Institute of Oceanology, Chinese Academy of Sciences
Article Information
- SHEN Lili, HUANG Tao, CHEN Yuanqing, CHU Zhuding, XIE Zhouqing
- Diverse transformations of sulfur in seabird-affected sediments revealed by microbial and stable isotope analyses
- Journal of Oceanology and Limnology, 41(1): 138-149
- http://dx.doi.org/10.1007/s00343-021-1173-z
Article History
- Received Jun. 2, 2021
- accepted in principle Nov. 12, 2021
- accepted for publication Dec. 16, 2021
2 Anhui Province Key Laboratory of Wetland Ecosystem Protection and Restoration, Anhui University, Hefei 230601, China;
3 Anhui Key Laboratory of Polar Environment and Global Change, Department of Environmental Science and Engineering, University of Science and Technology of China, Hefei 230026, China
Sulfur is one of the important nutrient elements, and its biogeochemical transformation and recycling play an important role in maintaining the function of aquatic ecosystems (Orem et al., 2015; Poulin et al., 2017; Chen et al., 2020b). For example, sulfur biogeochemistry is coupled closely with the remineralization of organic matter, acidification of water bodies, recycling of nutrients and control of trace metal bioavailability in aquatic environments (Pester et al., 2012; Sheng et al., 2013; Liu et al., 2017; Jørgensen et al., 2019; Chen et al., 2020a).
A variety of sulfur species occur in aquatic sediments, including sulfide, elemental sulfur, sulfate, and organic sulfur compounds. Transformations among these species are controlled by the microbial activity, redox condition, organic matter, and pH (Norman et al., 2002; Sánchez-Andrea et al., 2014; Chen et al., 2020b). Organic sulfur compounds can be degraded to sulfate by sulfur-degrading bacteria (Couture et al., 2016). Sulfate-reducing bacteria (SRB) reduce sulfate to sulfide under anoxic and anaerobic conditions (Luther III et al., 2003). Correspondingly, some sulfides could also be oxidized to sulfate and intermediate species of elemental sulfur, thiosulfate, sulfite, and pyrite by chemical oxidant and/or sulfur oxidizing bacteria (SOB) (Purcell et al., 2014; Jørgensen et al., 2019).
The composition of sulfur isotopes is a useful tool to trace sulfur biogeochemical processes in aquatic environments, since different isotope fractionations were observed during these transformations (Sela-Adler et al., 2016; Jørgensen et al., 2019). The remineralization of organic sulfur could result in sulfur isotope fractionation effects of 10‰–30‰ (Norman et al., 2002; Shawar et al., 2018). Dissimilatory sulfate reduction (DSR) in sediments driven by anaerobic microorganisms is the main process imparting sulfur isotope fractionation with a variation between 0 and 70‰ (Canfield, 2001). The assimilatory sulfate reduction produces very small sulfur isotope fractionation of 1‰–3‰ (Sela-Adler et al., 2016). Most of the sulfides are ultimately reoxidized back to sulfate with only negligible fractionations under the condition of considerable oxidants (Balci et al., 2012).
The oxygen isotope compositions of sulfate (δ18O) in sediments could also provide information on sulfur biogeochemistry. The intermediate sulfur species generated from the DSR inherit enriched δ18O in the residual sulfate (Brunner and Bernasconi, 2005). During the oxidation of sulfides, the formed sulfate inherits the depleted 18O from water in the cytoplasm (Poser et al., 2014). Dual stable sulfur and oxygen isotopes of sulfate in natural environments therefore have been used increasingly to study the net rate and pathway of DSR (Antler et al., 2013; Feng et al., 2016).
Studies of sulfur geochemistry in Antarctic aquatic ecosystems are limited. The sulfur isotope value of sulfate in the bottom water of the Ace Lake in Vestfold Hills, East Antarctica was as high as 67‰ and associated with sulfate reduction by microorganisms (Burton and Barker, 1979). Subsequent studies analyzed the microbial composition and metabolic function in waters of the adjacent Organic Lake, discussed the coupling relationship between carbon and sulfur transformations, and indicated how the microbial communities adapt to the specific Antarctic environment (Ng et al., 2010; Yau et al., 2013). The microbial compositions of SRB and SOB in the sediments of Subglacial Lake Whillans in Antarctica were also determined to study the sulfur transformations (Purcell et al., 2014). In the extremely dry and cold McMurdo valleys, geochemical and molecular microbial community analyses were performed to investigate the anaerobic oxidation of methane and associated sulfate reduction in Lake Fryxell (Karr et al., 2005; Sattley and Madigan, 2006; Saxton et al., 2016). Sulfur and oxygen isotope ratios of sulfate in lake waters from Deception Island, Antarctic Peninsula suggest mixing of sulfate from atmospheric deposition and from oxidation of local sulfide minerals (Kim et al., 2017, 2021).
In Polar Regions, as well as in the globe, seabirds transport and accumulate large amounts of nutrients and pollutants in the form of guano from ocean to lacustrine ecosystems (Sun et al., 2000, 2013; Blais et al., 2005; Michelutti et al., 2009; Emslie et al., 2014). Nine bio-elements in the ornithogenic sediments from Y2 Lake, one of our study sites, have been identified and used to reconstruct the penguin population change in the past 3 000 years at Ardley Island, West Antarctic Peninsula (Sun et al., 2000). High level of organic matter and nutrients including nitrogen, phosphorus, and sulfur in the ornithogenic waste products provide abundant nutrients that are often promoting the growth of microorganisms (Li et al., 2006). The bacterial richness and diversity in Y2 Lake is strongly associated with historical penguin activity (Zhu et al., 2015). In our previous study, we analyzed various sulfur species in a seabird-affected sediment core Y2 and a seabird-free sediment core YO from Ardley Island and Fildes Peninsula, discussed the indicated sulfate reduction in those sediments and concluded the main forms of organic sulfur for Y2 and organic sulfur and sulfate for YO (Chen et al., 2020b). The specific biogeochemical transformations of sulfur in Y2 and YO, however, remain unclear since sulfur species provides rare indication on microgeochemical processes. Therefore, in the present study, we analyze the compositions of sulfur and oxygen isotope for sulfate/sulfides and the microbial community in sediments to study and reveal the diverse transformations of sulfur in the seabirdaffected aquatic ecosystem.
