Institute of Oceanology, Chinese Academy of Sciences
Article Information
- ZHENG Guanchao, WU Haiyan, GUO Mengmeng, PENG Jixing, ZHAI Yuxiu, TAN Zhijun
- First observation of domoic acid and its isomers in shellfish samples from Shandong Province, China
- Journal of Oceanology and Limnology, 40(6): 2231-2241
- http://dx.doi.org/10.1007/s00343-022-2104-3
Article History
- Received Mar. 18, 2022
- accepted in principle May 25, 2022
- accepted for publication Jun. 6, 2022
2 Pilot National Laboratory for Marine Science and Technology(Qingdao), Qingdao 266237, China;
3 Collaborative Innovation Center of Seafood Deep Processing, Dalian Polytechnic University, Dalian 116034, China
With rapid economic development of China in the past 30 years, water eutrophication has become a serious issue, with frequent occurrence of harmful algal bloom (HAB) (Lu et al., 2014). At least 60 HAB events have been annually recorded in China (Li et al., 2017). Pseudo-nitzschia spp. are pennate chain-forming diatoms that are widely distributed in coastal waters around the world, and a number of these species have been reported to cause HAB (Bates et al., 2018). Domoic acid (DA), a HAB toxin produced by Pseudo-nitzschia spp., has attracted global attention owing to its frequent cause of amnesic shellfish poisoning (ASP) (Gibble et al., 2021). The first ASP incident was recorded in 1987 in Prince Edward Island, Canada, with at least 4 deaths and 143 hospitalizations (Bates et al., 1989). DA can accumulate in some marine animals such as shellfish, and can cause potential threat to seafood safety and human health through the food chain. To protect consumers from symptomatic acute exposure to DA, Canada first established a 20 000-μg/kg safety limit standard for DA in shellfish after the first ASP event, which has been adopted by other countries, such as European Union, USA, and New Zealand (Lefebvre and Robertson, 2010). In addition, some isomers have been found in shellfish in recent years (Regueiro et al., 2011; Ben Haddouch et al., 2016), which exhibited varying toxicity (Sawant et al., 2008, 2010).
China is the largest producer of seafood in the world (Szuwalski et al., 2020). Shellfish are filter feeders that can easily accumulate a variety of pollutants, including DA. Although no ASP event has been recorded in China, several studies have shown that long-term low dose DA exposure could also cause potential harm to human health. It has been demonstrated that chronic low level DA exposure could cause cognitive deficits in mice without any visible histopathological lesions in brain (Lefebvre et al., 2017). Besides, low dose DA exposure has also been noted to significantly alter zebrafish gene expression and disrupt myelin structure (Panlilio et al., 2020).
Shandong Province, located in the eastern coast of China, extends to the Bohai Sea (BS) and Yellow Sea (YS), with a coastline of approximately 3 345 km, covering one-sixth of China's coastline (Fu et al., 2018). With rapid industrialization and urbanization in these areas, many pollutants, including toxic metal pollutants (Zhou et al., 2020), perfluorinated alkyl substances (Guo et al., 2019), microplastics (Zhang et al., 2020), pesticides (Yin et al., 2015), as well as paralytic shellfish toxins (PSTs), diarrhetic shellfish toxins (DSTs) and DA (Chen et al., 2019; Gu et al., 2022; Liang et al., 2022), have been detected. Some investigations have found potential DA-producing algae such as Pseudo-nitzschia galaxiae (Xu and Li, 2015), Pseudo-nitzschia pungens var. averiensis, and Pseudo-nitzschia pungens var. pungens (Dong et al., 2018) in the coastal areas of Shandong Province. In recent years, DA-contaminated shellfish have been continuously detected in the areas around Shandong Province, such as Liaoning and Zhejiang Province (Li et al., 2017; Chen et al., 2019). Although the concentration of DA in shellfish had been reported to be relatively low (< 20 000 μg/kg), contamination and HAB incidences pose potential health risk to humans. Hence, knowledge on the distribution characteristics of DA in shellfish and health risk of human exposure to DA in Shandong Province is crucial.
Accordingly, the present study aimed to investigate the (1) general characterization of DA and its isomers concentrations in shellfish samples by employing liquid chromatography-tandem mass spectrometry (LC-MS/MS); (2) spatial and species variations of DA in shellfish samples; (3) relationships between DA concentrations and seasonal variation; and (4) health risk of human exposure to DA through aquatic food consumption. The results of this study could provide background data on DA contamination in Shandong Province.
2 MATERIAL AND METHOD 2.1 Sample collectionShellfish samples were collected from May to October 2019 from shellfish farms in the following cities along the coastal areas of the Shandong Province: Binzhou (BZ), Dongying (DY), Weifang (WF), Yantai (YT), Weihai (WH), Qingdao (QD), and Rizhao (RZ) (Fig. 1). A total of 133 shellfish samples were collected, which consisted of the following main cultured categories: 64 clams (Ruditapes philippinarum, Cyclina sinensis, Meretrix, and Mactra veneriformis), nine mussels (Mytilus edulis), 34 scallops (Chlamys farreri), and 26 oysters (Crassostrea gigas). The shellfish samples were collected randomly from the local shellfish culture area and sorted by location and type of specimens, washed with clean water, stored in a portable refrigerator at < 4 ℃, and transported to the laboratory within 48 h. The whole soft body tissue of the shellfish samples was homogenized with a T18 basic Ultra-Turrax mixer at 24 000 r/min (IKA, Germany), and the homogenates were stored at -20 ℃ until LC-MS/MS analysis.

