Institute of Oceanology, Chinese Academy of Sciences
Article Information
- LIU Qiqin, YANG Rui, SUN Xiaoxiao, ZHOU Xinqian, CHEN Haimin
- Biofilm formation under high temperature causes the commensal bacteria Bacillus cereus WPySW2 to shift from friend to foe in Neoporphyra haitanensis in vitro model
- Journal of Oceanology and Limnology, 41(1): 229-240
- http://dx.doi.org/10.1007/s00343-022-1339-3
Article History
- Received Oct. 15, 2021
- accepted in principle Dec. 13, 2021
- accepted for publication Jan. 10, 2022
2 Key Laboratory of Marine Biotechnology of Zhejiang Province, Ningbo University, Ningbo 315211, China
Marine ecosystems contain a large number of diverse and important microorganisms (Whitman et al., 1998; Dang et al., 2011). Many marine microorganisms form "biofilm" on the surfaces of marine life, such as seaweeds and corals, and help protect their hosts from predators, antibiotics, and environmental stress (Wahl et al., 2012). Biofilm formation is used by marine microorganisms to rapidly respond to environmental challenges (Watnick and Kolter, 1999).
The microorganisms that form biofilms differ from their planktonic counterparts in morphology, physiological metabolism, gene expression patterns, and genetic structure (Salaün et al., 2012). The bacteria in biofilms secrete large amounts of extracellular polymeric substances (EPSs), containing glycopeptides, lipids, and lipopolysaccharides that help the bacteria adhere together and form the biofilm frameworks (Flemming and Wingender, 2010). During biofilm formation, the characteristics of the microorganisms and their symbiotic role with the host may change dramatically. For example, the acid resistance of the biofilm cells of Streptococcus mutans was found to be 820 to 70 000 times higher than that of the planktonic cells (Welin-Neilands and Svensäter, 2007). Seneviratne et al. (2010) found that biofilm formation by plant growth-promoting rhizobacteria might further promote the growth and development of the plant.
However, biofilms are not always friendly to their hosts. For example, once intestinal pathogenic bacteria such as Vibrio cholerae colonize the human intestinal tract, they easily cause severe infectious intestinal diseases, and the characteristics of the biofilm make the pathogen difficult to kill with antibiotics (Costerton et al., 1995; Teschler et al., 2015).
Environmental changes may also cause biofilms to harm their hosts. Environmental conditions are the most important factor affecting biofilm formation (Lewis, 2001). Russell et al. (2013) found that ocean acidification and temperature rise increased the primary productivity of intertidal biofilms. Skowron et al. (2019) showed that temperature, pH, salinity, and nutrient availability all affected the biofilm formation and disinfectant susceptibility of Listeria monocytogenes. A change in the composition of the biofilm may affect its function and alter the ecology of its host (Burke et al., 2011). For instance, disruptions of the gut microbiota biofilm structure and function may cause human intestinal diseases (Buret et al., 2019). Zaura et al. (2004) found that nutrients could enhance the metabolic activity of dental plaque, resulting in cariogenicity at plaque biofilm retention sites in the mouth. Therefore, biofilms with composition, morphology, and functions that change under different environments often affect their hosts (Costerton et al., 1995).
Macroalgae (seaweeds) are a common habitat for marine microorganisms. Algae provide nutrients and shelter for other organisms and allow microbes to colonize the algal surface to form a biofilm (Wahl et al., 2012). We previously found that the epiphytic bacteria of Neoporphyra haitanensis (a seaweed cultivated in East Asia), Bacillus sp. WPySW2, promoted the growth of N. haitanensis at 20 ℃ but exacerbated algal decay when the temperature exceeded 28 ℃ (Xiong et al., 2018; Yang et al., 2018). Through genome identification, the strain was named Bacillus cereus WPySW2 (unpublished data; GenBank accession number: CP053289–CP053290). In this study, we found that the morphology of the biofilm formed by B. cereus WPySW2 on algal surfaces varied under different environments. Our hypothesis is that temperature plays a major role in this process. The bacteria shift from beneficial to antagonistic by affecting the biofilm formation process. To address this problem, we studied the biofilm formation characteristics of B. cereus WPySW2 and its morphology and the metabolome changes under different temperatures. The data demonstrated that the environment could modify the ecological functions of some algae-associated bacteria by changing their group aggregation forms and metabolic characteristics. These altered forms can have a detrimental effect on algal health.
2 MATERIAL AND METHOD 2.1 Dual effects of B. cereus WPySW2 on N. haitanensis at different temperatures 2.1.1 Bacterial strain cultivationStrain WPySW2 was isolated from seawater surrounding diseased Neopyropia yezoensis and preserved at the Key Laboratory of Marine Biotechnology of Zhejiang Province (Yang et al., 2008). The strain was activated and cultured in 2216E solid medium (Patrick, 1978) at 37 ℃ for 18–24 h. A single colony was selected and cultured in 2216E liquid medium to the logarithmic phase, and the concentration of bacterial solution was adjusted for subsequent experiments (an OD600 of bacterial solution ≈0.5 was used).
2.1.2 Co-culture of algae and bacteriaThe N. haitanensis thalli were pre-treated with antibiotics (final concentrations: ampicillin 300 μg/mL, kanamycin 100 μg/mL, and gentamicin 100 μg/mL) to remove the epiphytic microorganisms and cut into 1.0-cm2 fragments for further use (Zhou et al., 2012). Sterilized seawater (300 mL) was added to a 500-mL inflatable culture flask, and 0.2 g of pre-treated N. haitanensis thalli was co-inoculated with B. cereus WPySW2 (about 3×108 cells/mL) in the inflatable culture flask. The co-cultivation conditions were 20 ℃ or 28 ℃, 50–70 μmol photons/(m2·s), a 12-h꞉12-h (light꞉dark) photoperiod, and aerated culture. A pure culture of N. haitanensis thalli was used as the control group, and the culture conditions were the same as those used in the co-culture groups. There were three replications for each treatment.
