Institute of Oceanology, Chinese Academy of Sciences
Article Information
- WANG Jinxiu, KONG Fanzhou, WANG Yunfeng, JI Nanjing, SONG Minjie, HU Zhangxi, NIU Zhuang, LIU Chao, WANG Xin, SUN Yuanyuan, YU Rencheng, YAN Tian
- Newly recorded bloom-forming dinoflagellate Gymnodinium impudicum in Haizhou Bay, Yellow Sea, China
- Journal of Oceanology and Limnology, 40(6): 2430-2445
- http://dx.doi.org/10.1007/s00343-022-1402-0
Article History
- Received Nov. 30, 2021
- accepted in principle Jan. 14, 2022
- accepted for publication May 23, 2022
2 Laboratory for Marine Ecology and Environmental Science, Pilot National Laboratory for Marine Science and Technology(Qingdao), Qingdao 266237, China;
3 University of Chinese Academy of Sciences, Beijing 100049, China;
4 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China;
5 CAS Key Laboratory of Experimental Marine Biology, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China;
6 Jiangsu Key Laboratory of Marine Biological Resources and Environment, Jiangsu Ocean University, Lianyungang 222005, China
Harmful algal blooms (HABs) are intense proliferations of algae that can cause severe economic losses, alter ecosystems, or even endanger human health (Glibert et al., 2014). The frequency, intensity, duration, diversity, and impacts of HABs are increasing worldwide under the influence of intensified human activities and climate changes (Roelke et al., 2012; Glibert, 2017). Haizhou Bay, a typical open bay located in the South Yellow Sea, is a historically important fishing ground in China. However, with the development of local agriculture and mariculture industry, eutrophication has become a severe ecological and environmental concern in Haizhou Bay, leading to the frequent occurrence of HABs (Han et al., 2019). In the Haizhou Bay, these blooms show a trend of diversification, reduction in cell size and more severe impacts in the last two decades. The dominant species of bloom-forming microalga in the Haizhou Bay have changed from the dinoflagellate Noctiluca scintillans to small-sized (6–22 μm) diatom Skeletonema costatum and many harmful/toxic dinoflagellates (Gao et al., 2017), such as some species of gymnodinoids that are responsible for producing phycotoxins. Multiple blooms of Gymnodinium catenatum, Amphidinium carterae, and Karenia mikimotoi have been recorded in Haizhou Bay (Jiangsu Provincial Oceanic and Fishery Bureau, 2011–2017), as well as a Karlodinium veneficum bloom in 2020 (unpublished data), that pose severe threats to aquatic and human health. On September 9, 2020, a chain-forming gymnodinioid bloom was observed in Haizhou Bay co-occurring with a bloom of a single-celled gymnodinioid Takayama sp. (Zhang et al., 2022). The increasing occurrence of new gymnodinioid blooms pose a challenge to species identification and bloom monitoring in Haizhou Bay.
During HABs monitoring, traditional identification of unarmoured gymnodinioids was typically based on morphological characteristics, such as the length of the cingulum displacement, but this approach was clearly inadequate and often problematic because of the species' fragile cells and highly variable morphology (Reñé et al., 2015). Electron microscopy technology has alleviated this problem, and allowed proposing some new features (e.g., the apical groove) for the identification of gymnodinioids (Takayama, 1985). With the development of molecular biology, ribosomal DNA (rDNA) and its internal transcribed spacer (ITS) have been widely used in microalgae phylogeny and classification, especially for morphologically similar species. Thus, Gymnodiniales have been redefined using a combination of morphological and ultrastructural features along with biological phylogeny (Daugbjerg et al., 2000; Reñé et al., 2011; Luo et al., 2018). However, the routine monitoring of phytoplankton in the Haizhou Bay is based on typically algal morphological identification by light microscopy, which may lead to the lack of detection or misidentification of many gymnodinioids species.
To identify the causative species of the chainforming gymnodinioid bloom on September 9, 2020, a strain of the causative species was isolated and phytoplankton water samples were collected during the bloom. Morphological features, phylogenetic sequences, pigment composition, the putative paralytic shellfish toxins (PSTs) profile, and biotoxicity of the isolate, which were consistent with field results, confirmed a newly recorded bloom-forming species, Gymnodinium impudicum, in the Yellow Sea. Phytoplankton composition and spatial distribution during the bloom, as well as their relationships with environmental factors were also analyzed. This study aims to: 1) identify bloom microalgae to species level by applying morphological and molecular methods to the study of algal isolates from a bloom in Haizhou Bay, 2) determine pigment and toxin profiles of the isolated strain and its acute toxicity to invertebrates, and 3) elucidate the occurrence and ecological characteristics of the G. impudicum bloom and potential risk.
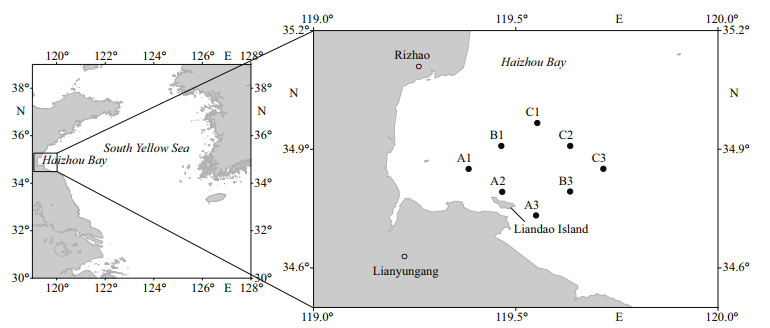
2 MATERIAL AND METHOD 2.1 Sample collection, and strain isolation and cultureSurface water samples were collected from eight sites in Haizhou Bay on September 9, 2020 (Fig. 1). Phytoplankton samples were preserved with Lugol iodine solution and stored in darkness until microscopic analysis. For phytoplankton pigment analysis, water samples were filtered onto precombusted (450 ℃, 6 h) 25-mm Whatman GF/F fiberglass filters with a 0.68-μm nominal pore size. The filters were frozen in liquid nitrogen and stored at -80 ℃ until pigment extraction. Filtrates were collected in polyethylene bottles and stored at -20 ℃ for subsequent analysis of dissolved nutrients. Water temperature and salinity were measured with a RBR concerto CTD (RBR Company, Canada).
|
| Fig.1 Study area and sampling sites in Haizhou Bay |
A single cell of the bloom-forming species was obtained from live algal blooms samples using an aseptic capillary under an inverted microscope. The isolate was initially cultured in a 48-well plate, and gradually transferred to larger culture volumes. Ultimately, the isolate was cultured in 100-mL Erlenmeyer flasks using L1-Si medium (Guillard and Hargraves, 1993) prepared with autoclaved seawater at a salinity of 32, at 20±1 ℃ with a light intensity of 100 μE/(m2·s) and a 14-h: 10-h light: dark cycle.
2.2 Laboratory analyses 2.2.1 Characterization of the isolated strain 2.2.1.1 Morphological characterizationThe morphology of the isolated strain was determined by light microscopy (LM) and scanning electron microscopy (SEM). Live cells were observed and photographed with an upright microscope (DM2500, Leica, Germany) at 400× magnification. Cells in mid-exponential growth stage for SEM observation were fixed with 2% OsO4 for 1 h, and gravity filtered through a 5-μm Millipore nylon membrane. The membrane sample was dehydrated through a gradient acetone series, treated with liquid CO2 for critical point-drying (EM CPD300, Leica, Austria), then sputter-coated with gold before examination using an S-3400 N SEM (Hitachi, Hitachinaka, Japan). Cell size of the strain was calculated from SEM micrographs.
2.2.1.2 Molecular phylogenetic analysisGenomic DNA was extracted from 10 mL of clonal culture using a modified cetyltrimethylammonium bromide (CTAB) method, as described by Winnepenninckx et al. (2002). The DNA was dissolved in 30-μL Tris-EDTA buffer and frozen at -80 ℃ until PCR reactions; D1–D2 regions of the 28S large-sub-unit (LSU) rDNA were amplified using the primers: LSU D12-F (5'-ACCCGCTGAATTTAAGCATA-3') and LSU D12-R (5'-CCTTGGTCCGTGTTTCAAGA-3') (Lenaers et al., 1989). Amplification was conducted with an initial denaturation at 94 ℃ for 5 min, 35 cycles at 94 ℃ for 20 s, 52 ℃ for 20 s, 72 ℃ for 20 s, and a final elongation step of 5 min at 72 ℃. Targeted DNA bands were electrophoresed in 1% agarose gel, purified and sequenced from both ends by Sangon Biotech (Shanghai, China). The obtained sequences were assembled with Vector NTI 11.5.3 ContigExpress (ThermoFisher, USA).
