Institute of Oceanology, Chinese Academy of Sciences
Article Information
- GAO Jian, DONG Yuelei, ZHOU Xiaoyu, CUI Lei, LÜ Songhui
- Biochemical composition of the brown tide causative species Aureococcus anophagefferens cultivated in different nitrogen sources
- Journal of Oceanology and Limnology, 40(6): 2189-2201
- http://dx.doi.org/10.1007/s00343-022-1343-7
Article History
- Received Oct. 18, 2021
- accepted in principle Dec. 21, 2021
- accepted for publication Feb. 25, 2022
2 Southern Marine Science and Engineering Guangdong Laboratory, Zhuhai 519000, China;
3 Key Laboratory of Eutrophication and Red Tide Prevention of Guangdong Higher Education Institutes, Jinan University, Guangzhou 510632, China
Summer algal blooms, caused by the rapid growth and proliferation of eukaryotic picoplankton Aureococcus anophagefferens, have become a recurring phenomenon in many coastal embayments, e.g., the east coast of the United States, since 1985 and Saldanha Bay, South Africa, since 1997 (Sieburth et al., 1988; Bricelj and Lonsdale, 1997; Probyn et al., 2001, 2010). In 2009, a large-scale bloom caused by a similar picoplankton (~2 μm), which was later confirmed to be A. anophagefferens, occurred along the coast of Qinhuangdao, China (Zhang et al., 2012). Since then, such blooms have recurred in this area, making China the third country to suffer from brown tides.
The maximum cell density of brown tide along the Qinhuangdao coast can reach over 106 cells/mL, covering more than 3 000 km2 and normally lasting for 1–3 months (Zhang et al., 2012; Zhen et al., 2016). Both the feeding activities and growth of shellfish, especially the bay scallop Argopecten irradians, the main maricultural species cultivated in this region, were severely affected (Zhang et al., 2012). From 2009 to 2016, the annual economic loss caused by brown tide exceeded USD 14.5 million (Ou et al., 2018b).
Brown tide has recurred in coastal ecosystems of the United States for over three decades and has also significantly damaged the agriculture industry along the East Coast. Extensive studies focusing on the adverse impacts and ecological consequences of A. anophagefferens have been conducted. It has been well confirmed that A. anophagefferens can severely impact the feeding activity, growth, survival, and reproduction of bivalve mollusks, such as Mercenaria mercenaria (Bricelj et al., 2001; Harke et al., 2011), Mytilus edulis (Tracey, 1988; Bricelj et al., 2004), and Argopecten irradians (Gallager et al., 1989; Griffith et al., 2019). Beyond that, detrimental effects on microzooplankton were also observed (Lonsdale et al., 1996; Caron et al., 2004; Smith et al., 2008; He et al., 2018). However, the exact reason and mechanism remain ambiguous thus far.
Hypothetical mechanisms have been proposed. One claimed that cellular toxicity contributes to noxious effects. According to Sieburth et al. (1988), organic material forms a diffuse exocellular layer associated with the cell membrane of A. anophagefferens. After being hydrolyzed by amylase, the reaction product of the outer polysaccharide layer could reduce the beating frequency of the excised lateral cilia of M. edulis (Gainey and Shumway, 1991). The effect was similar to that of dopamine and could also could be blocked by pretreatment with the dopamine antagonist ergometrine. Thus, the reduced grazing rates may be caused by the impact of a dopamine-mimetic compound associated with the cell membrane of A. anophagefferens (Gainey and Shumway, 1991). In addition, the ciliary movement of bivalves may also be physically affected by sticky outer polysaccharides (Sieburth et al., 1988; Liu and Buskey, 2000).
Nevertheless, to date, a precise chemical substance related to the noxious effect of brown tide has never been identified. In addition, Caron et al. (2004) found that when serving as a sole food source, A. anophagefferens could support the good growth of three protist species (Oxyrrhis marina, Uronema spp. and Euplotes spp.). Slipper limpets (Crepidula fornicata) can effectively clear A. anophagefferens at biomass-specific rates (Harke et al., 2011). In addition, according to Padilla et al. (2006), A. anophagefferens can support a faster development of certain bivalve mollusk species (i.e., M. mercenaria larvae) when mixed with Isochrysis galbana. Moreover, when moderate-density brown tide cells mixed with I. galbana served as the diet, there was no distinct effect compared with the effect of a single species-diet of I. galbana, and in certain cases, enhanced growth was generated (Padilla et al., 2006). These findings are all serve as counterevidence to the cellular toxicity of A. anophagefferens.
Another hypothesis, which was proposed after Lonsdale et al. (1996) proved A. anophagefferens to be a nutritionally inadequate food source for some copepods, declared that the nutritional inadequacy of A. anophagefferens might account for its detrimental effects. Smith et al. (2008) found that although the ingestion rates of nauplii of a calanoid copepod, Acartia tonsa, were not restrained by A. anophagefferens (CCMP 1708), the development of nauplii was severely depressed by A. anophagefferens cells. A similar case was discovered when the Chinese strain A. anophagefferens served as a monoalgal diet for nauplii of the copepod Pseudodiaptomus poplesia. Nauplii exhibited a high ingestion rate for brown tide cells, however, they did not metamorphose and died in the late naupliar phase, in a similar way to those under starvation (He et al., 2018). Evidence for the suspected nutritional inadequacy of A. anophagefferens was also found when it served as a food source for bivalves. Conspicuous brown lipid droplets in the hard clam M. mercenaria larvae were observed when fed I. galbana, while no visible lipid stores were found in larvae when A. anophagefferens served as the sole food source (Padilla et al., 2006).
However, A. anophagefferens cultured in f/2 medium was affirmed to contain eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which are highlighted as two vital nutritional constituents for the development of zooplankton and bivalves (Bricelj et al., 1989).
Although the total lipid content and fatty acid composition of A. anophagefferens cultured in f/2 culture medium were detected, the biochemical composition of brown tide cells has not been fully elucidated. In addition, no previous study has been conducted regarding the protein, amino acid, and carbohydrate composition of A. anophagefferens. These constituents are also important ingredients related to the nutritional quality of food sources for bivalve mollusks (Hemaiswarya et al., 2011). Additionally, the biochemical composition of microalgae is significantly affected by the source of nitrogen (Levasseur et al., 1993; Lourenco et al., 2002). The brown tides of A. anophagefferens often bloom when the ambient dissolved organic nitrogen (DON) level is elevated (Mulholland et al., 2009; Probyn et al., 2010; Gobler and Sunda, 2012), which was also observed along the coast of Qinhuangdao during bloom periods (Zhang et al., 2021). Thus, more information is needed on the biochemical composition of A. anophagefferens cultivated in different nitrogen sources.