2.1.3 Photosynthetic oxygen release and respiration rate of N. haitanensisThe photosynthetic oxygen release and respiration rates of N. haitanensis were measured after 0, 6, 12, 24, 48, and 72 h of culture. A Clark-type oxygen electrode (Chlorolab 2, Hansatech Instruments Ltd., King's Lynn, Norfolk, UK) was used in this experiment, and the measurement method was described by Liu (2016). Sterile seawater (2 mL) was pre-added into the reaction tank of the Clark-type oxygen electrode, and the temperature of the reaction tank was controlled at 20 ℃ or 28 ℃ using a constant-temperature water bath. After calibration using a two-point correction method, the seaweed thalli (about 0.015 g) were placed into the reaction tank to determine the photosynthetic oxygen release and respiration rates. The respiration rate was measured in complete darkness. The photosynthetic oxygen release rate of algae was measured under 50 μmol/(m2·s) light intensity (halogen light source). Each treatment was set with four parallels, and the experiment was repeated three times.
2.1.4 Morphological changes of algae co-cultured with bacteriaThe N. haitanensis thalli were sampled after culture for 0, 24, 48, and 72 h. The thalli were spread on a glass slide and stained with 200-μL fluorescent dye SYTO9. After 15 min of staining in darkness, the thalli were rinsed with sterile seawater three times, and then observed and photographed using a fluorescent microscope (OLYMPUS BX-60, Olympus Corporation, Tokyo, Japan).
2.1.5 Statistical analysisIBM SPSS Statistics 17.0 software was used for statistical analysis, and one-way ANOVA was used to determine the significance of differences between the treatment groups at each point in time followed by Duncan's and Dunnett's T3 tests. P < 0.05 represented a significant difference.
2.2 Morphological observation of biofilmTwenty milliliters of 2216E liquid medium were added to a 9-cm diameter dish. Each dish was inoculated with 500 μL of B. cereus WPySW2 (refer to 4.1 for the activation of culture) and cultured at 20 ℃, the optimum temperature for the growth of N. haitanensis, or 28 ℃ in order to study algal tolerance to a high temperature. The biofilm formation process was observed and photographed every 6 h.
2.3 Observation of biofilm by confocal laser scanning microscopy (CLSM)A sterile cover glass (24 mm×24 mm) was placed obliquely in a 9-cm petri dish. Then, 500 μL of the above bacterial solution was added to the surface of the cover glass slide and left undisturbed for 2 min to allow the bacteria to adhere. We then added 10 mL of 2216E liquid medium to the petri dish. Bacteria on the cover glass could absorb the nutrients from the liquid medium. Before observation, the cover glass was rinsed gently with 1 mL of distilled water to remove the non-adhering bacteria.
After 6, 12, 18, 24, 48, and 72 h of incubation, the cover glasses received 200 μL of fluorescent dye SYTO9 and PI (L-7012 LIVE/DEAD BacLight Bacterial Viability Kit, Invitrogen Corporation, California, USA). CLSM was observed after 15 min of incubation in darkness (Olympus FV 2000, Olympus Corporation, Tokyo, Japan). The observation conditions included an argon laser (488 nm), HeNe laser (543 nm), objective lens ×20, and water lens ×63. Four replicates were set at each time point, and a blank cover glass placed in 2216E medium was used as a background control.
2.4 Measurement of OD600 value of biofilm and planktonic bacteriaThe cultivation of biofilm refers to the method in Section 2.2. The OD600 values of biofilm bacteria and planktonic bacteria were detected after incubation for 0, 2, 4, 8, 12, 18, 24, 36, 40, 48, and 72 h. The culture medium was filtered with 250-μm and 38-μm sieve silks in succession, and the biofilm remaining on the sieve silks was collected. The absorbance value of the filtrate at 600 nm was detected and considered as the biomass of planktonic bacteria (OD600 (P)). Then the bacterial biofilm was dispersed and fully mixed with the filtrate to measure the total absorptivity OD600 (B+P) of the biofilm and planktonic bacteria. The biofilm biomass OD600 (B)=OD600(B+P)−OD600 (P). We conducted at least six repeated measurements at each time point, and the mean value was used.
2.5 Characteristics of metabolites of B. cereus WPySW2 biofilm 2.5.1 Comparison of metabolites of biofilm in different periodsThe cultivation of biofilm refers to the method in Section 2.1. After incubation for 18, 24, and 40 h, planktonic and biofilm samples of B. cereus WPySW2 in the aggregation, maturation, and disintegration periods were collected. The culture conditions were 28 ℃, with pH 8.0. Each treatment was set up with 10 replications.
2.5.2 Comparison of metabolites of biofilm at different temperaturesThe biofilm samples were collected for metabolite analysis after incubation at 20 ℃ and 28 ℃ for 24 h. Each treatment was set up with 10 replications.
2.5.3 Proton nuclear magnetic resonance (1H-NMR) sample collectionThe collection of biofilm bacteria samples referred to the method in Section 2.4. The filtrate obtained after two filtrations was used as the planktonic bacteria sample. Both collections were centrifuged at 4 ℃ and 4 800×g for 5 min to remove excess medium.
The samples were washed three times with ice-cold phosphate buffer (0.1-mol/L K2HPO4/NaH2PO4, 0.1% NaN3, 0.005% TSP, pH 7.4) and then frozen in liquid nitrogen. All the samples were stored at -80 ℃ until NMR analysis.
