the results of Wang et al. (2019).
4.3 Health risk assessmentOPEs mainly pass through air, drinking water, animals, and plants contaminated by OPEs, and food contaminated during processing, packaging, and storage (Poma et al., 2018). Studies have shown that OPEs dietary health risk assessment is necessary (Zhang et al., 2016; Poma et al., 2017; Ding et al., 2018). Different exposure pathways have different effects on individuals. The EDI results of the three age groups in this study showed that the EDI of children and adolescents was higher than that of adults, which was consistent with the results of Gbadamosi et al. (2021). The study of Shi et al. (2020) found that TPhP was one of the main risks in Zhujiang estuary, which was consistent with the results of this study. Compared with other OPEs, TPhP has higher toxicity and health risk. This study found that TPhP had higher concentration in organisms, so which need to be given continuous attention in future (Cristale et al., 2016; Shi et al., 2020). In addition, HI in this study(5.53×10-4–1.06×10-2) was higher than that in Beibu Gulf farms (2.1×10-4–5.3×10-4) and South China Sea tropical oceans (3.88×10-4–7.18×10-4), but all were still below 1 (Ding et al., 2020; Zhang et al., 2020). Although the HQ and HI of 9 OPEs were all lower than the safety threshold of 1, the use of OPEs has appeared an increasing trend year by year. We cannot ignore the fact that OPEs will continue to accumulate in the environment, organisms, and humans. Moreover, we still need to continue to pay close attention to OPEs and assess their acute and chronic health risks in order to avoid their harm to human health.
5 CONCLUSIONOPEs were detected in water samples, sediments, and organisms from Huanghe River delta aquaculture farms and the adjacent natural areas. Among the 13 OPEs, TDCP was the main contaminant in water samples, while TPrP was the main contributor in sediments and organisms. In addition, TPhP and TCPP showed higher bioaccumulation levels in prawns and holothurians. Based on the dietary exposure health risk assessment, we found that these 13 OPEs were of low health risk to all three age groups of human consumers (2–5-year-old, 6–18-year-old, and > 18-year-old). Due to the characteristics of bioaccumulation, persistence, toxicity, and comprehensive usage, the potential risks of OPEs to humans cannot be ignored.
6 DATA AVAILABILITY STATEMENTThe datasets supporting the conclusions of this article are included within the article.
Ali N, Dirtu A C, Van den Eede N, et al. 2012. Occurrence of alternative flame retardants in indoor dust from New Zealand: indoor sources and human exposure assessment. Chemosphere, 88(11): 1276-1282.
DOI:10.1016/j.chemosphere.2012.03.100 |
Arukwe A, Carteny C C, Eggen T, et al. 2018. Novel aspects of uptake patterns, metabolite formation and toxicological responses in Salmon exposed to the organophosphate esters—Tris(2-butoxyethyl)- and tris(2-chloroethyl) phosphate. Aquatic Toxicology, 196: 146-153.
DOI:10.1016/j.aquatox.2018.01.014 |
Aznar-Alemany ò, Aminot Y, Vilà-Cano J, et al. 2018. Halogenated and organophosphorus flame retardants in European aquaculture samples. Science of the Total Environment, 612: 492-500.
DOI:10.1016/j.scitotenv.2017.08.199 |
Bekele T G, Zhao H X, Wang Q Z, et al. 2019. Bioaccumulation and trophic transfer of emerging organophosphate flame retardants in the marine food webs of laizhou bay, North China. Environmental Science & Technology, 53(22): 13417-13426.
DOI:10.1021/acs.est.9b03687 |
Campone L, Piccinelli A L, Östman C, et al. 2010. Determination of organophosphorous flame retardants in fish tissues by matrix solid-phase dispersion and gas chromatography. Analytical and Bioanalytical Chemistry, 397(2): 799-806.
DOI:10.1007/s00216-010-3548-4 |
Cao D D, Guo J H, Wang Y W, et al. 2017. Organophosphate esters in sediment of the great lakes. Environmental Science & Technology, 51(3): 1441-1449.
