Institute of Oceanology, Chinese Academy of Sciences
Article Information
- TIAN Xiaoqing, FAN Chengqi, TANG Yunyu, ZHANG Haiyan, KANG Wei, CHEN Sha, LI Chongbin, LU Ya'nan
- A two-year (2020–2021) observation of marine phycotoxins in phytoplankton in typical mariculture areas of East China Sea
- Journal of Oceanology and Limnology, 40(6): 2256-2266
- http://dx.doi.org/10.1007/s00343-022-2297-5
Article History
- Received Aug. 15, 2022
- accepted in principle Sep. 13, 2022
- accepted for publication Oct. 19, 2022
2 Shanghai Ocean University, Shanghai 201306, China
Marine phycotoxins (MPTs), mainly produced by harmful marine microalgae, frequently accumulate in bivalves which make great losses in aquaculture and cause serious safety issues to human health (Grienke et al., 2014; Liang et al., 2022). East China Sea (ECS) is one of the most important aquaculture areas in China. An increasing number of harmful algal blooms (HABs) events have been recorded during the last two decades in the coastal waters of ECS. From 2002 to 2017, the cumulative size affected by HABs in ECS was 103 776 km2; from 2011 to 2017, there were 212 HABs in ECS; Zhejiang coastal area showed the most frequent HABs and was the most affected area by HABs (Zhang et al., 2020). On 20th century, the ominant microalgae species of HABs in ECS were diatoms. However, during the past 20 years of the 21th century, dinoflagellates, including Prorocentrum donghaiense, Karenia mikimotoi, and Alexandrium spp., have become the majority species of large-scale HABs (Zhou et al., 2008, 2022; Yu et al., 2017, 2018). For example, in the spring of 2005, a red tide caused by dinoflagellates was recorded which the size was more than 10 000 km2 and the economic loss was over 30 million RMB in the Changjiang (Yangtze) River estuary and adjacent waters; in 2012, a red tide caused by K. mikimotoi in Fujian Province resulted in at least 2 billion RMB losses (Yu et al., 2017). Pessimistically, researches showed that changes in nutrient composition of the Changjiang River discharge and global climate warming may make dinoflagellate HABs in ECS more frequently in future (Wang et al., 2021; Zhou et al., 2022).
Comprehensive and effective monitoring and early warning is an important method to prevent and control the contamination of MPTs in the mariculture areas. In China, research on MPTs in phytoplankton or seawater was mainly concentrated in the bays of the Yellow Sea and the Bohai Sea, while research on the East China Sea (ECS) was relative rare (Chen et al., 2019; Liang et al., 2022). In Changjiang River estuary and adjacent sea areas, according to a study of phytoplankton from the end of March to the end of May in 2011, N -sulfocarbamoyl toxins C1 and C2 were the dominated toxins accounting for 94% of the total toxins (Chen, 2013). A research about paralytic shellfish toxins (PSTs) in phytoplankton showed that May and June were the high-risk period of PSTs contamination (Liu et al., 2020). Another correlation analysis from results of three surveys in spring-autumn season during 2017–2018 showed that the lipophilic marine toxins (LMTs) levels in seawater were positively correlated with dissolved oxygen and salinity, but negatively correlated with temperature and nutrients (He et al., 2019). In Nanji Island, middle coast of ECS, Qu et al. (2016) studied LMTs in the mussels along coast of Nanji Island with solid phase adsorption toxin tracking (SPATT) technology and 7 types of LMTs were detected. In three locations of the south coast of ECS, Xiamen, Jinjiang, and Dongshan, results showed a quite low detection level (0.03–0.2 ng/L) of LMTs (Zhang et al., 2018).
To learn the status and regular pattern of MPTs contamination in the East China Sea, the net-concentrated phytoplankton samples from three typical mariculture areas in ECS were collected in two consecutive years (2020–2021), and the toxins were detected by high performance liquid chromatography-mass spectrometry (HPLC-MS) method. The research results will be helpful in learning knowledge of the outbreaks characteristics of marine microalgae toxins in ECS. Moreover, it will also be beneficial to protect marine ecological environment and ensure the safety of aquaculture seafood.
2 MATERIAL AND METHOD 2.1 Reagent and materialThirteen PSTs certified reference standards (saxitoxin (STX), decarbamoyl saxitoxin (dcSTX), neosaxitoxin (NEO), decarbamoyl neosaxitoxin (dcNEO), gonyatoxins 1–5 (GTX1–5), decarbamoyl gonyatoxins 2–3 (dcGTX2–3), N-sulfocarbamoyl toxins C1–2 (C1–2)), 10 LMTs certified reference standards (okadaic acid (OA), dinophysistoxins 1–2 (DTX1–2), pectenotoxin 2 (PTX2), yessotoxin (YTX), 13-desmethyl spirolide C (SPX1), gymnodimine (GYM) and azaspiracids 1–3 (AZA1–3)) and domoic acid (DA) were purchased from the National Research Council (NRC) of Canada. Formic acid (> 98%), ammonium acetate (> 97%), acetonitrile, and methanol (absolute, hypergrade) were purchased from J. T. Bake (USA). Deionized water was produced by a Milli-Q water purification system (Millipore, Billerica, MA, USA). Solid phase extraction (SPE) column Strata-X (60 mg3/mL) was purchased from Phenomenex (USA). Columns for HPLC were TSK-Amide-80 (3 μm, 2 mm×150 mm, TSKgel, Japan) and Kinetex XB-C18 (2.6 μm, 2.1 mm×100 mm, Phenomenex, America), respectively. GF/C filter membrane (Φ47 mm) was from Sangon Biotech (China). Temperature, salinity, chlorophyll a (Chl a), pH, and dissolved oxygen (DO) were measured with U-50 water quality monitor (HORIBA, Japan).