Each group of bacteria samples was randomly divided into 10 portions. Each portion (weight=0.4 g) was extracted with 600 μL of 66% aqueous methanol solution by shaking with a TissueLyser (Qiagen/Retsch, Germany) at 5 000 Hz for 30 s, followed by a 30-s ice bath. This procedure was repeated 30 times. After 10 min centrifugation (19 000×g, 4 ℃), the resultant supernatants were lyophilized following the removal of methanol in a vacuum. The extracts were then reconstituted into 600 μL of phosphate buffer containing 100% D2O, 0.005% sodium 3-trimethylsilyl [2, 2, 3, 3-d4] propionate (TSP), and 0.1% NaN3. After centrifugation, 550 μL of the supernatant from each extract was transferred into a 5-mm NMR tube (Norell, ST500-7, USA) for NMR detection.
2.5.4 1H-NMR spectroscopy and data analysisThe 1H NMR experiments were performed according to the procedure described by Shi et al. (2019). The 1H NMR spectra were manually corrected for phase and baseline distortion using TOPSPIN (version 2.0, Bruker Biospin) and referenced to the TSP signal (δ 0.0). After manual phase and baseline correction, the spectral 1H NMR (δ 0.6–9.8) was integrated into regions with equal widths of 0.004×10-6 (2.4 Hz), which excluded the imperfect water saturation (δ 5.02–4.75) and methanol signal (δ 3.38–3.36). Then the integral generated was normalized.
Principal component analysis (PCA), pair-wise orthogonal projection to latent structure discriminant analysis (OPLS-DA), and cross-validated residual (CV-ANOVA) were conducted as described previously (Lin et al., 2020). See the supplementary materials for detailed analysis method.
The specific metabolites were identified according to published NMR data information (Fan, 1996; Ye et al., 2012) and further confirmed with a range of two-dimensional NMR experiments, including homonuclear chemical shift correlation spectroscopy, total correlation spectroscopy, heteronuclear single quantum correlation, and heteronuclear multiple-bond correlation.
3 RESULT 3.1 Effect of high temperature on the co-culture system of algae and bacteriaBacillus cereus WPySW2 had a low colonization density on the surface of N. haitanensis within 48 h at 20 ℃, and only small aggregation areas were observed at 72 h (Fig. 1). This bacterium was more likely to form biofilm on the surface of N. haitanensis thalli under high temperatures. At 28 ℃, the biomass of the strain colonized on the surface of algae increased at 24 h and formed clear flaky areas at 48 h. At 72 h, the bacteria formed a thick biofilm, and it was difficult to observe the N. haitanensis cells under the bacterial biofilm. In addition, B. cereus WPySW2 preferentially gathered at the edges of the thallus, and the bacterial biomass was significantly higher there than in the other parts.
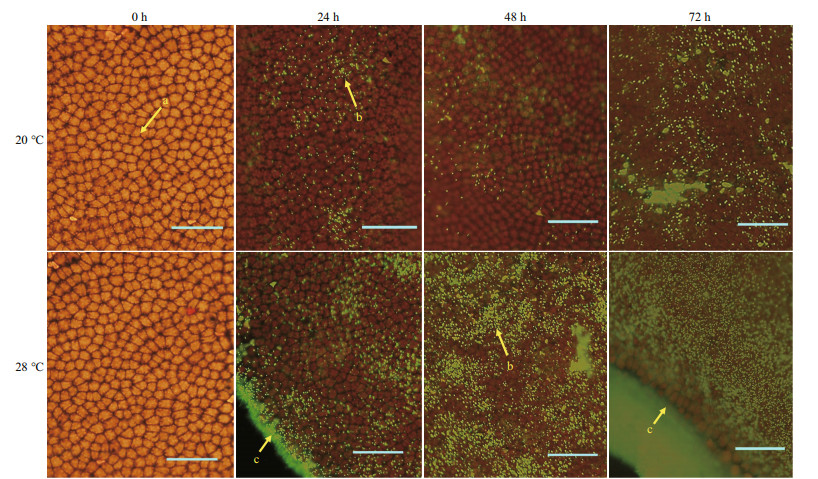
|
| Fig.1 Colonization of Bacillus cereus WPySW2 on the surface of Neoporphyra haitanensis at 20 ℃ and 28 ℃ a. N. haitanensis cells; b. B. cereus WPySW2 cells that emit green fluorescence after being dyed with SYTO9 fluorescent stain; c. edge of N. haitanensis |
Bacillus cereus WPySW2 promoted the photosynthesis of N. haitanensis (P > 0.05) at 20 ℃. High temperatures inhibited the photosynthesis of algae, and the combined effect of bacteria and high temperature produced the greatest damage to algal photosynthesis (72 h, P < 0.05) (Fig. 2a).
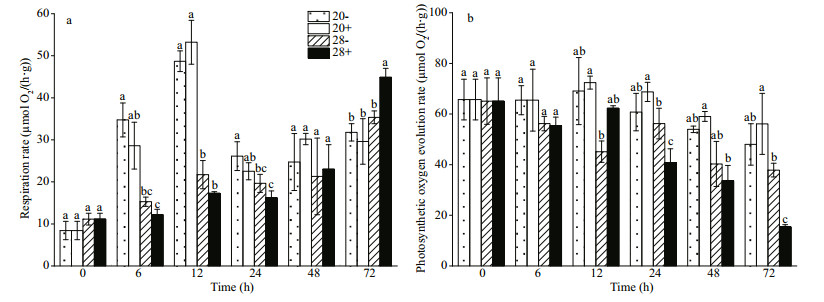
|
| Fig.2 Effect of Bacillus cereus WPySW2 on the photosynthetic oxygen evolution and respiratory rate of Neoporphyra haitanensis at 20 ℃ and 28 ℃ a. effect of B. cereus WPySW2 on the photosynthetic oxygen evolution rate of N. haitanensis at 20 ℃ and 28 ℃; b. effect of B. cereus WPySW2 on respiratory rate of N. haitanensis at 20 ℃ and 28 ℃. 20-: N. haitanensis cultured at 20 ℃, 20+: co-culture group at 20 ℃; 28-: N. haitanensis cultured at 28 ℃; 28+: co-culture group at 28 ℃. Different letters show significant difference at 5% level. |
At 20 ℃, the respiration rates of both the co-cultured (20+) and pure cultured (20−) N. haitanensis significantly increased during the first 12 h of the light period (P < 0.05) and decreased sharply in the following 12 h of the dark period (24 h, Fig. 2b). At 28 ℃, the co-culture (28+) and the pure culture group (28−) did not show a significant change in the first 48 h. However, at 72 h, the respiratory rate in the "28+" group reached the highest of all the experimental groups, and the difference was significant (P < 0.05).
