Institute of Oceanology, Chinese Academy of Sciences
Article Information
- CAO Pan, LIU De, LIU Yuhan, WANG Huming, ZHANG Chao, YUAN Chengqing, LIU Xiaodan
- Marine antifouling behavior of the surfaces modified by dopamine and antibacterial peptide
- Journal of Oceanology and Limnology, 41(1): 174-188
- http://dx.doi.org/10.1007/s00343-021-1270-z
Article History
- Received Aug. 21, 2021
- accepted in principle Nov. 4, 2021
- accepted for publication Dec. 13, 2021
2 Reliability Engineering Institute, National Engineering Research Center for Water Transport Safety, Wuhan University of Technology, Wuhan 430063, China;
3 College of Animal Science and Technology, Yangzhou University, Yangzhou 225009, China
Marine biofouling is a widespread biological phenomenon that refers to damage to underwater installations due to access and infestation by marine organisms (Xie et al., 2020; Yu et al., 2021) and can significantly harm the economic aspects, environmental and ecological aspects, and safety aspects of the marine industry (He et al., 2019; Chen et al., 2021). In economic aspects, bio-siltation attached to the hull surface increases the weight of the ship and surface friction resistance, leading to higher fuel consumption (Kirschner and Brennan, 2012; Brzozowska et al., 2014). In other areas of the marine industry, biofouling adheres to fishing nets, causing deformation of nets and thus reducing fish production with economic losses (Bloecher and Floerl, 2020). In the field of sensors used for marine monitoring, biofouling adherence reduces the precision of sensor performance (Brzozowska et al., 2014). Biofouling erodes various surfaces, which increases the maintenance costs with big economic losses (Gu et al., 2020). The overall cost of marine biofouling reached over $150 billion per year (Nurioglu et al., 2015). In environmental and ecological aspects, biofouling increases fiction of the ship sailing with more fuel consumption and more emissions (Liu et al., 2018). In terms of safety, biofouling adheres to surfaces of metal or concrete and release corrosive biogenic acids, affecting normal functioning of ships, marine equipment, and facilities, shortening their lifespan, and posing a safety hazard (Gu et al., 2020; Vishwakarma, 2020).
Various strategies have been proposed against biofouling. Application of antifouling coatings containing heavy metal ions such as tin, copper, and zinc is the most common practice; however, due to their non-targeted biological toxicity, heavy metal ions accumulate in marine organisms and have harmful effects on marine ecosystems (Sonak et al., 2009; Selim et al., 2018). Therefore, non-toxic silicon-based or fluorine-based materials are studied. However, the disadvantages are obvious which restrict their applications, because the release of silicon-based or fluorine-based coatings relies heavily on scouring by water current, and their mechanical properties are poor and easy to fall off from substrates (Silva et al., 2019). Thus, to develop green, strong, and wide-range adhesion anti-fouling coatings became an urgent task.
Compared with conventional antifouling coatings, natural antifouling agents are mostly bio-derived organic compounds with better biocompatibility and degradability. Several literature reviews and studies were recently published on the application of new green antifouling coatings based on natural antifouling agents in marine field (Saha et al., 2018; Jiang et al., 2020; Pinteus et al., 2020). Salam et al. (2018) extracted bioactive metabolites from three macroalgae. After three-month field test, extracts of Turbinaria ornata and Sargassum polycystum significantly reduced the development of biofouling on nylon plates, showing potential of the extracts against antifouling using natural products. Feng et al. (2018) investigated the antifouling properties of 18 alkaloids from terrestrial plants. They conducted a 1-year marine field antifouling study, showing that camptothecin could significantly inhibit marine biofouling; and through toxicity studies on Phaeodactylum tricornutum and Isochrysis galbana, camptothecin showed goodbiocompatibility. Thus, this alkaloid was shown with application potential for marine antifouling coatings. Surface functionalization of the material by coating green antifouling coatings on surface of the substrate enhances the antifouling performance and corrosion resistance of the material surface.
In using antifouling materials in harsh marine environments, the nature of the substrate material and the strength of adhesion of the surface coating are issues that must be considered in the design. Stainless steel (SS) features good biocompatibility, excellent corrosion resistance, and wide application in marine industry (Yuan et al., 2012; Kim et al., 2015; Cao et al., 2019). Furthermore, the self-polymerization product of dopamine (DA), polydopamine, has been shown to have strong adhesion to almost all types of organic and inorganic materials (Lee et al., 2009; Lee et al., 2011). More importantly, polydopamine includes many functional groups, such as catechols, amines, and imines. They can be used as reactive groups for further functionalization as well as for designing and obtaining desired functional materials (Liu et al., 2014), which inspired the design of a new composite marine antifouling coating. Yuan et al. (2011) prepared a novel antifouling surface with antifouling poly (ethylene glycol) monomethacrylate (PEGMA) brushes on 304 SS surface using dopamine and a natural defensive enzyme and this antifouling surface showed excellent antifouling properties and corrosion resistance. Zhang et al. (2019) have produced a functionalized SS substrate with good antifouling and anticorrosion properties by firmly anchoring controlled polycationic brushes to a steel substrate through a bionic polydopamine layer. Antimicrobial peptides (AMP) are biomolecules secreted by many organisms (microorganisms, vegetables, fish, and mammals) to protect themselves against microbial invasion (Gura, 2001). Studies have shown that antimicrobial peptides sterilize through initial electrostatic interactions between positively charged peptides and negatively charged bacterial cell membranes, thus consequently disrupt biofilms on the surface of metallic materials through the amphiphilic nature of peptides (Yeaman and Yount, 2003; Brogden, 2005). Formation of microbial films is the initial and critical stage of biofouling, and blocking the formation could inhibit microbial adhesion. In recent years, various methods have been reported for coating antimicrobial peptides onto the surface of materials, such as layer stacking (LBL), chemical coupling, and self-assembly of monomers (SAMS) polymer brushing methods (Love et al., 2005; Glinel et al., 2012). Under weak alkaline conditions, DA is readily deposited on the SS surface, providing binding sites for subsequent antimicrobial peptide immobilization (Lee et al., 2007; Jeong et al., 2018). Cao et al. (2020b) successfully fabricated antibacterial surfaces with excellent properties by coating antimicrobial peptide MAG Ⅱ onto 304 SS surfaces using dopamine as the coupling agent. Turgencin BMox2 (TB), a novel peptide from residues of N-terminal peptide sequence containing 35 residues, 6 cysteines, and 2 methionine that isolated from Arctic sea squirts, has broad-spectrum antibacterial activity against gram-positive and gram-negative bacteria (Hansen et al., 2020).
In contrast to previously reported surface coupling methods that requiring extensive surface modification and complex reaction steps (Gabriel et al., 2006; Mishra et al., 2013). This study describes a novel strategy for the effective immobilization of AMP (TB) onto 304 SS. Firstly, the surface of 304 SS was treated with dopamine and subsequently the dopamine-treated surface was modified with TB, thereby imparting strong antifouling properties to the 304 SS. The surface physicochemical properties of the functionalized material and the presence of the antimicrobial peptide were examined using surface characterization instruments. Vibrio natriegens and P. tricornutum were selected for adhesion tests to study the antifouling properties of the modified surfaces. Moreover, the corrosion resistance of the modified samples and the longevity of the coating were observed in a 14-day experiment, showing excellent corrosion resistance and good cytocompatibility of the antimicrobial peptide. This work may provide a promising strategy against antifouling on marine equipment surfaces.
