Institute of Oceanology, Chinese Academy of Sciences
Article Information
- YAN Tian
- The "harmful algae and algal toxins in coastal waters of China: investigation and database" project
- Journal of Oceanology and Limnology, 40(6): 2081-2093
- http://dx.doi.org/10.1007/s00343-022-2165-3
Article History
- Received Apr. 3, 2022
- accepted in principle May 8, 2022
- accepted for publication Jun. 30, 2022
2 Laboratory for Marine Ecology and Environmental Science, Pilot National Laboratory for Marine Science and Technology(Qingdao), Qingdao 266237, China;
3 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
Toxic and harmful algal blooms have frequently occurred and pose a serious problem in the marine environment. They were previously reported to have intensified in coastal regions globally in recent decades (Anderson et al., 2012). While recent statistical analysis has thrown doubt of previous assumptions of harmful algal bloom (HAB) increases at a global scale (Hallegraeff et al., 2021), rapid economic development and associated environmental impacts in Chinese waters, including benthos degradation (Xu et al., 2017) and increase in giant jellyfish blooms (Sun et al., 2015) suggests that such doubt on HAB increase should not yet be expressed for Chinese waters. Offshore HABs mainly include red tides (referred to as microalgal blooms here on in), brown tides caused by picoalgae Aureococcus sp., green tides caused by green macroalgae and golden tides caused by brown macroalgae (Cosper et al., 1990; Fletcher, 1996; Anderson, 1997b; Smetacek and Zingone, 2013). HABs are an algal bloom phenomenon that cause harm through massive proliferation or toxin contamination (Kudela et al., 2017). They have been increasing along the coast, and have had serious social and economic impacts in China over recent decades (Li et al., 2021). Moreover, algal toxins produced by toxic algae threaten food safety through the food chain, and increasing poisoning incidents such as paralytic shellfish poisoning (PSP) and diarrhetic shellfish poisoning (DSP) have been reported recently (Yan et al., 2002; Hallegraeff, 2021). Shellfish exports to the EU were banned for 19 years until 2018 due to algal toxin contamination problems, resulting in serious economic losses. HAB disaster occurrence has extended along the coast of China from the south to the north, endangering human health, coastal tourism, offshore aquaculture, and nuclear power safety (Wang et al., 2021b; Gu et al., 2022). Furthermore, HABs have become a significant challenge for recent development strategies, such as the construction of "Healthy China" and "Beautiful China", as well as the protection of "Blue Granary" and "Ecological Security".
Chinese scientists started to study the problem of HABs during the 1970s. From the 1990s, China successively established several national HAB projects: two major National Natural Science Foundation of China projects in the 1990s led by Prof. Yuzao Qi, two 973 Plan projects led by Prof. Mingjiang Zhou since 2000, and two special Research and Development projects for red and green tides starting from 2017. Each of these national projects focused on the causes and control mechanisms of HABs in specific sea areas along the Chinese coast, such as Daya Bay, Jiaozhou Bay, Changjiang estuary, and the Beibu Gulf. For national red tide monitoring, 19 zones, mainly in the estuaries and bays along the coast, were established by the State Oceanic Administration (SOA, now Ministry of Natural Resources) in China.
Through previous project implementation and other investigations, the causes, impacts, and mechanism of mitigation of major algal bloom disasters (such as blooms caused by Prorocentrum donghaiense, Karenia mikimotoi, Phaeocystis globasa, and Ulva prolifera), as well as the regional distribution pattern of typical harmful algae and algal toxins in coastal waters, have been reviewed (Yan et al., 2022). However, general background data for the whole offshore region of China remains insufficient, and basic investigative project support is urgently required.
Here, we summarize the main challenges related to harmful algae and algal toxins in China, and the "Harmful Algae and Algal Toxins in Coastal Waters of China: Investigation and Database" (HAATC) project, including its latest progress.
2 INCREASING MARINE DISASTERS RELATED TO HABS AND ALGAL TOXINS IN CHINAOver the past 20 years, algal blooms caused by toxic and harmful algae in China's coastal waters have been increasing (He et al., 2021). Toxic microalgae, such as Alexandrium sp., Dinophysis sp. and Pseudo-nitzschia sp., threatening human life and health have attracted much attention in China, since they produce various algal toxins affecting seafood safety; Other harmful algae, such as K. mikimotoi, Aureococcus anophagefferens, and U. prolifera, although without any known human toxins, have impacted marine aquaculture, ecology, and environments in China through large-scale, high biomass blooms and the generation of other toxic substances (Xiao et al., 2021; Gu et al., 2022). To date, the algal blooms caused by toxic and harmful algae in China's offshore waters have evolved, as described below:
Marine microalgae blooms in China are increasing in terms of both number of species, and toxic forms. Brown tides, which have previously only occurred in the United States and South Africa, have started to occur in the Bohai Sea of China almost annually, and have caused economic losses involving hundreds of millions of yuan in the scallop mariculture between 2007 and 2014 (Zhang et al., 2012). K. mikimotoi blooms, which had already caused a large number of fish deaths worldwide, had a devastating impact on the abalone mariculture industry in Fujian in 2012, causing about US$ 300 million economic losses (MNR, 2012). The fish-killing P. globosa has posed a major threat to the safety of the cold source system of the Guangxi nuclear power plant by blocking the filtration facilities in recent years (Wang et al., 2021b). The emergence of newly-recorded algae species and new harmful forms in coastal waters increases the necessity of obtaining knowledge about background algal species, and techniques for identifications and detections to support the early monitoring and warning of HABs.
