Institute of Oceanology, Chinese Academy of Sciences
Article Information
- NIU Zhuang, GUAN Weibing, WANG Jinxiu, YUAN Yongquan, KONG Fanzhou, LIU Chao, ZHANG Qingchun, YU Rencheng
- Dynamics of Phaeocystis globosa bloom and implications for its seed sources in the Beibu Gulf, China
- Journal of Oceanology and Limnology, 40(6): 2385-2400
- http://dx.doi.org/10.1007/s00343-022-1447-0
Article History
- Received Dec. 30, 2021
- accepted in principle Feb. 21, 2022
- accepted for publication Apr. 14, 2022
2 Laboratory for Marine Ecology and Environmental Science, Pilot National Laboratory for Marine Science and Technology(Qingdao), Qingdao 266237, China;
3 State Key Laboratory of Satellite Ocean Environment Dynamics, Second Institute of Oceanography, Ministry of Natural Resources, Hangzhou 310012, China;
4 School of Oceanography, Shanghai Jiao Tong University, Shanghai 200030, China;
5 Center of Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China;
6 University of Chinese Academy of Sciences, Beijing 100049, China
Haptophyte Phaeocystis globosa is a cosmopolitan bloom-forming species and often occurs in blooms of gelatinous colonies in temperate and tropical coastal waters (Schoemann et al., 2005), seriously threatening the coastal ecosystem and aquaculture fishery because of its toxic hemolytic substances and the formed foams after colony decay (Chen et al., 1999; Shen et al., 2018b). Since 1997, there have been frequent outbreaks of P. globosa blooms in the South China Sea (SCS) in the form of centimeter-sized giant colonies (Chen et al., 1999; Qi et al., 2004), much larger than those of P. globosa in other regions, such as the Atlantic North Sea, a region with high-frequency P. globosa blooms (Rousseau et al., 1990; Madhupratap et al., 2000). In the winter of 2011, a P. globosa bloom was firstly recorded in the Beibu Gulf (Qin et al., 2016) and has since become a recurrent phenomenon (He et al., 2019; Wang et al., 2019a), threatening the safe operation of power stations along the coastline in this region (Cao et al., 2017; He et al., 2019).
As a semi-closed gulf in the northwest of the SCS, the Beibu Gulf covers an area of 1.27×105 km2 in average depth of approximately 40 m, and borders Vietnam to the west, and Guangxi Zhuang Autonomous Region, Leizhou Peninsula, and Hainan Island of China to the east and north. With the rapid development of the population, industry, and economics in the coastal regions, the input of dissolved inorganic nitrogen (DIN) and phosphate (DIP) into the Beibu Gulf increased rapidly, which is conducive to the increase in harmful algal bloom (HAB) events in this area (Xu et al., 2019). The hydrodynamics of the Beibu Gulf are complex (Shen et al., 2018a). The direction of summer circulation is not fully understood, and winter circulation is confirmed to be counterclockwise (Hu et al., 2000), implying that the coastal water from Guangdong Province can enter the Beibu Gulf via the Qiongzhou Strait and enhance the formation of winter circulation (Chen et al., 2019). In addition, northeasterly monsoonal winds drive upwelling in the Beibu Gulf in winter (Tang et al., 2003; Shen et al., 2018). The complex hydrodynamics result in different temporal and spatial distribution of water masses from different sources in the Beibu Gulf (Cao et al., 2019). However, little is known of the relationship between the complex hydrodynamics and the distribution of P. globosa blooms in the Beibu Gulf.
Cumulative evidence suggests that different geographical strains of P. globosa have variable colony sizes and pigment profiles, and maintain a high level of genetic diversity (Zapata et al., 2004; Medlin and Zingone, 2007; Smith et al., 2014). Recently, two genotypes (Type Ⅰ and Type Ⅳ) of P. globosa have been demonstrated to co-exist in the Beibu Gulf (Song et al., 2020, 2021; Zhang et al., 2021; Niu et al., 2022). Type Ⅰ cells possess two marker pigments, 19ʹ-hexanoyloxyfucoxanthin (Hex-fuco) and 19ʹ-butanoyloxyfucoxanthin (But-fuco), and form small colonies (< 0.3 cm in diameter), and Type Ⅳ cells contain a unique marker pigment, But-fuco, and produce centimeter-sized giant colonies (Wang et al., 2019a; Niu et al., 2022; Zhang et al., 2022). Much less is known about the relationships between the spatiotemporal distribution of these two genotypes and the dynamics of P. globosa blooms in the Beibu Gulf. Recently, two genotype-specific quantitative polymerase chain reaction (qPCR) methods targeting the mitochondrial atp8 gene have been developed to quantitatively detect Type Ⅰ and Type Ⅳ P. globosa. Both qPCR assays have a wide detection range and high sensitivity, with the lowest quantitative detection limit of 30 cells/L (Niu et al., 2022), providing helpful tools to quantify the cell abundance of Type Ⅰ and Type Ⅳ in the Beibu Gulf.
Therefore, the aims of this study are to quantify the spatiotemporal distribution of Type Ⅰ and Type Ⅳ P. globosa in the Beibu Gulf during its bloom in winter 2018, to analyze the response of P. globosa to environmental factors, and to identify the potential seed sources of P. globosa triggering the intense blooms, based on the distribution of P. globosa cells in different water masses before bloom.
2 MATERIAL AND METHOD 2.1 Sample collectionThree investigations were performed in the Beibu Gulf during the P. globosa bloom in November 2018 and January 2019, and February 2019. The first investigation covered 31 sampling sites; the last two investigations covered 28 sampling sites, excluding three southern sampling sites (ZN0-1, ZN0-2, and ZN0-3) (Fig. 1). The seawater temperature, salinity, and excess water density were detected in situ using SBE-9 plus conductivity-temperature-depth (CTD) sensors (SBE 911, Sea-Bird Electronics). Approximately 2 L of seawater was collected from the surface, middle, and bottom of the water column, respectively; and then, 1-L and 0.5-L subsamples were filtered through a 0.4-μm polycarbonate (PC) membrane (Millipore, USA) and a combusted glass fiber membrane (GF/F, Whatman) under a 40-kPa vacuum, respectively. The filtered membranes were frozen in liquid nitrogen in situ and stored at -80 ℃ for DNA and chlorophyll-a (Chl-a) extraction. And 60-mL seawater filtered by a GF/F membrane was stored frozen at -20 ℃ for nutrient analysis.

|
| Fig.1 Map of the Beibu Gulf and sampling sites (black dots) Map review No. GS(2019)1708. |
The genomic DNA of seawater samples was extracted using a modified cetyltrimethylammonium bromide (CTAB) method (Winnepenninckx et al., 1993) and dissolved in 30-μL Tris-EDTA buffer solution (TE) before being stored at -20 ℃. The qPCR reactions with the specific primer pairs and probes (Supplementary Table S1) were performed to detect the two genotypes of P. globosa in a Bio-Rad C1000 thermal cycler (Bio-Rad, USA) following the previous study (Niu et al., 2022). The qPCR reaction of Type Ⅰ was performed in a total volume of 10 μL with 5 μL of 2× qPCR buffer (TaKaRa, Beijing, China), 3.1 μL of ddH2O, 0.3 μL of 10-nmol/L forward primer, 0.3 μL of 10-nmol/L reverse primer, 0.3 μL of 10-nmol/L probe, and 1 μL of DNA template. And the qPCR reaction of Type Ⅳ was done with 5 μL of 2× qPCR buffer (TaKaRa, Beijing, China), 3.4 μL of ddH2O, 0.2 μL of 10-nmol/L forward primer, 0.2 μL of 10-nmol/L reverse primer, 0.2 μL of 10-nmol/L probe, and 1 μL of DNA template in a final volume of 10 μL. Both the two qPCR assays were run at 95 ℃ for 30 s, followed by 40 cycles of 95 ℃ for 5 s and 60 ℃ for 30 s. The establishment of calibration curve and the limit of quantitative detection for the two assays were presented in the Supplementary materials (Supplementary Figs. S1 & S2).