Nitrate and urea are dominant nitrogen sources among the various forms of nitrogen that are available for phytoplankton communities in nature (Durmaz, 2007), including coastal ecosystems in which A. anophagefferens blooms. In this study, an elaborate analysis of the effect of different nitrogen resources (urea, nitrate, and a mixture of both) on the biochemical composition of the Chinese strain A. anophagefferens was conducted. The results were also compared with species that are frequently used to feed bivalves (values from the literature), to determine whether A. anophagefferens is nutritionally inadequate for filter predators.
2 MATERIAL AND METHOD 2.1 Algal strain and cultureThe specie of microalgae used for the experiment was the Chinese strain of A. anophagefferens (No. AA-1). The strain was originally isolated from the bloom water column of the Qinhuangdao coast in 2012 (119°37.911′E, 39°54.111′N) and preserved at the Research Center of Harmful Algae and Marine Biology, Jinan University, Guangzhou, China. It was grown in sterilized artificial sea water (ASW; Cavanaugh, 1956) enriched with f/2 culture medium (Guillard and Ryther, 1962) and subcultured routinely. The culture was grown at 20±0.5 ℃ in an illumination intensity of 100 μmol photons/(m2·s) along with a 12-h: 12-h light: dark cycle.
2.2 Nitrogen sourceThree conical flasks, containing 1 000-mL medium and each with nutrient added at a quarter of the f/2 culture medium concentration, were inoculated with approximately 0.6×106 cells/mL of A. anophagefferens. When the cultures grew to the mid-exponential phase, algal cells were harvested by centrifugation (4 500×g, 10 min), re-suspended in 3 000-mL medium containing different nitrogen sources. Nitrogen conditions were designed as follows: Treatment A: 220-μmol/L NaNO3; Treatment B: 110-μmol/L urea; and Treatment C: 110-μmol/L NaNO3 and 55-μmol/L urea. Each treatment was conducted in triplicate.
2.3 Growth phaseCell number counting was conducted every other day for cell growth rate calculation. After cultivation, algae biomass was harvested by centrifugation, freeze-dried, and grounded before biochemical analysis. Treatment A was harvested on Day 7, Treatments B and C were harvested on Day 5 for total lipid, fatty acid, amino acid, and carbohydrate analysis.
2.4 Ash, moisture, and total lipid contentThe total ash and moisture contents of Treatment A were detected according to Van Wychen and Laurens (2016).
The total lipid contents of all the treatments were measured by a modified method based on Bligh and Dyer (1959).
2.5 Amino acid, fatty acid, and carbohydrate analysisThe amino acid content of all treatments was determined by an external, commercial testing organization according to the standard GB 5009.124-2016 acid hydrolysis method.
Fatty acid analysis was adapted from Van Wychen et al. (2016), see Supplementary material for details.
Carbohydrate analysis was conducted using the method modified from Van Wychen and Laurens (2020), see Supplementary material for details.
2.6 Statistical analysisAll results are reported as the mean±standard deviation (SD) of three replicate groups. A comparison between means was analyzed by one-way ANOVA, followed by least-significant difference (LSD) test, and significance was accepted when P < 0.05. Statistical analysis was conducted by using the SPSS program, version 25 (SPSS Inc., Chicago, IL, USA).
3 RESULT 3.1 Growth rate of A. anophagefferensThe growth rates in the exponential phase (calculated from Days 1–5) are shown in Fig. 1. Growth rates for cultures grown in urea were highest (0.32/d), followed by the nitrate-urea mixture (0.30/d). There were no significant differences among growth rates (P > 0.05).
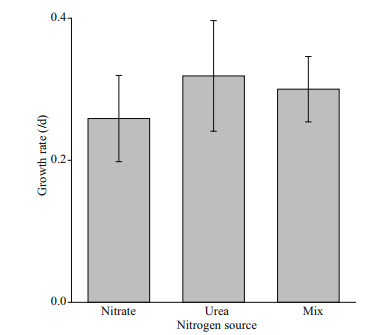
|
| Fig.1 Exponential phase growth rates of A. anophagefferens cultivated in nitrate, urea, and nitrate-urea mixture Error bars represent standard deviation of the means for three replicates. |
The total extractable lipid content is shown in Fig. 2. For A. anophagefferens cells grown on urea, the total lipid content was the highest (24.31%), followed by the cells grown on nitrate (21.99%). There were no significant differences between these two groups (P > 0.05). The total lipid content of the cells grown on the nitrate-urea mixture was the lowest (19.00%), which was significantly lower than that of other two groups (P < 0.05).

|
| Fig.2 Total extractable lipid contents (% of dry weight) of A. anophagefferens cultivated in nitrate, urea, and nitrate-urea mixture Error bars represent standard deviation of the means for three replicates. Asterisk indicates significantly difference: P < 0.05. |
The gross biochemical composition, expressed as a percentage of dry weight, showed the total extractable lipid, protein, and total carbohydrate contents of A. anophagefferens under different nitrogen cultivations (Table 1). According to Laurens (2016), the protein content was determined as the summation of amino acids, and the total carbohydrates (including starch) in algal biomass were determined as the summation of monosaccharides. The protein content of the culture on nitrate (39.12%) was not significantly different from that of the culture on urea (40.35%, P > 0.05) and was significantly lower than that of the culture on the nitrate-urea mixture (40.88%, P < 0.05). There was no significant difference between the two organic nitrogen addition groups (P > 0.05). The total carbohydrate contents of A. anophagefferens was significantly higher (10.25%) when cultured in nitrate (P < 0.05), and there was no significant difference between cultures on the urea (7.87%) and the nitrate-urea mixture (7.80%).