2 MATERIAL AND METHOD 2.1 MaterialDopamine hydrochloride (purity 98%), Tris (hydroxymethyl) aminomethane (Tris ≥99.9%), propidium iodide (PI, Sigma), and glutaraldehyde (purity 50%) were obtained from Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). 2216E liquid medium and 2216E agar were purchased from Qingdao Hope Bio-Technology Co., Ltd. (Qingdao, China). Phosphate buffered saline (PBS, pH=7.4) was supplied by Beijing Wokai Biotechnology Co., Ltd. (Beijing, China). Ethanol absolute (≥99.7%) was acquired from Sinopharm Group Chemical Reagent Co., Ltd. (Shanghai, China). TB (amino acid sequence: GIKEMLCNMACAQTVCKKSG GPLCDTCQAACKALG) was purchased from Dgpeptides Co., Ltd. (purity 93.63%, molecular weight: 3 548.76, its spatial structure is shown in Supplementary Fig.S1). All of the chemical reagents used in our work were analytically pure and were used with no further purification.
2.2 Preparation of SS-DA-TBThe surfaces of 304 SS Φ10 mm×1 mm were polished with sandpaper of #400, #800, #1 000, and #1 200 in turn for 2 min each, then polished on polishing cloth using silica polishing solution with a precision grade of 0.05 μm until it showed a bright luster. The samples were cleaned with anhydrous ethanol and deionized water for 20 min each ultrasonically in turn and finally dried in oven. Afterward, dopamine was dissolved in Tris-HCl buffer (pH=8.5) to a dopamine concentration of 2 mg/mL, and then the treated samples were immersed in dopamine solution and shaken slowly (40 r/min) for 6 h. Samples were removed and washed three times with deionized water. The dopamine-treated samples were then placed in 24-well cell culture plates and 1-mL TB (Tris-HCl buffer) in concentration of 0.5 mg/mL was added to each well and incubated at 37 ℃ for 6 h. And again, samples were removed, washed, and dried before use. The samples whose surfaces were modified with dopamine and TB are denoted as SS-DA-TB.
2.3 Characterization of surface physicochemical propertiesFourier transform infrared spectrometer (FTIR) measurements were carried out using an attenuated total reflection (ATR)-FTIR spectrometer (Nicolet Is50) in the scanning range from 4 000 cm-1 to 400 cm-1 for 128 scans in spectral resolution of 4 cm-1. The surface chemical compositions of the samples were analyzed in X-ray photoelectron spectroscopy (XPS, Escalab 250xi, USA). The water contact angle of sample surface was evaluated by a contact angle-measuring instrument (JC2000D, China). The surface morphology was characterized via an atomic force microscope (AFM, SPM 9700, Japan). The roughness was characterized by a 3D optical profilometer (Contour GT-K, Bruker, USA). Spectroscopic ellipsometry (SE 850 DUV, Sentech, Germany) was chosen to measure the thickness of organic films on the sample surfaces. The coating thickness was determined by previously reported methods (Bernsmann et al., 2009; Zhu and Edmondson, 2011). The wavelength range of 500–700 nm was selected to avoid strong light absorption of polydopamine at shorter wavelengths (Bernsmann et al., 2009). The refractive index of dopamine at all wavelengths is supposed n=1.6 with no optical absorption (i.e., the non-zero imaginary part of the refractive index k=0) (Zhu and Edmondson, 2011). Absorption with k=0.02 was added to all wavelengths, as measured previously for polydopamine at 589 nm (Bernsmann et al., 2009). V. natriegens and P. tricornutum were selected as thetarget fouling organisms. A confocal laser-scanning microscope (CLSM, Leica TCS SP8 STED, Germany) was used to evaluate the antifouling performance of the sample surfaces. Fluorescence inverted microscopy (FIM, Axiovert A1, Germany) was employed to study the morphology of cells on the sample surface and the toxicity of peptide to the cells was analyzed. To ensure the accuracy of data, three quantitative measurements were taken for each group.
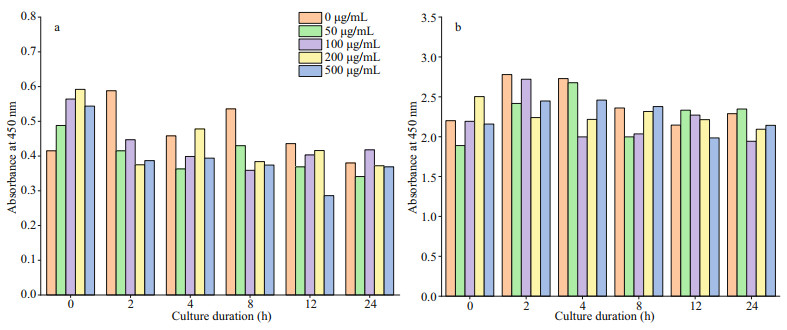
2.4 Cell compatibility assayThe CCK-8 kit was used to evaluate the biocompatibility of peptides after 0, 2, 4, 8, 12, and 24 h of co-culture with cells. Striated Channa cells (E11) and human renal epithelial cells (293T) were used to analyze the toxicity of the antimicrobial peptide to fish cells and human cells. The cell solution was added into 96-well plate (100 μL/well) in concentration of 2 000 cells/mL and incubated at 37 ℃ in 5% CO2 volume fraction in humidity-saturated incubator for 24 h. Then, 10 μL/well of different concentrations (0, 50, 100, 200, and 500 μg/mL) of antimicrobial peptide solution was added to continue incubation for the appropriate time. Three replicate wells were set up for each concentration group, and the blank control wells were set up without cytosol. Finally, CCK-8 solution was added to co-culture with the cell solution for a appropriate time. The absorbance at 450 nm was measured by an enzyme marker.
The morphology of cells incubated in the antimicrobial peptide solution was observed directly under natural light using an inverted fluorescent microscope.
2.5 Antifouling testing 2.5.1 Bacterial adhesion testVibrio natriegens, a common marine gram-negative bacterium (Cheng et al., 2009; Cao et al., 2020a), was used to evaluate the antibacterial capacity of SS-DA-TB. First, V. natriegens was cultured on 2216E Agar culture plate in incubator at 37 ℃ for 24 h, and then the monoclonal bacteria were picked to obtain V. natriegens solution in concentration of 1×106 colony-forming unit (CFU)/mL (37 ℃, 180 r/min, 48 h). Secondly, the untreated sample and SS-DA-TB were cultured in V. natriegens solution (37 ℃, 48 h), while the treated samples were washed with sterile PBS solution to remove unadhered or loosely attached bacteria. SS-DA-TB was then immersed into 2.5% glutaraldehyde solution (4 ℃, 12 h) and stained with a sterile PBS solution containing PI in concentration of 50 μg/mL for 30 min in dark. CLSM was chosen to take bacterial adhesion images after being washed in sterile PBS solution. The excitation wavelength in the experiment was 535 nm and the emission wavelength was 615 nm.
2.5.2 Marine algae attachment assaysPhaeodactylum tricornutum was selected to evaluate the anti-algae performance of SS-DA-TB. The untreated sample and SS-DA-TB was immersed individually in P.tricornutum solution with a concentration of 5×106 CFU/mL configured in artificial seawater (ASW), and then immersed in a light incubator at 25 ℃, 12 h꞉12 h cycle day and night (7 d). The sterilized ASW was used to wash incubated surfaces to remove any non-adherent or loosely adherent diatom. Next, the samples were immersed in 2.5% glutaraldehyde solution at 4 ℃ for 2 h to allow algae be fixed onto the sample surface. The chlorophyll of algae is self-fluorescent and can be used for CLSM testing without dyeing to observe the attachment of P. tricornutum at the sample surface.
