Institute of Oceanology, Chinese Academy of Sciences
Article Information
- TANG Wenjiao, GENG Huixia, XI Yanjuan, ZHANG Qingchun, TANG Xuexi, YU Rencheng
- Mapping the resting cysts of dinoflagellate Alexandrium catenella along the coast of Qinhuangdao, China
- Journal of Oceanology and Limnology, 40(6): 2312-2321
- http://dx.doi.org/10.1007/s00343-022-2190-2
Article History
- Received Apr. 14, 2022
- accepted in principle Apr. 29, 2022
- accepted for publication Jun. 29, 2022
2 CAS Key Laboratory of Marine Ecology and Environmental Sciences, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China;
3 Key Laboratory for Marine Ecology and Environmental Science, Pilot National Laboratory for Marine Science and Technology(Qingdao), Qingdao 266237, China;
4 University of Chinese Academy of Sciences, Beijing 100049, China;
5 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China;
6 Hebei Academy of Ocean and Fishery Science, Qinhuangdao 066200, China
Paralytic shellfish toxins (PSTs), a group of potent neurotoxins, are widely distributed around the world (Asakawa et al., 1995; Lassus et al., 2016). PSTs are mainly produced by marine dinoflagellates, such as Alexandrium spp., Gymnodinium catenatum, and Pyrodinium behamense var. compressum), and freshwater cyanobacteria in the genera Anabaena, Aphanizomenon, Cylindrospermopsis, Lyngbya, Planktothrix, and Scytonema (Anderson et al., 2012a; Christensen and Khan, 2020). In marine environments, PSTs produced by toxic dinoflagellates can accumulate in fish and shellfish at the high trophic levels via marine food web. Consumption of contaminated animals poses a serious threat to human health, and sometimes leads to mortality of victims. About 1 600 paralytic shellfish poisoning (PSP) episodes were recorded prior to the 1970s, mainly in Europe, East Asia, and North America. The distribution of PSP events has expanded gradually around the world since then, and more than 900 poisoning episodes were recorded globally from 1970 to 1984 (U. S. National Office for Harmful Algal Blooms, https://hab.whoi.edu/). It is estimated that about 2 000 PSP incidents occur worldwide every year (Berdalet et al., 2016; Wang et al., 2016; Brown et al., 2020; Yu et al., 2020).
In China, more than 600 suspected PSP cases have been reported so far, and PSTs were detected in many shellfish samples (FAO, 2004; Liu et al., 2016; Liang et al., 2019). In Daya Bay, for example, PST toxicity of the scallops' digestive gland screened by mouse bioassay reached 227 340 μg STX eq./kg in 1999 (Jiang et al., 2000). In another study in the northern Yellow Sea, the maximum PST toxicity of scallops reached 65 140 μg STX eq./kg in 2007 (Xia et al., 2010). Recently, PSP episodes were reported in Fujian province and Hebei province. Toxic dinoflagellate Gymnodinium catenatum has been identified as the causative agent for PSP incidents in Fujian province (Chen et al., 2018). In Hebei province, several PSP episodes were reported in Qinhuangdao, a coastal city located near the Bohai Sea (Ding et al., 2017). The maximum level of PST content in a sample of mussel Mytilus galloprovincialis reached 40 561 μg/kg in the spring of 2016 (Liang et al., 2019). PSTs detected in shellfish samples were mainly composed of carbamate toxins, and A. catenella producing only carbamate toxins was proposed recently as the major causative species of the PSP events in this region (Yu et al., 2021). The follow-up studies found that PST contamination in shellfish collected from Qinhuangdao occurred shortly after the bloom of A. catenella in April (Tang et al., 2022).
Dinoflagellate A. catenella has a complex life cycle and diverse modes of reproduction (Anderson et al., 2012a), and the formation of A. catenella bloom is closely associated with its resting cysts (Ribeiro et al., 2011; Lundholm et al., 2011; Miyazono et al., 2012; Dai et al., 2020). Vegetative cells of A. catenella in seawater mainly reproduce asexually by binary fission. When exposed to sudden environmental changes or other stimuli, the vegetative cells of A. catenella may lose their flagella and form temporary cysts (Anderson et al., 2012b). The temporary cysts can return to the stage of vegetative cells quickly under optimal environmental conditions. More importantly, the vegetative cells of A. catenella can undergo sexual reproduction to form thick-walled, non-motile resting cysts, which significantly improves its adaptability to environmental changes and the capability of population dispersal (Erdner et al., 2010; Genovesi et al., 2015). The resting cysts of A. catenella formed in the water column deposit to the bottom and reserve in sediments. Therefore, the distribution of resting cysts in sediments is generally consistent with the distribution of vegetative cells in seawater (Yamaguchi et al., 1995; Shimada and Miyazono, 2005), and the variation of resting cyst abundance in a sediment core can reflect the long-term changes of A. catenella population in a specific region (He et al., 2008; Anderson et al., 2014; Tang et al., 2016). Moreover, the germination of A. catenella resting cysts releases vegetative cells into seawater, which could proliferate rapidly under optimal environmental conditions to form a bloom (Bravo et al., 2006; Kim et al., 2020). Based on the data of cyst abundance and distribution in surface sediments, numerical models have been successfully developed to predict the blooms of A. catenella (previously named as A. fundyense) in the Gulf of Maine, America (Anderson et al., 2005, 2014; He et al., 2008).
