Institute of Oceanology, Chinese Academy of Sciences
Article Information
- SHI Dawei, JIA Houlei
- Transport and behavior of marine oil spill containing polycyclic aromatic hydrocarbons in mesocosm experiments
- Journal of Oceanology and Limnology, 41(1): 166-173
- http://dx.doi.org/10.1007/s00343-022-1388-7
Article History
- Received Nov. 17, 2021
- accepted in principle Dec. 22, 2021
- accepted for publication Mar. 2, 2022
2 Southern Marine Science and Engineering Guangdong Laboratory(Guangzhou), Guangzhou 511458, China
Oil pollution in the sea arises from multiple sources including accidental oil spill from tankers, leakages from drilling operations, industrial wastewater, and others (Zhang et al., 2019). Polycyclic aromatic hydrocarbons (PAHs) are one of the most important group of chemicals in oil spills with various biological effects such as acute toxicity, carcinogenicity, mutagenicity, teratogenicity, and endocrine disrupting activity (Abdel-Shafy and Mansour, 2016; Wang et al., 2018), leading to significant toxic and/or carcinogenic effects towards humans and wildlife (Allan et al., 2012; Zhu et al., 2019). In addition, some of these PAHs are reportedly persistent components after oil spills, aggregating their harmfulness to human health and ecosystem (Peacock et al., 2007; Kim et al., 2017; Duan et al., 2018).
Therefore, it is important to understand the behavior of PAHs in marine environment. PAHs in seawater can be removed via either physical interactions (such as evaporation, photo oxidation, and sedimentation) or biodegradation (Yamada et al., 2003; Haritash and Kaushik, 2009). Some laboratory experiments have been carried out to study the degradation process of PAHs in seawater (Gearing et al., 1980; Yamada et al., 2003; Zhou et al., 2013). It was estimated that at least 3%–4% of the oil ended up on the seafloor in Deepwater Horizon oil spill incident in 2010 (Chanton et al., 2015). Marine snow aggregates are known to carry organic carbon to deep water (Joye et al., 2014); studies characterized the formation of "marine oil snow" aggregates as an important process to transfer petroleum hydrocarbons to the deep sea (Passow, 2016). However, little is known about the interaction of PAHs, as a typical representative of persistent organic pollutants (POPs), with microbial communities in marine environment (Quigg et al., 2016; Brakstad et al., 2018).
The distribution, transport, and destiny of petroleum hydrocarbons in marine environment are dependent of their physiochemical properties, i.e., water solubility, volatility, and photochemical/ biological degradability (Tarr et al., 2016). PAHs are known able to be preferentially adsorbed by particulate matter, and oil droplets containing PAHs may be adsorbed on particulate surface or even incorporated into marine snow particles and sink to the bottom (Wirth et al., 2018). Bottom sediments may act as a reservoir for these hydrophobic PAHs in aquatic environment (Budzinski et al., 1997). However, application of dispersants in marine oil spill incident could complicated this course because dispersion may lead to the formation of small oil droplets in the water column and promotes oil dissolution, which may significantly increase the PAH concentration in the water column (Yamada et al., 2003; Couillard et al., 2005) and make the oil more accessible during biodegradation (Lessard and DeMarco, 2000). However, the application of dispersants and their effects on oil distribution and degradation are often controversial (Kleindienst et al., 2015; Rahsepar et al., 2016). At present, contribution of marine snow sedimentation to the removal of oil hydrocarbons has not been well understood (Brakstad et al., 2018).
To address this knowledge gap, Wade et al. (2017) conducted mesocosm experiments to investigate the role of microbial exopolymers during aggregation, sedimentation, and degradation of oil. The experiments simulated natural conditions in a controlled fashion, enabling the study of marine oil snow formation. Correspondingly, we conducted similar mesocosm experiments focusing on the determination of changes of PAHs concentrations and distribution, providing information about the removal rates of PAHs from seawater containing water-accommodated oil (WAF) and chemically-enhanced water-accommodated fraction of oil (CEWAF). The objective of this study is to monitor temporal changes of PAH concentrations in replicate mesocosm experiments to further understand the transport and behavior of PAHs in marine oil spill and document effect on dispersant use.
2 MATERIAL AND METHOD 2.1 Mesocosm setupThe seawater used in the mesocosm experiment was collected on January 25, 2021 near Qi'ao Island, Zhuhai, China (22°24.265 6′N, 113°39.519 3′E) in salinity of ~30. The water samples were then transferred to a tank in laboratory in the South China Sea Institute of Planning and Environmental Research, and stored overnight at room temperature for experimental setup.