The attachment rate of algae and bacteria can intuitively reflect the attachment result of seaweed and bacteria to the material surface, of which the calculation formula is:
Adhesion rate (%)=bacterial attachment area ÷ total area of the view field×100%.
The adhesion rate was analyzed by ImageJ. All quantitative measurements were conducted in triplication per group.
2.6 Corrosion resistance testingThe corrosion resistance of samples before and after modification was evaluated in the electrochemical workstation CHI600E with Gamry software. The sterilized original samples and modified samples were encapsulated with epoxy resin. The circular working surface with an area of 1.0 cm2 was polished and soaked in 2216E culture solution for cultivating V. natriegens, and then placed on a shaking table for culture (30 ℃, 120 r/min). On Days 1, 7, and 14, samples were taken out for the corrosion experiment.
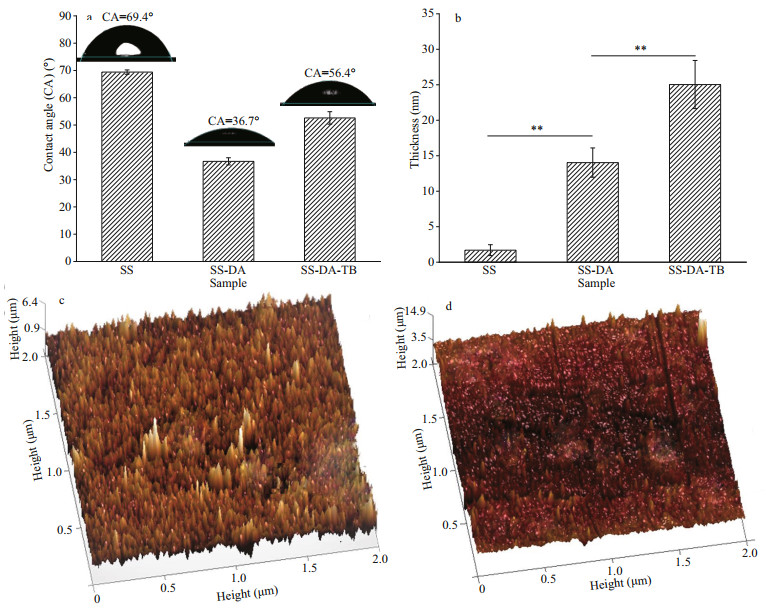
In our electrochemical experiment, platinum electrode was the auxiliary electrode, and the saturated glycerol electrode was the reference electrode. The sample tested was the working electrode, and the working surface was 1.0 cm2. To obtain the polarization curve and electrochemical impedance spectroscopy (EIS), samples were scanned in the range of -0.75 V to 0.5 V at the rate of 5 mV/s at room temperature. By fitting and analyzing the polarization curve (Tafel diagram), the cathode (βc) and anode (βa) Tafel slopes of the samples before and after modification, corrosion potential (Ecorr), corrosion current density (icorr), and instantaneous corrosion rate (CR) were obtained. The inhibition efficiency (IE) of the surface-functionalized coupons was calculated as per Zhang et al. (2019):
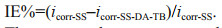
The icorr-SS and icorr-SS-DA-TB are the corrosion current densities of the original SS and modified samples, respectively, as determined by the Tafel polarization curve analysis.
2.7 Robustness assaySS-DA-TB samples were soaked in sterilized artificial seawater for 1, 7, and 14 days with gentle shaking (30 r/min). Afterward, samples were taken and were placed in super-clean platform for natural air drying. Later, the SS-DA-TB samples were tested for bacterial resistance and algae resistance determination using the method stated in Sections 2.5.1 and 2.5.2.
2.8 Statistical analysisTo ensure the accuracy of the data, three quantitative measurements were taken for each group and all the difference between SS, SS-DA, and SS-DA-TB was determined by a standard student t-test (P 0.05 stands for no significance, 0.01≤P < 0.05 is considered statistically significant, and P < 0.01 is considered statistically very significant).
3 RESULT AND DISCUSSION 3.1 Coating TB onto 304 SSModification of 304 SS with dopamine and antimicrobial peptide is illustrated in Fig. 1. Under weak alkaline condition, the catechol group of dopamine is easily oxidized to form a dopamine quinone compound with a phylloquinone structure. The anti-disproportionation reaction between dopamine and dopamine quinone produces semiquinone free radicals, which are then coupled to form cross-linked bonds; and at the same time, a tightly attached polydopamine composite layer is formed on the surface of the matrix material (Lee et al., 2007; Kim et al., 2018). After immersing into the dopamine solution, dopamine molecules underwent oxidative polymerization and bonded to the surface of the 304 SS to obtain a dopamine-treated metal surface (SS-DA). In addition, the active NH2/-NH-on the molecular chain of TB is easy to covalently bond with the quinone compound formed by the oxidative polymerization of dopamine (Jiang et al., 2010; Ozaltin et al., 2016). The surface of SS-DA was modified with TB to obtain a new surface of SS-DA-TB with antifouling properties.
|
| Fig.1 Schematic illustration of the synthesis of modified surfaces |
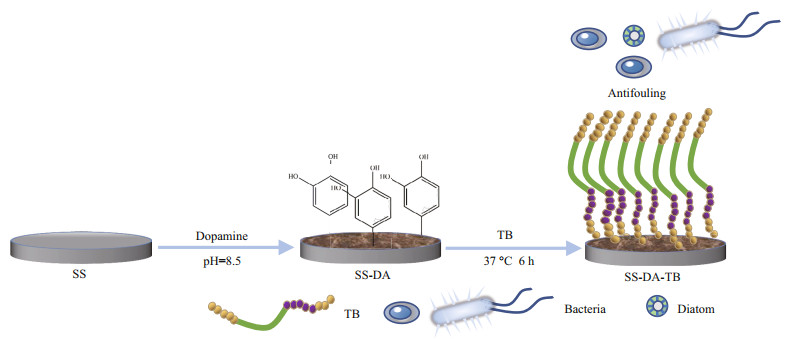
The chemical structure of the coatings was analyzed with ATR-FTIR, and the results are illustrated in Fig. 2. After surface modification, the peak at 3 300–3 500 cm-1 was contributed to Amide A of peptide, which demonstrated the existence of -NH2, -NH, -OH, and other related functional groups. The two peaks at 2 800–3 000 cm-1 indicate the presence of alkyl groups on the sample surface. The peak at 1 717–1 777 cm-1 reflects symmetric or asymmetric C=O vibration. The peak at 1 460 cm-1 shows the bending vibration of -CH, and the peak at 1 050– 1 085 cm-1 is the stretching vibration of -CO (Wang et al., 2002; Chen et al., 2017). The presence of these functional groups proved that dopamine is attached to the surface of 304 SS. The basis of the two peaks at 3 300–3 500 cm-1 and 2 800–3 000 cm-1, the peaks near 1 690 cm-1 and 1 612 cm-1 are β-overlapping symmetry or asymmetric C=O vibration (Amide I). The peaks at 1 480–1 575 cm-1 are C-N stretching vibrations or N-H bending vibrations (Amide II), 1 250–1 410 cm-1 are C-N stretching vibrations or N-H bending vibrations (Amide III) (Carbonaro and Nucara, 2010), while the peak at 1 050–1 085 cm-1 is C-O stretching of C-OH. The presence of these functional groups demonstrates the existence of antimicrobial peptide in the modified samples. Furthermore, the sharp peaks in 1 300–1 400 cm-1 and the strong absorption peaks around 1 100 cm-1 in the SS-DA-TB sample are different from those in the SS-DA sample and are caused by the presence of organic sulfides on the surface (Cao et al., 2018). These peaks that only appear in the SS-DA-TB sample are additional evidence that the antimicrobial peptide had been coated onto the surface of the 304 SS samples.