|
| Fig.1 Sampling stations along the Shandong Province in 2019 |
Sample extraction and cleanup were conducted according to a previously described method with minor modifications (Furey et al., 2001). A total of 5.00±0.02 g of homogenized shellfish tissue were added to a 50-mL polypropylene centrifuge tube, and extracted with 12.0 mL of 50% MeOH. Then, the sample was vortexed for 1 min, ultrasonicated for 10 min, vortexed for 1 min, and centrifuged at 4 000 r/min for 10 min. The resulting supernatant was transferred into a new 50-mL polypropylene tube and the pellet was re-extracted with 5.0 mL of 50% MeOH. The volume of the combined supernatants was adjusted to 25.0 mL with the extracting solvent, vortexed for 1 min, and centrifuged for 15 min at 10 000 r/min.
The Bond Elut SAX cartridge (3 mL, 500 mg, Agilent Technologies, USA) was preconditioned prior to sample loading with MeOH (6.0 mL), H2O (3.0 mL), and 50% MeOH (3.0 mL), respectively, and 5.0 mL of the sample extract were loaded on the cartridge. Then, the cartridge was washed with 5.0 mL of 10% v/v acetonitrile and eluted with 4.0 mL of 0.3% v/v formic acid at a constant flow rate of 2 mL/min. The eluate was adjusted to 4.0 mL with eluting solvent, filtered through a 0.22-μm mixed cellulose filtration membrane (Agela Technologie, China), and transferred into autosampler vials prior to LC-MS/MS analysis.
LC-MS/MS analysis was performed using an U3000 HPLC system (Thermo Scientific, USA) linked to a Thermo TSQ Endura mass spectrometer equipped with an ESI source operated in positive ion mode. The chromatographic system was equipped with a binary pump and an autosampler with a 100-μL sample loop. By employing flow injection analysis at 10 μL/min, the mass spectrometer was tuned using CRM-DA-f DA standard solution (1.0 μg/mL), and the final ion source conditions were as follows: spray voltage of +3.50 kV, sheath gas flow of 25 arb, auxiliary gas flow of 15 arb, sweep gas flow of 0 arb, ion transfer tube temperature of 300 ℃, and vaporizer temperature of 250 ℃. Full-scan MS in the positive ion mode produced predominant peak at [M+H]+m/z 312.0, which was selected as the precursor ion. The SRM transitions from the protonated DA ion were m/z 312.0 > 266.0 ([M-HCOOH+H] +) at a collision energy (CE) of 15 V used for quantitative analysis, and confirmatory transition was m/z 312.0 > 248.0 ([M−HCOOH–H2O+H]+) at CE of 16 V, under the conditions of RF lens of 142 V, dwell time of 100 ms, and collision-induced dissociation gas setting of 1.5 mTorr.
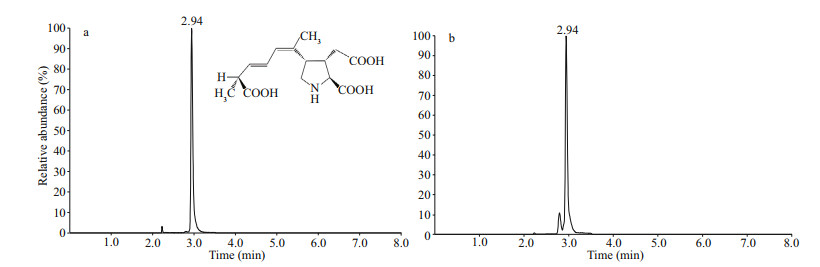
A total of 10 μL of the sample were injected into a LC Kinetex C18 column (2.1×100 mm, 2.6 μm, Phenomenex, USA). Before the detection of isomers, we first used a fast gradient method to screen the total DA in the shellfish samples. The system was operated under the following gradient elution program: solution A (2-mmol/L ammonium formate in H2O) and solution B (100% MeOH) at a flow rate of 0.3 mL/min: 0–1 min, 20% B; 1–3 min, 20%–90% B; 3.0–3.1 min, 90%–20% B; and 3.1–8.0 min, 20% B. The DA retention time was around 2.94 min. If it was a positive sample (detection limit = 0.08 μg/kg), the isomers were detected. The system was operated under the following gradient elution program: solution A (0.1% formic acid in H2O) and solution B (0.1% formic acid in acetonitrile) at a flow rate of 0.3 mL/min: 0–1 min, 5% B; 1–20 min, 5%–20% B; 20–22 min, 20%–5% B; 22–25 min, 5% B. Both the certified calibration solution (CRM-DA-f) and mussel tissue reference material (CRM-ASP-Mus-b) for DA were purchased from Certified Reference Materials Program, National Research Council (Halifax, NS, Canada). Individual stock solutions of DA were prepared in acetonitrile/water (1:9, v/v) and stored in amber glass vials at -20 ℃. Further dilutions and mixtures were prepared by following appropriate procedures. The average recovery of DA from CRM-ASP-Mus-b was 90% (n=3). Figure 2 shows a typical chromatogram of total DA standard solution (10.0 ng/mL) and real oyster samples with fast gradient method, respectively.
|
| Fig.2 Chromatogram of total DA standard solution (10.0 ng/mL) (a) and real oyster samples (b) |
To evaluate the health risk of human exposure to DA following consumption of different shellfish produced in coastal Shandong Province, Estimated Daily Intake (EDI, ng/(kg·d)) and Hazard Index (HR) were determined based on the measured DA concentration in shellfish and oral reference dose (RfD). A HR < 1.0 indicated acceptable risk and HR > 1.0 represented potential health risk of human exposure to DA. The EDI and HR were calculated as follows (Wang et al., 2021):
EDI=(C×Q)/BW,
HR=EDI/RfD,
where C is the mean DA concentration (μg/kg, wet weight) in shellfish samples, Q is the average daily consumption of aquatic food (g/d), and BW is the average body weight (kg) of Chinese in different age and gender groups. An RfD of 30 μg DA/kg body weight was considered in accordance with the European Food Safety Authority (EFSA, 2009).