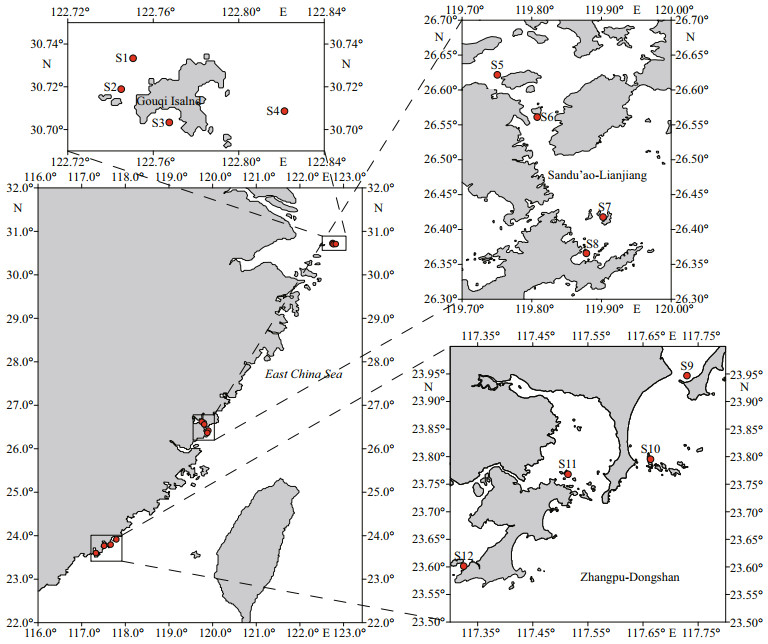
2.2 Sampling stations and proceduresTwelve stations were selected in three typical coastal aquaculture areas (4 stations for each) covering the East China Sea (Fig. 1). Phytoplankton samples were collected in May, August, November of 2020, and March, May, August, October of 2021. From each station, 200-L surface seawater was pumped and filtered through 20-μm mesh in length of 100 cm, then rinsed with seawater and diluted to 1 L for phytoplankton collection. An amount of 300-mL concentrated liquid was filtered with GF/C filter membrane (Φ47 mm) and the membranes were frozen at -20 ℃.
|
| Fig.1 Sampling locations in three typical mariculture areas of East China Sea |
Frozen membranes with phytoplankton cells mentioned in Section 2.2 were cut into small pieces and put in the centrifuge tubes. For PSTs detection, the tubes were ultrasonically extracted for 5 min with 5-mL 1% acetic acid solution in ice bath, 2-mL extractions was centrifuged for 10 min at 10 000 r/min and filtered through 0.22-μm filter, then kept at -80 ℃ for further analysis. For LMTs and DA detection, tubes were ultrasonically extracted for 5 min with 5-mL methanol in ice bath, then were centrifuged and filtered as PSTs procedures and kept in the same conditions before analysis.
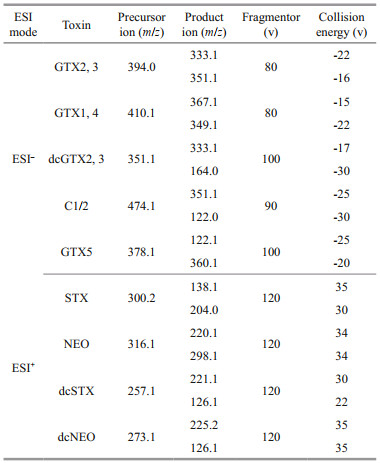
2.4 Liquid chromatography tandem mass spectrometry (LC-MS/MS) analysis 2.4.1 Detection of Extraction of PSTsFor common analogues of PSTs, multiple reaction monitoring (MRM) was used. The Shimadzu DGU-20A5R HPLC (Shimadzu Corporation, Japan) was coupled to the Sciex Qtrap 5500 tandem quadrupole mass spectrometer (Danaher Corporation, USA) with an electrospray ionization interface. The chromatographic separation was performed on a TSK-gel Amide-80® HILIC column (150 mm×2 mm i.d., 3 μm) (Tosoh Bioscience LLC, Montgomeryville, PA, USA) using a flow rate of 1.2 mL/min at 40 ℃. The binary mobile phase were water (solvent A) and 95% acetonitrile (solvent B), each of them containing 2-mmol/L ammonium formate and 50-mmol/L formic acid. The gradient ran from 90% B to 80% B over 3.6 min, decreased to 60% B over an additional 2.4 min, held for 1.5 min at 60% B, increased to 90% B over an additional 1 min, held for 1.5 min at 90% B before re-equilibration for the next run. High resolution mass spectrometry conditions included spray voltage of 4.5 kV and -4.5 kV for positive and negative ion, respectively, curtain gas (CUR) pressure of 20 psi, ion source gas1 (GS1) and gas2 (GS2) pressure of 30 psi, ion source temperature of 550 ℃ and collision gas (CAD) of medium. Common analogues were scanned using the selective reaction monitoring (SRM) transitions shown in Table 1.
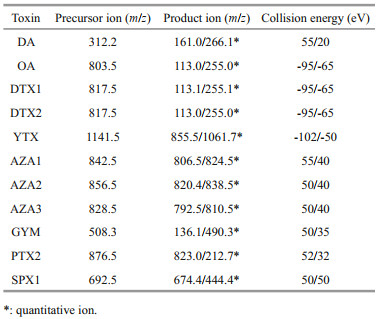
Toxins were analyzed by LC-MS/MS system composed of a Thermo Scientific HPLC system and a Thermo TSQ Quantum Ultra triple-quadrupole mass spectrometer with electrospray ionization (ESI) source (Thermo Fisher Scientific, Waltham, MA USA). The chromatographic separation was performed on a KinetexXB-C-18 column (2.6 μm, 2.1 mm×100 mm, Phenomenex, America) at flow rate of 1.2 mL/min at 40 ℃. The binary mobile phases were water (solvent A) and 95% acetonitrile (solvent B), both mixed with 2-mmol/L ammonium formate and 50-mmol/L formic acid. From start to 7.00 min, the gradient shifted from 20% B to 90% B, and was kept at this proportion for 3 min, then recovered to 20% B in 1/10 min and was maintained for 2 min for re-equilibration for the next run.
