Institute of Oceanology, Chinese Academy of Sciences
Article Information
- JIANG Maowang, CHEN Huan, ZHOU Shuangnan, HAN Qingxi, PENG Ruibing, JIANG Xiamin
- Changes in embryonic development, juvenile growth and physiological adaptation of the cuttlefish Sepia pharaonis in response to photoperiod manipulation
- Journal of Oceanology and Limnology, 40(5): 2012-2027
- http://dx.doi.org/10.1007/s00343-021-1243-2
Article History
- Received Jul. 29, 2021
- accepted in principle Oct. 12, 2021
- accepted for publication Nov. 17, 2021
During the spawning season from February to April, adult cuttlefish migrate to shore, congregate in offshore waters for mating, and lay eggs in the shallow coastal waters (5−40-m deep) (Boletzky, 1983; Tehranifard and Dastan, 2011), where egg cases are not only attached but also oxygenated by ocean currents to satisfy embryonic development and light can regulate the development gonads of the reproductive stock (Roper et al., 1984). In recent years, numerous studies have been conducted on the effects of biotic and abiotic factors that influence embryonic development (Peng et al., 2015; Lee et al., 2016; O'Brien et al., 2018) and juvenile growth performance (Minton, 2004; Anil et al., 2005). Photoperiod is one of the key environmental factors affecting the growth, development, and survival of aquatic organisms (Gehrke, 1994). During evolution, organisms have developed physiological and behavioral mechanisms that allow them to adapt to the circadian rhythm of light. However, photoperiod may be one of the critical factors for embryonic development and juvenile growth, as well as one of the most controllable variables in the hatchery. Currently, little attention has been given to the influence of "unnatural" light conditions (constant light or darkness), although they are known to affect the development of physiological disturbances in the cuttlefish and their circadian rhythms (Koueta and Boucaud-Camou, 2001).
The light-dark cycle is one of the most important environmental challenge for organisms to survive in the wild. As a result, light-sensitive circadian clocks have changed in most animals including vertebrates and invertebrates. The photoperiod, an internal "zeitgeber", is known to affect the endogenous rhythms in teleosteas and has been shown to be controlled by a well-developed photoreceptive structure in the pineal gland (Simensen et al., 2000; Tuckey and Smith, 2001). Likewise, transcriptions of opsin have been found in the skin of cephalopods, similar to the retina, which is an effector-epistellar photoreceptive body (Cobb et al., 1995; Mäthger et al., 2010). Photoperiod has a notable effect on the intrinsic aspects of body's ontogenesis, including metamorphosis, physiological activity, and sexual maturation (Forsythe et al., 1994; Kamler, 2002; Dong et al., 2011; Qiu et al., 2015). Moreover, it also has several external effects such as on hatching rate (Shi et al., 2012), the incubation period of embryos (Downing and Litvak, 2002), body weight of newly hatched larvae (Villamizar et al., 2009), juvenile swimming activity and behaviour (Trotter et al., 2003), and subsequent on survival and growth (Moustakas et al., 2004).
It is well-known that most cuttlefish depend on sight to detect predators and prey, and are strongly influenced by lighting (Groeger et al., 2005; Serb and Eernisse, 2008). For each species, there is a minimum light intensity that allows juveniles to catch prey. However, the light intensities that permit the normal development of juveniles vary considerably between species, from 1 lx in striped bass (Morone saxatilis) to 3 000 lx in Leopard coral grouper (Plectropomus leopardus) (Boeuf and Le Bail, 1999; Yoseda et al., 2008). Sykes et al. (2014) showed that low light intensity (100 lx) improved better growth and survival (vs. 350 and 1 200 lx) of juvenile cuttlefish for the first 50 days after hatching (DAH). In addition to the light intensity, the photoperiod affects the behaviour of juvenile cuttlefish, and they show high levels of nocturnal activity at the age of 30 days, while at the age of 6 months old, prolonged light had no impact on growth performance (Richard, 1971). In conditions of constant light, Sepia officinalis, which has been cultivated for several generations, has induced a longer life span, and has grown into larger individuals by inhibiting gonad development and sexual maturation (Forsythe et al., 1994). To date, studies on the effect of photoperiod on embryonic development are still rare, and the growth performance of juveniles remains limited to a single study (Koueta and Boucaud-Camou, 2003). However, the fertilized cuttlefish eggs are transparent, soft, easy to observe with the naked eye, and sensitive to external stimuli (inking in the egg capsule), it is necessary to understand how the photoperiod affects the embryonic development of those fertilized eggs to reveal changes in the photosensitivity of the species in the course of growth and development.
The purpose of this study was to explore the effects of light: dark cycles (L: D) on the performance of embryonic development and newly hatched juvenile, including physiological factors related to stress on embryonic development, digestive enzyme and metabolic enzyme relative to digestive gland and muscles, tissue glycogen content relative to energy marker. This research provides the theoretical basis for optimizing the light environment of fertilized cuttlefish eggs and newly hatched juveniles.
2 MATERIAL AND METHOD 2.1 AnimalThe experiment was carried out at the Laifa Aquaculture Co. Ltd. (29°59′N, 121°99′E) (Zhejiang Province, China). Cuttlefish (Sepia pharaonis) from a stock of first generation broodstock (Jiang et al., 2019). A total of 389 broodstock individuals with a male: female ratio close to 1:2 was placed in square concrete tanks (7.8 m×3.8 m×1.6 m, length×width× depth; area: 30 m2), at a culture density of 5–8 inds./m2 during the spawning season from February to April in 2020. The broodstock was reared in a closed system, using natural seawater that was filtered through a filter bed and preheated to 23±0.8 ℃ before being pumped into the tank. These cuttlefish were fed with frozen fishes (Larimichthys polyactis and Pampus argenteus) and white shrimp (Penaeus vannamei) twice a day (fed ad libitum), with a feeding rate of 3%–5% body weight (BW)/d (Jiang et al., 2018). Eggs were laid in February–April and the same batch of eggs cases was used for this study. Nylon nets with a diameter of 1–1.5 cm were used to collect fertilized eggs. The broodstock was cultivated under the following environmental conditions: salinity 28.7±1.2, temperature 23.8±0.6, pH 8.13±0.86, dissolved oxygen 5.77±1.13 mg/L, and a natural day-night photoperiod with intensity below 1 000 lx.
2.2 Experimental design 2.2.1 Experiment 1: effect of photoperiod on embryonic developmentAll systems used in this study were composed of fifteen blue fiberglass cylindrical tanks (diameter: 1 m; water depth: 0.65 m; and volume: 500 L), as described in Jiang et al. (2020). Round plastic baskets (diameter of 0.55 m, height of 0.18 m) with a series of small holes (diameter of 0.3 cm) floated over the water in each fiberglass cylindrical tank; the eggs were incubated in these suspended baskets, as shown in Fig. 1a. Fertilized eggs of S. pharaonis were obtained from same batch which hatched on the same day (i.e., being the same age), and distributed into round plastic basket. Eggs were removed from the cluster individually, with 300 eggs assigned to each of basket and 3 replicates per treatment group (Fig. 1a). Natural seawater was filtered through a filter bed and ultraviolet sterilizers prior to being pumped into the tank. The water temperature was controlled at 24.6±0.5 ℃ (Samuel and Patterson, 2015; Jiang et al., 2020) using a water bath equipped (1.5 kW) with a digital thermoregulator and immersion heaters (SUNSUN GD-1000, PID). Each tank was supplied with constant gentle ventilation with an air stone and an air lift. During the incubation period, salinity was 29.1±0.4, dissolved oxygen was 6.17±0.26 mg/L, and pH was 8.07±0.25. The salinity, pH, and dissolved oxygen were measured using the YSI Pro Plus instrument (YSI; www.ysi.com ), and 30% of the seawater was renewed every third day under the same conditions.