|
| Fig.2 FTIR spectra of SS-DA and SS-DA-TB |
XPS was used to portray the elements information of SS, SS-DA, and SS-DA-TB on surface, illustrating the changes in the surface before and after modification (Fig. 3). Sample 304 SS had almost no N or S in its fraction. As can be seen from Fig. 3, the dopamine-treated sample SS-DA showed a clear peak of N1s at 400 eV, the ratio of the content of element C to element N decreased from 40.45 to 1.94, the content of element N increased significantly, and the original sample itself contains only a small amount of N (Fig. 3a), proving that the surface of the SS was covered with a dopamine layer after dopamine treatment. The presence of the dopamine layer increased the content of N on sample surface by an order of magnitude, and the ratio of C to O increased from 0.58 to 6.03. The above analysis confirms the successful coating of dopamine onto the SS surface. After coating with the antimicrobial peptide, the ratio of C to N increased from 1.94 to 4.01 and the ratio of C to O decreased from 6.03 to 2.85 on the surface of the SS-DA-TB sample. It is possible that after treatment with dopamine and antimicrobial peptide, the surface of the sample was covered by catechol group formed by the autoxidation of dopamine, which then underwent esterification with the carboxylic acid root of antimicrobial peptide (Lee et al., 2007), resulting in the increase of N content and decrease of O content. Figure 3b shows the S2p peaks of the samples, in which no S was detected on the SS and SS-DA samples due to the absence of S in both the 304 SS and dopamine fractions, but had distinct peaks at 164.5 and 167.9 eV caused by C-S-C and C-SOX-C on sample surface after being coated with antimicrobial peptide, respectively (Gerin et al., 1995; Wirde et al., 1999). Figure 3c shows three C1s peaks at 287.9, 285.6, and 284.6 eV in the SS-DA-TB sample, corresponding to carbon atoms in the O=C-N, C-C-O, and C-C/C-H bonds in antimicrobial peptide (Minier et al., 2005); and other three peaks at 287.4, 285.6, and 284.6 eV in the SS-DA sample correspond to carbon atoms in the C=N, C-N, and C-H bonds in dopamine. The N1s peaks of the samples are shown in Fig. 3d. No ester groups were found on the surfaces of SS and SS-DA, and the NH/CONH peak was found on the surface of SS-DA-TB, indicating successful coating of antimicrobial peptide on surface of SS-DA. The peak at 399.2 eV corresponds to the nitrogen atom in O=C-N and the peak at 400.8 eV corresponds to the nitrogen atom in -NH3+/-NH2 (Kong et al., 2001). The above analysis confirmed the successful coating of antimicrobial peptide onto the surface of 304 SS via dopamine.

|
| Fig.3 XPS spectrum of samples' surfaces a. full spectrum of SS, SS-DA, and SS-DA-TB surfaces; b. S2p spectrum of SS, SS-DA, and SS-DA-TB surfaces; c. C1s spectrum of SS, SS-DA, and SS-DA-TB surface; d. N1s spectrum of SS, SS-DA, and SS-DA-TB surfaces. |
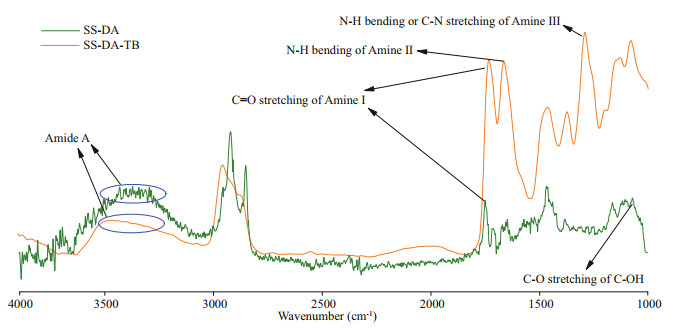
The contact angle of sample was studied to evaluate the wettability of modified surface. The surface contact angles of the SS, SS-DA, and SS-DA-TB are illustrated in Fig. 4a. After dopamine treatment, the contact angle of the sample surface decreased from 69.4°±0.8° to 36.7°±1.3°, which is the result of good hydrophilic properties of dopamine and its oxidative polymerized intermediates. The contact angle of sample surface increased to 56.4°±2.3° after TB treatment due to the presence of some hydrophobic amino acids in the TB sequence of peptide, such as glycine and alanine, which increased the surface hydrophobicity and thus slightly increased the water contact angles on sample surface. The change in contact angle on surfaces of different samples indicate that dopamine was successful in coating TB onto the surface of 304 SS as a coupling agent.
|
| Fig.4 Physicochemical properties and surface morphology characterization of samples a. contact angles of three substrate surfaces; b. surface thickness of different samples (**: P < 0.01); c. AFM 3D image of SS surface; d. AFM 3D image of SS-DA-TB surface. |
The surface morphology of samples was characterized via AFM. The surface morphology of the untreated 304 SS was smooth, with a maximum vertical height of 6.4 nm (Fig. 4c). In contrast, the surface of SS-DA-TB was rougher and irregular (Fig. 4d), with a maximum vertical height of 14.9 nm due to the coating of dopamine and antimicrobial peptide TB, as the surface of the modified sample captured some macromolecular particles. In addition, the surface roughness before and after modification was characterized by a 3D optical profilometer. The 3D profile of the original samples ranged -2.91– 1.38 μm (Supplementary Fig.S2a), while modified samples ranged -2.358–1.644 μm (Supplementary Fig.S2b). Moreover, the roughness values (Ra) did not change significantly. This could be due to the successful coating of antimicrobial peptides, but the thickness of the modified films was too small to affect the roughness, which would not have an effect V.natriegens on the adhesion of on the sample surfacebefore and after modification (Zhong et al., 2021).
3.2.5 Ellipsometry analysisEllipsometry is widely used to measure the thickness of coating (Zhu and Edmondson, 2011; Kang et al., 2013; Wei et al., 2019). The thickness of the surface layer of SS, SS-DA, and SS-DA-TB was measured in ellipsometry. As indicated in Fig. 4b, the surface thickness of the untreated samples was 1.697± 0.771 nm, the layer thickness of SS-DA and SS-DA-TB was 14.033±2.065 nm and 25.033±3.39 nm respectively, which demonstrated that the thickness of the surface layer increased significantly after treatment with dopamine and antimicrobial peptide due to the oxidative polymerization of dopamine after dopamine soaking, which formed a layer of polydopamine (PDA) on 304 SS surface and increased layer thickness on the surface (Ball et al., 2012). After modification with TB, antimicrobial peptide was well-coated onto the sample surface via dopamine, resulting in a further increase in the thickness of the sample surface coating.