The ESI source was set on the positive and negative ion mode with 3 500-V spray voltage and 350-℃ capillary temperature. The sheath gas and auxiliary gas flow rates were 30 and 10 psi, respectively. The MS parameters of retention time, optimized ion transitions, and collision energies are listed in Table 2.
Concentration of toxins in phytoplankton samples was calculated with Eq.1.
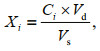 (1)
(1)in which Xi: toxin concentration in phytoplankton sample i, unit: ng/L, i means the certain sample in 80 samples, i=1, 2, ∙∙∙, 80; Ci: the corresponding toxin concentration of sample i shown on the standard curve, unit: μg/mL; Vd: dilution volume, unit: mL; Vs: volume of filtered seawater, unit: L.
3 RESULT 3.1 Toxin detection rate and diversityToxins were detected in 24 samples from 9 stations, which accounted for 30.0% of the total 80 samples and 75.0% of total 12 stations (Fig. 2 and Supplementary Fig.S1). The majority of samples were found only one type of toxin except for 2 samples for having 2 different toxins (8.3% of total samples with toxins). Stations 1 and 7 showed the highest toxin detection rate (57.1%) among all stations. Stations 5, 9, and 12 showed no toxins in the two years. In three mariculture areas, Gouqi Island showed the highest toxin detection rate (39.3%), in which toxins were detected from 11 samples of the total 28 samples. Meanwhile, the samples with 2 toxins were all in Gouqi Island area. Toxin detection rate in Sandu'ao-Lianjiang area was 28.6%. Although the toxin detection rate in Zhangpu-Dongshan area was the lowest at 20.8%, it did not present that this area was better than the other two because there was a lack of data for August in 2021.
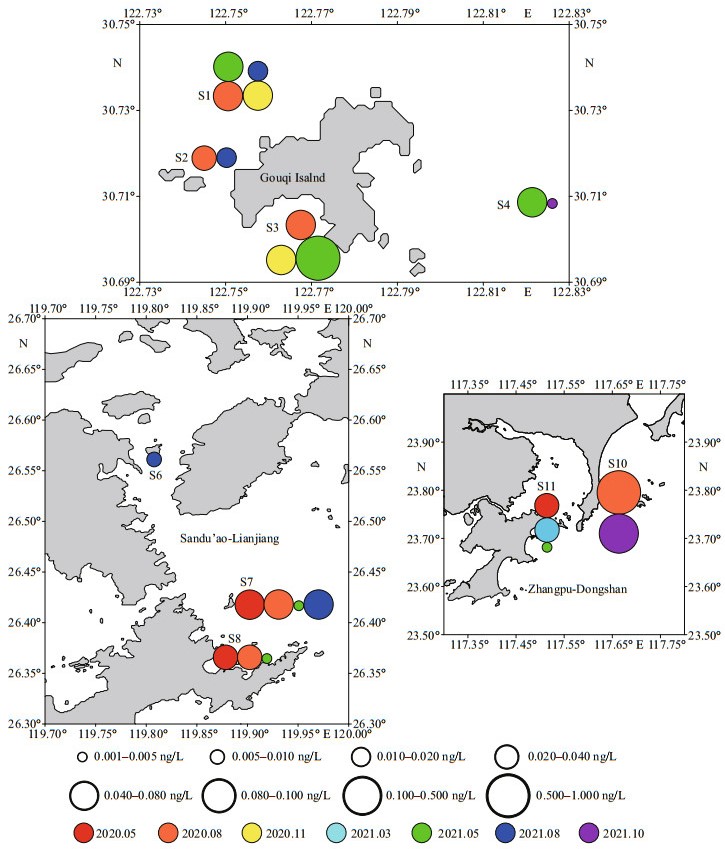
|
| Fig.2 Distribution of MPTs in three mariculture areas of ECS |
Six types of toxins were detected, including 2 PSTs (C2 and dcGTX3) and 4 LMTs (PTX2, SPX1, GYM, and DA). The toxin with the highest detection rate was PTX2, which was detected in 20 samples (25.0%). The highest concentration was 0.117 ng/L at S10 station in October 2021. The other three LMTs were only detected once, respectively, and toxin detection rate was all 0.01%. Regarding to PSTs, C2 was detected twice while dcGTX3 was only once.
The toxin diversity in Gouqi Island areas was the highest where 2 PSTs and 3 LMTs were detected. In Sandu'ao-Lianjiang area, only PTX2 was detected. In Zhangpu-Dongshan area, except for PTX2, DA had been detected from sample of S10 in August 2020 with a high concentration of 0.991 ng/L.
Although there was less data, it showed trends that the types of toxins were different according to seasons in different areas. In Gouqi Island area, PSP toxins only occurred in spring-summer season, while LMTs were detected mainly in summer and autumn-winter (October and November). In Sandu'ao-Lianjiang area, toxins were mainly detected in May and August. In Zhangpu-Dongshan area, because the data of August 2021 was lost, it was not easy to summarize the trend.
3.2 Trends of PTX2In this research, only PTX2 was found in all three mariculture areas. The concentration in 2021 was higher than that in 2020 (Fig. 3). However, the highest concentration was detected in S10 in October 2021 with 0.117 ng/L, which was almost double than the second highest concentration which detected in S3 in November 2020 (0.062 ng/L).

|
| Fig.3 Distribution trend of PTX2 in mariculture areas of ECS |
The detection of PTX2 presented seasonal trend in all areas. In Gouqi Island area, although PTX2 was detected only one time in spring with a low concentration (S3, May 2021, 0.013 ng/L), it was mainly found in August and November. In 2020, the PTX2 concentrations of samples from S1 and S3 were higher than that from S2. In 2021, the concentrations were almost the same. PTX2 had never been detected in samples from S4. The possible reason might be that, compared with other three stations, S4 was located in the open sea.