Macroalgal bloom disasters in China's coastal waters have shown an increasing trend. A massive U. prolifera green tide in the South Yellow Sea has occurred annually since 2007, resulting in more than US$ 15 million economic losses each year (Zhou et al., 2015). Moreover, a Sargassum horneri golden tide appeared offshore at the end of 2016, and impacted the area of laver culture in the shoal of northern Jiangsu for the first time (Xing et al., 2017). At that time, the laver industry in Yancheng and Nantong alone suffered a direct loss of as much as US$ 75 million (MNR, 2017). Furthermore, a co-occurrence of green, golden, and red tides has been observed during the spring and summer 2017 in the Southern Yellow Sea, which is relatively rare along coasts globally (Kong et al., 2018). Compared with microalgal blooms, the social and economic losses associated with macroalgal blooms are greater due to the higher biomass and the large floating algae, which can be transported with ocean currents causing disasters in different places, making their prediction and prevention difficult (Xiao et al., 2020).
Marine algal toxins in China threaten food safety and human health. Recently, there has been a significant increase in seafood poisoning incidents caused by algal toxins (Yan et al., 2022). For example, PSP incidents were reported in Fujian Province in June 2017; more than 30 poisoned patients developed symptoms, such as dizziness, vomiting and numbness of limbs, after ingestion of contaminated mussels in Shishi (Quanzhou) and Longhai and Zhangpu (Zhangzhou) (Fan et al., 2021). Another PSP incident was caused by the consumption of purple mussels in Qinhuangdao City at the end of April 2016, where nine people were hospitalized (Yu and Luo, 2016). At the end of May 2011, more than 200 people were hospitalized for DSP caused by purple mussels in Ningde (Fujian) and Ningbo (Zhejiang) (Li et al., 2012). Since the poisoning symptoms of algal toxins are easily confused with other diseases, a considerable number of algal toxin poisoning events have not been identified or counted. Toxin events can cause market panic, a large amount of seafood has been destroyed or banned for sale, and earnings of foreign exchange has been severely and repeatedly impacted (Yan et al., 2022). In recent years, some new records of algal toxins, such as azaspiracid, cycloimines and neurotoxins β-N-methylamino-l-alanine have often been detected in shellfish in China (Gu et al., 2013; Liu et al., 2019; Wang et al., 2021a).
In summary, the issues of HAB and algal toxins in China's offshore waters are becoming increasingly complex and diverse. There is therefore an urgent need to further investigate harmful algae and algal toxins in coastal China.
3 INTERNATIONAL HAB RESEARCHHABs have long been an important marine ecological environment problem and frontier issue in international marine scientific research. The Intergovernmental Oceanographic Commission (IOC) of the United Nations Educational, Scientific and Cultural Organization (UNESCO) and the Scientific Committee on Oceanic Research (SCOR) have jointly supported two international plans for HABs, the "Global Study of Harmful Algal Bloom Ecology and Oceanography" from 1998 to 2013 and the "Global Change and Harmful Algal Bloom Plan" launched in 2016 (GEOHAB, 2001; Kudela et al., 2017). The new plan advocates focusing attention on the occurrence of HABs, and the evolution of algal disasters under the accelerated impacts of global climate change and human activities. Harmful algae and algal toxins have always been one of the core issues of international marine research.
3.1 Progress in research and technologyIn the study of harmful algae in offshore waters, the identification and classification of algae species forms the basis of all research and management. Researchers have performed extensive research and sorting relating to their taxonomy and geographic distribution. Since the 1990s, based on the morphological identification of traditional light and electron microscopy, molecular markers have been gradually used for algae species identification (Scholin and Anderson, 1994). Some algal species have been screened for genetic information in order to distinguish between subspecies and geographical populations, which has been convenient and has led to new understanding for the classification of algae species (Costas, 1997). Therefore, the classification of some microalgae and macroalgae has changed. For example, the Karenia family was established in 2006 based on morphological characteristics, ribosomal large subunit DNA sequence, and pigment composition. Its species previously were assigned to Gymnodiniales Gymnodinium, and a few species belonged to Gyrodinium and Karenia (Bergholtz et al., 2006; Wang et al., 2017). The phylogenetic tree of the Alexandrium complex species was established based also on the sequence of large subunits. The species complex was divided into five distinct groups. Group Ⅰ and group Ⅳ are toxic strains, while groups Ⅱ, Ⅲ, and Ⅴ are nontoxic strains (Wang et al., 2014). Enteromorpha was classified into Ulva based on nuclear-encoded and chloroplast-encoded ribulose-bisphosphate carboxylase gene sequences (Hayden et al., 2003).
In recent years, DNA barcoding technology has become an important technical support in taxonomy and biodiversity research (Saunders, 2005). In contrast to animals, it is more difficult to determine a single bar code gene in plants (Fazekas et al., 2009). Similarly, a unified bar code gene suitable for algae has not been found at present. However, for diatoms, red algae, brown algae, and green algae, DNA bar code gene combinations suitable for this category have been determined through extensive comparison (Saunders and McDevit, 2012). In addition, in the application of the amplicon high-throughput sequencing method for the investigation of genetic diversity of marine phytoplankton, the species of microalgae identified include common toxic and harmful species, as well as many species that are difficult to identify through traditional investigation methods, highlighting the technical advantages (Xiao et al., 2014). There remains much diversity in harmful microalgae to be discovered.