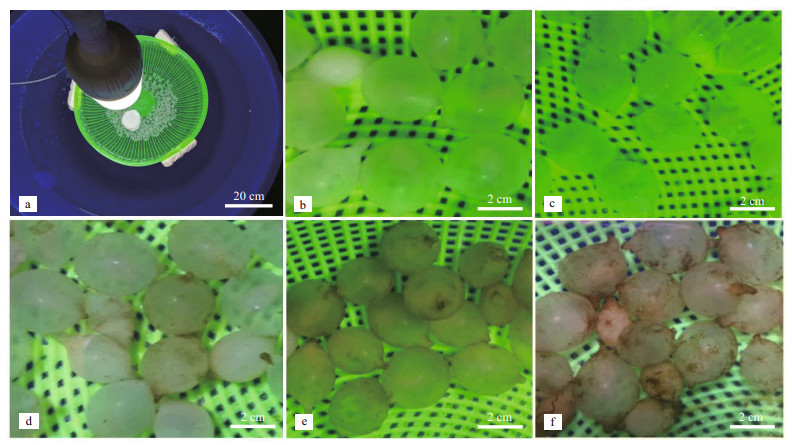
|
| Fig.1 The experimental device and the embryo observation (15 days post spawning) exposure to different photoperiods a. the photoperiod device; b. 0L: 24D treatment; c. 6L: 18D treatment; d. 12L: 12D treatment; e. 18L: 6D treatment; f. 24L: 0D treatment. |
The photoperiod (light: dark, L: D) treatments were as follows: 1) constant light (24L: 0D), 2) 18 h light: 6 h dark (18L: 6D), 3) 12 h light: 12 h dark (12L: 12D), 4) 6 h light: 18 h dark (6L: 18D), and 5) constant darkness (0L: 24D). The photoperiod was maintained in each treatment with timers. Previous studies have suggested that cuttlefish acclimatize to artificial light with no response to stress (Boletzky, 1974; Koueta and Boucaud-Camou, 2003). Recently, we tested the influence of light intensity on embryo development by using cool-white fluorescent lamps as a light source and found that they were sensitive to strong light (Zhou et al., 2018). Consequently, an artificial light (cool-white fluorescent lamps) of low intensity (250−300 lx) was used in this experiment. All tanks were placed in a dark room equipped with one 20W cool-white fluorescent lamp of SEEBEST (QZ130-20W, Xiamen SEEBEST Technology Co. Ltd., Xiamen, China), mounted 0.50 m above the water surface, and given a range of light intensity between 250 and 300 lx at 0.18 m above the water surface. The light intensity of each treatment was measured at the air-water interface using a ZDS-10 luxmeter (Shanghai, China). Each tank was enclosed in a box of black plastic sheets to prevent the light from leaking to the surrounding tanks. This study was performed in accordance with the recommendations of the Guidelines for the Care and Welfare of Cephalopods in Research (Fiorito et al., 2015). The National Standards for Laboratory Animals of the People's Republic of China were followed by the authors.
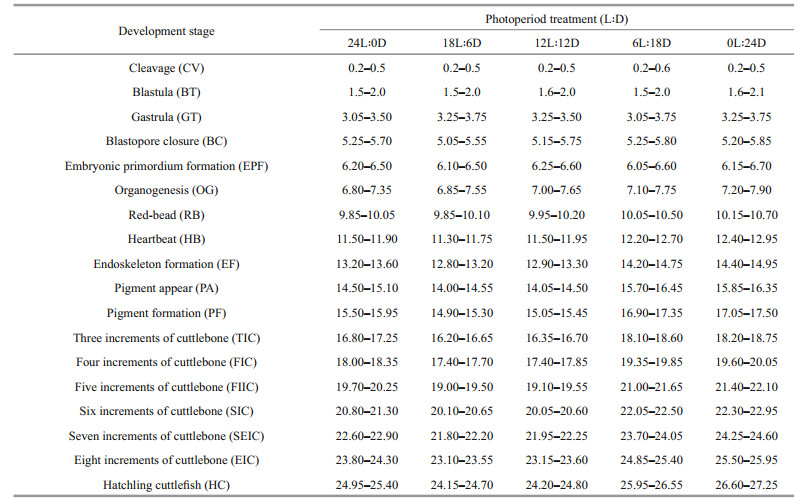
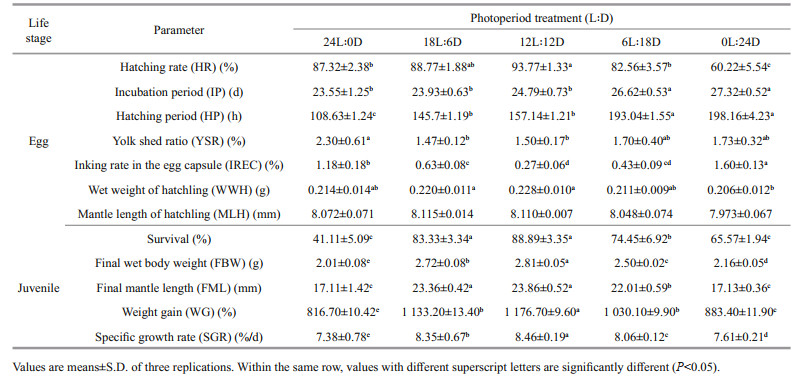
Effects of photoperiod on embryonic development were assessed according to the following criteria: 1) chronological comparison of the embryo development at different photoperiods, embryonic development of S. pharaonis was observed from the first day post spawning (hours post spawning, HPS) to the day of hatching under laboratory conditions, and the embryos were photographed with a digital camera (HDR-CX450, SONY, Japan) for determining the stages of embryonic development; to observe in a more intuitive and efficient way the effects of the photoperiod on embryonic development, 18 stages (cleavage, blastula, gastrula, blastopore closure, embryonic primordium formation, organogenesis, red-bead, heartbeat, endoskeleton formation, pigment appear, pigment formation, three increments of cuttlebone, four increments of cuttlebone, five increments of cuttlebone, six increments of cuttlebone, seven increments of cuttlebone, eight increments of cuttlebone, and nine increments of cuttlebone (i.e., hatchling cuttlefish) (Fig. 2) were adopted observation points in this experiment; 2) the hatching rate (HR), the incubation period (IP), the hatching period (HP), the yolk shed rate (YSR), the inking rate in the egg capsule (IREC), the mean wet weight and mantle length of the new hatchlings (Gracia-López et al., 2004), were calculated at the end of the experiment. The HR was determined as the percentage of stocked embryos that hatched hatchlings. The IP, which was the time interval between the activation period of the eggs and the period during which all the fertilized eggs hatched. The HP, which was defined as the total duration from the first hatchling to the last one. The YSR was determined as the ratio of the number of yolks shed by different factors to the total number of eggs. The IREC was determined as the ratio of the number of pre-hatchings inked by any mechanical impact to the total number of eggs. The total wet weight of each basket of new hatchlings was quantified on one scale (AE AUW120D, maximum 200 g, accuracy 0.000 1 g). Mantle length of hatchling were measured with digital Vernier calipers (0.001 mm) (n=6).

|
| Fig.2 The 18 stages of S. pharaonis embryonic development a. cleavage (CV); b. blastula (BT); c. gastrula (GT); d. blastopore closure (BC); e. embryonic primordium formation (EPF); f. organogenesis (OG); g. red-bead (RB); h. heartbeat (HB); i. endoskeleton formation (EF); j. pigment appear (PA); k. pigment formation (PF); l. three increments of cuttlebone (TIC); m. four increments of cuttlebone (FIC); n. five increments of cuttlebone (FIIC); o. six increments of cuttlebone (SIC); p. seven increments of cuttlebone (SEIC); q. Eight increments of cuttlebone (EIC); r. nine increments of cuttlebone (hatchling cuttlefish, HC). The embryonic developmental stage was modified according to Samuel and Patterson (2015) and Peng et al. (2015). The embryos were observed every 3 h on the first day of the experiment, then observed and recorded them at irregular intervals each day. Microscopic observations of samples of two embryos in each basket were performed using a dissection microscope (SZX 7, Olympus, Tokyo, Japan). Before observation, the egg capsule was removed and the mucosubstances was gently washed with seawater to clearly see the embryo, and images were taken directly from the eyepieces (HDR-CX450, SONY, Japan). Scale bars: a–k=2 mm, l–r=1 mm. |
The culture system and the photoperiod treatments were identical to the above experiment. A total of 750 juveniles (1DAH, weight 0.221±0.011 g, dorsal mantle length 8.058±0.164 mm, n=50) were randomly divided into fifteen 500-L fiberglass cylindrical tanks, including 50 juveniles in each basket and 3 replicates per treatment group. Initial stocking density was 150 inds./m2 (Correia et al., 2005). New hatchlings were fed with enriched live Artemia (Artemia nauplii) and mysids (Hyperacanthomysis brevirostris) during the first 3 days of post-hatching, and then fed with live mysids twice a day ad libitum (Jiang et al., 2020). Feeding rates were set at 20% BW/d, which is considered a satisfactory proportion (Domingues et al., 2004). Feed provided was offered were adjusted every 7 days to reflect the new total biomass in each basket, ensure that the prey remained in the basket until the total duration of the test. Tanks and baskets were thoroughly cleaned as required, and the cuttlefish were placed under dark conditions. The trial period lasted 30 days. Previous researchers have suggested that cuttlefish are sensitive to intense light (Richard, 1971; Sykes et al., 2014). As a result, we used a low-intensity light (250–300 lx) in this test. The water temperature was controlled at 24±0.5 ℃ using a water bath equipped (1.5 kW) with digital thermoregulator and immersion heaters, and 30% of the seawater was refreshed every day under the same conditions. The water conditions were as follows: salinity was 29.4±0.5, dissolved oxygen was 5.69±0.18 mg/L, and pH was 7.97±0.31. During the experiment, all cuttlefish from each basket were enumerated and measured every 10 days to calculate the survival and growth parameters.