2.3 Measurement of nutrientsFive nutrients (ammonium, nitrite, nitrate, phosphate, and silicate) were analyzed using a continuous flow analyzer (SKALAR, Breda, Netherlands) according to the protocol in Yuan et al. (2019).
2.4 Water mass characterizationTo explore the effects of water masses on the cell abundance and distribution of two genotypes of P. globosa in the Beibu Gulf, the classification and distribution of water masses, including the coastal water (CW), SCS bottom water (BW), SCS mixed water (MW) in this area from November 2018 to February 2019, were analyzed based on CTD data, using the K-mean clustering method as described in the previous study (Cao et al., 2019). The CW water mass with low salinity (< 32) from the river discharges is limited to a small strip on the coast of Guangxi Zhuang Autonomous Region, and the BW water mass with high salinity (> 33) and low temperature is mainly formed by seawater intrusion from SCS offshore waters outside the Gulf. And according to the source of mixed water, the MW is subdivided into two parts from the Qiongzhou Strait (MW-Q) and the southern Beibu Gulf (MW-S).
2.5 Data analysisStatistical analysis of raw data was performed using IBM SPSS Statistics 26 (IBM, Armonk, NY, USA). The horizontal and profile distribution of two P. globosa genotypes was determined using Surfer 13.0 (Golden Software Inc., USA). The CANOCO 5.0 (Microcomputer Power, USA) was used to explore the relationship between environmental factors and two genotypes of P. globosa. The result of detrended correspondence analysis (DCA) performed in CANOCO 5.0 revealed that the Type Ⅰ and Type Ⅳ abundance exhibited a linear response to environmental factors, thus redundancy analysis (RDA) combined with Monte Carlo test (499 permutations) as a linear constrained ordination method was chosen for data analysis. In addition, Pearson correlation coefficients and two-tailed test were also used to evaluate correlation between environmental factors and the distribution of two genotypes of P. globosa.
3 RESULT 3.1 Dynamics of temperature, salinity, nutrients and Phaeocystis globosa abundance during the bloomDuring the three investigations, the average temperature of water column was 26.2 ℃ in Nov. 2018, decreased sharply to 19.3 ℃ in Jan. 2019, and increased to 21.2 ℃ in Feb. 2019. The average salinity gradually increased from 31.59 to 32.31 during the three investigations. The excess water density exhibited the lowest average value of 20.7 kg/m3, ranging from 14.2 to 22.8 kg/m3 in Nov. 2018, and increased markedly to 22.9 kg/m3 in Jan. 2019 and 22.6 kg/m3 in Feb. 2019 (Table 1).

|
The average concentrations of nitrate and silicate showed a similar change trend, peaking in Jan. 2019 and then decreasing to the lowest level in Feb. 2019. Nitrite slightly decreased from Nov. 2018 to Jan. 2019, and peaked 0.61 μmol/L in Feb. 2019. Ammonium decreased from 0.78 to 0.52 μmol/L and then sharply increased to 2.90 μmol/L in Feb. 2019. Phosphate increased to 0.61 μmol/L in Jan. 2019, then down to the same level in Feb. 2019 as in Nov. 2018 (Table 1).
The abundance of Type Ⅳ cells was higher than that of Type Ⅰ cells during the three investigations. In Nov. 2018, the average abundances of Type Ⅰ and Type Ⅳ were 8.7×103 and 1.3×104 cells/L, and subsequently increased to 3.9×104 and 1.2×105 cells/L in Jan. 2019, and then decreased to 1.1×104 cells/L and 5.1×104 cells/L in Feb. 2019, respectively (Table 1).
3.2 Horizontal distribution of hydrological factors and Phaeocystis globosaThe variations in the sea surface temperature (SST) and the sea bottom temperature (SBT) were similar during the three surveys (Fig. 2). In Nov. 2018, the average SST was 26.3 ℃, with the highest value of 27.8 ℃ at the site ZN0-2, and it decreased gradually toward inshore (Fig. 2a). The SBT ranged from 23.9 ℃ to 27.6 ℃ in the survey area in Nov. 2018, and there was a high-temperature (> 27.0 ℃) zone in the middle of southern section that extended from site ZN0-3 to site ZN3-3 (Fig. 2b). The lowest average seawater temperature was in Jan. 2019 (Table 1). In this month, the high-temperature zone (22.5 ℃ at the surface and 22.4 ℃ at the bottom) was in the south of the Gulf and gradually decreased toward the north (Fig. 2c–d). During the last survey in Feb. 2019, the temperature did not change significantly throughout the study area (Fig. 2e–f), and the surface and bottom average temperatures were 21.3 ℃ and 20.8 ℃, respectively.

|
| Fig.2 Distribution of temperature at surface (Sur) and bottom (Bot) of the Beibu Gulf from November 2018 to February 2019 |
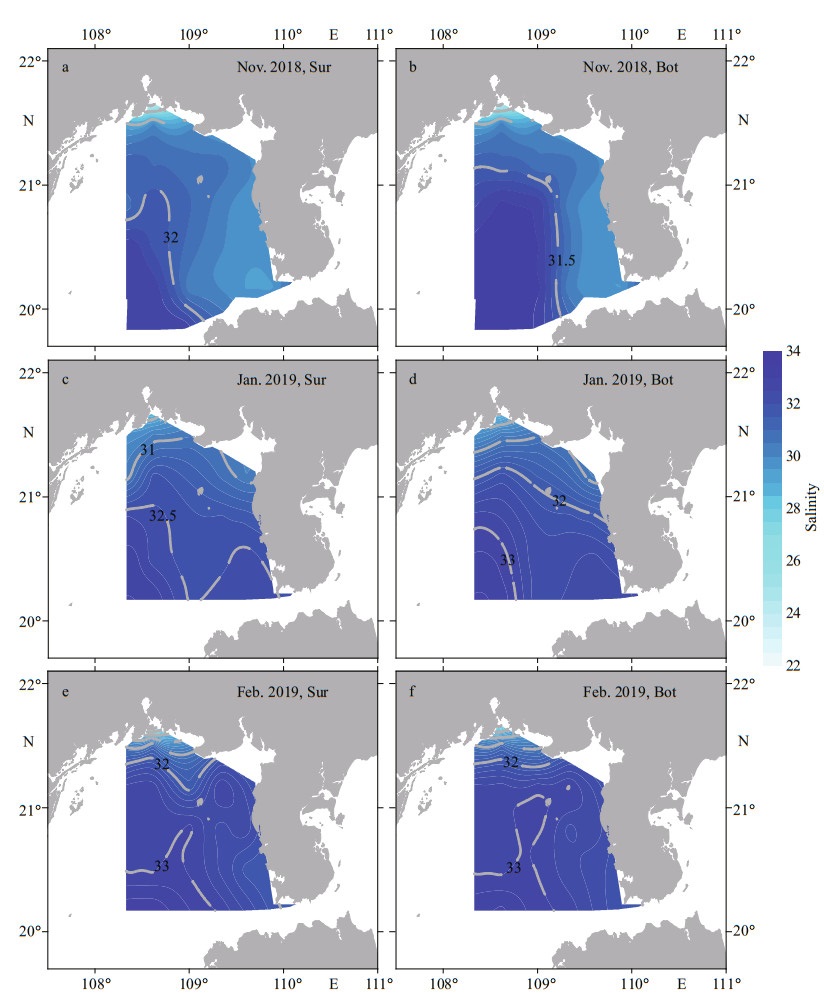
The variations in sea surface salinity (SSS) and sea bottom salinity (SBS) were similar, ranging 22.8–33.5 and 23.0–33.9, respectively, in the three investigations. In Nov. 2018, the high-salinity water mass (> 31.5) was located in the southwest waters and intrude northward and northeastward into the inshore. Notably, this high-salinity water mass separated the low-salinity water in Qinzhou Bay and southwest of the Leizhou Peninsula (Fig. 3a–b). Subsequently, SSS and SBS increased and reached higher average values of 31.9 and 32.0 in Jan. 2019, respectively. High-salinity water (> 32.5) occurred at the mouth of the Qiongzhou Strait and southwest of survey area and gradually decreased toward the coastline of Guangxi Zhuang Autonomous Region (Fig. 3c–d). Seawater salinity continued to increase during the last investigation in Feb. 2019 (Table 1). High-salinity water (> 32.5) filled the survey area, excluding the low-salinity zones in the waters of Qinzhou Bay and the mouth of the Qiongzhou Strait (Fig. 3e–f).
