Institute of Oceanology, Chinese Academy of Sciences
Article Information
- WAN Lingling, SONG Chunlei, ZHOU Yiyong, CAO Xiuyun
- Cyanobacterial extracellular alkaline phosphatase: detection and ecological function
- Journal of Oceanology and Limnology, 40(5): 1840-1854
- http://dx.doi.org/10.1007/s00343-022-2112-3
Article History
- Received Mar. 11, 2022
- accepted in principle Apr. 15, 2022
- accepted for publication Jul. 1, 2022
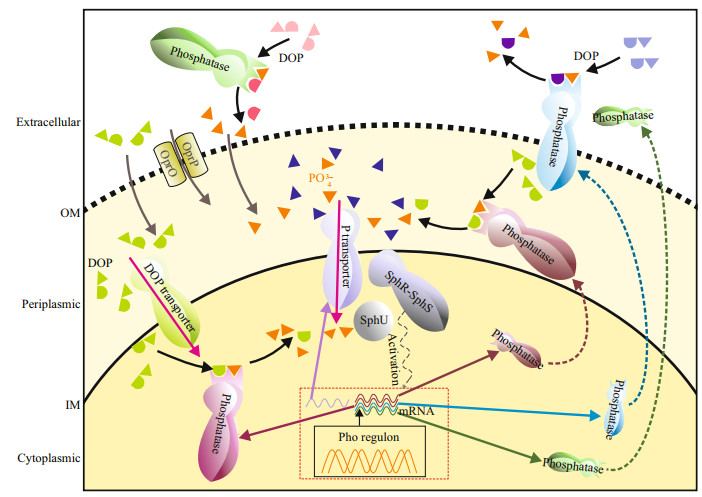
Harmful cyanobacterial blooms in lakes are serious aquatic environmental problems. Phosphorus (P) is one of the limiting elements of phytoplankton growth in natural waters (Schindler, 1977; White et al., 2003). Dissolved inorganic P (DIP) is the preferred form of directly bioavailable P for phytoplankton (Cotner and Wetzel, 1992; Björkman and Karl, 1994; Baken et al., 2014). Dissolved organic P (DOP) maintains the supply of DIP via alkaline phosphatase catalyzing (Berman, 1970; Cao et al., 2018; Lim et al., 2018; Feng et al., 2020). Alkaline phosphatase (APase, EC 3.1.3.1) is a type of organophosphate hydrolase, which could catalyze the hydrolysis of phosphate ester bonds containing C-O-P ester bond to release orthophosphate (Pi) (Hoppe, 2003). The substrates of APases include phosphate glycosides, phosphomonoesters, and phosphate diesters, etc. (Bentzen et al., 1992; Yamaguchi et al., 2005), which contributed over 75% of DOP in the water (Kolowith et al., 2001; Young and Ingall, 2010; Yates et al., 2019). In addition to phototrophic and heterotrophic prokaryotes, protozoa and zooplankton, phytoplankton was found to be able to produce extracellular APases (Hoppe, 2003). Despite there have been numerous studies on the eco-physiological responses to P and increasing researches on genomics in bloom-forming algae in the past decades, few attempts to synthesize information in terms of extracellular APases produced by cyanobacteria were reported. Generally, extracellular enzymes are principally located outside the cell membrane and no longer in contact with their producers, while ecto-enzymes were defined as the enzymes located outside the cellular outer membrane, but still in contact with the cell (Chróst, 1991). Here, we consider that extracellular phosphatase includes extracellular (or free) and ecto-phosphatase (Fig. 1). Previously, Jansson et al. (1988) and Hoppe (2003) have systematically reviewed the origin, characteristics, and function of phosphatases in lake and ocean. Physiological ecology and molecular mechanisms responding to P status of ecto-APases were subsequently summarized in marine typical bloom-forming eukaryotic algae (Dyhrman, 2005; Lin et al., 2016). In this paper, we give a brief review of the determination method development in cyanobacterial extracellular phosphatase and its ecological function both in fresh and marine water.