3.2 Characteristics of B. cereus WPySW2 biofilm formation 3.2.1 Morphological characteristics and formation process of B. cereus WPySW2 biofilmThe single colony phenotype was nest-like, white, with a rough dry edge, expanding and protruding upward, and nearly round (Fig. 3a). After incubation in liquid medium for 24 h, a thin milky pellicle formed where the liquid was in contact with the air (Fig. 3b). The biofilm phenotype was dense and complete with a wavy surface texture.
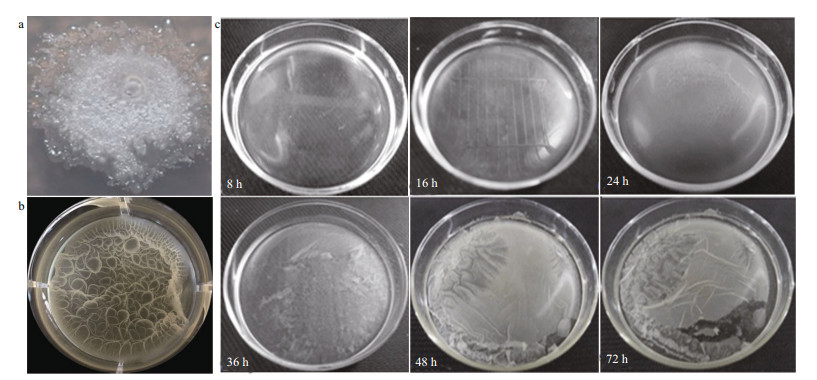
|
| Fig.3 Morphological characteristics and formation process of Bacillus cereus WPySW2 biofilm a. single colony of B. cereus WPySW2 growing on 2216E solid medium at 28 ℃ and pH 8 for 72 h; b. biofilm morphology of B. cereus WPySW2; c. morphological observation during biofilm formation process of B. cereus WPySW2. |
Bacillus cereus WPySW2 was cultured in 2216E liquid medium at 28 ℃ for about 16 h, and the surface of the medium showed an insignificant membrane-like structure (Fig. 3c). At 24 h, the biofilm had thickened to become elastic and viscous, and the biofilm was easily removed from the culture dish. After 48 h, the elasticity and viscosity of the biofilm decreased, and the biofilm was easy to rupture.
The biofilm formation process of B. cereus WPySW2 at 28 ℃ was divided into four periods based on the biofilm phenotype (Fig. 3c), CLSM (Fig. 4a), and biomass detection (Fig. 4b).
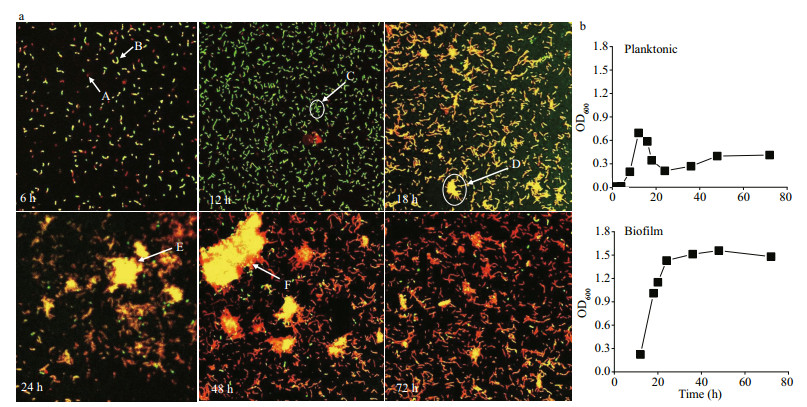
|
| Fig.4 Phenotype and biomass detection of Bacillus cereus WPySW2 during biofilm formation process at 28 ℃ a. confocal laser scanning microscopy (CLSM) observation of the biofilm formation process of B. cereus WPySW2 at 28 ℃. Note: A: the dead bacteria were dyed with red fluorescence; B: the living bacteria were dyed with green fluorescence; C: B. cereus WPySW2 accumulated; D: a large number of bacteria aggregated into the initial biofilm; E: the mature biofilm; F: bacterial death at the edge of the biofilm in the early stage of disintegration; b. OD600 value of planktonic and biofilm bacteria (OD600 value of biofilm was measured from 12 h). |
1) Preparatory period: Most bacteria were in a planktonic state and grew rapidly (Fig. 4b).
2) Aggregation period: When incubated for 12 h to 18 h, the biomass of planktonic bacteria decreased sharply, and the biofilm bacteria accumulated rapidly (Fig. 4b). From 18 h to 24 h, the biofilm thickened (Fig. 3c), and the biomass increased steadily (Fig. 4b). As shown by the yellow fluorescence in Fig. 4a, B. cereus WPySW2 had more living bacteria than dead bacteria when cultured for 18 h. A large amount of bacteria adhered and accumulated, and a bacterial biofilm was initially formed. At 24 h, the mature biofilm structure was formed and the number of dead bacteria increased.
3) Mature period: The dead and living bacteria in the biofilm were in equilibrium (Fig. 4a). A large area of fluorescence appeared in the field of CLSM after 24 h, and the biomass of the biofilm began to stabilize (Fig. 4b).