|
| Fig.3 Distribution of salinity at surface (Sur) and bottom (Bot) of the Beibu Gulf from November 2018 to February 2019 |
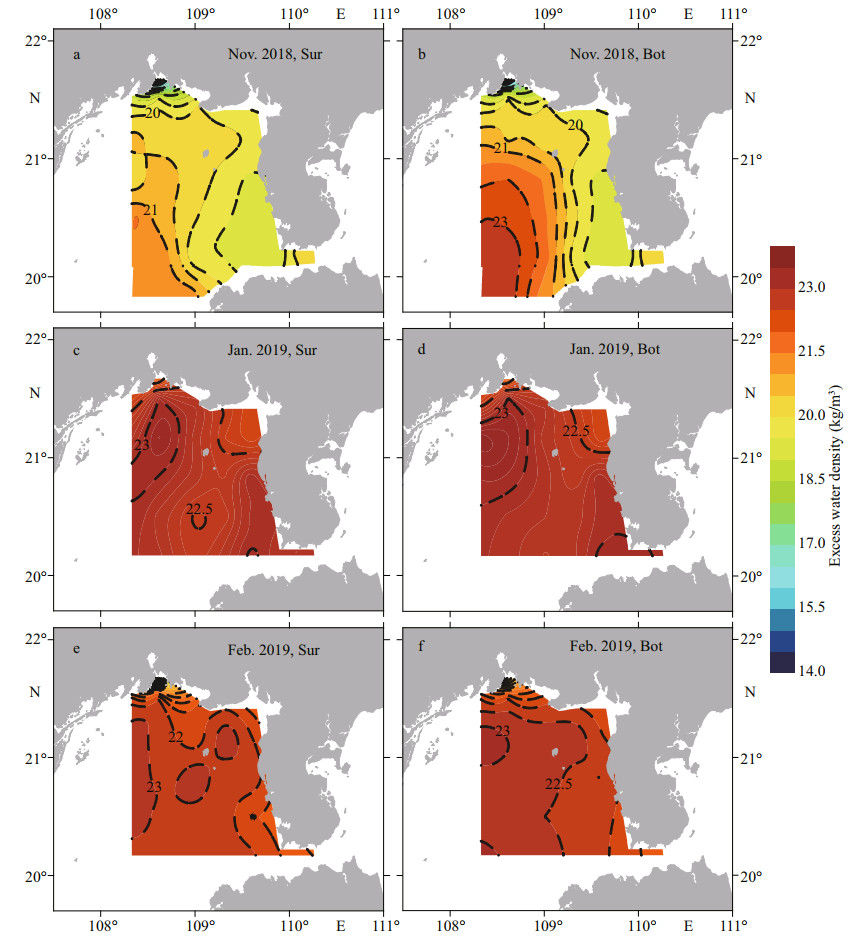
The surface and bottom distribution of excess water density was similar to that of salinity during the three surveys. In Nov. 2018, the high-density water intruded from the southwest of study area to the inshore of Beihai City and separated the low-density water in Qinzhou Bay and the southwest of Leizhou Peninsula (Fig. 4a–b). In Jan. 2019, the high excess water density was located uniformly in the survey area, and there was a strip with a slightly lower density from the center of section ZN Ⅰ (22.7 kg/m3) to the coast of Beihai City (Fig. 4c–d). The excess water density in February was slightly lower than that in January and gradually decreased from the southwest of the Beibu Gulf to the inshore area (Fig. 4e–f).
|
| Fig.4 Distribution of excess water density at surface (Sur) and bottom (Bot) of the Beibu Gulf from November 2018 to February 2019 |
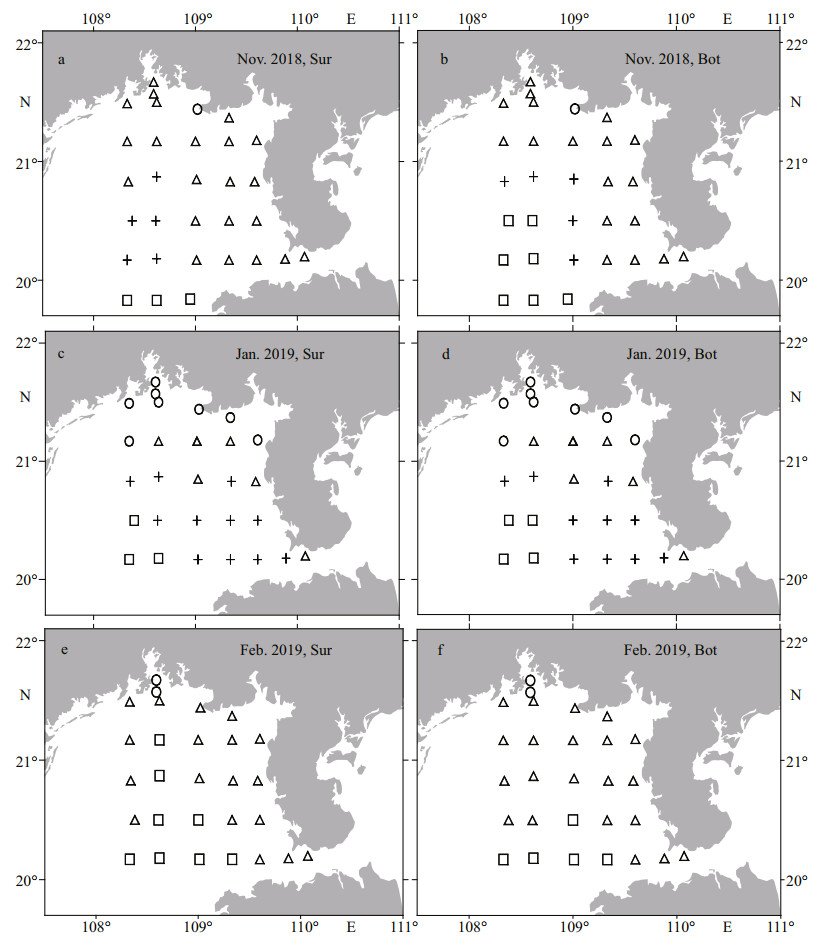
In Nov. 2018, the inshore area was dominated by the MW-Q, except for the CW at site ZN5-3, and the BW and MW-S on the southwest offshore surface and bottom (Fig. 5a–b). In Jan. 2019, the MW-S expanded toward the coast of Guangxi Zhuang Autonomous Region and the Leizhou Peninsula, and occupied most of the region formerly controlled by the MW-Q; the CW control region was significantly expanded larger than that in Nov. 2018; the MW-Q was only preserved in a small area between the CW and MW-S control regions (Fig. 5c–d). During the last survey, the MW-Q reoccupied most of the survey area, except for the mouth of Qinzhou Bay located by the CW and the southwest waters located by the BW (Fig. 5e–f).