Pectenotoxin 2 was the only toxin detected from the 28 samples in Sandu'ao-Lianjiang area and showed obvious seasonal trend, which was detected only in May and August. Among them, S7 and S8 showed worse status than the other two stations. In 2020, the concentrations of samples from S7 in both May (0.051 ng/L) and August (0.050 ng/L) were obviously higher than those from S8 (0.031 and 0.028 ng/L, respectively). In 2021, the concentrations in May were all quite low (S7 with 0.002 ng/L and S8 with 0.003 ng/L), but in August, the concentration of S7 increased rapidly to 0.042 ng/L, while the toxin disappeared in S8. PTX2 was found only once with low concentration at 0.006 ng/L at S6 in August 2021, while it was never found at S5.
In Zhangpu-Dongshan area, PTX2 was not found at S9 and S12. At S11, PTX2 was first detected from samples collected in March with a concentration at 0.026 ng/L, and then the concentration went down to 0.003 ng/L in May. The highest concentration (0.117 ng/L) was recorded at S10 in October 2021, and because the data of August in the same year was missing, it was difficult to know whether it happened coincidentally.
4 DISCUSSION 4.1 Contamination risks of PSTs in mariculture areas in ECSParalytic shellfish toxin contamination is common in gastropods and bivalves of ECS, and showed the trends that the toxicity of samples collected from offshore waters was generally higher than that of samples from inshore, as well as that PSTs concentration was typically the highest in spring of all bivalves (Liang et al., 2022). Liu et al. (2020) found that April to June was the peak season of PSTs in Zhoushan Islands, where C1 and C2 showed the highest concentration in this period. Meanwhile, low concentration of GTXs, dcGTX, and NEO were also detected in the same period. In our research, the detection of high concentration of C2 (highest at 1.409 ng/L) and low concentration of dcGTX3 in Gouqi Island (part of Zhoushan Islands) in May showed the same tendency as Liu's research.
Dinoflagellate of genus Alexandrium was the common microalgae that could produce PSTs in coastal areas of China. From 2002 to 2017, 24 Alexandrium blooms were reported in China, among which 14 occurred in the East China Sea, and most of them were complex of Alexandrium spp. and Prorocentrum donghaiens (Liang et al., 2019). A research about the diversity of Alexandrium in China showed that Atama complex Group IV (A. pacificum) was the major reason corresponding to PSTs producing in ECS area, and C1and C2 were predominant in almost all strains (Gu et al., 2013; Zou et al., 2014). In the north ECS area, spring was a season with more dinoflagellate blooms (Liu et al., 2020). Therefore, in mariculture area of the north ECS, Alexandrium microalgae blooms and PSTs contamination monitoring should be enhanced.
4.2 Pectenotoxins contamination risks and suggested countermeasuresPectenotoxin 2 (PTX2) was the main toxin that could be detected in ECS mariculture areas. In Gouqi Island, the north of ECS, PTX2 was found in samples from August and November; In Sandu'ao-Lianjiang, the middle coast of ECS, PTX2 was found in samples of May and August. In Zhangpu-Dongshan, the south coast of ECS, PTX2 was found first time in March. In general, the outbreak time of PTX2 showed a trend of gradually advancing from north to south, which may be attributed to that the seawater temperature was higher gradually from the north to the south.
PTXs, a type of marine toxin with polyether lactone structures produced from Dinophysis microalgae, was first found from Japanese cultured scallop (Patinopecten yessoensis) (Liu and Liang, 2010; Grienke et al., 2014). Now, over 20 PTXs have been found and identified from shellfish and algae all over the world (Liu and Liang, 2010; Paredes et al., 2011; Díaz et al., 2020). Some studies showed that only four PTXs, PTX2, PTX11, PTX12, and PTX13, originated from algae Dinophysis, while the others were metabolic compounds in shellfish or derivative compounds during chemical extraction (Dominguez et al., 2010). In China, Dinophysis species distributed widely along coast (Gu et al., 2022), D. rotundata and D. fortii were reported in Dalian and Qingdao in the Yellow Sea (Luo, 2011; Gao et al., 2017) and Hongkong in South China Sea (Gu et al., 2022); D. caudata was recorded in Hainan Province of the South China Sea (Chen and Ni, 1988) and East China Sea (ECS) (Li et al., 2015).
In microalgae samples from Qingdao (Luo, 2011) and Qinzhou Bay in the South China Sea (Xu et al., 2021), PTX2 was detected. In the East China Sea, Li et al. (2015) collected and identified D. caudata in Gouqi Island seawater in July and September 2013, and found PTX2 in them. In our research, PTX2 was found in phytoplankton samples in August, which was the same season as Li's research. It might show that the summer-autumn (August and September) was the incident time for PTX2 toxin in Gouqi Island. However, Dinophysis genus cells were not found in our research. One of the possible reasons was that the abundance of Dinophysis cells was not high enough to be collected. The other possible reason was that the cells were attached on the inner side of trawl net and could not to be washed into the sample bottle. In future research, the phytoplankton collecting time should be prolonged and the net should be rinsed for more times to ensure the sample number of microalgae.
Pectenotoxins were firstly considered as family of diarrheal shellfish poisons (DSP), because they were often detected with OA. However, later studies showed that PTXs did not induce diarrhea (Nicolas et al., 2017). Although PTXs was found to cause gastrointestinal disorders, for example, the middle-lower intestine eroding and gastric organs injury (Ito et al., 2008), more adverse effects were shown as liver, spleen, and kidneys injuries (Terao et al., 1986; Yoon and Kim, 1997; Ito et al., 2008). Moreover, some researches showed that PTX2 could inhibit the actin (Hori et al., 1999; Allingham et al., 2007). Butler et al. (2012) found that PTX2 caused a dose-dependent decrease in both rate and yield of skeletal muscle actin polymerization, but its analog, PTX2 seco acid, did not cause the same effect, suggesting that the intact lactone ring was necessary for bioactivity. Some research reported the in vitro hepatic biotransformation path in Wistar rats of PTX2, and found that PTX2 transferred rapidly to two major and several minor oxidized metabolites (Sandvik et al., 2020). It may explain why PTX2 seldom induces serious oral toxicity.