3 RESULT 3.1 General characterization of DA concentrations in shellfish samplesA total of 133 shellfish samples, including seven shellfish species, were collected from the coastal areas of Shandong Province. Among them, 48 shellfish samples contained detectable DA. The DA detection rate was relatively high (36.1% of the samples), which indicated widespread DA contamination in Shandong Province. The mean DA concentration in all the shellfish samples was 7.33 μg /kg and ranged from 0 to 102 μg/kg.
3.2 Spatial variation of DA in shellfish samplesIn this study, shellfish samples were collected from shellfish culture areas in seven cities along the coastline of Shandong Province. The number of samples collected from WH, QD, DY, WF, YT, RZ, and BZ was 30, 26, 25, 20, 12, 12, and 8, respectively. At least one sample was contaminated with DA in each city, and the DA concentrations varied among different locations (Fig. 3). The DA detection rate in different cities presented the following trend: WH (50.0%), BZ (50.0%) > DY (44.0%) > WF (40.0%) > YT (33.3%) > QD (19.2%) > RZ (8.33%). Although the highest mean DA concentration was found in shellfish samples from YT (22.0 μg/kg), the maximum DA concentration (102 μg/kg) was noted in shellfish samples from WH. Besides, shellfish samples from BZ (mean DA concentration, 8.85 μg/kg) and DY (mean DA concentration, 1.76 μg/kg) showed relatively high DA detection rate; however, their mean DA concentrations were comparatively low. The lowest mean DA concentration and detection rate were observed in RZ (10.2 μg/kg, DA was detected in only one shellfish sample in this city).

|
| Fig.3 Spatial variation of DA in shellfish samples collected from seven cities along the Shandong Province Different colored circles represent samples detected in different cities. The curve represents the distribution of samples DA concentrations. |
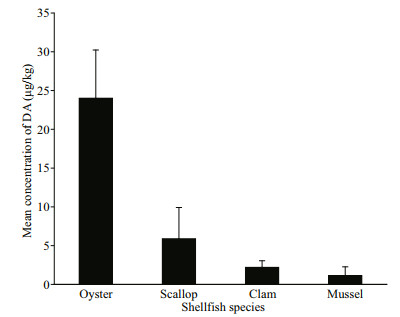
The DA concentrations varied among different shellfish species (Fig. 4). Among the samples presenting detectable DA concentration, the highest DA concentration was noted in C. gigas (102 μg/kg), whereas the lowest DA concentration was found in M. veneriformis (0.15 μg /kg). As indicated in Fig. 4, oysters showed higher mean DA concentration than the other shellfish species examined. The mean DA concentration in different shellfish species exhibited the following trend: oysters (24.0 μg/kg) > scallops (5.89 μg/kg) > clams (2.20 μg/kg) > mussels (1.14 μg/kg). Oysters presented the highest DA detection rate (80.8%, 21 of 26 shellfish samples), followed by clams (35.9%, 23 of 64 shellfish samples), mussels (11.1%, one of nine shellfish samples), and scallops (8.82%, three of 34 shellfish samples). Although scallops showed a relatively
|
| Fig.4 Contamination of DA in difference shellfish species low DA detection rate, the DA concentrations in two C. farreri samples (101 and 96.1 μg/kg, respectively) were higher than that in most of the shellfish samples, close to the highest DA concentration in oysters. In contrast, DA contamination in mussels was not serious, and was detected in only one M. edulis sample. |
A total of 42, 54, and 37 shellfish samples were collected from the coastline of Shandong Province in 2019 spring (March–May), summer (June–August), and autumn (September–November), respectively. The DA concentrations in the shellfish samples were strongly related to seasonal variation (Fig. 5). The DA detection rate and mean concentration in shellfish samples increased with seasonal variation and the toxin was detected in 14.3%, 40.7%, and 54.0% of the shellfish samples collected in spring, summer, and autumn, respectively. Shellfish samples collected in autumn (mean DA concentration, 16.5 μg/kg) and summer (mean DA concentration, 6.05 μg/kg) presented higher DA concentrations in the range of 0–102 and 0–74.8 μg/kg, respectively, when compared with those collected in spring (mean DA concentration, 0.90 μg/kg). In contrast, low DA levels were detected in shellfish samples collected in spring, with DA concentrations ranging from 0 to 17.0 μg/kg.