3.3 Cytocompatibility evaluationThe adhesion and value-added properties of cells in antimicrobial peptide solution allowed the analysis of the toxicity of TB to the cells. Results show the potential to the evaluation of the toxicity of the samples modified with dopamine and antimicrobial peptides to other marine organisms. The concentration of this product was linearly correlated with the absorbance value at 450 nm. The relative activity of cells can be compared by measuring the absorbance values in the system. The CCK-8 results show that the optical density (OD) value of cells decreased slightly as the concentration of antimicrobial peptide increased (Fig. 5), probably due to the strong positive charge of antimicrobial peptide itself, which is toxic to the cells and would deactivate the cells. The results are consistent with a previous study by Wang et al. (2015). After 24-h incubation, both E11 and 293T presented a high survival rate of over 90%. Moreover, study about cell morphology stated below demonstrated the effectiveness of TB on cell compatibility. It can be seen that the cells stick and spread well in antimicrobial peptide solution and demonstrated superior cytocompatibility of TB (Supplementary Fig.S3), which shows that the peptide is environmental friendly to marine fish and human body.
|
| Fig.5 Value-added of cells after incubation with different concentrations of antimicrobial peptides for 0, 2, 4, 8, 12, and 24 h a. E11; b. 293T. |
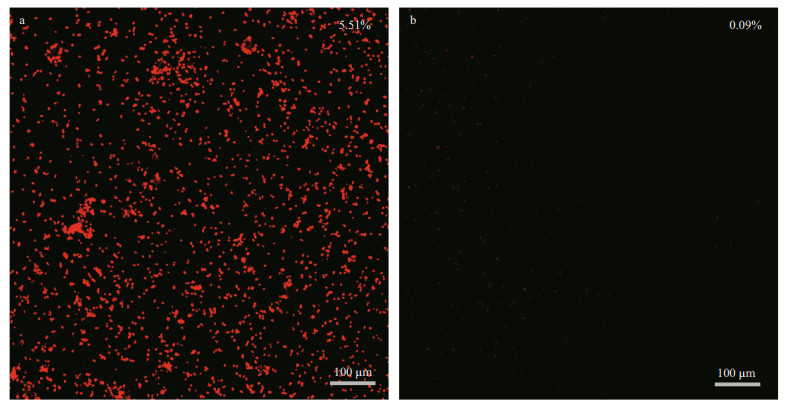
Vibrio natriegens was selected for bacterial adhesion experiments on SS and SS-DA-TB surfaces. After 24-h incubation in V. natriegens solution, the antibacterial properties of different sample surfaces were evaluated via CLSM images (Fig. 6). As illustrated in the image, the untreated SS surface showed pervasive bacterial adherence but nearly none for SS-DA-TB due to the antibacterial effect of TB. The antimicrobial properties reflect the interaction between the cationic antimicrobial peptide and the bacterial cell membrane. After modification with dopamine and the antimicrobial peptide, the surface of the samples formed a hydrated layer that disrupted the negatively charged V. natriegens outer membrane (Lim et al., 2013; Yoo et al., 2018). The derivatives of the antimicrobial peptide even entered the bacterial cells and ripped their integrity (Cao et al., 2020b). To evaluate the antibacterial effect of the SS-DA-TB surface, the bacterial coverage of the SS-DA-TB surface was calculated by ImageJ software. The results illustrated a 99.85% reduction of adherent bacteria on the SS-DA-TB surface, indicating a perfect antibacterial efficiency.
|
| Fig.6 CLSM images of V. natriegens attached to surfaces Red color represents adherent bacteria. a. SS surface; b. SS-DA-TB surface. |
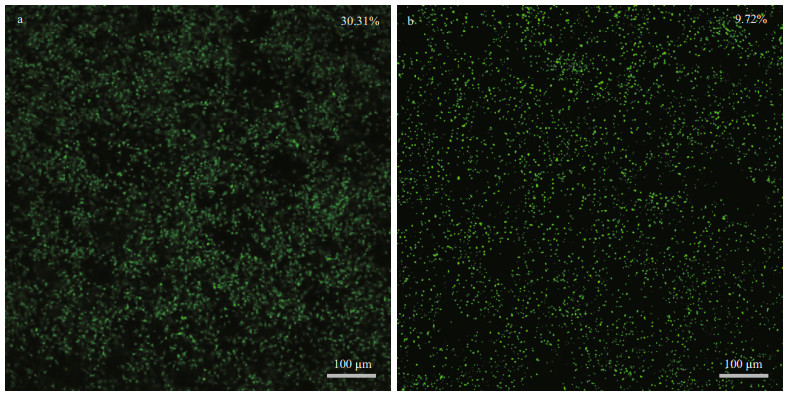
The antifouling properties of the samples surfaces before and after modification were evaluated by examining the adhesion of P. tricornutum, one of the typical algae in the East China Sea. P. tricornutum is often used for related studies because of its involvement in the early stages of biofouling. The anti-algae properties of different sample surfaces were evaluated via CLSM images after 7-day incubation in P.tricornutum solution (Fig. 7). As can be seen fromthe CLSM images, there was a significant amount of diatom adherence on the surface of the untreated samples and significantly less diatom adherence on the surface of the modified samples. Statistical analysis of the diatom adhesion rate demonstrated that the antibacterial peptide reduced the adhesion rate of P. tricornutum on the surface of 304 SS by 67.93% and the adhesion rate of P. tricornutum on the surface of the samples was reduced from 30.31% to 9.73%. This is attributed to the antimicrobial peptide, which inhibited the adhesion of proteins and polysaccharides to the sample surface to form a conditional film, making the fouling P. tricornutum less likely to adhere to the sample surface (Wang et al., 2020).
|
| Fig.7 CLSM images of P. tricornutum attached on sample surface Green dots are adherent P. tricornutum. a. SS surface; b. SS-DA-TB surface. |
Tafel polarization curves and electrochemical impedance spectroscopy (EIS) measurements are widely used to evaluate the efficiency of polymer coatings on metal surfaces against biocorrosion (Wan et al., 2010). From the Nyquist plot, it can be seen that the impedance arc radius of SS samples immersed in inoculated V. natriegens culture solution varied greatly at different time points (Supplementary Fig.S4), showing a trend of gradual increase in the impedance arc radius at Days 14 was the largest, which is consistent with the Bode modulus plot of the total value of low-frequency impedance. Comparatively, the impedance arc radius of SS-DA-TB samples showed a trend of decreasing and then increasing at different time nodes, but the impedance radius of SS-DA-TB samples was larger than that of SS samples during the immersion period, indicating that the modification of antimicrobial peptides enhanced the corrosion resistance of 304 SS (Cao et al., 1990). This probably due to the growth and metabolism of V. natriegens and biofilm production accelerated the corrosion process (Cheng et al., 2009), but the samples coated with antimicrobial peptide had excellent antimicrobial properties, which reduced the adhesion of V. natriegens, and the corrosion rate was significantly reduced (Table 1).
|
To clarify the corrosion resistance of modified stainless steel samples, the Tafel plots of SS and SS-DA-TB samples exposed in V. natriegens solutions for 1, 7, and 14 days were analyzed, from which inhibition efficiency (IE) values were calculated (Fig. 8). It is evident that the shapes of the specimen polarization curves of the SS and SS-DA-TB samples are largely the same in the experiment period, indicating that the cathode was still controlled by oxygen diffusion (Cheng et al., 2009). The polarization curves of the SS and SS-DA-TB samples shifted negatively after 14 days of immersion, indicating that the corrosion potential (Ecorr) of the samples decreased with increasing immersion time and the anticorrosion performance decreased. Meanwhile, the polarization curves of the SS samples shifted first to the left and then to the right on Days 7 and 14 compared to that of Day 1, indicating that the corrosion current decreased first and then increased. The polarization curves of the SS-DA-TB samples shifted rightward on Days 7 and 14 from that of Day 1, indicating that the corrosion current increased. The polarization rate in the cathodic part of the polarization curve decreased, indicating that the cathodic process was facilitated and the corrosion resistance weakened.