|
| Fig.5 Distribution of water masses at surface (Sur) and bottom (Bot) of the Beibu Gulf from November 2018 to February 2019 □: the SCS bottom water of the South China Sea (BW); ○: the coastal water (CW); Δ: the mixed water from Qiongzhou Strait (MW-Q); +: the mixed water from the southern Beibu Gulf (MW-S). |
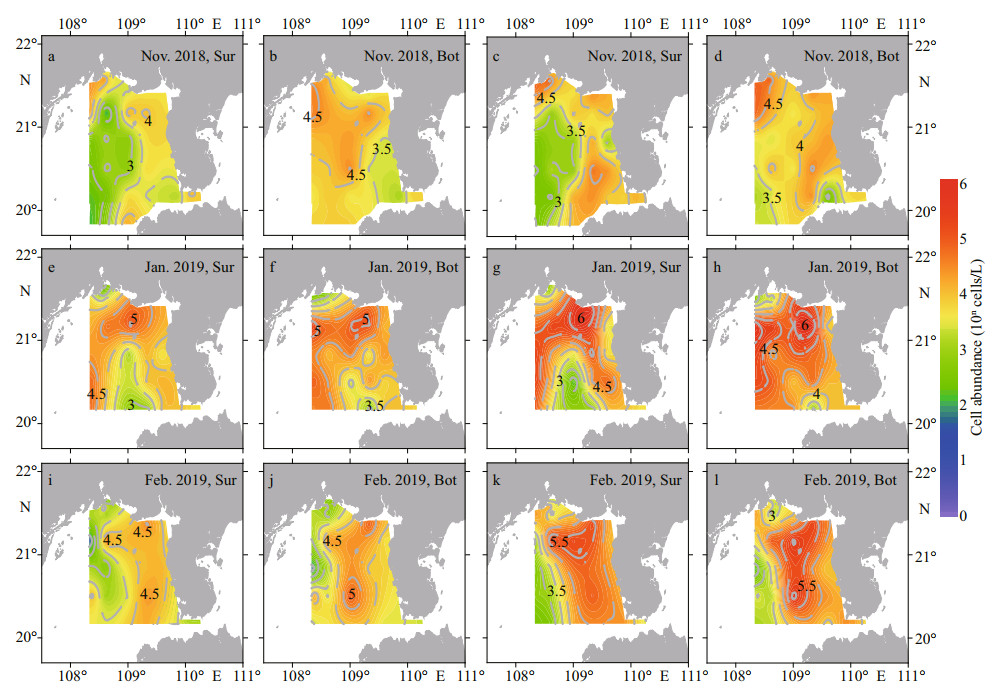
Apart from the differences in cell abundance(Table 1), the distribution patterns of Type Ⅰ and Type Ⅳ P. globosa were similar during the three surveys. The distribution of P. globosa was different at the surface and bottom in Nov. 2018. The western and central surface waters of survey area were distributed with a low abundance of P. globosa, while the high abundance (> 5.0×103 cells/L) of Type Ⅰ and Type Ⅳ was discontinuously distributed from the mouth of Qinzhou Bay to the west of the Leizhou Peninsula and Qiongzhou Strait. Notably, there was a low-abundance area between the Qinzhou Bay estuary and the coastline of Beihai City (Fig. 6a & c). The high abundance of two genotypes was widespread at the bottom. The Type Ⅳ abundance was greater than that of Type Ⅰ in the waters west of the Qiongzhou Strait and the mouth of Qinzhou Bay (Fig. 6b & d). With the development of P. globosa blooms, the abundance of both genotypes increased rapidly, and their high-abundance cells (> 1.0×104 cells/L) were distributed in most surface and bottom waters of the surveyed area, except for the estuary of Qinzhou Bay and the waters adjacent station ZN1-4 in Jan. 2019 (Fig. 6e–h). With the decline in P. globosa bloom, the cell abundance of two genotypes gradually decreased, and their distribution decreased in the western part of survey area in Feb. 2019 (Fig. 6i–l).
|
| Fig.6 Distribution of Type Ⅰ (a, b, e, f, i, and j) and Type Ⅳ (c, d, g, h, k, and l) of Phaeocystis globosa at surface (Sur) and bottom (Bot) of the Beibu Gulf from November 2018 to February 2019 |
Before the bloom in Nov. 2018, the profile distribution of hydrological factors and P. globosa was observed at north-south section Ⅰ from site ZN6-2 to site ZN1-2, and east-west section Ⅱ from site ZN1-7 to site ZN1-1 (Fig. 1). In north-south section Ⅰ, a high-density water mass with low temperature and high salinity was located at the south bottom and intruded the inshore of the Qinzhou Bay estuary along the slope (Fig. 7a, c, & e). Similarly, the high abundance (> 5.0×103 cells/L) of two genotypes was distributed from the south bottom to the inshore (Fig. 7g & i). Unlike section Ⅰ, there was a shoal in the middle of east-west section Ⅱ. The distribution patterns of hydrological factors differed on the eastern and western slopes. There was a high-density water mass with low temperature and high salinity at the western bottom with a water depth greater than 25 m (Fig. 7b, d, & f). Low-density mixed seawater with high temperature and low salinity resided on the eastern slope and slightly intruded the western slope over the central shoal (Fig. 7d & f). Similar to north-south section Ⅰ, there was no obvious difference in the distribution of two genotypes in section Ⅱ, and the high-abundance cells (> 5.0×103 cells/L) were distributed at the western bottom and in eastern mixed water (Fig. 7h & j).
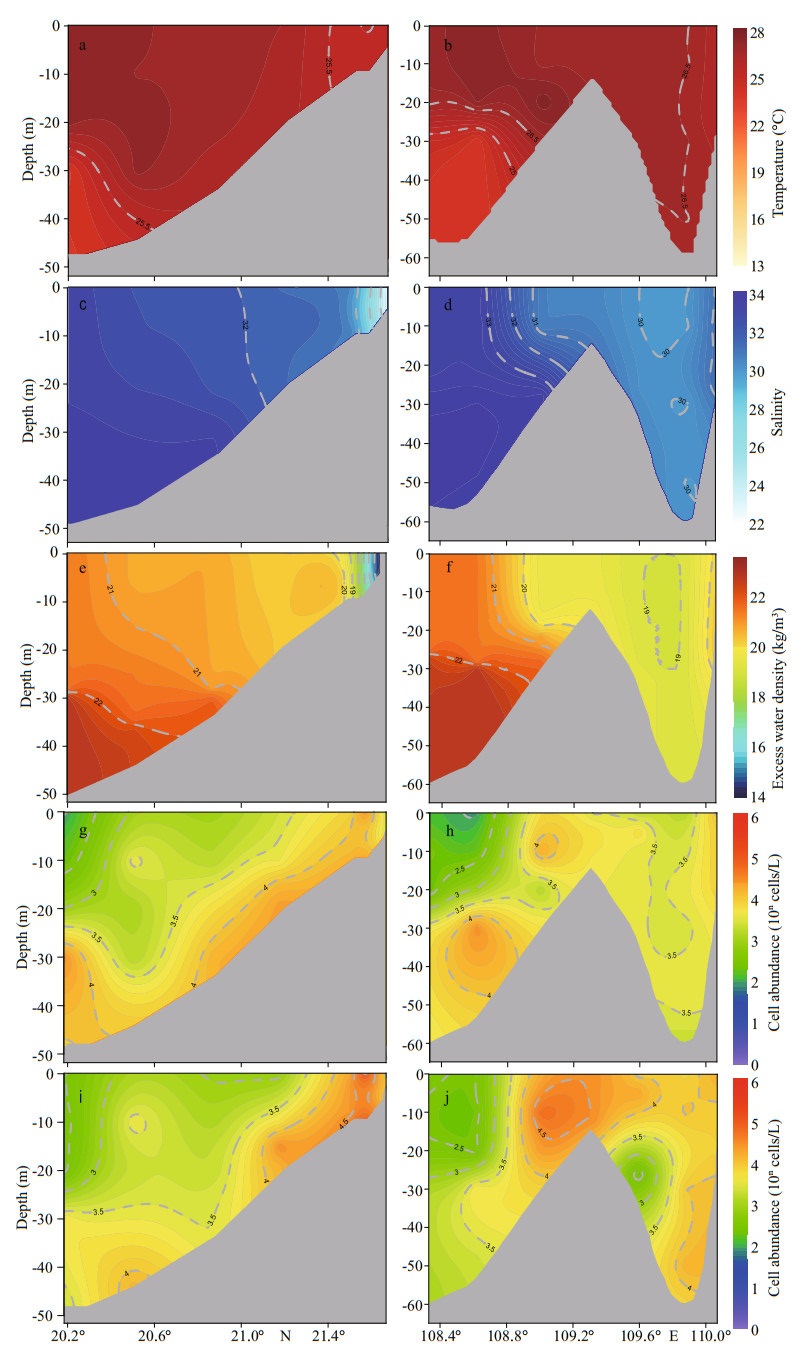
|
| Fig.7 Profile distribution of temperature (a, b), salinity (c, d), excess water density (e, f), and abundance of Type Ⅰ (g, h) and Type Ⅳ (i, j) of Phaeocystis globosa at section Ⅰ and section Ⅱ |
RDA and Pearson correlation analysis were used to analyze the relationships between the distribution of two genotypes of P. globosa and environmental factors (Fig. 8; Supplementary Table S2). The permutation tests showed that the RDA model including all environmental variables was extremely significant (P < 0.01). RDA axes 1 and 2 explained 19.24% and 2.54% of total variation, respectively. Temperature (0.58), and ammonium (0.46) were positively correlated with the RDA Axis 1. By contrast, salinity (-0.12), phosphate (-0.37), silicate (-0.18), nitrite (-0.31), and nitrate (-0.14) were negatively correlated with the RDA Axis 1. Temperature was the most important response variable, and both genotypes of P. globosa had extremely negative correlations with temperature (P < 0.01). In addition, the abundance of two genotypes also showed a negative correlation with nitrite, nitrate, ammonium (P < 0.01 for Type Ⅰ), and DIN (P < 0.01 for Type Ⅰ, 0.01≤P < 0.05 for Type Ⅳ). Notably, the correlations between the abundance of two genotypes and salinity, silicate, and phosphate differed. Salinity was positively correlated with the abundance of Type Ⅰ, but was not correlated with Type Ⅳ. The correlation between phosphate and Type Ⅰ was positive, while negative correlations were found between silicate, phosphate, and Type Ⅳ.