4) Disintegration period: The proportion of dead bacteria in the B. cereus WPySW2 biofilm greatly increased at 48 h. At 72 h, the bacterial biofilm completely disintegrated, and the dead bacteria outnumbered the living bacteria (Fig. 4a).
3.2.2 Characteristics of metabolites in B. cereus WPySW2 biofilm formationThe 1H-NMR spectra of extracellular metabolites in different periods of B. cereus WPySW2 biofilm formation are shown in Supplementary Fig.S1. A total of 38 small molecule metabolites of B. cereus WPySW2 were identified, including two unknown substances. The substance number, metabolite identification, and attribution information are shown in Supplementary Table S1.
The overall metabolic level of biofilm in the aggregation period was higher than that of planktonic bacteria, while metabolism in the mature and disintegration periods was lower than that of planktonic bacteria (Fig. 5). At the aggregation stage, the content of three amino acids (4, leucine; 16, glutamate; 19, lysine) and dGTP (30) in biofilm was significantly higher than that of free bacteria (P < 0.05). However, the content of most amino acids (4, leucine; 5, valine; 11, alanine; 19, lysine; 28, tyrosine; 29, phenylalanine in Fig. 5b; 10, threonine; 28, tyrosine; 29, phenylalanine in Fig. 5c) and nucleic acid derivatives (24, ribose-5-phosphate; 25, uracil; 27, uridine; 32, hypoxanthine in Fig. 5b, C; 33, adenosine in Fig. 5b) in biofilm was significantly lower than that of planktonic bacteria in the mature and disintegration stages of biofilm (P < 0.05). In addition, the contents of 3-hydroxybutyrate (8) and betaine (20) in the biofilm during the disintegration period were significantly lower than that of planktonic bacteria, whereas the content of adenosine (33) was significantly higher than that of planktonic bacteria (P < 0.05). The content of acetamide (14) in the biofilm was significantly higher than that of the planktonic bacteria in all three stages (P < 0.05).

|
| Fig.5 Orthogonal projection to latent structure discriminant analysis (OPLS-DA) score plots (left) and corresponding color-coded correlation coefficient loading plots (right) generated by comparisons of intracellular extract spectra between biofilm (B1/B2/B3) and planktonic (P1/P2/P3) cells of B. cereus WPySW2 a. aggregation period (18 h); b. mature period (24 h); c. disintegration period (40 h). The R2X value describes the goodness of fit of the model, and the Q2 value describes the predictive capacity of the model. The line color shows the significance of metabolite variations between the two groups. See Supplementary Fig.S1 and Table S1 for a key to the metabolite identification and number. |
As shown in Fig. 6, temperature had a great influence on the formation time and morphology of biofilm. High temperature was more conductive to the rapid maturation of the biofilm. The stability and the biomass of mature biofilm at 28 ℃ were higher than those at 20 ℃ (Fig. 6b).

|
| Fig.6 Effects of temperature on the morphology (a) and biomass (b) of Bacillus cereus WPySW2 biofilms |
The 1H-NMR spectra of the extracellular metabolites of B. cereus WPySW2 biofilm at 24 h under different temperatures are shown in Supplementary Fig.S2. Levels of amino acids such as alanine (11), glutamate (16), glycine (22), nucleic acid metabolites such as ribose 5-phosphate (24) and uracil (25), and other substances like acetamide (14), succinate (17), and betaine (20) in the biofilm at 20 ℃ were significantly higher than those at 28 ℃ (Fig. 7, P < 0.05). These results indicated that the overall metabolic level of biofilm formed at 28 ℃ was lower than that of biofilm formed at 20 ℃.
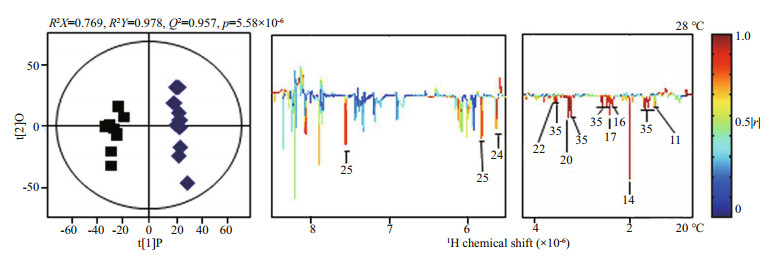
|
| Fig.7 Effects of temperature on and metabolites of Bacillus cereus WPySW2 biofilms Orthogonal projection to latent structure discriminant analysis (OPLS-DA) score plots (left) and corresponding color-coded correlation coefficient loading plots (right) generated by comparisons of the intracellular extract spectra of B. cereus WPySW2 biofilm under different temperatures at pH 8.0. The R2X value describes the goodness of fit of the model, and the Q2 value describes the predictive capacity of the model. The line color shows the significance of metabolite variations between the two groups. See Supplementary Fig.S2 and Table S1 for a key to the metabolite identification and number. |
This study found that high temperature could promote the formation of B. cereus WPySW2 biofilm on the host surface, increase the biomass of biofilm, and have a significant impact on cellular metabolism in biofilm. Thick biofilm inhibited the photosynthesis and respiration of N. haitanensis under high temperature, while the increase in respiratory rate at 72 h might be due to the amplification of bacteria on the algal surface (Fig. 2). These changes eventually led to the transformation of the bacterial role.
Compared with planktonic bacteria, biofilm allows microorganisms to better respond to environmental changes and occupy the advantageous position in the interaction with their host (Costerton et al., 1995). Our discovery of biofilm formation by the seaweed epiphytic bacteria B. cereus WPySW2 supported this finding.