|
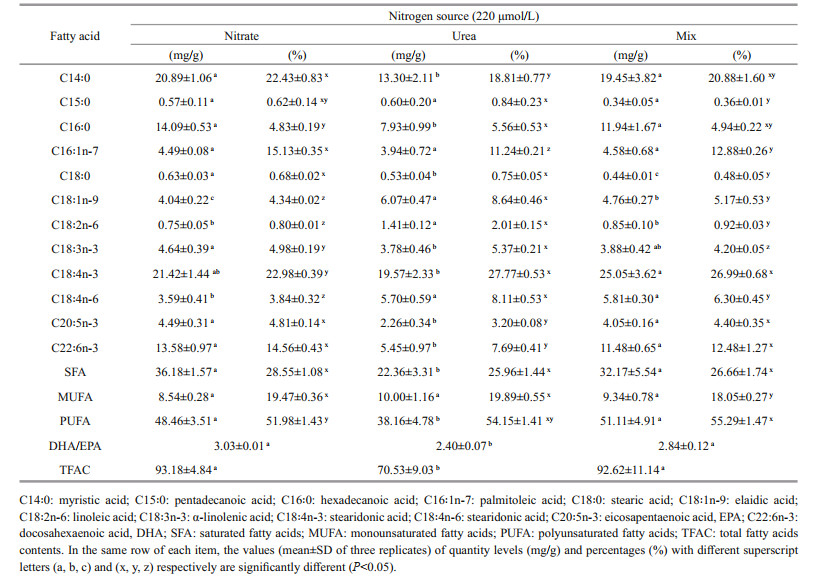
The fatty acid compositions of A. anophagefferens cultivated in different nitrogen conditions are shown in Table 2.
|
Gas chromatography-mass spectrometry analysis revealed that, as a percent of total fatty acids, A. anophagefferens cultured in different nitrogen sources contained 25.96%–28.55% saturated fatty acids (SFAs), 18.05%–19.89% monounsaturated fatty acids (MUFAs), and 51.98%–55.29% PUFAs. The major fatty acids identified in A. anophagefferens were C14:0 (myristic acid, 18.81%–22.43%), C16:1n-7 (palmitoleic acid, 11.24%–15.13%), C18:4n-3 (stearidonic acid, 22.98%–27.77%), and C22:6n-3 (DHA, 7.69%–14.56%). C14:0 and C16:1n-7 comprised 22.43% and 15.13% of the total fatty acids of the culture on nitrate and were significantly higher than those of the culture in urea (18.81% and 11.24% respectively). The culture in the nitrate and the nitrate-urea mixture contained a higher percentage of EPA and DHA than the culture in urea (P < 0.05). However, regarding the percentages of all C18 mono- and poly-unsaturated fatty acids, culture in the urea had significantly higher concentrations than both cultures in the nitrate and nitrate-urea mixture, except for C18:4n-3 in which no significant difference between the culture in the urea and nitrate-urea mixture was shown. The culture in urea contained the highest percentage of MUFAs. The culture in the nitrate-urea mixture contained the highest percentage of PUFAs. There was no significant difference among the three cultures in the percentage of SFAs. For all three treatments, no eicosatetraenoic acid (C20:4n-6, ARA) or docosapentaenoic acid (C22:5n-6, DPA) was detected.
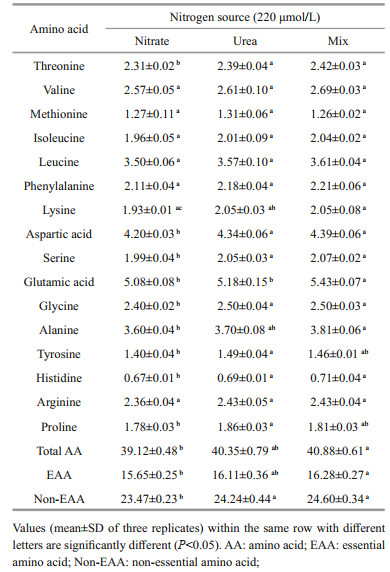
3.4 Amino acid profilesThe amino acid content (% by dry weight) of A. anophagefferens cultivated in different nitrogen conditions is shown in Table 3.
|
The percentage composition of nonessential amino acids varied from 23.47% to 24.60%. The percentage composition of essential amino acids varied from 15.65% to 16.28%. The percentage composition of total amino acids varied from 39.12% to 40.88%. The principal fraction of amino acids in the treatment consisted of leucine (3.50%–3.61%), aspartic acid (4.20%–4.39%), and glutamic acid (5.08%–5.43%).
The nonessential amino acid content of the culture in the nitrate was significantly lower than that of the cultures in the urea and the nitrate-urea mixture (P < 0.05). Both the essential amino acid and total amino acid contents of the culture in nitrate were not significantly different from those of the contents of the culture in the urea (P > 0.05) and were significantly lower than those of the contents of the culture in the nitrate-urea mixture (P < 0.05).
3.5 Carbohydrate profilesThe monomeric carbohydrate compositions of A. anophagefferens cultivated in different nitrogen conditions are shown in Table 4.

|
Eleven monomeric carbohydrates were detected in A. anophagefferens, and the major monomeric carbohydrates identified in A. anophagefferens were galacturonic acid (1.60%–2.90%), glucose (1.30%– 1.70%), and galactose (0.96%–1.59%). Except for glucose and galactose, the contents of nine other monomeric carbohydrates were significantly higher in the cultures in nitrate (P < 0.05). The glucose content was highest in the culture in urea, and the galactose content was highest in the culture in the nitrate-urea mixture.
4 DISCUSSION 4.1 Growth of A. anophagefferens cultured in different nitrogen sourcesIn this study, A. anophagefferens grew well when urea served as the sole nitrogen supplement. Cultures with urea and the nitrate-urea mixture were associated with higher growth rates and entered the stationary phase two days earlier than the culture with nitrate. A similar phenomenon occurred during A. anophagefferens blooms in the coast of Qinhuangdao, China: inorganic nitrogen was negatively correlated with the abundance of A. anophagefferens, and half of the total dissolved nutrient pools were contributed by DON and DOP (Yao et al., 2019; Zhang et al., 2021). The results also matched previous nutrient enrichment studies in Quantuck Bay and Narragansett Bay, USA, in which, both mesocosms and field measurements showed that dissolved inorganic phosphorus (DIN) concentration was inversely correlated with A. anophagefferens cell density, and that the initiation of A. anophagefferens blooms was usually associated with increasing DON levels (Keller and Rice, 1989; Kana et al., 2004; Gobler and Sunda, 2012).
4.2 Assessment of gross biochemical compositionAccording to Guedes and Malcata (2012), microalgae grown to the late exponential growth phase usually consist of 5%–15% carbohydrate, 10%–20% lipid, and 30%–40% protein. The A. anophagefferens cultured in different nitrogen sources contained 7.80%–10.25% carbohydrate, a relatively high amount of protein (39.12%–40.88%, around the upper limit of the average value), and a high amount of total extractable lipid (up to 24.31% in urea cultivation). The protein and carbohydrate contents were basically within the range, but the total extractable lipid content was higher than that of the average range value. Bricelj et al. (1989) also found that when cultured in f/2 medium, the lipid content of A. anophagefferens was quite high (26.4%±6.4% of dry weight). The result of the present study is consistent with Bricelj's work.