DOI:10.1021/acs.est.6b05484 |
Castro Ó, Pocurull E, Borrull F. 2020. Determination of organophosphate ester flame retardants and plasticisers in fish samples by QuEChERs followed by gas chromatography-tandem mass spectrometry. Exposure and risk assessment through fish consumption. Journal of Chromatography A, 1626: 461356.
DOI:10.1016/j.chroma.2020.461356 |
Chen Y Q, Zhang Q, Luo T W, et al. 2019a. Occurrence, distribution and health risk assessment of organophosphate esters in outdoor dust in Nanjing, China: urban vs. rural areas. Chemosphere, 231: 41-50.
DOI:10.1016/j.chemosphere.2019.05.135 |
Chen Y, Jiang L, Lu S Y, et al. 2019b. Organophosphate ester and phthalate ester metabolites in urine from primiparas in Shenzhen, China: implications for health risks. Environmental Pollution, 247: 944-952.
DOI:10.1016/j.envpol.2019.01.107 |
Choi W, Lee S, Lee H K, et al. 2020. Organophosphate flame retardants and plasticizers in sediment and bivalves along the Korean coast: occurrence, geographical distribution, and a potential for bioaccumulation. Marine Pollution Bulletin, 156: 111275.
DOI:10.1016/j.marpolbul.2020.111275 |
Cristale J, Katsoyiannis A, Chen C E, et al. 2013. Assessment of flame retardants in river water using a ceramic dosimeter passive sampler. Environmental Pollution, 172: 163-169.
DOI:10.1016/j.envpol.2012.08.014 |
Cristale J, Ramos D D, Dantas R F, et al. 2016. Can activated sludge treatments and advanced oxidation processes remove organophosphorus flame retardants?. Environmental Research, 144: 11-18.
DOI:10.1016/j.envres.2015.10.008 |
Ding J J, Deng T Q, Xu M M, et al. 2018. Residuals of organophosphate esters in foodstuffs and implication for human exposure. Environmental Pollution, 233: 986-991.
DOI:10.1016/j.envpol.2017.09.092 |
Ding J J, Shen X L, Liu W P, et al. 2015. Occurrence and risk assessment of organophosphate esters in drinking water from Eastern China. Science of the Total Environment, 538: 959-965.
DOI:10.1016/j.scitotenv.2015.08.101 |
Ding Y, Han M W, Wu Z Q, et al. 2020. Bioaccumulation and trophic transfer of organophosphate esters in tropical marine food web, South China Sea. Environment International, 143: 105919.
DOI:10.1016/j.envint.2020.105919 |
Dishaw L V, Hunter D L, Padnos B, et al. 2014. Developmental exposure to organophosphate flame retardants elicits overt toxicity and alters behavior in early life stage zebrafish (Danio rerio). Toxicological Sciences, 142(2): 445-454.
DOI:10.1093/toxsci/kfu194 |
Gadelha J R, Rocha A C, Camacho C, et al. 2019. Persistent and emerging pollutants assessment on aquaculture oysters (Crassostrea gigas) from NW Portuguese coast (Ria De Aveiro). Science of the Total Environment, 666: 731-742.
DOI:10.1016/j.scitotenv.2019.02.280 |
Gbadamosi M R, Abdallah M A E, Harrad S. 2021. A critical review of human exposure to organophosphate esters with a focus on dietary intake. Science of the Total Environment, 771: 144752.
DOI:10.1016/j.scitotenv.2020.144752 |
Giulivo M, Capri E, Kalogianni E, et al. 2017. Occurrence of halogenated and organophosphate flame retardants in sediment and fish samples from three European river basins. Science of the Total Environment, 586: 782-791.
DOI:10.1016/j.scitotenv.2017.02.056 |
Greaves A K, Letcher R J. 2017. A review of organophosphate esters in the environment from biological effects to distribution and fate. Bulletin of Environmental Contamination and Toxicology, 98(1): 2-7.