Most studies use the method developed by Chemical Response to Oil Spills Ecological Effects Research Forum (CROSERF) to prepare WAF (Singer et al., 2000). In this study, a system similar to baffed recirculation tanks (BRTs) as per Wade et al. (2017) was utilized to prepare WAF and CEWAF. A commercial oil spill dispersant (JFT-001) was used to treat oil/water mixtures to be applied in CEWAF treatment.
Correspondingly, 120-L collected seawater was transferred to each BRT in which WAF and CEWAF were produced. In the BRTs, oil and dispersant (JFT-001)were physically dispersed by a flow that generated by a polytetrafluoroethylene (PTFE)-diaphragm pump to circulate the seawater at ~250 mL/min. For WAF, 2-mL oil was added to BRT every 30 min to a total volume of 12 mL. CEWAF was prepared in the same way using 12-mL premixed 1꞉10 (dispersant꞉oil by volume) dilution. The dispersant-to-oil ratio was determined based on the application guideline of JFT-001. An electric stirrer was placed in the first chamber of the BRT system at mixing rate of 200 r/min to simulate current flow without causing vortex in water. The total mixing time was 20 hours.
2.2 Mesocosm experimentBefore the WAF and CEWAF were moved to mesocosm tanks from the BRT system, Time 0 samples were collected for both treatments. Later, the WAF prepared in BRT was transferred to the WAF mesocosm tanks (36 L), and the CEWAF prepared in BRT was transferred to the CEWAF mesocosm tanks (36 L). Each treatment (WAF and CEWAF) was prepared in triplicate, plus a control (with no oil added), which sums to a total of 7 mesocosm tanks. A sediment container was placed at the bottom of each mesocosm tank in advance to collect marine oil snow (MOS) aggregates. Natural light was introduced to all the mesocosms. A round glass container in diameter of 110 mm was placed at the bottom of tank as a sediment trap in each mesocosm. The experiment was run for 7 days and the sediment was collected on Day 7.
2.3 PAHs and estimated oil equivalents (EOE) analysesBulk water samples (1 L) were collected on Days 1, 2, 3, and 7 from the control and each of the triplicate treatments (WAF and CEWAF). An amount of 20-mL dichloromethane (DCM) was added to each sample immediately after it was taken to cease biological activity. The sample taken from water and sediment container was transferred into a 1-L separator funnel and extracted twice with 100-mL and 50-mL DCM, respectively. The DCM extract was combined and concentrated to 2 mL. For water samples, fluorescence analysis was carried out to determine the estimated oil equivalents (EOE) with Hitachi F-4500FL Spectrophotometer in the Department of Chemistry, Sun Yat-Sen University. The EOE values were calculated by fitting the fluorescence reading to a calibration curve generated by a series of standard petroleum samples, thus, EOE might give a rough estimate on the overall petroleum content in the water samples (Wade et al., 2011). The linear calibration curve with R2 > 0.92 was used to calculate EOE concentrations for DCM extracts of mesocosm water samples. The DCM fraction was transferred into cuvettes and analyzed for EOE by total scanning fluorescence (TSF). The optimum excitation (λex) and emission (λem) wavelengths for EOE were λex=260 nm and λem=370 nm.
After fluorescence analysis, the DCM fraction of water and the sediment trap samples were purified with an alumina/silica gel (Al/Si, 10 g/20 g) chromatographic column (300 mm×13 mm i.d.). The hydrocarbons in the sample were eluted from the column using 200 mL of DCM/pentane mixture (1/1 by volume). The eluted faction was then concentrated and exchanged into hexane to a final volume of 1 mL and spiked with PAH internal standards (containing naphthalene-d8, phenanthrene-d10, fluorene-d10, and chrysene-d12) for gas chramatography with mass spectrometry detector (GC/MS) analysis with Hewlett-Packard 6890 gas chromatograph (GC) coupled with Hewlett-Packard 5973N mass selective detector. PAHs were separated with a DB-5 MS fused silica capillary column (30 m×0.25 mm i.d., 0.25-μm film thickness). The GC temperature program was as follows: injection at 300 ℃, oven at 60 ℃ for 5 min isothermal hold, ramp to 110 ℃ at 40 ℃ /min, ramp to 300 ℃ at 10 ℃/min, and isothermal hold at 300 ℃ for 5 min. Target compounds were obtained by comparing the gas chromatographic peaks of the sample with those of the standard. PAHs were identified by comparing the retention time and mass spectrum of selected ions with calibration standards.