Several strains of A. catenella have been established using the resting cysts isolated from the coastal region of Qinhuangdao (Gu et al., 2013; Yu et al., 2021), but the knowledge on the distribution of A. catenella resting cysts is still quite limited in this region. In previous studies, a sensitive quantitative PCR (qPCR) assay specific for A. catenella has been established and applied successfully to detect vegetative cells and resting cysts of A. catenella in the Bohai Sea and Yellow Sea (Gao et al., 2015; Dai et al., 2020). In this study, the qPCR assay was used to detect A. catenella resting cysts in surface sediments, as well as its vegetative cells in seawater, during the three surveys in 2020 and 2021. The study aims to map the distribution of A. catenella resting cysts along the coast of Qinhuangdao and to explore their relationship with A. catenella blooms. The results would be helpful to understand the mechanisms of A. catenella blooms and to develop monitoring strategies accordingly.
2 MATERIAL AND METHOD 2.1 Sample collectionThree cruises were carried out in June and October 2020, and March 2021. Each cruise covered about 40 sampling sites along 8 sections (Fig. 1). Environmental parameters including temperature and salinity were measured using a self-contained STD profiling system (RBR, Linkocean Technologies Ltd., Canada). Surface sediment samples were collected using a grab sampler (DDC1-2, GuoKe). Approximately 200-g sediment was stored in a freezer at -20 ℃ to detect A. catenella resting cysts and to measure sediment grain size. A total of 111 sediment samples (32, 40, and 39 samples in June and October 2020, and March 2021, respectively) were collected during the three cruises. In June 2020 and March 2021, phytoplankton samples were also collected to determine the abundance and distribution of A. catenella vegetative cells. Surface seawater was collected using a submersible pump at each sampling site, and 1-L seawater was passed through a sieve (mesh size 200 μm) to remove zooplankton. The filtrate was then filtered through a 0.4-μm polycarbonate fiber membrane (HTTP, Millipore, Ireland) to collect phytoplankton. The membranes were stored at -80 ℃ for qPCR assay. A total of 32 phytoplankton samples were collected in June 2020, and 40 samples were collected in March 2021.
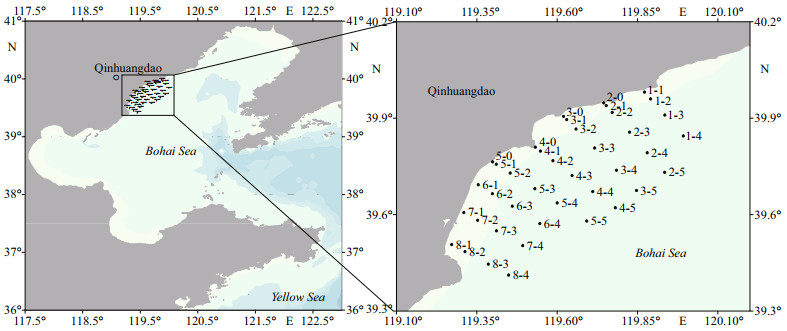
|
| Fig.1 The sampling sites along the coast of Qinhuangdao in the Bohai Sea |
Extraction of the genomic DNA of resting cysts was performed according to the protocol described by Kamikawa et al. (2007) and Dai et al. (2020). Briefly, the frozen sediment samples were thawed at room temperature, and about 5-g sediment was weighed and put into a beaker. The sediment was mixed with seawater filtered through cellulose-ester membranes (pore diameter 0.4 μm), and further treated in an ultrasonic cleaner (Branson, 2510, USA). The mixture was then filtered successively through 120-μm and 20-μm sieves, and the fraction between 20 and 120 μm was collected and centrifuged at 3 000×g for 10 min using a high-speed refrigerated centrifuge (Sigma, 3-16 K, Germany). The precipitate was carefully weighed to extracted DNA using the PowerSoil DNA Isolation Kit (Qiagen, USA), following the instruction manual. The DNA recovered from the silica gel filter membrane was used for qPCR assay.
The genomic DNA of phytoplankton samples was extracted using a cetyltrimethyl ammonium bromide (CTAB) method described by Winnepenninckx et al. (1993). The DNA was dissolved in 30-μL TE buffer and stored at -20 ℃ for qPCR assay.
2.3 qPCR assay for Alexandrium catenellaA qPCR assay developed by Gao et al.(2013, 2015) was used to determine the abundance of A. catenella vegetative cells in seawater and resting cysts in sediments. A Bio-Rad CFX96 TouchTM real-time PCR detection system was used to perform the analysis. The primers were AtI-F (5′-GCTTGGTGGGAGTGTTGCAC-3′) and AtI-R (5′-TAAGTCCAAGGAAGGAAGCATC-3′), and the TaqMan probe was AtI-P (5′-AGAGCTTTGGGCT-GTGGGTGTA-3′). The volume of qPCR reaction mixture was 10 μL, containing 5-μL 2× qPCR buffer (TaKaRa, China), 0.4 μL of 10-nmol/L forward primer, 0.4 μL of 10-nmol/L reverse primer, 0.4 μL of 10-nmol/L probes labeled with Texas Red and BHQ2 at the 5′ and 3′ ends, 2.8 μL of ddH2O, and 1 μL of DNA template. The qPCR reaction started from an initial denaturing at 95 ℃ for 30 s, followed by 40 cycles of denaturing at 95℃ for 5 s and annealing-elongating at 60 ℃ for 30 s.
