Institute of Oceanology, Chinese Academy of Sciences
Article Information
- CHE Xingkai, LI Hu, ZHANG Litao, LIU Jianguo
- Effect of high light and desiccation on photosystem Ⅱ in the seedlings and mature plants of tropical seagrass Enhalus acoroides during low tide
- Journal of Oceanology and Limnology, 41(1): 241-250
- http://dx.doi.org/10.1007/s00343-021-1170-2
Article History
- Received Jun. 14, 2021
- accepted in principle Sep. 13, 2021
- accepted for publication Dec. 4, 2021
2 Laboratory for Marine Biology and Biotechnology, Pilot National Laboratory for Marine Science and Technology(Qingdao), Qingdao 266237, China
Seagrasses are the only monocotyledonous angiosperms that live completely submerged in shallow seawater along coastal areas (Orth et al., 2006a; Short et al., 2011). Tropical seagrass meadows, dominated by Enhalus acoroides in Southeast Asia, have declined rapidly in recent decades, which has become a serious ecological problem (Yang and Yang, 2009; Yu et al., 2018) and has also become a global concern. Natural stresses during low tide threaten the survival of seagrasses. Stresses during low tide decrease biomass accumulation and nutrient content of seagrass meadows (Erftemeijer and Herman, 1994; Stapel et al., 1997; Jiang et al., 2013). A combination of stresses during low tide can also result in seagrass meadow loss, for instance, loss of 13 000 hectares of seagrass meadows in South Australia (Seddon et al., 2000). Tolerance to the stresses during low tide determines the vertical distribution of seagrasses (Tanaka and Nakaoka, 2004; Shafer et al., 2007). The damage of low tide to seagrasses is complex, because seagrasses suffer from several types of stresses during low tide, such as high light, desiccation, and high temperature (Erftemeijer and Lewis Ⅲ, 2006; van Katwijk et al., 2009; Unsworth et al., 2012). High light and desiccation are the two major stresses during low tide. Seagrasses suffer from desiccation stress when exposed to the air during low tide. Seagrasses will also suffer from high-light stress when low tide occurs during the day, but it will not during the night. Some seagrass leaves in deeper water float on the sea surface without exposure to the air during daytime low tide. At this time, the upper surface of the seagrass leaves suffer from high-light stress but not from desiccation stress. In addition, low tide is accompanied by an increase in temperature during the daytime, which accelerates the dehydration rate of seagrasses. However, as the water evaporates from wet beaches, cooling them or when seagrasses float on the sea surface, the change in leaf temperature is less dramatic. Moreover, the negative effects of the increase in temperature on seagrasses are mainly due to the accelerated desiccation process. Therefore, we chose high light and desiccation as the variables to study the effects of low-tide stresses on seagrasses. Previous research has focused on the effect of low tide on mature seagrasses (Tanaka and Nakaoka, 2004; Shafer et al., 2007; Jiang et al., 2014). However, some seagrasses, such as E. acoroides, produce large seeds for sexual reproduction (Kendrick et al., 2017), but it is difficult to find seedlings developed from seeds of E. acoroides in the wild. In addition to the seedlings being eaten by animals, stresses during low tide could impair survival of these seedlings. Studying the effect of low tide across development stages of seagrasses would improve the understanding of the main causes for the decline of seagrass meadows, help maintain an abundance of seagrass genomes, and improve the success of transplantation to restore seagrass meadows by seedlings or seeds.
Photosynthesis is one of the most important primary metabolic pathways of plants, which provides oxygen, energy, and substrates for plant growth and development (Yan et al., 2007). Photosystem Ⅱ (PSII), as an important component of the light reaction, is often a limiting factor of photosynthesis under stress. Therefore, it is significant to study the effect of the stresses during low tide on the PSII of seagrasses to explore the causes of the decline of seagrass meadows and to develop strategies to restore them. Previous research has indicated that the stresses during low tide limit photosynthetic capacity and decreases biomass of seagrasses (Erftemeijer and Herman, 1994; Björk et al., 1999; Shafer et al., 2007; Unsworth et al., 2012; Jiang et al., 2013, 2014; Kaewsrikhaw et al., 2016). However, the resistance of photosynthesis to the stresses during low tide and the recovery of damaged photosynthetic apparatus after low tide are equally important for maintaining the normal photosynthetic capacity of seagrasses, but the effects of low tide on the recovery of photosynthesis of seagrasses are rarely reported (Shafer et al., 2007).
To explore these questions, we used the intertidal seagrass E. acoroides as experimental material and addressed how high light and desiccation affected both the immediate response of PSII and its capacity for recovery after rehydration, to provide a theoretical basis for the development of effective seagrass protection strategies.