Scientists from various countries have performed a large amount of research into the distributions of harmful algae and algal toxins in different sea areas (Meyer et al., 1928; Sournia, 1995; Yan et al., 2022). The United States and some European countries have determined relatively clear distribution patterns of the main toxic and harmful microalgae and algal toxins in different sea areas, such as Alexandrium and its paralytic shellfish toxins (PSTs) in the Northeast sea area of the United States (Anderson, 1997a), Karenia brevis and its neurotoxic shellfish toxins (NSTs)in the southeast sea area of the United States (Soto et al., 2018), and Pseudo-nitzschia sp. and its amnesic shellfish toxins (ASTs) along the West coast of the US (McCabe et al., 2016), providing a basic background for in-depth research and management. International research on dormant algal cells, especially dinoflagellate cysts, began in the 1960s (Wall and Dale, 1966). The United States, Europe, Japan, and other countries have carried out surveys of dinoflagellate cysts in sediments (Wall and Dale, 1968; Matsuoka, 1999; Dale, 2001). Recently, scientists from Canada, the United Kingdom, Belgium, Germany, and France have jointly investigated dinoflagellate cysts in the sediments of the Black Sea corridor (Mudie et al., 2017).
There are approximately 300 algal species that can cause HABs in the coastal sea, of which about 80 kinds are toxic (Hallegraeff, 2003). According to the intermediate vectors and toxic symptoms of algal toxins, the main algal toxins can be divided into NSTs, PSTs, ASTs, diarrhetic shellfish toxins (DSTs) and ciguatera fish toxins (CFTs) (Hallegraeff, 2003). Among them, PSTs and DSTs are the two most widely distributed and harmful algal toxins. They have been listed as routine detection items in the United Kingdom, the United States, Japan, South Korea, and China. According to statistics, more than 200 types of algal toxins and their derivatives have been found (Gerssen et al., 2011). Algal toxins can accumulate in filter feeding organisms, resulting in potential risks to the food safety of aquatic products (Hallegraeff, 1993). Worldwide, human poisonings and even death, are occasionally caused by algal toxins. There are about 60 000 poisoning incidents in the world every year related to marine algal toxins, mainly PSP and DSP (Gill et al., 2003). At present, the international detection and analysis methods of algal toxins mainly include biological test methods, chemical analysis methods and other methods (Stewart and McLeod, 2014). Among these, the mouse method is the most commonly used biological method. Although its operation is simple, its sensitivity and accuracy are poor. Chemical analysis methods can provide accurate qualitative and quantitative analysis of algal toxins, but need to be calibrated against standard toxins. The main chemical analysis methods include high performance liquid chromatography, thin layer chromatography, capillary electrophoresis, high performance liquid chromatography-mass spectrometry and nuclear magnetic resonance. In addition, the detection methods of algal toxins also include protein phosphatase inhibition test, cytotoxicity test, immunological detection technology, and the biosensor method, amongst others (Carmichael and An, 1999; Heresztyn and Nicholson, 2001; Pierce and Kirkpatrick, 2001).
3.2 HAB guidelines and databasesThe international community attaches great importance to the basic research methods on harmful algae. The manual on harmful marine microalgae (IOC manuals and guide No. 33) and its revision were published in 1995 and 2004, respectively. The manual, illustrating sampling, identification, toxin analysis, and monitoring management, was prepared by 46 well-known scientists with the support of the IOC of UNESCO.
To improve basic research and better protect the ecological environment, long-term preservation of toxic and harmful algae species resources has been carried out. The National Center for Marine Algae and Microbiota (formerly National Center for Culture of Marine Phytoplankton) of the United States has preserved more than 2 000 species of microalgae. Countries such as Australia and Canada have built algae banks involving thousands of strains. Japan has isolated more than 1 000 algal species through the project of "Earth Research Renewal Technology Plan", and established the algal species bank of the National Institute of Environment of Japan.
During this century, international marine science organizations have begun to pay attention to the collection of harmful algal blooms and algal toxin pollution data. The 15th Working Group of the North Pacific Marine Science Organization (PICES) summarized HABs in Japan, China, South Korea, Russia, Canada, the United States, and Mexico, and published a scientific report (Taylor and Trainer, 2002) on HABs in the North Pacific in 2002.
Recently, databases and internet information platforms have been used to collect and release HAB information. In the action plan for the environmental protection, management and development of the Northwest Pacific Marine and coastal area of the United Nations Environment Program (Northwest Pacific Action Plan), the third working group of the Special Monitoring and Coastal Environmental Assessment Regional Activity Centre developed an integrated website related to HABs of Northwest Pacific Action Plan members in China, Japan, South Korea, and Russia (http://www.cearac-project.org/HAB_Integrated_Website/). It publishes information and data on the distribution of major harmful algae, algal bloom events, and toxin distribution in the aforementioned countries.
In 2009, nine internationally renowned microalgae classification experts established a toxic and harmful microalgae classification information platform (http://www.marinespecies.org/hab/index.php). At present, 176 toxic and harmful species have been collected and updated, and species morphological information, photographs, classification, and simple toxin information have been listed. This website is mainly used for the species identification of toxic and harmful microalgae, clarification of classification information, change of name, and accurate classification.
The "Toxic and Harmful Microalgae of the World Ocean" published in 2016, updated the list of toxic and harmful phytoplankton species in the international database (Lassus et al., 2016). This included location, cell density, toxin level in HAB outbreak areas, extraction and analysis protocols of harmful algal active substances and toxins, and the geographical distribution of typical harmful algae and their possible distribution trend in the past 30 years. HAB outbreaks in European countries have shown a downward trend due to intensive monitoring and management, as well as global change and environmental restoration programs (https://ec.europa.eu/info/research-and-innovation/research-area/environment/oceans-and-seas/eu-marine-strategy-framework-directive_en).