A strain of A. catenella (MEL91) isolated from the coastal waters of Qinhuangdao was cultured in laboratory (Yu et al., 2021), and the resting cysts were induced by nutrient starve. The cysts were collected using the sodium polytungstate gradient centrifugation method (Bolch, 1997) and picked out under a microscope. The cysts were used to optimize qPCR conditions and to establish a standard calibration curve for the qPCR assay. Vegetative cells of the strain MEL91 were also used to establish the calibration curve to determine A. catenella cells in seawater.
2.4 Sediment analysisSediment samples collected in March 2021 were thawed at room temperature, and approximately 0.2-g sediment were placed into a beaker and mixed with the seawater filtered through cellulose-ester membranes (pore diameter 0.4 μm). After ultrasonic treatment for 1 min in an ultrasonic cleaner, the samples were analyzed using a laser particle size analyzer (1190, Cilas, France). The measurement range of particle size was from 0.04 to 2 500 μm, the accuracy was less than 3%, and the repeatability was less than 1%. Following the protocol of Folk-Walker classification (Folk et al., 1970), the sediment particles were divided into three parts based on the measured grain sizes, namely clay (< 4 μm), silt (4 63 μm), and sand (> 63 μm). Among them, the clay and silt were named collectively as fine-grained particles (< 63 μm).
2.5 Statistical analysisStatistical analyses were performed using IBM SPSS 26.0 (IBM, Armonk, NY, USA), and the correlation between two factors was analyzed using the Pearson's chi-squared test. The correlation was considered significant when P < 0.05 (2-sided significance testing). The difference analysis between two data was analyzed using t-test, and the difference was considered significant when P < 0.05 (2-sided significance testing). The distribution map of A. catenella resting cysts, vegetative cells, and environmental parameters in the study region were prepared using Surfer 13.0 (Golden Software, Inc., USA).
3 RESULT 3.1 Distribution and abundance of Alexandrium catenella resting cysts in surface sedimentsThe abundance of A. catenella resting cysts in surface sediment samples collected in June and October 2020 and March 2021 are illustrated in Fig. 2. In June 2020, the cyst abundance in 32 samples was in a range of ND (not detected) to 1 300 cysts/g sediment (wet weight, (WW)), with the average abundance of 162 cysts/g sediment (WW). In October 2020, the cyst abundance in 40 samples ranged from ND to 424 cysts/g sediment (WW), and with the average abundance of 39 cysts/g sediment (WW). In March 2021, the cyst abundance of 39 samples was in a range of ND to 459 cysts/g sediment (WW) with the average abundance of 59 cysts/g sediment (WW).
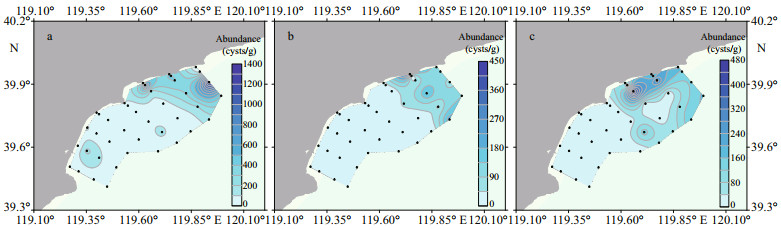
|
| Fig.2 Distribution and abundance of Alexandrium catenella resting cysts in the coastal waters of Qinhuangdao in June (a), October (b) 2020, and March 2021 (c) Color bar means abundance of Alexandrium catenella resting cysts (cysts/g sediment (wet weight)). |
Distribution patterns of A. catenella resting cysts during the three surveys were very similar. Cyst abundance in the northern part of the study region (sections 1 4) was significantly higher than the southern part (sections 5 8) (P < 0.05). In June 2020, the abundance of resting cysts on average in the northern and southern parts of the study region was 238 and 33 cysts/g sediment (WW), respectively. In October 2020, the values decreased to 68 and 4 cysts/g sediment (WW), respectively. The abundance of resting cysts in the two parts was 109 and 0 cysts/g sediment (WW), respectively, in March 2021.
3.2 Distribution and abundance of Alexandrium catenella vegetative cells in seawaterThe abundance of A. catenella vegetative cells in seawater collected in June 2020 and March 2021 was determined by qPCR assay. The abundance was extremely low in the whole survey region in June 2020, and A. catenella cells were only detected at the site 1-1 (7 cells/L) and the site 3-0 (4 cells/L) (Fig. 3a). In March 2021, A. catenella cells were widely detected in the study region, and the abundance ranged from 1 to 206 cells/L, averaged 45 cells/L. The maximum abundance of A. catenella was recorded at the site 2-0 (Fig. 3b). The average abundance of A. catenella cells in the northern part (57 cells/L, sections 1 4) was higher than that in the southern part (31 cells/L, sections 5 8).