|
| Fig.1 Subcellular localizations of alkaline phosphatase and its regulation by Pho regulons in cyanobacteria This diagram represents a composite of known cyanobacterial organic phosphate esters acquisition and hydrolysis pathways, which are likely to be present in some cyanobacterial species or bacteria. Grey arrows indicate possible pathways by which inorganic and organic P is transported into periplasm through a phosphate-selective porin OprO-P, which has been identified in bacteria (Rao and Torriani, 1990; Modi et al., 2013). Magenta arrows indicate the transmembrane transport of inorganic and organic P, and these sites of transmembrane transporters reference to Rao and Torriani (1990) and Luo et al. (2009). Dashed arrows (brown, blue, and green) indicate secretion systems of alkaline phosphatases. Subcellular locations of alkaline phosphatases of cyanobacteria reference to Luo et al. (2009). Two component systems regulate the organophosphate metabolism, which is made up with the sensor kinase (SphR) and the reponse regulator (SphS). The specific activation process is: P-limitation conditions imply the unsaturated P transporter, which will prompt the detachment (or conformational changes) of a negative regulator (SphU). Then, Pho regulons will be activated by the phosphorylation of SphS (Juntarajumnong et al., 2007; Su et al., 2007; Hsieh and Wanner, 2010; Tiwari et al., 2015). IM: inner membrane; OM: outer membrane. |
Since Steiner (1938) first discovered that DOP could be enzymatically hydrolyzed in lakes, the research on extracellular phosphatases in water bodies have been a rapid development (Berman, 1970; Zhou and Zhou, 1997; Luo et al., 2009; Zhang et al., 2016; Wang et al., 2021). Tracing the origin or producer of extracellular phosphatase is of great ecological significance. The accuracy mainly depends on the advances in the analytical methods, while extracellular phosphatase from cyanobacteria could be detected by axenic culture and enzyme purification. In-situ detection by enzyme labelled fluoresce (ELF) technology and molecular biological methods could give more solid evidence for the origin of extracellular phosphatase.
2.1 Extracellular phosphatase activity analysis from cyanobacteriaCyanobacteria can produce APases attached to the cellular outer membrane or dissolved in the medium (Healey and Hendzel, 1979a, b; Smith and Kalff, 1981; Vaitomaa et al., 2002; Luo et al., 2009). Measurements of APase activity (APA) were effective means to quantitatively characterize APases in the field investigations and indoor experiments (Lehman et al., 2013; Yuan et al., 2016; Wan et al., 2019; Zhou et al., 2021). Determination of APA in the lab experiment can further prove the relationship between axenic cultured cyanobacteria and the origins of phosphatases to a certain degree, such as the contribution of cyanobacteria to soluble extracellular APases. For example, in the Anacystis nidulans and Synechococcus culture medium, APA was detected using para-nitro-pheneye phosphate (pNPP) as substrates (Ihlenfeldt and Gibson, 1975). APA detected using pNPP as substrates were related to cyanobacterial colonies (Rivularia) in the British rivers (Livingstone and Whitton, 1984). The same method was later used to detect APA from Nostoc linckia, Nostoc muscorum, and Trichodesmium in the field survey and lab culture (Kumar et al., 1992; Stihl et al., 2001). Besides pNPP, other organic P compound could also be used as substrates. For example, bis-p-nitrophenyl phosphate (bis-pNPP) was used to determine cell membrane attached and dissolved APA in Nostoc commune UTEX 584 culture (Whitton et al., 1990). Later on, 50 species of cyanobacteria were found to secrete extracellular APases using the same assay protocal (Whitton et al., 1991). Since the new century, 4-methylumbelliferyl phosphate (MUFP) has been used as substrate to determine APA in the field survey and lab cyanobacterial culture (Vaitomaa et al., 2002). pNPP and MUFP methods mainly focused on the liberation of Pi hydrolyzed by phosphomonoesterase, while less attentions were paid to phosphodiesterase (Sirová et al., 2013), which is a non-negligible enzyme involved in the organophosphorus hydrolysis pathway (Accoroni et al., 2017; Srivastava et al., 2021). Therefore, bis-pNPP or bis-(4-methylumbelliferyl) phosphate (bis-MUFP) was still suggested to access the phosphodiesterase activity and thus indicate the sources of organic P (Sirová et al., 2013). The accuracy of APA was also constrained by several factors, e.g. assay substrate, incubation time, pH, and sample sources, etc. (Hernández and Whitton, 1996). The peak month of APA of Rivularia colonies was observed to be different by using pNPP and MUFP as the sole substrate in intertidal pools at Tyne Sands, Scotland (Yelloly and Whitton, 1996). The comparison experimental results showed that the maximum release of the product (p-nitrophenol) appeared in over 20 min and at slightly acid pH, while the maximum 4-MUF appeared in more than 40 min and at neutral pH (Hernández and Whitton, 1996). Cyanobacterial blooms always occurred in alkalescent water bodies, and higher growth rates of cyanobacteria were also observed in the alkalescent culture mediums (Hong and Lee, 2008; de Souza Santos et al., 2011; Fang et al., 2018; Wei et al., 2022). Therefore, the MUFP method may be more suitable for the detection of APA from cyanobacteria.