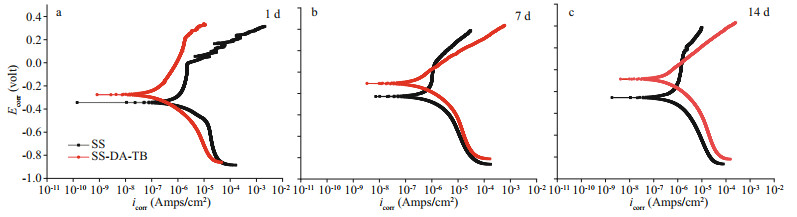
|
| Fig.8 Tafel polarization plots of the SS and SS-DA-TB samples in Vibrio natriegens inoculated medium for different days Ecorr refers to corrosion potential, icorr refers to corrosion current density. a. 1 d; b. 7 d; c. 14 d. |
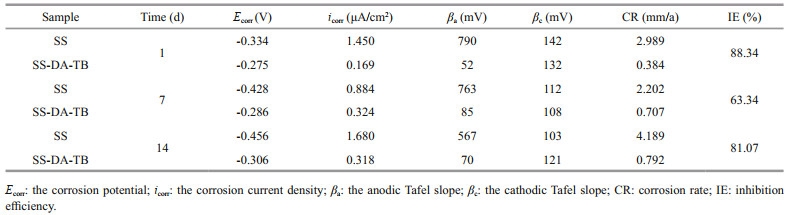
The parameters of the polarization curve for samples exposed in V. natriegens solutions were obtained by extrapolating the anodic and cathodic branches to the intersection point (Table 1) by Tafel extrapolation method (Lv et al., 2014). The Ecorr of the SS samples showed a decreasing trend from -0.334 V on Day 1 to -0.428 V on Day 7 and then to -0.456 V on Day 14. The corrosion current tended to decrease and then increased, from 1.450 μA/cm2 on Day 1 to 0.884 μA/cm2 on Day 7 and then bounce back to 1.680 μA/cm2, showing first decreasing and then increasing. In contrast, the Ecorr of the SS-DA-TB samples showed only a small negative shift from -0.275 V on Day 1 to -0.286 V on Day 7 and -0.306 V on Day 14, indicating that Ecorr remained relatively constant throughout the exposure period in a trend of first increasing and then stabilizing. Therefore, as the immersion time increased, the sample surface was corroded and broken, the Ecorr decreased, the icorr increased and the corrosion of the sample surface accelerated (Wan et al., 2010; Dong et al., 2011; Lv et al., 2014). However, the Ecorr of the samples coated with antimicrobial peptide was always greater than that of the unmodified samples, and the icorr was significantly lower. The icorr values of SS-DA-TB samples were reduced 3-fold and 5-fold relative to the unmodified samples exposed for 7 and 14 days, respectively, in medium inoculated with V.natriegens. Furthermore, the SS-DA-TB samples showed an inhibition efficiency (IE) of over 63.34% and 81.07% for SS samples exposed for 7 and 14 days, respectively. Successful coating of antimicrobial peptides increases the electron escape work on the sample surface and reduces the electron activity on the sample surface (Bhushan and Goldade, 2000), thus reducing the corrosion energy on the material surface. The results indicate that the samples have superior corrosion resistance after coating with antimicrobial peptides.
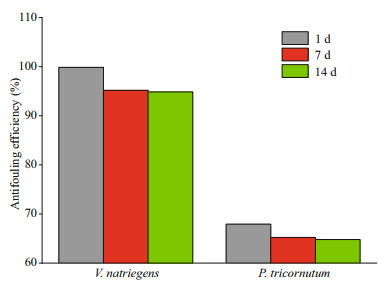
3.6 Robustness analysisThe stability of surface coatings on marine equipment can be undermined by shear forces in the flow of seawater in the ocean. To characterize the stability of the modified sample surfaces, the stability of the samples was assessed and antibacterial and anti-algae efficiencies were evaluated by simulating the marine environment by immersing the samples in ASW for 1, 7, and 14 days, and by gently shaking (30 r/min). The inhibition of V. natriegens and P. tricornutum by SS-DA-TB decreased after 7 daysof immersion from that of Day 1 from 99.85% and 67.93% to 94.89% and 66.53%, respectively. After 14 days of soaking, the anti-fouling effect stabilized, and the inhibition of V. natriegens and P. tricornutum reched over 95% and 65%, respectively (Fig. 9). These results indicate that most of the antimicrobial peptides were firmly bound to the surface of the samples via dopamine and maintained excellent antifouling ability over a significant time. This highlights that this dopamine-antibacterial peptide coating has great potential for application on the surface of marine equipment.
|
| Fig.9 Robustness of antibacterial effciency of SS-DA-TB surfaces |
The surface of 304 SS was modified with dopamine and TB to obtain SS-DA-TB with excellent antifouling properties. FTIR and XPS results demonstrate that dopamine was successfully used as a coupling agent to coat TB onto the surface of 304 SS. Contact angles and surface morphology showed that the morphology and wettability of the sample surface changed considerably after modification with dopamine and TB. Cytocompatibility assays revealed that E11 and 293T cells had stable proliferation in the antimicrobial peptide solution, which demonstrated that the peptide had good cytocompatibility. Antifouling test results indicated that the SS-DA-TB exhibited excellent antifouling properties with 99.85% inhibition of V. natriegens and 67.93% inhibition of P. tricornutum.Electrochemical corrosion analysis demonstrated a slow corrosion rate on the surface of the modified samples, indicating that the antimicrobial peptide layer provided excellent corrosion resistance. This finding could offer a promising strategy for solving the surface fouling problem of ships and marine constructions.
5 DATA AVAILABILITY STATEMENTAll data generated and/or analyzed during this study are available from the corresponding author upon reasonable request.
Electronic supplementary material
Supplementary material (Supplementary Figs.S1–S4) is available in the online version of this article at
Ball V, Frari D D, Toniazzo V, et al. 2012. Kinetics of polydopamine film deposition as a function of pH and dopamine concentration: insights in the polydopamine deposition mechanism. Journal of Colloid and Interface Science, 386(1): 366-372.
DOI:10.1016/j.jcis.2012.07.030 |
Bernsmann F, Ponche A, Ringwald C, et al. 2009. Characterization of dopamine-melanin growth on silicon oxide. The Journal of Physical Chemistry C, 113(19): 8234-8242.
DOI:10.1021/jp901188h |
Bhushan B, Goldade A V. 2000. Measurements and analysis of surface potential change during wear of single-crystal silicon (100) at ultralow loads using Kelvin probe microscopy. Applied Surface Science, 157(4): 373-381.
DOI:10.1016/S0169-4332(99)00553-X |
Bloecher N, Floerl O. 2020. Efficacy testing of novel antifouling coatings for pen nets in aquaculture: how good are alternatives to traditional copper coatings?. Aquaculture, 519: 734936.
DOI:10.1016/j.aquaculture.2020.734936 |
Brogden K A. 2005. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria?. Nature Reviews Microbiology, 3(3): 238-250.
DOI:10.1038/nrmicro1098 |
Brzozowska A M, Parra-Velandia F J, Quintana R, et al. 2014. Biomimicking micropatterned surfaces and their effect on marine biofouling. Langmuir, 30(30): 9165-9175.
DOI:10.1021/la502006s |
Cao C N. 1990. On the impedance plane displays for irreversible electrode reactions based on the stability conditions of the steady-state—I. One state variable besides electrode potential. Electrochimica Acta, 35: 831-836.
DOI:10.1016/0013-4686(90)90077-D |
Cao P, Cao Z M, Yuan C Q. 2019. Stainless steel coated by Cu NPs via dopamine coupling for antifouling application. Surface and Interface Analysis, 51(8): 809-816.