In view of the possible hazards of PTXs to human health, the European parliament promulgated the regulatory limits of PTXs (Regulation (EC) No. 853/2004) in 2004. The limit of the total amount of OA, DTXs, and PTXs in the edible bivalve mollusks was no more than 160-μg/kg OA equivalents (Li et al., 2009; Chen et al., 2014). However, there has been no limit about PTXs in China yet. In previous toxin monitoring and analysis in phytoplankton from the Bohai Sea to the Changjiang River estuary, it was found that PTXs were common in these areas and PTX2 was the main structure of PTXs (Liu, 2017). In fact, there have been many reports on PTX2 in shellfish in China. Guo et al. (2012) found it in Chlamys ferreri, Argopectens irradias, and Crassostrea gigas in culture farms of Lingshan, Qingdao; Deng (2017) reported PTX2 in shells from retail markets in Qingdao. In the north Yellow Sea, PTX2 was identified from cultured scallop Chlamys ferreri (Chen, 2013) and it was considered as one of the major toxins in that area (Wu et al., 2018). In the East China Sea, PTX2 was found in oyster Crassostrea gigas samples in September 2012, April, May and June 2013, and mussel Mytilus galloprovincialis samples in September 2012, May and June 2013, in Gouqi Island area (Li et al., 2012, 2015). A study in 2008 showed that PTX2 was the most frequently detected toxin in coastal shellfish in China, with a detection rate of 44% and the highest value of 53.2 μg/kg (Liu et al., 2014).
In our research, it seems that risks of PTXs pollution in the East China Sea mariculture areas are possible in specific seasons. The cultured shellfish in China are in great risk of contamination by PTXs. It is recommended that PTXs detecting standards in seafood should be formulated as soon as possible. Meanwhile, the monitoring of algae Dinophysis in China coastal aquaculture area is not complete or systematic. The knowledge about the main toxic-producing species of Dinophysis, toxin types and the outbreak pattern of Dinophysis red tide is still insufficient. To understand the regulation of PTXs outbreaks and the cultured shellfish situation of contamination by PTXs along China coastline, regular monitoring should be conducted on the Dinophysis species, toxin-producing characteristics, and the PTXs detection of cultured shellfish in future. It will provide a theoretical basis for finding prevention and control countermeasures of PTXs contamination.
4.3 Cyclic imine toxins and DA contamination risksGymnodimine and 13-desmethyl spirolide C were chemical characterized by a macrocycle and two conserved features that include the cyclic imine group and spiroketal ring system. In our research, these two cyclic imine toxins, GYM and SPX1 were only detected in very low concentration both at 0.001 ng/L. However, reports showed that they had been detected in shellfish for many times in China (Liang et al., 2022). Liu et al. (2014) had found high concentration (4.2 μg/kg) of SPX1 in oyster Alectryonella plicatula from the middle coast of ECS. Li et al. (2015) detected GYM in three species of shellfish from ECS and found that GYM could be detected almost in samples of all seasons, and the highest levels occurred in winter. The highest concentration of GYM in ECS was recorded at 36.09 μg/kg in Batillaria zonalis, while GYM and/or SPX1 were detected from different species of bivalves and gastropods collected ECS in spring 2016 (Ji et al., 2018). From previous study, Gouqi Island was a high-risk place of GYM in ECS, where the detection rate in shellfish samples was high to 32.3% (Li et al., 2015). In research of LMTs in surface water of Changjiang River and adjacent sea area, GYM was detected in 9 stations of total 25 stations with a detection rate at 36.0% (He et al., 2019). In our research, although GYM was only detected in very low concentration in a sample from Gouqi Island in October 2021, it still suggested the risk of cyclic imine toxins in autumn and winter season in the north ECS.
Gymnodimine was first isolated from dinoflagellate of genus Karenia (formerly Gymnodinium) in 1995 (Seki et al., 1995), then it was found in several different species of the same genus, e.g., K. mokimotoi, K. brevisulcata, K. selliformis (Mountfort et al., 2006; Tan et al., 2013; Li et al., 2018). Although K. selliformis had not been recorded in Chinese water area (Liang et al., 2022), K. mokimotoi was confirmed as the dominant species of HABs in ECS. Early back to 1986, K. mokimotoi was discovered in the south of ECS (Yu et al., 2017). In 2012, even 12 HABs caused by K. mokimotoi were recorded with a whole size of 627.7 km2 and economic losses up to 2 billion RMB (Chen et al., 2015). Toxin SPX1 could be produced by Alexandrium ostenfeldii (Tillmann et al., 2014), a species of toxic dinoflagellate found in Chinese water (Gu, 2011; Liang et al., 2022). Although the same microalgae has not been found in ECS area till now, the former reports about HABs of uncertain species, Alexandrium spp. (Yu et al., 2017), indicated the possible threat of A. ostenfeldii. To avoid contamination risks of these two kinds of toxins, not only the seafood should be detected on schedule, but also the toxic dinoflagellates should be monitored in special mariculture area of the north ECS, especially in summer and autumn-winter.
Domoic acid is a kind of toxin which mainly produced by genus diatoms Pseudo-nitzschia (Yu et al., 2020). Although there were 24 potentially toxic Pseudo-nitzschia species that had been recorded in China (Li et al., 2017), it was found that this genus of diatoms was mostly distributed in the South China Sea (SCS) of China (Yu et al., 2020). DA was not often found in culture seafood (Liang et al., 2022). In ECS, DA had ever been detected in shellfish from Zhejiang with low concentration (Song et al., 2008; Wang, 2011). Wang et al. (2019) had found DA in seawater from ECS by using a disk-based SPE technology. In our research, high concentration DA (0.991 ng/L) was detected in samples from the south ECS, indicating that DA pollution risk could not be ignored in the south of ECS.