2 MATERIAL AND METHOD 2.1 Culture and treatment of plant materialsThe distribution of E. acoroides was observed and photographed during low tide in October 2019 from the mid-intertidal zone in Li'an Bay, Hainan Province, China (18°24′N, 110°03′E). Whole E. acoroides plants and seeds were collected, then washed twice to remove impurities, and cultured in the laboratory in aerated seawater at 28 ℃, 30 salinity and 100 μmol/(m2·s) light꞉dark (12 h꞉12 h). The mature E. acoroides plants were planted in the laboratory for 5-d acclimatization, and then were used for subsequent experiment. After cultured in the laboratory for 8 d, the seeds became to seedlings with 4–6-cm leaves, and then the seedlings were used for subsequent experiment.
To eliminate the interference of the external complex environment on the experiment and ensure a single variable, we used 8-d seedlings and in vitro leaves of mature plants indoor and simulated high-light and desiccation stresses during low tide to study their effects on PSII. Mature plant leaves of E. acoroides were more than 20-cm long and 1.5-cm wide. Middle portions were cut into leaf segments (4-cm long and 1.5-cm wide). Leaf segments cut from living plants were immediately placed in four environments (i.e., four treatments). The experiment was carried out with six replicate leaf segments in each treatment and all treatments were carried out at the same time. The light-water (LW) treatment simulates the situation of leaves floating on the sea during low tide in the day (i.e., high light stress). In LW treatment, leaf segments floated on the seawater at high light (1 600 μmol/(m2·s)). The dark-water (DW) treatment simulates the situation of leaves floating on the sea during low tide in the night (i.e., no stress). In DW treatment, leaf segments floated on the seawater under darkness. The light-air (LA) treatment simulate the situation of leaves exposed to air during low tide in the day (i.e., high light×desiccation stress). In LA treatment, leaf segments were exposed to the air at high light (1 600 μmol/(m2·s)). The dark-air (DA) treatment simulate the situation of leaves exposed to air during low tide in the night (i.e., only desiccation stress). In DA treatment, leaf segments were exposed to the air under darkness. The time of four treatment was 3 h. The four treatments occurred at constant air and water temperature (28 ℃) and the relative air humidity was maintained at 75%. The high light was provided by a 1-m×1-m light emitting diode (LED) lamp in LW and LA treatments. Light intensity of LED lamp was measure by illuminance meter (Hansatech, Norfolk, UK). The leaves of 8-d seedlings were 4–6-cm long and 0.3-cm wide. The seedling leaves were cut into leaf segments (4-cm long and 0.3-cm wide) and given the same four treatments (LW, DW, LA, and DA). In the LA and DA treatments, in order to simulate the desiccation of seedlings and mature plants on a wet beach during low tide, we put a wet gauze under the seedling and mature plant leaves during the time the leaves were exposed to air. During the recovery, the seedling and mature plant leaves from different treatments (LW, LA, and DA) were maintained in aerated seawater at 28 ℃ and 30 salinity and dim light (20 μmol/(m2·s)). Since the DW treatment experienced neither high light nor desiccation, PSII activity was not impaired, so there was no need to consider the PSII recovery for the DW treatment. In addition, when the leaves were dehydrated to different degrees, the initial photosynthetic activity differed across treatments, which would influence the recovery rate of photosynthesis. Therefore, to control for the fact that the initial photosynthetic activity differed across treatments, when examining the recovery of PSII activity, the PSII activity of all the different treatments was reduced to the same level before measuring the level of recovery. To determine which damage (dehydrate damage or hypersaline toxicity) is the main cause of the decrease of PSII activity of seedling and mature plant leaves caused by desiccation, leaf segments of seedling and mature plant were treated with different concentrations of polyethylene glycol (PEG) and different salinities. PEG was used to dehydrate seagrasses to simulate dehydration damage during desiccation. The leaf segments (seedling and mature plant) were submerged in 30% and 40% PEG seawater solution (25 salinity) in darkness for 3 h, respectively. Hyposaline seawater is to balance the hypersaline environment inside the cells due to dehydration by PEG. For hypersaline treatments, the leaf segments (seedling and mature plant) were submerged at salinity of 50 and 60 seawater in darkness for 3 h, respectively.
The usage of leaf segments in this study was due to the experimental space and logistical constraints of using whole plants. This methodology might not fully reflect the behavior of intact plants growing rooted in their sediment, but it is widely used and can be considered valid for seagrass photosynthetic activity measurements (Invers et al., 2001; Burnell et al., 2014; Schubert et al., 2015).