DOI:10.1007/s00128-016-1898-0 |
Guo J H, Venier M, Salamova A, et al. 2017. Bioaccumulation of dechloranes, organophosphate esters, and other flame retardants in Great Lakes fish. Science of the Total Environment, 583: 1-9.
DOI:10.1016/j.scitotenv.2016.11.063 |
Guo J Y, Wu F C, Shen R L, et al. 2010. Dietary intake and potential health risk of DDTs and PBDEs via seafood consumption in South China. Ecotoxicology and Environmental Safety, 73(7): 1812-1819.
DOI:10.1016/j.ecoenv.2010.08.009 |
Han M, Niu X R, Tang M, et al. 2020. Distribution of microplastics in surface water of the lower Yellow River near estuary. Science of the Total Environment, 707: 135601.
DOI:10.1016/j.scitotenv.2019.135601 |
Hu M Y, Li J, Zhang B B, et al. 2014. Regional distribution of halogenated organophosphate flame retardants in seawater samples from three coastal cities in China. Marine Pollution Bulletin, 86(1-2): 569-574.
DOI:10.1016/j.marpolbul.2014.06.009 |
Huang Y, Mu X Y, Li Y R. 2021. A framework building of aquaculture pollution total load control in China. Chinese Fishery Quality and Standards, 11(2): 63-70.
(in Chinese with English abstract) DOI:10.3969/j.issn.2095-1833.2021.02.009 |
Ji Y, Wang Y, Yao Y M, et al. 2019. Occurrence of organophosphate flame retardants in farmland soils from Northern China: primary source analysis and risk assessment. Environmental Pollution, 247: 832-838.
DOI:10.1016/j.envpol.2019.01.036 |
Kim J W, Isobe T, Muto M, et al. 2014. Organophosphorus flame retardants (PFRs) in human breast milk from several Asian countries. Chemosphere, 116: 91-97.
DOI:10.1016/j.chemosphere.2014.02.033 |
Lai N L S, Kwok K Y, Wang X H, et al. 2019. Assessment of organophosphorus flame retardants and plasticizers in aquatic environments of China (Pearl River Delta, South China Sea, Yellow River Estuary) and Japan (Tokyo Bay). Journal of Hazardous Materials, 371: 288-294.
DOI:10.1016/j.jhazmat.2019.03.029 |
Li J F, He J H, Li Y N, et al. 2019a. Assessing the threats of organophosphate esters (flame retardants and plasticizers) to drinking water safety based on USEPA oral reference dose (RfD) and oral cancer slope factor (SFO). Water Research, 154: 84-93.
DOI:10.1016/j.watres.2019.01.035 |
Li J, Li F D, Liu Q. 2017. PAHs behavior in surface water and groundwater of the Yellow River estuary: evidence from isotopes and hydrochemistry. Chemosphere, 178: 143-153.
DOI:10.1016/j.chemosphere.2017.03.052 |
Li J, Tang J H, Mi W Y, et al. 2018. Spatial distribution and seasonal variation of organophosphate esters in air above the Bohai and Yellow Seas, China. Environmental Science & Technology, 52(1): 89-97.
DOI:10.1021/acs.est.7b03807 |
Li J, Wang J, Taylor A R, et al. 2019b. Inference of organophosphate ester emission history from marine sediment cores impacted by wastewater effluents. Environmental Science & Technology, 53(15): 8767-8775.
DOI:10.1021/acs.est.9b01713 |
Li W W, Zhang Z M, Zhang R R, et al. 2021. Spatiotemporal occurrence, sources and risk assessment of polycyclic aromatic hydrocarbons in a typical mariculture ecosystem. Water Research, 204: 117632.
DOI:10.1016/j.watres.2021.117632 |
Liang C, Peng B, Wei G L, et al. 2021. Organophosphate diesters in urban river sediment from South China: call for more research on their occurrence and fate in field environment. ACS ES&T Water, 1(4): 871-880.
DOI:10.1021/acsestwater.0c00217 |
Liao C Y, Kim U J, Kannan K. 2020. Occurrence and distribution of organophosphate esters in sediment from northern Chinese coastal waters. Science of the Total Environment, 704: 135328.