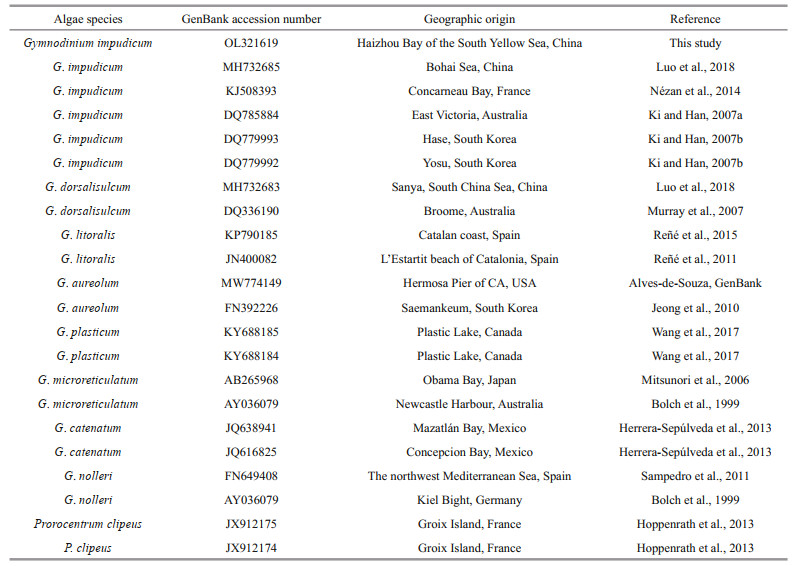
Sequences obtained and 21 other closely related species and outgroup taxa downloaded from GenBank were used for phylogenetic analysis (Table 1). Maximum likelihood analysis was performed using MEGA 7.0 with 500 bootstrap replicates and the K2+G was chosen as the bestfitting nucleotide substitution model. The sequence of this strain was submitted to GenBank (http://www.ncbi.nlm.nih.gov/), with the accession number OL321619.
|
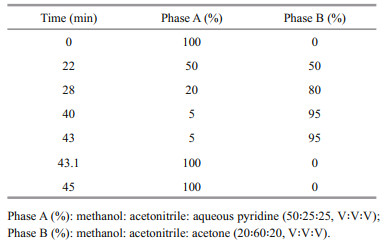
Clonal cultures in exponential growth (40 mL) were filtered onto a 25-mm Whatman GF/F fiberglass filter (0.68-μm nominal pore size), and then extracted and analyzed following the method of Zapata et al. (2000) with slight modification of the extraction volume and elution gradient profile. Pigments were extracted in 1.4-mL 95% methanol, with 100-μL 8'-apo-β, ψ-carotaldehyde (750 μg/L) (Sigma, USA) as internal standard (IS), and analyzed on a Waters E2695 high-performance liquid chromatography (HPLC) system using a Waters Symmetry C8 column (3.5 μm, 4.6 mm×150 mm) with binary gradient elution. The mobile phase and modified elution gradient profile are shown in Table 2. Pigments were detected with a Waters 2998 diode array detector (DAD) at a wavelength of 440 nm, and identified by comparison of their retention time and spectra with pigment standards (DHI Water and Environment, Hørsholm, Denmark).
Clonal culture in exponential growth (100 mL) was filtered onto a Whatman GF/C fiberglass filter (1.2-μm nominal pore size), and paralytic shellfish toxins (PSTs) were extracted following the method of Costa et al. (2015), with some modifications (Lin et al., 2022). The filter was extracted in 3 mL of 0.05-mol/L acetic acid and disrupted with a probe sonicator (Scientz Biotechnology Co. Ltd., Ningbo, China) for 5 min on ice. The extract was centrifuged and the supernatant cleaned by an octadecyl bonded silica cartridge (Supelclean LC-18 SPE cartridge, 3 mL, Supelco, Sigma Aldrich, USA). Water (1.5 mL) was added onto the column to elute residual PSTs. The cartridge was subjected to a second elution with 0.5-mL 20% acetonitrile (V: V), followed by 0.5-mL 80% acetonitrile to capture the hydroxybenzoate analogues or GC toxins. The eluates were then reconstituted and filtered through 0.22-μm syringe membrane filters before analysis.
Toxin analysis was performed using a Thermo Fisher Ultimate 3000 HPLC system coupled to an AB SCIEX Q-Trap 4500 mass spectrometer (Applied Biosystems, Darmstadt, Germany). The HPLC-tandem mass spectrometry (MS/MS) analysis of PSTs including the GC toxins employed during this study was established following the protocol of Costa et al. (2015), with little modification (Lin et al., 2022). The elution procedure was modified and set as 65% B to 45% B at a flow rate of 0.2 mL/min in 20 min, and then maintained at 65% B for 5 min. Due to the lack of GC toxin standards, extract of G. catenatum known to contain GC toxins was used as a reference. The strain of G. catenatum (MEL11) was isolated from the coastal waters of Fujian Province, and contains four GC toxins (GC2, GC3, GC5, and GC6) (Lin et al., 2022).
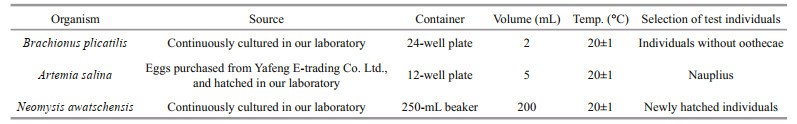
2.2.1.5 Acute toxicity testsAcute toxicity tests of the isolated strain were performed with the rotifer Brachionus plicatilis, the brine shrimp Artemia salina and the eurytopic mysid species Neomysis awatschensis as test organisms. The initial cell density of the isolated strain for acute toxicity tests was 1.0×103 cells/mL (slightly higher than the bloom cell density), with Chlorella sp. of the same cell density as the negative control and sterilized seawater as the blank. Chlorella sp. was provided by the Algal Culture Center of the Institute of Oceanology, Chinese Academy of Sciences. Both the isolated strain and Chlorella sp. used for acute toxicity tests were cultured in L1-Si medium at 20±1 ℃ under a light intensity of 100 μE/(m2·s) and a 14-h: 10-h light: dark cycle. Each treatment was conducted in triplicate with 10 test organisms. The tests were conducted for 24 h without renewing solutions. Mortalities of test organisms were measured at 0, 3, 6, 12, and 24 h after the beginning of the tests (see Table 3 for the source and test conditions of the three test organisms).
Phytoplankton identification and enumeration of preserved water samples were performed using an inverted optical microscope (Axiovert, Zeiss, Germany) at 200× or 400× magnification. Each sample was counted using a Sedgewick Rafter counting chamber (1 mm2), ensuring that the total number of counted cells was not less than 300. The community taxonomic composition was determined down to the genus and species levels.
2.2.2.2 Pigment analysis and CHEMTAX analysisField samples for pigment analysis were treated in the same manner as the cultured strain above. Data were analyzed using CHEMTAX software to calculate the contribution of distinct phytoplankton taxa based on the ratio of diagnostic pigments to chlorophyll a (Chl a). The initial pigment ratios (Table 4) for CHEMTAX calculation were approximated base on Mackey et al. (1996). Dinoflagellates were divided into two sub-groups. Peridinin (Peri) was used as a diagnostic pigment to represent most dinoflagellates (Dino-peri), while the group "Dino-fuco" represented Takayama sp. (Zhang et al., 2022), dominant species with 19'-butanoyloxyfucoxanthin (But-fuco), fucoxanthin (Fuco) and gyroxanthin-diester (Gyro) as diagnostic pigments. The initial ratio of each diagnostic pigment to Chl a for the "Dino-fuco" group was determined based on the pigment ratio of Takayama sp. at the bloom site and the cultured Takayama sp. strain (data not shown). The selection of other phytoplankton groups (i.e., prasinophytes, cryptophytes, haptophytes (containing the Type 8 pigment (T8) described by Zapata et al. (2004)), chlorophytes, cyanobacteria, and diatoms) was mainly based on the detection of their diagnostic pigments in Haizhou Bay. The optimized output matrix of pigment ratios is provided in Supplementary Table S1.