2.2 Determination of relative water content (RWC)Fresh weight changed during emersion treatments and was measured at the same time as photosynthesis. The water saturated weight was the initial leaf weight before treatment. The dry weight was weighted after the leaves were dried at 80 ℃ in an oven for 72 h. RWC was calculated according to RWC=(fresh weight–dry weight)/(initial weight–dry weight).
2.3 Chlorophyll-a fluorescence measurementThe chlorophyll-a fluorescence transient was measured using an m-PEA system (Hansatech, Norfolk, UK), after leaves were acclimated to the dark for 15 min in aerated seawater at 28 ℃, and 30 salinity. Saturating red light at 5 000 μmol/(m2·s) was produced by an array of LED (peak 650 nm). The chlorophyll-a fluorescence transients were obtained by 1-s saturating red light and analyzed with the OJIP-test (Strasser and Strasser, 1995): maximum quantum yield of PSII (or PSII activity), Fv/Fm=1–(Fo/Fm). Fm and Fo were obtained automatically by the m-PEA system. Fv/Fm (relative value)=(Fv/Fm)-treated/(Fv/Fm)-intitial; RC/CSM (reaction center per cross section (at t=tFm)) was also calculated automatically by the m-PEA system.
2.4 Statistical analysisThe IBM SPSS 17.0 software package was used for statistical analyses. The variation of each parameter was tested by the one-way analysis of variance (ANOVA). The experiment was carried out with six replicate leaves in each treatment and the data presented are means±standard deviation (SD) for n=6. Least significant difference was used to analyze the differences between different treatments. Differences were considered significant at a probability level of P < 0.05. All analyses and graphs were generated using SigmaPlot 10.0 software.
3 RESULT 3.1 The adult E. acoroides in natural habitat during low tide and its seedings cultivated indoorsBased on field observations, leaves of E. acoroides growing in Li'an Bay were exposed to the air (Fig. 1a) or floated on the sea surface (Fig. 1b) during low tide. When low tide occurs during the night, E. acoroides may suffer from desiccation stress. However, when low tide occurs during the day, E. acoroides suffers from both desiccation stress and high-light stress, or suffers from high-light stress but not from desiccation stress because the upper parts of leaves of E. acoroides in deeper water floated on the sea surface without exposure to the air during low tide in the day. Depending on the degree of low tide, the duration of stresses ranges from 30 min to 3 h. There are only sporadic E. acoroides plants in the zone where they suffer from both desiccation stress and high-light stress; in contrast there are many plants in the zone with only high-light stress (Fig. 1c). This finding indicates that the combined stresses of high light and desiccation seriously affect the survival of E. acoroides. Figure 1d shows the 8-d seedlings cultured in the laboratory. However, we did not find any seedlings of E. acoroides in Li'an Bay. Therefore, we speculated that the seedlings of E. acoroides may be more sensitive to the stresses during low tide than mature plants, which resulted in their low survival rate in the wild and thus it was difficult to found them.

|
| Fig.1 The mature E. acoroides exposed to the air (a) or floating on the sea surface (b) during low tide in its natural habitat; the distibution of E. acoroides during low tide in intertidal zone (c); the morphological characteristics of the 8-d seedlings (d) |
When leaves were exposed to the air, whether in the high light or in the dark, the RWC decline rate of seedling leaves was significantly slower than that of mature plant leaves (Fig. 2a & b). The result indicated that seedling leaves have better water retention than mature plant leaves. For both seedling leaves and mature plant leaves, the decline rate of RWC in high light was faster than that in the dark (Fig. 2a & b), which indicates that high light accelerates the dehydration rate of leaves in the air.