When served as food for bivalve mollusks, the nutritional value of the algal diet depends largely on the gross biochemical components (Marshall et al., 2010). Saucedo et al. (2013) discovered that elevated algal diet protein levels were associated with enhanced growth and respiration of juvenile lion-paw scallop, Nodipecten subnodosus. Uriarte and Farías (1999) discovered that both the growth and survival of the Chilean scallop, Argopecten purpuratus, are positively correlated with dietary diet protein levels. Tang et al. (2006) proved that the feeding behavior of hard clam Meretrix meretrix is positively correlated with the total lipid content of algal diet. Wikfors et al. (1992) found that the gross biochemical composition of algae affects the growth of hard clams M. mercenaria, one of the most adversely affected species by A. anophagefferens. To support rapid growth, both dietary protein and lipids must be present in sufficient quantities. For bivalve mollusks, neutral lipids are the principal energy-providing constituent in algal diets, followed by protein (Fernández-Reiriz et al., 2011; Matias et al., 2011). From this perspective, the relatively high amount of lipids and proteins should make A. anophagefferens an appropriate bite.
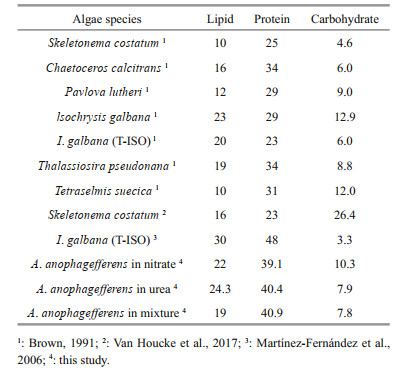
Skeletonema costatum, Chaetoceros calcitrans, Pavlova lutheri, lsochrysis galbana, Thalassiosira pseudonana, and Tetraselmis suecica are some of the commonly used microalgae species in bivalve rearing (Kaparapu, 2018). When compared with these species, the gross biochemical composition of A. anophagefferens is comparable with all (Table 5). Among them, Isochrysis sp. (T-ISO), P. lutheri, and C. calcitrans, are the most commonly used species when feeding the larva, early juvenile, and broodstock stages of bivalve mollusks (Brown, 2002). Moreover, S. costatum, T. pseudonana, and I. galbana support excellent growth of a great number of bivalve mollusks, including M. mercenaria (Wikfors et al., 1992; Greenfield et al., 2004; Bricelj and MacQuarrie, 2007).
|
Fatty acids, especially PUFAs, are vital constituents in membrane fluidity and function maintenance and serve as precursors to some bioactive molecules involved in metabolism and reproduction (Da Costa et al., 2015; Cheng et al., 2020). Most animals, including bivalve mollusks, cannot synthesize either n-3 or n-6 family PUFAs de nova (Zhukova, 2019). The ability to produce long-chain PUFAs from shorter chain precursors is also limited (Langdon and Waldock, 1981; Delaunay et al., 1993). The primary source of long-chain PUFAs for bivalves in the field is through the ingestion of algae (Cheng et al., 2020). Therefore, for most bivalves, the content of long-chain PUFAs is emphasized when evaluating the nutritional value of algae.
Bricelj et al. (1989) detected the fatty acid composition of A. anophagefferens isolated from the East Coast of the United States. They reported that the amount and composition of fatty acids were remarkably constant between the exponential growth phase and the early stationary phase when cultured in nitrate. The levels of saturated fatty acids (SFAs), monounsaturated fatty acids (MUFAs), and polyunsaturated fatty acids (PUFAs) in cells in the early stationary phase were 23.2%, 17.5%, and 59.2% respectively. EPA and DHA accounted for 5.1% and 14.0% of the total fatty acids, respectively. In the present study, the SFA level was higher (28.55%), and the PUFA level was lower (51.98%). The MUFA, EPA, and DHA levels of the present study were all comparable to the values of Bricelj's results.
When compared with five favorably used microalgae species in bivalve feeding, A. anophagefferens is similar to, and, in certain cases higher than, these algal species regarding their content of stearidonic acid (C18:4n-3), α-linolenic acid (C18:3n-3), DHA, and total PUFA (Table 6). Although the EPA content of A. anophagefferens is lower than that of most of these species, the content of stearidonic acid (SDA, C18:4n-3), which is an important precursor for EPA (Jónasdóttir, 2019), surpassed those of all the species.
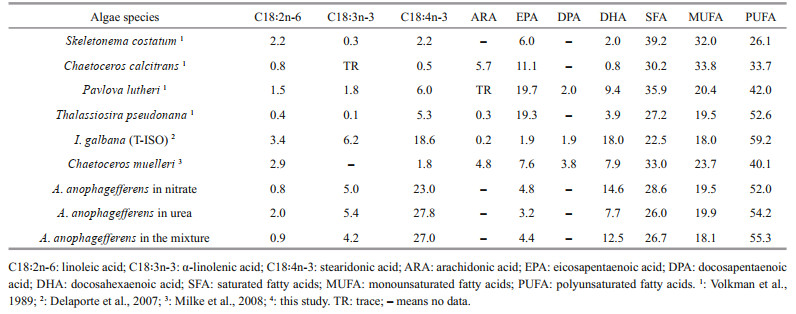
|
Hard clams (M. mercenaria), marine mussels (M. edulis) and bay scallops (A. irradians) are three bivalve mollusk species that are severely affected by brown tide. Since bivalve nutritional requirements are different among species, a detailed comparison is indispensable. When served as a solo food supplement, both S. costatum and I. galbana (T-ISO) consistently supported rapid growth of M. mercenaria (Helm and Laing, 1987; Wikfors et al., 1992; Bricelj and MacQuarrie, 2007). The C18-PUFA (except C18:2n-6) and DHA contents of A. anophagefferens in the nitrate and nitrate-urea mixture were higher than those of S. costatum, whereas the EPA level was lower. When compared with I. galbana (T-ISO), the C18:4n-3 and EPA levels were higher, while the C18:3n-3 and DHA contents were lower. Portilla et al. (2015) found that an exogenous supplement of EPA and DHA is indispensable for hard calms acclimation to declining temperatures during the overwinter period. The present study proved that these two PUFAs are contained in A. anophagefferens cultured in different nitrogen sources.