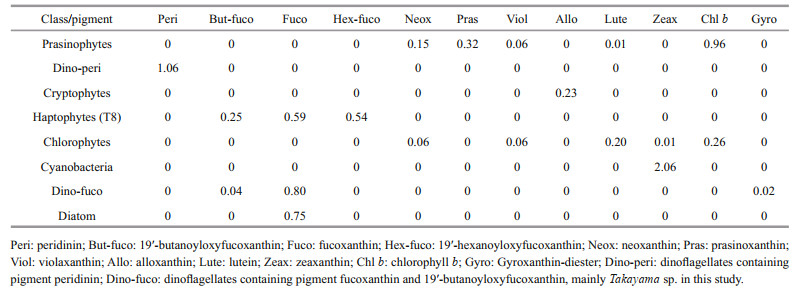
|
Nutrients in seawater, including the concentration of nitrate (NO3-), nitrite (NO2-), ammonium (NH4+), phosphate (PO43-), silicate (SiO32-), dissolved total nitrogen (DTN), and dissolved total phosphorus (DTP), were measured with a QUAATRO Continuous Flow Analyzer (QuAAtro 39, Seal, Germany). The principle of nutrient analysis is based on the "Specification for oceanographic survey" (GB/T 12763.4-2007). The concentration of NO3-, NO2-, NH4+, PO43-, and SiO32- were analyzed by the copper-cadmium reduction method, diazo azo method, sodium bromate oxidation method, molybdenum blue colorimetry, and silicon-molybdenum blue method, respectively. DTN and DTP were analyzed following persulfate oxidation (120 ℃, 30 min). The sum of NO3-, NO2-, and NH4+ was considered as dissolved inorganic nitrogen (DIN), and PO43- was considered as dissolved inorganic phosphorus (DIP). The concentrations of dissolved organic nitrogen (DON) and dissolved organic phosphorus (DOP) were determined by subtracting DIN and DIP from DTN and DTP, respectively.
2.3 Statistical analysisStatistical significance of the difference between controls and treatments in acute toxicity tests was determined using a two-tailed paired t-test and results were considered statistically significant at P < 0.05. The correlation analysis between microscopic enumeration and CHEMTAX calculation was performed by Pearson correlation tests. Principal component analysis (PCA) performed with CANOCO 5.1 software was used to examine the relationships between phytoplankton groups and environmental factors.
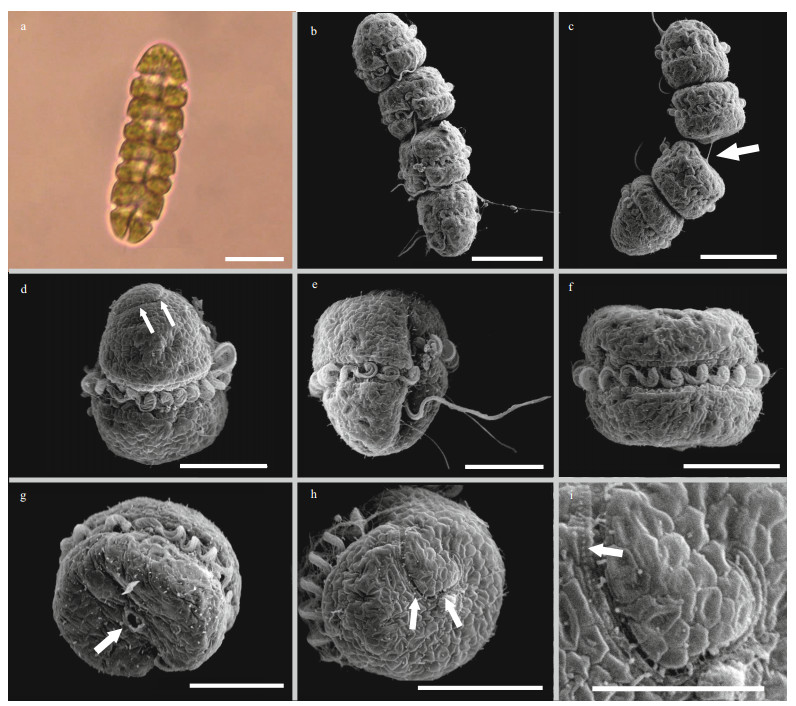
3 RESULT 3.1 Biological characteristics of algal bloom causative species 3.1.1 MorphologyLight microscope (Fig. 2a) and SEM (Fig. 2b–i) images of Gymnodinium impudicum reveal that the unarmoured cells were ovoid, from 10.5- to 20.3-μm long (15.0±2.8 μm standard error, n=27) and from 15.1- to 20.0-μm wide (17.3±1.3 μm standard error, n=27). Chains of 2, 4, and 8 cells were observed, although the most common were chains of 4 cells (Fig. 2a–c). The anterior and posterior cells of the chains were longer than the intermediate cells. The anterior cells had a dome-shaped epicone and flattened hypocone, while the posterior cells showed the opposite shape (Fig. 2d–e). The epicone and hypocone of intermediate cells in the chain were flattened (Fig. 2f). There were round pores in the middle apex and antapex (Fig. 2g), and the pores seemed to be connected by filaments in some cells (Fig. 2c), through which the cells form chains. The cingulum was deeply incised with a displacement of ca. 1/3–1/4 of the cell length. The sulcus was narrow, penetrated into the epicone directly to the apex forming the apical groove. The latter was loopshaped, running anticlockwise viewed from the apex (Fig. 2d & h). It contained three elongated vesicles, and possessed a string of knobs on the outer row of vesicles (Fig. 2i).
|
| Fig.2 Light microscope (a) and scanning electron microscope (b–i) micrographs of Gymnodinium impudicum a. chain composed of four cells under light microscopy; b. ventral view of a four-celled chain under scanning electron microscopy; c. dorsal view of the chain showing connecting filaments between cells (white arrow); d. dorsal view of the anterior cell showing the apical groove (white arrows); e. right ventrolateral view of the posterior cell of the chain; f. dorsal view of the intermediate cell in the chain; g. antapical view of the intermediate cell in the chain showing the pore in the middle antapex (white arrow); h. apical view of the cell showing the loop-shaped apical groove (white arrows); i. detail of the apical groove comprising three rows of vesicles with a string of knobs on the outer row (white arrow). Scale bars: (a–c) 20 μm, (d–h) 10 μm, (i) 5 μm. |
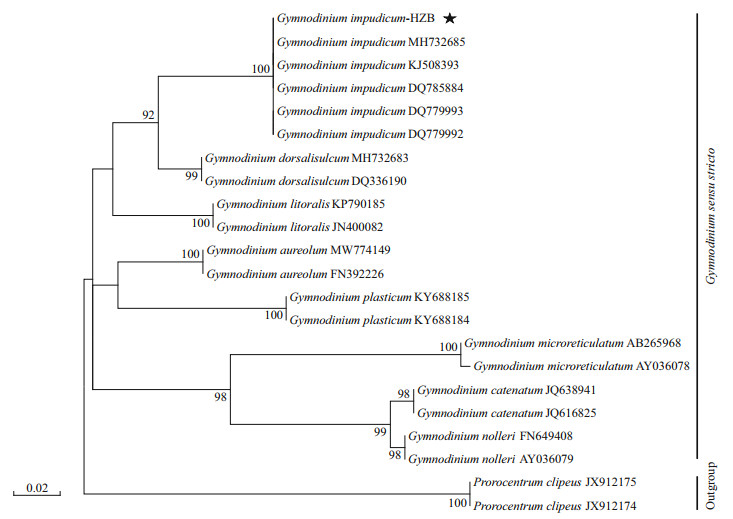
The 28S rDNA sequence of the strain isolated from the Haizhou Bay was aligned with other related microalgae belonging to the Gymnodinium genus selected from GenBank and using Prorocentrumclipeus as an outgroup (Fig. 3). The well resolved Gymnodinium clade sensu stricto comprised G. impudicum as well as other species, such as G. dorsalisulcum, G. litoralis, G. aureolum, G. plasticum, G. microreticulatum, G. catenatum, and G. nolleri. The strain in this study grouped well with five strains of G. impudicum isolated from other sea areas (the Bohai Sea, coastal waters of France, Australia, and South Korea) to form a single phylogenic clade with a posterior probability of 100%. All other Gymnodinium sequences formed distinct clades. Gymnodinium impudicum species were closely related to a clade of G. dorsalisulcum with moderate ML bootstrap support (92). Molecular phylogeny was also analyzed using 18S rDNA and ITS rDNA sequences with a subset of species, and these results were not significantly different from those of 28S rDNA phylogenetic analysis (data not shown).