|
| Fig.8 Redundancy analysis (RDA) ordination plots for the relationships between environmental factors and the cell abundance of two genotypes of Phaeocystis globosa |
Phaeocystis globosa blooms, characterized by giant colonies (> 1 cm in diameter), have been continually occurring in the SCS, including the coastal waters of Guangdong Province, Hainan Island, and the Beibu Gulf (Qi et al., 2004; Qin et al., 2016). Previous studies have found that multiple genotypes of P. globosa reside in the SCS, among which the Type Ⅰ and Type Ⅳ are the two dominant genotypes in the Beibu Gulf (Song et al., 2020; Zhang et al., 2021, 2022). As a result, determining the dynamics of dominant genotype in the Beibu Gulf will advance the understanding of formation mechanisms of P. globosa blooms in this area.
In this study, the abundance of two genotypes of P. globosa was determined by the two sensitive genotype-specific qPCR assays (Niu et al., 2022). The cell abundance of Type Ⅳ was extremely significantly higher than that of Type Ⅰ (Table 1, P < 0.01) in the Beibu Gulf during the three surveys, especially at the bloom peak in Jan. 2019; the abundance of Type Ⅳ was three times greater than that of Type Ⅰ. The similar results were found in the winter of 2016 before the P. globosa bloom in this area (Niu et al., 2022). These results are consistent with the results based on the CHEMTAX analysis of phytoplankton assemblages in the Beibu Gulf in winter 2016 (Wang et al., 2021a). Some recent studies have found that centimeter-sized giant colonies of P. globosa were mainly formed by Type Ⅳ cells during P. globosa blooms from 2016 to 2021 in the coastal waters of Guangdong Zhuang Autonomous Region and the Beibu Gulf, using a high-resolution chloroplast molecular marker (Zhang et al., 2021, 2022). The appearance of a large number of colonies is often regarded as an indicator of P. globosa bloom (Rousseau et al., 1994; Qi et al., 2004). During the three surveys in the winter of 2018, the P. globosa colonies were collected from the water column by vertical trawling using a phytoplankton sampling net (mesh aperture of 76 μm) during the bloom (Supplementary Fig. S3) (He, 2021). The distribution patterns of colonies and Type Ⅳ P. globosa were similar in Jan. and Feb. 2019. Therefore, it is suggested that the Type Ⅳ cells account for the P. globosa blooms in the Beibu Gulf.
4.2 Response of Phaeocystis globosa to environmental factorsExcept for the lower cell abundance, the distribution patterns of Type Ⅰ and Type Ⅳ were similar from Nov. 2018 to Feb. 2019 (Table 1; Fig. 6). In Nov. 2018, the high abundance of Type Ⅰ and Type Ⅳ cells mainly distributed in the inshore surface and the broader bottom; in Jan. 2019, the high abundance of P. globosa cells was found throughout the survey area, except for a low-abundance zone in the middle of southern section Ⅱ; subsequently, the abundance of both genotypes decreased obviously in the western surveyed area in Feb. 2019 (Fig. 6). The dynamics of P. globosa bloom may be associated with multiple environmental factors, including temperature, salinity, and nutrient loading in the Beibu Gulf. Temperature is a crucial environmental factor affecting the growth and blooms of P. globosa (He et al., 2019). Some experimental studies suggested that the optimal temperature is approximately 20–24 ℃ for the growth and colony formation of P. globosa in the SCS (Xu et al., 2017; Wang et al., 2021b), which is in accord with survey results of previous and this studies. In this study, the temperature ranged 18.0– 23.9 ℃ in the region with a high abundance of Type Ⅳ cells (Table 1). The temperature ranged from 18.5 to 24.0 ℃ in the Beibu Gulf from January to March 2017 when large numbers of colonies appeared (He et al., 2019), and 18.0 to 24.0 ℃ on the Guangdong coastal waters during the P. globosa bloom in 1998 (Chen et al., 1999). The RDA and Pearson correlation results showed that the abundance of both P. globosa genotypes was extremely negatively associated with temperature, implying that low temperature in the winter is advantageous for the growth of P. globosa in the Beibu Gulf. During the last two surveys, the temperature in the most of study area was 18.0–24.0 ℃ within the range of optimum growth temperature for P. globosa, while that of the whole sea area was greater than 25.0 ℃ during the first survey in Nov. 2018 (Table 1 & Fig. 2), which helps explain the variation of P. globosa abundance from Nov. 2018 to Feb. 2019. In addition, the variation of seawater temperature during the surveys may also be responsible for the higher abundance of P. globosa colonies in Jan. and Feb 2019 than in Nov. 2018 (Supplementary Fig.S3) (He, 2021), which is also in agreement with previous studies (Tian and Lv, 2010; Zhang et al., 2020). Therefore, the low temperature in winter created a time window for the outbreaks of P. globosa blooms in Beibu Gulf.
Seawater salinity is another environmental factor affecting the spatial and temporal distribution of P. globosa blooms (Tian and Lv, 2010). P. globosa can grow under a wide range of salinity (20–40) (Madhupratap et al., 2000; Xu et al., 2003), and the optimal salinity for the growth and bloom of P. globosa is suggested to 25–33 (Schoemann et al., 2005; Tian and Lv, 2010). The Beibu Gulf winter salinity is generally within the optimum range for the growth of P. globosa, which agree the widespread distribution of P. globosa in this area. The relationships between the two P. globosa genotypes and salinity were different based on the RDA and Pearson correlation results (Fig. 8; Supplementary Table S2). Salinity was positively correlated with Type Ⅰ, but no correlation with Type Ⅳ (Fig. 8). Our further experiments show that the optimal salinity range of Type Ⅰ was 30–35, and Type Ⅳ had a wider optimal salinity ranging from 25 to 40 (data not shown). These results imply that Type Ⅳ cells may be more habituated to the low-salinity inshore environments than Type Ⅰ cells.