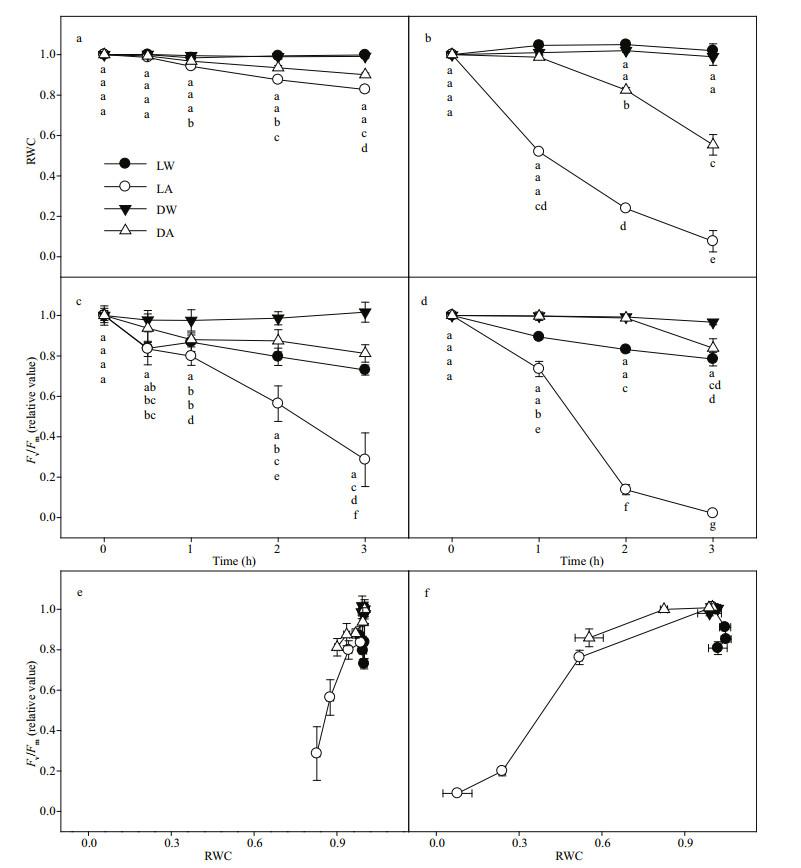
|
| Fig.2 The change in RWC and Fv/Fm of seedling (a, c, e) and mature plant (b, d, f) leaves with light-water (LW), light-air (LA), dark-water (DW), or dark-air (DA) treatment Different letters indicate significant differences between leaves with different treatments (P < 0.05). Values are the mean ± SE (n=6). |
Regardless of seedling leaves or mature plant leaves, the PSII activity of the LA treatment decreased fastest, followed by that of the LW treatment, and finally that of the DA treatment (Fig. 2c & d).
The PSII activity of seedling leaves was lower than that of mature plant leaves at the same RWC (Fig. 2e & f). This indicates that the PSII of mature plant leaves is more resistant to desiccation than that of seedling leaves. However, there is no significant difference in the high-light resistance of PSII of seedling and mature plant leaves without desiccation (Fig. 2c & d). In addition, slight desiccation (RWC > 80%) in the dark did not result in the decline of PSII activity of mature plant leaves (Fig. 2b, d, & f).
3.3 The effect of dehydrated and hypersaline treatment on RWC and PSII activity in seedling and mature plant leaves of E. acoroidesThe desiccation damage to leaves includes not only dehydration damage but also hypersaline toxicity due to water loss. To determine which damage is the main cause of the decrease of PSII activity of seedling and mature plant leaves caused by desiccation, seedling and mature plant leaves were treated with different salinities and different concentrations of PEG.
In seedling leaves, the PSII activity and RWC of the hypersaline treatment did not change, but the PSII activity of PEG treatment markedly decreased with the RWC decreasing (Fig. 3a & c). The result indicates the damage of desiccation to young leaves is mainly dehydration damage. In contrast, in mature plant leaves, the PSII activity of the hypersaline treatment markedly decreased with the RWC not changing, but the PSII activity of the PEG treatment did not change with the RWC markedly decreasing (Fig. 3b & d). This result indicates that the desiccation damage of to mature plant leaves is mainly hypersaline toxicity.

|
| Fig.3 The change in RWC and Fv/Fm of seedling (a, c) and mature plant (b, d) leaves with PEG or hypersaline treatment Different letters indicate significant differences between leaves with different treatments (P < 0.05). Values are the mean±SE (n=6). |
During the recovery process, the RWC of the different treatments rapidly returned to the normal level and the recovery rate of RWC of the different treatments were similar (Fig. 4a & b), but the recovery rate of PSII activity in treatments with desiccation was slower than that in treatments without desiccation (Fig. 4c & d). Moreover, the greater the dehydration at desiccation, the slower the recovery rate of PSII activity. When the dehydration was severe (17% RWC), PSII was unable to recover. These results indicate that the dehydration at desiccation inhibits the recovery of PSII activity even after full hydration is restored.