Also of note is that, the sources of APases cannot be distinguished by pNPP and MUFP methods both in field investigations and indoor experiments, indicating that the contribution of APA from algae will be overestimated if the contribution of bacteria cannot be ruled out. Reducing the growth of bacteria by adding antibiotic or using UV light is an effective way to exclude its interference to algal APA in the cultivations (Gerloff et al., 1950; Harke et al., 2012; Li et al., 2015b). Purification of APases could relate to the APA and its producers partially (Martland and Robison, 1929; McComb et al., 1979). Jansson (1976) separated and purified the phosphatase in the cell and culture medium. Dissolved extracellular phosphatase in water might be derived from a zooplankton, Bosmina obtusirostris, by comparing the physical and chemical characteristics of the enzymes. Phosphatase isolated and purified from cyanobacteria (Anacystis nidulans) had different characteristics from other phosphatases described previously (Ihlenfeldt and Gibson, 1975). Doonan and Jensen (1980) extracted attached and free APases from eighteen cyanobacteria and demonstrated the inducibility of eleven of them. APases, purified from cyanobacteria (Arthrospira platensi) using Triton X-114, was proven to be a calcium-dependent protein (Asencio et al., 2012). The purification method has not been widely used in the field surveys of phytoplankton ecology or environmental science, since it is complex and inefficient to distinguish between intracellular and extracellular phosphatases attached to the cell (Malherbe et al., 2019). Size fraction measurement of APA was a rough way to evaluate the contributor of particle (including cyanobacteria) APases (Dyhrman and Ruttenberg, 2006; Lim et al., 2018). If the large particle size contributed most to the total APA, extracellular APases were assumed being mainly contributed by the dominant or cultured cyanobacteria (Raoui et al., 2002; Wan et al., 2019).
2.2 Visualization of extracellular phosphataseEnzyme labelled fluorescence (ELF) method provided an artificial substrate to visually detect extracellular APases in cyanobacteria or algae. The basic principle of this method is as follows: ELFTM97 phosphate (ELFP) is a specific substrate of phosphatase, which could be hydrolyzed by APase into Pi and fluorescent organic residues (ELFA). The insolubility of organic residues makes the precipitate attach to the enzymatic reaction, so the fluorescence (or confocal) microscope or flow cytometer can not only determine the presence of extracellular phosphatase of cyanobacteria or algae, but also mark the occurrence site (Dyhrman and Palenik, 1999). Extracellular phosphatase was firstly determined by ELF method in the culture of marine algae (González-Gil et al., 1998). ELF method was introduced and developed to detect algal and cyanobacterial extracellular phosphatase in Czech reservoirs by Štrojsová et al. (2003, 2005). Nedoma et al. (2007) further proved that the ELF assay could ensure a high saturation of extracellular phosphatases in the marine (> 99%) and fresh (> 90%) waters. Since then, this method has been widely used in the monitor of extracellular phosphatase of the marine and freshwater cyanobacteria, which has led the utilization of fluorescence microscopy into the visualization of APases in the cyanobacterial cells (Table 1). Significantly, not all tested cyanobacteria displayed fluorescent precipitates of ELFA (Table 1, Dignum et al., 2004b; Rychtecký et al., 2015; Wan et al., 2019). Possible reasons include: firstly, the evoked threshold concentration of P is variable for initiating the secretion of APases by different cyanobacteria. The same P concentration or environmental condition might not trigger the secretion of APases for some cyanobacteria (Nausch, 1998; Dyhrman and Palenik, 1999; Sebastián et al., 2004; Cao et al., 2007; Girault et al., 2013; Zhang et al., 2021). Secondly, some cyanobacteria may secrete dissolved APases, which cannot trace back to the producers by ELF assay (Wang et al., 2021). Overall, there were still some limitations in analyzing cyanobacterial extracellular APA: firstly, absolute axenic conditions were difficult to achieve, as a result, the contribution of bacteria to enzyme activity cannot be ruled out. Secondly, the physiological, biochemical, and external conditions of cyanobacteria grown were not consistent. Therefore, the chemical measurements of APA have many objective disadvantages, and multiple methods must be taken into consideration to comprehensively evaluate the cyanobacterial APases.