2 MATERIAL AND METHOD 2.1 Study area and sample collectionArdley Island is a special ecological reserve designated by the Scientific Committee on Antarctic Research, which is connected to Fildes Peninsula with a sandbar (Fig. 1). The island covers an area of about 2 km2 with a flat and stable terrain, where lichens and mosses populated therein. Ardley Island is famous for the breeding colonies of penguin populations, with those occupied the eastern part of the island nowadays and the western part in the past (Yang et al., 2019). During the summer breeding period, abundant penguin guano is washed into nearby lakes and ponds and leaves distinct ornithogenic signatures in the sediments. In contrast, the lakes and ponds on Fildes Peninsula, including our study site YO, are seabird-free and not affected by penguin activity (Chu et al., 2019). The YO lake covers an area of about 9 000 m2 and located about 200 m from the nearest coastline of the Great Wall Bay. Sediment cores Y2 (60 cm) and YO (30 cm) were collected in austral summer 2012/2013 during the 29th Chinese Antarctic Expedition. In laboratory, Y2 and YO were sectioned at 1-cm intervals and obtained 60 and 30 subsamples, respectively. The penguin-affected Y2 core discharges a strong and unpleasant smell of guano, especially in the bottom layer; and the seabird-free YO core was characterized by a dominance of greyish deposits.
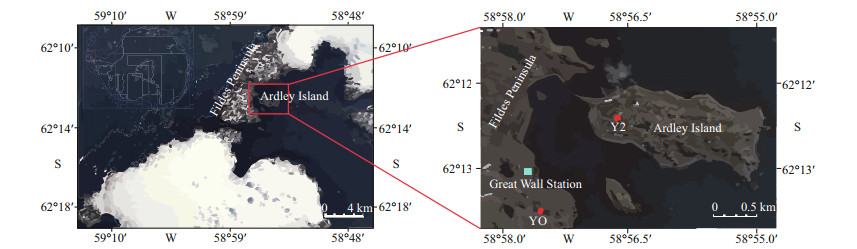
|
| Fig.1 Study area and sampling sites on Fildes Peninsula and Ardley Island (based on Chen et al., 2020b) |
Sub-sediments of Y2 and YO analyzed in the present study were parallel samples to those reported in Chen et al. (2020b). Before chemical analyses and DNA extraction, one part of each subsample was stored at -20 ℃, and the other part was centrifugalized to extract the pore water prior to freeze-dried and powdered. The final powder was passed through a 200-mesh sieve, placed in a drying apparatus, and then prepared to barite for stable sulfur and oxygen isotope analyses.
2.3 Analyses of microbial community compositions 2.3.1 Sediment DNA extractionDNA was extracted from the sediments by the sodium dodecyl sulfate (SDS) high salt method (Zhou et al., 1996). Sediment samples of 0.5 g were mixed with 600 μL of DNA extraction buffer (1-mol/L Tris-HCl, 0.5-mol/L EDTA, 0.5-mol/L phosphate, 3-mol/L NaCl, 1% CTAB) and 50 μL of proteinase K (20 mg/mL) in Oakridge tubes by horizontal shaking at 225 r/min for 30 min at 37 ℃. After the shaking treatment, 600-μL 20% SDS was added, and the samples were incubated in a 65-℃ water bath for 30 min. The supernatants were collected after centrifugation at 8 000 r/min for 10 min at room temperature and transferred into 2-mL centrifuge tubes. Supernatants from extractions were combined and mixed with an equal volume of Phenol-chloroform-isoamyl alcohol (25꞉24꞉1). The aqueous phase was recovered by centrifugation and precipitated with PEG-NaCl at 4 ℃ for 2 h. The pellet of crude nucleic acids was obtained by centrifugation at 14 000 r/min for 10 min at 4 ℃, washed with 75% ethanol, add 100-μL Tris-EDTA buffer, and store at -20 ℃.
2.3.2 PCR amplification and 16S rDNA sequencingThe V3–V4 region of the bacterial 16S rRNA gene was amplified using the 341F (5ʹ-CCTACGGGNGGCWGCAG-3ʹ)/805R (5ʹ-GACTACHVGGGTATCTAATCC-3ʹ) primers. PCR reaction was done for each sample under the following conditions: 98 ℃ for 30 s; 35 cycles of denaturation at 98 ℃ for 10 s, annealing at 54 ℃ or 52 ℃ for 30 s, and extension at 72 ℃ for 45 s; followed by a final extension at 72 ℃ for 10 min. The PCR products were collected and purified using the Agarose Gel DNA purification kit (TaKaRa, Japan), and then sequencing was conducted using the Illumina MiSeq PE300 Sequencer (Illumina, Inc., CA, USA) at LCBio Technologies (Hangzhou, China) Co., Ltd.
2.3.3 Data processingPaired-end reads were merged using the FLASH program. Chimeric sequences were filtered using Vsearch software (v2.3.4). Sequences with ≥97% similarity were assigned to the same operational taxonomic units (OTUs) using Vsearch. Representative sequences were selected for each OTU, and taxonomic data were then assigned to each representative sequence using the Ribosomal Database Project (RDP Release 11.5) classifier. OTU abundance information was normalized using the sequence number of the sample with the fewest sequences as a standard. The microbial communities at the class level and the sulfur-cycle related microbial genus in Y2 and YO sediments were obtained by using the above method.
2.4 Sulfur and oxygen isotope analysesGeochemical analysis was not performed for pore water due to the low content. The sulfates in Y2 and YO sediments were extracted by solution of NaH2PO4 (pH=6, 0.016 mol/L), collected through centrifugation and filtration, purified by dissolution and re-precipitation in a chelating solution of diethylene triamine penta acetic acid (DTPA) (Bao, 2006), and to precipitated as barite by adding saturated BaCl2 solution. The collected BaSO4 was washed repeatedly by Milli-Q water and then heated in an oven at 105 ℃. The dried BaSO4 was powdered and placed into a 2-mL centrifuge tube in drying apparatus.
Since the main sulfides in Y2 and YO sediments are acid volatile sulfur (AVS) and pyrite sulfur (CRS), respectively (Chen et al., 2020b), and the precipitation of sulfides is associated with only negligible fractionation (Böttcher et al., 1998). The precipitation of AVS and CRS in Y2 and YO for stable sulfur isotope analyses was prepared according to Habicht and Canfield (1997). The detail steps were as follows: AVS in Y2 and CRS in YO were extracted into ZnS; the ZnS was rinsed sequentially by NaOH (2 mol/L, 15 mL) and weakly alkaline water (pH=8.0, 15 mL); then the ZnS was converted to Ag2S by adding AgNO3 solution (0.1 mol/L, 10 mL); finally, the precipitated Ag2S was separated by centrifugation, washed twice by 15-mL distilled water, dried in an oven at 105 ℃ and placed in a 2-mL centrifuge tube in a drying dish.