DOI:10.1002/sia.6654 |
Cao P, Du C W, He X Y, et al. 2020a. Modification of a derived antimicrobial peptide on steel surface for marine bacterial resistance. Applied Surface Science, 510: 145512.
DOI:10.1016/j.apsusc.2020.145512 |
Cao P, Liu K W, Liu X D, et al. 2020b. Antibacterial properties of Magainin II peptide onto 304 stainless steel surfaces: a comparison study of two dopamine modification methods. Colloids and Surfaces B: Biointerfaces, 194: 111198.
DOI:10.1016/j.colsurfb.2020.111198 |
Cao P, Yang Y, Uche F I, et al. 2018. Coupling plantderived cyclotides to metal surfaces: an antibacterial and antibiofilm study. International Journal of Molecular Sciences, 19(3): 793.
DOI:10.3390/ijms19030793 |
Carbonaro M, Nucara A. 2010. Secondary structure of food proteins by Fourier transform spectroscopy in the midinfrared region. Amino Acids, 38(3): 679-690.
DOI:10.1007/s00726-009-0274-3 |
Chen L R, Duan Y Y, Cui M, et al. 2021. Biomimetic surface coatings for marine antifouling: natural antifoulants, synthetic polymers and surface microtopography. Science of the Total Environment, 766: 144469.
DOI:10.1016/j.scitotenv.2020.144469 |
Chen T P, Liu T C, Su T L, et al. 2017. Self-polymerization of dopamine in acidic environments without oxygen. Langmuir, 33(23): 5863-5871.
DOI:10.1021/acs.langmuir.7b01127 |
Cheng S, Tian J T, Chen S G, et al. 2009. Microbially influenced corrosion of stainless steel by marine bacterium Vibrio natriegens: (I) Corrosion behavior. Materials Science and Engineering: C, 29(3): 751-755.
DOI:10.1016/j.msec.2008.11.013 |
Dong Z H, Liu T, Liu H F. 2011. Influence of EPS isolated from thermophilic sulphate-reducing bacteria on carbon steel corrosion. Biofouling, 27(5): 487-495.
DOI:10.1080/08927014.2011.584369 |
Feng D Q, He J, Chen S Y, et al. 2018. The plant alkaloid camptothecin as a novel antifouling compound for marine paints: laboratory bioassays and field trials. Marine Biotechnology, 20(5): 623-638.
DOI:10.1007/s10126-018-9834-4 |
Gabriel M, Nazmi K, Veerman E C, et al. 2006. Preparation of LL-37-grafted titanium surfaces with bactericidal activity. Bioconjugate Chemistry, 17(2): 548-550.
DOI:10.1021/bc050091v |
Gerin P A, Dengis P B, Rouxhet P G. 1995. Performance of XPS analysis of model biochemical compounds. Journal de Chimie Physique et de Physico-Chimie Biologique, 92: 1043-1065.
DOI:10.1051/jcp/1995921043 |
Glinel K, Thebault P, Humblot V, et al. 2012. Antibacterial surfaces developed from bio-inspired approaches. Acta Biomaterialia, 8(5): 1670-1684.
DOI:10.1016/j.actbio.2012.01.011 |
Gu Y Q, Yu L Z, Mou J G, et al. 2020. Research strategies to develop environmentally friendly marine antifouling coatings. Marine Drugs, 18(7): 371.
DOI:10.3390/md18070371 |
Gura T. 2001. Ancient system gets new respect. Science, 291(5511): 2068-2071.
DOI:10.1126/science.291.5511.2068 |
Hansen I K Ø, Isaksson J, Poth A G, et al. 2020. Isolation and characterization of antimicrobial peptides with unusual disulfide connectivity from the colonial ascidian Synoicum turgens. Marine Drugs, 18(1): 51.
DOI:10.3390/md18010051 |
He X Y, Cao P, Tian F, et al. 2019. Infused configurations induced by structures influence stability and antifouling performance of biomimetic lubricant-infused surfaces. Surface and Coatings Technology, 358: 159-166.
DOI:10.1016/j.surfcoat.2018.11.035 |
Jeong Y, Thuy L T, Ki S H, et al. 2018. Multipurpose antifouling coating of solid surfaces with the marine-derived polymer fucoidan. Macromolecular Bioscience, 18(10): 1800137.
DOI:10.1002/mabi.201800137 |
Jiang B, Zeng Q Z, Hou Y, et al. 2020. Quorum quenching bacteria bioaugmented GO/PPy modified membrane in EMBR for membrane antifouling. Science of the Total Environment, 718: 137412.
DOI:10.1016/j.scitotenv.2020.137412 |
Jiang J H, Zhu L P, Li X L, et al. 2010. Surface modification of PE porous membranes based on the strong adhesion of polydopamine and covalent immobilization of heparin. Journal of Membrane Science, 364(1-2): 194-202.
DOI:10.1016/j.memsci.2010.08.017 |
Kang J, Tada S, Kitajima T, et al. 2013. Immobilization of bone morphogenetic protein on DOPA- or dopaminetreated titanium surfaces to enhance osseointegration. Biomed Research International, 2013: 265980.
DOI:10.1155/2013/265980 |
Kim S, Gim T, Jeong Y, et al. 2018. Facile construction of robust multilayered PEG films on polydopamine-coated solid substrates for marine antifouling applications. ACS Applied Materials & Interfaces, 10(9): 7626-7631.
DOI:10.1021/acsami.7b07199 |
Kim S, Gim T, Kang S M. 2015. Versatile, tannic acid-mediated surface PEGylation for marine antifouling applications. ACS Applied Materials & Interfaces, 7(12): 6412-6416.
DOI:10.1021/acsami.5b01304 |
Kirschner C M, Brennan A B. 2012. Bio-inspired antifouling strategies. Annual Review of Materials Research, 42: 211-229.
DOI:10.1146/annurev-matsci-070511-155012 |
Kong X X, Kawai T, Abe J, et al. 2001. Amphiphilic polymer brushes grown from the silicon surface by atom transfer radical polymerization. Macromolecules, 34(6): 1837-1844.
DOI:10.1021/ma001152h |
Lee B P, Messersmith P B, Israelachvili J N, et al. 2011. Musselinspired adhesives and coatings. Annual Review of Materials Research, 41: 99-132.
DOI:10.1146/annurev-matsci-062910-100429 |
Lee H, Dellatore S M, Miller W M, et al. 2007. Musselinspired surface chemistry for multifunctional coatings. Science, 318(5849): 426-430.
DOI:10.1126/science.1147241 |
Lee H, Rho J, Messersmith P B. 2009. Facile conjugation of biomolecules onto surfaces via mussel adhesive protein inspired coatings. Advanced Materials, 21(4): 431-434.
DOI:10.1002/adma.200801222 |
Lim K, Chua R R Y, Saravanan R, et al. 2013. Immobilization studies of an engineered arginine-tryptophan-rich peptide on a silicone surface with antimicrobial and antibiofilm activity. ACS Applied Materials & Interfaces, 5(13): 6412-6422.
DOI:10.1021/am401629p |
Liu H, Chen S Y, Guo J Y, et al. 2018. Effective natural antifouling compounds from the plant Nerium oleander and testing. International Biodeterioration & Biodegradation, 127: 170-177.
DOI:10.1016/j.ibiod.2017.11.022 |
Liu Y, Ai K, Lu L. 2014. Polydopamine and its derivative materials: synthesis and promising applications in energy, environmental, and biomedical fields. Chemical Reviews, 114: 5057-5115.