Development of molecular biological methods provided deep insights into the origin of extracellular phosphatase, if combined with the quantification and ELF technique. The dominant molecular model, used to describe transformations and assimilations of P, has been the Pho regulon of the gram-negative bacterium Escherichia coli (Vershinina and Znamenskaya, 2002). So far, the most important APases in prokaryotes include PhoA, PhoD, PhoV, PhoX, and atypical phosphatases (Luo et al., 2009; Kageyama et al., 2011; Lin et al., 2016). The general elements of the Pho regulon in cyanobacteria and its regulatory mechanisms might be similar to heterotrophic bacteria (Fig. 1). The full-length of APase genes were sequenced and expressions of these genes were quantified under different environment in the past two decades. The corresponding complementary DNA and RNA sequencing was coupled to a comprehensive metabolomics survey afterwards. Gene sequence analysis has revealed that phoD encodes APase in Anabaena (Singh et al., 2015). In addition, the genes encoding APases in Anabaena (later named Dolichospermum) include phoA, phoD, and phoS (Liu and Wu, 2012). phoA and phoX have been also reported in pico-cyanobacteria or cyanobacteria (Vershinina and Znamenskaya, 2002; Su et al., 2003; Moore et al., 2005; Sebastian and Ammerman, 2009; Tetu et al., 2009; Kathuria and Martiny, 2011; Harke et al., 2012). Atypical APase genes like other cyanobacterial phoA and a classical phoA gene are predicted in the genome of Anabaena sp. PCC 7120 (Luo et al., 2010).
The development of protein sequencing technology has further revealed the structures and functions of cyanobacterial APases. Ray et al. (1991) found an atypical APase in Synechococcus sp. PCC7942, whose size (145 kDa) is larger than the previously reported one (47–87 kDa). The PhoA-type APase has a Zn2+ cofactor, while PhoX associated with uncultured Prochlorococcus is an active phosphatase with a Ca2+ cofactor (Kathuria and Martiny, 2011). PhoX is a monomeric enzyme activated by Ca2+ and Fe3+ (Majumdar et al., 2005; Monds et al., 2006; Yong et al., 2014) with a lower substrate specificity for C-O-P bonds, such as nucleotides, phosphorylated carbohydrates, and amino acids (Zaheer et al., 2009). PhoX in Microcystis aeruginosa FACHB7806 is strongly activated by Mg2+, followed by other divalent ions (like Co2+, Ca2+, Zn2+, and Mn2+), but it is inhibited by Ni2+ (Hong et al., 2021). Proteome results suggest that PhoA (Mg2+ and Zn2+) and PhoX (Ca2+) has been expressed in Synechococcus sp. WH 8102 (Cox and Saito, 2013). PhoD from Aphanothece halophytica is a hydrolase activated by Ca2+ that can hydrolyze phosphomonoesters and phosphodiesters (Kageyama et al., 2011). PhoD from a unicellular N2-fixing cyanobacteria (Halothece sp. PCC 7418) shows the connection between Ca2+ and Fe3+, which harbors eight copies of APase encoding genes (Fernández-Juárez et al., 2019). PhoV is a hydrolase activated by Zn2+, which can hydrolyze phosphate monoesters and has a wide range of adaptation to pH, but it is inhibited by Mn2+ (Wagner et al., 1995).
The detection of APase encoding genes and their expression levels enable the interpretation of APA and visualization of APases more reasonable. For example, the increased expression of putative APase gene (phoX) in M. aeruginosa under low P supply evidenced that the extracellular phosphatases originated from M. aeruginosa, together with a significantly positive correlation between P concentrations and APA in a monocultural experiment (Harke et al., 2012). Similarly, up-regulated phoX gene of the predominant Microcystis was observed in low-P regions of Lake Erie (Harke et al., 2016). In respond to the decreasing P concentrations, bloom-forming Hydrocoleum sp. elevated the expression level of phoA gene (Moisander et al., 2022). The changes in the expression levels of phoA-like and phoD genes well explained the increase of APA of Raphidiopsis mediterranea and Planktothrix agardhii in the P-starvation conditions (Aguilera et al., 2019). Increasing the expression level of cyanobacterial APase genes in response to low P supply is species specific in terms of the differences in genes copy numbers and gene types (Harke et al., 2012; Liu and Wu, 2012; Lin et al., 2018; Aguilera et al., 2019; Willis et al., 2019). For example, phoX gene was not identified in Microcystis weisenbergii but in M. aeruginosa (Harke et al., 2012). PhoA, phoD, and phoX genes were identified in Anabaena cylindrica FACHB-170 (Lin et al., 2018). Even though Raphidiopsis raciborskii CS-505 and CS-506 harbored the same gene type, the copy numbers of their phoA gene were also different (Willis et al., 2019). Furthermore, the combined observations in transcriptional changes and metabolic homeostasis in M. aeruginosa provide novel and extensive insights into the complex cellular interactions that take place in this important bloom-forming organism (Steffen et al., 2014). Therefore, comprehensively assess molecular aspects of phosphatase encoding gene and its expression, transcriptional patterns in the important bloom-forming cyanobacteria might expand our knowledge in understanding its bloom mechanism.