|
| Fig.3 Distribution and abundance of Alexandrium catenella vegetative cells in the coastal waters of Qinhuangdao in June 2020 (a) and March 2021 (b) Color bar means abundance of Alexandrium catenella vegetative cells (cells/L). |
Seawater temperature and salinity in the coastal waters of Qinhuangdao were measured in June 2020 and March 2021 (Fig. 4). Seawater temperature was in a range of 18.7 24.2 ℃ in June 2020, and the salinity was in a range of 31.9 32.3. In the southern part of the study region, there was an apparent onshore intrusion of warm seawater with relatively high salinity along the sections 7 & 8. In the central part of the study region, there was a similar coastward intrusion of high salinity tongue along the sections 3 & 4, but the temperature of seawater was much lower. In March 2021, seawater temperature was in a range of 2.4 7.4 ℃, and salinity was in a range of 31.4 32.3. Seawater with relatively high salinity and low temperature occupied the northern part of the study region.
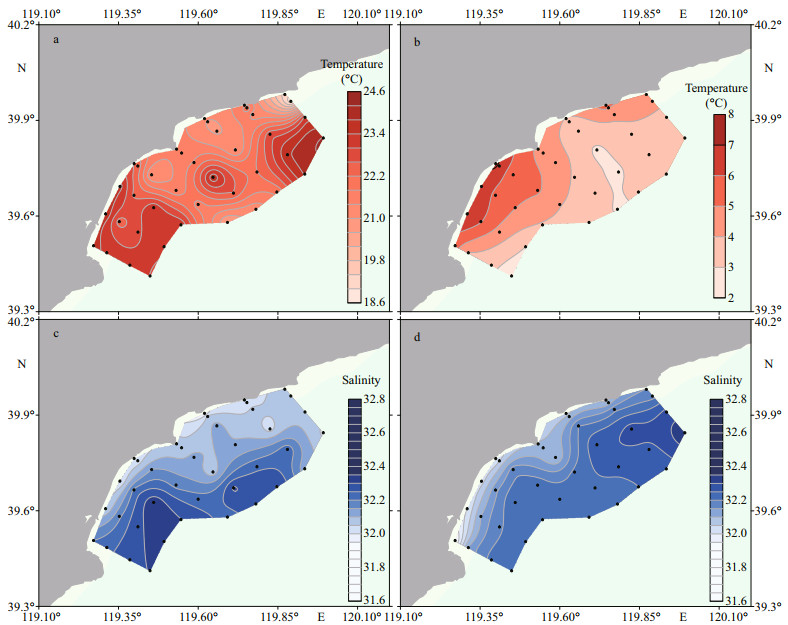
|
| Fig.4 Temperature (a, b) and salinity (c, d) of surface seawater in the coastal waters of Qinhuangdao in June 2020 (a, c) and March 2021 (b, d) |
In the study region, approximately one third (32.5%) of sediment samples were composed of silt and clay (grain size≤63 μm), and the others were mainly composed of sand (grain size > 63 μm) (Fig. 5). Silt and clay were primarily distributed in the northern part of the study region (sections 1-4), and the percentage of fine-grained particles in the northern part was higher than 50%. In contrast, grain size of sediments in the sections 5 8 was much coarser, and the percentage of fine-grained particles was generally less than 40%, except for the sampling sites 5-4 and 8-2.
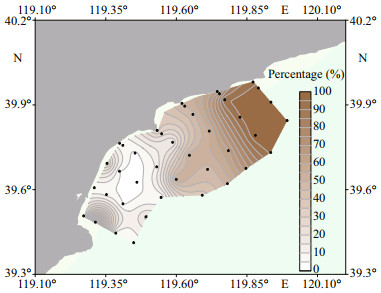
|
| Fig.5 Percentage of fine-grained particles in surface sediments in the coastal waters of Qinhuangdao in March 2021 |
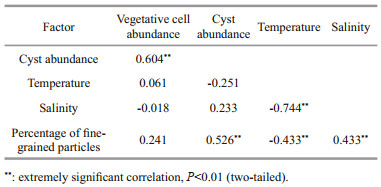
The abundance of A. catenella resting cysts had a significant positive correlation with the percentage of fine-grained particles in both June 2020 and March 2021 (P < 0.05 and P < 0.01) (Tables 1 & 2). Resting cysts of A. catenella were mainly detected at the sites with the percentage of fine-grained sediment above 50%. Almost no A. catenella resting cysts were detected in the region with the percentage of fine-grained particles lower than 50%. The abundance of A. catenella vegetative cells was extremely low in June 2020, and it had a significant negative correlation with temperature (P < 0.05). Prior to the formation of A. catenella bloom in March 2021, the abundance of A. catenella vegetative cells had little correlation with seawater temperature and salinity, but it had an extremely significant positive correlation with resting cyst abundance in surface sediment (P < 0.01) (Table 2).

|
|
In our previous studies, three isolates of A. catenella have been established by germination of resting cysts collected from surface sediment along the coast of Qinhuangdao (Yu et al., 2021). The results could confirm the presence of A. catenella resting cysts in this region, but there is little information available about their distribution characteristics. In this study, the distribution of A. catenella resting cysts along the coast of Qinhuangdao was investigated for the first time using a qPCR assay specific for A. catenella. The resting cysts of A. catenella were detected in surface sediments during all three cruises, suggesting that the resting cysts exist all year round in the coastal waters of Qinhuangdao. High abundance of A. catenella resting cysts were found in the northern part of the study region (119.62°E 119.99°E, 39.67°N 39.98°N), which is northeast to the coastal waters of Qinhuangdao.