DOI:10.1016/j.scitotenv.2019.135328 |
Liu J, He L X, Zeng X Y, et al. 2016. Occurrence and distribution of organophosphorus flame retardants/plasticizer in surface sediments from the Pearl River and Dongjiang River. Asian Journal of Ecotoxicology, 11(2): 436-443.
(in Chinese with English abstract) DOI:10.7524/AJE.1673-5897.20150915001 |
Liu X S, Cai Y, Wang Y, et al. 2019a. Effects of tris(1, 3-dichloro-2-propyl) phosphate (TDCPP) and triphenyl phosphate (TPP) on sex-dependent alterations of thyroid hormones in adult zebrafish. Ecotoxicology and Environmental Safety, 170: 25-32.
DOI:10.1016/j.ecoenv.2018.11.058 |
Liu Y E, Luo X J, Huang L Q, et al. 2019b. Organophosphorus flame retardants in fish from rivers in the Pearl River Delta, South China. Science of the Total Environment, 663: 125-132.
DOI:10.1016/j.scitotenv.2019.01.344 |
Liu Y H, Song N H, Guo R X, et al. 2018. Occurrence and partitioning behavior of organophosphate esters in surface water and sediment of a shallow Chinese freshwater lake (Taihu Lake): implication for eco-toxicity risk. Chemosphere, 202: 255-263.
DOI:10.1016/j.chemosphere.2018.03.108 |
Luo K, Aimuzi R, Wang Y Q, et al. 2020. Urinaryorganophosphate esters metabolites, glucose homeostasis and prediabetes in adolescents. Environmental Pollution, 267: 115607.
DOI:10.1016/j.envpol.2020.115607 |
Ma Y X, Xie Z Y, Lohmann R, et al. 2017. Organophosphate ester flame retardants and plasticizers in ocean sediments from the North Pacific to the Arctic Ocean. Environmental Science & Technology, 51(7): 3809-3815.
DOI:10.1021/acs.est.7b00755 |
Marklund A, Andersson B, Haglund P. 2003. Screening of organophosphorus compounds and their distribution in various indoor environments. Chemosphere, 53(9): 1137-1146.
DOI:10.1016/S0045-6535(03)00666-0 |
Meeker J D, Stapleton H M. 2010. House dust concentrations of organophosphate flame retardants in relation to hormone levels and semen quality parameters. Environmental Health Perspectives, 118(3): 318-323.
DOI:10.1289/ehp.0901332 |
Möller A, Sturm R, Xie Z Y, et al. 2012. Organophosphorus flame retardants and plasticizers in airborne particles over the Northern Pacific and Indian Ocean toward the polar regions: evidence for global occurrence. Environmental Science & Technology, 46(6): 3127-3134.
DOI:10.1021/es204272v |
Ou Y X. 2011. Developments of organic phosphorus flame retardant industry in China. Chemical Industry and Engineering Progress, 30(1): 210-215.
(in Chinese with English abstract) DOI:10.16085/j.issn.1000-6613.2011.01.033 |
Pantelaki I, Voutsa D. 2019. Organophosphate flame retardants (OPFRs): a review on analytical methods and occurrence in wastewater and aquatic environment. Science of the Total Environment, 649: 247-263.
DOI:10.1016/j.scitotenv.2018.08.286 |
Pantelaki I, Voutsa D. 2020. Occurrence, analysis and risk assessment of organophosphate esters (OPEs) in biota: a review. Marine Pollution Bulletin, 160: 111547.
DOI:10.1016/j.marpolbul.2020.111547 |
Pantelaki I, Voutsa D. 2021. Organophosphate esters in inland and coastal waters in northern Greece. Science of the Total Environment, 800: 149544.
DOI:10.1016/j.scitotenv.2021.149544 |
Poma G, Glynn A, Malarvannan G, et al. 2017. Dietary intake of phosphorus flame retardants (PFRs) using Swedish food market basket estimations. Food and Chemical Toxicology, 100: 1-7.