For A. irradians, Milke et al. (2006) reported that, to support fast growth of postlarval and juvenile bay scallops, in addition to EPA and DHA, n-6 PUFAs such as ARA and DPA are also essential. A Pavlova sp. (CCMP 459) and Chaetoceros muelleri combination diet containing high amounts of ARA and DPA, outperformed all other diets. Similarly, excellent growth of marine mussel M. edulis larvae was supported by C. muelleri, an algae that contains a high amount of ARA (Leonardos and Lucas, 2000). Although n-6 PUFAs were not detected in the present study or in Bricelj's report (Bricelj et al., 1989), the presence of both ARA (2.35%) and DPA (4.55%) in A. anophagefferens was detected by Bigelow et al. (2013), upon culture in L1 medium. Moreover, the fast growth of M. edulis larvae was also supported by S. costatum, which contained no ARA or DPA (Leonardos and Lucas, 2000).
The fatty acid composition of algae is significantly affected by the culture medium. Bigelow et al. (2013) reported that A. anophagefferens contained less C14:0 (10.99%), C16:1n-7 (4.55%), and MUFAs (7.38%), and more C18:2n-6 (3.92%), C18:4n-3 (28.57%), and PUFAs (68.76%) when cultured in L1 medium. The present study found that both EPA and DHA levels were significantly lower when cultured in urea (P < 0.05). Moreover, when expressed as per unit of algal dry weight (mg/g), cultures in urea contained significantly lower levels of EPA, DHA, SFA, PUFA, and TFAC than cultures in nitrate and the nitrateurea mixture (P < 0.05). The nutritional value of A. anophagefferens dropped when cultured in urea; nevertheless, both EPA and DHA contents were still comparable with S. costatum, which support good growth of M. mercenaria and M. edulis larvae. The lack of n-6 PUFAs may make A. anophagefferens a nutritionally inadequate food source for A. irradians. Paradoxically, if this is true, instead of expecting feeding cessation and starvation, a low growth rate and delayed development of the bivalve should be expected.
4.4 Amino acid composition of A. anophagefferensIn total, 11 amino acids are essential for bivalve mollusks: threonine, valine, methionine, isoleucine, leucine, phenylalanine, lysine, histidine, arginine, proline and tryptophan (Brown, 1991). Except for tryptophan, which was destroyed by hydrolysis during analysis, the other 10 essential amino acids were detected in A. anophagefferens. Moreover, when cultivated in urea, the levels of all 10 amino acids were either not significantly or significantly higher than those of cells cultured in nitrate. Ou et al. (2018a) found that, compared with nitrate-cultivated cells, A. anophagefferens cultured in urea contained significantly higher amounts of protein in the stationary phase. Dong et al. (2014) reported that many transcripts encoding enzymes involved in amino acid synthesis increased when A. anophagefferens was cultured in urea. The results of the present study are in agreement with Ou et al. (2018a) and Dong et al. (2014)'s observations.
When compared with favorably used species in mariculture, the amino acid composition of A. anophagefferens is similar to that of these species. Unlike the amount of protein, the quality of the protein is unlikely to be an element that contributes to differences in nutritional value among microalgae, since it is generally accepted that the amino acid composition is rather similar among species (Guedes and Malcata, 2012). Therefore, when applied to bivalve feeding, the amino acid composition of algae is unlikely to be responsible for differential growth performance.
4.5 Carbohydrate composition of A. anophagefferensGlucose is the principal sugar in most species used in mariculture (Brown, 1991). However, the present study showed that galacturonic acid is the primary monomeric carbohydrate in the A. anophagefferens hydrolysate. Although available research does not suggest a close connection between the monomeric carbohydrate composition and the nutritional value of microalgae (Wikfors et al., 1992), the proportion of glucose in the readily hydrolysable carbohydrate may be related to the nutritional quality of microalgae (Knauer and Southgate, 1999). The glucose content is 21%–87% in species that are frequently used in aquaculture, when expressed as a percent of the total carbohydrates (Brown, 1991). Compared with them, A. anophagefferens contained relatively lower amounts of glucose (12.65%–21.74%).
The best growth of juvenile oysters and larval scallops was associated with high levels of carbohydrates provided in algal diets, when sufficient protein and essential fatty acids were also supplied (Guedes and Malcata, 2012). Compared with cultures in nitrate, the carbohydrate concentration of A. anophagefferens significantly dropped when cultured in urea and nitrate-urea mixture, which may reduce the nutritional value. Nevertheless, it is still comparable with some of the favorably used species in bivalve feeding (Table 5).
4.6 The speculated reason for bivalve starvation caused by A. anophagefferensTo serve as an appropriate diet for bivalves, apart from having high nutritional value, microalgae should be easily digested, nontoxic and of adequate size (Cheng et al., 2020). Recruitment failure, reduced filter feeding rate, and cessation of feeding were observed when A. anophagefferens reached a certain cell density (Tracey, 1988; Bricelj et al., 2001; Gobler and Sunda, 2012). The retention efficiency of bivalves usually decreases with decreasing particle size (Cranford, 2019). The small size of A. anophagefferens may adversely affect bivalve growth. However, it seems not be an adequate explanation that accounts for starvation. In addition, the high absorbance efficiency (up to 90%) (Bricelj and Kuenstner, 1989) of A. anophagefferens by M. edulis and A. irradians apparently rules out indigestibility. Moreover, the relatively low nutritional value of A. anophagefferens cultured in urea is still comparable with algae such as S. costatum that supports fast bivalve growth. A plausible cause of detrimental effects is the toxicity of cells, which should be seriously evaluated.
5 CONCLUSIONThis study found that the gross biochemical compositions of A. anophagefferens are comparable with the values found in the literature for species that are frequently used to feed bivalves. When cultured in nitrate and a nitrate-urea mixture, fatty acid levels, especially PUFAs, including EPA and DHA, are comparable to and in certain cases higher than, the values found in the literature. Although the DHA, EPA, and PUFA contents were significantly decreased when cultivated in urea, they are still comparable to those of S. costatum, which supports good growth of M. mercenaria and M. edulis larvae. In summary, we found that A. anophagefferens is not a nutritionally inadequate food source for bivalve mollusks. Cytotoxicity is the more likely cause of detrimental effects.
6 DATA AVAILABILITY STATEMENTThe datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Supplementary material is available in the online version of this article at https://doi.org/10.1007/s00343-022-1343-7.
Bigelow N, Barker J, Ryken S, et al. 2013. Chrysochromulina sp.: a proposed lipid standard for the algal biofuel industry and its application to diverse taxa for screening lipid content. Algal Research, 2(4): 385-393.
DOI:10.1016/j.algal.2013.07.001 |
Bligh E G, Dyer W J. 1959. A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology, 37(8): 911-917.