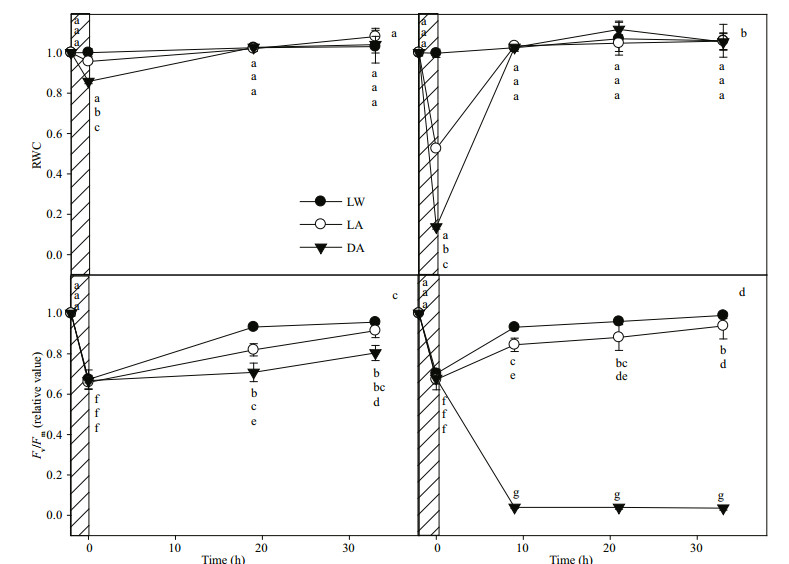
|
| Fig.4 Fv/Fm of LW, LA, and DA treatment reached the same level (shadow) and then floated on the seawater to recover under low light (20 μmol/(m2·s)), during which changes in RWC and Fv/Fm of seedling (a, c) and mature plant (b, d) leaves of E. acoroides were determined Different letters indicate significant differences between leaves with different treatments (P < 0.05). Values are the mean±SE (n=6). |
The recovery rate of PSII activity of seedling leaves was significantly slower than that of mature plant leaves after the stresses disappeared (Fig. 4c & d). We do not consider comparing the recovery rate of PSII with DA treatment between seedling leaves and mature plant leaves, because in order to reach the same degree of photoinhibition as that of the LW or LA treatment, the DA treatment took a long time (about 5 h) and such a long time of desiccation does not exist in nature. Therefore, DA treatment is only a theoretical exploration of the effects of desiccation in the night on PSII recovery.
3.5 The effect of high light and desiccation on the recovery of RC/CSM of PSII in seedling and mature plant leaves of E. acoroidesRC/CSM represents the density of active reaction centers. When the Fv/Fm of different treatments reached the same level (Fig. 4c & d), the value of RC/CSM for the different treatments in seedling and mature plant leaves was almost the same (Fig. 5a & b). However, the recovery of RC/CSM during the recovery process in treatments with desiccation was slower than that in treatments without desiccation (Fig. 5a & b). This result indicates that desiccation significantly inhibits the recovery of RC/CSM.

|
| Fig.5 Fv/Fm of LW, LA, and DA treatment reached the same level (shadow) and then floated on the seawater to recover under low light (20 μmol/(m2·s)), during which changes in RC/CSM of seedling (a) and mature plant (b) leaves of E. acoroides were determined Different letters indicate significant differences between leaves with different treatments (P < 0.05). Values are the mean±SE (n=6). |
High light and desiccation seriously damaged PSII of E. acoroides decreasing photosynthetic activity during low tide. The resistance of seedling and mature plant leaves to high light was similar but they differed with regard to desiccation. Seedling and mature plant leaves adopt different strategies to resist the damage of desiccation to PSII. These different strategies to protect PSII also match their living environment. Tanaka and Nakaoka (2004) found that the large leaves were exposed to the air longer. The leaves of mature plants are significantly larger than those of seedlings, which mean that the desiccation duration of mature plant leaves is longer. Therefore, mature plant leaves can not only better adapt to this condition by increasing the resistance of PSII to desiccation, but slight desiccation does not even result in the decline of PSII activity (Fig. 2b, d, & f). The short leaves of seedlings are less exposed to the air during low tide and thus the duration of their exposure to desiccation is much shorter. Increasing the water-retention ability in seedling leaves would be a favorable survival option (Fig. 2a, c, & e). Single stressors (desiccation in the dark, or high light on the surface of water) had less negative effect than the two combined. Tidal exposure during the day poses the greatest threat to E. acoroides, because the combined stresses of high light and desiccation during tidal exposure in the day significantly reduced PSII activity and inhibited the recovery of PSII activity (Figs. 2c, 2d, 4c, & 4d), which severely decreased the overall photosynthetic activity of E. acoroides. In addition, high light accelerated the dehydration rate of leaves in the air exacerbating the damage of desiccation to PSII (Fig. 2). Due to these combined effects, there are only sporadic E. acoroides plants in the intertidal zone that is most exposed to the air during low tide (Fig. 1a).