Stable sulfur and oxygen isotope analyses of barite and sulfides were performed in the State Key Laboratory of Ore Deposit Geochemistry. Weighed samples of barite as well as V2O5 (1꞉3) and Ag2S, respectively, were compacted into tin cups for sulfur isotope analysis and the weighed BaSO4 was compacted into silver cups for oxygen isotope analysis. Stable sulfur and oxygen isotope ratios were determined by isotope ratio mass spectrometer (Thermo Fisher Delta V Advantage) coupled to an elemental analyzer (Flash 2000 for sulfur and thermal conversion eElemental analyzer (TC/EA) for oxygen). The instrument precision was ±0.2‰ for δ34S and 0.30‰ for δ18O. IAEA-SO-5 (δ34S, 0.5‰), IAEA-SO-6 (-34.1‰), and NBS-127 (δ34S, 20.3‰) were used as the standard samples for sulfur isotope analysis of barite and IAEA S1 (-0.3‰), IAEA S2 (+22.6‰) and IAEA S3 (-32.5‰) for sulfur isotope analysis of sulfides. NBS- 127 (δ18O, 8.59‰) was used as the standard sample for the oxygen isotope analysis. Stable isotope results were presented in δ (‰) and expressed relative to the vienna canyon diablo troilite (VCDT) for δ34S and VSMOW for δ18O according to the equation of δ (‰)=[(Rsample-Rstandard)/Rstandard]×1 000, where δ (‰) represents the δ34S or δ18O, Rsample is the isotopic ratio of the sample, and Rstandard, the isotopic ratio of VCDT and vienna standard mean ocean water (VSMOW).
3 RESULT 3.1 Compositions of microbial communityAmounts of 1 045 366 and 811 921 high-quality microbial sequences were obtained from Y2 and YO sediments, with a range of 43 123–81 825 and 39 185–93 981 sequences per sample. At the class level, the dominant microbial groups in Y2 sediments were Betaproteobacteria (23.99%), Gemmatimonadetes (14.98%), Actinobacteria (11.83%), Gammaproteobacteria (9.33%), and Alphaproteobacteria (7.13%). The average abundance of each of other groups was less than 5%. The dominant bacteria in YO sediments were Actinobacteria (41.23%), Thermoleophilia (15.17%), and Gammaproteobacteria (12.01%).
The vertical distribution of microorganisms in Y2 and YO was plotted in Fig. 2 at class level. In Y2 sediment profile, bacteria of Gammaproteobacteria, Gemmatimonadetes, Actinobacteria, and Alphaproteobacteria show higher relative abundance in 0–19 cm and 48–56 cm and lower in 23–45 cm; while Betaproteobacteria and Deltaproteobacteria show an opposite vertical trend with those above. The dominant bacteria of Actinobacteria, Thermoleophilia, and Gammaproteobacteria in YO show high relative abundance in the vertical profile except for the layer of 5 cm, where the relative abundance of Cyanobacteriia is as high as 70.34%.

|
| Fig.2 Relative abundance of microorganisms at the class level in Y2 and YO sediments |
Relative abundance of the sulfur-cycle related microbial communities at genus level in Y2 and YO sediments were plotted in Fig. 3. In Y2 sediments, Desulfotalea was the dominant SRB genus (0.37%– 13.40%), with the highest abundance at depth of 48 cm; the abundance of Pseudomonas in the section of 0–23 cm was higher than that of 23–56 cm, while the abundance of Thermomonas was very low in the up 30-cm layer in contrast to those high in 34–56 cm. Other sulfur cycle-related microbial groups in Y2 included Desulfosporosinus (0.48%), Gallionella (0.40%), and Sulfuriferula (0.36%). In YO sediments, Sulfuriferula (0.30%–6.19%) and Desulfotalea (0.22%–8.84%) were the primary groups of SOB and SRB, and high abundance of them was observed in 16 cm and 28 cm, respectively. Other sulfur cyclerelated microbial groups in YO include Pseudomonas (1.63%), Gallionella (0.64%), and Ferruginibacter (0.90%).
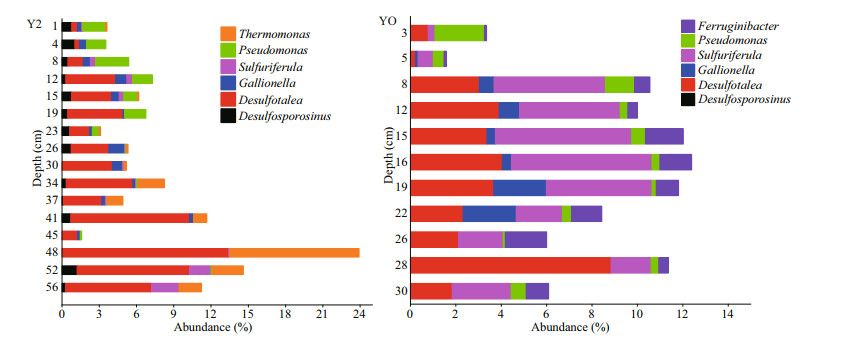
|
| Fig.3 Relative abundance of the sulfur cycle-related microbial genus in Y2 and YO sediments |
The vertical distributions of AVS, CRS, and sulfate and the corresponding δ34SAVS, δ34SCRS, δ34SSO4 and δ18OSO4 in Y2 and YO sediments were plotted in Fig. 4. The δ34SSO4 values in the 0–23-cm layer in Y2 sediments ranged between -4.5‰ and 4.7‰, while those below 30 cm showed a fluctuated trend, with large enrichment in 30, 41, and 52 cm, and much depletion in 34–37- and 45–48-cm layers. The δ34SAVS in Y2 sediments depleting from 5 to 12 cm and enriching between 12 and 56 cm, with much enriched values in 48–56-cm layer. The δ18OSO4 values in Y2 sediments ranged from -2.09‰ to 2.78‰ with a fluctuation. In YO sediments, almost of the δ34SSO4 ranged narrowly between 11.6‰ and 18.6‰, except the depleted values in 3–5 cm and the most enriched in 28 cm. The δ34SCRS in YO sediments enriching from 5 cm to 22 cm; the most enriched value in 28 cm was similar to that of δ34SSO4. The δ18OSO4 values in YO sediments ranged from 0.20‰ to 3.96‰ with a fluctuation.