|
| Fig.5 Seasonal variation of DA in shellfish collected from the Shandong Province |
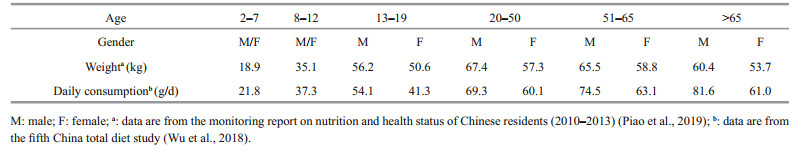
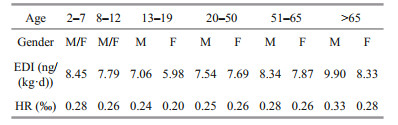
Table 1 shows the daily aquatic food consumption by Chinese residents in different age and sex groups based on the monitoring report on nutrition and health status of Chinese residents (2010–2013) (Piao et al., 2019) and the fifth China total diet study (Wu et al., 2018). Aquatic food consumption was divided into 10 age/sex groups and ranged from 21.8 to 81.6 g/d. The average weight of males was higher than that of females in the age group of > 13 years old. The EDI and HR were calculated based on the average weight, daily aquatic food consumption for each group and RfD of DA. The highest EDI (9.90 ng/(kg·d)) was noted in the age group of > 65 years, whereas the lowest EDI (5.98 ng/(kg·d)) was determined in the 13–19 years old female group (Table 2). The HR of DA exposure through aquatic food consumption for different age and gender groups ranged from 0.20‰ to 0.33‰, which were lower than the risk value of 1.
|
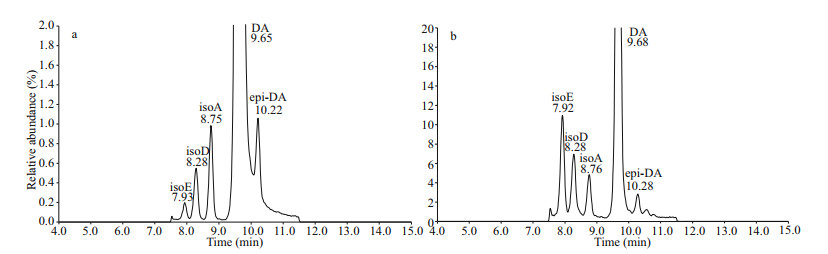
The detection rates of isoE, isoD, isoA, and epi-DA in all collected shellfish samples were calculated to be 15.8%, 15.8%, 14.3%, and 3.00%, respectively. The ratio of isomers to DA was around 18.0% (Fig. 6). Figure 7a shows the LC-MS/MS chromatogram of DA and its isomers standard solution (DA, 1 007 ng/mL; isoA, 5.2 ng/mL; isoD, 4.1 ng/mL; isoE, 0.13 ng/mL; and epi-DA, 11 ng/mL) with slow gradient method. One large peak and several smaller peaks were observed, whose retention times coincided with those of isoE (7.93 min), isoD (8.28 min), isoA (8.75 min), DA (9.65 min), and epi-DA (10.22 min) of the standard (CRM-DA-f). Although the isomers concentrations were low, five DA and isomers were also detected in some scallop and oyster samples (Fig. 7b). No DA or isomers were detected in C. sinensis and R. philippinarum, and small amounts of DA and isomers were found in other bivalve species collected from different cities. The mean isoE (1.35 μg/kg) and isoD (1.27 μg/kg) concentrations in shellfish samples were higher than mean isoA (0.63 μg/kg) and epi-DA (0.12 μg/kg). The ratio of isomers in oysters was similar to that in scallops and clams.

|
| Fig.6 Ratio of DA and isomers in shellfish collected from the Shandong Province |
|
| Fig.7 Chromatogram of DA and its isomers standard solution (a) and real oyster samples (b) |
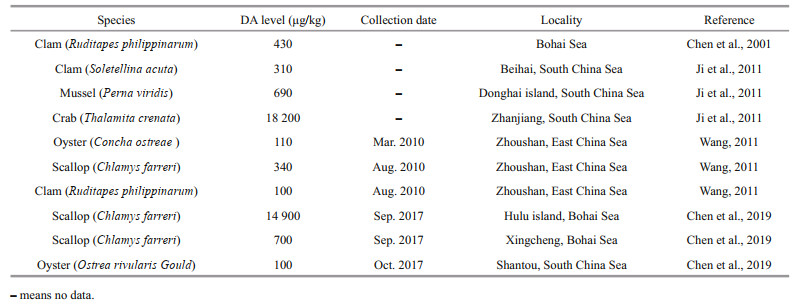
To accomplish high-quality protein production in China, mariculture has become one of the fastest growing industries since the 21st century (Yu and Han, 2020). Shandong Province is an important region of China in aquaculture and fishery with a gross ocean product of > 1 220 billion CNY (Chinese Yuan) in 2015, ranking the second among all provinces and accounting for 17% of China's gross ocean product (Su and Yang, 2018). Although there are no relevant studies on DA in shellfish along the coastal areas of Shandong Province, relatively low or negligible DA concentrations have been detected in several shellfish in nearby provinces or sea areas, such as in scallop (C. farreri) in Liaoning Province, clam (R. philippinarum) in Bohai Sea, and oyster (Ostrea rivularis Gould) in Shantou (Chen et al., 2001, 2019). The highest DA concentration (14 900 μg/kg) was noted in shellfish collected from a local market in Hulu Island, Liaoning Province in 2017, which is higher than that detected in this study (100 μg/kg). Besides, DA has also been detected in other marine organisms, such as crab (Thalamita crenata, 18 200 μg/kg) (Ji et al., 2011). Although the DA concentrations detected in shellfish and other marine organisms in China have not exceeded the safety limit of 20 000 μg/kg and only few samples have been found to contain relatively high DA concentrations, more attention must be paid to DA contamination (Table 3).