Many processes, such as cyst formation and germination, sediment composition, hydrodynamic processes, and animal predation, could affect the distribution of A. catenella resting cysts (Persson, 2000; Anderson et al., 2005; Richlen et al., 2016). Sediment composed of fine-grained particles can provide a better condition for the preservation of resting cysts, while the cysts in sediment composed of sand are easily lost under the external impacts (Anderson et al., 2003). A previous study in the northern Yellow Sea found that there was a strong positive correlation between the abundance of Alexandrium cysts and the percentage of fine-grained particles, while a negative correlation between cyst abundance and sand percentage (Shi et al., 2011). Similarly, the investigations in the Yellow Sea and the Bohai Sea also found strong correlation between the abundance of A. catenella resting cysts and the percentage of fine-grained particles in surface sediments (Dai et al., 2020). In this study, the region with high abundance of A. catenella resting cysts is also featured by a high percentage of fine-grained particles. It can be implied that sediment composition is a critical factor affecting the distribution of resting cysts (Anderson et al., 2005; Shin et al., 2013). Besides, hydrodynamic processes also affect the distribution of resting cysts. The Bohai Sea is strongly affected by tidal currents, but there is an amphidromic point close to Qinhuangdao (Zhang and Yang, 2013; Luo and Liu, 2015). Therefore, the coastal waters of Qinhuangdao could offer a stable environment for the growth of A. catenella vegetative cells, and for the deposition and preservation of its resting cysts formed during the bloom.
4.2 The relationship between Alexandrium catenella resting cysts and its bloomsMany studies demonstrate the important role of resting cysts in the initiation of A. catenella blooms (Kim et al., 2002; Genovesi et al., 2009; Natsuike et al., 2017; Shin et al., 2021). Our study in the coastal waters of Qinhuangdao found that A. catenella bloomed in April 2021 (Tang et al., 2022). Prior to the formation of the bloom, vegetative cells of A. catenella were detected in the water column during the cruise in March 2021. It was found that vegetative cells were mainly distributed in the northern part of the study region, similar to the resting cysts in surface sediment. There is a significant positive correlation between the abundance of vegetative cells and the abundance of resting cysts, while no significant correlation was found between vegetative cells and other environmental parameters. It can be deduced that vegetative cells of A. catenella in seawater prior to the bloom formation were mainly resulted from the germination of resting cysts in surface sediment. The vegetative cells could then grow rapidly under optimal conditions to form the bloom. Therefore, resting cysts preserved in sediments could serve as a seed bank to modulate the dynamics of A. catenella blooms in the coastal waters of Qinhuangdao.
Temperature is a critical factor for the germination of resting cysts and the formation of blooms (Anderson and Morel, 1979; Anderson et al., 1990; Kim et al., 2002; Natsuike et al., 2017). A. catenella is a cold-water species, which forms blooms generally in the temperature range of 5 15 ℃. In the Gulf of Maine, for example, recurrent blooms of A. catenella were found in a temperature range of 10 13 ℃, and the germination of A. catenella cysts mainly occurred in a range of 7 15 ℃ (Tobin et al., 2019). In a study in the coastal waters of South Korea, it was also found that up to 73% of the A. catenella resting cysts germinated in November. The vegetative cells of A. catenella germinated from resting cysts had low cell density in winter, and grew rapidly to form a bloom (about 4×104 cells/L) in mid-April when seawater temperature reached 15 ℃ (Kim et al., 2020). The results in the coastal waters of Qinhuangdao are in accordance with the findings in the Gulf of Maine and the coastal waters of South Korea. From winter to spring, the gradual increase of seawater temperature promotes the germination of A. catenella resting cysts and drives the bloom formation. The studies also demonstrate impacts of A. catenella vegetative cells on the resting cysts in sediment. Among the three cruises, the distribution patterns of resting cysts were similar in the study region, but the abundance of resting cysts in June 2020 was much higher than March 2021 and October 2020. Our study found that the bloom of A. catenella declined in May (Tang et al., 2022), probably due to the inhibition of rising temperature on the growth of A. catenella. Water temperature along the coast of Qinhuangdao exceeds 20 ℃ in summer, beyond the optimal temperature range for the growth of A. catenella (Tobin et al., 2019). The resting cysts formed at the late stage of bloom deposit to the bottom and lead to the relatively higher abundance of resting cysts in June. In the Gulf of Maine, it was also found that remaining cells after the bloom of A. catenella formed resting cysts, which deposited to sediment and contributed to the seed bed of resting cysts (Anderson, 1998).
5 CONCLUSIONIn this study, three surveys were carried out in the coastal waters of Qinhuangdao to map the distribution of A. catenella resting cysts in surface sediments and to explore their relationships with A. catenella blooms. The distribution pattern of A. catenella cysts along the coast of Qinhuangdao was revealed for the first time using a species-specific qPCR assay. A region with high abundance of resting cysts (119.62°E 119.99°E, 39.67°N 39.98°N) was identified northeast to the coastal waters of Qinhuangdao, where surface sediments were mainly composed of fine-grained particles (percentage above 50%). Prior to the formation of the A. catenella bloom in March 2021, the abundance of A. catenella vegetative cells in seawater had a significant correlation with the abundance of resting cysts in surface sediments, reflecting the important role of resting cysts in the initiation of A. catenella blooms. The results will offer a sound basis for the future monitoring and mitigation of toxic A. catenella blooms and PSP events in this region.