The growth parameters in this experiment were calculated as follows: survival (%)=100×(Nt/N0), weight gain (WG, %)=100×(Wt–W0)×100/W0, and specific growth rate (SGR, %/d)=(lnWt–lnW0)×100/T. In these equations, N0 is the number of cuttlefish in the basket at the start of experiment, Nt is the number of cuttlefish in the basket at the end of experiment, W0 is the initial mean wet body weight (g), Wt is the final mean wet body weight (g), and T is the experimental period (days).
2.3 Sampling and biochemical analysesAt the end of the experiment, six cuttlefish per basket were anaesthetized in diluted ethanol (1:20, Sinopharm Chemical Reagent, Shanghai, China). Samples of digestive glands and muscles were immediately frozen in liquid nitrogen and stored at -80 ℃ until analysis. The procedures for preparing glycogen and enzyme extract were as follows: 1) the frozen samples were thawed, weighed (~100 mg), and homogenized (3 min) in an ice-cold 50-mmol/L citrate phosphate buffer (pH 7.0) at a ratio of 1:9 (w/v) using Ultraturrax (T-25) homogenizer; 2) the homogenate was centrifuged at 4 000 r/min (4 ℃) for 10 min, and then the supernatant was centrifuged at 12 000 r/min (4 ℃) for 15 min; 3) the supernatants were collected and used for further testing within 4 h and kept refrigerated at 4 ℃; 4) the total protein content of the crude extracts was measured at 30 ℃ using bovine serum albumin as a standard at 595 nm using the Bradford (1976). All assay kits were purchased from Nanjing Jiancheng Bioengineering Institute (http://www.njjcbio.com/, Nanjing, China), testing procedures following the manufacturer's instructions. The assays are briefly described below.
The glycogen content for digestive gland and muscle samples was measured using the glycogen assay kit. Glycogen concentration was determined using the anthrone reaction method according to Kohyama-Koganeya et al. (2015), free glucose was subtracted from the total glucosyl equivalents for glycogen levels, and sample absorption was read at 620 nm. The following test procedures as our previously described (Jiang et al., 2020). Glycogen level was expressed as per mg of total protein (specific level).
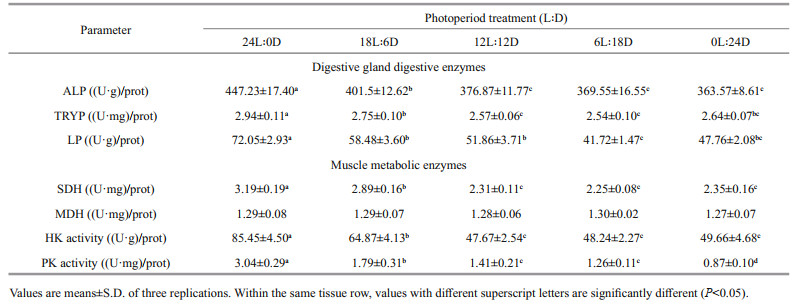
The activities of digestive gland alkaline phosphatase (ALP), trypsin (TRYP), and lipase (LIP) were determined according to the procedures described by Principato et al. (1982), Tsunematsu et al. (1985), and Pinsirodom and Parkin (2001), respectively. The absorption of ALP (EC 3.1.3.1), TRYP (EC 3.4.4.4), and LP (EC 3.1.1.3) was recorded at 520 nm, 253 nm, and 420 nm, respectively. Enzyme activities were expressed as per mg of total protein (specific activity).
The activities of muscle tissue succinate dehydrogenase (SDH), malate dehydrogenase (MDH), hexokinase (HK), and pyruvate kinase (PK) were determined in accordance with procedures described by Palmer et al. (1977), Baldwin and Reed (1976), Tranulis et al. (1996), and Foster and Moon (1986), respectively. The absorption of SDH (EC 1.3.5.1), MDH (EC 1.1.1.37), HK (EC 2.7.1.1), and PK (EC 2.7.1.40) was read at 600 nm, 340 nm, 340 nm, and 340 nm, respectively. Enzyme activities were expressed as per g or/and mg of total protein (specific activity).
2.4 Statistical analysisData are expressed as mean±standard deviation (means±S.D.). The effects of photoperiod on the indices were analyzed using a one-way ANOVA, followed by the Duncan's test with P<0.05 taken as the statistically significant threshold. Analyses were performed using the SPSS program version 20.0 (SPSS for Windows). Histograms were drawn in GraphPad Prism 8.0.2 (https://www.graphpad.com/scientific-software/prism/). Spearman's correlation analysis and principal component analysis (PCA) were visualized using the R 4.1.0 packages (https://www.r-project.org/) corrplot and ggord, respectively.
3 RESULT 3.1 Effect of photoperiod on the embryonic development time and egg capsuleThe embryonic development process and hatching time varied considerably according to the photoperiod during incubation (Table 1). The time from cleavage to red-bead was 9.85–10.70 d at photoperiods treatment, but the difference in development time was aggravated after the red-bead stage. The period of embryonic development was similar in the 18L: 6D treatment and the 12L: 12D treatment. Hatching occurred 24.15–24.80 d at 18L: 6D and 12L: 12D treatments, which was 0.7–1.0 d, 1.5–2.0 d, and 2.5– 3.0 d shorter than that at 24L: 0D, 6L: 18D, and 0L: 24D treatments, respectively.
|
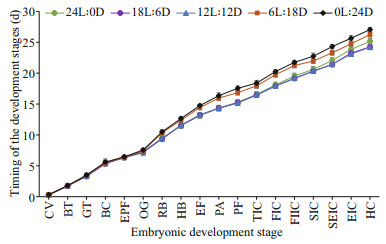
Timing of embryo development stages exposed to different photoperiods is shown in Fig. 3. The difference in embryo development timing did not appear until the red-bead stage, with the red-bead stage appeared earlier in 12L: 12D to 24L: 0D treatments than 6L: 18D to 0L: 24D treatments. Subsequently, the difference in the development timing of embryo was more evident, as shown that the seven layers of cuttlebone stage appeared earlier in 12L: 12D and 18L: 6D treatment than 24L: 0D treatment. Until cuttlefish hatched, the incubation time from 24L: 0D to 0L: 24D treatment were 24.95– 25.40, 24.15–24.70, 24.20–24.80, 25.95–26.55, and 26.60–27.25 d, respectively.
|
| Fig.3 Comparison of embryo development exposure to different photoperiods |
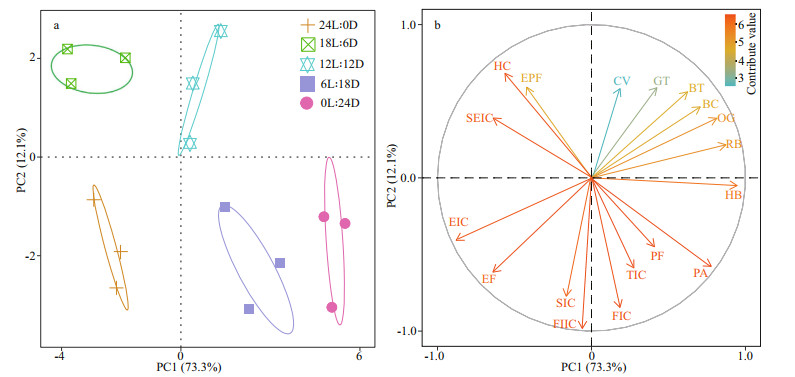
The PCA plots for the embryonic development timing (Fig. 4), in which each point represents a sample from a different photoperiod treatment, clearly highlights the differences between the different light-dark cycles. The first and second principal components accounted for 85.4% of the total variation (73.3% and 12.1%, respectively) (Fig. 4a). The profiles of the embryonic development timing were categorized into five groups corresponding to each photoperiod treatment. S. pharaonis embryonic development timing was most similar between 12L: 12D and 18L: 6D treatments, whereas 24L: 0D was the furthest away from the other treatments. In Fig. 4b, combined with the factor analysis of the principal components of SPSS, showed that the main contributing factors to the first principal component were the red-bead stage, heartbeat, endoskeleton formation, pigment appear, six increments of cuttlebone, and their score coefficients are all higher than 0.95.
|
| Fig.4 Principal component analysis (PCA) based on the different developmental timing of S. pharaonis embryos exposure to different photoperiods a. the factor coefficients plot; b. the loading plot for the corresponding developmental timing contribution to the scores plot. |
The observation of the egg capsule (15 days post spawning) as shown in Fig. 1. During the photoperiods, the egg capsule surface varied greatly with the prolonged incubation time. At 12L: 12D treatment, the algae began to adhere to the surface of the egg capsule, and the amount of algae adhesion increased with the prolonged exposure to light (Fig. 1e & f), but it did not appear at 0L: 24D and 6L: 18D treatments (Fig. 1b & c).