To the best of our knowledge, there is no study on DA contamination of shellfish in Shandong Province. The DA concentration in shellfish (Table 3) collected from the coast of China's other provinces was found consistent with that we observed in this study. Besides, investigation of the DA concentration in different shellfish in Zhoushan City, located in East China Sea, revealed 54%–69% DA detection rate from different sampling batches (Wang, 2011), which is higher than that determined in shellfish samples from all the cities examined in the present study. However, it must be noted that most of the DA concentrations in shellfish from Zhoushan City were < 1 000 μg/kg, which is only slightly higher than those detected in this study. Pseudo-nitzschia spp. are distributed worldwide in marine waters, and are known to produce the toxin, DA. According to a recent research, 58 Pseudo-nitzschia spp. have been identified around the world, of which 31 species have been reported in Chinese coastal waters, including the coastal region of Shandong Province (Chen et al., 2021). Since the identification of the first toxigenic diatom species (Pseudo-nitzschia simulans sp. nov.) in Chinese waters (Li et al., 2017), several other Chinese Pseudo-nitzschia strains have been confirmed to produce DA (Huang et al., 2017, 2019; Pang et al., 2018). In the present study, shellfish products from each of the examined city were found to be contaminated with DA, although the toxin concentration widely varied among the cities. In particular, P. galaxiae, P. pungens var. averiensis, and P. pungens var. pungens have been detected in Qingdao, located in the coast of Shandong Province (Xu et al., 2015; Dong et al., 2018), which are potentially toxic diatom species that can produce DA under suitable growth conditions (Bates et al., 2018). The spatial variation of DA accumulation in the seven cities examined may be related to different DA-producing species. Although this was expected in nature, it must also be emphasized that the sampling was opportunistic, and differences might have coincided with the sampling date. While most of the previous studies on DA contamination of shellfish in China have focused on the Chinese southeast coastal waters (Huang et al., 2019; Chen et al., 2021) and have detected many Pseudo-nitzschia spp., only a relatively few studies have investigated on Pseudo-nitzschia spp. occurrence in Shandong Province, which requires more attention.
It has been reported that the DA depuration rates obviously varied among different shellfish species. Many bivalve species, including mussels such as Mytilus galloprovicialis (Blanco et al., 2002) and M. edulis (Novaczek et al., 1992), depurate DA from their bodies within a few hours or days. However, scallops (Placopecten magellanicus) require a few weeks to depurate DA from their tissues (Douglas et al., 1997), while razor clams (Siliqua patula) depurate DA over many months (Trainer and Bill, 2004). Similarly, in the present study, the accumulated DA concentrations in different shellfish samples significantly varied. Although many studies have shown that DA accumulation and elimination rates widely vary in different shellfish species, the detoxification mechanism is still unknown. Nevertheless, a previous study has demonstrated that Pacific razor clams (S. patula) may contain more than one subtype of glutamate receptor, and could tolerate high concentrations of DA (Trainer and Bill, 2004). DA is a hydrophilic molecule with three carboxyl groups and an amine group (Shum et al., 2018), and mainly exists in a water-soluble state in shellfish cells and is excreted from the body without being metabolized (Novaczek et al., 1991). Analysis of the digestive gland transcriptional response of the mussel M. galloprovicialis to DA exposure indicated overexpression of several membrane transporters that belong to the family of solute carriers (Pazos et al., 2017). These membrane transporters may be efficient transmembrane transporters that can depurate DA outside the tissues; thus, the slow depuration of DA in some shellfish species may be owing to the lack of an efficient efflux transporter.
Seasonal variation and levels of toxins in shellfish are strongly related (Dursun, 2021). Pseudo-nitzschia spp. have a wide range of growth temperature from 8.4 ℃ to 23.5 ℃, and the highest DA production by P. australis in laboratory culture was observed between 13.5 ℃ and 18.6 ℃ (Thorel et al., 2014). Besides, seawater surface temperatures (14–17 ℃) have been found to have a positive correlation with the abundance of Pseudo-nitzschia spp. (Walz et al., 1994). It has been reported that autumn and summer seawater surface temperatures are more suitable for Pseudo-nitzschia growth, with the highest DA concentration of 2 671 ng/net tow observed in September phytoplankton samples and Pseudo-nitzschia cell density of > 6.0×105 cells/net tow noted in August and November in Daya Bay, South China Sea (Jiang et al., 2017). Similarly, in the present study, the DA detection rate and mean concentration were also higher in autumn and summer than those in spring. In China, HABs caused by Pseudo-nitzschia spp. have been reported in Dalian (Liaoning Province) and Qingdao (Shandong Province), and may occur throughout the year, especially in summer and autumn (Lu et al., 2014). It has been reported that monsoon climate could significantly influence the hydrodynamic environment in these areas around Shandong Province and cause substantial seasonal changes (Yuan et al., 2020). A large amount of freshwater from Huanghe (Yellow) River and Changjiang (Yangtze) River gets discharged into these areas during the summer monsoon season, which leads to lower salinity and higher nutrients, thus significantly affecting the marine ecosystem, especially, phytoplankton growth in these areas (Yang et al., 2015).
Dietary aquatic food intake is an important approach to evaluate human exposure to DA. According to the data from the fifth China total diet study (Wu et al., 2018), significant differences in aquatic food consumption were noted among different age and gender groups, ranging from 21.8 to 81.6 g/d. In the present study, the highest HR value was 0.33‰, indicating that DA exposure through different shellfish consumption had an acceptable risk for both adults and children. Males faced higher health risk through aquatic food consumption owing to their higher body weight and shellfish consumption than females. A major limitation of the present study is evaluation of the outcomes based on a single contamination factor. It has been reported that dietary intake of aquatic food may be higher among fish farmers and islanders, when compared with that for the general population (Wu et al., 2014). Moreover, although the average daily intake of seafood by urban residents has been found to be 42.3 g/d (Li et al., 2013), seafood consumption can also be related to the geographic location, because coastal residents generally consume more seafood. Hence, future studies must also consider other risk factors when evaluating the health risks of DA exposure through aquatic food consumption.