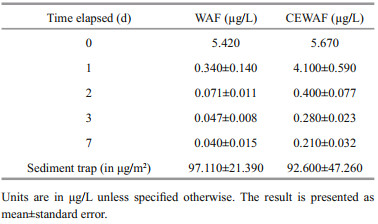
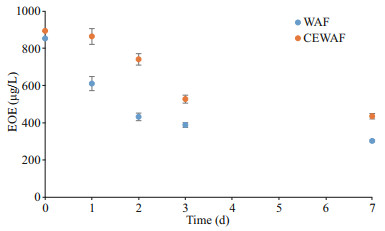
3 RESULT AND DISCUSSION 3.1 Estimated oil equivalenceThe measured EOE concentrations are shown in Fig. 1. All the fluorescence signals for the control treatment were below detection limit; thus, the EOE concentration for the control was determined as 0 μg/L. Initially, the CEWAF and WAF EOE concentrations were 983 and 853 μg/L, respectively. The EOEs in CEWAF were noticeably higher than that in WAF, indicating that with the application of chemical dispersant, more spilled oil entered the water column than that in WAF.
|
| Fig.1 The estimated oil equivalents (EOE) vs. time in day Error bars indicate standard deviation among triplicates. |
Furthermore, the EOEs decreased with time in all treatments, which roughly followed an exponential decay model below:
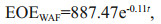
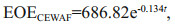
where EOEWAF is the EOE in WAF, EOECEWAF is the EOE in CEWAF, and t is the number of days. The R2 for modeling vs. measurement data was 0.90 and 0.81 for CEWAF and WAF, respectively. The exponentially decay pattern for EOE was also observed in earlier mesocosm experiments (Morales-McDevitt et al., 2020; Shi et al., 2020). Accordingly, the half-life of EOE was calculated to be 5.6 d for CEWAF and 4.7 d for WAF. The half-lives were longer in both treatments than those in a similar case in Texas using Gulf of Mexico seawater (Morales-McDevitt et al., 2020) due probably to that the source oil used in our study was more weathered than the Texas case, which is discussed in the next section.
3.2 TPAH in water columnThe total concentrations of 10 PAHs (TPAH) (i.e., naphthalene[Naph], acenaphthylene[Acy], acenaphthene[Ace], fluorene[Flu], phenanthrene[Phe], anthracene[Anth], fluoranthene[Flt], pyrene[Pyr], benzo[a]anthracene[BaA], chrysene[Chry]) were measured daily in water column (Table 1). Similar to EOE values, TPAH was higher in CEWAF than that in WAF due to chemical dispersion. Pearson's correlation coefficient (R) between EOE and TPAH in each mesocosm was calculated to be 0.9 for WAF treatment and 0.86 for CEWAF treatment, indicating a significant correlation between EOE and TPAH.
The initial TPAH concentration was close to that in Texas case (Morales-McDevitt et al., 2020) in magnitude. However, the TPAH in CEWAF treatment were about 8 times higher than that in WAF treatment in this study, while the TPAH in CEWAF were about 1.16 times higher than those in WAF in this study. This indicates that this study using JFT-001 had a considerably lower amplification effect on PAHs in water phase compared to previous study using Corexit-9500. The reason may be that the dispersant utilized in this study (JFT-001) may have a lower efficiency in dispersing the oil under the given dosage. On the other hand, the mixing energy in the WAF generator was designed to provide optimal mixing effect on Corexit, and JFT-001 could behave below expectation under such situation.
Nevertheless, WAF and CEWAF treatment showed distinctively different PAH removal patterns. Specific concentrations of individual PAHs are presented in Fig. 2. All PAHs, especially the concentrations of low molecular weight (LMW) ones (e.g., Naph and Phe) drastically decreased on the first 1–2 days. High molecular weight (HMW) PAHs (pyrene and chrysene) were rapidly removed in the first two days in WAF treatment, while in CEWAF treatment, longer time were taken. The reasons may be due to the differences in solubility and degradability of LMW and HMW PAH components, as well as the dispersion rates with WAF and CEWAF treatments.