DOI:10.1021/cr400407a |
Love J C, Estroff L A, Kriebel J K, et al. 2005. Selfassembled monolayers of thiolates on metals as a form of nanotechnology. Chemical Reviews, 105(4): 1103-1170.
DOI:10.1021/cr0300789 |
Lv L, Yuan S J, Zheng Y, et al. 2014. Surface modification of mild steel with thermally cured antibacterial poly(vinylbenzyl chloride)-polyaniline bilayers for effective protection against sulfate reducing bacteria induced corrosion. Industrial & Engineering Chemistry Research, 53(31): 12363-12378.
DOI:10.1021/ie501654b |
Minier M, Salmain M, Yacoubi N, et al. 2005. Covalent immobilization of lysozyme on stainless steel. Interface spectroscopic characterization and measurement of enzymatic activity. Langmuir, 21(13): 5957-5965.
DOI:10.1021/la0501278 |
Mishra B, Basu A, Saravanan R, et al. 2013. Lasioglossin-III: antimicrobial characterization and feasibility study for immobilization applications. RSC Advances, 3(24): 9534-9543.
DOI:10.1039/c3ra40887f |
Nurioglu A G, Esteves A C C, de With G. 2015. Non-toxic, nonbiocide-release antifouling coatings based on molecular structure design for marine applications. Journal of Materials Chemistry B, 3(32): 6547-6570.
DOI:10.1039/C5TB00232J |
Ozaltin K, Lehocký M, Humpolíček P, et al. 2016. A new route of fucoidan immobilization on low density polyethylene and its blood compatibility and anticoagulation activity. International Journal of Molecular Sciences, 17(6): 908.
DOI:10.3390/ijms17060908 |
Pinteus S, Lemos M F L, Freitas R, et al. 2020. Medusa polyps adherence inhibition: a novel experimental model for antifouling assays. Science of the Total Environment, 715: 136796.
DOI:10.1016/j.scitotenv.2020.136796 |
Saha M, Goecke F, Bhadury P. 2018. Minireview: algal natural compounds and extracts as antifoulants. Journal of Applied Phycology, 30(3): 1859-1874.
DOI:10.1007/s10811-017-1322-0 |
Salama A J, Satheesh S, Balqadi A A. 2018. Antifouling activities of methanolic extracts of three macroalgal species from the Red Sea. Journal of Applied Phycology, 30(3): 1943-1953.
DOI:10.1007/s10811-017-1345-6 |
Selim M S, Shenashen M A, Hashem A I, et al. 2018. Linseed oil-based alkyd/Cu2O nanocomposite coatings for surface applications. New Journal of Chemistry, 42(12): 10048-10058.
DOI:10.1039/C7NJ03440G |
Silva E R, Ferreira O, Ramalho P A, et al. 2019. Eco-friendly non-biocide-release coatings for marine biofouling prevention. Science of the Total Environment, 650: 2499-2511.
DOI:10.1016/j.scitotenv.2018.10.010 |
Sonak S, Pangam P, Giriyan A, et al. 2009. Implications of the ban on organotins for protection of global coastal and marine ecology. Journal of Environmental Management, 90(Suppl 1): S96-S108.
DOI:10.1016/j.jenvman.2008.08.017 |
Vishwakarma V. 2020. Impact of environmental biofilms: industrial components and its remediation. Journal of Basic Microbiology, 60(3): 198-206.
DOI:10.1002/jobm.201900569 |
Wan D, Yuan S J, Neoh K G, et al. 2010. Surface Functionalization of copper via oxidative graft polymerization of 2, 2'-bithiophene and immobilization of silver nanoparticles for combating biocorrosion. ACS Applied Materials & Interfaces, 2(6): 1653-1662.
DOI:10.1021/am100186n |
Wang F, Zhang H, Yu B, et al. 2020. Review of the research on anti-protein fouling coatings materials. Progress in Organic Coatings, 147: 105860.
DOI:10.1016/j.porgcoat.2020.105860 |
Wang L, Chen J J, Cai C Z, et al. 2015. Multi-biofunctionalization of a titanium surface with a mixture of peptides to achieve excellent antimicrobial activity and biocompatibility. Journal of Materials Chemistry B, 3(1): 30-33.
DOI:10.1039/C4TB01318B |
Wang X Y, Jin B K, Lin X Q. 2002. In-situ FTIR spectroelectrochemical study of dopamine at a glassy carbon electrode in a neutral solution. Analytical Sciences, 18(8): 931-933.
DOI:10.2116/analsci.18.931 |
Wei Q B, Liu X Q, Yue Q Y, et al. 2019. Mussel-inspired onestep fabrication of ultralow-friction coatings on diverse biomaterial surfaces. Langmuir, 35(24): 8068-8075.
DOI:10.1021/acs.langmuir.9b00421 |
Wirde M, Gelius U, Nyholm L. 1999. Self-assembled monolayers of cystamine and cysteamine on gold studied by XPS and voltammetry. Langmuir, 15(19): 6370-6378.
DOI:10.1021/la9903245 |
Xie C H, Guo H S, Zhao W Q, et al. 2020. Environmentally friendly marine antifouling coating based on a synergistic strategy. Langmuir, 36(9): 2396-2402.
DOI:10.1021/acs.langmuir.9b03764 |
Yeaman M R, Yount N Y. 2003. Mechanisms of antimicrobial peptide action and resistance. Pharmacological Reviews, 55(1): 27-55.
DOI:10.1124/pr.55.1.2 |
Yoo J, Birke A, Kim J, et al. 2018. Cooperative catecholfunctionalized polypept(o)ide brushes and Ag nanoparticles for combination of protein resistance and antimicrobial activity on metal oxide surfaces. Biomacromolecules, 19(5): 1602-1613.
DOI:10.1021/acs.biomac.8b00135 |
Yu W F, Wang Y X, Gnutt P, et al. 2021. Layer-by-layer deposited hybrid polymer coatings based on polysaccharides and zwitterionic silanes with marine antifouling properties. ACS Applied Bio Materials, 4(3): 2385-2397.
DOI:10.1021/acsabm.0c01253 |
Yuan S J, Tang S W, Lv L, et al. 2012. Poly(4-vinylaniline)-polyaniline bilayer-modified stainless steels for the mitigation of biocorrosion by sulfate-reducing bacteria(SRB) in seawater. Industrial & Engineering Chemistry Research, 51(45): 14738-14751.
DOI:10.1021/ie302303x |
Yuan S J, Wan D, Liang B, et al. 2011. Lysozyme-coupled poly(poly(ethylene glycol) methacrylate)-stainless steel hybrids and their antifouling and antibacterial surfaces. Langmuir, 27(6): 2761-2774.
DOI:10.1021/la104442f |
Zhang B, Yan Q, Yuan S J, et al. 2019. Enhanced antifouling and anticorrosion properties of stainless steel by biomimetic anchoring PEGDMA-cross-linking polycationic brushes. Industrial & Engineering Chemistry Research, 58(17): 7107-7119.
DOI:10.1021/acs.iecr.8b05599 |
Zhong L J, Song Y B, Zhou S F. 2021. Covalent grafting of sodium p-styrene sulfonate to stainless steel for antibacterial applications. Materials Chemistry and Physics, 268: 124753.
DOI:10.1016/j.matchemphys.2021.124753 |
Zhu B C, Edmondson S. 2011. Polydopamine-melanin initiators for surface-initiated ATRP. Polymer, 52(10): 2141-2149.
DOI:10.1016/j.polymer.2011.03.027 |
 2023, Vol. 41
2023, Vol. 41