3.2 Effect of photoperiod on hatching rate, incubation period, hatching period, yolk shed ratio, inking rate in the egg capsule, and mean wet weight of hatchlingPhotoperiod had a significant effect on the hatching rate, incubation period, and hatching period, although the hatching period seemed to increase as the length of darkness increased (Table 2). A relatively high hatching rate (> 85%), a lower incubation period (< 25 d), and an embryos hatching period (< 6 d) of the were found in the 12L: 12D to 24L: 0D treatments. These results regarding the incubation period and the hatching period of embryos at different photoperiods, indicated that the photoperiod had no significant influence between the 18L: 6D and 12L: 12D treatments (P>0.05). However, the incubation period and hatching period were longer at 0L: 24D treatment than that at other treatments (P<0.05). The highest hatching rate (93.77%±1.33%), the shortest incubation period (23.55±1.25 d), and hatching period (108.63±1.24 h), were observed in the 12L: 12D, 24L: 0D, and 24L: 0D treatments, respectively.
|
Moreover, the photoperiod also influenced the yolk shed ratio and inking rate in the egg capsule of the embryos. The yolk shed ratio and the inking rate in the egg capsule significantly increased (P<0.05) with constant light and/or darkness, whereas there was no significant difference (P>0.05) between 18L: 6D, 12L: 12D, and 6L: 18D treatments (Table 2). The highest yolk shed ratio (2.30%±0.61%) and inking rate in the egg capsule (1.60%±0.13%) was found in the 24L: 0D and 0L: 24D treatments. Photoperiod had a modest effect on the mean mantle length of hatchling, but it was not significant (P>0.05). As with the mean wet weight of hatchling, no significant difference in the mean mantle length between photoperiod treatments (P>0.05).
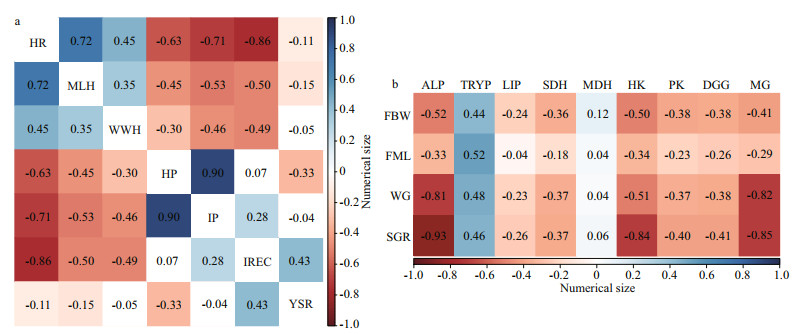
Spearman was used to analyze correlations between incubation parameters and physiological response values. Figure 5a shows that the hatching rate was strongly positive correlated with the mantle length of hatchling, while a strong negative correlated with the incubation period, and a very strong negative correlation was observed for inking rate in the egg capsule. The mantle length of hatchling showed a moderately negative correlation with the incubation period and the inking rate in the egg capsule. A very strong positive correlation was found between the hatching period and the incubation period.
|
| Fig.5 The correlation analysis of incubation parameters, physiological response values during embryonic development stage (a); the correlation analysis of growth performance and the levels of digestive enzymes from the digestive gland and muscle of juveniles (b) HR: hatching rate; MHL: mantle length of hatchling; WWH: wet weight of hatchling; HP: hatching period; IP: incubation period; IREC: inking rate in the egg capsule; YSR: yolk shed ratio; FBW: final wet body weight; FML; final mantle length; WG: weight gain; SGR: specific growth rate; ALP: alkaline phosphatase; TRYP: trypsin; LIP: lipase; SDH: succinate dehydrogenase; MDH: malate dehydrogenase; HK: hexokinase; PK: pyruvate kinase; DGG: digestive gland glycogen; MG: muscle glycogen. The color shades represent the numerical size of the correlation coefficient. |r|≥0.8: very strong correlation; 0.6≤|r|<0.8: strong correlation; 0.4≤|r|<0.6: moderate correlation; 0.2≤|r|<0.4: weak correlation; |r|<0.2: no correlation. |
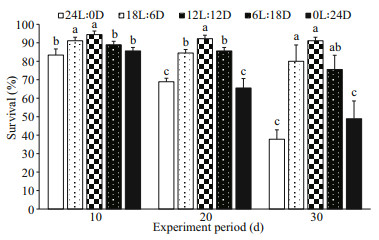
Over the first 10 d of rearing, the survival of the 12L: 12D and 18L: 6D treatments were not significantly different (P>0.05), but were significantly higher than those of the 0L: 24D and 24L: 0D treatments (P<0.05), and the minimum level was observed in the 24L: 0D treatment. Subsequently, at the age of 20 d, the survival of the 12L: 12D treatment was significantly higher than those of the other groups (P<0.05), and the lowest value was observed in the 24L: 0D and 0L: 24D treatments. After 30 d of rearing, significant differences in survival were observed among all groups (P<0.05), as follows: 12L: 12D≥6L: 18D ≥18L: 6D>0L: 24D≥24L: 0D (Fig. 6).
|
| Fig.6 Effect of photoperiods on survival of S. pharaonis Values are means±S.D. of three replications. Means within in the same group of bars with different lower-case letters are significantly different (P<0.05). |
Growth of juvenile cuttlefish (30 DAH) under photoperiod treatments are shown in Table 2. Changes in weight gain and specific growth rate corresponded to exposure to different photoperiods. After 30 days of the experiment, significant differences in the specific growth rate were observed among all groups (P<0.05), as follows: 12L: 12D>18L: 6D>6L: 18D>0L: 24D>24L: 0D, were 8.46%±0.19%, 8.35%±0.67%, 8.06%±0.12%, 7.61%±0.21%, and 7.38%±0.78% /d, respectively.
3.4 Effect of photoperiod on glycogen concentrations and enzyme activities of tissuesGlycogen levels in the digestive gland and muscles were significantly affected by the photoperiod (Fig. 7). The glycogen content of the 18L: 6D, 12L: 12D, and 6L: 18D treatments was not significantly different (P>0.05), but was significantly lower than that of the 24L: 0D treatment (P<0.05) in the digestive gland, and the minimum value was found in the 0L: 24D treatment. Muscle glycogen levels of 24L: 0D treatment was significantly higher than in the other groups, and no significant differences were observed in the other treatments. Moreover, the glycogen levels of the digestive gland (~3.5 mg/g) was twice as high as that of the muscle (~1.5 mg/g).

|
| Fig.7 Effect of photoperiod on glycogen level in the digestive gland and muscle tissues of S. pharaonis Values are means±S.D. of three replications. Means within in the same tissue of bars with different lower-case letters are significantly different (P<0.05). |
Table 3 shows the digestive enzyme (alkaline phosphatase, trypsin, and lipase) in the digestive gland of juvenile exposed to photoperiod. Alkaline phosphatase activity did not differ significantly (P>0.05) between 12L: 12D, 6L: 18D, and 0L: 24D treatments, whereas the maximum value appeared at 24L: 0D treatment. Photoperiod had a modest effect on trypsin, which was significantly higher at 24L: 0D treatment than that of other treatments. Changes in lipase and trypsin were consistent with exposure to different light-dark cycles.
|
Muscle metabolic enzyme of juvenile cuttlefish are presented in Table 3. Muscle malate dehydrogenase did not differ between treatments (P>0.05), whereas succinate dehydrogenase, hexokinase, and pyruvate kinase activities were significantly affected by the photoperiod. Succinate dehydrogenase increased significantly with the increase in illumination time from 0 to 24 h (P<0.05), the minimum and maximum values appeared at 0L: 24D and 24L: 0D treatments, respectively. Hexokinase activity first kept stabilized and then increased, with the maximum value appearing at 24L: 0D treatment, but showed no statistical difference (P>0.05) between 0L: 24D, 6L: 18D, 12L: 12D, and 18L: 6D treatments. The variation in pyruvate kinase activity was similar to the that of hexokinase activity, the highest level was observed in the 24L: 0D treatment, followed by the 18L: 6D treatment, the minimum value at the 0L: 24D treatment, but no significant differences between 6L: 18D, and 12L: 12D treatments (P>0.05).