5 CONCLUSIONEighty net-concentrated phytoplankton samples were collected from 12 stations in three ECS mariculture areas and 6 types of toxins were detected in 24 samples from 9 stations. LMTs were more common and diverse in these areas. PTX2 was the main LMT in the concentrated phytoplankton samples and the occurrence showed seasonal differences from north to south. According to the potential risks of PTXs to seafood safety, it is suggested to execute regular monitoring on PTXs in ECS and a mandatory standard should be formulated based on the comprehensive analysis of in-situ monitoring and lab research. Meanwhile, contamination risks of cyclic imine toxins in the north and DA in the south of ECS should be taken into consideration, too.
There were only 2 PSTs, C2, and dcGTX3, found from spring samples in the north of ECS, which suggested that PSTs was the main risk in spring in this area.
Gouqi Island showed higher toxin diversity in this research and the toxin detection rate was higher than the other two areas. PSTs were the serious potential threats in spring, and LMTs instead of PSTs became the main risk in summer-autumn season. As the biggest mussel culture county of China, the MPTs pollution in this area should be paid high attention to.
To ensure the safety of seafood and marine environmental health, it is recommended to have long-term targeted tracking and monitoring of MPTs in ECS mariculture areas.
6 DATA AVAILABILITY STATEMENTThe datasets generated and/or analyzed during the current study are not publicly available due to the requirement of the project but are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENTMany thanks to Professor Lin SUN of Xiamen University and two postgraduates in her research group, Anqi ZHANG and Honghan LIU, for sample collecting and environmental analysis.
Electronic supplementary material
Supplementary material (Supplementary Fig.S1) is available in the online version of this article at https://doi.org/10.1007/s00343-022-2297-5.
Allingham S J, Miles C O, Rayment I. 2007. A structural basis for regulation of actin polymerization by pectenotoxins. Journal of Molecular Biology, 371(4): 959-970.
DOI:10.1016/j.jmb.2007.05.056 |
Butler S C, Miles C O, Karim A, et al. 2012. Inhibitory effects of pectenotoxins from marine algae on the polymerization of various actin isoforms. Toxicology in Vitro, 26(3): 493-499.
DOI:10.1016/j.tiv.2011.12.015 |
Chen B H, Xie E Y, Gao Y H, et al. 2015. Toxic effects of red tide caused by Karenia mikimotoi on marine organisms. Journal of Fujian Fisheries, 37(3): 241-250.
(in Chinese with English abstract) |
Chen G W, Ni S D. 1988. Taxonomic studies on three genera of Dinophysiaceae in South China Sea. Oceanologia et Limnologia Sinica, 19(3): 238-248.
(in Chinese with English abstract) |
Chen J H. 2013. Composition, Distribution of Phycotoxins and Contamination status of Shellfish in Two Representative Agriculture Zones of Shellfish in China. Institute of Oceanology, Chinese Academy of Sciences, Qingdao.p: 47-92.
(in Chinese with English abstract) |
Chen J H, Wu D N, He X P, et al. 2019. The research advances in detection technology and distribution characteristics of algae toxins in marine water environment. Advances in Marine Science, 37(3): 355-373.
(in Chinese with English abstract) DOI:10.3969/j.issn.1671-6647.2019.03.001 |
Chen J H, Yu R C, Kong F Z, et al. 2014. Detection of lipophilic phycotoxin in Patinopecten yessoensis in the northern Yellow Sea. Oceanologia et Limnologia Sinica, 45(4): 855-863.
(in Chinese with English abstract) DOI:10.11693/hyhz20130400016 |
Deng S. 2017. Surveillance and Risk Assessments of Marine Biotoxins in the Qingdao Market. Qingdao University, Qingdao. p: 26-33.
(in Chinese with English abstract) |
Díaz P A, Álvarez G, Seguel M, et al. 2020. First detection of pectenotoxin-2 in shellfish associated with an intense spring bloom of Dinophysis acuminata on the central Chilean coast. Marine Pollution Bulletin, 158: 111414.
DOI:10.1016/j.marpolbul.2020.111414 |
Dominguez H, Paz B, Daranas A, et al. 2010. Dinoflagellate polyether within the yessotoxin, pectenotoxin and okadaic acid toxin groups: Characterization, analysis and human health implications. Toxicon, 56: 191-217.
DOI:10.1016/j.toxicon.2009.11.005 |
Gao H, An X L, Liu L, et al. 2017. Characterization of Dinophysis acuminata from the Yellow Sea, China, and its response to different temperatures and Mesodinium prey. Oceanological and Hydrobiological Studies, 46(4): 439-450.
DOI:10.1515/ohs-2017-0043 |
Grienke U, Silke J, Tasdemir D. 2014. Bioactive compounds from marine mussels and their effects on human health. Food Chemistry, 142: 48-60.
DOI:10.1016/j.foodchem.2013.07.027 |
Gu H F. 2011. Morphology, phylogenetic position, and ecophysiology of Alexandrium ostenfeldii (Dinophyceae)from the Bohai Sea, China. Journal of systematics and Evolution, 49(6): 606-616.
DOI:10.1111/j.1759-6831.2011.00160.x |
Gu H F, Zeng N, Liu T T, et al. 2013. Morphology, toxicity, and phylogeny of Alexandrium (Dinophyceae) species along the coast of China. Harmful Algae, 27: 68-81.
DOI:10.1016/j.hal.2013.05.008 |
Gu H F, Wu Y R, Lü S H, et al. 2022. Emerging harmful algal bloom species over the last four decades in China. Harmful Algae, 111: 102059.
DOI:10.1016/j.hal.2021.102059 |
Guo M M, Tan Z J, Wu H Y, et al. 2012. Simultaneous determination of okadaic acid, dinophysistoxin, pectenotoxin and yessotoxin in shellfish by liquid chromatography-tandem mass spectrometry. Chinese Journal of Chromatography, 30(3): 256-261.
(in Chinese with English abstract) DOI:10.3724/SP.J.1123.2011.11032 |
He X P, Chen J H, Wu D N, et al. 2019. Distribution characteristics and environmental control factors of lipophilic marine algal toxins in Changjiang estuary and the adjacent East China Sea. Toxins, 11(10): 596.