|
| Fig.3 Maximum-likelihood phylogenetic tree of Gymnodinium impudicum and selected species inferred from the D1–D2 region of 28S LSU rDNA Prorocentrum clipeus is used as an outgroup. Numbers indicate the bootstrap values from maximum-likelihood analysis. Only bootstrap values > 50 are shown. Gymnodinium impudicum strains sequenced in this study are labeled with stars. |
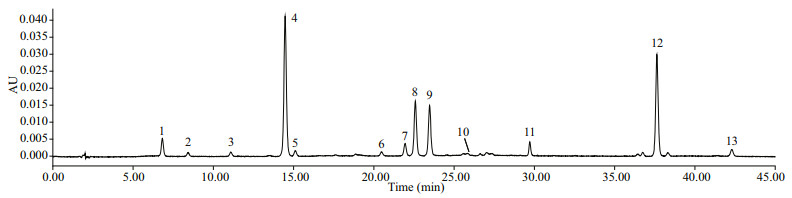
Pigments detected in the cultured G. impudicum isolate included peridinin (Peri), diadinoxanthin (Diad), dinoxathin (Dino), Chl a, and a small amount of peridininol, diatoxanthin, zeaxanthin, β, β-carotene and some unknown carotenoids (Fig. 4). Results showed that G. impudicum has a peridinin-type chloroplast, with Peri (21.2 pg/cell) as the diagnostic pigment, which was the most abundant and slightly higher than Chl a (18.6 pg/cell). The molar ratio of Peri to Chl a was about 1.6:1. Diadinoxanthin (Diad) and Dino were major accessory carotenoids, contributing almost equally to the carotenoid pool.
|
| Fig.4 Chromatogram of Gymnodinium impudicum pigments 1, 2, 6, 7: unknown carotenoids; 3: chlorophyllide a; 4: peridinin; 5: peridininol; 8: diadinoxanthin; 9: dinoxanthin; 10: diatoxanthin; 11: internal standard; 12: chlorophyll a; 13: β, β-carotene. |
Compared with HPLC-MS/MS product ion spectra of the PST standards (including the gonyautoxins GTX1-6, neosaxitoxin (NEO), saxitoxin (STX), and decarbamoyl toxins dcGTX2, dcGTX3, dcSTX) and GC toxins from G. catenatum (including the GC2, GC3, GC5, and GC6), no toxin was detected in G. impudicum, which confirmed that G. impudicum did not contain PSTs (Supplementary Fig.S1).
Acute toxicity tests showed that G. impudicum had no obvious biological toxicity towards B. plicatilis, A. salina and N. awatschensis (Fig. 5). Brachionus plicatilis and A. salina were insensitive to G. impudicum, and experienced negligible mortalities when exposed to this alga during 24 h. The mortality of N. awatschensis was higher than that of B. plicatilis and A. salina. Neomysis awatschensis began to die after 3 h, but there was no significant difference between the treatments and controls.
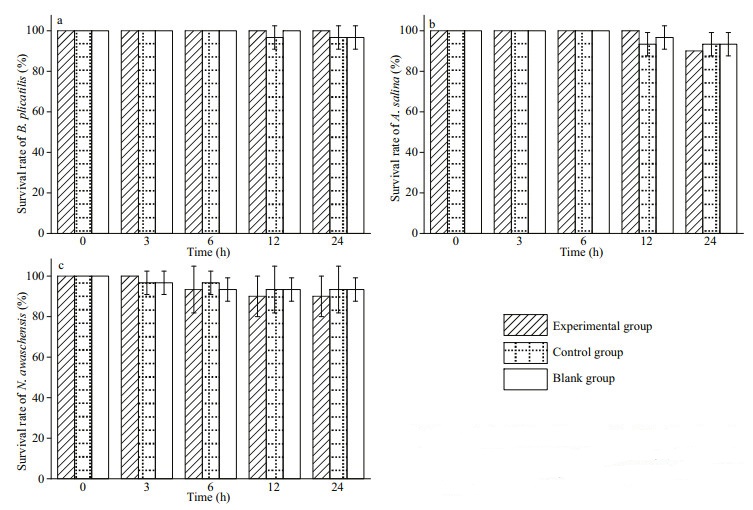
|
| Fig.5 Survival of Brachionus plicatilis (a), Artemia salina (b), and Neomysis awatshcensis (c) after exposure to Gymnodinium impudicum Mean±SE, n=3. |
A total of 30 phytoplankton taxa were detected and identified from this Haizhou Bay algal bloom. The phytoplankton community was mainly dominated by dinoflagellate and diatom species. Gymnodinium impudicum and Takayama sp. were the two dominant dinoflagellate species, with a peak abundance of 2.6×105 cells/L (site A1) and 1.7×107 cells/L (site B1), respectively, which jointly caused the heterogeneous bloom. Ceratium furca, C. tripos and C. fusus were sub dominant groups, with highest cell abundances of 7.8×104, 8.8×103, and 5.0×103 cells/L, respectively. Protoperidinium spp. was another relatively abundant dinoflagellate group. The abundance of diatoms was lower than that of dinoflagellates, and the major diatom species belonged to the genera Chaetoceros, Coscinodiscus, and Nitzschia. Distribution patterns of these species are shown in Fig. 6. The distribution of G. impudicum, C. furca, and Chaetoceros spp. were similar, with highest abundance in the area closest to Liandao Island. Ceratium fusus and Protoperidinium spp. were also distributed in nearshore waters, mainly at sites A1 and A3. Both Takayama sp. and C. tripos were concentrated at site B1 in the northwest of the study area. The distributions of Coscinodiscus spp. and Nitzschia spp. were opposite to that of Takayama sp. in mainly the southeast site B3.

|
| Fig.6 Distribution of major phytoplankton groups in the Haizhou Bay |
The contribution of different phytoplankton groups to total Chl-a biomass estimated using CHEMTAX is shown in Fig. 7. Consistent with the results of microscopic examination, Dino-peri (mainly G. impudicum) and Dino-fuco (mainly Takayama sp.) were the dominant phytoplankters, exceeding diatoms at almost all sites. There was a high and significant correlation between the results of microscopic cell counts and CHEMTAX analysis for Dino-peri (Pearson correlation coefficient, R=0.89, P < 0.01) and Dino-fuco (Pearson correlation coefficient, R=0.88, P < 0.01). Site A2 with the greatest abundance of Dino-peri had the lowest proportion of the Dino-fuco group, while the inverse was observed at site B1. The phytoplankton communities were more complex at sites relatively far from the coast and the algal bloom area, such as B3, C1, and C3. Site C1 in the north had a relatively high abundance of chlorophytes, cyanobacteria, and prasinophytes.