|
| Fig.2 Remaining percentages of selected PAHs over time in WAF (a) and CEWAF (b) treatment |
In addition, a higher chrysene concentration was observed on Day 1 compared to initial value in the CEWAF treatment. This could be due to heterogeneity in the CEWAF generation and sampling process; part of the oil introduced to the system may be adsorbed to the wall of the mesocosm or stayed in the surface slick and be redispersed into the system later. On Day 7, the TPAH had been lowered to ~0.7% in WAF treatment and ~3.7% of the initial value in CEWAF treatment. However, percentages of remained HMW PAHs were much higher in CEWAF treatment: about 10% of pyrene and 25% of chrysene were still present in the water column on Day 7 of CEWAF treatments, while in WAF treatment only 2% of pyrene and 6% of chrysene remained.
The PAH composition in mesocosm tanks is plotted in pie chart (Fig. 3). The major constituents of PAH were 2-ring PAHs such as naphthalene at the beginning of experiment, which were rapidly removed. The 2-ring PAHs only added up to ~50% of the TPAH in both treatments at the initial time. Comparatively, in an earlier study using Macundo surrogate oil in Texas (Shi et al., 2020), the percentage of 2-ring PAH components may reach as high as 80% of TPAH at the beginning. This indicates that the source oil used in this study might be more weathered than the Macundo surrogate oil, thus leading to a lower dispersant efficiency as stated earlier. The 3-ring PAHs including fluorene, phenanthrene, and anthracene made up a relatively stable proportion through the 7-day experiment, while the percentage of 4-ring PAHs gradually increased towards the end of experiment, especially for the CEWAF treatment, where 4-ring PAHs can reach up to ~20% of the sum of PAHs at Day 7. This probably could be credited to the dispersants allowing for better dissolution to these hydrophobic PAH components. These compositional changes in PAHs are consistent with previous studies (Wang and Fingas, 1995; Yamada et al., 2003).

|
| Fig.3 PAHs composition (by rings) changes over time in WAF and CEWAF treatments |
The average removal rate for certain PAH species can be determined by fitting the PAH concentration over time to the first-order exponential model:
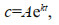
where c is the concentration; A is the initial concentration; t is time in day; and k is the calculated exponential (removal) rate constant. The half-lives of each individual PAH species can be calculated by (assuming exponential decay pattern):

Half-lives of PAHs measured in this study is provided in Table 2. The causes of PAHs removal in mesocosm tanks are complicated: evaporation, photo-oxidation, sedimentation, and biodegradation could all contribute to this process differently. Thus, the removal pattern was deviated from classical exponential decay. To evaluate the degree of deviation, R2 value was also shown in the table.

|
Overall, the half-lives of PAHs agreed well with previous studies using similar experimental methods (Morales-McDevitt et al., 2020; Shi et al., 2020). Basically, PAHs' half-lives increased with the number of rings. This is expected since lower molecular weight PAHs is easily affected by evaporation and usually removed rapidly. The PAHs in CEWAF treatments had higher half-lives than PAHs in WAF treatments. The benzo[a]anthracene and chrysene of CEWAF treatment had the longest half-lives across all the PAHs investigated in this study. For lighter PAHs, the R2 values ranged 0.47–0.71, suggesting the concentration change of these PAHs over time was only a moderate fit to exponential curve. This is expected since the removal of lighter PAHs can be resulted from multiple processes as mentioned above. The exceptions are benzo[a]anthracene and chrysene (especially in CEWAF treatments) with R2 values as high as 0.97, which are two PAHs with the suggesting that the removal of these two PAHs may be likely due to a single process rather than mixed paths like the lighter species. For these HMW PAHs, sedimentation may play a more important role than other processes in their removal.
3.3 PAHs in sedimentThe sediment TPAH concentration (in μg/m2) was calculated by c=c0×Atrap/Atank, where c0 is the PAH concentration in extracted samples injected to GC-MS; Atrap and Atank are the base area of sediment traps and mesocosm tanks, respectively. The sediment TPAH concentration presented in Table 1 is close in magnitude to the sedimental PAH samples collected in Gulf of Mexico after the Deepwater Horizon oil spill (Adhikari et al., 2016).