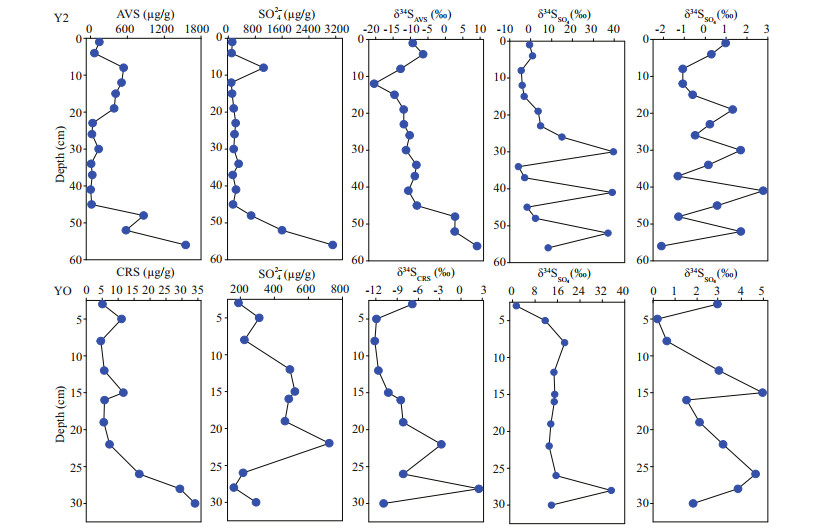
|
| Fig.4 Vertical distribution of sulfur species of AVS, CRS, and sulfate (data from Chen et al., 2020b) and the corresponding δ34SAVS, δ34SCRS, δ34SSO4, and δ18OSO4 in Y2 and YO sediments |
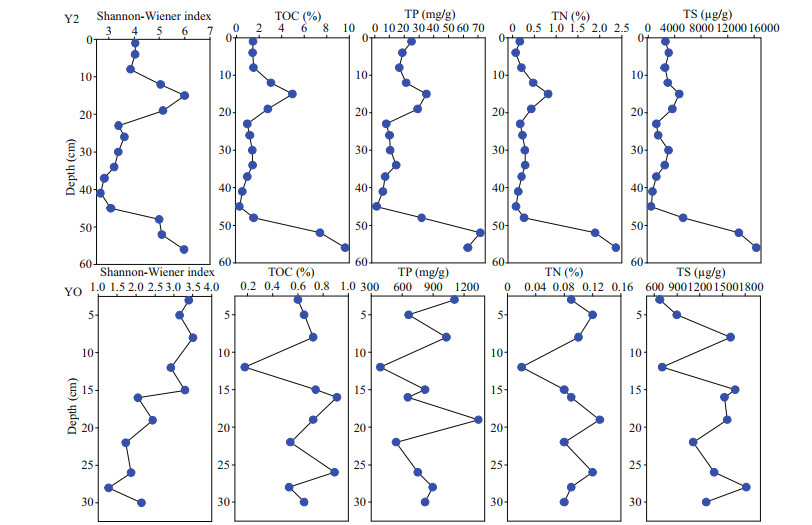
Shannon-Wiener index was calculated in this study to evaluate the microbial diversity in Y2 and YO sediments. Seabird-derived nutrients such as phosphorus, nitrogen, and sulfur, as well as associated increases in organic matter content in Y2 sediments have been used to reconstruct penguin population dynamics over the past 3 000 years (Sun et al., 2000). Significant and positive correlations between the Shannon-Wiener index and total organic carbon (TOC), total phosphorus (TP), total nitrogen (TN), and total sulfur (TS) in Y2 sediments (Table 1), as well as their consistent vertical distributions (Fig. 5) indicated that the microbial communities at the class level were associated tightly with penguin population changes in the past, similar to those reported in phylum level in Zhu et al. (2015). The nutrients in seabird-free YO sediments were very low in contrast to those in Y2, and the microbial diversity showed a decreasing trend from the top down (Fig. 5).
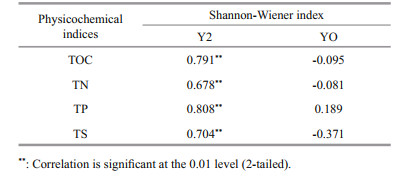
|
|
| Fig.5 Vertical distribution of Shannon-Wiener index and the reported TOC, TP, TN, and TS in Y2 and YO sediments (Chen et al., 2020b) |
The sulfur cycle-related bacterial genus plotted in Fig. 3 includes SRB, SOB, and the sulfur-degrading bacteria. Sulfate-reducing bacteria are a diverse group of anaerobic microorganisms that use sulfate as terminal electron acceptor to obtain energy through catabolism, electron transfer, and oxidation of organic matter (Mußmann et al., 2005; Bhattarai et al., 2018), and reduce sulfate to sulfide and/or elemental sulfur. Therefore, high organic matter in sediments promotes the growth of SRB community (Brodersen et al., 2019). Two SRB of Desulfosporosinus and Desulfotalea were observed in Y2 sediments but only Desulfotalea in YO. Desulfosporosinus inhabited in acid sediments (Sen and Johnson, 1999), while Desulfotalea was maninly found from marine sediments in cold regions (Rabus et al., 2004). High abundance of SRB has been reported in subsurface sediments (Leloup et al., 2005; Finke et al., 2007). In Y2 sediments, the abundance of Desulfotalea in deep layer of 48 cm was the highest, being 3.6 times as abundant as that in subsurface layer (8–19 cm). This is likely due to the high level of organic matter inputs from penguin guano and strictly anaerobic conditions therein, which promote the growth of SRB community. The abundance of Desulfotalea was low in 3–5-cm layer in YO sediments (Fig. 3), corresponding to the low level of organic matter and nutrients. The organic matter in sediments is the electron donor for sulfate during the DSR; it correlated positively with the sulfate reduction rate (Taketani et al., 2010). The highest abundance of Desulfotalea in 28 cm indicated strongest sulfate reduction in YO sediments, which coincides with the results concluded by the ratios of reduced inorganic sulfur and sulfate (RIS/SO42ˉ) in Chen et al. (2020b).