DOI:10.1139/o59-099 |
Bricelj V M, Fisher N S, Guckert J B et al. 1989. Lipid composition and nutritional value of the brown tide alga Aureococcus anophagefferens. In: Cosper E M, Bricelj V M, Carpenter E J eds. Novel Phytoplankton Blooms. Springer, Berlin. p. 85-100.
|
Bricelj V M, Kuenstner S H. 1989. Effects of the "brown tide" on the feeding physiology and growth of bay scallops and mussels. In: Cosper E M, Bricelj V M, Carpenter E J eds. Novel Phytoplankton Blooms. Springer, Berlin. p. 491-509.
|
Bricelj V M, Lonsdale D J. 1997. Aureococcusanophagefferens: causes and ecological consequences of brown tides in U.S. mid‐Atlantic coastal waters. Limnology and Oceanography, 42(5 Pt 2): 1023-1038.
DOI:10.4319/lo.1997.42.5_part_2.1023 |
Bricelj V M, MacQuarrie S P, Smolowitz R. 2004. Concentration-dependent effects of toxic and non-toxic isolates of the brown tide alga Aureococcus anophagefferens on growth of juvenile bivalves. Marine Ecology Progress Series, 282: 101-114.
DOI:10.3354/meps282101 |
Bricelj V M, MacQuarrie S P. 2007. Effects of brown tide (Aureococcus anophagefferens) on hard clam Mercenaria mercenaria larvae and implications for benthic recruitment. Marine Ecology Progress Series, 331: 147-159.
DOI:10.3354/meps331147 |
Bricelj V, MacQuarrie S, Schaffner R. 2001. Differential effects of Aureococcus anophagefferens isolates ("brown tide") in unialgal and mixed suspensions on bivalve feeding. Marine Biology, 139(4): 605-616.
DOI:10.1007/s002270100612 |
Brown M R. 1991. The amino-acid and sugar composition of 16 species of microalgae used in mariculture. Journal of Experimental Marine Biology and Ecology, 145(1): 79-99.
DOI:10.1016/0022-0981(91)90007-J |
Brown M R. 2002. Nutritional value and use of microalgae in aquaculture. In: Cruz-Suárez L E, Ricque-Marie D, Tapia-Salazar M et al. eds. Avances en Nutrición Acuicola VI. Memorias del VI Simposium Internacional de Nutrición Acuícola. Cancún.
|
Caron D A, Gobler C J, Lonsdale D J, et al. 2004. Microbial herbivory on the brown tide alga, Aureococcus anophagefferens: results from natural ecosystems, mesocosms and laboratory experiments. Harmful Algae, 3(4): 439-457.
DOI:10.1016/j.hal.2004.06.011 |
Cavanaugh G M. 1956. Formulae and Methods IV of the Marine Biological Laboratory Chemical Room. Marine Biological Laboratory, Woods Hole. p. 67-69.
|
Cheng P F, Zhou C X, Chu R R, et al. 2020. Effect of microalgae diet and culture system on the rearing of bivalve mollusks: nutritional properties and potential cost improvements. Algal Research, 51: 102076.
DOI:10.1016/j.algal.2020.102076 |
Cranford P J. 2019. Magnitude and extent of water clarification services provided by bivalve suspension feeding. In: Smaal A C, Ferreira J G, Grant J et al. eds. Goods and Services of Marine Bivalves. Springer, Cham. p. 119-141.
|
Da Costa F, Robert R, Quéré C, et al. 2015. Essential fatty acid assimilation and synthesis in larvae of the bivalve Crassostrea gigas. Lipids, 50(5): 503-511.
DOI:10.1007/s11745-015-4006-z |
Delaporte M, Chu F L, Langdon C, et al. 2007. Changes in biochemical and hemocyte parameters of the Pacific oysters Crassostrea gigas fed T-ISO supplemented with lipid emulsions rich in eicosapentaenoic acid. Journal of Experimental Marine Biology and Ecology, 343(2): 261-275.
DOI:10.1016/j.jembe.2006.12.021 |
Delaunay F, Marty Y, Moal J, et al. 1993. The effect of monospecific algal diets on growth and fatty acid composition of Pecten maximus (L.) larvae. Journal of Experimental Marine Biology and Ecology, 173(2): 163-179.
DOI:10.1016/0022-0981(93)90051-O |
Dong H P, Huang K X, Wang H L, et al. 2014. Understanding strategy of nitrate and urea assimilation in a Chinese strain of Aureococcus anophagefferens through RNA-Seq analysis. PLoS One, 9(10): e111069.
DOI:10.1371/journal.pone.0111069 |
Durmaz Y. 2007. Vitamin E (α-tocopherol) production by the marine microalgae Nannochloropsis oculata (Eustigmatophyceae) in nitrogen limitation. Aquaculture, 272(1-4): 717-722.
DOI:10.1016/j.aquaculture.2007.07.213 |
Fernández-Reiriz M J, Pérez-Camacho A, Peteiro L G, et al. 2011. Growth and kinetics of lipids and fatty acids of the clam Venerupis pullastra during larval development and postlarvae. Aquaculture Nutrition, 17(1): 13-23.
DOI:10.1111/j.1365-2095.2009.00701.x |
Gainey Jr L F, Shumway S E. 1991. The physiological effect of Aureococcus anophagefferens ("brown tide") on the lateral cilia of bivalve mollusks. The Biological Bulletin, 181(2): 298-306.
DOI:10.2307/1542101 |
Gallager S M, Stoecker D K, Bricelj V M. 1989. Effects of the brown tide alga on growth, feeding physiology and locomotory behavior of scallop larvae (Argopecten irradians). In: Cosper E M, Bricelj V M, Carpenter EJ eds. Novel Phytoplankton Blooms. Springer, Berlin. p. 511-541.
|
Gobler C J, Sunda W G. 2012. Ecosystem disruptive algal blooms of the brown tide species, Aureococcus anophagefferens and Aureoumbra lagunensis. Harmful Algae, 14: 36-45.
DOI:10.1016/j.hal.2011.10.013 |
Greenfield D I, Lonsdale D J, Cerrato R M, et al. 2004. Effects of background concentrations of Aureococcus anophagefferens (brown tide) on growth and feeding in the bivalve Mercenaria mercenaria. Marine Ecology Progress Series, 274: 171-181.
DOI:10.3354/meps274171 |
Griffith A W, Harke M J, DePasquale E, et al. 2019. The harmful algae, Cochlodinium polykrikoides and Aureococcus anophagefferens, elicit stronger transcriptomic and mortality response in larval bivalves (Argopecten irradians) than climate change stressors. Ecology and Evolution, 9(8): 4931-4948.