Previous research found that the shallow-water seagrass species, often suffering from the stresses during low tide, are more sensitive to desiccation than deeper-water species (Björk et al., 1999), but this b phenomenon defies common sense. Previous studies f on the damage of desiccation during low tide to seagrasses focused on dehydration damage but neglected hypersaline toxicity caused by dehydration (Stapel et al., 1997; Björk et al., 1999; Shafer et al., 2007; Jiang et al., 2014). However, both dehydration and hypersalinity occur during desiccation. Which factor actually damages seedling and mature leaves? This research found that the damage of desiccation during low tide to seedling leaves is mainly caused by dehydration but to mature plant leaves is mainly caused by hypersaline toxicity. This is why slight desiccation does not result in the decrease of PSII activity of mature plant leaves (Fig. 2e & f). Sandoval-Gil (2014) found that hypersalinity has deleterious effects on Cymodocea nodosa plants from the shallow meadows but not on C. nodosa plants from the deeper meadows. Therefore, we inferred that the stronger resistance of deeper-water seagrass species to desiccation, compared to shallow-water species, might be related to the stronger resistance of deeper seagrass species to hypersaline toxicity.
The photosynthetic consequences of many stresses depend not only on the resistance of photosynthetic apparatus to stresses, but also on the recovery rate of a damaged photosynthetic apparatus after these stresses disappear. Although many studies reported the effect of the stresses during low tide on photosynthesis of seagrasses, the recovery of photosynthesis of seagrasses was rarely studied. Shafer et al. (2007) studied the effect of different levels of dehydration on the recovery of photosynthesis of seagrasses. However, when the leaves were dehydrated to different degrees, the initial photosynthetic activity differed across treatments, which would influence the recovery rate of photosynthesis. Therefore, in our research, the PSII activity of the different treatments was reduced to the same level and then allowed to recover (Fig. 4). The result showed that the recovery rate of seedling leaves was significantly slower than that of mature plant leaves after the stresses of low tide were removed (Fig. 4), which may be the one of reasons why seedlings are rare in the field. In addition, the recovery rate of PSII in leaves undergoing a desiccation treatment was significantly slower than that of only high-light treatment, although the RWC of both treatments rapidly returned to the normal level (Fig. 4). This result indicates that desiccation will inhibit the recovery rate of PSII even after the desiccation has been reversed, which is one of the reasons why only sporadic E. acoroides plants in the intertidal zone that is most exposed to the air during low tide. Desiccation inhibited the recovery rate of RC/CSM, which will decrease the number of electrons going into the electron transport chain and thus decrease the production of assimilatory power. The decrease of assimilatory power will inhibit the recovery rate of PSII, because the repair of damaged PSII requires assimilation power (Nishiyama et al., 2011), which may be the cause of the slower recovery after desiccation disappears.
The global seagrass meadows are rapidly declining (Short et al., 2011). Seagrass transplantation is currently one of the important methods for seagrass meadow restoration (Orth et al., 2006b; van Katwijk et al., 2009; Irawan, 2018). Seagrass cannot grow normally when the light intensity of the seafloor is less than 11% of the light intensity incident on the sea surface (Duarte, 1991; Ralph et al., 2007). Therefore, in the process of transplanting E. acoroides to restore seagrass meadows, the plants should be placed as close to the upper intertidal zone as possible for a better light environment. However, the research showed that excessive low tide exposure time in the intertidal zone will significantly reduce the survival rate of transplanted seagrasses and the length of their leaves and roots (Tanaka and Nakaoka, 2004). We inferred that the significant decrease in photosynthetic activity and the slower recovery of PSII activity caused by desiccation during low tide are important causes for the decline in the survival rate and biomass of seagrasses in the intertidal zone, rather than high light. Therefore, E. acoroides transplanting should choose an appropriate transplanting depth where E. acoroides may be exposed to high light but not to the air during low tide. In addition, we established a new method for obtaining large numbers of the healthy E. acoroides seedling in the laboratory (Li et al., 2021). In the subsequent transplanting of seedlings to rebuild the seagrass meadows, considering that seedling leaves are more sensitive to desiccation than mature plant leaves, the transplanting depth of seedlings may be different from that of mature plants.
5 CONCLUSIONIn summary, the study showed that the resistance of seedling and mature plant leaves to high light was quite similar, but to desiccation was very different. Seedling leaves were more sensitive to desiccation than mature plant leaves, but had better water retention. The damage of desiccation to seedling leaves was mainly caused by dehydration, whereas that to mature plant leaves was caused by hypersaline toxicity. The recovery rate of PSII of seedling leaves was significantly slower than that of the mature plants after the stresses during low tide disappeared, which may at least partly contribute to seedling mortality in the wild. In addition, compared to high light, desiccation seriously inhibited the recovery rate of PSII activities even if the leaves become fully rehydrated to their normal relative water content (RWC) in the following re-immersion. Desiccation inhibited the recovery rate of RC/CSM to decrease the production of assimilatory power, which may be the cause of the slower PSII recovery in desiccation treatments. Clearly, high light and particularly desiccation have a very negative effect on the PSII of E. acoroides during low tide and the sensitivity of seedlings and mature plants to desiccation was significantly different, which have important reference significance to choose an appropriate transplanting depth where seedlings and mature plant of E. acoroides not only receive sufficient light for growth, but also that minimize desiccation stress during low tide.