Sulfur-oxidizing bacteria are a group of microorganisms who oxidize the sulfide, elemental sulfur, thiosulfate, and sulfite to sulfate or intermediates. In Y2 sediments, SOB of Gallionella, Sulfuriferula, and Thermomonas was observed, and the abundance of Gallionella and Sulfuriferula was very low; Thermomonas is a strict anaerobic bacteria that could drive denitrification coupling with the oxidation of reduced inorganic sulfur compounds (He et al., 2017; Ucar et al., 2020). Gallionella, Sulfuriferula, and Ferruginibacter were observed in YO sediments. High abundance of Sulfuriferula as well as Ferruginibacter increased rapidly below 8 cm in YO indicates that they are anaerobic SOB and can use nitrate and ferric iron as electron acceptors and oxidize sulfide to sulfate under anaerobic and/or anoxic conditions (Sugio et al., 1985; Mahmood et al., 2009).
Pseudomonas is one of the sulfur-degrading bacteria that could produce sulfatase and degrade the sulfuric acid ester to sulfate (Wallner et al., 2004; Hagelueken et al., 2006). High abundance of Pseudomonas indicated active remineralization of organic sulfur compounds in 0–23-cm layer of Y2 sediments. The abundance of Pseudomonas was the highest in surface layer (3–5 cm) and the third abundant in other layer indicated the strong and moderate degradation of organic sulfur compounds in YO sediments.
4.3 Remineralization of organic sulfur compoundsThe sulfur in Y2 sediments originated primarily from inputs of penguin guano with a main form of organic sulfur compounds (Chen et al., 2020b), which would degrade to sulfate by microbial activity. This degradation, also known as remineralization of organic sulfur compounds, would produce ~10‰ sulfur isotope fractionation (Norman et al., 2002). Organic sulfur compounds are formed through the assimilatory sulfate reduction and sulfurization of organic matter (Werne et al., 2008; Rosenberg et al., 2018), and the δ34S of them inherits those of their precursor, sulfate, and sulfide (Aizenshtat and Amrani, 2004). Therefore, the δ34S of sulfate that degraded from organic sulfur compounds ranges between those of sulfate and sulfide.
Very high abundance of Pseudomonas indicates strong remineralization of organic sulfur compounds in the layer of 0–23 cm in Y2 sediments (Fig. 3), consistent with those indicated by sulfur species in Chen et al. (2020b). Although the high abundance of Desulfotalea (SRB) observed in the section of 12– 19 cm in Y2 indicated remarkable sulfate reduction, the sulfur isotopic depletion of -4.5‰–4.7‰ for sulfate suggested that the intensity of remineralization of organic sulfur compounds was much higher than that of sulfate reduction in this section. The very low level of Pseudomonas in the layer below 23 cm in Y2 suggested weak degradation of organic sulfur compounds. The organic sulfur-degrading bacteria Pseudomonas observed from the top down indicates remineralization of organic sulfur compounds in every layer in YO, with high intensity in 3–5 cm and considerable in other layer.
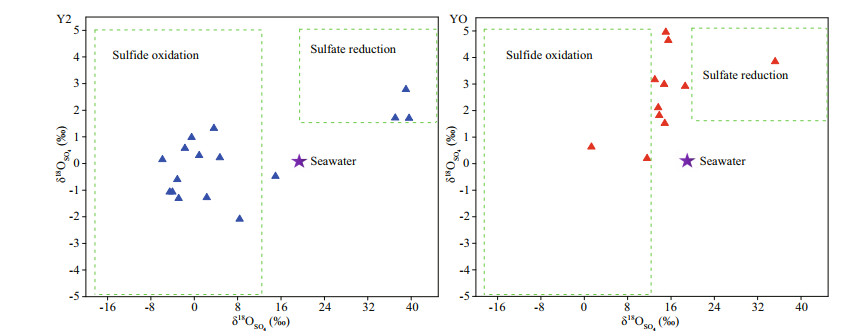
4.4 Concurrence of sulfate reduction and sulfur oxidationDissimilatory sulfate reduction by SRB is a key step of the sulfur biogeochemical transformations in aquatic ecosystems (Orem et al., 2015; Wasmund and Mußmann, 2017), which would led to a large enrichment in δ34S and δ18O for the residual sulfate and depletion in δ34SAVS and δ34SCRS for the reduced products (Antler et al., 2013). As the sulfur isotope fractionation for oxidation of sulfides was negligible, thus the formed sulfate inherits the depleted sulfur isotopic ratio of sulfides (Zerkle et al., 2009; Balci et al., 2012; Jørgensen et al., 2019). The observed large enrichment in δ34SSO4 and δ18OSO4 (Fig. 4) and high abundance of SRB (Fig. 3) in Y2 sediments in the 30-, 41-, and 52-cm layer indicated a strong sulfate reduction therein (Fig. 6). This indication, however, is inconsistent with the strong sulfate reduction in 12–19-cm layer as suggested by high proportion of RIS/SO42ˉ in Chen et al. (2020b). This inconsistency is likely because sulfur and oxygen isotope compositions of sulfate were affected simultaneously by sulfate reduction, sulfur oxidation, and remineralization of organic sulfur compounds, because sulfate reduction results in large enrichment in δ34SSO4 and δ18OSO4, whereas sulfur oxidation and remineralization of organic sulfur compounds would deplete δ34SSO4 and δ18OSO4 in system. Similar high abundance of SRB in 12–19 cm and 30 cm in Y2 sediments (Fig. 3) indicated strong sulfate reduction there, while high abundance of SOB and sulfur-degrading bacteria Pseudomonas in 12–19-cm layer indicated concurrent strong sulfur oxidation and remineralization of organic sulfur compounds and consequently deplete the δ34SSO4 in system. High abundance of SRB and Thermomonas (SOB) observed in 34–37, 48, and 56 cm in Y2 sediments (Fig. 3) indicated concurrent sulfate reduction and sulfur oxidation, and thus resulted in depletion of δ34SSO4 and δ18OSO4 (Fig. 6). In addition, the comparative δ34SAVS and δ34SSO4 in 56 cm in Y2 may also be due to the high rate of sulfate reduction in the bottom layer with a very high level of sulfate. It was reported that high concentration of substrate promotes faster sulfate reduction and results smaller fractionation effects (Habicht and Canfield, 1997; Habicht et al., 2005; Canfield et al., 2010).
|
| Fig.6 Bivariate plots of δ34SSO4 and δ18OSO4 for sulfate in sediments in this study and the worldwide seawater (data from Tostevin et al., 2014) |
In YO sediments, the large enriched δ34SSO4, δ34SCRS and highest abundance of Desulfotalea in 28 cm consistent with the highest ratio of RIS/SO42ˉ reported in Chen et al. (2020b) and indicated strongest sulfate reduction. The medium δ34SSO4 value of 11.6‰–18.6‰ and high abundance of SRB and SOB in 8–26 cm and 30 cm indicated concurrence of sulfate reduction and sulfur oxidation.