Water eutrophication is a notable factor for the occurrence of HABs in the coastal waters worldwide (Anderson et al., 2002). Nutrient composition and stoichiometry are shown to influence the duration and succession of HABs (Glibert, 2020). The DIN concentrations averaged 3.9 μmol/L with the range from 0.4 to 25.4 μmol/L in the winter of 2018, lower than 7.3 μmol/L during the P. globosa bloom in the winter of 2016 (Yuan et al., 2019), and the DIP concentrations in the winters of 2016 and 2018 were relatively stable and averaged 0.64 and 0.56 μmol/L (Yuan et al., 2019). It is implied that the Beibu Gulf is potentially nitrogen-limited with low inorganic N/P ratios during the P. globosa blooms (Redfield et al., 1963). By contrast, the most China's coastal waters, particularly the estuary area of the Changjiang (Yangtze) River, in which HAB events are frequent, are primarily DIN-rich and phosphate-limited (Zhou et al., 2008). The distribution and dynamics of P. globosa blooms in the Beibu Gulf are strongly affected by the concentration and composition of nitrogen nutrients (Yuan et al., 2019). In general, the nitrate is the dominant composition in the DIN pools in the coastal waters. High concentration of nitrate can promote the formation of colonies and outbreak of Phaeocystis blooms (Lancelot et al., 2007; Wang et al., 2021b). In this study, the average nitrate concentrate was 4.5 μmol/L at the bloom peak outbreak in Jan. 2019; subsequently, the nitrate content was consumed by the high abundance of P. globosa cells and colonies during the bloom maintenance; in Feb. 2019, the nitrate concentrations decreased obviously accelerated the decrease of P. globosa colonies (Table 1). The similar phenomenon was also observed near the coast of Vietnam, where a P. globosa bloom occurred from August to September 2007 (Hai et al., 2010). Moreover, the distribution patterns of Phaeocystis blooms are affected by the nitrate concentration (Lancelot et al., 2007). This study also shows that the high abundance of P. globosa cells and colonies distributed in several high-nitrate areas in the Beibu Gulf, including the coast of Guangxi Zhuang Autonomous Region, western waters of the Leizhou Peninsula, and southwestern waters of the Beibu Gulf, similar to other studies in this area (He et al., 2019; Liu et al., 2020). Therefore, the nitrate may be very crucial for duration and distribution of P. globosa blooms in the Beibu Gulf.
The dissolved organic nitrogen (DON) has been significantly elevated in many coastal waters in the last two decades (Glibert, 2020). Notably, urea content in seawater has increased significantly with the increasing use of urea as a nitrogen fertilizer (Zheng et al., 2001; Glibert et al., 2006). Previous studies have recognized the critical role of urea in the growth and formation of P. globosa colonies (Chen et al., 2011; Liang et al., 2018). Thus, the role of DON in P. globosa blooms cannot be ignored. Unfortunately, the dissolved organic substances were not included in this study. Further studies are necessary to elucidate the relationship between dissolved organic nutrients and P. globosa blooms in this area.
Phaeocystis blooms require high concentration of phosphate, and 0.5 μmol/L is essential for the formation of Phaeocystis colonies (Cariou, 1991; Egge and Heimdal, 1994; Wang et al., 2007). During the three investigations, the average phosphate concentration was higher than 0.5 μmol/L in the Beibu Gulf, and the similar result was reported in the Beibu Gulf during the P. globosa bloom in the winter of 2016 (He et al., 2019). These results indicate that phosphate is not a limiting factor that affects the distribution and dynamics of P. globosa booms in the Beibu Gulf.
Providing the attachment substrates for the colony formation, diatoms also play an important role in the formation of Phaeocystis blooms (Rousseau et al., 1994, 2002; Peperzak et al., 1998). As an essential nutrient for diatom growth, high content of silicate is consumed to increase the biomass of diatoms, which is conducive to the formation of P. globosa colonies, and the initiation and duration of Phaeocystis blooms (Tréguer and De La Rocha, 2013; He et al., 2019). In this study, silicate shared a similar variation with nitrate, implying the role of silicates and diatoms in P. globosa blooms in the Beibu Gulf.
4.3 Implications for the potential seed sources of Phaeocystis globosa bloom in Beibu GulfHydrological factors strongly regulate the distribution of bloom-forming species at the initial stage of a bloom and further affect the dynamics of subsequent intense bloom (Zhou and Zhu, 2006). In the early stage of P. globosa bloom in Nov. 2018, the high abundance of P. globosa cells was discontinuously distributed in the inshore waters along Qinzhou Bay and Beihai City and the western waters of the Leizhou Peninsula. A distinctly low-abundance region was observed between Qinzhou Bay and Beihai City (Fig. 6a–d), which was also observed in Nov. 2016 (Niu et al., 2022). This unique phenomenon may reflect the multiple seed sources of P. globosa in the Beibu Gulf.
Prior to the formation of bloom in Nov. 2016 and 2018, the high-density water mass with low temperature and high salinity was located at the southwest bottom (Fig. 7a, c, & e; Cao et al., 2019). Affected by the northeast monsoon, the surface water of Beibu Gulf moves southward from nearshore in autumn and winter (Liu et al., 2002). As part of compensation current, the SCS offshore bottom water outside the Gulf invades the inshore area via upwelling (Cao et al., 2019; Gao et al., 2019), which help to explain the high-density water mass located at the southwest bottom of the Gulf originates from the SCS offshore waters (Cao et al., 2019). The high abundance of P. globosa cells was in this high-density water mass and extended along the slope from the southern bottom water layer to the northern surface water in the Qinzhou Bay estuary (Figs. 5b, 7a, 7c, 7e, 7g, & 7i). Previous studies also demonstrated that phytoplankton assemblages at the southern bottom of the Gulf could extend to the coastline of Guangxi Zhuang Autonomous Region in autumn (Zhao et al., 2019). Therefore, the high abundance P. globosa cells in the Qinzhou Bay estuary more likely to originate from the SCS offshore waters outside the Beibu Gulf via upwelling.
There are two possible seed sources of P. globosa cells causing the bloom along the western waters of Leizhou Peninsula. Many studies agree that the cyclonic circulation is strongest in the Beibu Gulf in winter (Gao et al., 2013). Under the control of winter circulation, the seawater derived from Guangdong Province enters the western waters of Leizhou Peninsula via the Qiongzhou Strait (Sun and Huang, 2001). The local phytoplankton assemblies and high nutrient content along the coastal waters of Guangdong Province were found to be imported into the Beibu Gulf via the Qiongzhou Strait (Tang et al., 2003; Wang et al., 2014). This study showed that a high abundance of P. globosa cells was continuously distributed in the Qiongzhou Strait and the western waters of the Leizhou Peninsula resided with the MW-Q featuring low salinity and water density in Nov. 2018 (Figs. 3, 4, 5a, 5b, 7d, & 7f). Further studies have found that P. globosa genotypes have the same diversity in the coastal waters of Guangdong Province and the Beibu Gulf (Zhang et al., 2022), providing helpful evidence for the genetic connectivity of P. globosa in these two areas. Therefore, the P. globosa cells in the Guangdong waters with high frequently of P. globosa blooms in autumn and winter can be transported into the western waters of the Leizhou Peninsula via the Qiongzhou Strait.
The high-abundance region of P. globosa in the western waters of the Leizhou Peninsula extended to the northwestern waters of Hainan Island prior to the boom in Nov. 2018 (Fig. 6a–d). The similar phenomena were observed in the winters of 2012 and 2016 (Xu et al., 2015; Niu et al., 2022). The phenomena may be related to the strong upwelling along the southwestern waters of Hainan Island and the tidal front in the northeastern waters of the Gulf (Tang et al., 2003; Wang et al., 2019b). Previous studies have demonstrated that the nutrients can be transported from the bottom to the surface in the northwest waters of Hainan Island, and the phytoplankton assemblies can be transported from this area to the tidal mixing area in the western waters of the Leizhou Peninsula via the upwelling (Tang et al., 2003; Shen et al., 2018a; Wang et al., 2019b). Thus, it is implied that the southwestward current in the northwest waters of Hainan Island could likely transport P. globosa cells to the western waters of the Leizhou Peninsula in winter.
In summary, the P. globosa blooms in the Beibu Gulf may have different seed sources. The P. globosa cells from the SCS offshore waters outside the Gulf are more likely to be transported to the mouth of Qinzhou Bay via upwelling in winter. The P. globoa cells distributed along the western waters of the Leizhou Peninsula are likely to originate from the coastal waters of Guangdong via the Qiongzhou Gulf, and the northwestern waters of Hainan Island via upwelling.
5 CONCLUSIONHaving quantified the distribution and dynamics of P. globosa bloom using two sensitive genotype-specific qPCR methods in the Beibu Gulf in the winter of 2018, and analyzed the relationships between the two types of P. globosa and environmental factors, we found that the Type Ⅳ cells of P. globosa dominated the bloom; low temperature in winter was conducive to the occurrence of P. globosa blooms; and nitrate was one of the key factors influencing the bloom dynamics. The P. globosa cells triggering intense P. globosa bloom in the Beibu Gulf are likely originated from the mixed water via the Qiongzhou Strait, and the northwestern waters of Hainan Island and the SCS offshore waters outside the Beibu Gulf via upwelling.