The effciency of PAHs sedimentation in WAF and CEWAF treatments, which is the ratio in percentage of PAHs incorporated into sediment to the initial amount of PAHs, is shown in Table 3. Generally, the CEWAF mesocosm had lower PAH sedimentation effciency. Since PAHs concentration was higher in the water phase of CEWAF treatment, the total amount of PAHs sinking to sediment for both WAF and CEWAF treatments were relatively similar. The sedimentation effciency of individual PAHs varied with their number of rings. The 2-ring component of naphthalene (2.7% for WAF and 2.3% for CEWAF) and 3-ring component of phenanthrene (7.9% for WAF and 6.5% for CEWAF) were poorly incorporated into sediment. In contrast, as high as 72.4% of chrysene in WAF treatment and 45.8% of chrysene in CEWAF treatment were transported to sediment phase. Two-ring PAHs like naphthalene and its alkylated homologues are volatile and dissolved in water, making them more available for biodegradation and evaporation (Ghosal et al., 2016; Bacosa et al., 2020). As their molecular weights grow higher, the PAHs usually tend to be adsorbed to particle phase and move towards the sediment; the recalcitrant HMW PAHs are among the least degraded and the slowest leaving a weathered oil containing numerous HMWs PAHs at elevated concentrations (Gong et al., 2014). These HMW PAHs are known to be scavenged and sink to the bottom of water column (Hinga et al., 1986; Wirth et al., 2018). However, the CEWAF treatment saw a significant decrease on the sedimentation effciency of HMW PAHs, which could be due to the presence of dispersants providing additional buoyancy to marine snow particles, thus decreasing their sinking velocity (Xu et al., 2019). Overall, it seemed that the addition of dispersant did not increase the sediment uptake of PAHs in the 7-day window; however, the drop of removal rate of PAHs in CEWAF treatment indicated that PAHs tend to stay in the water column for a longer time.

|
Having conducted a series of Mesocosm experiments, the transport and behavior of marine oil spill-containing PAHs were simulated and analyzed. The estimated oil equivalents (EOE) as well as the total PAH concentration of 10 PAHs were determined for seawater containing water accommodated oil (WAF) and chemically-enhanced water-accommodated fraction of oil (CEWAF). The application of dispersants in CEWAF treatment resulted in 16% increase in the concentration of total PAHs in water column compared to WAF, which was mainly due to the significant increased concentration of high molecular weight (HMW) PAHs. The CEWAF treatment also had higher half-lives of PAHs, especially HMW ones, compared to WAF treatment. In both treatments, significant amount of HMW PAHs were finally accumulated in sediment. In general, the application of dispersant did not increase the sediment uptake of PAHs and did increase the PAHs concentration in water column.
5 DATA AVAILABILITY STATEMENTThe data that support the findings of this study are available from the corresponding author upon reasonable request.
Abdel-Shafy H I, Mansour M S M. 2016. A review on polycyclic aromatic hydrocarbons: source, environmental impact, effect on human health and remediation. Egyptian Journal of Petroleum, 25(1): 107-123.
DOI:10.1016/j.ejpe.2015.03.011 |
Adhikari P L, Maiti K, Overton E B, et al. 2016. Distributions and accumulation rates of polycyclic aromatic hydrocarbons in the northern Gulf of Mexico sediments. Environmental Pollution, 212: 413-423.
DOI:10.1016/j.envpol.2016.01.064 |
Allan S E, Smith B W, Anderson K A. 2012. Impact of the deepwater horizon oil spill on bioavailable polycyclic aromatic hydrocarbons in Gulf of Mexico coastal waters. Environmental Science & Technology, 46(4): 2033-2039.
DOI:10.1021/es202942q |
Bacosa H P, Steichen J, Kamalanathan M, et al. 2020. Polycyclic aromatic hydrocarbons (PAHs) and putative PAH-degrading bacteria in Galveston Bay, TX (USA), following Hurricane Harvey (2017). Environmental Science and Pollution Research, 27(28): 34987-34999.
DOI:10.1007/s11356-020-09754-5 |
Brakstad O G, Lewis A, Beegle-Krause C J. 2018. A critical review of marine snow in the context of oil spills and oil spill dispersant treatment with focus on the Deepwater Horizon oil spill. Marine Pollution Bulletin, 135: 346-356.
DOI:10.1016/j.marpolbul.2018.07.028 |
Budzinski H, Jones I, Bellocq J, et al. 1997. Evaluation of sediment contamination by polycyclic aromatic hydrocarbons in the Gironde estuary. Marine Chemistry, 58(1-2): 85-97.