During the aggregation period, the metabolic level of B. cereus WPySW2 biofilm was higher than the level in the planktonic cells. However, during the maturation period, the metabolic level of biofilm was lower than that of the free-living individuals. This tendency toward static stability is a common feature of biofilms (Watnick and Kolter, 2000). There are many persistent cells in a stable biofilm that grow slowly and do not divide, so the metabolism of these cells is low (Lewis, 2005). This group behavior ensures a supply of energy and materials to cope with the poor environment and improve the adaptability of the group (Pysz et al., 2004). For example, 3-hydroxybutyric acid participates in the synthesis and degradation of poly-β-hydroxybutyrate (PHB), which serves as both an electron and a carbon sink in regulating NADH and NAD+ levels in some bacteria (Pötter and Steinbüchel, 2005; Khosravi-Darani et al., 2013). In this study, the content of 3-hydroxybutyric acid in the B. cereus WPySW2 biofilm during the disintegration period was significantly lower than that of planktonic bacteria (P < 0.05). This result indicated that the energy demand of biofilm decreased during the disintegration period. Adenosine was reported to be abolished in the swarming of Pseudomonas aeruginosa during biofilm formation, and this prevented the production of rhamnolipids. This was considered to be an inter-kingdom signal (Sheng et al., 2012).
In this study, the level of acetamide in biofilm in different periods was significantly higher than the level in planktonic bacteria (P < 0.05). Extracellular polymeric substances are major components of the biofilm matrix and are critical for biofilm formation (Itoh et al., 2005). The polysaccharide N-acetyl-D-glucosamine (GlcNAc) has been implicated in biofilm adhesion during biofilm formation (Itoh et al., 2005). GlcNAc can produce acetamide (Sato et al., 1998), which demonstrates that GlcNAc may play a role in the biofilm formation of B. cereus WPySW2. In mature biofilms, cells may divide infrequently, but excess energy is used to produce edible scaffold-extracellular polysaccharides, which cells can digest when needed (Kjelleberg and Molin, 2002). This may explain why the level of overall metabolites in mature biofilms formed by B. cereus WPySW2 was significantly reduced, while the content of acetamide, which may be related to extracellular polysaccharide synthesis, remained high.
Based on the characteristics of low metabolism in the mature stage of biofilm, these findings further supported the view that high temperature accelerates the formation process of biofilm. The results of the metabolome analysis showed that after 24 h of incubation, the level of metabolites in the biofilm under high temperature was significantly lower than that in the low-temperature group. This indicated that the high temperature caused the bacteria to enter the biofilm maturation stage of a low metabolic level more quickly, which made the bacteria more competitive. In aquatic systems, temperature is important in biological deposition and biofilm formation (Bott, 1995). In a natural water environment, increased temperature increases microorganism metabolism, and biofilm formation is more rapid at high temperatures (Rao, 2010; Villanueva et al., 2011).
It should be noted that compared with the low-temperature group, high temperature did not cause the production or increased abundance of some toxic metabolites in B. cereus WPySW2. This showed that the stress caused by B. cereus WPySW2 on seaweed was mainly due to changes in the morphology and biomass of its biofilm, rather than toxins.
Under the background of global climate change, seawater warming and acidification pose increasing risks to marine life. For example, many studies have been conducted on the effect of temperature on kelp and coral (Fulton et al., 2014; Gaitán-Espitia et al., 2014; Tait, 2014). Song et al. (2014) found that, under high-temperature and low-pH conditions, the inhibitory effect of Pseudomonas sp. NPyS3 on algae was greater than that under natural conditions. This means that when the host is under abiotic stress, it may also suffer from stress due to algal microorganisms, and the stress caused by microorganisms is much greater than environmental stress.
The ability of marine microorganisms to adapt to the environment is greater than that of their hosts (Rosenberg et al., 2007). The bacterial biofilm demands more nutrition to promote biomass. Meanwhile, high temperature may cause some algae, such as N. haitanensis, to suffer damage and even cause algal decay (Yang et al., 2013), which initially promotes the formation, growth, and stability of bacterial biofilm. The stable and thick biofilm covers the surface of the algae and blocks the exchange of nutrients and air within the host. This change transforms the interaction between the bacteria and its host. Their ecological function also changed from "ally" to "foe" in the in vitro model used in this study. This further confirms that changes in the number and morphology of microorganisms under high temperature conditions are important factors that exacerbate imbalance in the algae population.
Research on the mechanism of opportunistic pathogenicity has been a consistent focus of pathologists. Virulence factors, including adhesion, toxins, and biofilms, are all important for bacterial pathogens (Dinges et al., 2000; Coppa et al., 2006; Teschler et al., 2015). In regard to the relationship between biofilm and disease, many case studies have focused on animal and plant diseases, while little research has been conducted on algal disease. This study reported that the ecological function of B. cereus WPySW2 changed due to environmental factors and verified that the form of its biofilm may have been the main cause of this change. This will provide new directions for the comprehensive analysis of opportunistic pathogenic algal mechanisms, especially in the discussion of "environment-pathogen-host" relationships.
5 CONCLUSIONBacillus cereus WPySW2 can form a thick, stable biofilm under high temperature, which causes its ecological function to change from being a probiotic to accelerating disease in algae. In this study, using B. cereus and N. haitanensis for the in vitro model, methods including morphological observation, dynamic tracking, and metabolomic analysis were used to investigate the interaction between epiphytic bacteria and algae, especially for the biofilm on the surface of the algae. In the future, these methods can be combined with genome and transcriptome analysis technology to further explore the mechanisms of "algae-bacteria" and "bacteria-bacteria" interactions under the action of environmental factors.
6 DATA AVAILABILITY STATEMENTThe datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Supplementary material (Supplementary Table S1 and Figs.S1–S2) is available in the online version of this article at https://doi.org/10.1007/s00343-022-1339-3.
Bott T R. 1995. Fouling of Heat Exchangers. Elsevier, Amsterdam, Netherlands. 315p.
|
Buret A G, Motta J P, Allain T, et al. 2019. Pathobiont release from dysbiotic gut microbiota biofilms in intestinal inflammatory diseases: a role for iron?. Journal of Biomedical Science, 26(1): 1.