6 DATA AVAILABILITY STATEMENTThe datasets analyzed during the current study are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENTWe acknowledge all the researchers who worked on survey cruises for the collection of field samples. We deeply miss the late Professor Weibing GUAN, a co-author of this article.
Electronic supplementary material
Supplementary material (Supplementary Tables S1–S2 & Figs.S1–S3) is available in the online version of this article at https://doi.org/10.1007/s00343-022-1447-0.
Anderson D M, Glibert P M, Burkholder J M. 2002. Harmful algal blooms and eutrophication: nutrient sources, composition, and consequences. Estuaries, 25(4): 704-726.
DOI:10.1007/BF02804901 |
Cao X H, Yu Z M, Qiu L X. 2017. Field experiment and emergent application of modified clays for Phaeocystis globosa blooms mitigation. Oceanologia et Limnologia Sinica, 48(4): 753-759.
(in Chinese with English abstract) |
Cao Z Y, Bao M, Guan W B, et al. 2019. Water-mass evolution and the seasonal change in northeast of the Beibu Gulf, China. Oceanologia et Limnologia Sinica, 50(3): 532-542.
(in Chinese with English abstract) |
Cariou V. 1991. Etude des Mécanismes de Formation des Colonies de Phaeocystis: Influence des Phosphates. University of Pierre et Marie Curie, Paris.
|
Chen B, Xu Z X, Ya H Z, et al. 2019. Impact of the water input from the eastern Qiongzhou Strait to the Beibu Gulf on Guangxi coastal circulation. Acta Oceanologica Sinica, 38(9): 1-11.
DOI:10.1007/s13131-019-1472-2 |
Chen J F, Xu N, Jiang T J, et al. 1999. A report of Phaeocystis globosa bloom in coastal water of southeast China. Journal of Jinan University (Natural Science), 20(3): 124-129.
(in Chinese with English abstract) |
Chen Y, Xu N, Duan S S. 2011. Effects of the ratios of organic nitrogen and light intensity on the growth and photosynthesis of the brown tide Phaeocystis globosa. Ecology and Environmental Sciences, 20(3): 499-504.
(in Chinese with English abstract) |
Egge J K, Heimdal B R. 1994. Blooms of phytoplankton including Emiliania huxleyi (Haptophyta).Effects of nutrient supply in different N: P ratios. Sarsia, 79(4): 333-348.
DOI:10.1080/00364827.1994.10413565 |
Gao J S, Wu G D, Nguyen K C, et al. 2019. Counter-wind deep current in the northern Beibu Gulf in boreal winter. Journal of Ocean University of China, 18(1): 57-68.
DOI:10.1007/s11802-019-3711-2 |
Gao J S, Xue H J, Chai F, et al. 2013. Modeling the circulation in the Gulf of Tonkin, South China Sea. Ocean Dynamics, 63: 979-993.
DOI:10.1007/s10236-013-0636-y |
Glibert P M, Harrison J, Heil C, et al. 2006. Escalating worldwide use of urea-a global change contributing to coastal eutrophication. Biogeochemistry, 77(3): 441-463.
DOI:10.1007/s10533-005-3070-5 |
Glibert P M. 2020. Harmful algae at the complex nexus of eutrophication and climate change. Harmful Algae, 91: 101583.
DOI:10.1016/j.hal.2019.03.001 |
Hai D H, Lam N N, Dippner J W. 2010. Development of Phaeocystis globosa blooms in the upwelling waters of the South central coast of Viet Nam. Journal of Marine Systems, 83(3-4): 253-261.
DOI:10.1016/j.jmarsys.2010.04.015 |
He C, Song S Q, Li C W. 2019. The spatial-temperal distribution of Phaeocystis globosa colonies and related affecting factors in Guangxi Beibu Gulf. Oceanologia et Limnologia Sinica, 50(3): 630-643.
(in Chinese with English abstract) |
He C. 2021. The Spatial-temporal distribution of Phaeocystis globosa colonies and prokaryotic communities and their responses to environmental factors in the Beibu Gulf, China. Institute of Oceanology, Chinese Academy of Sciences, Qingdao. (in Chinese)
|
Hu J Y, Kawamura H, Hong H S, et al. 2000. A review on the currents in the South China Sea: seasonal circulation, South China Sea warm current and Kuroshio intrusion. Journal of Oceanography, 56(6): 607-624.
DOI:10.1023/A:1011117531252 |
Lancelot C, Gypens N, Billen G, et al. 2007. Testing an integrated river-ocean mathematical tool for linking marine eutrophication to land use: the Phaeocystis-dominated Belgian coastal zone (Southern North Sea) over the past 50 years. Journal of Marine Systems, 64(1-4): 216-228.
DOI:10.1016/j.jmarsys.2006.03.010 |
Liang D Y, Wang X D, Wang Y. 2018. Effects of different nitrogen on the growth and the formation of colony in Phaeocystis globosa. Advances in Marine Science, 36(2): 272-278.
(in Chinese with English abstract) DOI:10.3969/j.issn.1671-6647.2018.02.012 |
Liu K K, Chao S Y, Shaw P T, et al. 2002. Monsoon-forced chlorophyll distribution and primary production in the South China Sea: observations and a numerical study. Deep Sea Research Part I: Oceanographic Research Papers, 49(8): 1387-1412.
DOI:10.1016/S0967-0637(02)00035-3 |
Liu L, Li Y, Sun P, et al. 2020. Seasonal changes of phytoplankton community structure and its influencing factors in Qinzhou Bay. Marine Environmental Science, 39(5): 776-784, 790.
(in Chinese with English abstract) |
Madhupratap M, Sawant S, Gauns M. 2000. A first report on a bloom of the marine prymnesiophycean, Phaeocystis globosa from the Arabian Sea. Oceanologica Acta, 23(1): 83-90.
DOI:10.1016/S0399-1784(00)00109-2 |
Medlin L K, Zingone A. 2007. A taxonomic review of the genus Phaeocystis. Biogeochemistry, 83: 3-18.
DOI:10.1007/s10533-007-9087-1 |
Niu Z, Liu C, Zhang Q C, et al. 2022. Development of sensitive genotype-specific quantitative polymerase chain reaction methods for detection of Phaeocystis globosa in the South China Sea. Limnology and Oceanography: Methods, 20(3): 131-145.
DOI:10.1002/lom3.10476 |
Peperzak L, Colijn F, Gieskes W W C, et al. 1998. Development of the diatom- Phaeocystis spring bloom in the Dutch coastal zone of the North Sea: the silicon depletion versus the daily irradiance threshold hypothesis. Journal of Plankton Research, 20(3): 517-537.
DOI:10.1093/plankt/20.3.517 |
Qi Y Z, Chen J F, Wang Z H, et al. 2004. Some observations on harmful algal bloom (HAB) events along the coast of Guangdong, southern China in 1998. Hydrobiologia, 512(1-3): 209-214.
DOI:10.1023/B:HYDR.0000020329.06666.8c |
Qin X L, Lai J X, Chen B, et al. 2016. Molecular identification of Phaeocystis from Beibu Gulf based on 18S rDNA sequences. Journal of Tropical and Subtropical Botany, 24(2): 176-181.
(in Chinese with English abstract) |
Redfield A C, Ketchum B H, Richards F A. 1963. The influence of organisms on the composition of sea-water. In: Hill M N ed. The Sea. Wiley Interscience, New York. p. 26-77.
|
Rousseau V, Leynaert A, Daoud N, et al. 2002. Diatom succession, silicification and silicic acid availability in Belgian coastal waters (Southern North Sea). Marine Ecology Progress Series, 236: 61-73.
DOI:10.3354/meps236061 |
Rousseau V, Mathot S, Lancelot C. 1990. Calculating carbon biomass of Phaeocystis sp.from microscopic observations. Marine Biology, 107(2): 305-314.
DOI:10.1007/BF01319830 |
Rousseau V, Vaulot D, Casotti R, et al. 1994. The life cycle of Phaeocystis (Prymnesiophycaea): evidence and hypotheses. Journal of Marine Systems, 5(1): 23-39.