To date, DA, along with its isoforms A–F, its 5′-epimer, and its two lactone derivatives, have been identified in algae or shellfish, and have been noted to be highly water-soluble. Previous studies have shown that the genera Pseudo-nitzschia and Nitzschia could produce different isomers (Bates et al., 2018), and that shellfish can accumulate isomers from different toxic-producing algae through filter-feeding. UV-exposed dilute DA solutions photoisomerize reversibly into isoD, isoE, and isoF, and decarboxylate irreversibly and these isomers might act as photointermediates in the photochemical degradation of DA in the ocean (Bouillon et al., 2008), which may also be the reason for the accumulation of different isomers in shellfish. Although there have been reports on DA-producing toxic algae and contaminated shellfish along the coast of China, no further analyses on the occurrence of isomers have been conducted, which are crucial for future studies. Assessment of acute seizurogenic potencies of isomers and their binding affinities to heterogeneous populations of both kainate and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptors via in vivo seizure induction revealed that the toxicity of different isomers widely varied (Sawant et al., 2008, 2010). Although the concentrations of isomers in the present study were relatively low, accurate quantification of different isomers allows scientific evaluation of the magnitude of DA toxicity and provides essential data for DA risk assessment in shellfish.
5 CONCLUSIONThe present study provided the baseline concentration of DA in marine shellfish collected from the coastal areas of Shandong Province in China. The overall DA detection rate in the shellfish samples was 36.1%, indicating ubiquitous DA contamination in Shandong Province. The results obtained highlight the spatial and species variation of DA concentration in shellfish. The relationship between seasonal changes and DA concentration revealed that environmental changes could significantly affect DA accumulation in shellfish samples. Although the highest DA concentration determined in the present study was much lower than the permitted limit of 20 000 μg/kg, DA contamination might be significantly underestimated owing to its high detection rate and different toxic isomers. Furthermore, the lower health risk of DA exposure for the general population was found to be associated with seafood consumption in the coastal areas of Shandong Province. As Pseudo-nitzschia spp. is very common in the Chinese coastal waters, they may be a potential threat to aquatic food consumers, and further studies in the coastal areas around Shandong Province could lead to the identification of new DA-producing algal species. Besides, analyses of the trend in DA and its isomers concentration in shellfish in Shandong Province could provide valuable data for risk assessment and monitoring strategy in future.
6 DATA AVAILABILITY STATEMENTThe datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Bates S S, Bird C J, de Freitas A S W, et al. 1989. Pennate diatom Nitzschia pungens as the primary source of domoic acid, a toxin in shellfish from eastern Prince Edward Island, Canada. Canadian Journal of Fisheries and Aquatic Sciences, 46(7): 1203-1215.
DOI:10.1139/f89-156 |
Bates S S, Hubbard K A, Lundholm N, et al. 2018. Pseudo-nitzschia, Nitzschia, and domoic acid: New research since 2011. Harmful Algae, 79: 3-43.
DOI:10.1016/j.hal.2018.06.001 |
Ben Haddouch A, Taleb H, Elmortaji H, et al. 2016. Accumulation and tissue distribution of domoic acid in the common cuttlefish, SȦpia officinalis from the South Moroccan Coast. American Scientific Research Journal for Engineering, Technology, and Sciences, 15(1): 252-264.
|
Blanco J, de la Puente M B, ArȦvalo F, et al. 2002. Depuration of mussels (Mytilus galloprovincialis) contaminated with domoic acid. Aquatic Living Resources, 15(1): 53-60.
DOI:10.1016/S0990-7440(01)01139-1 |
Bouillon R C, Kieber R J, Skrabal S A, et al. 2008. Photochemistry and identification of photodegradation products of the marine toxin domoic acid. Marine Chemistry, 110(1-2): 18-27.
DOI:10.1016/j.marchem.2008.02.002 |
Chen S, Zhang X J, Yan Z Y, et al. 2019. Development and application of immunoaffinity column purification and ultrahigh performance liquid chromatography-tandem mass spectrometry for determination of domoic acid in shellfish. Toxins, 11(2): 83.
DOI:10.3390/toxins11020083 |
Chen X M, Pang J X, Huang C X, et al. 2021. Two new and nontoxigenic Pseudo-nitzschia species (Bacillariophyceae) from Chinese southeast coastal waters. Journal of Phycology, 57(1): 335-344.
DOI:10.1111/jpy.13101 |
Chen X P, Wang C B, Hu J M, et al. 2001. Determination of domoic acid in water and aquatic animals by high performance liquid chromatography. Journal of Hygiene Research, 30(4): 247-248.
(in Chinese with English abstract) DOI:10.3969/j.issn.1000-8020.2001.04.022 |
Dong H C, Huang C X, Xu G S, et al. 2018. Studies on intraspecies diversity of Pseudo-nitzschia pungens from Chinese coastal waters. Journal of Tropical Oceanography, 31(1): 12-19.
(in Chinese with English abstract) DOI:10.5670/oceanog.2018.104 |
Douglas D J, Kenchington E R, Bird C J, et al. 1997. Accumulation of domoic acid by the sea scallop (Placopecten magellanicus) fed cultured cells of toxic Pseudo-nitzschia multiseries. Canadian Journal of Fisheries and Aquatic Sciences, 54(4): 907-913.