4 NATIONAL PROGRAMS OF HARMFUL ALGAE AND ALGAL TOXIN RESEARCH IN CHINASince the 1990s, the National Natural Science Foundation of China has established two major funds successively, "Research on the occurrence mechanism of red tides in the southeast coast of China" and "Research on the dynamics and control mechanism of harmful red tides in typical coastal aquaculture areas of China". Since 2002, the Ministry of Science and Technology has successively organized and implemented two National Key Basic Research Program of China (973 Program); "Ecology and Oceanology, prediction and control of harmful red tides in China's offshore" and "Evolution mechanism of algal bloom disaster and ecological safety in China's offshore", and two recently launched research and development projects of harmful algal blooms; "U. prolifera green tide formation mechanism and comprehensive prevention and control technology" and "formation mechanism, monitoring, prediction and evaluation prevention and control technology of disaster causing red tide in China's offshore". In 2004, the SOA established 19 red tide monitoring areas nationwide. These projects and other related work have effectively improved HAB research in China.
Chinese researchers have obtained experience in the classification of phytoplankton and macroalgae; therefore, the list of diatoms and dinoflagellates has been continuously updated in the books of "Chinese Algae Records", "Marine Species and Their Distributions in China's Seas", and "Marine Organisms in China" (Huang, 2008). Light and electron microscope photographs of algae are included in the books of "Dinoflagellates in the South China Sea" (Lin and Zhou, 1993) and "Dinoflagellates in China's Sea" (Yang et al., 2014). The species and geographic distribution information of some disaster causative macroalgae in China's coastal areas are included in "China's Economic Algae Chronicle" (IOCAS, 1962), "China's Yellow and Bohai Sea Algae" (Zeng, 2009), and "Volume IV of China's Algae Chronicle" (Ding, 2013). However, these catalogues and maps were not complied from the perspective of toxic and harmful algae.
The "Atlas of red tide organisms in China's coastal waters" published in 2004, and "The investigation and evaluation of red tide disasters in China (1933– 2009)" reported the status of red tide organisms, red tide events, environmental factors, and algal toxins in some sea areas in China, and included pictures of red tide organisms. The SOA reports on the red tides and green tides in the bulletin of China's Marine Environmental Quality and the Bulletin of China's Marine Disasters annually. This includes the occurrence times and cumulative area of red tides in each sea area every year, the total monthly area of red tides, the dominant species and timings of red tide outbreaks, a brief description of the dominant species and maximum area, start and end times of some large-scale red tide outbreaks, and the distribution and coverage area of green tides (MNR, 2009–2020).
Chinese researchers have isolated toxic and harmful algae species from China's coastal waters, and maintained a collection of algal cultures including microalgae and macroalgae in their respective laboratories for HAB research.
5 OVERALL DATA ON HARMFUL ALGAE AND ALGAL TOXINS IN CHINA'S OFFSHORE WATERS ARE URGENTLY NEEDEDThere has been a considerable amount of research into the biology, ecology, and oceanography of HABs to date; however, it is often limited to specific sea areas or specific algal bloom disasters (Qi et al., 2017; Zhang et al., 2019; Li et al., 2021). Data on toxic and harmful algae, and algal toxins in China's offshore are insufficient for understanding the distribution pattern, seafood safety risk, and pattern shift under the intensified influence of human activity and global climate change.
The HAATC project aims to obtain basic data on HAB species (microalgae and macroalgae, and cysts) and algal toxin distribution in China's offshore waters, and the temporal and spatial dynamic changes of HAB species, algal toxins, and environmental factors in typical HAB zones. It intends to build a multi-information open platform of basic scientific data to support further HAB research, monitoring, and seafood safety management in China's offshore waters. It will also be useful for understanding the long-term evolution of HAB occurrence under the influence of intensified human activity and global change.
6 HAATC IMPLEMENTATION PLANThe HAATC research plan objectives are to: (1) investigate the large-scale distribution of toxic and harmful microalgae, and cysts in China's offshore waters, and their temporal and spatial dynamic changes in key aquaculture areas and red tide areas; (2) investigate the overall distribution of algal toxins in shellfish in China's coastal waters, and their temporal and spatial distribution in key aquaculture areas and red tide areas; (3) investigate the distribution of disaster causative macroalgae along the coastline and in green tide areas of China; (4) collect environmental data from typical HAB zones; (5) collect typical harmful algae isolates, and study the morphological, genetic, pigment, and toxicity data of each species; and (6) establish an open database and multi information query platform of HABs.
The overall distribution of more than 100 HAB species and their cysts (if any), and 20 algal toxins will be studied in more than 50% of the waters off the coast of China, as well as that of more than 20 macroalgae species along the coast. The temporal and spatial variation of harmful algae and algal toxins (both in phytoplankton and shellfish), and biotic and abiotic factors will be collected under bloom dynamics in eight typical aquaculture areas and HAB zones. More than 60 typical harmful algae species will be isolated and their biological features including morphology, molecular, pigments, and toxicity will be recorded.
Figure 1 shows the HAATC sampling sites along the coast of China. The field cruise, site sampling, laboratory analysis, and data processing are combined in the research. Research vessels are used for overall distribution surveys of microalgae, cysts, and algal toxins in offshore water. Fishing boats are used in eight key zones for red tides and aquaculture, and in one key zone for green tides, where sampling will be carried out in three successive years. The annual plan of the 5-year project is to standardize the investigation protocols and calibrate the methods in the first year, conduct sampling and analysis from the second year to the fourth year, and then focus on the atlas and database construction in the final year.

|
| Fig.1 Large-scale sampling sites (a) and key zones for successive sampling (b) along the coast of China |
Nearly 100 scientists and PhD students from the Institute of Oceanology, Chinese Academy of Sciences, Wuhan Document and Information Center, Jinan University, Xiamen University, Ocean University of China, South China Normal University, Ningbo University, Yellow Sea Fisheries Research Institute, East China Sea Fisheries Research Institute (Chinese Academy of Fishery Sciences), and the First Institute of Oceanography (Ministry of Natural Resources) participate in seven tasks:
Ⅰ. Investigation of the distribution of toxic and harmful microalgae, and algal toxins in the South China Sea.