DOI:10.1016/0924-7963(94)90014-0 |
Schoemann V, Becquevort S, Stefels J, et al. 2005. Phaeocystis blooms in the global ocean and their controlling mechanisms: a review. Journal of Sea Research, 53(1-2): 43-66.
DOI:10.1016/j.seares.2004.01.008 |
Shen C Y, Yan Y R, Zhao H, et al. 2018a. Influence of monsoonal winds on chlorophyll-α distribution in the Beibu Gulf. PLoS One, 13(1): e0191051.
DOI:10.1371/journal.pone.0191051 |
Shen P P, Qi Y Z, Ou L J. 2018b. Phaeocystis globosa in coastal China: taxonomy, distribution, and its blooms. Marine Sciences, 42(10): 146-162.
(in Chinese with English abstract) |
Smith W O Jr, Liu X, Tang K W, et al. 2014. Giantism and its role in the harmful algal bloom species Phaeocystis globosa. Deep Sea Research Part II: Topical Studies in Oceanography, 101: 95-106.
DOI:10.1016/j.dsr2.2012.12.005 |
Song H Y, Chen Y, Gibson K, et al. 2021. High genetic diversity of the harmful algal bloom species Phaeocystis globosa revealed using the molecular marker COX1. Harmful Algae, 107: 102065.
DOI:10.1016/j.hal.2021.102065 |
Song H Y, Liu F, Li Z L, et al. 2020. Development of a high-resolution molecular marker for tracking Phaeocystis globosa genetic diversity through comparative analysis of chloroplast genomes. Harmful Algae, 99: 101911.
DOI:10.1016/j.hal.2020.101911 |
Sun H L, Huang W M. 2001. Three-dimensional numerical simulation for tide and tidal current in the Beibu Gulf. Acta Oceanologica Sinica, 23(2): 1-8.
(in Chinese with English abstract) DOI:10.3321/j.issn:0253-4193.2001.02.001 |
Tang D L, Kawamura H, Lee M A, et al. 2003. Seasonal and spatial distribution of chlorophyll-α concentrations and water conditions in the Gulf of Tonkin, South China Sea. Remote Sensing of Environment, 85(4): 475-483.
DOI:10.1016/S0034-4257(03)00049-X |
Tian J J, Lv S H. 2010. Effects of temperature and salinity on the colony formation of Phaeocystis globosa. Journal of Anhui Agricultural Sciences, 38(18): 9750-9752.
(in Chinese with English abstract) DOI:10.3969/j.issn.0517-6611.2010.18.137 |
Tréguer P J, De La Rocha C L. 2013. The world ocean silica cycle. Annual Review of Marine Science, 5: 477-501.
DOI:10.1146/annurev-marine-121211-172346 |
Wang J X, Kong F Z, Chen Z F, et al. 2019a. Characterization of pigment composition of six strains of Phaeocystis globosa. Oceanologia et Limnologia Sinica, 50(3): 611-620.
(in Chinese with English abstract) |
Wang J X, Kong F Z, Geng H X, et al. 2021a. CHEMTAX analysis of phytoplankton assemblages revealed potential indicators for blooms of haptophyte Phaeocystis globosa. Ecological Indicators, 131: 108177.
DOI:10.1016/j.ecolind.2021.108177 |
Wang Q, Guan W B, Cao Z Y, et al. 2019b. Observations and analysis of tidal front in northeast of Beibu Gulf, South China Sea. Oceanologia et Limnologia Sinica, 50(3): 543-552.
(in Chinese with English abstract) |
Wang X D, Song H Y, Wang Y, et al. 2021b. Research on the biology and ecology of the harmful algal bloom species Phaeocystis globosa in China: progresses in the last 20 years. Harmful Algae, 107: 102057.
DOI:10.1016/j.hal.2021.102057 |
Wang Y B, Zhang W J, Lin Y S, et al. 2014. Phosphorus, nitrogen and chlorophyll-α are significant factors controlling ciliate communities in summer in the northern Beibu Gulf, South China Sea. PLoS One, 9(7): e101121.
DOI:10.1371/journal.pone.0101121 |
Wang Y, Qi Y Z, Li S S. 2007. Nutritional requirements for the growth of Phaeocystis globosa scherffel. Acta Hydrobiologica Sinica, 31(1): 24-29.
(in Chinese with English abstract) DOI:10.3321/j.issn:1000-3207.2007.01.004 |
Winnepenninckx B, Backeljau T, De Wachter R. 1993. Extraction of high molecular weight DNA from molluscs. Trends in Genetics, 9(12): 407.
DOI:10.1016/0168-9525(93)90102-N |
Xu N, Huang B Z, Hu Z X, et al. 2017. Effects of temperature, salinity, and irradiance on the growth of harmful algal bloom species Phaeocystis globosa Scherffel (Prymnesiophyceae) isolated from the South China Sea. Chinese Journal of Oceanology and Limnology, 35(3): 557-565.
DOI:10.1007/s00343-017-5352-x |
Xu N, Qi Y Z, Chen J F, et al. 2003. Analysis on the cause of Phaeocystis globosa Scherffel red tide. Acta Scientiae Circumstantiae, 23(1): 113-118.
(in Chinese with English abstract) DOI:10.3321/j.issn:0253-2468.2003.01.022 |
Xu S N, Lin H J, Gong Y Y, et al. 2015. Ecological characteristics of phytoplankton community structure in northwest Hainan coastal areas. Marine Environmental Science, 34(5): 661-668, 685.
(in Chinese with English abstract) |
Xu Y X, Zhang T, Zhou J. 2019. Historical occurrence of algal blooms in the northern Beibu Gulf of China and implications for future trends. Frontiers in Microbiology, 10: 451.
DOI:10.3389/fmicb.2019.00451 |
Yuan Y Q, Lü X N, Wu Z X, et al. 2019. Temporal and spatial distribution of main environmental factors in typical sea area of the Beibu Gulf and its influencing factors. Oceanologia et Limnologia Sinica, 50(3): 579-589.
(in Chinese with English abstract) |
Zapata M, Jeffrey S W, Wright S W, et al. 2004. Photosynthetic pigments in 37 species (65 strains) of Haptophyta: implications for oceanography and chemotaxonomy. Marine EcologyProgress Series, 270: 83-102.
DOI:10.3354/meps270083 |
Zhang Q C, Liu C, Wang J X, et al. 2022. Intense blooms of Phaeocystis globosa in the South China Sea are caused by a unique "giant-colony" ecotype. Harmful Algae, 114(2022): 102227.
|
Zhang Q C, Niu Z, Wang J X, et al. 2021. Development of high-resolution chloroplast markers for intraspecific phylogeographic studies of Phaeocystis globosa. Journal of Oceanology and Limnology, 39(2): 508-524.
DOI:10.1007/s00343-020-9304-5 |
Zhang S F, Zhang K, Cheng H M, et al. 2020. Comparative transcriptomics reveals colony formation mechanism of a harmful algal bloom species Phaeocystis Globosa. Science of the Total Environment, 719: 137454.
DOI:10.1016/j.scitotenv.2020.137454 |
Zhao Y, Yu R C, Zhang Q C, et al. 2019. Relationship between seasonal variation of pico-and nano-phytoplankton assemblages and Phaeocystis red tides in Beibu Gulf. Oceanologia et Limnologia Sinica, 50(3): 590-600.
(in Chinese with English abstract) |
Zheng A R, Shen H W, Liu J X, et al. 2001. The mechanism of low nutrients-high productivity in Daya Bay. Marine Sciences, 25(11): 48-52.
(in Chinese with English abstract) DOI:10.3969/j.issn.1000-3096.2001.11.014 |
Zhou M J, Shen Z L, Yu R C. 2008. Responses of a coastal phytoplankton community to increased nutrient input from the Changjiang (Yangtze) River. Continental Shelf Research, 28(12): 1483-1489.
DOI:10.1016/j.csr.2007.02.009 |
Zhou M J, Zhu M Y. 2006. Progress of the project "Ecology and oceanography of harmful algal blooms in China". Advances in Earth Science, 21(7): 673-679.
(in Chinese with English abstract) DOI:10.3321/j.issn:1001-8166.2006.07.003 |
 2022, Vol. 40
2022, Vol. 40