DOI:10.1139/f96-333 |
Dursun F. 2021. Occurence and variability of domoic acid in mussel (Mytilus galloprovincialis) samples from the Golden Horn Estuary, Sea of Marmara (Turkey). Bulletin of Environmental Contamination and Toxicology, 106(2): 318-326.
DOI:10.1007/s00128-020-03082-7 |
EF SA. 2009. Scientific opinion: marine biotoxins in shellfish jDomoic acid. Scientific opinion of the panel on contaminants in the food chain. The EFSA Journal, 1181: 1-61.
|
Fu X F, Wang N, Jiang S S, et al. 2018. Value evaluation of marine bioresources in Shandong offshore area in China. Ocean & Coastal Management, 163: 296-303.
DOI:10.1016/j.ocecoaman.2018.07.002 |
Furey A, Lehane M, Gillman M, et al. 2001. Determination of domoic acid in shellfish by liquid chromatography with electrospray ionization and multiple tandem mass spectrometry. Journal of Chromatography A, 938(1-2): 167-174.
DOI:10.1016/S0021-9673(01)01385-1 |
Gibble C M, Kudela R M, Knowles S, et al. 2021. Domoic acid and saxitoxin in seabirds in the United States between 2007 and 2018. Harmful Algae, 103: 101981.
DOI:10.1016/j.hal.2021.101981 |
Gu H F, Wu Y R, Lȹ S H, et al. 2022. Emerging harmful algal bloom species over the last four decades in China. Harmful Algae, 111: 102059.
DOI:10.1016/j.hal.2021.102059 |
Guo M M, Zheng G C, Peng J X, et al. 2019. Distribution of perfluorinated alkyl substances in marine shellfish along the Chinese Bohai Sea coast. Journal of Environmental Science and Health, Part B, 54(4): 271-280.
DOI:10.1080/03601234.2018.1559570 |
Huang C X, Dong H C, Lundholm N, et al. 2019. Species composition and toxicity of the genus Pseudo-nitzschia in Taiwan Strait, including P. chiniana sp. nov. and P. qiana sp. nov. Harmful Algae, 84: 195-209.
DOI:10.1016/j.hal.2019.04.003 |
Huang C X, Dong H C, Wu H Y, et al. 2017. Pseudo-nitzschia fukuyoija new record of toxic Pseudo-nitzschia taxa from China. Oceanologia et Limnologia Sinica, 48(5): 1014-1021.
(in Chinese with English abstract) |
Ji W, Zheng J Y, Zeng X P, et al. 2011. HPLC analysis of domoic acid poisoning in South China Sea. Modern Food Science & Technology, 27(1): 120-122, 116.
(in Chinese with English abstract) |
Jiang T, Liu L, Li Y, et al. 2017. Occurrence of marine algal toxins in oyster and phytoplankton samples in Daya Bay, South China Sea. Chemosphere, 183: 80-88.
DOI:10.1016/j.chemosphere.2017.05.067 |
Lefebvre K A, Kendrick P S, Ladiges W, et al. 2017. Chronic low-level exposure to the common seafood toxin domoic acid causes cognitive deficits in mice. Harmful Algae, 64: 20-29.
DOI:10.1016/j.hal.2017.03.003 |
Lefebvre K A, Robertson A. 2010. Domoic acid and human exposure risks: a review. Toxicon, 56(2): 218-230.
DOI:10.1016/j.toxicon.2009.05.034 |
Li P, Feng X B, Liang P, et al. 2013. Mercury in the seafood and human exposure in coastal area of Guangdong Province, South China. Environmental Toxicology and Chemistry, 32(3): 541-547.
DOI:10.1002/etc.2113 |
Li Y, Huang C X, Xu G S, et al. 2017. Pseudo-nitzschia simulans sp. nov. (Bacillariophyceae), the first domoic acid producer from Chinese waters. Harmful Algae, 67: 119-130.
DOI:10.1016/j.hal.2017.06.008 |
Liang Y B, Li A F, Chen J H, et al. 2022. Progress on the investigation and monitoring of marine phycotoxins in China. Harmful Algae, 111: 102152.
DOI:10.1016/j.hal.2021.102152 |
Lu D D, Qi Y Z, Gu H F, et al. 2014. Causative species of harmful algal blooms in Chinese coastal waters. Algological Studies, 145-146: 145-168.
DOI:10.1127/1864-1318/2014/0161 |
Novaczek I, Madhyastha M S, Ablett R F, et al. 1991. Uptake, disposition and depuration of domoic acid by blue mussels (Mytilus edulis). Aquatic Toxicology, 21(1-2): 103-118.
DOI:10.1016/0166-445X(91)90009-X |
Novaczek I, Madhyastha M S, Ablett R F, et al. 1992. Depuration of domoic acid from live blue mussels (Mytilus edulis). Canadian Journal of Fisheries and Aquatic Sciences, 49(2): 312-318.
DOI:10.1139/f92-035 |
Pang J X, Dong H C, Li Y. 2018. New record and toxic species of the genus Pseudo-nitzschia from southeastern China waters. Acta Oceanologica Sinica, 40(12): 120-128.
(in Chinese with English abstract) |
Panlilio J M, Aluru N, Hahn M E. 2020. Developmental neurotoxicity of the harmful algal bloom toxin domoic acid: cellular and molecular mechanisms underlying altered behavior in the zebrafish model. Environmental Health Perspectives, 128(11): 117002.
DOI:10.1289/EHP6652 |
Pazos A J, Ventoso P, MartȪnez-Escauriaza R, et al. 2017. Transcriptional response after exposure to domoic acid-producing Pseudo-nitzschia in the digestive gland of the mussel Mytilus galloprovincialis. Toxicon, 140: 60-71.