Ⅱ. Investigation of the distribution of toxic and harmful microalgae, and algal toxins in the East China Sea.
Ⅲ. Investigation of the distribution of toxic and harmful microalgae, and algal toxins in the Yellow Sea and Bohai Sea in China.
Ⅳ. Investigation of the distribution of toxic and harmful microalgae dormant cells in the offshore sediments of China.
Ⅴ. Investigation of the species and distribution of marine harmful macroalgae in China's coastal waters.
Ⅵ. Investigation of the morphological, genetic, pigment, and toxicity features of harmful algae species.
Ⅶ. Construction of a HAB database and information platform.
A Consultation Committee is led by Prof. Yiyu CHEN (Academician), with Profs. Yuzao QI, Mingjiang ZHOU, Songhui LÜ, Rencheng YU, Song SUN, Ping XIE, Wenjun DING, Zhongjie YOU, Xiaoyong SHI, and Tian YAN from the Chinese Academy of Sciences, universities, and other institutes, as well as the Administration of Fishery and Marine Disasters.
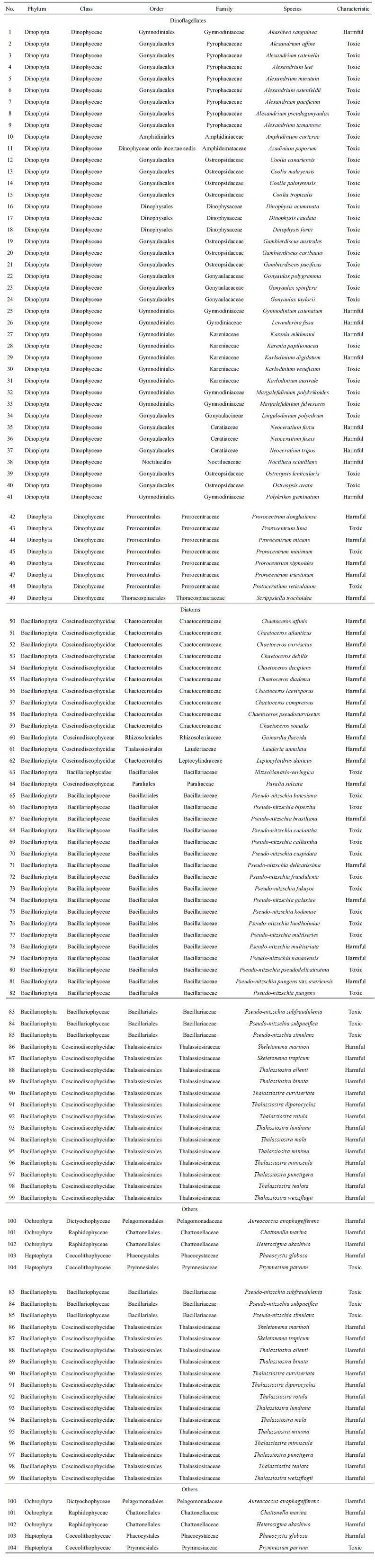
8 SUMMARY OF LATEST HAATC PROGRESSA list of 104 HAB species (shown in Table 1) has been proposed after several discussion meetings, determined by both morphology and genetic parameters measured in the study. Protocols for the sampling and analysis of algae (microalgae and macroalgae, and cysts), toxins (PSTs, lipid-soluble shellfish toxins and domoic acid in phytoplankton and shellfish samples), toxicity test methods (biology and hemolytic toxicity) for harmful algal species, and environmental data were recommended to the research teams, and generally followed national or international guidelines or references. Toxin analysis calibration among six research groups has been carried out using phytoplankton and shellfish samples.
|
After more than 2 years of work, in November 2021, 4 066 samples were obtained, and 10 169 algae data, 14 630 toxin data, and 3 055 environmental data were analyzed. Furthermore, 7 731 microalgae operational taxonomic units have been obtained by molecular sequence analysis, and 24 440 toxic and harmful algae species have been annotated. The results have revealed that the distribution of HAB algae and algal toxins in China's coastal waters has increased, of which 20 newly recorded species of harmful microalgae, seven species of Ulva, and 17 species of dinoflagellate cysts, as well as three new dinoflagellate species, have been found. Domoic acid toxins have started to become widely distributed. The investigation results of toxic algae and algal toxins causing mussel poisoning in Qinhuangdao sea areas have provided an important basis for seafood safety management, the reports of which have been submitted, and applied by national and local government departments. The field data of the key processes of U. prolifera green tides in the South Yellow Sea in 2019, 2020, and 2021 have provided a basis for the scientific evaluation of the national green tide mitigation, and the scientific and technological consultation report to the national and local governments. Over this timeframe, 67 papers have been published, including 56 papers indexed by Science Citation Index, 34 of which acknowledged HAATC as the first funder, as well as one monograph, nine patents, and one software copyright. A special issue of "Harmful Algae and Algal toxins in the Coastal Waters of China" is being organized for publication to the Journal of Oceanology and Limnology in November, 2022.
9 DATA AVAILABILITY STATEMENTAll data generated and/or analyzed during this study are included in this published article.
10 ACKNOWLEDGMENTSpecial appreciation to Profs. Yuzao QI (Jinan University), Mingjiang ZHOU (IOCAS), Songhui LÜ (Jinan University), and Rencheng YU (IOCAS) for valuable discussion, suggestion, and other comments during the project application and implementation. Thanks also to Mr. Minjie SONG (IOCAS) for his help with editing and the figures.