6 DATA AVAILABILITY STATEMENTAll data generated and/or analyzed during this study are available from the first author on request via chexingkai@qdio.ac.cn.
7 ACKNOWLEDGMENTThe authors thank Dr. John van der Meer (Pan-American Marine Biotechnology Association) for his assistance in proofreading.
Björk M, Uku J, Weil A, Beer S. 1999. Photosynthetic tolerances to desiccation of tropical intertidal seagrasses. Marine Ecology Progress Series, 191: 121-126.
DOI:10.3354/meps191121 |
Burnell O W, Connell S D, Irving A D, Watling J R, Russell B D. 2014. Contemporary reliance on bicarbonate acquisition predicts increased growth of seagrass Amphibolis antarctica in a high-CO2 world. Conservation Physiology, 2(1): cou052.
DOI:10.1093/conphys/cou052 |
Duarte C M. 1991. Seagrass depth limits. Aquatic Botany, 40(4): 363-377.
DOI:10.1016/0304-3770(91)90081-F |
Erftemeijer P L A, Herman P M J. 1994. Seasonal changes in environmental variables, biomass, production and nutrient contents in two contrasting tropical intertidal seagrass beds in South Sulawesi, Indonesia. Oecologia, 99(1): 45-59.
|
Erftemeijer P L A, Lewis Ⅲ R R R. 2006. Environmental impacts of dredging on seagrasses: a review. Marine Pollution Bulletin, 52(12): 1553-1572.
DOI:10.1016/j.marpolbul.2006.09.006 |
Invers O, Zimmerman R C, Alberte R S, Pérez M, Romero J. 2011. Inorganic carbon sources for seagrass photosynthesis: an experimental evaluation of bicarbonate use in species inhabiting temperate waters. Journal of Experimental Marine Biology and Ecology, 265(2): 203-217.
|
Irawan A. 2018. Transplantation of Enhalus acoroides on a sedimentary beach in Ambon Bay. IOP Conference Series: Earth and Environmental Science, 118: 012054.
DOI:10.1088/1755-1315/118/1/012054 |
Jiang Z J, Huang X P, Zhang J P, Zhou C Y, Lian Z L, Ni Z X. 2014. The effects of air exposure on the desiccation rate and photosynthetic activity of Thalassia hemprichii and Enhalus acoroides. Marine Biology, 161(5): 1051-1061.
DOI:10.1007/s00227-014-2398-6 |
Jiang Z J, Huang X P, Zhang J P. 2013. Dynamics of nonstructural carbohydrates in seagrass Thalassia hemprichii and its response to shading. Acta Oceanologica Sinica, 32(8): 61-67.
DOI:10.1007/s13131-013-0342-0 |
Kaewsrikhaw R, Ritchie R J, Prathep A. 2016. Variations of tidal exposures and seasons on growth, morphology, anatomy and physiology of the seagrass Halophila ovalis (R. Br.) Hook. f. in a seagrass bed in Trang Province, Southern Thailand. Aquatic Botany, 130: 11-20.
DOI:10.1016/j.aquabot.2015.12.006 |
Kendrick G A, Orth R J, Statton J, Hovey R, Montoya L R, Lowe R J, Krauss S L, Sinclair E A. 2017. Demographic and genetic connectivity: the role and consequences of reproduction, dispersal and recruitment in seagrasses. Biological Reviews, 92(2): 921-938.
DOI:10.1111/brv.12261 |
Li H, Liu J G, Che X K. 2021. Establishing healthy seedlings of Enhalus acoroides for the tropical seagrass restoration. Journal of Environmental Management, 286: 112200.
DOI:10.1016/j.jenvman.2021.112200 |
Nishiyama Y, Allakhverdiev S I, Murata N. 2011. Protein synthesis is the primary target of reactive oxygen species in the photoinhibition of photosystem Ⅱ. Physiologia Plantarum, 142(1): 35-46.
DOI:10.1111/j.1399-3054.2011.01457.x |
Orth R J, Carruthers T J B, Dennison W C, Duarte C M, Fourqurean J W, Heck K L, Hughes A R, Kendrick G A, Kenworthy W J, Olyarnik S, Short F T, Waycott M, Williams S L. 2006a. A global crisis for seagrass ecosystems. Bioscience, 56(12): 987-996.
DOI:10.1641/0006-3568(2006)56[987:AGCFSE]2.0.CO;2 |
Orth R J, Luckenbach M L, Marion S R, Moore K A, Wilcox D J. 2006b. Seagrass recovery in the Delmarva coastal bays, USA. Aquatic Botany, 84(1): 26-36.