3 ECOLOGICAL FUNCTIONS OF CYANOBACTERIAL EXTRACELLULAR PHOSPHATASES 3.1 Indicating P deficiencyPhytoplankton will produce extracellular phosphatase when suffering from P deficiency in natural population and lab experiment (Schindler, 1977; Healey, 1978; White et al., 2003; Cao et al., 2018; Lim et al., 2018; Feng et al., 2020). Therefore, algal APA or total APA was inversely proportional to P concentration (Smith and Kalff, 1981; Chróst et al., 1984; Francko, 1984; Pettersson, 1985; Vrba et al., 1993). APA of cyanobacterial mats in the Caribbean showed significantly negative correlation to the P concentrations, as well as the P content of the mat (Rejmánková and Komárková 2005). Furthermore, APA would be inhibited in the presence of sufficient inorganic P supply (Kuenzler and Perras, 1965). This relationship was summarized as "induction-repression" mechanism (Jansson et al., 1988). As a result, APA has often been recommended as an indicator of P starvation in planktonic systems (Healey and Hendzel, 1980; Istánovics et al., 1992; Rose and Axler, 1997; Thingstad et al., 1998; Jamet et al., 2001) or cyanobacterial mats (Rejmánková and Komárková 2000).
Normalized APA, such as chlorophyll-specific total APA (Istánovics et al., 1992), surface-area-specific APA (Newman et al., 2003) was more sensitive enough to show changes in the P status of algae than total APA (Zhang et al., 2021). The specific activity divided by algal biomass can substantially reflect the intrinsic catalytic efficiency of the enzyme, and thus serve as a suitable indicator for the P nutrient status of water and algae (Kuenzler and Perras, 1965; Perry, 1972; Fitzgerald and Nelson, 1975; Moegenburg and Vanni, 1991; Istánovics et al., 1992). On the other hand, the reliability of using APase as an indicator of P deficiency in phytoplankton was questioned (Cembella et al., 1982). For example, in Florida Bay, APA was highest during cyanobacterial blooms and particulate APA was related to the bloom, but APA/chlorophyll a and dissolved APA showed no correlation to the bloom (Koch et al., 2009). Factors interfering APA as the indicator of P deficiency include variety of extracellular phosphatase producers such as bacteria and zooplankton (Koch et al., 2009), enzyme substrate diversity (Sharma et al., 2014; Zhang et al., 2020b) and its effect on APA (Fonseca-De-Souza et al., 2008; Harke et al., 2012; Li et al., 2015a), extracellular secretions such as extracellular toxins (Bar-Yosef et al., 2010; Dobronoki et al., 2019; Lu et al., 2021), species specific responses (Olsen et al., 1989; Wan et al., 2019), external P threshold concentrations for activated response (Pick, 1987; Ruiz et al., 1997), environmental factors, such as light (Giraudet et al., 1997; Rychtecký et al., 2015; Yadav et al., 2016) and temperature (Bai et al., 2021; Ivančić et al., 2021), and the level of intracellular P content (Olsson, 1983; Whitton et al., 1990; Zhang et al., 2020a). Furthermore, the activity of endogenous enzymes might be overestimated because of the exogenous input (Stevens and Parr, 1977).