The light cycle has an impact on the physiological activity of the body, Spearman correlation analysis was used to further analyze the correlation analysis between growth and physiological indicators. As shown in Fig. 5b, weight gain has a moderate negative correlation with muscle hexokinase, while it has a strong negative correlation with alkaline phosphatase and muscle glycogen content of the digestive glands. The specific growth rate was negatively correlated with alkaline phosphatase, hexokinase, and glycogen levels of the digestive glands. A moderate positive correlation of trypsin with growth parameters was observed.
4 DISCUSSIONIn cephalopods, encapsulation of egg is essential, as it provides a physical barrier and chemical defence during embryonic development, and allows exchanges with the environment (Benkendorff et al., 2001; Boletzky, 2003). Once a fertilized egg is formed, embryo development is a continuous process. The whole process of embryonic development involves a series of processes of cell differentiation, division, and morphogenesis, which are influenced internally by genetic expression and externally by environmental factors (Boletzky, 2003; Cinti et al., 2004). Light has an important effect on the embryonic development of many marine species, such as increasing the hatching rate, shortening the time to hatch, and improving the viability of the 24 h post hatching larvae (Downing and Litvak, 2002; Shi et al., 2012). In nature, S. pharaonis fertilized eggs in the South China Sea are mostly found in the shallow waters offshore during late spring in February and March, and a natural light cycle was measured approximately 14L: 10D. In the present study, the prolonged photoperiod induced an acceleration of the rate of embryonic development was noted in S. pharaonis embryos, particularly in the red bead stage began to show differences. It could also be attested to the short development time, where a difference may have appeared but was not found to be significant. Prior to retina formation, the pineal gland was shown functional (Andrew et al., 2009). Studies have shown that light stimulation of the pineal gland can affect the physiological state of teleost fish through neuro-modulation or humoralregulation (Falcón et al., 2010). In addition, from the 12L: 12D to 24L: 0D treatments, the individual development speed was obviously accelerated from the red-bead stage to seven layers of cuttlebone stage. It is highly probable that the retinal visual acuity is sensitive to light stimulation after the red-bead stage formation, and the duration of light/day length may accelerate the embryonic development process (Evans et al., 2015). At this moment, the visual function of the cuttlefish has been fully developed; the retinal photoreceptor is equipped with a complete photosensitive pathway. We inferred from the development rate that the 24-h day length at the late stage of development had an adverse effect on the embryos, and the incubation period of 24L: 0D treatment was greater than that of 12L: 12D treatment and 18L: 6D treatment. However, the cornea closure times for different species are not consistent. Lee et al. (2016) showed that the cornea enclosed the eyes of S. pharaonis at the pigment-formation stage, whereas enclosed corneas occurred in four layers of cuttlebone in S. officinalis and 2 DAH in Sepioteuthis australis (Bozzano et al., 2009). It remains to be determined whether the timing of corneal closure is related to species phototaxis, brain photosensitive vesicles, or retinal visual acuity. The hatching occurred 24−25 d at day lengths of 12−24 h, which was 42−58 h earlier than the day lengths of 0−6 h. PCA analysis showed that the main contributing factors of the first principal component were red-bead stage, heartbeat, endoskeleton formation, pigment appear, and six increments of cuttlebone. The development time and duration of these five stages varies different with exposure to different photoperiods, which may determine the length of the incubation period.
Incubation and hatching periods are important parameters for evaluating embryonic development. The incubation period refers to the rate of fertilized egg development under certain conditions, and the hatching period provides information on the consistency of the development rate. This study found that the shortest incubation and hatching periods occurred in the 24L: 0D treatment, and that medium-to-long light cycles tended to shorten the incubation and hatching periods. The earliest hatchling was observed at 20-d post spawning in 24L: 0D treatment. However, a decrease in the rate of embryonic development and the timing of hatching were observed with the 0L: 24D and 6L: 24D treatments. The hatching rate is an important indicator in the study of embryonic development for large-scale reproduction. In this study, we found that changes in the hatching rate were not consistent with the photoperiod, and a reduction in the hatching rate was observed with the 0L: 24D and 24L: 0D treatments. This may be related to the extent of environmental adaptation and the adjustment of the endogenous rhythm of the species (Bromage et al., 2001). During embryonic development, the outer membrane becomes more transparent by absorbing water and reduces the thickness of the egg capsule. It is observed that the algae start to adhere to the egg capsule surface 15 d post spawning, and more algae adhere with increased light exposure. However, algal attachment had no significant effect on hatching, and new hatchlings could break out from the membrane. Egg yolk is greatly reduced as the size of the developing embryo increases. In the present study, we found that egg yolk shedding occurred primarily in the later stages of embryonic development, especially under constant light or darkness. Some hatchlings possessed an external incompletely absorbed yolk sac even after hatching, which they immediately released, if necessary (Anil et al., 2005). We observed that premature hatchlings in the egg capsule are capable of releasing ink when handled vigorously or when changes are detected in the external environment and the ink gland was found to be functional even during the pigmentation stage (Samuel and Patterson, 2015). Twenty-four hours of continuous darkness may not be conducive to embryonic development and, on exposure to light stimulation during the 0L: 24D treatment, premature hatchling inking rate in the egg capsule was found to be three to five times higher than that of other treatments. As a result, we conclude that the optimal photoperiods for the optimal S. pharaonis embryonic development are 12-h day lengths.
Our findings did not only show that S. pharaonis possesses the characteristic of high growth rates, but also contribute to the optimization of the cuttlefish culture method to ensure more consistent growth and survival based on a welfare approach to the problem. In contrast to the embryonic development stage, during which the cuttlefish grows based on endogenous nutrition, juvenile cuttlefish gain energy from hunting at the newly hatched stage, when they are in high demand for food and nutrition. S. pharaonis, similar to most other cephalopods, are highly dependent on vision for prey capture, predator avoidance, and intraspecific communication (Hanlon, 2007). In this study, the new hatchlings were less sensitive to light and were able to hunt all the time for the first few days of their life. In general, the long photoperiod benefits increased feeding, probably because of increased food availability (Boeuf and Le Bail, 1999). This is consistent with the present study, which revealed that increased day length (12−24 h) produced significantly higher growth rates with respect to wet weight compared to day lengths of 0−6 h. However, over the first 10 d of rearing, the survival rates of juveniles exposed to the 0L: 24D and 18L: 6D treatments did not differ significantly, but were significantly higher than those of juveniles in 24L: 0D treatment. Speers-Roesch et al. (2016) showed that cuttlefish juveniles could tolerate starvation for up to 12 d; however, we found that the specific growth rate in 0L: 24D group was much lower than that of other treatments. As a result, constant light and darkness were not beneficial to juvenile growth and survival; constant light had a negative effect on juvenile growth, and constant darkness was detrimental to survival (Fig. 5). As Richard (1975) indicates, cuttlefish in total darkness had low growth and high mortality as a cumulative effect of starvation, which was most common at an early stage. The visual functions of cuttlefish are limited in the dark and therefore depend more on other organs (such as the developed nervous system and the sense organs for olfactory and light sensitivity) to promote predation, which leads to decrease in food intake due to imprecise location, orientation, and low success rate of capture prey during hunting (Jereb and Roper, 2005). In the present study, the findings of 0L: 24D and 6L: 18D treatments conformed this interpretation. Specific growth rates of juveniles cultivated in 0L: 24D and 6L: 18D treatments were significantly lower than those of juveniles grown in 12L: 12D and 18L: 6D treatments. Subsequently, the specific growth rate and survival gradually increased as the day length was extended from 6 h to 18 h. Under these photoperiods, cuttlefish may have greater opportunities to feed, which should result in better growth and survival rates based on access to sufficient food (Litvak, 1999). Combined with the life habits of S. pharaonis, which inhabited low latitudes in the Indo-Pacific region, it is reasonable to assume that the cuttlefish preferred a natural photoperiod or a medium-to-high amount of sunlight. Our results indicated that specific growth rate and survival of juvenile cuttlefish had optimal values under a 12L: 12D light cycle, which was consistent with the data obtained from S. officinalis (Koueta and Boucaud-Camou, 2003).