DOI:10.3390/toxins11100596 |
Hori M, Matsuura Y, Yoshimoto R, et al. 1999. Actin depolymerizing action by marine toxin, Pectenotoxin-2. Folia Pharmacologica Japonica, 114(S1): 225-229.
DOI:10.1254/fpj.114.supplement_225 |
Ito E, Suzuki T, Oshima Y, et al. 2008. Studies of diarrhetic activity on pectenotoxin-6 in the mouse and rat. Toxicon, 51(4): 707-716.
DOI:10.1016/j.toxicon.2007.12.006 |
Ji Y, Hu Y, Song J L, et al. 2018. Characteristics of components and regional distribution of lipophilic shellfish toxins in bivalves cultured along the Chinese Coast in spring. Chinese Fishery Quality and Standards, 8(4): 15-24.
(in Chinese with English abstract) DOI:10.3969/j.issn.2095-1833.2018.04.003 |
Li A F, Ma J G, Cao J J, et al. 2012. Toxins in mussels (Mytilus galloprovincialis) associated with diarrhetic shellfish poisoning episodes in China. Toxicon, 60(3): 420-425.
DOI:10.1016/j.toxicon.2012.04.339 |
Li A F, Sun G, Qiu J B, et al. 2015. Lipophilic shellfish toxins in Dinophysis caudata picked cells and in shellfish from the East China Sea. Environmental Science and Pollution Research, 22(4): 3116-3126.
DOI:10.1007/s11356-014-3595-z |
Li S, Cen J Y, Wang J Y, et al. 2018. Study on acute toxicity of two Karenia (Dinophyceae)Species to rotifer Brachinous plicatilis. Marine Environmental Science, 37(1): 28-32, 69.
(in Chinese with English abstract) |
Li Y, Huang C X, Xu G S, et al. 2017. Pseudo-nitzschia simulans sp. nov. (Bacillariophyceae), the first domoic acid producer from Chinese waters.. Harmful Algae, 67: 119-130.
DOI:10.1016/j.hal.2017.06.008 |
Li Z X, Guo M M, Yang S G, et al. 2009. A review on the study of pectenotoxins. Progress in Fishery Sciences, 30(4): 131-141.
(in Chinese with English abstract) DOI:10.3969/j.issn.1000-7075.2009.04.020 |
Liang Y B, Li A F, Chen J H, et al. 2022. Progress on the investigation and monitoring of marine phycotoxins in China. Harmful Algae, 111: 102152.
DOI:10.1016/j.hal.2021.102152 |
Liang Y B, Li D M, Yao J Y, et al. 2019. Progresses in investigation and research on phycotoxins and toxic microalgaes in the coastal waters of China. Oceanologia et Limnologia Sinica, 50(3): 511-524.
(in Chinese with English abstract) DOI:10.11693/hyhz20181000233 |
Liu R Y, Liang Y B. 2010. Advances in pectenotoxins studies: a review. Acta Ecologica Sinica, 30(19): 5355-5370.
(in Chinese with English abstract) |
Liu R Y, Liang Y B, Liu L, et al. 2014. The lipophilic phycotoxins profile and distribution in bivalve shellfish of Chinese coasts by high performance liquid chromatography coupled with mass spectrometry. Ecology and Environmental Sciences, 23(8): 1320-1326.
(in Chinese with English abstract) DOI:10.16258/j.cnki.1674-5906.2014.08.010 |
Liu Y. 2017. Studies of Phycotoxin Contamination in the Coastal Waters of China and Preparation of Toxin Reference Materials. Institute of Oceanology, Chinese Academy of Sciences, Qingdao. p: 55-158.
(in Chinese with English abstract) |
Liu Y, Dai L, Chen Z F, et al. 2020. Spatiotemporal variation of paralytic shellfish toxins in the sea area adjacent to the Changjiang River estuary. Environmental Pollution, 259: 113730.
DOI:10.1016/j.envpol.2019.113730 |
Luo X. 2011. Population Dynamics and Toxin Production of Dinophysis Species in the Coastal Waters of Qingdao. Institute of Oceanology, Chinese Academy of Sciences, Qingdao. p: 44-133.
(in Chinese with English abstract) |
Mountfort D, Beuzenberg V, MacKenzie L, et al. 2006. Enhancement of growth and gymnodimine production by the marine dinoflagellate, Karenia selliformis. Harmful Algae, 5(6): 658-664.
DOI:10.1016/j.hal.2006.02.001 |
Nicolas J, Hoogenboom R L A P, Hendriksen P J M, et al. 2017. Marine biotoxins and associated outbreaks following seafood consumption: prevention and surveillance in the 21st century. Global Food Security, 15: 11-21.
DOI:10.1016/j.gfs.2017.03.002 |
Paredes I, Rietjens I M C M, Vieites J M, et al. 2011. Update of risk assessments of main marine biotoxins in the European Union. Toxicon, 58(4): 336-354.
DOI:10.1016/j.toxicon.2011.07.001 |
Qu P P, Yang J J, Xu Y X, et al. 2016. Application of SPATT for toxin detection in the mussels along coast of Nanji Island, East China Sea. Oceanologia et Limnologia Sinica, 47(4): 795-803.
(in Chinese with English abstract) DOI:10.11693/hyhz20160100007 |
Sandvik M, Miles C O, Wilkins A L, et al. 2020. In vitro hepatic biotransformation of the algal toxin pectenotoxin-2. Toxicon: X, 6: 100031.
DOI:10.1016/j.toxcx.2020.100031 |
Seki T, Satake M, Mackenzie L, et al. 1995. Gymnodimine, a new marine toxin of unprecedented structure isolated from New Zealand oysters and the dinoflagellate, Gymnodinium sp. Tetrahedron Letters, 36(39): 7093-7096.