Biochemical components unrelated to P can also cause increase or decrease in APA (Wilkins, 1972; Francko and Wetzel, 1982). In addition to differences in gene homology, the metal ions, located in the active sites of phosphatases, are also different (Luo et al., 2009). Therefore, the lack of external related metal elements will also affect the synthesis and function of cyanobacterial extracellular APA (Singh et al., 2006; Cox and Saito, 2013). APA contributed by dissolved APases could be spontaneously released during cyanobacterial blooms, other than driven by the P limitation. This explained coexistences of high Pi concentrations and high potential APA in water observed previously (Wang et al., 2021). The activity of dissolved phosphatases can be maintained for several weeks (Jansson, 1981; Olsson, 1983), which means that enzymes produced in one place may function in a wider range of waters. Shortly, the relationships between APA and ambient bioavailable P concentration are complicated. There are several limitations to use extracellular APA as a general indicator for describing P deficiency of phytoplankton (Cao et al., 2010). The multiple complex regulatory factors make it cautious when using extracellular APA as indicators of P deficiency.
3.2 Alleviating P stressAlgae could use extracellular phosphatases to obtain nutrients and grow normally with a different organophosphates supply (Ruiz et al., 1997). It was found that 17%–82% of the P absorbed into phytoplankton was from organophosphorus in the Sargasso Sea (McLaughlin et al., 2013). In the eutrophic Lake Nantua, the Pi released from DOP via APases could temporarily maintain the P supply for the algae dominated by Oscillatoria rubescens while DIP was deficiency in the summer (Feuillade et al., 1990). Moreover, the growth of cyanobacteria could be mainly supported by hydrolyzing dissolved organophosphate via APase in Lake Taihu and other warm-monomictic lake (Gao et al., 2006; Prentice et al., 2019). While in the absence of inorganic P, organophosphorus, e.g. pesticides, can be used by filamentous-heterocystous cyanobacteria as the only source of P (Subramanian et al., 1994). In the Grangent reservoir, cyanobacteria often relied on the APase to mineralize organic P (Giraudet et al., 1999). The aggregates dominated by Nodularia spumigena in the Baltic Sea were the key contributor to P regeneration (Stoecker et al., 2005). The increasing biomass of Aphanizomenon in a P-limited summer and autumn was accompanied by high enzymatic activity of APase (Hadas et al., 1999). Filamentous cyanobacteria, Nodularia and Aphanizomenon, from the Baltic Sea, showed higher APA with the decline of ambient Pi concentrations (Degerholm et al., 2006). Both ELF methods and molecular biology approaches supported the view that Cylindrospermopsis raciborskii could use different organophosphates to sustain growth when P was limited (Bai et al., 2014). Besides increasing potential APA, cyanobacteria may compensate for P stress by lowering Michaelis constant (Km) values of APases in the meanwhile (Zhang et al., 2021). During a Microcystis bloom outbreak, a large number of P sources was hydrolyzed by extracellular APases and released to sustain the growth of Microcystis (Chuai et al., 2011), but the extracellular APases might be excreted mainly by bacteria rather than Microcystis (Dai et al., 2018). Therefore, APase plays a crucial role in the process of cyanobacterial growth and blooms, although it might be secreted by microorganisms rather than cyanobacteria themselves (Zhao et al., 2012).
4 FUTURE RESEARCH 4.1 To what extent can extracellular phosphatases alleviate P stressResearchers, mainly focusing on bloom-forming cyanobacteria, hold a hypothesis that secreting extracellular phosphatase to use DOP is their key competitive advantage, when they realized that APA was regulated by P supply (Kelly et al., 2019; Rabouille et al., 2022; Zhang et al., 2022). Unexpectedly, cells that secrete extracellular phosphatase are often not cyanobacteria even dominated in phytoplankton assemblages (Rengefors et al., 2001; Štrojsová et al., 2003), which makes the above hypothesis difficult to understand. If we can answer the question to what extent extracellular APase can alleviate P stress, the puzzle could be solved. Unfortunately, the current detection method cannot achieve the goal because of obvious deficiencies. Firstly, it is difficult to determine to what extent it can be able to represent a natural substrate for APase when using artificial synthetic organophosphates as the substrate. Therefore, the cyanobacterial extracellular APA, strictly speaking, can only reflect the qualitative rather than quantitative relationship between APase and environmental variables (Siuda, 1984). To overcome this uncertainty, ecologists have used the radiometric method to determine the availability of P in the organic P pool of water bodies (Bentzen et al., 1992). Hernández et al. (1996) used 32P-G6P as a substrate to explore a more sensitive method for determining APA while there is still a problem since the radiometric element method might be selectively absorbed by phytoplankton. As a result, the function of phosphatase to alleviate P limitation may be overestimated for cyanobacteria. So, a combination study of cyanobacteria from molecular biology, biochemistry, field investigation, or indoor culture experiments with a more accurate test method is urgently needed.