Cephalopods are highly developed marine molluscs with digestive physiological functions that share many similarities to teleosts. The process of maturation of the digestive gland has been described in cephalopods as the progressive development of intracellular and extracellular digestion enzymes during the first month of life (Safi et al., 2018). Several studies have shown that these enzyme activities are related to diet and growth (Perrin et al., 2004; Rosas et al., 2011), or are localized to describe their function in the digestive system (Boucaud-Camou and Roper, 1995). The onset of juvenile growth is profoundly affected by the transition period from endogenous to exogenous feeding, which is primarily affected by digestive enzymes of the digestive glands. Boucaud-Camou et al. (1985) reported that digestive enzymes have shifted from a predominantly acidic intracellular digestion to extracellular alkaline digestion in newly hatched cuttlefish, and the change/ratio of these enzyme activities could be used as an indicator of the maturation of digestive glands in Sepia during the early life stages (Safi et al., 2018). Trypsin is a member of serine protease that specifically hydrolyzes proteins, which are located in the secretory organs of the digestive tract in S. officinalis (Jellouli et al., 2009). Alkaline phosphatase (ALP) catalyzes the hydrolysis of various phosphate-containing compounds and acts as a transphosphorylase at alkaline pH, and is found in the digestive system of cephalopods (Mazorra et al., 2002). In the present study, the photoperiod strongly affected the digestive enzyme activity of juveniles from the 1 to 30 DAH. No significant difference was observed as the day length increased from 6 to 18 h, and a high level of ALP and trypsin activities were observed during 24 h of continuous light exposure. Trypsin and ALP activities were stabilized from 14 DAH in the digestive glands of cuttlefish, indicating that both two enzymes function as exogenous digestive enzymes (Safi et al., 2018). It is possible that juveniles need to consider nutrient digestion, absorption, and assimilation of nutritions to meet metabolic needs, rather than the length of the day or prey supply. In other words, continuous light exposure may interfere with digestive physiological controls and physiological rhythms of juvenile (Shi et al., 2012).
Photoperiod manipulation may affect food intake, physiological activity, individual behaviour and/or activity. Generally, a long day length can promote better growth because a longer feeding time allows for more food consumption and better absorption (Giri et al., 2002). In this study, however, it was observed that continuous light exposure may have caused the animals to remain in a constant state of activity. High levels of glycogen was found in muscles after 24L: 0D treatment may be due to the high metabolic rate of juveniles. Sykes et al. (2014) reported that in the active portion of the day, the metabolic rate of cuttlefish was 2.5 to 3 times higher than that during the inactive portion. In the cephalopod muscle, carbohydrates are important fuels, and hexokinase (HK) and pyruvate kinase (PK) enzymes play a major role in the energetic regulation of the glycolysis pathway (Panserat et al., 2001), whereas succinate dehydrogenase (SDH) is involved in both the citric acid cycle and the respiratory electron transfer chain, and roughly reflects the level of aerobic metabolism (Rutter et al., 2010). In the present study, high levels of HK, PK, and SDH activity were observed in muscles after the 24L: 0D treatment, indicating an increase in the aerobic metabolism of the organism. The constant light on juveniles may have led to suboptimal absorption and assimilation, and thus reduced growth performance compared to that in the 18L: 6D and 12L: 12D treatments where the juveniles were in the "dark" phase. Therefore, the optimal photoperiod is species-specific, maximum feeding time is not necessarily the optimal condition, and continuous light may disturb an organism's endogenous controls. This has been proven in other species, such as the southern flounder Paralichthys lethostigma (Tuckey and Smith, 2001), the catfish Wallago attu (Giri et al., 2002), the green turtles Chelonia mydas (Southwood et al., 2003), and the tawny puffer Takifugu flavidus (Shi et al., 2012). Moreover, the continuous darkness did not favour the development of juveniles. In the present study, the growth, survival, digestive gland/system development, and physiological activity of juveniles reared in constant darkness have deteriorated. These results are supported by Villamizar et al. (2009) and Shi et al. (2010), who found that total darkness strongly affected the digestive system development of fish and inhibited animal feeding. Alternatively, juvenile S. pharaonis may display a circadian feeding pattern. Inappropriate light cycles resulted in disturbances of their physiological activity that have compromised the growth and development of cuttlefish.
5 CONCLUSIONIn conclusion, the embryonic development of S. pharaonis was strongly affected by light-dark cycles.The 12L: 12D cycles provided the best incubation conditions for embryos and produced a large number of high-quality new hatchlings. Furthermore, increases in day length (12L: 12D and 18L: 6D cycles) contributed to improved growth and survival of S. pharaonis juveniles from 1 to 30 DAH. Inappropriate light cycles (constant light and darkness) have led to disturbances of physiological activity that have compromised the welfare and survival of cuttlefish. The results of this study are helpful in increasing the production of this species during embryo incubation and juveniles rearing in aquaculture practice.
6 DATA AVAILABILITY STATEMENTThe data that support the findings of this study are available from the corresponding author upon reasonable request.
7 ACKNOWLEDGMENTThe authors are grateful to Laifa Aquaculture Co. Ltd. (Zhejiang Province, China) for supplying the experimental S. pharaonis cuttlefish hatchlings and providing logistical support throughout the experiment.
Andrew R J, Osorio D, Budaev S. 2009. Light during embryonic development modulates patterns of lateralization strongly and similarly in both zebrafish and chick. Philosophical Transactions of the Royal Society B: Biological Sciences, 364(1519): 983-989.
DOI:10.1098/rstb.2008.0241 |
Anil M K, Andrews J, Unnikrishnan C. 2005. Growth, behavior, and mating of pharaoh cuttlefish (Sepia pharaonis ehrenberg) in captivity. The Israeli Journal of Aquaculture-Bamidgeh, 57(1): 25-31.
DOI:10.46989/001c.20400 |
Baldwin J, Reed K C. 1976. Cytoplasmic sources of NADPH for fat synthesis in rainbow trout liver: effect of thermal acclimation on enzyme activities. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry, 54(4): 527-529.
DOI:10.1016/0305-0491(76)90134-6 |
Benkendorff K, Davis A R, Bremner J B. 2001. Chemical defense in the egg masses of benthic invertebrates: an assessment of antibacterial activity in 39 mollusks and 4 polychaetes. Journal of Invertebrate Pathology, 78(2): 109-118.
DOI:10.1006/jipa.2001.5047 |
Boeuf G, Le Bail P Y. 1999. Does light have an influence on fish growth?. Aquaculture, 177(1-4): 129-152.
DOI:10.1016/S0044-8486(99)00074-5 |
Boletzky S V. 1974. Elevage de Céphalopodes en aquarium. Vie et Milieu, 24(2A): 304-309.
|
Boletzky S V. 1983. Sepia officinalis. In: Boyle P R ed. CephalopodLifeCycles. AcademicPress, London. p. 31-52.
|
Boletzky S V. 2003. Biology of early life stages in cephalopod molluscs. Advances in Marine Biology, 44: 143-203.
DOI:10.1016/S0065-2881(03)44003-0 |
Boucaud-Camou E, Roper C F E. 1995. Digestive enzymes in paralarval cephalopods. Bulletin of Marine Science, 57(2): 313-327.
|
Boucaud-Camou E, Yim M, Tresgot A. 1985. Feeding and digestion of young Sepia officinalis L. (Mollusca: Cephalopoda) during post-hatching development. Vie et Milieu, 35(3): 263-266.
|
Bozzano A, Pankhurst P M, Moltschaniwskyj N A, Villanueva R. 2009. Eye development in southern calamary, Sepioteuthis australis, embryos and hatchlings. Marine Biology, 156(7): 1359-1373.
DOI:10.1007/s00227-009-1177-2 |
Bradford M M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1-2): 248-254.
DOI:10.1016/0003-2697(76)90527-3 |
Bromage N, Porter M, Randall C. 2001. The environmental regulation of maturation in farmed finfish with special reference to the role of photoperiod and melatonin. Aquaculture, 197(1-4): 63-98.
DOI:10.1016/S0044-8486(01)00583-X |
Cinti A, Barón P J, Rivas A L. 2004. The effects of environmental factors on the embryonic survival of the Patagonian squid Loligo gahi. Journal of Experimental Marine Biology and Ecology, 313(2): 225-240.
DOI:10.1016/j.jembe.2004.05.017 |
Cobb C S, Pope S K, Williamson R. 1995. Circadian rhythms to light‐dark cycles in the lesser octopus, Eledone clrrhosa. Marine and Freshwater Behaviour and Physiology, 26(1): 47-57.
DOI:10.1080/10236249509378927 |
Correia M, Domingues P M, Sykes A, Andrade J P. 2005. Effects of culture density on growth and broodstock management of the cuttlefish, Sepia officinalis (Linnaeus, 1758). Aquaculture, 245(1-4): 163-173.