DOI:10.1016/j.aquabot.2005.07.007 |
Ralph P J, Durako M J, Enríquez S, Collier C J, Doblin M A. 2007. Impact of light limitation on seagrasses. Journal of Experimental Marine Biology and Ecology, 350(1-2): 176-193.
DOI:10.1016/j.jembe.2007.06.017 |
Sandoval-Gil J M, Ruiz J M, Marín-Guirao L, Bernardeau-Esteller J, Sánchez-Lizaso J L. 2014. Ecophysiological plasticity of shallow and deep populations of the Mediterranean seagrasses Posidonia oceanica and Cymodocea nodosa in response to hypersaline stress. Marine Environmental Research, 95: 39-61.
DOI:10.1016/j.marenvres.2013.12.011 |
Schubert N, Colombo-Pallota M F, Enríquez S. 2015. Leaf and canopy scale characterization of the photoprotective response to high-light stress of the seagrass Thalassia testudinum. Limnology and Oceanography, 60(1): 286-302.
DOI:10.1002/lno.10024 |
Seddon S, Connolly R M, Edyvane K S. 2000. Large-scale seagrass dieback in northern Spencer Gulf, South Australia. Aquatic Botany, 66(4): 297-310.
DOI:10.1016/S0304-3770(99)00080-7 |
Shafer D J, Sherman T D, Wyllie-Echeverria S. 2007. Do desiccation tolerances control the vertical distribution of intertidal seagrasses?. Aquatic Botany, 87(2): 161-166.
DOI:10.1016/j.aquabot.2007.04.003 |
Short F T, Polidoro B, Livingstone S R, Carpenter K E, Bandeira S, Bujang J S, Calumpong H P, Carruthers T J B, Coles R G, Dennison W C, Erftemeijer P L A, Fortes M D, Freeman A S, Jagtap T G, Kamal A H M, Kendrick G A, Judson Kenworthy W, La Nafie Y A, Nasution I M, Orth R J, Prathep A, Sanciangco J C, van Tussenbroek B, Vergara S G, Waycott M, Zieman J C. 2011. Extinction risk assessment of the world's seagrass species. Biological Conservation, 144(7): 1961-1971.
DOI:10.1016/j.biocon.2011.04.010 |
Stapel J, Manuntun R, Hemminga M A. 1997. Biomass loss and nutrient redistribution in an Indonesian Thalassia hemprichii seagrass bed following seasonal low tide exposure during daylight. Marine Ecology Progress Series, 148: 251-262.
DOI:10.3354/meps148251 |
Strasser B J, Strasser R J. 1995. Measuring fast fluorescence transients to address environmental questions: the JIP test. In: Mathis P ed. Photosynthesis: from Light to Biosphere. Kluwer Academic, Dordrecht. p. 977-980.
|
Tanaka Y, Nakaoka M. 2004. Emergence stress and morphological constraints affect the species distribution and growth of subtropical intertidal seagrasses. Marine Ecology Progress Series, 284: 117-131.
DOI:10.3354/meps284117 |
Unsworth R K F, Rasheed M A, Chartrand K M, Roelofs A J. 2012. Solar Radiation and tidal exposure as environmental drivers of Enhalus acoroides dominated seagrass meadows. PLoS One, 7(3): e34133.
DOI:10.1371/journal.pone.0034133 |
van Katwijk M M, Bos A R, de Jonge V N, Hanssen L S A M, Hermus D C R, de Jong D J. 2009. Guidelines for seagrass restoration: importance of habitat selection and donor population, spreading of risks, and ecosystem engineering effects. Marine Pollution Bulletin, 58(2): 179-188.
DOI:10.1016/j.marpolbul.2008.09.028 |
Yan X F, Wang Y, Li Y M. 2007. Plant secondary metabolism and its response to environment. Acta Ecologica Sinica, 27(6): 2554-2562.
(in Chinese with English abstract) DOI:10.3321/j.issn:1000-0933.2007.06.050 |
Yang D T, Yang C Y. 2009. Detection of seagrass distribution changes from 1991 to 2006 in Xincun Bay, Hainan, with satellite remote sensing. Sensors, 9(2): 830-844.
DOI:10.3390/s90200830 |
Yu S, Liu S L, Jiang K, Zhang J P, Jiang Z J, Wu Y C, Huang C, Zhao C Y, Huang X P, Trevathan-Tackett S M. 2018. Population genetic structure of the threatened tropical seagrass Enhalus acoroides in Hainan Island, China. Aquatic Botany, 150: 64-70.
DOI:10.1016/j.aquabot.2018.07.005 |
 2023, Vol. 41
2023, Vol. 41