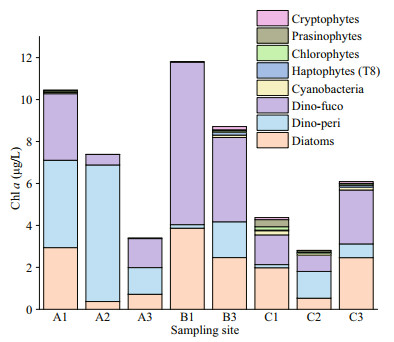
|
| Fig.7 Chlorophyll-a biomass contributed by different phytoplankton groups during the Haizhou Bay bloom on September 9, 2020 |
The spatial distributions of temperature, salinity, and major nutrients are given in Fig. 8. Water temperatures ranged between 26.45 and 28.81 ℃. It is noteworthy that water temperatures were measured at different times during the same day, so they may not effectively reflect the spatial temperature pattern. Salinity was lower in nearshore waters and highest in the north of the study area. The distribution of SiO32-, NO3- and NO2- was consistent, showing the highest concentrations at sites A1 and A2 near the west coast. Concentrations of NO3- and NO2- were extremely low at site B1, which was the area of high densities of Takayama sp. The ratio of DIN to DIP ranged from 12:1 (at the B1 site) to 258:1, with an average of 144:1, thus far exceeding 16:1 Redfield ratio (Redfield et al., 1963). In contrast to NO3- and NO2-, PO43-, DON, and DOP concentrations were the highest at site B1. Ammonium concentration was maximal at site C1 in the north, but was lower in nearshore waters.
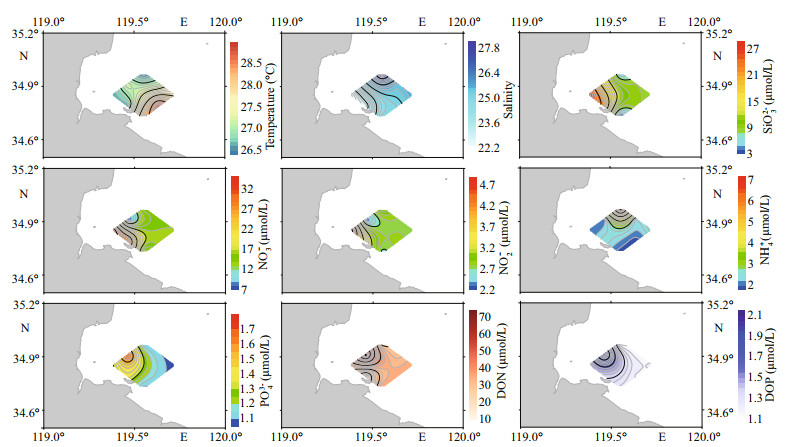
|
| Fig.8 Distribution of temperature, salinity, and major nutrients in the Haizhou Bay |
Principal component analysis (PCA) was performed to discern the relationship between major phytoplankton groups and environmental parameters (Fig. 9). Gymnodinium impudicum, C. furca, C. fusus, Chaetoceros, and Protoperidinium spp. were positively correlated with NO3-, NO2-, and SiO32-, but negatively correlated with temperature and salinity, which were mainly attributable to their nearshore distribution. In contrast, Takayama sp., C. tripos, Nitzschia and Coscinodiscus spp. were positively correlated with DON, DOP, and PO43-, negatively correlated with inorganic nitrogen and were not significantly affected by temperature and salinity. Ammonium was negatively correlated with most phytoplankton groups, but had little effect.
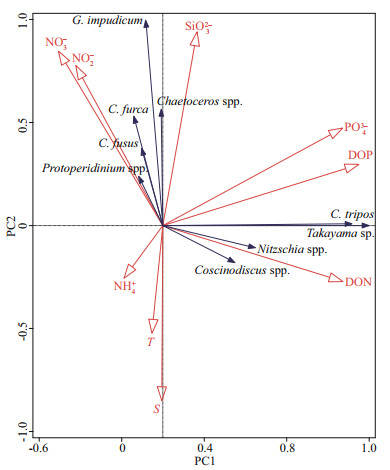
|
| Fig.9 Principal component analysis (PCA) of the association of major phytoplankton groups with environmental parameters T: temperature; S: salinity; NO3-: nitrate; NO2-: nitrite; PO43-: phosphate; NH4+: ammonium; SiO32-: silicate; DON: dissolved organic nitrogen; DOP: dissolved organic phosphorus. |
Naked dinoflagellates are difficult to differentiate under the light microscope, especially from fixed samples. Gymnodinium impudicum was initially named Gyrodinium impudicum based on morphological features (Fraga et al., 1995). Gymnodinium species were originally characterized by a cingulum displacement ≤20% of the cell length, while Gyrodinium species have a cingulum displacement ≥20% of the cell length. However, variation of the cingulum displacement within the same species often hindered generic identification. Therefore, additional morphological features, such as the apical structure complex (ASC), have been proposed as new characteristics for the taxonomy of gymnodiniods. Thus, Gyrodinium impudicum was renamed Gymnodinium impudicum based on an anticlockwise loop-shaped apical groove and molecular analyses (Daugbjerg et al., 2000). Other gymnodinioid species were also transferred to either known or newly described genera based on the shape of the apical groove and other important morphological criteria. For example, Gymnodinium fusus was successively transferred to Ceratoperidinium (Reñé et al., 2013) and Pseliodinium (Gómez, 2018). The new genera Levanderina (Moestrup et al., 2014), Pellucidodinium (Onuma et al., 2015), Wangodinium (Luo et al., 2018) etc., were established to incorporate Gymnodinium-like species.
Gymnodinium impudicum has been confused with toxigenic G. catenatum due to their similar chainlike morphology based on which the differentiation in light microscopy is difficult (Carrada et al., 1991; Gómez, 2003). Compared with G. catenatum (isolated from coastal waters of Fujian Province) (Fig. 10a & d), G. impudicum can be distinguished by smaller cell size and shorter chains of eight cells at the most, while G. catenatum can form chains of up to 64 cells. Chain cells of G. impudicum are closely attached, while in G. catenatum there are elongated connections (Fraga et al., 1995). In terms of cell shape, the hypocone of G. impudicum is "plump" whereas that of G. catenatum is trapezoidal. However, all these distinguishing features are vague and often inaccurate, especially for inexperienced phytoplankton identification personnel. Additionally, fixation with Lugol solution (Fig. 10b & e) and formaldehyde solution (Fig. 10c & f) cause deformation and degradation of G. impudicum and G. catenatum. Fixed cells are nearly spherical, and the taxonomic characteristics are almost lost, making species identification more difficult. Gymnodinium catenatum has become the second dominant algal bloom species in Haizhou Bay in addition to S. costatum in the past two decades (Gao et al., 2017), but detailed species identification has not been undertaken, and no associated poisoning event has been reported. Water samples from the routine monitoring of algal blooms in Haizhou Bay are typically fixed with Lugol solution or formaldehyde solution and identified by light microscopy, which is known to be insufficient for species identification. Additionally, G. impudicum and G. catenatum overlap ecologically (Band-Schmidt et al., 2020), and sometimes co-occur in the same bloom (Lee et al., 2001; Glibert et al., 2002). It is thus probable that G. catenatum blooms have been misidentified and that G. impudicum has been underrepresented in the Haizhou Bay in the past.

|
| Fig.10 Light microscope micrographs of live cells (a, d) and cells fixed with Lugol solution (b, e) and formaldehyde solution (c, f) of Gymnodinium impudicum (a, b, c) and Gymnodinium catenatum (d, e, f) Scale bars=20 μm. |
To overcome these confounding factors, pigment profiling, DNA sequencing and more sophisticated microscopy techniques, such as scanning or transmission electron microscopy, are essential to distinguish unarmoured dinoflagellates. Results of the analyses conducted in this study support the conclusion that the bloom occurred on September 9, 2020 in Haizhou Bay was formed by G. impudicum rather than G. catenatum. Most of the morphological features and the pigment profile of G. impudicum in this study are consistent with those reported in other sea areas (Fraga et al., 1995; Yim and Lee, 2004; Luo et al., 2018). Phylogenetic analysis of 28S rDNA showed that G. impudicum differed significantly from G. catenatum in terms of molecular phylogeny with a nucleotide sequence similarity of 86%, although they have similar morphological characteristics. Gymnodinium impudicum isolated in this study was non-toxic, and did not cause significant mortality of marine test organisms both in acute toxicity tests and in the bloom area, which was consistent with the description of Fraga et al. (1995). Given that G. catenatum is a PSTs producer, to distinguish G. catenatum and G. impudicum in the future algal bloom monitoring in Haizhou Bay, it is firstly recommended by microscopic observation combined with rapid toxin kits or HPLC-MS/MS toxin analysis, secondly by accurate molecular analysis at the same time for species verification if conditions permit.