DOI:10.1016/S0304-4203(97)00028-5 |
Chanton J, Zhao T T, Rosenheim B E, et al. 2015. Using natural abundance radiocarbon to trace the flux of petrocarbon to the seafloor following the deepwater horizon oil spill. Environmental Science & Technology, 49(2): 847-854.
DOI:10.1021/es5046524 |
Couillard C M, Lee K, Légaré B, et al. 2005. Effect of dispersant on the composition of the water-accommodated fraction of crude oil and its toxicity to larval marine fish. Environmental Toxicology and Chemistry, 24(6): 1496-1504.
DOI:10.1897/04-267R.1 |
Duan J, Liu W, Zhao X, et al. 2018. Study of residual oil in Bay Jimmy sediment 5 years after the Deepwater Horizon oil spill: persistence of sediment retained oil hydrocarbons and effect of dispersants on desorption. Science of the Total Environment, 618: 1244-1253.
DOI:10.1016/j.scitotenv.2017.09.234 |
Gearing P J, Gearing J N, Pruell R J, et al. 1980. Partitioning of No.2 fuel oil in controlled estuarine ecosystems. Sediments and suspended particulate matter. Environmental Science & Technology, 14(9): 1129-1136.
DOI:10.1021/es60169a011 |
Ghosal D, Ghosh S, Dutta T K, et al. 2016. Current state of knowledge in microbial degradation of polycyclic aromatic hydrocarbons (PAHs): a review. Frontiers in Microbiology, 7: 1369.
DOI:10.3389/fmicb.2016.01369 |
Gong Y Y, Zhao X, Cai Z Q, et al. 2014. A review of oil, dispersed oil and sediment interactions in the aquatic environment: influence on the fate, transport and remediation of oil spills. Marine Pollution Bulletin, 79(1-2): 16-33.
DOI:10.1016/j.marpolbul.2013.12.024 |
Haritash A K, Kaushik C P. 2009. Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): a review. Journal of Hazardous Materials, 169(1-3): 1-15.
DOI:10.1016/j.jhazmat.2009.03.137 |
Hinga K R, Pilson M E Q, Almquist G, et al. 1986. The degradation of 7, 12-dimethylbenz(a)anthracene in an enclosed marine ecosystem. Marine Environmental Research, 18(2): 79-91.
DOI:10.1016/0141-1136(86)90001-2 |
Joye S B, Teske A P, Kostka J E. 2014. Microbial dynamics following the Macondo oil well blowout across Gulf of Mexico environments. Bioscience, 64(9): 766-777.
DOI:10.1093/biosci/biu121 |
Kim M, Jung J H, Ha S Y, et al. 2017. Long-term monitoring of PAH contamination in sediment and recovery after the Hebei Spirit oil spill. Archives of Environmental Contamination and Toxicology, 73(1): 93-102.
DOI:10.1007/s00244-017-0365-1 |
Kleindienst S, Paul J H, Joye S B. 2015. Using dispersants after oil spills: impacts on the composition and activity of microbial communities. Nature Reviews Microbiology, 13(6): 388-396.
DOI:10.1038/nrmicro3452 |
Lessard R R, DeMarco G. 2000. The significance of oil spill dispersants. Spill Science & Technology Bulletin, 6(1): 59-68.
DOI:10.1016/S1353-2561(99)00061-4 |
Morales-Mcdevitt M E, Shi D W, Knap A H, et al. 2020. Mesocosm experiments to better understand hydrocarbon half-lives for oil and oil dispersant mixtures. PLoS One, 15(1): e0228554.
DOI:10.1371/journal.pone.0228554 |
Passow U. 2016. Formation of rapidly-sinking, oil-associated marine snow. Deep Sea Research Part II: Topical Studies in Oceanography, 129: 232-240.
DOI:10.1016/j.dsr2.2014.10.001 |
Peacock E E, Hampson G R, Nelson R K, et al. 2007. The 1974 spill of the Bouchard 65 oil barge: Petroleum hydrocarbons persist in Winsor Cove salt marsh sediments. Marine Pollution Bulletin, 54(2): 214-225.
DOI:10.1016/j.marpolbul.2006.10.007 |
Quigg A, Passow U, Chin W C, et al. 2016. The role of microbial exopolymers in determining the fate of oil and chemical dispersants in the ocean. Limnology and Oceanography Letters, 1(1): 3-26.