DOI:10.1186/s12929-018-0495-4 |
Burke C, Thomas T, Lewis M, et al. 2011. Composition, uniqueness and variability of the epiphytic bacterial community of the green alga Ulva australis. The ISME Journal, 5(4): 590-600.
DOI:10.1038/ismej.2010.164 |
Coppa G V, Zampini L, Galeazzi T, et al. 2006. Human milk oligosaccharides inhibit the adhesion to Caco-2 cells of diarrheal pathogens: Escherichia coli, Vibrio cholerae, and Salmonella fyris. Pediatric Research, 59(3): 377-382.
DOI:10.1203/01.pdr.0000200805.45593.17 |
Costerton J W, Lewandowski Z, Caldwell D E, et al. 1995. Microbial Biofilms. Annual Review of Microbiology, 49: 711-745.
DOI:10.1146/annurev.mi.49.100195.003431 |
Dang H Y, Chen R P, Wang L, et al. 2011. Molecular characterization of putative biocorroding microbiota with a novel niche detection of Epsilon- and Zetaproteobacteria in Pacific Ocean coastal seawaters. Environmental Microbiology, 13(11): 3059-3074.
DOI:10.1111/j.1462-2920.2011.02583.x |
Dinges M M, Orwin P M, Schlievert P M. 2000. Exotoxins of Staphylococcus aureus. Clinical Microbiology Reviews, 13(1): 16-34.
DOI:10.1128/CMR.13.1.16-34.2000 |
Fan T W M. 1996. Metabolite profiling by one- and two-dimensional NMR analysis of complex mixtures. Progress in Nuclear Magnetic Resonance Spectroscopy, 28(2): 161-219.
DOI:10.1016/0079-6565(95)01017-3 |
Flemming H C, Wingender J. 2010. The biofilm matrix. Nature Reviews Microbiology, 8(9): 623-633.
DOI:10.1038/nrmicro2415 |
Fulton C J, Depczynski M, Holmes T H, et al. 2014. Sea temperature shapes seasonal fluctuations in seaweed biomass within the Ningaloo coral reef ecosystem. Limnology and Oceanography, 59(1): 156-166.
DOI:10.4319/lo.2014.59.1.0156 |
Gaitán-Espitia J D, Hancock J R, Padilla-Gamiño J L, et al. 2014. Interactive effects of elevated temperature and pCO2 on early-life-history stages of the giant kelp Macrocystis pyrifera. Journal of Experimental Marine Biology and Ecology, 457: 51-58.
DOI:10.1016/j.jembe.2014.03.018 |
Itoh Y, Wang X, Hinnebusch B J, et al. 2005. Depolymerization of β-1, 6-N-Acetyl-D-glucosamine disrupts the integrity of diverse bacterial biofilms. Journal of Bacteriology, 187(1): 382-387.
DOI:10.1128/JB.187.1.382-387.2005 |
Khosravi-Darani K, Mokhtari Z B, Amai T, et al. 2013. Microbial production of poly(hydroxybutyrate) from C1 carbon sources. Applied Microbiology and Biotechnology, 97(4): 1407-1424.
DOI:10.1007/s00253-012-4649-0 |
Kjelleberg S, Molin S. 2002. Is there a role for quorum sensing signals in bacterial biofilms?. Current Opinion in Microbiology, 5(3): 254-258.
DOI:10.1016/S1369-5274(02)00325-9 |
Lewis K. 2001. Riddle of biofilm resistance. Antimicrobial Agents and Chemotherapy, 45(4): 999-1007.
DOI:10.1128/AAC.45.4.999-1007.2001 |
Lewis K. 2005. Persister cells and the riddle of biofilm survival. Biochemistry (Moscow), 70(2): 267-274.
DOI:10.1007/s10541-005-0111-6 |
Lin W C, Ren Z M, Mu C K, et al. 2020. Effects of elevated pCO2 on the survival and growth of Portunus trituberculatus. Frontiers in Physiology, 11: 750.
DOI:10.3389/fphys.2020.00750 |
Liu T. 2016. Experimental technology of macroalgae. China Ocean Press, China. p. 90-93. (in Chinese)
|
Patrick F M. 1978. The use of membrane filtration and marine agar 2216E to enumerate marine heterotrophic bacteria. Aquaculture, 13(4): 369-372.
DOI:10.1016/0044-8486(78)90186-2 |
Pötter M, Steinbüchel A. 2005. Poly(3-hydroxybutyrate) granule-associated proteins: impacts on poly(3-hydroxybutyrate) synthesis and degradation. Biomacromolecules, 6(2): 552-560.
DOI:10.1021/bm049401n |
Pysz M A, Conners S B, Montero C I, et al. 2004. Transcriptional analysis of biofilm formation processes in the anaerobic, hyperthermophilic bacterium Thermotoga maritima. Applied and Environmental Microbiology, 70(10): 6098-6112.
DOI:10.1128/AEM.70.10.6098-6112.2004 |
Rao T S. 2010. Comparative effect of temperature on biofilm formation in natural and modified marine environment. Aquatic Ecology, 44(2): 463-478.
DOI:10.1007/s10452-009-9304-1 |
Rosenberg E, Koren O, Reshef L, et al. 2007. The role of microorganisms in coral health, disease and evolution. Nature Reviews Microbiology, 5(5): 355-362.
DOI:10.1038/nrmicro1635 |
Russell B D, Connell S D, Findlay H S, et al. 2013. Ocean acidification and rising temperatures may increase biofilm primary productivity but decrease grazer consumption. Philosophical Transactions of the Royal Society Series B: Biological Sciences, 368(1627): 20120438.