Compared to those of YO, the sulfur isotope compositions of Y2 varied widely due to much stronger sulfate reduction activity (Chen et al., 2020b; this study). Other potential mechanism such as the local terrestrial inputs from sulfide oxidation may be responsible for some of the observed δ34SSO4, since the depleted δ34S of volcanic rocks and sulfides were reported in King George Island (Kim et al., 2017, 2021). This process, however, may not be a meaningful contribution to the observed δ34SSO4 in the present study, because the sulfur inputs from terrestrial runoff was negligible in comparison with the guano depositions in Y2. The consistent associations between sulfur cycle-related bacteria and sulfur isotope compositions in both Y2 and YO indicated that internal bio-transformations of sulfur species were the main trigger for the change of sulfur isotope compositions.
5 CONCLUSIONThe microbial communities in Y2 sediments were associated with elevated sediment organic matter content and track the nutrient-rich inputs from past penguin activities. Stable sulfur and oxygen isotope ratios of sulfate and the sulfur cycle-related bacteria indicated remarkable and diverse transformations of sulfur in the seabird-affected sediments. Our study indicates that microbial community and stable sulfur isotope analysis in ornithogenic sediments could provide potential tracking of penguin activities around freshwater systems in Antarctica.
6 DATA AVAILABILITY STATEMENTThe datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENTSamples in this study were provided by the BIRDSSediment system (Hefei). We thank the Chinese Arctic and Antarctic Administration and Polar Research Institute of China for logistical support in the field work.
Aizenshtat Z, Amrani A. 2004. Significance of δ34S and evaluation of its imprint on sedimentary organic matter: I. The role of reduced sulfur species in the diagenetic stage: a conceptual review. The Geochemical Society Special Publications, 9: 15-33.
|
Antler G, Turchyn A V, Rennie V, et al. 2013. Coupled sulfur and oxygen isotope insight into bacterial sulfate reduction in the natural environment. Geochimica et Cosmochimica Acta, 118: 98-117.
DOI:10.1016/j.gca.2013.05.005 |
Balci N, Mayer B, Shanks III W C, et al. 2012. Oxygen and sulfur isotope systematics of sulfate produced during abiotic and bacterial oxidation of sphalerite and elemental sulfur. Geochimica et Cosmochimica Acta, 77: 335-351.
DOI:10.1016/j.gca.2011.10.022 |
Bao H M. 2006. Purifying barite for oxygen isotope measurement by dissolution and reprecipitation in a chelating solution. Analytical Chemistry, 78(1): 304-309.
DOI:10.1021/ac051568z |
Bhattarai S, Cassarini C, Naangmenyele Z, et al. 2018. Microbial sulfate-reducing activities in anoxic sediment from Marine Lake Grevelingen: screening of electron donors and acceptors. Limnology, 19(1): 31-41.
DOI:10.1007/s10201-017-0516-0 |
Blais J M, Kimpe L E, McMahon D, et al. 2005. Arctic seabirds transport marine-derived contaminants. Science, 309(5733): 445.
DOI:10.1126/science.1112658 |
Böttcher M E, Smock A M, Cypionka H. 1998. Sulfur isotope fractionation during experimental precipitation of iron(II) and manganese(II) sulfide at room temperature. Chemical Geology, 146(3-4): 127-134.
DOI:10.1016/S0009-2541(98)00004-7 |
Brodersen K E, Trevathan-Tackett S M, Nielsen D A, et al. 2019. Oxygen consumption and sulfate reduction in vegetated coastal habitats: effects of physical disturbance. Frontiers in Marine Science, 6: 14.
DOI:10.3389/fmars.2019.00014 |
Brunner B, Bernasconi S M. 2005. A revised isotope fractionation model for dissimilatory sulfate reduction in sulfate reducing bacteria. Geochimica et Cosmochimica Acta, 69(20): 4759-4771.
DOI:10.1016/j.gca.2005.04.015 |
Burton H R, Barker R J. 1979. Sulfur chemistry and microbiological fractionation of sulfur isotopes in a saline Antarctic lake. Geomicrobiology Journal, 1(4): 329-340.
DOI:10.1080/01490457909377739 |
Canfield D E, Farquhar J, Zerkle A L. 2010. High isotope fractionations during sulfate reduction in a low-sulfate euxinic ocean analog. Geology, 38(5): 415-418.
DOI:10.1130/G30723.1 |
Canfield D E. 2001. Isotope fractionation by natural populations of sulfate-reducing bacteria. Geochimica et Cosmochimica Acta, 65(7): 1117-1124.
DOI:10.1016/S0016-7037(00)00584-6 |
Chen Y Q, Ge J W, Huang T, et al. 2020a. Restriction of sulfate reduction on the bioavailability and toxicity of trace metals in Antarctic lake sediments. Marine Pollution Bulletin, 151: 110807.
DOI:10.1016/j.marpolbul.2019.110807 |
Chen Y Q, Shen L L, Huang T, et al. 2020b. Transformation of sulfur species in lake sediments at Ardley Island and Fildes Peninsula, King George Island, Antarctic Peninsula. Science of the Total Environment, 703: 135591.
DOI:10.1016/j.scitotenv.2019.135591 |
Chu Z D, Yang Z K, Wang Y H, et al. 2019. Assessment of heavy metal contamination from penguins and anthropogenic activities on Fildes Peninsula and Ardley Island, Antarctic. Science of the Total Environment, 646: 951-957.
DOI:10.1016/j.scitotenv.2018.07.152 |
Couture R M, Fischer R, Van Cappellen P, et al. 2016. Nonsteady state diagenesis of organic and inorganic sulfur in lake sediments. Geochimica et Cosmochimica Acta, 194: 15-33.
DOI:10.1016/j.gca.2016.08.029 |
Emslie S D, Polito M J, Brasso R, et al. 2014. Ornithogenic soils and the paleoecology of pygoscelid penguins in Antarctica. Quaternary International, 352: 4-15.
DOI:10.1016/j.quaint.2014.07.031 |
Feng D, Peng Y B, Bao H M, et al. 2016. A carbonate-based proxy for sulfate-driven anaerobic oxidation of methane. Geology, 44(12): 999-1002.