DOI:10.1016/j.aquaculture.2004.12.017 |
Domingues P, Sykes A, Sommerfield A, Almansa E, Lorenzo A, Andrade J P. 2004. Growth and survival of cuttlefish (Sepia officinalis) of different ages fed crustaceans and fish. Effects of frozen and live prey. Aquaculture, 229(1-4): 239-254.
DOI:10.1016/S0044-8486(03)00351-X |
Dong G C, Dong S L, Tian X L, Wang F. 2011. Effects of photoperiod on daily activity rhythm of juvenile sea cucumber, Apostichopus japonicus (Selenka). Chinese Journal of Oceanology and Limnology, 29(5): 1015-1022.
DOI:10.1007/s00343-011-0204-6 |
Downing G, Litvak M K. 2002. Effects of light intensity, spectral composition and photoperiod on development and hatching of haddock (Melanogrammus aeglefinus) embryos. Aquaculture, 213(1-4): 265-278.
DOI:10.1016/S0044-8486(02)00090-X |
Evans A B, Acosta M L, Bolstad K S. 2015. Retinal development and ommin pigment in the cranchiid squid Teuthowenia pellucida (Cephalopoda: Oegopsida). PLoS One, 10(5): e0123453.
DOI:10.1371/journal.pone.0123453 |
Falcón J, Migaud H, Muñoz-Cueto J A, Carrillo M. 2010. Current knowledge on the melatonin system in teleost fish. General and Comparative Endocrinology, 165(3): 469-482.
DOI:10.1016/j.ygcen.2009.04.026 |
Fiorito G, Affuso A, Basil J, Cole A, de Girolamo P, D'Angelo L, Dickel L, Gestal C, Grasso F, Kuba M, Mark F, Melillo D, Osorio D, Perkins K, Ponte G, Shashar N, Smith D, Smith J, Andrews P L. 2015. Guidelines for the care and welfare of cephalopods in research—a consensus based on an initiative by CephRes, FELASA and the Boyd Group. Laboratory Animals, 49(S2): 1-90.
DOI:10.1177/0023677215580006 |
Forsythe J W, DeRusha R H, Hanlon R T. 1994. Growth, reproduction and life span of Sepia officinalis (Cephalopoda: Mollusca) cultured through seven consecutive generations. Journal of Zoology, 233(2): 175-192.
DOI:10.1111/j.1469-7998.1994.tb08582.x |
Foster G D, Moon T W. 1986. Enzyme activities in the Atlantic hagfish, Myxine glutinosa: changes with captivity and food deprivation. Canadian Journal of Zoology, 64(5): 1080-1085.
DOI:10.1139/z86-162 |
Gehrke P C. 1994. Influence of light intensity and wavelength on phototactic behaviour of larval silver perch Bidyanus bidyanus and golden perch Macquana ambigua and the effectiveness of light traps. Journal of Fish Biology, 44(5): 741-751.
DOI:10.1111/j.1095-8649.1994.tb01252.x |
Giri S S, Sahoo S K, Sahu B B, Sahu A K, Mohanty S N, Mukhopadhyay P K, Ayyappan S. 2002. Larval survival and growth in Wallago attu (Bloch and Schneider): effects of light, photoperiod and feeding regimes. Aquaculture, 213(1-4): 151-161.
DOI:10.1016/S0044-8486(02)00012-1 |
Gracia-López V, Kiewek-Martı́nez M, Maldonado-Garcı́a M. 2004. Effects of temperature and salinity on artificially reproduced eggs and larvae of the leopard grouper Mycteroperca rosacea. Aquaculture, 237(1-4): 485-498.
DOI:10.1016/j.aquaculture.2004.04.018 |
Groeger G, Cotton P A, Williamson R. 2005. Ontogenetic changes in the visual acuity of Sepia officinalis measured using the optomotor response. Canadian Journal of Zoology, 83(2): 274-279.
DOI:10.1139/z05-011 |
Hanlon R. 2007. Cephalopod dynamic camouflage. Current Biology, 17(11): R400-R404.
DOI:10.1016/j.cub.2007.03.034 |
Jellouli K, Bougatef A, Daassi D, Balti R, Barkia A, Nasri M. 2009. New alkaline trypsin from the intestine of grey triggerfish (Balistes capriscus) with high activity at low temperature: purification and characterisation. Food Chemistry, 116(3): 644-650.
DOI:10.1016/j.foodchem.2009.02.087 |
Jereb P, Roper C F E. 2005. Cephalopods of the World Vol. 1. FAO Species Catalogue for Fishery Purposes No. 4, Vol. 1 An Annotated and Illustrated Catalogue of Cephalopod Species Known to Date Volume 1: Chambered Nautiluses and Sepioids (Nautilidae, Sepiidae, Sepiolidae, Sepiadariidae, Idiosepiidae and Spirulidae). FAO, Rome.
|
Jiang M W, Peng R B, Wang S J, Zhou S N, Chen Q C, Huang C, Han Q X, Jiang X M. 2018. Growth performance and nutritional composition of Sepia pharaonis under artificial culturing conditions. Aquaculture Research, 49(8): 2788-2798.
DOI:10.1111/are.13741 |
Jiang M W, Ruan P, Peng R B, Han Q X, Jiang X M. 2020. Effects of size dominance on the survival, growth and physiological activities of juvenile pharaoh cuttlefish (Sepia pharaonis). Journal of Experimental Marine Biology and Ecology, 525: 151318.
DOI:10.1016/j.jembe.2020.151318 |
Jiang M W, Zhao C X, Yan R X, Li J P, Song W W, Peng R B, Han Q X, Jiang X M. 2019. Continuous inking affects the biological and biochemical responses of cuttlefish Sepia pharaonis. Frontiers in Physiology, 10: 1429.
DOI:10.3389/fphys.2019.01429 |
Kamler E. 2002. Ontogeny of yolk-feeding fish: an ecological perspective. Reviews in Fish Biology and Fisheries, 12(1): 79-103.
DOI:10.1023/A:1022603204337 |
Kohyama-Koganeya A, Kurosawa M, Hirabayashi Y. 2015. Differential effects of tissue-specific deletion of BOSS on feeding behaviors and energy metabolism. PLoS One, 10(7): e0133083.
DOI:10.1371/journal.pone.0133083 |
Koueta N, Boucaud-Camou E. 2001. Basic growth relations in experimental rearing of early juvenile cuttlefish Sepia officinalis L.(Mollusca: Cephalopoda). Journal of Experimental Marine Biology and Ecology, 265(1): 75-87.
DOI:10.1016/S0022-0981(01)00319-7 |
Koueta N, Boucaud-Camou E. 2003. Combined effects of photoperiod and feeding frequency on survival and growth of juvenile cuttlefish Sepia officinalis L. in experimental rearing. Journal of Experimental Marine Biology and Ecology, 296(2): 215-226.
DOI:10.1016/S0022-0981(03)00322-8 |
Lee M F, Lin C Y, Chiao C C, Lu C C. 2016. Reproductive behavior and embryonic development of the pharaoh cuttlefish, Sepia pharaonis (Cephalopoda: Sepiidae). Zoological Studies, 55: 41.
DOI:10.6620/ZS.2016.55-41 |
Litvak M K. 1999. The development of winter flounder (Pleuronectes americanus) for aquaculture in Atlantic Canada: current status and future prospects. Aquaculture, 176(1-2): 55-64.
DOI:10.1016/S0044-8486(99)00050-2 |
Mäthger L M, Roberts S B, Hanlon R T. 2010. Evidence for distributed light sensing in the skin of cuttlefish, Sepia officinalis. Biology Letters, 6(5): 600-603.
DOI:10.1098/rsbl.2010.0223 |
Mazorra M T, Rubio J A, Blasco J. 2002. Acid and alkaline phosphatase activities in the clam Scrobicularia plana: kinetic characteristics and effects of heavy metals. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 131(2): 241-249.
DOI:10.1016/S1096-4959(01)00502-4 |
Minton J W. 2004. The pattern of growth in the early lifecycleof individual Sepia pharaonis. Marine and Freshwater Research, 55(4): 415-422.
DOI:10.1071/MF03204 |
Moustakas C T, Watanabe W O, Copeland K A. 2004. Combined effects of photoperiod and salinity on growth, survival, and osmoregulatory ability of larval southern flounder Paralichthys lethostigma. Aquaculture, 229(1-4): 159-179.
DOI:10.1016/S0044-8486(03)00366-1 |
O'Brien C E, Bellanger C, Jozet-Alves C, Mezrai N, Darmaillacq A S, Dickel L. 2018. Stressful conditions affect reproducing cuttlefish (Sepia officinalis), reducing egg output and quality. ICES Journal of Marine Science, 75(6): 2060-2069.