6 DATA AVAILABILITY STATEMENTThe datasets analyzed during the current study are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENTWe acknowledge all the researchers who worked on survey cruises for the collection of field samples, and Professors Jiabo ZHANG and Gang WANG from Marine Geological Resources Survey Center of Hebei Province for providing seawater temperature and salinity data.
Anderson D M, Alpermann T J, Cembella A D, et al. 2012a. The globally distributed genus Alexandrium: multifaceted roles in marine ecosystems and impacts on human health. Harmful Algae, 14: 10-35.
DOI:10.1016/j.hal.2011.10.012 |
Anderson D M, Cembella A D, Hallegraeff G M. 2012b. Progress in understanding Harmful Algal Blooms: paradigm shifts and new technologies for research, monitoring, and management. Annual Review of Marine Science, 4: 143-176.
DOI:10.1146/annurev-marine-120308-081121 |
Anderson D M, Fukuyo Y, Matsuoka K. 2003. Cyst methodologies. Manual on Harmful Marine Microalgae: 165-189.
|
Anderson D M, Keafer B A, Kleindinst J L, et al. 2014. Alexandrium fundyense cysts in the Gulf of Maine: long-term time series of abundance and distribution, and linkages to past and future blooms. Deep Sea Research Part II: Topical Studies in Oceanography, 103: 6-26.
DOI:10.1016/j.dsr2.2013.10.002 |
Anderson D M, Kulis D M, Sullivan J J, et al. 1990. Dynamics and physiology of saxitoxin production by the dinoflagellates Alexandrium spp. Marine Biology, 104(3): 511-524.
DOI:10.1007/BF01314358 |
Anderson D M, Morel F M M. 1979. The seeding of two red tide blooms by the germination of benthic Gonyaulax tamarensis hypnocysts. Estuarine and Coastal Marine Science, 8(3): 279-293.
DOI:10.1016/0302-3524(79)90098-7 |
Anderson D M, Stock C A, Keafer B A, et al. 2005. Alexandrium fundyense cyst dynamics in the Gulf of Maine. Deep Sea Research Part II: Topical Studies in Oceanography, 52(19-21): 2522-2542.
DOI:10.1016/j.dsr2.2005.06.014 |
Anderson D M. 1998. Physiology and bloom dynamics of toxic Alexandrium species, with emphasis on life cycle transitions. Nato ASI Series G: Ecological Sciences, 41: 29-48.
|
Asakawa M, Miyazawa K, Takayama H, et al. 1995. Dinoflagellate Alexandrium tamarense as the source of paralytic shellfish poison (PSP) contained in bivalves from Hiroshima Bay, Hiroshima Prefecture, Japan. Toxicon, 33(5): 691-697.
DOI:10.1016/0041-0101(94)00177-A |
Berdalet E, Fleming L E, Gowen R, et al. 2016. Marine Harmful Algal Blooms, human health and wellbeing: challenges and opportunities in the 21st century. Journal of the Marine Biological Association of the United Kingdom, 96(1): 61-91.
DOI:10.1017/S0025315415001733 |
Bolch C J S. 1997. The use of sodium polytungstate for the separation and concentration of living dinoflagellate cysts from marine sediments. Phycologia, 36(6): 472-478.
DOI:10.2216/i0031-8884-36-6-472.1 |
Bravo I, Garcés E, Diogène J, et al. 2006. Resting cysts of the toxigenic dinoflagellate genus Alexandrium in recent sediments from the Western Mediterranean coast, including the first description of cysts of A of A. kutnerae and A. peruvianum. European Journal of Phycology, 41(3): 293-302.
DOI:10.1080/09670260600810360 |
Brown A R, Lilley M, Shutler J, et al. 2020. Assessing risks and mitigating impacts of harmful algal blooms on mariculture and marine fisheries. Reviews in Aquaculture, 12(3): 1663-1688.
DOI:10.1111/raq.12403 |
Chen H R. 2018. Emergency treatment and reflection of red tide event of Gymnodinium catenatum in Fujian sea area in 2017. Journal of Fisheries Research, 40(4): 308-314.
(in Chinese with English abstract) DOI:10.14012/j.cnki.fjsc.2018.04.008 |
Christensen V G, Khan E. 2020. Freshwater neurotoxins and concerns for human, animal, and ecosystem health: a review of anatoxin-a and saxitoxin. Science of the Total Environment, 736: 139515.
DOI:10.1016/j.scitotenv.2020.139515 |
Dai L, Yu R C, Geng H X, et al. 2020. Resting cysts of Alexandrium catenella and A.pacificum (Dinophyceae) in the Bohai and Yellow Seas, China: abundance, distribution and implications for toxic algal blooms. Harmful Algae, 93: 101794.
DOI:10.1016/j.hal.2020.101794 |
Ding L, Qiu J B, Li A F. 2017. Proposed biotransformation pathways for new metabolites of paralytic shellfish toxins based on field and experimental mussel samples. Journal of Agricultural and Food Chemistry, 65(27): 5494-5502.