Anderson D M, Cembella A D, Hallegraeff G M. 2012. Progress in understanding harmful algal blooms: paradigm shifts and new technologies for research, monitoring, and management. Annual Review of Marine Science, 4(1): 143-176.
DOI:10.1146/annurev-marine-120308-081121 |
Anderson D M. 1997a. Bloom dynamics of toxic Alexandrium species in the northeastern U.S.. Limnology and Oceanography, 42(5part2): 1009-1022.
DOI:10.4319/lo.1997.42.5_part_2.1009 |
Anderson D M. 1997b. Turning back the harmful red tide. Nature, 388(6642): 513-514.
DOI:10.1038/41415 |
Bergholtz T, Daugbjerg N, Moestrup Ø, et al. 2006. On the identity of Karlodinium veneficum and description of Karlodinium armiger sp. nov. (Dinophyceae), based on light and electron microscopy, nuclear-encoded LSU rDNA, and pigment composition. Journal of Phycology, 42(1): 170-193.
DOI:10.1111/j.1529-8817.2006.00172.x |
Carmichael W W, An J S. 1999. Using an enzyme linked immunosorbent assay (ELISA) and a protein phosphatase inhibition assay (PPIA) for the detection of microcystins and nodularins. Natural Toxins, 7(6): 377-385.
DOI:10.1002/1522-7189(199911/12)7:6<377::AID-NT80>3.0.CO;2-8 |
Cosper E M, Lee C, Carpenter E J. 1990. Novel "Brown Tide" blooms in Long Island embayments: a search for the causes. In: Granéli E, Sundström B, Edler L et al eds. Toxic Marine Phytoplankton. Proceedings of the Fourth International Conference on Toxic Marine Phytoplankton. Elsevier, Lund, Sweden. p. 17-28.
|
Costas E. 1997. Red tides and toxic algal blooms: who's to blame?. Lagascalia, 19(1-2): 165-178.
|
Dale B. 2001. The sedimentary record of dinoflagellate cysts: looking back into the future of phytoplankton blooms. Scientia Marina, 65(S2): 257-272.
DOI:10.3989/scimar.2001.65s2257 |
Ding L P. 2013. Volume IV of China's Algae Chronicle. Science Press, Beijing.
(in Chinese)
|
Fan L Q, Zheng G C, Wu H Y, et al. 2021. Research progress on the accumulation and metabolism of paralytic shellfish toxin in mussels. Marine Sciences, 45(4): 201-212.
(in Chinese with English abstract) |
Fazekas A J, Kesanakurti P R, Burgess K S, et al. 2009. Are plant species inherently harder to discriminate than animal species using DNA barcoding markers?. Molecular Ecology Resources, 9(S1): 130-139.
DOI:10.1111/j.1755-0998.2009.02652.x |
Fletcher R L. 1996. The occurrence of "Green Tides"— a review. In: Schramm W, Nienhuis P H eds. Marine Benthic Vegetation: Recent Changes and the Effects of Eutrophication. Springer, Berlin, Heidelberg. p. 7-43, https://doi.org/10.1007/978-3-642-61398-2_2.
|
GEOHAB. 2001. Global Ecology and Oceanography of Harmful Algal Blooms, Science Plan. IOC and SCOR, Baltimore and Paris. 87p.
|
Gerssen A, Mulder P P J, de Boer J. 2011. Screening of lipophilic marine toxins in shellfish and algae: development of a library using liquid chromatography coupled to orbitrap mass spectrometry. Analytica Chimica Acta, 685(2): 176-185.
DOI:10.1016/j.aca.2010.11.036 |
Gill S, Murphy M, Clausen J, et al. 2003. Neural injury biomarkers of novel shellfish toxins, Spirolides: a pilot study using immunochemical and transcriptional analysis. Neurotoxicology, 24(4-5): 593-604.
DOI:10.1016/S0161-813X(03)00014-7 |
Gu H F, Luo Z H, Krock B, et al. 2013. Morphology, phylogeny and azaspiracid profile of Azadinium poporum (Dinophyceae) from the China Sea. Harmful Algae, 21-22: 64-75.
DOI:10.1016/j.hal.2012.11.009 |
Gu H F, Wu Y R, Lü S H, et al. 2022. Emerging harmful algal bloom species over the last four decades in China. Harmful Algae, 111: 102059.
DOI:10.1016/j.hal.2021.102059 |
Hallegraeff G M, Anderson D M, Belin C, et al. 2021. Perceived global increase in algal blooms is attributable to intensified monitoring and emerging bloom impacts. Communications Earth & Environment, 2(1): 117.
DOI:10.1038/s43247-021-00178-8 |
Hallegraeff G M. 1993. A review of harmful algal blooms and their apparent global increase. Phycologia, 32(2): 79-99.
DOI:10.2216/i0031-8884-32-2-79.1 |
Hallegraeff G M. 2003. Harmful algal blooms: a global overview. In: Hallegraeff G M, Anderson D M, Cembella A D eds. Manual on Harmful Marine Microalgae. UNESCO, Paris. p. 25-49.
|
Hallegraeff G M. 2021. Global Harmful Algal Bloom: Status Report 2021. UNESCO, Paris.
|
Hayden H S, Blomster J, Maggs C A, et al. 2003. Linnaeus was right all along: Ulva and Enteromorpha are not distinct genera. European Journal of Phycology, 38(3): 277-294.
DOI:10.1080/1364253031000136321 |
He X Y, Chen C, Lu C, et al. 2021. Spatial-temporal distribution of red tide in coastal China. IOP Conference Series: Earth and Environmental Science, 783(1): 012141.