DOI:10.1093/icesjms/fsy115 |
Palmer J W, Tandler B, Hoppel C L. 1977. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. Journal of Biological Chemistry, 252(23): 8731-8739.
DOI:10.1016/S0021-9258(19)75283-1 |
Panserat S, Capilla E, Gutierrez J, Frappart P O, Vachot C, Plagnes-Juan E, Aguirre P, Brèque J, Kaushik S. 2001. Glucokinase is highly induced and glucose-6-phosphatase poorly repressed in liver of rainbow trout (Oncorhynchus mykiss) by a single meal with glucose. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 128(2): 275-283.
DOI:10.1016/S1096-4959(00)00322-5 |
Peng R B, Le K X, Jiang X M, Wang Y, Han Q X. 2015. Effects of different diets on the growth, survival, and nutritional composition of juvenile cuttlefish, Sepia pharaonis. Journal of the World Aquaculture Society, 46(6): 650-664.
DOI:10.1111/jwas.12235 |
Perrin A, Le Bihan E, Koueta N. 2004. Experimental study of enriched frozen diet on digestive enzymes and growth of juvenile cuttlefish Sepia officinalis L.(Mollusca Cephalopoda). Journal of Experimental Marine Biology and Ecology, 311(2): 267-285.
DOI:10.1016/j.jembe.2004.05.012 |
Pinsirodom P, Parkin K L. 2001. Lipase assays. In: Wrolstad R E, Acree T E, Decker E A, Penner M H, Reid D S, Schwartz S J, Shoemaker C F, Smith D M, Sporns P eds. Current Protocols in Food Analytical Chemistry. John Wiley & Sons, Inc., New Jersey. p. 315-319.
|
Principato G B, Cristina Aisa M, Biagioni M, Giovannini E. 1982. Partial purification and characterization of an alkaline phosphatase in Helix nemoralis and in Octopus vulgaris. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry, 72(2): 325-328.
DOI:10.1016/0305-0491(82)90055-4 |
Qiu D G, Xu S H, Song C B, Chi L, Li X, Sun G X, Liu B L, Liu Y. 2015. Effects of spectral composition, photoperiod and light intensity on the gonadal development of Atlantic salmon Salmo salar in recirculating aquaculture systems (RAS). Chinese Journal of Oceanology and Limnology, 33(1): 45-56.
DOI:10.1007/s00343-015-4011-3 |
Richard A. 1971. Contribution à L'étude Expérimentale de la Croissance et de la Maturation Sexuelle de Sepia officinalis L. (Mollusque Céphalopode). Université Lille, Lille.
|
Roper C F E, Sweeney M J, Nauen C E. 1984. Cephalopods of the World. An Annotated and Illustrated Catalogue of Species of Interest to Fisheries. FAO Species Catalogue No. 125, FAO.
|
Rosas C, Sánchez A, Pascual C, Aguila J, Maldonado T, Domingues P. 2011. Effects of two dietary protein levels on energy balance and digestive capacity of Octopus maya. Aquaculture International, 19(1): 165-180.
DOI:10.1007/s10499-010-9350-7 |
Rutter J, Winge D R, Schiffman J D. 2010. Succinate dehydrogenase- assembly, regulation and role in human disease. Mitochondrion, 10(4): 393-401.
DOI:10.1016/j.mito.2010.03.001 |
Safi G, Martinez A S, Le Pabic C, Le Bihan E, Robin J P, Koueta N. 2018. Digestive enzyme ratios are good indicators of hatchling yolk reserve and digestive gland maturation in early life stages of cuttlefish Sepia officinalis L.: application of these new tools in ecology and aquaculture. Journal of Comparative Physiology B, 188(1): 57-76.
DOI:10.1007/s00360-017-1115-4 |
Samuel D V, Patterson J. 2015. Studies on the embryonic development of pharaoh's cuttlefish Sepia pharaonis Ehrenberg, 1831 under laboratory conditions. Indian Journal of Geo-Marine Sciences, 44(4): 519-527.
|
Serb J M, Eernisse D J. 2008. Charting evolution's trajectory: using molluscan eye diversity to understand parallel and convergent evolution. Evolution: Education and Outreach, 1(4): 439-447.
DOI:10.1007/s12052-008-0084-1 |
Shi Y H, Zhang G Y, Liu J Z, Zhu X D. 2012. Effects of photoperiod on embryos and larvae of tawny puffer, Takifugu flavidus. Journal of the World Aquaculture Society, 43(2): 278-285.
DOI:10.1111/j.1749-7345.2012.00561.x |
Shi Y H, Zhang G Y, Zhu Y Z, Liu J Z. 2010. Effects of photoperiod, temperature, and salinity on growth and survival of obscure puffer Takifugu obscurus larvae. Aquaculture, 309(1-4): 103-108.
DOI:10.1016/j.aquaculture.2010.09.004 |
Simensen L M, Jonassen T M, Imsland A K, Stefansson S O. 2000. Photoperiod regulation of growth of juvenile Atlantic halibut (Hippoglossus hippoglossus L.). Aquaculture, 190(1-2): 119-128.
DOI:10.1016/S0044-8486(00)00397-5 |
Southwood A L, Darveau C A, Jones D R. 2003. Metabolic and cardiovascular adjustments of juvenile green turtles to seasonal changes in temperature and photoperiod. Journal of Experimental Biology, 206(24): 4521-4531.
DOI:10.1242/jeb.00689 |
Speers-Roesch B, Callaghan N I, MacCormack T J, Lamarre S G, Sykes A V, Driedzic W R. 2016. Enzymatic capacitiesof metabolic fuel use in cuttlefish (Sepia officinalis) and responses to food deprivation: insight into the metabolic organization and starvation survival strategy of cephalopods. Journal of Comparative Physiology B, 186(6): 711-725.
DOI:10.1007/s00360-016-0991-3 |
Sykes A V, Quintana D, Andrade J P. 2014. The Effects of light intensity on growth and survival of cuttlefish (Sepia officinalis) hatchlings and Juveniles. Aquaculture Research, 45(12): 2032-2040.
DOI:10.1111/are.12150 |
Tehranifard A, Dastan K. 2011. General morphological characteristics of the Sepia pharaonis (cephalopoda) from Persian Gulf, Bushehr region. In: Proceedings of 2011 International Conference on Biomedical Engineering and Technology. IACSIT Press, Singapore. p. 120-126.
|
Tranulis M A, Dregni O, Christophersen B, Krogdahl Å, Borrebaek B. 1996. A glucokinase-like enzyme in the liver of Atlantic salmon (Salmo salar). Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 114(1): 35-39.
DOI:10.1016/0305-0491(95)02119-1 |
Trotter A J, Battaglene S C, Pankhurst P M. 2003. Effects of photoperiod and light intensity on initial swim bladder inflation, growth and post-inflation viability in cultured striped trumpeter (Latris lineata) larvae. Aquaculture, 224(1-4): 141-158.
DOI:10.1016/S0044-8486(03)00212-6 |
Tsunematsu H, Nishimura H, Mizusaki K, Makisumi S. 1985. Kinetics of hydrolysis of amide and anilide substrates of p-Guanidino-L-Phenylalanine by bovine and porcinetrypsins. The Journal of Biochemistry, 97(2): 617-623.
DOI:10.1093/oxfordjournals.jbchem.a135097 |
Tuckey L M, Smith T I J. 2001. Effects of photoperiod and substrate on larval development and substrate preference of juvenile southern flounder, Paralichthys lethostigma. Journal of Applied Aquaculture, 11(1-2): 1-20.
DOI:10.1300/J028v11n01_02 |
Villamizar N, García-Alcazar A, Sánchez-Vázquez F J. 2009. Effect of light spectrum and photoperiod on the growth, development and survival of European sea bass (Dicentrarchus labrax) larvae. Aquaculture, 292(1-2): 80-86.
DOI:10.1016/j.aquaculture.2009.03.045 |
Yoseda K, Yamamoto K, Asami K, Chimura M, Hashimoto K, Kosaka S. 2008. Influence of light intensity on feeding, growth, and early survival of leopard coral grouper (Plectropomus leopardus) larvae under mass-scale rearing conditions. Aquaculture, 279(1-4): 55-62.
DOI:10.1016/j.aquaculture.2008.04.002 |
Zhou S N, Lyu T T, Chen Q C, Peng R B, Han Q X, Jiang X M. 2018. Effects of light intensity and photoperiod on the embryonic development of Sepia pharaonis. Chinese Journal of Applied Ecology, 29(6): 2059-2067.
(in Chinese with English abstract) DOI:10.13287/j.1001-9332.201806.033.(inChinesewithEnglishabstract) |
 2022, Vol. 40
2022, Vol. 40