DOI:10.1098/rstb.2012.0438 |
Salaün S, La Barre S, Santos-Goncalvez M D, et al. 2012. Influence of exudates of the kelp Laminaria Digitata on biofilm formation of associated and exogenous bacterial epiphytes. Microbial Ecology, 64(2): 359-369.
DOI:10.1007/s00248-012-0048-4 |
Sato H, Mizutani S I, Tsuge S, et al. 1998. Determination of the degree of acetylation of chitin/chitosan by pyrolysis-gas chromatography in the presence of oxalic acid. Analytical Chemistry, 70(1): 7-12.
DOI:10.1021/ac9706685 |
Seneviratne G, Weerasekara M L M A W, Seneviratne K A C N et al. 2010. Importance of biofilm formation in plant growth promoting rhizobacterial action. In: Maheshwari D K ed. Plant Growth and Health Promoting Bacteria. Springer Berlin Heidelberg, Berlin, Heidelberg. p. 81-95.
|
Sheng L L, Pu M M, Hegde M, et al. 2012. Interkingdom adenosine signal reduces Pseudomonas aeruginosa pathogenicity. Environmental Microbiology, 5(4): 560-572.
DOI:10.1111/j.1751-7915.2012.00338.x |
Shi C, Xia M J, Li R H, et al. 2019. Vibrio alginolyticus infection induces coupled changes of bacterial community and metabolic phenotype in the gut of swimming crab. Aquaculture, 499: 251-259.
DOI:10.1016/j.aquaculture.2018.09.031 |
Skowron K, Wałecka-Zacharska E, Grudlewska K, et al. 2019. Disinfectant susceptibility of biofilm formed by Listeria monocytogenes under selected environmental conditions. Microorganisms, 7(9): 280.
DOI:10.3390/microorganisms7090280 |
Song D D, Yang R, Ren J R, et al. 2014. Effects of environmental pH and Psuedoalteromonas sp. NPyS3 on the Pleurochrysis carterae. Journal of Biology, 31(5): 50-54.
(in Chinese with English abstract) DOI:10.3969/j.issn.2095-1736.2014.05.050 |
Tait L W. 2014. Impacts of natural and manipulated variations in temperature, pH and light on photosynthetic parameters of coralline-kelp assemblages. Journal of Experimental Marine Biology and Ecology, 454: 1-8.
DOI:10.1016/j.jembe.2014.01.016 |
Teschler J K, Zamorano-Sánchez D, Utada A S, et al. 2015. Living in the matrix: assembly and control of Vibrio cholerae biofilms. Nature Reviews Microbiology, 13(5): 255-268.
DOI:10.1038/nrmicro3433 |
Villanueva V D, Font J, Schwartz T, et al. 2011. Biofilm formation at warming temperature: acceleration of microbial colonization and microbial interactive effects. Biofouling, 27(1-2): 59-71.
DOI:10.1080/08927014.2010.538841 |
Wahl M, Goecke F, Labes A, et al. 2012. The second skin: ecological role of epibiotic biofilms on marine organisms. Frontiers in Microbiology, 3: 292.
DOI:10.3389/fmicb.2012.00292 |
Watnick P I, Kolter R. 1999. Steps in the development of a Vibrio cholerae El Tor biofilm. Molecular Microbiology, 34(3): 586-595.
DOI:10.1046/j.1365-2958.1999.01624.x |
Watnick P, Kolter R. 2000. Biofilm, city of microbes. Journal of Bacteriology, 182(10): 2675-2679.
DOI:10.1128/JB.182.10.2675-2679.2000 |
Welin-Neilands J, Svensäter G. 2007. Acid tolerance of biofilm cells of Streptococcus mutans. Applied and Environmental Microbiology, 73(17): 5633-5638.
DOI:10.1128/AEM.01049-07 |
Whitman W B, Coleman D C, Wiebe W J. 1998. Prokaryotes: the unseen majority. Proceedings of the National Academy of Sciences of the United States of America, 95(3): 6578-6583.
DOI:10.1073/pnas.95.12.6578 |
Xiong Y Q, Yang R, Yang H T, et al. 2018. Effects of phycosphere Bacillus sp. WPySW2 on physiology of Pyropia haitanensis. Journal of Biology, 35(1): 37-41.
(in Chinese with English abstract) DOI:10.3969/j.issn.2095-1736.2018.01.037 |
Yang H T, Xiong Y Q, Yang R. 2018. Effects of Bacillus sp. on Pyropia haitanensis at high temperature. Journal of Fisheries of China, 42(7): 1009-1018.
(in Chinese with English abstract) |
Yang R, Fang W Y, Shan Y Y, et al. 2008. Genetic diversity of epiphytic bacteria in Porphyra yezoensis. Acta Oceanologica Sinica, 30(4): 161-168.
(in Chinese with English abstract) DOI:10.3321/j.issn:0253-4193.2008.04.020 |
Yang R, Liu W, Zhang X L, et al. 2013. Sequences of Mn-sod gene from Pyropia haitanensis (Bangiales, Rhodophyta) and its expression under heat shock. Botanica Marina, 56(3): 249-259.
DOI:10.1515/bot-2012-0178 |
Ye Y F, Zhang L M, Hao F H, et al. 2012. Global metabolomic responses of Escherichia coli to heat stress. Journal of Proteome Research, 11(4): 2559-2566.
DOI:10.1021/pr3000128 |
Zaura E, ten Cate J. 2004. Dental plaque as a biofilm: a pilot study of the effects of nutrients on plaque pH and dentin demineralization. Caries Research, 38(S1): 9-15.
DOI:10.1159/000074357 |
Zhou J M, Yang R, Fang W Y, et al. 2012. Study on the axenic culture and application of Porphyra haitanensis thallus. Journal of Biology, 29(3): 83-87.
(in Chinese with English abstract) DOI:10.3969/j.issn.2095-1736.2012.03.083 |
 2023, Vol. 41
2023, Vol. 41