4.2 Determine ecological function of extracellular phosphatases on species levelExcretion of extracellular APases is species specific among cyanobacteria (Rengefors et al., 2003; Dignum et al., 2004b). Cyanobacteria respond to low P supply by combining with other physiological process. Several perspectives deserve noting, including interspecific response between toxin and non-toxin producing cyanobacteria; the connection between toxin and extracellular APases producing; combining study among the different response mechanisms (e.g., P uptake, storage, regulating P demand, etc.). Since APases can be active, which are sometimes irrelevant to ambient P concentrations, other functions of APases are indeed worthy of attention at this moment, e.g. to constrain pigment biosynthesis, photosynthesis, fatty acid biosynthesis and cell division, which are of great significance in phytoplankton ecology. Although single-cell phosphatase of phytoplankton could be visualized and quantified by the ELF technology (Diaz-de-Quijano et al., 2014, 2020). Relation between transcriptional levels of APase encoding genes and the functional microbial compositions might give deep insight to the enzyme producers or origin (Bai et al., 2014; Dai et al., 2018). Integration of molecular biology and ELF assay is advised to give a comprehensive and reasonable result for the contributors of extracellular phosphatases at the species level. In summary, future research on specific species ecophysiology will benefit from a growing suite of tools available for assessing the activity and subcellular location of APase in field populations or cultures and ultimately the work done with specific species will be useful for studies of other harmful ones.
5 DATA AVAILABILITY STATEMENTAll data generated and/or analyzed during this study are included in this published article.
Accoroni S, Totti C, Razza E, et al. 2017. Phosphatase activities of a microepiphytic community during a bloom of Ostreopsis cf. ovata in the northern Adriatic Sea. Water Research, 120: 272-279.
DOI:10.1016/j.watres.2017.05.004 |
Aguilera A, Aubriot L, Echenique R O, et al. 2019. Raphidiopsis mediterranea (Nostocales) exhibits a flexible growth strategy under light and nutrient fluctuations in contrast to Planktothrix agardhii (Oscillatoriales). Hydrobiologia, 839(1): 145-157.
DOI:10.1007/s10750-019-04002-5 |
Asencio A D, Morte A, García-Carmona F, et al. 2012. Partial purification and characterization of a calcium-dependent alkaline phosphatase from the cyanobacterium Arthrospira platensis. Journal of Phycology, 48(2): 347-354.
DOI:10.1111/j.1529-8817.2012.01119.x |
Bai F, Liu R, Yang Y J, et al. 2014. Dissolved organic phosphorus use by the invasive freshwater diazotroph cyanobacterium, Cylindrospermopsis raciborskii. Harmful Algae, 39: 112-120.
DOI:10.1016/j.hal.2014.06.015 |
Bai X L, Zhou Y K, Ye W N, et al. 2021. Response of organic phosphorus in lake water to environmental factors: a simulative study. Science of the Total Environment, 785: 147275.
DOI:10.1016/j.scitotenv.2021.147275 |
Baken S, Nawara S, Van Moorleghem C, et al. 2014. Iron colloids reduce the bioavailability of phosphorus to the green alga Raphidocelis subcapitata. Water Research, 59: 198-206.
DOI:10.1016/j.watres.2014.04.010 |
Bar-Yosef Y, Sukenik A, Hadas O, et al. 2010. Enslavement in the water body by toxic Aphanizomenon ovalisporum, inducing alkaline phosphatase in phytoplanktons. Current Biology, 20(17): 1557-1561.
DOI:10.1016/j.cub.2010.07.032 |
Bentzen E, Taylor W D, Millard E S. 1992. The importance of dissolved organic phosphorus to phosphorus uptake by limnetic plankton. Limnology and Oceanography, 37(2): 217-231.
DOI:10.4319/lo.1992.37.2.0217 |
Berman T. 1970. Alkaline phosphatases and phosphorus availability in Lake Kinneret. Limnology and Oceanography, 15(5): 663-674.
DOI:10.4319/lo.1970.15.5.0663 |
Björkman K, Karl D M. 1994. Bioavailability of inorganic and organic phosphorus compounds to natural assemblages of microorganisms in Hawaiian coastal waters. Marine Ecology Progress Series, 111(3): 265-273.
DOI:10.3354/meps111265 |
Cao X Y, Song C L, Li Q M, et al. 2007. Dredging effects on P status and phytoplankton density and composition during winter and spring in Lake Taihu, China. Hydrobiologia, 581: 287-295.