DOI:10.1130/G38233.1 |
Finke N, Vandieken V, Jørgensen B B. 2007. Acetate, lactate, propionate, and isobutyrate as electron donors for iron and sulfate reduction in Arctic marine sediments, Svalbard. FEMS Microbiology Ecology, 59(1): 10-22.
DOI:10.1111/j.1574-6941.2006.00214.x |
Habicht K S, Canfield D E. 1997. Sulfur isotope fractionation during bacterial sulfate reduction in organic-rich sediments. Geochimica et Cosmochimica Acta, 61(24): 5351-5361.
DOI:10.1016/S0016-7037(97)00311-6 |
Habicht K S, Salling L, Thamdrup B, et al. 2005. Effect of low sulfate concentrations on lactate oxidation and isotope fractionation during sulfate reduction by Archaeoglobus fulgidus strain Z. Applied and Environmental Microbiology, 71(7): 3770-3777.
DOI:10.1128/AEM.71.7.3770-3777.2005 |
Hagelueken G, Adams T M, Wiehlmann L, et al. 2006. The crystal structure of SdsA1, an alkylsulfatase from Pseudomonas aeruginosa, defines a third class of sulfatases. Proceedings of the National Academy of Sciences of the United States of America, 103(20): 7631-7636.
DOI:10.1073/pnas.0510501103 |
He Z H, Long X X, Li L Y, et al. 2017. Temperature response of sulfide/ferrous oxidation and microbial community in anoxic sediments treated with calcium nitrate addition. Journal of Environmental Management, 191: 209-218.
DOI:10.1016/j.jenvman.2017.01.008 |
Jørgensen B B, Findlay A J, Pellerin A. 2019. The biogeochemical sulfur cycle of marine sediments. Frontiers in Microbiology, 10: 849.
DOI:10.3389/fmicb.2019.00849 |
Karr E A, Sattley W M, Rice M R, et al. 2005. Diversity and distribution of sulfate-reducing bacteria in permanently frozen Lake Fryxell, McMurdo Dry Valleys, Antarctica. Applied and Environmental Microbiology, 71(10): 6353-6359.
DOI:10.1128/AEM.71.10.6353-6359.2005 |
Kim Y, Lee I, Mayer B, et al. 2021. Sulphur and oxygen isotope signatures of dissolved sulphate in freshwater from King George Island, Antarctic Peninsula. Antarctic Science, 33(4): 415-417.
DOI:10.1017/S0954102021000286 |
Kim Y, Lee I, Seo J H, et al. 2017. Multiple oxygen (16O, 17O and 18O) and sulfur (32S, 33S, 34S and 36S) isotope signatures of the dissolved sulfate from Deception Island, Antarctic Peninsula: implications on sulfate formation, transportation and deposition in the Antarctic region. Chemical Geology, 466: 762-775.
DOI:10.1016/j.chemgeo.2017.07.029 |
Leloup J, Petit F, Boust D, et al. 2005. Dynamics of sulfatereducing microorganisms (dsrAB genes) in two contrasting mudflats of the Seine Estuary (France). Microbial Ecology, 50(3): 307-314.
DOI:10.1007/s00248-004-0034-6 |
Li S K, Xiao X, Yin X B, et al. 2006. Bacterial community along a historic lake sediment core of Ardley Island, West Antarctica. Extremophiles, 10(5): 461-467.
DOI:10.1007/s00792-006-0523-2 |
Liu J, Jiang T, Huang R, et al. 2017. A simulation study of inorganic sulfur cycling in the water level fluctuation zone of the Three Gorges Reservoir, China and the implications for mercury methylation. Chemosphere, 166: 31-40.
DOI:10.1016/j.chemosphere.2016.09.079 |
Luther III G W, Glazer B, Ma S F, et al. 2003. Iron and sulfur chemistry in a stratified lake: evidence for iron-rich sulfide complexes. Aquatic Geochemistry, 9(2): 87-110.
DOI:10.1023/B:AQUA.0000019466.62564.94 |
Mahmood Q, Hu B L, Cai J, et al. 2009. Isolation of Ochrobactrum sp. QZ2 from sulfide and nitrite treatment system. Journal of Hazardous Materials, 165(1-3): 558-565.
DOI:10.1016/j.jhazmat.2008.10.021 |
Michelutti N, Keatley B E, Brimble S, et al. 2009. Seabirddriven shifts in Arctic pond ecosystems. Proceedings of the Royal Society B: Biological Sciences, 276(1656): 591-596.
DOI:10.1098/rspb.2008.1103 |
Mußmann M, Ishii K, Rabus R, et al. 2005. Diversity and vertical distribution of cultured and uncultured Deltaproteobacteria in an intertidal mud flat of the Wadden Sea. Environmental Microbiology, 7(3): 405-418.
DOI:10.1111/j.1462-2920.2005.00708.x |
Ng C, DeMaere M Z, Williams T J, et al. 2010. Metaproteogenomic analysis of a dominant green sulfur bacterium from Ace Lake, Antarctica. The ISME Journal, 4(8): 1002-1019.
DOI:10.1038/ismej.2010.28 |
Norman A L, Giesemann A, Krouse H R, et al. 2002. Sulphur isotope fractionation during sulphur mineralization: results of an incubation-extraction experiment with a Black Forest soil. Soil Biology and Biochemistry, 34(10): 1425-1438.
DOI:10.1016/S0038-0717(02)00086-X |
Orem W, Newman S, Osborne T Z, et al. 2015. Projecting changes in Everglades soil biogeochemistry for carbon and other key elements, to possible 2060 climate and hydrologic scenarios. Environmental Management, 55(4): 776-798.
DOI:10.1007/s00267-014-0381-0 |
Pester M, Knorr K H, Friedrich M W, et al. 2012. Sulfatereducing microorganisms in wetlands-fameless actors in carbon cycling and climate change. Frontiers in Microbiology, 3: 72.
|
Poser A, Vogt C, Knöller K, et al. 2014. Stable sulfur and oxygen isotope fractionation of anoxic sulfide oxidation by two different enzymatic pathways. Environmental Science & Technology, 48(16): 9094-9102.
|
Poulin B A, Ryan J N, Nagy K L, et al. 2017. Spatial dependence of reduced sulfur in Everglades dissolved organic matter controlled by sulfate enrichment. Environmental Science& Technology, 51(7): 3630-3639.
|
Purcell A M, Mikucki J A, Achberger A M, et al. 2014. Microbial sulfur transformations in sediments from Subglacial Lake Whillans. Frontiers in Microbiology, 5: 594.
|
Rabus R, Ruepp A, Frickey T, et al. 2004. The genome of Desulfotalea psychrophila, a sulfate-reducing bacterium from permanently cold Arctic sediments. Environmental Microbiology, 6(9): 887-902.