DOI:10.1088/1755-1315/783/1/012141 |
Heresztyn T, Nicholson B C. 2001. Determination of cyanobacterial hepatotoxins directly in water using a protein phosphatase inhibition assay. Water Research, 35(13): 3049-3056.
DOI:10.1016/S0043-1354(01)00018-5 |
Huang Z G. 2008. Marine Species and Their Distribution in China. China Ocean Press, Beijing.
(in Chinese)
|
Institute of Oceanology Chinese Academy of Sciences (IOCAS). 1962. China's Economic Algae Chronicle. Science Press, Beijing.
(in Chinese)
|
Kong F Z, Jiang P, Wei C J, et al. 2018. Co-occurence of greentide, golden tide and red tides along the 35°N transect in the Yellow Sea during Spring and Summer in 2017. Oceanologia et Limnologia Sinica, 49(5): 1021-1030.
(in Chinese with English abstract) |
Kudela R M, Berdalet E, Enevoldsen H, et al. 2017. GEOHAB-The global ecology and oceanography of harmful algal blooms program: motivation, goals, and legacy. Oceanography, 30(1): 12-21.
DOI:10.5670/oceanog.2017.106 |
Lassus P, Chomérat N, Hess P et al. 2016. Toxic and harmful microalgae of the World Ocean / Micro-algues toxiques et nuisibles de l'océan mondial. International Society for the Study of Harmful Algae / Intergovernmental Oceanographic Commission of UNESCO, Denmark. (in Bilingual English/French)
|
Li A F, Ma J G, Cao J J, et al. 2012. Toxins in mussels (Mytilus galloprovincialis) associated with diarrhetic shellfish poisoning episodes in China. Toxicon, 60(3): 420-425.
DOI:10.1016/j.toxicon.2012.04.339 |
Li X Y, Yu R C, Geng H X, et al. 2021. Increasing dominance of dinoflagellate red tides in the coastal waters of Yellow Sea, China. Marine Pollution Bulletin, 168: 112439.
DOI:10.1016/j.marpolbul.2021.112439 |
Lin Y S, Zhou J M. 1993. Dinoflagellates in the South China Sea. Science Press, Beijing. 115p.
(in Chinese)
|
Liu Y, Yu R C, Kong F Z, et al. 2019. Contamination status of lipophilic marine toxins in shellfish samples from the Bohai Sea, China. Environmental Pollution, 249: 171-180.
DOI:10.1016/j.envpol.2019.02.050 |
Matsuoka K. 1999. Eutrophication process recorded in dinoflagellate cyst assemblages—a case of Yokohama Port, Tokyo Bay, Japan. Science of the Total Environment, 231(1): 17-35.
DOI:10.1016/S0048-9697(99)00087-X |
McCabe R M, Hickey B M, Kudela R M, et al. 2016. An unprecedented coastwide toxic algal bloom linked to anomalous ocean conditions. Geophysical Research Letters, 43(19): 10366-10376.
DOI:10.1002/2016GL070023 |
Meyer K F, Sommer H, Schoenholz P. 1928. Mussel poisoning. Journal of Preventive Medicine, 2: 365-394.
|
Ministry of Natural Resources (MNR). 2009-2020. The Bulletin of China Marine Disaster. (in Chinese)
|
Ministry of Natural Resources (MNR). 2012. The Bulletin of China Marine Disaster. (in Chinese)
|
Ministry of Natural Resources (MNR). 2017. The Bulletin of China Marine Disaster. (in Chinese)
|
Mudie P J, Marret F, Mertens K N, et al. 2017. Atlas of modern dinoflagellate cyst distributions in the Black Sea Corridor: from Aegean to Aral Seas, including Marmara, Black, Azov and Caspian Seas. Marine Micropaleontology, 134: 1-152.
DOI:10.1016/j.marmicro.2017.05.004 |
Pierce R H, Kirkpatrick G J. 2001. Innovative techniques for harmful algal toxin analysis. Environmental Toxicology and Chemistry, 20(1): 107-114.
DOI:10.1002/etc.5620200110 |
Qi L, Hu C M, Wang M Q, et al. 2017. Floating algae blooms in the East China Sea. Geophysical Research Letters, 44(22): 11501-11509.
|
Saunders G W, McDevit D C. 2012. Methods for DNA Barcoding Photosynthetic Protists Emphasizing the Macroalgae and Diatoms. In: Kress W J, Erickson D L eds. DNA Barcodes: Methods and Protocols. Humana Press, Totowa, NJ. p. 207-222, https://doi.org/10.1007/978-1-61779-591-6_10.
|
Saunders G W. 2005. Applying DNA barcoding to red macroalgae: a preliminary appraisal holds promise for future applications. Philosophical Transactions of the Royal Society B: Biological Sciences, 360(1462): 1879-1888.
DOI:10.1098/rstb.2005.1719 |
Scholin C A, Anderson D M. 1994. Identification of group- and strain-specific genetic markers for globally distributed Alexandrium (Dinophyceae). I. RFLP analysis of SSU rRNA genes.. Journal of Phycology, 30(4): 744-754.
DOI:10.1111/j.0022-3646.1994.00744.x |
Smetacek V, Zingone A. 2013. Green and golden seaweed tides on the rise. Nature, 504(7478): 84-88.
DOI:10.1038/nature12860 |
Soto I M, Cambazoglu M K, Boyette A D, et al. 2018. Advection of Karenia brevis blooms from the Florida Panhandle towards Mississippi coastal waters. Harmful Algae, 72: 46-64.