DOI:10.1002/lol2.10030 |
Rahsepar S, Smit M P J, Murk A J, et al. 2016. Chemical dispersants: oil biodegradation friend or foe?. Marine Pollution Bulletin, 108(1-2): 113-119.
DOI:10.1016/j.marpolbul.2016.04.044 |
Shi D W, Bera G, Knap A H, et al. 2020. A mesocosm experiment to determine half-lives of individual hydrocarbons in simulated oil spill scenarios with and without the dispersant, Corexit. Marine Pollution Bulletin, 151: 110804.
DOI:10.1016/j.marpolbul.2019.110804 |
Singer M M, Aurand D, Bragin G E, et al. 2000. Standardization of the preparation and quantitation of wateraccommodated fractions of petroleum for toxicity testing. Marine Pollution Bulletin, 40(11): 1007-1016.
DOI:10.1016/S0025-326X(00)00045-X |
Tarr M A, Zito P, Overton E B, et al. 2016. Weathering of oil spilled in the marine environment. Oceanography, 29(3): 126-135.
DOI:10.5670/oceanog.2016.77 |
Wade T L, Morales-Mcdevitt M, Bera G, et al. 2017. A method for the production of large volumes of WAF and CEWAF for dosing mesocosms to understand marine oil snow formation. Heliyon, 3(10): e00419.
DOI:10.1016/j.heliyon.2017.e00419 |
Wade T L, Sweet S T, Sericano J L et al. 2011. Analyses of water samples from the Deepwaterhorizonoil spill: documentation of the subsurface plume. In: Liu Y G, Macfadyen A, Ji Z G eds. Monitoring and Modeling the Deepwater Horizon Oil Spill: a Record-Breaking Enterprise. p. 77-82, https://www.researchgate.net/publication/235737679_Analyses_of_Water_Samples_From_the_Deepwater_Horizon_Oil_Spill_Documentation_of_the_Subsurface_Plume. Accessed on May 7, 2022.
|
Wang D, Ma J, Li H, et al. 2018. Concentration and potential ecological risk of PAHs in different layers of soil in the petroleum-contaminated areas of the Loess Plateau, China. International Journal of Environmental Research and Public Health, 15(8): 1785.
DOI:10.3390/ijerph15081785 |
Wang Z D, Fingas M. 1995. Differentiation of the source of spilled oil and monitoring of the oil weathering process using gas chromatography-mass spectrometry. Journal of Chromatography A, 712(2): 321-343.
DOI:10.1016/0021-9673(95)00546-Y |
Wirth M A, Passow U, Jeschek J, et al. 2018. Partitioning of oil compounds into marine oil snow: insights into prevailing mechanisms and dispersant effects. Marine Chemistry, 206: 62-73.
DOI:10.1016/j.marchem.2018.09.007 |
Xu C, Lin P, Zhang S J, et al. 2019. The interplay of extracellular polymeric substances and oil/Corexit to affect the petroleum incorporation into sinking marine oil snow in four mesocosms. Science of the Total Environment, 693: 133626.
DOI:10.1016/j.scitotenv.2019.133626 |
Yamada M, Takada H, Toyoda K, et al. 2003. Study on the fate of petroleum-derived polycyclic aromatic hydrocarbons(PAHs) and the effect of chemical dispersant using an enclosed ecosystem, mesocosm. Marine Pollution Bulletin, 47(1-6): 105-113.
DOI:10.1016/S0025-326X(03)00102-4 |
Zhang B, Matchinski E J, Chen B et al. 2019. Chapter 21 - Marine oil spills—oil pollution, sources and effects. In: Sheppard C ed. World Seas: an EnvironmentalEvaluation. 2nd edn. Elsevier, Amsterdam. 391p, https://doi.org/10.1016/B978-0-12-805052-1.00024-3.
|
Zhou Z Z, Liu Z F, Guo L D. 2013. Chemical evolution of Macondo crude oil during laboratory degradation as characterized by fluorescence EEMs and hydrocarbon composition. Marine Pollution Bulletin, 66(1-2): 164-175.
DOI:10.1016/j.marpolbul.2012.09.028 |
Zhu Y Y, Duan X L, Qin N, et al. 2019. Health risk from dietary exposure to polycyclic aromatic hydrocarbons (PAHs) in a typical high cancer incidence area in southwest China. Science of the Total Environment, 649: 731-738.
DOI:10.1016/j.scitotenv.2018.08.157 |
 2023, Vol. 41
2023, Vol. 41