DOI:10.1002/ece3.5100 |
Guedes A C, Malcata F X. 2012. Nutritional value and uses of microalgae in aquaculture. In: Muchlisin Z ed. Aquaculture. IntechOpen. p. 59-78.
|
Guillard R R L, Ryther J H. 1962. Studies of marine planktonic diatoms: I. Cyclotella nana Hustedt, and Detonula confervacea (Cleve) Gran. Canadian Journal of Microbiology, 8(2): 229-239.
|
Harke M J, Gobler C J, Shumway S E. 2011. Suspension feeding by the Atlantic slipper limpet (Crepidula fornicata) and the northern quahog (Mercenaria mercenaria) in the presence of cultured and wild populations of the harmful brown tide alga, Aureococcus anophagefferens. Harmful Algae, 10(5): 503-511.
DOI:10.1016/j.hal.2011.03.005 |
He X J, Han D D, Han L Y, et al. 2018. Grazing and performance of the copepod Pseudodiaptomus poplesia on a Chinese strain of Aureococcus anophagefferens. Acta Oceanologica Sinica, 37(4): 69-76.
DOI:10.1007/s13131-018-1168-6 |
Helm M M, Laing I. 1987. Preliminary observations on the nutritional value of 'Tahiti Isochrysis' to bivalve larvae. Aquaculture, 62(3-4): 281-288.
DOI:10.1016/0044-8486(87)90170-0 |
Hemaiswarya S, Raja R, Kumar R R, et al. 2011. Microalgae: a sustainable feed source for aquaculture. World Journal of Microbiology and Biotechnology, 27(8): 1737-1746.
DOI:10.1007/s11274-010-0632-z |
Jónasdóttir S H. 2019. Fatty acid profiles and production in marine phytoplankton. Marine Drugs, 17(3): 151.
DOI:10.3390/md17030151 |
Kana T M, Lomas M W, MacIntyre H L, et al. 2004. Stimulation of the brown tide organism, Aureococcus anophagefferens, by selective nutrient additions to in situ mesocosms. Harmful Algae, 3(4): 377-388.
DOI:10.1016/j.hal.2004.06.008 |
Kaparapu J. 2018. Application of microalgae in aquaculture. Phykos, 48(1): 21-26.
|
Keller A A, Rice R L. 1989. Effects of nutrient enrichment on natural populations of the brown tide phytoplankton Aureococcus anophagefferens (Chrysophyceae). Journal of Phycology, 25(4): 636-646.
DOI:10.1111/j.0022-3646.1989.00636.x |
Knauer J, Southgate P C. 1999. A review of the nutritional requirements of bivalves and the development of alternative and artificial diets for bivalve aquaculture. Reviews in Fisheries Science, 7(3-4): 241-280.
DOI:10.1080/10641269908951362 |
Langdon C J, Waldock M J. 1981. The effect of algal and artificial diets on the growth and fatty acid composition of Crassostrea gigas spat. Journal of the Marine Biological Association of the United Kingdom, 61(2): 431-448.
DOI:10.1017/S0025315400047056 |
Laurens L M L. 2016. Summative Mass Analysis of Algal Biomass-Integration of Analytical Procedures: Laboratory Analytical Procedure (LAP). National Renewable Energy Lab. (NREL), Golden, CO, USA.
|
Leonardos N, Lucas I A N. 2000. The nutritional value of algae grown under different culture conditions for Mytilus edulis L. larvae. Aquaculture, 182(3-4): 301-315.
DOI:10.1016/S0044-8486(99)00269-0 |
Levasseur M, Thompson P A, Harrison P J. 1993. Physiological acclimation of marine phytoplankton to different nitrogen sources. Journal of Phycology, 29(5): 587-595.
DOI:10.1111/j.0022-3646.1993.00587.x |
Liu H B, Buskey E J. 2000. The exopolymer secretions (EPS) layer surrounding Aureoumbra lagunensis cells affects growth, grazing, and behavior of protozoa. Limnology and Oceanography, 45(5): 1187-1191.
DOI:10.4319/lo.2000.45.5.1187 |
Lonsdale D J, Cosper E M, Kim W S, et al. 1996. Food web interactions in the plankton of Long Island bays, with preliminary observations on brown tide effects. Marine Ecology Progress Series, 134: 247-263.
DOI:10.3354/meps134247 |
Lourenco S O, Barbarino E, Mancini-Filho J, et al. 2002. Effects of different nitrogen sources on the growth and biochemical profile of 10 marine microalgae in batch culture: an evaluation for aquaculture. Phycologia, 41(2): 158-168.
DOI:10.2216/i0031-8884-41-2-158.1 |
Marshall R, McKinley S, Pearce C M. 2010. Effects of nutrition on larval growth and survival in bivalves. Reviews in Aquaculture, 2(1): 33-55.
DOI:10.1111/j.1753-5131.2010.01022.x |
Martínez-Fernández E, Acosta-Salmón H, Southgate P C. 2006. The nutritional value of seven species of tropical microalgae for black-lip pearl oyster (Pinctada margaritifera, L.) larvae. Aquaculture, 257(1-4): 491-503.
DOI:10.1016/j.aquaculture.2006.03.022 |
Matias D, Joaquim S, Ramos M, et al. 2011. Biochemical compounds' dynamics during larval development of the carpet-shell clam Ruditapes decussatus (Linnaeus, 1758): effects of mono-specific diets and starvation. Helgoland Marine Research, 65(3): 369-379.
DOI:10.1007/s10152-010-0230-3 |
Milke L M, Bricelj V M, Parrish C C. 2006. Comparison of early life history stages of the bay scallop, Argopecten irradians: effects of microalgal diets on growth and biochemical composition. Aquaculture, 260(1-4): 272-289.
DOI:10.1016/j.aquaculture.2006.06.004 |
Milke L M, Bricelj V M, Parrish C C. 2008. Biochemical characterization and nutritional value of three Pavlova spp. in unialgal and mixed diets with Chaetoceros muelleri for postlarval sea scallops, Placopecten magellanicus. Aquaculture, 276(1-4): 130-142.
DOI:10.1016/j.aquaculture.2008.01.040 |
Mulholland M R, Boneillo G E, Bernhardt P W, et al. 2009. Comparison of nutrient and microbial dynamics over a seasonal cycle in a mid-Atlantic coastal lagoon prone to Aureococcus anophagefferens (brown tide) blooms. Estuaries and Coasts, 32(6): 1176-1194.
DOI:10.1007/s12237-009-9218-0 |
Ou L J, Cai Y Y, Jin W Y, et al. 2018a. Understanding the nitrogen uptake and assimilation of the Chinese strain of Aureococcus anophagefferens (Pelagophyceae). Algal Research, 34: 182-190.