DOI:10.1021/acs.jafc.7b02101 |
Erdner D L, Percy L, Keafer B, et al. 2010. A quantitative realtime PCR assay for the identification and enumeration of Alexandrium cysts in marine sediments. Deep Sea Research Part II: Topical Studies in Oceanography, 57(3-4): 279-287.
DOI:10.1016/j.dsr2.2009.09.006 |
FAO (Food and Agriculture Organization of the United Nations). 2004. Marine Biotoxins. FAO, Rome. p. 5-52.
|
Folk R L, Andrews P B, Lewis D W. 1970. Detrital sedimentary rock classification and nomenclature for use in New Zealand. New Zealand Journal of Geology and Geophysics, 13(4): 937-968.
DOI:10.1080/00288306.1970.10418211 |
Gao Y, Yu R C, Chen J H, et al. 2015. Distribution of Alexandrium fundyense and A pacificum (Dinophyceae) in the Yellow Sea and Bohai Sea. Marine Pollution Bulletin, 96(1-2): 210-219.
DOI:10.1016/j.marpolbul.2015.05.025 |
Gao Y, Yu R C, Zhang Q C, et al. 2013. Application of qPCR method in detection of Alexandrium tamarense species complex in China. Acta Scientiae Circumstantiae, 33(8): 2256-2263.
(in Chinese with English abstract) |
Genovesi B, Berrebi P, Nagai S, et al. 2015. Geographic structure evidenced in the toxic dinoflagellate Alexandrium pacificum Litaker (A.catenella – group IV (Whedon & Kofoid) Balech) along Japanese and Chinese coastal waters. Marine Pollution Bulletin, 98(1-2): 95-105.
DOI:10.1016/j.marpolbul.2015.07.009 |
Genovesi B, Laabir M, Masseret E, et al. 2009. Dormancy and germination features in resting cysts of Alexandrium tamarense species complex (Dinophyceae) can facilitate bloom formation in a shallow lagoon (Thau, southern France). Journal of Plankton Research, 31(10): 1209-1224.
DOI:10.1093/plankt/fbp066 |
Gu H F, Zeng N, Liu T T, et al. 2013. Morphology, toxicity, and phylogeny of Alexandrium (Dinophyceae) species along the coast of China. Harmful Algae, 27: 68-81.
DOI:10.1016/j.hal.2013.05.008 |
He R Y, McGillicuddy Jr D J, Keafer B A, et al. 2008. Historic 2005 toxic bloom of Alexandrium fundyense in the western Gulf of Maine: 2. Coupled biophysical numerical modeling. Journal of Geophysical Research, 113(C7): C07040.
DOI:10.1029/2007JC004602 |
Jiang T J, Yin Y W, Huang W J, et al. 2000. The characteristics of paralytic shellfish toxins in shellfish cultured in Daya Wan, Shenzhen. Journal of Jinan University (Natural Science & Medicine Edition), 21(5): 65-69.
(in Chinese with English abstract) DOI:10.3969/j.issn.1000-9965.2000.05.013 |
Kamikawa R, Nagai S, Hosoi-Tanabe S, et al. 2007. Application of real-time PCR assay for detection and quantification of Alexandrium tamarense and Alexandrium catenella cysts from marine sediments. Harmful Algae, 6(3): 413-420.
DOI:10.1016/j.hal.2006.12.004 |
Kim Y O, Choi J, Baek S H, et al. 2020. Tracking Alexandrium catenella from seed-bed to bloom on the southern coast of Korea. Harmful Algae, 99: 101922.
DOI:10.1016/j.hal.2020.101922 |
Kim Y O, Park M H, Han M S. 2002. Role of cyst germination in the bloom initiation of Alexandrium tamarense (Dinophyceae) in Masan Bay, Korea. Aquatic Microbial Ecology, 29: 279-286.
DOI:10.3354/ame029279 |
Lassus P, Chomérat N, Hess P, et al. 2016. Toxic and Harmful Microalgae of the World Ocean. International Society for the Study of Harmful Algae/Intergovernmental Oceanographic Commission of UNESCO, Denmark, p: 68.
|
Liang Y B, Li D M, Yao J Y, et al. 2019. Progresses in investigation and research on phycotoxins and toxic microalgaes in the coastal waters of China. Oceanologia et Limnologia Sinica, 50(3): 511-524.
(in Chinese with English abstract) |
Liu R Y, Liu L, Liang Y B, et al. 2016. The distribution, impacts and risks of toxic microalgae and phycotoxins in China. Marine Environmental Science, 35(5): 787-800.
(in Chinese with English abstract) DOI:10.13634/j.cnki.mes.2016.05.025 |
Lundholm N, Ribeiro S, Andersen T J, et al. 2011. Buried alive – germination of up to a century-old marine protist resting stages. Phycologia, 50(6): 629-640.
DOI:10.2216/11-16.1 |
Luo D, Liu H. 2015. Numerical study on the tides and tidal currents in the bohai sea. Journal of Shanghai Ocean University, 24(3): 457-464.
(in Chinese with English abstract) |
Miyazono A, Nagai S, Kudo I, et al. 2012. Viability of Alexandrium tamarense cysts in the sediment of Funka Bay, Hokkaido, Japan: over a hundred year survival times for cysts. Harmful Algae, 16: 81-88.