4.2 Trophic modes of Gymnodinium impudicum and concurrent Takayama sp.Dinoflagellates are characterized by different trophic modes, including exclusively autotrophic, mixotrophic, and heterotrophic, which contribute to the proliferation of different types of algae under varying nutrient proportions and forms (Heisler et al., 2008). Haizhou Bay is affected by multiple nutrient sources, such as industrial effluents, agricultural runoff, municipal sewage, and intensive mariculture, creating a complex nutritional environment via the introduction of organic and inorganic nutrients that can support a variety of algal species (Zhang et al., 2022).
Gymnodinium impudicum has been described as an autotrophic species (Yahia-Kéfi et al., 2005). It showed a significant positive correlation with nitrate concentration in the Gulf of Tunis (Aissaoui et al., 2012), and its growth rate increased significantly with increasing nitrate concentration below 40 μmol/L in a laboratory experiment (Lee et al., 2001). In the present study, the abundance of G. impudicum in the Haizhou Bay was highest in coastal waters near Liandao Island with high nitrate levels (33 μmol/L) and a high N/P ratio (128:1), which may be related to its autotrophic characteristics. Based on literature descriptions and field observations, the red seaweed Porphyra yezoensis is intensively cultured in this area (Su et al., 2020). Porphyra yezoensis showed high absorption of nitrate and ammonium during its growth period (from October to April of the following year), resulting in the decrease of inorganic nitrogen concentration in seawater (Wang and Cao, 2020). In September, four months after the harvest of P. yezoensis, nutrient accumulation coupled with increased input from runoff may provide sufficient nitrogen for G. impudicum bloom.
Species of all three genera of the family Kareniaceae have been found to adopt a mixotrophic strategy, with the ability to assimilate dissolved organic nutrients or consume prey. In contrast to G. impudicum, the highest abundance of Takayama sp. appeared in the area with the lowest nitrate but the highest organic nitrogen, which is close to an oyster and mussel culture area. It has long been known that Karenia can utilize a wide variety of organic N and P substrates (Yamaguchi et al., 2004; Killberg-Thoreson et al., 2014), but little is known about the nutrient requirements of Takayama. In the Johor Strait, Singapore, the occurrence of a variety of algal blooms including Takayama sp. is considered to be supported by the high concentration of dissolved organic material (Leong et al., 2015). The close relationship between Takayama sp. and DON and DOP concentrations in Haizhou Bay indicates that Takayama sp. may be able to remain competitive by utilizing organic compounds.
Phagotrophy has been noted for T. helix and T. tasmanica, and both of them can feed on algae much larger than themselves (Jeong et al., 2016; Lim et al., 2018). Ceratium tripos, which was closely related to Takayama sp., is considered a better food for Fragilidium subglobosum than C. furca and C. fusus, supporting higher growth rates of F. subglobosum (Hansen and Nielsen, 1997). Takayama sp. in Haizhou Bay may prey on large C. tripos cells by sucking intracellular contents, or perhaps mixotrophic C. tripos have similar ecological characteristics to those of Takayama sp. Since these suggestions are based only on one-day studies, the specific relationship between algae and nutrients needs further investigation via laboratory and field results.
4.3 Occurrence of novel algal blooms associated with eutrophication in the Haizhou BayReports of G. impudicum have usually been localized, the species has been reported to form blooms in Korea (Park and Park, 1999; Lee et al., 2001), the Arabian Gulf (Glibert et al., 2002), and different areas of the Mediterranean Sea (Spain, Italy, Tunisia, and Egypt coastal waters) (Fraga et al., 1995; Yahia-Kéfi et al., 2005; Mikhail et al., 2020). Most of these blooms develop in eutrophic coastal waters with high levels of nitrate or phosphate and usually occur in summer and autumn at high temperatures (22–31 ℃). In China, G. impudicum with Alexandrium sp. and other algal species caused large-scale blooms (630 km2) along the coast of Tianjin in the Bohai Sea from August to October 2016 (Ocean Administration of Tianjin, 2017). Cysts of G. impudicum have been detected in the North Yellow Sea and in Zhejiang and Fujian coastal waters (Sun et al., 2006; Lin et al., 2016; Weng, 2017), indicating the potential of this species to form blooms throughout a wide range of China's coastal waters, but detailed studies of G. impudicum in China have rarely been carried out.
The present study provides the first record of a G. impudicum bloom in the Haizhou Bay, and in the South Yellow Sea. Like most other G. impudicum blooms in warm and eutrophic waters, that occurring in Haizhou Bay shared similar environment characteristics. With socioeconomic development and accelerated industrialization, various anthropogenically produced nutrients are continuously discharged into Haizhou Bay, leading to eutrophication and frequent occurrence of HABs. Intensive shellfish mariculture activity in the Haizhou Bay is another potential cause of eutrophication by altering the natural cycling of nutrients as point sources of regenerated nutrients. Due to the low assimilation efficiency, shellfish can act as pumps along the coast to transform the nutrients in algal biomass into dissolved and particulate detrital nutrients (Bouwman et al., 2013). Since the 1980s, nitrate concentrations in Haizhou Bay increased markedly, from 1.30 to about 20–30 μmol/L in 2015, and the N/P ratio also increased from 8.3 to 91.9 (Zhu et al., 2017). Nitrate concentrations influenced by river flood discharge, are usually highest in August, September, and October (Tian et al., 2006). The weak water exchange capacity of this area aggravates the accumulation of nutrients, creating a favorable environment for the occurrence of various algal blooms, which extend from hundreds to thousands of square kiliometers in area.
In recent decades, toxic and harmful dinoflagellates, including presumed G. catenatum, Gonyaulax polygramma, A. carterae and K. mikimotoi, have gradually replaced diatoms as the dominant taxonomic group blooming in Haizhou Bay under high N/P conditions. From June to November 2020 alone, successive blooms caused by K. veneficum, Takayama sp., G. impudicum and the raphidophyte Heterosigma akashiwo were observed in the Haizhou Bay (unpublished data), i.e., without including other bloom events that may have been missed. Given the high bloom species variety and frequency in Haizhou Bay, we need to be vigilant of new algal blooms, which present new challenges for algal bloom monitoring and prevention, as well as unknown ecological and socioeconomic risks.
5 CONCLUSIONA newly-recorded algal bloom caused by G. impudicum in the Haizhou Bay was scrutinized based on morphological features and 28S rDNA gene analyses. Typical dinoflagellate pigments were found and no paralytic shellfish toxins were detected. The non-toxic G. impudicum tended to be distributed in coastal waters rich in nitrate, while the concurrent Takayama sp. preferred to organic nutrients, which might indicate differential trophic modes of these dinoflagellates. Complex eutrophication of Haizhou Bay has led to the occurrence of a variety of novel algal blooms, which needs to be considered to better manage the unexpected risks and impacts.
6 DATA AVAILABILITY STATEMENTThe datasets analyzed during the current study are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENTThe authors greatly appreciate the constructive advice of anonymous reviewers. They also thank Wei LIU, Institute of Oceanology, Chinese Academy of Sciences (IOCAS), for his help in the use of SEM, Xiuqi YU, IOCAS, for her assistance in performing acute toxicity tests, and Zhuoru LIN, IOCAS, for her assistance in the analysis of paralytic shellfish toxins.
Electronic supplementary material
Supplementary material (Supplementary Fig.S1 and Table S1) is available in the online version of this article at https://doi.org/10.1007/s00343-022-1402-0.
Aissaoui A, Turki S, Ben Hassine O K. 2012. Occurrence of harmful dinoflagellates in the punic harbors of Carthage (Gulf of Tunis, Tunisia) and their correlations with the physicochemical parameters. Bulletin de l'Institut National des Sciences et Technologies de la Mer, 39: 127-148.
|
Band-Schmidt C J, Zumaya-Higuera M G, López-Cortés D J, et al. 2020. Allelopathic effects of Margalefidinium polykrikoides and Gymnodinium impudicum in the growth of Gymnodinium catenatum. Harmful Algae, 96: 101846.