DOI:10.1016/j.toxicon.2017.10.002 |
Piao J H, Huo J S. 2019. The monitoring report on nutrition and health status of Chinese residents (2010-2013) (Ⅱ). People's Medical Publishing House, Beijing. p. 54-55. (in Chinese)
|
Regueiro J, Álvarez G, Mauriz A, et al. 2011. High throughput analysis of amnesic shellfish poisoning toxins in bivalve molluscs by dispersive solid-phase extraction and high-performance liquid chromatography using a monolithic column. Food Chemistry, 127(4): 1884-1891.
DOI:10.1016/j.foodchem.2011.02.039 |
Sawant P M, Holland P T, Mountfort D O, et al. 2008. In vivo seizure induction and pharmacological preconditioning by domoic acid and isodomoic acids A, B and C. Neuropharmacology, 55(8): 1412-1418.
DOI:10.1016/j.neuropharm.2008.09.001 |
Sawant P M, Tyndall J D A, Holland P T, et al. 2010. In vivo seizure induction and affinity studies of domoic acid and isodomoic acids-D, -E and -F. Neuropharmacology, 59(3): 129-138.
DOI:10.1016/j.neuropharm.2010.03.019 |
Shum S, Kirkwood J S, Jing J, et al. 2018. Validated HPLC-MS/MS method to quantify low levels of domoic acid in plasma and urine after subacute exposure. ACS Omega, 3(9): 12079-12088.
DOI:10.1021/acsomega.8b02115 |
Su M, Yang Y. 2018. Evolution of district marine policies in China: the case of Shandong Province. Marine Policy, 89: 124-131.
DOI:10.1016/j.marpol.2017.12.028 |
Szuwalski C, Jin X S, Shan X J, et al. 2020. Marine seafood production via intense exploitation and cultivation in China: Costs, benefits, and risks. PLoS One, 15(1): e0227106.
DOI:10.1371/journal.pone.0227106 |
Thorel M, Fauchot J, Morelle J, et al. 2014. Interactive effects of irradiance and temperature on growth and domoic acid production of the toxic diatom Pseudo-nitzschia australis (Bacillariophyceae). Harmful Algae, 39: 232-241.
DOI:10.1016/j.hal.2014.07.010 |
Trainer V L, Bill B D. 2004. Characterization of a domoic acid binding site from Pacific razor clam. Aquatic Toxicology, 69(2): 125-132.
DOI:10.1016/j.aquatox.2004.04.012 |
Walz P M, Garrison D L, Graham W M, et al. 1994. Domoic acid-producing diatom blooms in Monterey Bay, California: 1991-1993. Natural Toxins, 2(5): 271-279.
DOI:10.1002/nt.2620020505 |
Wang H. 2011. An investigation of domoic acid content in shellfish in Zhoushan islands. Chinese Journal of Health Laboratory Technology, 21(12): 2986-2988, 2992.
(in Chinese with English abstract) |
Wang X F, Mo M S, Chen H G, et al. 2021. Distribution and exposure risk assessment of perfluorinated alkyl substances in aquatic products along the coastal region of the South China Sea. Exposure and Health, 13(3): 505-515.
DOI:10.1007/s12403-021-00399-4 |
Wu X, Gao M, Wang L, et al. 2014. The arsenic content in marketed seafood and associated health risks for the residents of Shandong, China. Ecotoxicology and Environmental Safety, 102: 168-173.
DOI:10.1016/j.ecoenv.2014.01.028 |
Wu Y N, Zhao Y F, Li J G. 2018. The Fifth China Total Diet Study. Science Press, Beijing. 68p. (in Chinese with English abstract)
|
Xu G S, Li Y. 2015. Two new records of diatom genus Pseudo-nitzschia from Chinese waters and analysis of their domoic acid production. Journal of Tropical and Subtropical Botany, 23(6): 614-624.
(in Chinese with English abstract) |
Yang J, Yang G P, Zhang H H, et al. 2015. Spatial distribution of dimethylsulfide and dimethylsulfoniopropionate in the Yellow Sea and Bohai Sea during summer. Chinese Journal of Oceanology and Limnology, 33(4): 1020-1038.
|
Yin G, Asplund L, Qiu Y L, et al. 2015. Chlorinated and brominated organic pollutants in shellfish from the Yellow Sea and East China Sea. Environmental Science and Pollution Research, 22(3): 1713-1722.
DOI:10.1007/s11356-014-3198-8 |
Yu J K, Han Q C. 2020. Food security of mariculture in China: Evolution, future potential and policy. Marine Policy, 115: 103892.
DOI:10.1016/j.marpol.2020.103892 |
Yuan P, Wang H J, Wu X, et al. 2020. Grain-size distribution of surface sediments in the Bohai Sea and the northern yellow sea: sediment supply and hydrodynamics. Journal of Ocean University of China, 19(3): 589-600.
DOI:10.1007/s11802-020-4221-y |
Zhang Z Y, Mamat Z, Chen Y G. 2020. Current research and perspective of microplastics (MPs) in soils (dusts), rivers (lakes), and marine environments in China. Ecotoxicology and Environmental Safety, 202: 110976.
DOI:10.1016/j.ecoenv.2020.110976 |
Zhou Q F, Liu L H, Liu N, et al. 2020. Determination and characterization of metal nanoparticles in clams and oysters. Ecotoxicology and Environmental Safety, 198: 110670.
DOI:10.1016/j.ecoenv.2020.110670 |
 2022, Vol. 40
2022, Vol. 40