DOI:10.1016/0040-4039(95)01434-J |
Song L L, Zhang H Q, Hou J D, et al. 2008. High-performance liquid chromatography-tandem mass spectrometry for the determination of residue of domoic acid in shellfish. Journal of Fisheries of China, 32(6): 950-956.
(in Chinese with English abstract) DOI:10.3321/j.issn:1000-0615.2008.06.018 |
Suzuki T, Mitsuya T. 2001. Comparison of dinophysistoxin-1 and esterified dinophysistoxin-1 (dinophysistoxin-3) contents in the scallop Patinopecten yessoensis and the mussel Mytilus galloprovincialis. Toxicon, 39(6): 905-908.
DOI:10.1016/S0041-0101(00)00205-1 |
Tan Z J, Wu H Y, Guo M M, et al. 2013. Progresses in risk assessment and detection method of lipophilic phycotoxins. Journal of Fishery Sciences of China, 20(2): 467-479.
(in Chinese with English abstract) DOI:10.3724/SP.J.1118.2013.00467 |
Terao K, Ito E, Yasumoto T. 1986. Histopathological studies on experimental marine toxin poisoning I. Ultrastructural changes in the small intestine and liver of suckling mice induced by dinophysistoxin-1 and pectenotoxin-1. Toxicon, 24(11-12): 1141-1151.
DOI:10.1016/0041-0101(86)90140-6 |
Tillmann U, Kremp A, Tahvanainen P, et al. 2014. Characterization of spirolide producing Alexandrium ostenfeldii (Dinophyceae) from the western Arctic. Harmful Algae, 39: 259-270.
DOI:10.1016/j.hal.2014.08.008 |
Wang H. 2011. An investigation of domoic acid content in shellfish in Zhoushan Islands. Chinese Journal of Health Laboratory Technology, 21(12): 2986-2988, 2992.
(in Chinese with English abstract) |
Wang J M, Chen J H, He X P, et al. 2019. Separation and enrichment of domoic acid in seawater using disk-based solid phase extraction. Chinese Journal of Analysis Chemistry, 47(7): 1075-1081.
(in Chinese with English abstract) |
Wang J J, Bouwman A F, Liu X C, et al. 2021. Harmful algal blooms in Chinese coastal waters will persist due to perturbed nutrient ratios. Environmental Science & Technology Letters, 8(3): 276-284.
DOI:10.1021/acs.estlett.1c00012 |
Wang J M, Chen J H, He X P, et al. 2019. Separation and enrichment of domoic acid in seawater using disk-based solid phase extraction. Chinese Journal of Analytical Chemistry, 47(7): 1075-1081.
(in Chinese with English abstract) DOI:10.19756/j.issn.0253-3820.181761 |
Wu H Y, Luan Q S, Guo M M, et al. 2018. Phycotoxins in scallops (Patinopecten yessoensis) in relation to source, composition and temporal variation of phytoplankton and cysts in North Yellow Sea, China. Marine Pollution Bulletin, 135: 1198-1204.
DOI:10.1016/j.marpolbul.2018.08.045 |
Xu Y X, Wei G L, Wang Y, et al. 2021. Pollution of lipophilic shellfish toxins in Qinzhou Bay: seawater and Crassostrea hongkongensis. Oceanologia et Limnologia Sinica, 52(1): 144-152.
(in Chinese with English abstract) DOI:10.11693/hyhz20200400126 |
Yoon M Y, Kim Y C. 1997. Acute toxicity of pectenotoxin 2 and its effects on hepatic metabolizing enzyme system in mice. Toxicological Research, 13(3): 183-186.
|
Yu R C, Lü S H, Liang Y B. 2018. Harmful algal blooms in the coastal waters of China.In: Glibert P M, Berdalet E, Burford M A et al eds.. Global Ecology and Oceanography of Harmful Algal Blooms. Springer, Cham. p: 309-316.
DOI:10.1007/978-3-319-70069-4_15 |
Yu R C, Lü S H, Qi Y Z, et al. 2020. Progress and perspectives of harmful algal bloom studies in China. Oceanologia et Limnologia Sinica, 51(4): 768-788.
(in Chinese with English abstract) DOI:10.11693/hyhz20200400127 |
Yu R C, Zhang Q C, Kong F Z, et al. 2017. Status, impacts and long-term changes of harmful algal blooms in the sea area adjacent to the Changjiang River estuary. Oceanologia et Limnologia Sinica, 48(6): 1178-1186.
(in Chinese with English abstract) |
Zhang S F, Wang Q, Guan C Y, et al. 2020. Study on the occurrence law of red tide and its influencing factors in the offshore waters of China from 2001 to 2017. Acta Scientiarum Naturalium Universitatis Pekinensis, 56(6): 1129-1140.
(in Chinese with English abstract) DOI:10.13209/j.0479-8023.2020.110 |
Zhang Y P, Chen D W, Hong Z, et al. 2018. Polymeric ion exchange material based dispersive micro solid-phase extraction of lipophilic marine toxins in seawater followed by the Q Exactive mass spectrometer analysis using a scheduled high resolution parallel reaction monitoring. Microchemical Journal, 138: 526-532.
DOI:10.1016/j.microc.2018.02.005 |
Zhou M J, Shen Z L, Yu R C. 2008. Responses of a coastal phytoplankton community to increased nutrient input from the Changjiang (Yangtze) River. Continental Shelf Research, 28(12): 1483-1489.
DOI:10.1016/j.csr.2007.02.009 |
Zhou Z X, Yu R C, Zhou M J. 2022. Evolution of harmful algal blooms in the East China Sea under eutrophication and warming scenarios. Water Research, 221: 118807.
DOI:10.1016/j.watres.2022.118807 |
Zou C, Ye R M, Zheng J W, et al. 2014. Molecular phylogeny and PSP toxin profile of the Alexandrium tamarense species complex along the coast of China. Marine Pollution Bulletin, 89(1-2): 209-219.
DOI:10.1016/j.marpolbul.2014.09.056 |
 2022, Vol. 40
2022, Vol. 40