DOI:10.1016/j.fct.2016.12.011 |
Poma G, Sales C, Bruyland B, et al. 2018. Occurrence of organophosphorus flame retardants and plasticizers (PFRs) in Belgian foodstuffs and estimation of the dietary exposure of the adult population. Environmental Science & Technology, 52(4): 2331-2338.
DOI:10.1021/acs.est.7b06395 |
Regnery J, Püttmann W. 2010. Occurrence and fate of organophosphorus flame retardants and plasticizers in urban and remote surface waters in Germany. Water Research, 44(14): 4097-4104.
DOI:10.1016/j.watres.2010.05.024 |
Sala B, Giménez J, de Stephanis R, et al. 2019. First determination of high levels of organophosphorus flame retardants and plasticizers in dolphins from Southern European waters. Environmental Research, 172: 289-295.
DOI:10.1016/j.envres.2019.02.027 |
Shi Q P, Wang M, Shi F Q, et al. 2018. Developmental neurotoxicity of triphenyl phosphate in zebrafish larvae. Aquatic Toxicology, 203: 80-87.
DOI:10.1016/j.aquatox.2018.08.001 |
Shi Y F, Zhang Y, Du Y M, et al. 2020. Occurrence, composition and biological risk of organophosphate esters (OPEs) in water of the Pearl River Estuary, South China. Environmental Science and Pollution Research, 27(13): 14852-14862.
DOI:10.1007/s11356-020-08001-1 |
Sühring R, Diamond M L, Scheringer M, et al. 2016. Organophosphate esters in Canadian Arctic air: occurrence, levels and trends. Environmental Science & Technology, 50(14): 7409-7415.
DOI:10.1021/acs.est.6b00365 |
Sun R, Luo X, Tang B, et al. 2017. Bioaccumulation of short chain chlorinated paraffins in a typical freshwater food web contaminated by e-waste in south china: Bioaccumulation factors, tissue distribution, and trophic transfer. Environmental Pollution, 222: 165-174.
|
van der Veen I, de Boer J. 2012. Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis. Chemosphere, 88(10): 1119-1153.
DOI:10.1016/j.chemosphere.2012.03.067 |
Wang G W, Shi H H, Du Z K, et al. 2017a. Bioaccumulation mechanism of organophosphate esters in adult zebrafish (Danio rerio). Environmental Pollution, 229: 177-187.
DOI:10.1016/j.envpol.2017.05.075 |
Wang X L, Zhong W J, Xiao B W, et al. 2019. Bioavailability and biomagnification of organophosphate esters in the food web of Taihu Lake, China: impacts of chemical properties and metabolism. Environment International, 125: 25-32.
DOI:10.1016/j.envint.2019.01.018 |
Wang X L, Zhu L Y, Zhong W J, et al. 2018. Partition and source identification of organophosphate esters in the water and sediment of Taihu Lake, China. Journal of Hazardous Materials, 360: 43-50.
DOI:10.1016/j.jhazmat.2018.07.082 |
Wang Y, Hou M M, Zhang Q N, et al. 2017b. Organophosphorus flame retardants and plasticizers in building and decoration materials and their potential burdens in newly decorated houses in China. Environmental Science & Technology, 51(19): 10991-10999.
DOI:10.1021/acs.est.7b03367 |
Wang Y, Wu X W, Zhang Q N, et al. 2017c. Organophosphate esters in sediment cores from coastal Laizhou Bay of the Bohai Sea, China. Science of the Total Environment, 607-608: 103-108.
DOI:10.1016/j.scitotenv.2017.06.259 |
Wei G L, Li D Q, Zhuo M N, et al. 2015. Organophosphorus flame retardants and plasticizers: sources, occurrence, toxicity and human exposure. Environmental Pollution, 196: 29-46.
DOI:10.1016/j.envpol.2014.09.012 |
Woudneh M B, Benskin J P, Wang G, et al. 2015. Quantitative determination of 13 organophosphorous flame retardants and plasticizers in a wastewater treatment system by high performance liquid chromatography tandem mass spectrometry. Journal of Chromatography A, 1400: 149-155.