DOI:10.1016/j.algal.2018.07.019 |
Ou L J, Liu X H, Li J J, et al. 2018b. Significant activities of extracellular enzymes from a brown tide in the coastal waters of Qinhuangdao, China. Harmful Algae, 74: 1-9.
DOI:10.1016/j.hal.2018.03.005 |
Padilla D K, Doall M H, Gobler C J, et al. 2006. Brown tide alga, Aureococcus anophagefferens, can affect growth but not survivorship of Mercenaria mercenaria larvae. Harmful Algae, 5(6): 736-748.
DOI:10.1016/j.hal.2006.03.004 |
Portilla S E, Branco B F, Tanacredi J T. 2015. Preliminary investigation into the effects of two dietary fatty acids, 20:5n-3 and 22:6n-3, on mortality of juvenile Mercenaria mercenaria during the approach to winter. Aquaculture International, 23(6): 1357-1376.
DOI:10.1007/s10499-015-9889-4 |
Probyn T A, Bernard S, Pitcher G C, et al. 2010. Ecophysiological studies on Aureococcus anophagefferens blooms in Saldanha Bay, South Africa. Harmful Algae, 9(2): 123-133.
DOI:10.1016/j.hal.2009.08.008 |
Probyn T, Pitcher G, Pienaar R, et al. 2001. Brown tides and mariculture in Saldanha bay, South Africa. Marine Pollution Bulletin, 42(5): 405-408.
DOI:10.1016/S0025-326X(00)00170-3 |
Saucedo P E, González-Jiménez A, Acosta-Salmón H, et al. 2013. Nutritional value of microalgae-based diets for lions-paw scallop (Nodipecten subnodosus) juveniles reared at different temperatures. Aquaculture, 392-395: 113-119.
DOI:10.1016/j.aquaculture.2013.02.001 |
Sieburth J M, Johnson P W, Hargraves P E. 1988. Ultrastructure and ecology of Aureococcus anophageferens gen. et sp. nov. (Chrysophyceae): the Dominant picoplankter during a bloom in Narragansett bay, Rhode Island, summer 1985. Journal of Phycology, 24(3): 416-425.
DOI:10.1111/j.1529-8817.1988.tb04485.x |
Smith J K, Lonsdale D J, Gobler C J, et al. 2008. Feeding behavior and development of Acartia tonsa nauplii on the brown tide alga Aureococcus anophagefferens. Journal of Plankton Research, 30(8): 937-950.
DOI:10.1093/plankt/fbn050 |
Tang B J, Liu B Z, Wang G D, et al. 2006. Effects of various algal diets and starvation on larval growth and survival of Meretrix meretrix. Aquaculture, 254(1-4): 526-533.
DOI:10.1016/j.aquaculture.2005.11.012 |
Tracey G A. 1988. Feeding reduction, reproductive failure, and mortality in Mytilus edulis during the 1985 'brown tide' in Narragansett Bay, Rhode Island. Marine Ecology Progress Series, 50: 73-81.
|
Uriarte I, Farías A. 1999. The effect of dietary protein content on growth and biochemical composition of Chilean scallop Argopecten purpuratus (L.) postlarvae and spat. Aquaculture, 180(1-2): 119-127.
DOI:10.1016/S0044-8486(99)00145-3 |
Van Houcke J, Medina I, Maehre H K, et al. 2017. The effect of algae diets (Skeletonema costatum and Rhodomonas baltica) on the biochemical composition and sensory characteristics of Pacific cupped oysters (Crassostrea gigas) during land-based refinement. Food Research International, 100(Pt 1): 151-160.
DOI:10.1016/j.foodres.2017.06.041 |
Van Wychen S, Laurens L M L. 2016. Determination of Total Solids and Ash in Algal Biomass: Laboratory Analytical Procedure (LAP). National Renewable Energy Lab. (NREL), Golden, CO, USA.
|
Van Wychen S, Laurens L M L. 2020. Total carbohydrate content determination of microalgal biomass by acid hydrolysis followed by spectrophotometry or liquid chromatography. In: Spilling K ed. Biofuels from Algae: Methods and Protocols. Humana, New York. p. 191-202, https://doi.org/10.1007/7651_2017_106.
|
Van Wychen S, Ramirez K, Laurens L M L. 2016. Determination of Total Lipids as Fatty Acid Methyl Esters (FAME) by in Situ Transesterification: Laboratory Analytical Procedure (LAP). National Renewable Energy Lab. (NREL), Golden, CO, USA.
|
Volkman J K, Jeffrey S W, Nichols P D, et al. 1989. Fatty acid and lipid composition of 10 species of microalgae used in mariculture. Journal of Experimental Marine Biology and Ecology, 128(3): 219-240.
DOI:10.1016/0022-0981(89)90029-4 |
Wikfors G H, Ferris G E, Smith B C. 1992. The relationship between gross biochemical composition of cultured algal foods and growth of the hard clam, Mercenaria mercenaria (L.). Aquaculture, 108(1-2): 135-154.
DOI:10.1016/0044-8486(92)90324-E |
Yao P, Lei L, Zhao B, et al. 2019. Spatial-temporal variation of Aureococcus anophagefferens blooms in relation to environmental factors in the coastal waters of Qinhuangdao, China. Harmful Algae, 86: 106-118.
DOI:10.1016/j.hal.2019.05.011 |
Zhang Q C, Qiu L M, Yu R C, et al. 2012. Emergence of brown tides caused by Aureococcus anophagefferens Hargraves et Sieburth in China. Harmful Algae, 19: 117-124.
DOI:10.1016/j.hal.2012.06.007 |
Zhang Q C, Yu R C, Zhao J Y, et al. 2021. Distribution of Aureococcus anophagefferens in relation to environmental factors and implications for brown tide seed sources in Qinhuangdao coastal waters, China. Harmful Algae, 109: 102105.
DOI:10.1016/j.hal.2021.102105 |
Zhen Y, Qiao L, Gu B, et al. 2016. Characteristics of eukaryotic microalgal community and its abiotic influencing factors during brown tide blooms near Qinhuangdao, China. Harmful Algae, 57: 1-12.
DOI:10.1016/j.hal.2016.05.001 |
Zhukova N V. 2019. Fatty acids of marine mollusks: impact of diet, bacterial symbiosis and biosynthetic potential. Biomolecules, 9(12): 857.
DOI:10.3390/biom9120857 |
 2022, Vol. 40
2022, Vol. 40