DOI:10.1111/j.1462-2920.2004.00665.x |
Rosenberg Y O, Kutuzov I, Amrani A. 2018. Sulfurization as a preservation mechanism for the δ13C of biomarkers. Organic Geochemistry, 125: 66-69.
DOI:10.1016/j.orggeochem.2018.08.010 |
Sánchez-Andrea I, Sanz J L, Bijmans M F M, et al. 2014. Sulfate reduction at low pH to remediate acid mine drainage. Journal of Hazardous Materials, 269: 98-109.
DOI:10.1016/j.jhazmat.2013.12.032 |
Sattley W M, Madigan M T. 2006. Isolation, characterization, and ecology of cold-active, chemolithotrophic, sulfuroxidizing bacteria from perennially ice-covered Lake Fryxell, Antarctica. Applied and Environmental Microbiology, 72(8): 5562-5568.
DOI:10.1128/AEM.00702-06 |
Saxton M A, Samarkin V A, Schutte C A, et al. 2016. Biogeochemical and 16S rRNA gene sequence evidence supports a novel mode of anaerobic methanotrophy in permanently ice‐covered Lake Fryxell, Antarctica. Limnology and Oceanography, 61(S1): S119-S130.
DOI:10.1002/lno.10320 |
Sela-Adler M, Said-Ahmad W, Sivan O, et al. 2016. Isotopic evidence for the origin of dimethylsulfide and dimethylsulfoniopropionate-like compounds in a warm, monomictic freshwater lake. Environmental Chemistry, 13(2): 340-351.
DOI:10.1071/EN15042 |
Sen A M, Johnson B. 1999. Acidophilic sulphate-reducing bacteria: candidates for bioremediation of acid mine drainage. Process Metallurgy, 9: 709-718.
|
Shawar L, Halevy I, Said-Ahmad W, et al. 2018. Dynamics of pyrite formation and organic matter sulfurization in organic-rich carbonate sediments. Geochimica et Cosmochimica Acta, 241: 219-239.
DOI:10.1016/j.gca.2018.08.048 |
Sheng Y Q, Sun Q Y, Bottrell S H, et al. 2013. Anthropogenic impacts on reduced inorganic sulfur and heavy metals in coastal surface sediments, North Yellow Sea. Environmental Earth Sciences, 68(5): 1367-1374.
DOI:10.1007/s12665-012-1835-4 |
Sugio T, Domatsu C, Munaka O, et al. 1985. Role of a ferric ion- reducing system in sulfur oxidation of Thiobacillus ferrooxidans. Applied and Environmental Microbiology, 49(6): 1401-1406.
DOI:10.1128/aem.49.6.1401-1406.1985 |
Sun L G, Emslie S D, Huang T, et al. 2013. Vertebrate records in polar sediments: biological responses to past climate change and human activities. Earth-Science Reviews, 126: 147-155.
DOI:10.1016/j.earscirev.2013.08.004 |
Sun L G, Xie Z Q, Zhao J L. 2000. A 3, 000-year record of penguin populations. Nature, 407(6806): 858.
DOI:10.1038/35038163 |
Taketani R G, Yoshiura C A, Dias A C F, et al. 2010. Diversity and identification of methanogenic archaea and sulphatereducing bacteria in sediments from a pristine tropical mangrove. Antonie van Leeuwenhoek, 97(4): 401-411.
DOI:10.1007/s10482-010-9422-8 |
Tostevin R, Turchyn A V, Farquhar J, et al. 2014. Multiple sulfur isotope constraints on the modern sulfur cycle. Earth and Planetary Science Letters, 396: 14-21.
DOI:10.1016/j.epsl.2014.03.057 |
Ucar D, Yilmaz T, Di Capua F, et al. 2020. Comparison of biogenic and chemical sulfur as electron donors for autotrophic denitrification in sulfur-fed membrane bioreactor (SMBR). Bioresource Technology, 299: 122574.
DOI:10.1016/j.biortech.2019.122574 |
Wallner S R, Pogorevc M, Trauthwein H, et al. 2004. Biocatalytic enantio-convergent preparation of secalcohols using sulfatases. Engineering in Life Sciences, 4(6): 512-516.
DOI:10.1002/elsc.200402151 |
Wasmund K, Mußmann M, Loy A. 2017. The life sulfuric: microbial ecology of sulfur cycling in marine sediments. Environmental Microbiology Reports, 9(4): 323-344.
DOI:10.1111/1758-2229.12538 |
Werne J P, Lyons T W, Hollander D J, et al. 2008. Investigating pathways of diagenetic organic matter sulfurization using compound-specific sulfur isotope analysis. Geochimica et Cosmochimica Acta, 72(14): 3489-3502.
DOI:10.1016/j.gca.2008.04.033 |
Yang L J, Gao Y S, Sun L G, et al. 2019. Enhanced westerlies drove penguin movement at 1000 yr BP on Ardley Island, West Antarctic Peninsula. Quaternary Science Reviews, 214: 44-53.
DOI:10.1016/j.quascirev.2019.04.026 |
Yau S, Lauro F M, Williams T J, et al. 2013. Metagenomic insights into strategies of carbon conservation and unusual sulfur biogeochemistry in a hypersaline Antarctic lake. The ISME Journal, 7(10): 1944-1961.
DOI:10.1038/ismej.2013.69 |
Zerkle A L, Farquhar J, Johnston D T, et al. 2009. Fractionation of multiple sulfur isotopes during phototrophic oxidation of sulfide and elemental sulfur by a green sulfur bacterium. Geochimica et Cosmochimica Acta, 73(2): 291-306.
DOI:10.1016/j.gca.2008.10.027 |
Zhou J, Bruns M A, Tiedje J M. 1996. DNA recovery from soils of diverse composition. Applied and Environmental Microbiology, 62(2): 316-322.
DOI:10.1128/aem.62.2.316-322.1996 |
Zhu R B, Shi Y, Ma D W, et al. 2015. Bacterial diversity is strongly associated with historical penguin activity in an Antarctic lake sediment profile. Scientific Reports, 5(1): 17231.
DOI:10.1038/srep17231 |
 2023, Vol. 41
2023, Vol. 41