DOI:10.1016/j.chroma.2015.04.026 |
Xing L Q, Tao M, Zhang Q, et al. 2020. Occurrence, spatial distribution and risk assessment of organophosphate esters in surface water from the lower Yangtze River Basin. Science of the Total Environment, 734: 139380.
DOI:10.1016/j.scitotenv.2020.139380 |
Ya M L, Yu N Y, Zhang Y Y, et al. 2019. Biomonitoring of organophosphate triesters and diesters in human blood in Jiangsu Province, eastern China: occurrences, associations, and suspect screening of novel metabolites. Environment International, 131: 105056.
DOI:10.1016/j.envint.2019.105056 |
Yadav I C, Devi N L, Li J, et al. 2018. Concentration and spatial distribution of organophosphate esters in the soil-sediment profile of Kathmandu Valley, Nepal: implication for risk assessment. Science of the Total Environment, 613-614: 502-512.
DOI:10.1016/j.scitotenv.2017.09.039 |
Yang X G, Li Y P, Ma G S, et al. 2005. Study on weight and height of the Chinese people and the differences between 1992 and 2002. Chinese Journal of Epidemiology, 26(7): 489-493.
(in Chinese with English abstract) DOI:10.3760/j.issn:0254-6450.2005.07.006 |
Yin Y R, Li Y. 2021. SWOT analysis and strategy suggestions for international development of petroleum equipment manufacturing industry of DongYing. Foreign Economic Relations & Trade, (2): 59-63.
(in Chinese with English abstract) DOI:10.3969/j.issn.2095-3283.2021.02.013 |
Zeng X Y, Hu Q P, He L X, et al. 2018a. Occurrence, distribution and ecological risks of organophosphate esters and synthetic musks in sediments from the Hun River. Ecotoxicology and Environmental Safety, 160: 178-183.
DOI:10.1016/j.ecoenv.2018.05.034 |
Zeng X Y, Xu L, Hu Q P, et al. 2020. Occurrence and distribution of organophosphorus flame retardants/plasticizers in coastal sediments from the Taiwan Strait in China. Marine Pollution Bulletin, 151: 110843.
DOI:10.1016/j.marpolbul.2019.110843 |
Zeng X Y, Xu L, Liu J, et al. 2018b. Occurrence and distribution of organophosphorus flame retardants/plasticizers and synthetic musks in sediments from source water in the Pearl River Delta, China. Environmental Toxicology and Chemistry, 37(4): 975-982.
DOI:10.1002/etc.4040 |
Zhang R J, Yu K F, Li A, et al. 2020. Occurrence, phase distribution, and bioaccumulation of organophosphate esters (OPEs) in mariculture farms of the Beibu Gulf, China: a health risk assessment through seafood consumption. Environmental Pollution, 263: 114426.
DOI:10.1016/j.envpol.2020.114426 |
Zhang X L, Zou W, Mu L, et al. 2016. Rice ingestion is a major pathway for human exposure to organophosphate flame retardants (OPFRs) in China. Journal of Hazardous Materials, 318: 686-693.
DOI:10.1016/j.jhazmat.2016.07.055 |
Zhang Y, Zheng X B, Wei L F, et al. 2018. The distribution and accumulation of phosphate flame retardants (PFRs) in water environment. Science of the Total Environment, 630: 164-170.
DOI:10.1016/j.scitotenv.2018.02.215 |
Zhong M Y, Tang J H, Mi L J, et al. 2017. Occurrence and spatial distribution of organophosphorus flame retardants and plasticizers in the Bohai and Yellow Seas, China. Marine Pollution Bulletin, 121(1-2): 331-338.
DOI:10.1016/j.marpolbul.2017.06.034 |
Zhong M Y, Wu H F, Mi W Y, et al. 2018. Occurrences and distribution characteristics of organophosphate ester flame retardants and plasticizers in the sediments of the Bohai and Yellow Seas, China. Science of the Total Environment, 615: 1305-1311.
DOI:10.1016/j.scitotenv.2017.09.272 |
 2023, Vol. 41
2023, Vol. 41