DOI:10.1016/j.hal.2012.02.001 |
Natsuike M, Yokoyama K, Nishitani G, et al. 2017. Germination fluctuation of toxic Alexandrium fundyense and A. pacificum cysts and the relationship with bloom occurrences in Kesennuma Bay, Japan. Harmful Algae, 62: 52-59.
DOI:10.1016/j.hal.2016.11.018 |
Persson A. 2000. Possible predation of cysts—a gap in the knowledge of dinoflagellate ecology?. Journal of Plankton Research, 22(4): 803-809.
DOI:10.1093/plankt/22.4.803 |
Ribeiro S, Berge T, Lundholm N, et al. 2011. Phytoplankton growth after a century of dormancy illuminates past resilience to catastrophic darkness. Nature Communications, 2: 311.
DOI:10.1038/ncomms1314 |
Richlen M L, Zielinski O, Holinde L, et al. 2016. Distribution of Alexandrium fundyense (Dinophyceae) cysts in Greenland and Iceland, with an emphasis on viability and growth in the Arctic. Marine Ecology Progress Series, 547: 33-46.
DOI:10.3354/meps11660 |
Shi Y J, Liu D Y, Shao H B, et al. 2011. Distribution of dinoflagellate cysts in the surface sediments from the northern Yellow Sea, China. Marine Science Bulletin, 30(3): 320-327.
(in Chinese with English abstract) DOI:10.3969/j.issn.1001-6392.2011.03.014 |
Shimada H, Miyazono A. 2005. Horizontal distribution of toxic Alexandrium spp. (Dinophyceae) resting cysts around Hokkaido, Japan. Plankton Biology and Ecology, 52(2): 76-84.
|
Shin H H, Li Z, Kim H J, et al. 2021. Alexandrium catenella (Group I) and A.pacificum (Group IV) cyst germination, distribution, and toxicity in Jinhae-Masan Bay, Korea. Harmful Algae, 110: 102122.
DOI:10.1016/j.hal.2021.102122 |
Shin H H, Lim D, Park S Y, et al. 2013. Distribution of dinoflagellate cysts in Yellow Sea sediments. Acta Oceanologica Sinica, 32(9): 91-98.
DOI:10.1007/s13131-013-0356-7 |
Tang W J, Lin Z R, Zhang Q C, et al. 2022. An investigation on bloom dynamics of Alexandrium catenella and A. pacificum and toxin accumulation in shellfish along the coast of Qinhuangdao, China. Marine Pollution Bulletin, 183: 114058.
DOI:10.1016/j.marpolbul.2022.114058 |
Tang Y Z, Hu Z X, Deng Y Y. 2016. Characteristical life history (resting cyst) provides a mechanism for recurrence and geographic expansion of harmful algal blooms of dinoflagellates: a review. Studia Marina Sinica, (51): 132-154.
(in Chinese with English abstract) DOI:10.12036/hykxjk20160730001 |
Tobin E D, Wallace C L, Crumpton C, et al. 2019. Environmental drivers of paralytic shellfish toxin producing Alexandrium catenella blooms in a fjord system of northern Southeast Alaska. Harmful Algae, 88: 101659.
DOI:10.1016/j.hal.2019.101659 |
Wang D Z, Zhang S F, Zhang Y, et al. 2016. Paralytic shellfish toxin biosynthesis in cyanobacteria and dinoflagellates: a molecular overview. Journal of Proteomics, 135: 132-140.
DOI:10.1016/j.jprot.2015.08.008 |
Winnepenninckx B, Backeljau T, De Wachter R. 1993. Extraction of high molecular weight DNA from molluscs. Trends in Genetics, 9(12): 407.
DOI:10.1016/0168-9525(93)90102-N |
Xia Y Z, Wang S S, Xin Q Y, et al. 2010. Pollution survey of paralytic shellfish poison (PSP) from aquaculture zones of Changhai's sea area in Dalian. Food & Machinery, 26(2): 54-56.
(in Chinese with English abstract) DOI:10.13652/j.issn.1003-5788.2010.02.025 |
Yamaguchi M, Itakura S, Imai I, et al. 1995. A rapid and precise technique for enumeration of resting cysts of Alexandrium spp. (Dinophyceae) in natural sediments. Phycologia, 34(3): 207-214.
DOI:10.2216/i0031-8884-34-3-207.1 |
Yu R C, Lü S H, Qi Y Z, et al. 2020. Progress and perspectives of harmful algal bloom studies in China. Oceanologia et Limnologia Sinica, 51(4): 768-788.
(in Chinese with English abstract) DOI:10.11693/hyhz20200400127 |
Yu R C, Zhang Q C, Liu Y, et al. 2021. The dinoflagellate Alexandrium catenella producing only carbamate toxins may account for the seafood poisonings in Qinhuangdao, China. Harmful Algae, 103: 101980.
DOI:10.1016/j.hal.2021.101980 |
Zhang C, Yang T J. 2013. Distribution and variation law of mean tidal range in eastern coastal China. Port & Waterway Engineering, (1): 60-65.
(in Chinese with English abstract) DOI:10.16233/j.cnki.issn1002-4972.2013.01.001 |
 2022, Vol. 40
2022, Vol. 40