DOI:10.1016/j.hal.2020.101846 |
Bolch C J S, Negri A P, Hallegraeff G M. 1999. Gymnodinium microreticulatum sp. nov. (Dinophyceae): a naked, microreticulate cyst-producing dinoflagellate, distinct from Gymnodinium catenatum and Gymnodinium nolleri. Phycologia, 38(4): 301-313.
DOI:10.2216/i0031-8884-38-4-301.1 |
Bouwman L, Beusen A, Glibert P M, et al. 2013. Mariculture: significant and expanding cause of coastal nutrient enrichment. Environmental Research Letters, 8(4): 044026.
DOI:10.1088/1748-9326/8/4/044026 |
Carrada G C, Casotti R, Modigh M, et al. 1991. Presence of Gymnodinium catenatum (Dinophyceae) in a coastal Mediterranean lagoon. Journal of Plankton Research, 13(1): 229-238.
DOI:10.1093/plankt/13.1.229 |
Costa P R, Robertson A, Quilliam M A. 2015. Toxin profile of Gymnodinium catenatum (Dinophyceae) from the Portuguese coast, as determined by liquid chromatography tandem mass spectrometry. Marine Drugs, 13(4): 2046-2062.
DOI:10.3390/md13042046 |
Daugbjerg N, Hansen G, Larsen J, et al. 2000. Phylogeny of some of the major genera of dinoflagellates based on ultrastructure and partial LSU rDNA sequence data, including the erection of three new genera of unarmoured dinoflagellates. Phycologia, 39(4): 302-317.
DOI:10.2216/i0031-8884-39-4-302.1 |
Fraga S, Bravo I, Delgado M, et al. 1995. Gyrodinium impudicum sp. nov. (Dinophyceae), a non-toxic, chain-forming, red tide dinoflagellate. Phycologia, 34(6): 514-521.
DOI:10.2216/i0031-8884-34-6-514.1 |
Gao Q Q, Cao B, Yang B, et al. 2017. Characteristics of the red tide in the sea area of Jiangsu. Marine Science Bulletin, 36(2): 217-221, 229.
(in Chinese with English abstract) |
Glibert P M, Allen J I, Artioli Y, et al. 2014. Vulnerability of coastal ecosystems to changes in harmful algal bloom distribution in response to climate change: projections based on model analysis. Global Change Biology, 20(12): 3845-3858.
DOI:10.1111/gcb.12662 |
Glibert P M, Landsberg J H, Evans J J, et al. 2002. A fish kill of massive proportion in Kuwait Bay, Arabian Gulf, 2001: the roles of bacterial disease, harmful algae, and eutrophication. Harmful Algae, 1(2): 215-231.
DOI:10.1016/S1568-9883(02)00013-6 |
Glibert P M. 2017. Eutrophication, harmful algae and biodiversity—challenging paradigms in a world of complex nutrient changes. Marine Pollution Bulletin, 124(2): 591-606.
DOI:10.1016/j.marpolbul.2017.04.027 |
Gómez F. 2003. The toxic dinoflagellate Gymnodinium catenatum: an invader in the Mediterranean Sea. Acta Botanica Croatica, 62(2): 65-72.
|
Gómez F. 2018. Redefinition of Ceratoperidinium and Pseliodinium (Ceratoperidiniaceae, Dinophyceae) including reassignment of Gymnodinium fusus, Cochlodinium helix and C. pirum to Pseliodinium. CICIMAR Oceánides, 33(1): 1-11.
DOI:10.37543/oceanides.v33i1.218 |
Guillard R R L, Hargraves P E. 1993. Stichochrysis immobilis is a diatom, not a chrysophyte. Phycologia, 32(3): 234-236.
DOI:10.2216/i0031-8884-32-3-234.1 |
Han B, Lin F X, Ding Y, et al. 2019. Quality survey and risk assessment of the coastal waters of Haizhou Bay. Rock and Mineral Analysis, 38(4): 429-437.
(in Chinese with English abstract) |
Hansen P J, Nielsen T G. 1997. Mixotrophic feeding of Fragilidium subglobosum (Dinophyceae) on three species of Ceratium: effects of prey concentration, prey species and light intensity. Marine Ecology Progress Series, 147: 187-196.
DOI:10.3354/meps147187 |
Heisler J, Glibert P M, Burkholder J M, et al. 2008. Eutrophication and harmful algal blooms: a scientific consensus. Harmful Algae, 8(1): 3-13.
DOI:10.1016/j.hal.2008.08.006 |
Herrera-Sepúlveda A, Hernandez-Saavedra N Y, Medlin L K, et al. 2013. Capillary electrophoresis finger print technique (CE-SSCP): an alternative tool for the monitoring activities of HAB species in Baja California Sur Costal. Environmental Science and Pollution Research, 20(10): 6863-6871.
DOI:10.1007/s11356-012-1033-7 |
Hoppenrath M, Chomérat N, Horiguchi T, et al. 2013. Taxonomy and phylogeny of the benthic Prorocentrum species (Dinophyceae)—a proposal and review. Harmful Algae, 27: 1-28.
DOI:10.1016/j.hal.2013.03.006 |
Jeong H J, Ok J H, Lim A S, et al. 2016. Mixotrophy in the phototrophic dinoflagellate Takayama helix (family Kareniaceae): predator of diverse toxic and harmful dinoflagellates. Harmful Algae, 60: 92-106.
DOI:10.1016/j.hal.2016.10.008 |
Jeong H J, Yoo Y Y D, Kang N S, et al. 2010. Ecology of Gymnodinium aureolum. I. Feeding in western Korean waters. Aquatic Microbial Ecology, 59(3): 239-255.
DOI:10.3354/ame01394 |
Jiangsu Provincial Oceanic and Fishery Bureau. 2011-2017. Marine Environment Quality Bulletin of Jiangsu, 2011-2017. (in Chinese)
|
Ki J S, Han M S. 2007a. Informative characteristics of 12 divergent domains in complete large subunit rDNA sequences from the harmful dinoflagellate genus, Alexandrium (Dinophyceae). The Journal of Eukaryotic Microbiology, 54(2): 210-219.
DOI:10.1111/j.1550-7408.2007.00251.x |
Ki J S, Han M S. 2007b. Cryptic long internal repeat sequences in the ribosomal DNA ITS1 gene of the dinoflagellate Cochlodinium polykrikoides (Dinophyceae): a 101 nucleotide six-repeat track with a palindrome-like structure. Genes & Genetic Systems, 82(2): 161-166.
|
Killberg-Thoreson L, Mulholland M R, Heil C A, et al. 2014. Nitrogen uptake kinetics in field populations and cultured strains of Karenia brevis. Harmful Algae, 38: 73-85.
DOI:10.1016/j.hal.2014.04.008 |
Lee C K, Kim H C, Lee S G, et al. 2001. Abundance of harmful algae, Cochlodinium polykrikoides, Gyrodinium impudicum and Gymnodinium catenatum in the coastal area of South Sea of Korea and their effects of temperature, salinity, irradiance and nutrient on the growth in culture. Korean Journal of Fisheries and Aquatic Sciences, 34(5): 536-544.
|
Lenaers G, Maroteaux L, Michot B, et al. 1989. Dinoflagellates in evolution. A molecular phylogenetic analysis of large subunit ribosomal RNA. Journal of Molecular Evolution, 29(1): 40-51.
DOI:10.1007/BF02106180 |
Leong S, Yew C, Peng L L, et al. 2015. Three new records of dinoflagellates in Singapore's coastal waters, with observations on environmental conditions associated with microalgal growth in the Johor Straits. Raffles Bulletin of Zoology, 31: 24-36.
|
Lim A S, Jeong H J, Ok J H, et al. 2018. Feeding by the harmful phototrophic dinoflagellate Takayama tasmanica (Family Kareniaceae). Harmful Algae, 74: 19-29.
DOI:10.1016/j.hal.2018.03.009 |
Lin S L, Shui B N, You S P, et al. 2016. Study on distribution of Dinoflagellate cysts in Nanji islands in the summer of 2014. Journal of Zhejiang Ocean University (Natural Science), 35(2): 99-104.
(in Chinese with English abstract) |
Lin Z R, Geng H X, Zhang Q C, et al. 2022. Toxin production of dinoflagellate Gymnodinium catenatum isolated from the East China Sea. Harmful Algae, 113: 102188.