DOI:10.1016/j.hal.2017.12.008 |
Sournia A. 1995. Red tide and toxic marine phytoplankton of the world ocean: an inquiry into biodiversity. In: Lassus P, Arzul G, Erard E et al eds. Harmful Marine Algal Blooms. Lavoisier, Paris. p. 103-112.
|
Stewart I, McLeod C. 2014. The laboratory mouse in routine food safety testing for marine algal biotoxins and harmful algal bloom toxin research: past, present and future. Journal of AOAC International, 97(2): 356-372.
DOI:10.5740/jaoacint.SGEStewart |
Sun S, Sun X X, Jenkinson I R. 2015. Preface: giant jellyfish blooms in Chinese waters. Hydrobiologia, 754(1): 1-11.
DOI:10.1007/s10750-015-2320-3 |
Taylor F J R, Trainer V L. 2002. Harmful algal blooms in the PICES region of the North Pacific. PICES Scientific Report No. 23, https://meetings.pices.int/publications/scientific-reports/Report23/Rep_23_full.pdf.
|
Wall D, Dale B. 1966. "Living Fossils" in western Atlantic Plankton. Nature, 211(5053): 1025-1026.
DOI:10.1038/2111025a0 |
Wall D, Dale B. 1968. Modern dinoflagellate cysts and evolution of the Peridiniales. Micropaleontology, 14(3): 265-304.
DOI:10.2307/1484690 |
Wang C, Yan C, Qiu J B, et al. 2021a. Food web biomagnification of the neurotoxin β-N-methylamino-L-alanine in a diatom-dominated marine ecosystem in China. Journal of Hazardous Materials, 404: 124217.
DOI:10.1016/j.jhazmat.2020.124217 |
Wang J Y, Ho K C, Qi Y Z, et al. 2017. Progress in taxonomy study on Kareniaceae (Dinophyta). Oceanologia et Limnologia Sinica, 48(4): 786-797.
(in Chinese with English abstract) |
Wang L, Zhuang Y Y, Zhang H, et al. 2014. DNA barcoding species in Alexandrium tamarense complex using ITS and proposing designation of five species. Harmful Algae, 31: 100-113.
DOI:10.1016/j.hal.2013.10.013 |
Wang X D, Song H Y, Wang Y, et al. 2021b. Research on the biology and ecology of the harmful algal bloom species Phaeocystis globosa in China: progresses in the last 20 years. Harmful Algae, 107: 102057.
|
Xiao J, Fan S L, Wang Z L, et al. 2020. Decadal characteristics of the floating Ulva and Sargassum in the Subei Shoal, Yellow Sea. Acta Oceanologica Sinica, 39(10): 1-10.
DOI:10.1007/s13131-020-1655-4 |
Xiao J, Wang Z L, Liu D Y, et al. 2021. Harmful macroalgal blooms (HMBs) in China's coastal water: green and golden tides. Harmful Algae, 107: 102061.
DOI:10.1016/j.hal.2021.102061 |
Xiao X, Sogge H, Lagesen K, et al. 2014. Use of high throughput sequencing and light microscopy show contrasting results in a study of phytoplankton occurrence in a freshwater environment. PLoS One, 9(8): e106510.
DOI:10.1371/journal.pone.0106510 |
Xing Q G, Guo R H, Wu L L, et al. 2017. High-resolution satellite observations of a new hazard of golden tides caused by floating Sargassum in winter in the Yellow Sea. IEEE Geoscience and Remote Sensing Letters, 14(10): 1815-1819.
DOI:10.1109/LGRS.2017.2737079 |
Xu Y, Sui J, Yang M, et al. 2017. Variation in the macrofaunal community over large temporal and spatial scales in the southern Yellow Sea. Journal of Marine Systems, 173: 9-20.
DOI:10.1016/j.jmarsys.2016.11.006 |
Yan T, Li X D, Tan Z J, et al. 2022. Toxic effects, mechanisms, and ecological impacts of harmful algal blooms in China. Harmful Algae, 111: 102148.
DOI:10.1016/j.hal.2021.102148 |
Yan T, Zhou M J, Zou J Z. 2002. A national report on harmful algal blooms in China. In: Taylor F J R, Trainer V L eds. Harmful algal blooms in the PICES region of the North Pacific. PICES Scientific Report No. 23, p. 21-38. https://meetings.pices.int/publications/scientific-reports/Report23/Rep_23_full.pdf
.
|
Yang S M, Li R X, Dong S G. 2014. Dinoflagellates in the China's Seas. China Ocean Press, Beijing.
(in Chinese)
|
Yu R C, Luo X. 2016. Status and research perspectives on toxic algae and phycotoxins in the coastal waters of China. Studia Marina Sinica, (1): 155-166.
(in Chinese with English abstract) |
Zeng C K. 2009. Seaweeds in Yellow Sea and Bohai Sea of China. Science Press, Beijing..
(in Chinese)
|
Zhang Q C, Qiu L M, Yu R C, et al. 2012. Emergence of brown tides caused by Aureococcus anophagefferens Hargraves et Sieburth in China. Harmful Algae, 19: 117-124.
DOI:10.1016/j.hal.2012.06.007 |
Zhang Y Y, He P M, Li H M, et al. 2019. Ulva prolifera green-tide outbreaks and their environmental impact in the Yellow Sea, China. National Science Review, 6(4): 825-838.
DOI:10.1093/nsr/nwz026 |
Zhou M J, Liu D Y, Anderson D M, et al. 2015. Introduction to the Special Issue on green tides in the Yellow Sea. Estuarine, Coastal and Shelf Science, 163: 3-8.
DOI:10.1016/j.ecss.2015.06.023 |
 2022, Vol. 40
2022, Vol. 40