DOI:10.1016/j.hal.2022.102188 |
Luo Z H, Hu Z X, Tang Y Z, et al. 2018. Morphology, ultrastructure, and molecular phylogeny of Wangodinium sinense gen. et sp. nov. (Gymnodiniales, Dinophyceae) and revisiting of Gymnodinium dorsalisulcum and Gymnodinium impudicum. Journal of Phycology, 54(5): 744-761.
DOI:10.1111/jpy.12780 |
Mackey M D, Mackey D J, Higgins H W, et al. 1996. CHEMTAX-a program for estimating class abundances from chemical markers: application to HPLC measurements of phytoplankton. Marine Ecology Progress Series, 144: 265-283.
DOI:10.3354/meps144265 |
Mikhail S, Khalil N, El-Hadary M. 2020. A new recorded of red tide forming species; Heterocapsa triquetra, Gymnodinium impudicum, Heterosigma akashiwo and Thalassiosira rotula in Alexandria Waters, Egypt. Egyptian Journal of Aquatic Biology and Fisheries, 24(6): 207-223.
DOI:10.21608/ejabf.2020.117258 |
Mitsunori I, Hisae T, Haruyoshi T, et al. 2006. First report of Gymnodinium microreticulatum (Gymnodiniales, Dinophyceae) in Japanese coastal waters. Japanese Journal of Phycology, 54(2): 77-83.
|
Moestrup Ø, Hakanen P, Hansen G, et al. 2014. On Levanderina fissa gen. & comb. nov. (Dinophyceae) (syn. Gymnodinium fissum, Gyrodinium instriatum, Gyr. uncatenum), a dinoflagellate with a very unusual sulcus. Phycologia, 53(3): 265-292.
DOI:10.2216/13-254.1 |
Murray S, De Salas M, Luong-Van J, et al. 2007. Phylogenetic study of Gymnodinium dorsalisulcum comb. nov. from tropical Australian coastal waters (Dinophyceae). Phycological Research, 55(2): 176-184.
DOI:10.1111/j.1440-1835.2007.00460.x |
Nézan E, Siano R, Boulben S, et al. 2014. Genetic diversity of the harmful family Kareniaceae (Gymnodiniales, Dinophyceae) in France, with the description of Karlodinium gentienii sp. nov.: a new potentially toxic dinoflagellate. Harmful Algae, 40: 75-91.
DOI:10.1016/j.hal.2014.10.006 |
Ocean Administration of Tianjin. 2016. Report on Marine Environmental Quality of Tianjin, 2016. (in Chinese)
|
Onuma R, Watanabe K, Horiguchi T. 2015. Pellucidodinium psammophilum gen. & sp. nov. and Nusuttodinium desymbiontum sp. nov. (Dinophyceae), two novel heterotrophs closely related to kleptochloroplastidic dinoflagellates. Phycologia, 54(2): 192-209.
DOI:10.2216/14-103.1 |
Park J G, Park Y S. 1999. Comparison of morphological characteristics and the 24S rRNA sequences of Cochlodinium polykrikoides and Gyrodinium impudicum. The Sea: Journal of the Korean Society of Oceanography, 4(4): 363-370.
|
Redfield A C, Ketchum B H, Richards F A. 1963. The influence of organisms on the composition of sea-water. The Sea, 2: 26-77.
|
Reñé A, Camp J, Garcés E. 2015. Diversity and phylogeny of Gymnodiniales (Dinophyceae) from the NW Mediterranean Sea revealed by a morphological and molecular approach. Protist, 166(2): 234-263.
DOI:10.1016/j.protis.2015.03.001 |
Reñé A, De Salas M, Camp J, et al. 2013. A new Clade, based on partial LSU rDNA sequences, of unarmoured dinoflagellates. Protist, 164(5): 673-685.
DOI:10.1016/j.protis.2013.07.002 |
Reñé A, Satta C T, Garcés E, et al. 2011. Gymnodinium litoralis sp. nov. (Dinophyceae), a newly identified bloom-forming dinoflagellate from the NW Mediterranean Sea. Harmful Algae, 12: 11-25.
DOI:10.1016/j.hal.2011.08.008 |
Roelke D L, Brooks B W, Grover J P, et al. 2012. Anticipated human population and climate change effects on algal blooms of a toxic haptophyte in the south-central USA. Canadian Journal of Fisheries and Aquatic Sciences, 69(8): 1389-1404.
DOI:10.1139/f2012-019 |
Sampedro N, Fraga S, Penna A, et al. 2011. Barrufeta bravensis gen. nov. sp. nov. (Dinophyceae): A new bloom-forming species from the northwest Mediterranean Sea. Journal of Phycology, 47(2): 375-392.
DOI:10.1111/j.1529-8817.2011.00968.x |
Su J L, Fan W, Wang F. 2020. Correlation analysis of spatial distribution change and driving factors of laver cultivation in Haizhou bay. China Agricultural Information, 32(6): 22-31.
(in Chinese with English abstract) |
Sun A M, Li C, Lan D Z, et al. 2006. Dinoflagellate cysts records from core samples of modern marine sediment at the Luoyuan Bay mouth. Journal of Oceanography in Taiwan Strait, 25(1): 10-18.
(in Chinese with English abstract) |
Takayama H. 1985. Apical grooves of unarmored dinoflagellates. Bulletin of Plankton Society of Japan, 32: 129-140.
|
Tian H J, Ge X J, Lv H B. 2006. Water quality investigation and assessment on seawater in Lianyungang inshore. Environmental Science and Management, 31(9): 164-167.
(in Chinese with English abstract) |
Wang J, Cao L. 2020. Effect of laver culture on water quality in Haizhou Bay. Environmental Science and Technology, 33(5): 54-58, 64.
(in Chinese with English abstract) |
Wang N, Luo Z H, Mertens K N, et al. 2017. Cyst-motile stage relationship and molecular phylogeny of a new freshwater dinoflagellate Gymnodinium plasticum from Plastic Lake, Canada. Phycological Research, 65(4): 312-321.
|
Weng T T. 2017. Population characteristics and environmental indications of dinoflagellate cysts from the northern Yellow Sea. Xiamen University, Xiamen. (in Chinese with English abstract)
|
Winnepenninckx B, Backeljau T, De Wachter R. 2002. Extraction of high molecular weight DNA from molluscs. Trends in Genetics, 9(12): 407.
|
Yahia-Kéfi O D, Souissi S, Gomez F, et al. 2005. Spatio-temporal distribution of the dominant diatom and dinoflagellate species in the Bay of Tunis (SW Mediterranean Sea). Mediterranean Marine Science, 6(1): 17-34.
|
Yamaguchi H, Nishijima T, Nishitani H, et al. 2004. Organic phosphorus utilization and alkaline phosphatase production of 3 red tide phytoplankton. Nippon Suisan Gakkaishi, 70(2): 123-130.
|
Yim J H, Lee H K. 2004. Axenic culture of Gyrodinium impudicum strain KG03, a marine red-tide microalga that produces exopolysaccharide. The Journal of Microbiology, 42(4): 305-314.
|
Zapata M, Jeffrey S W, Wright S W, et al. 2004. Photosynthetic pigments in 37 species (65 strains) of Haptophyta: implications for oceanography and chemotaxonomy. Marine Ecology Progress Series, 270: 83-102.
|
Zapata M, Rodríguez F, Garrido J L. 2000. Separation of chlorophylls and carotenoids from marine phytoplankton: a new HPLC method using a reversed phase C8 column and pyridine-containing mobile phases. Marine Ecology Progress Series, 195: 29-45.
|
Zhang Q C, Wang Y F, Song M J, et al. 2022. First record of a Takayama bloom in Haizhou Bay in response to dissolved organic nitrogen and phosphorus. Marine Pollution Bulletin, 178: 113572.
|
Zhu X Y, Xu H H, Xu X, et al. 2017. Community composition of net-collected phytoplankton and its relation to environmental factors near Lianyungang sea area. Journal of Applied Oceanography, 36(3): 385-394.
(in Chinese with English abstract) |
 2022, Vol. 40
2022, Vol. 40