DOI:10.1007/s10750-006-0516-2 |
Cao X Y, Song C L, Zhou Y Y. 2010. Limitations of using extracellular alkaline phosphatase activities as a general indicator for describing P deficiency of phytoplankton in Chinese shallow lakes. Journal of Applied Phycology, 22(1): 33-41.
DOI:10.1007/s10811-009-9422-0 |
Cao X Y, Štrojsová, Znachor P, et al. 2005. Detection of extracellular phosphatases in natural spring phytoplankton of a shallow eutrophic lake (Donghu, China). European Journal of Phycology, 40(3): 251-258.
DOI:10.1080/09670260500192760 |
Cao X Y, Wan L L, Xiao J, et al. 2018. Environmental effects by introducing Potamogeton crispus to recover a eutrophic lake. Science of the Total Environment, 621: 360-367.
DOI:10.1016/j.scitotenv.2017.11.267 |
Cembella A D, Antia N J, Harrison P J. 1982. The utilization of inorganic and organic phosphorous compounds as nutrients by eukaryotic microalgae: a multidisciplinary perspective: part Ⅰ. CRC Critical Reviews in Microbiology, 10(4): 317-391.
DOI:10.3109/10408418209113567 |
Chen X Y, Dolinova I, Sevcu A, et al. 2020. Strategies adopted by Aphanizomenon flos-aquae in response to phosphorus deficiency and their role on growth. Environmental Sciences Europe, 32(1): 45.
DOI:10.1186/s12302-020-00328-3 |
Chróst R J, Siuda W, Halemejko G Z. 1984. Longterm studies on alkaline phosphatase activity (APA) in a lake with fish-aquaculture in relation to lake eutrophication and phosphorus cycle. Archiv für Hydrobiologie, 70(1): 1-32.
|
Chróst R J. 1991. Environmental control of the synthesis and activity of aquatic microbial ectoenzymes. In: Chróst R J ed. Microbial Enzymes in Aquatic Environments. Springer, New York. p. 29-59, https://doi.org/10.1007/978-1-4612-3090-8_3.
|
Chuai X M, Ding W, Chen X F, et al. 2011. Phosphorus release from cyanobacterial blooms in Meiliang Bay of Lake Taihu, China. Ecological Engineering, 37(6): 842-849.
DOI:10.1016/j.ecoleng.2011.01.001 |
Cotner J B, Wetzel R G. 1992. Uptake of dissolved inorganic and organic bphosphorus compounds by phytoplankton and bacterioplankton. Limnology and Oceanography, 37(2): 232-243.
DOI:10.4319/lo.1992.37.2.0232 |
Cox A D, Saito M A. 2013. Proteomic responses of oceanic Synechococcus WH8102 to phosphate and zinc scarcity and cadmium additions. Frontiers in Microbiology, 4: 387.
DOI:10.3389/fmicb.2013.00387 |
Dai J Y, Chen D, Wu S Q, et al. 2018. Dynamics of phosphorus and bacterial phoX genes during the decomposition of Microcystis blooms in a mesocosm. PLoS One, 13(5): e0195205.
DOI:10.1371/journal.pone.0195205 |
de Souza Santos K R, Jacinavicius F R, Sant'Anna C L. 2011. Effects of the pH on growth and morphology of Anabaenopsis elenkinii Miller (Cyanobacteria) isolated from the alkaline shallow lake of the Brazilian Pantanal. Fottea, 11(1): 119-126.
DOI:10.5507/fot.2011.012 |
Degerholm J, Gundersen K, Bergman B, et al. 2006. Phosphorus-limited growth dynamics in two Baltic Sea cyanobacteria, Nodularia sp. and Aphanizomenon sp. FEMS Microbiology Ecology, 58(3): 323-332.
DOI:10.1111/j.1574-6941.2006.00180.x |
Diaz-de-Quijano D, Palacios P, Horňák K, et al. 2014. 3D restoration microscopy improves quantification of enzyme-labeled fluorescence-based single-cell phosphatase activity in plankton. Cytometry Part A, 85(10): 841-853.
DOI:10.1002/cyto.a.22486 |
Diaz-de-Quijano D, Stratmann C N, Berger S A. 2020. DIY enzyme labelled fluorescence alcohol (ELFA) standard production protocol to quantify single-cell phosphatase activity (SCPA) of microplankton. Heliyon, 6(11): e05582.
DOI:10.1016/j.heliyon.2020.e05582 |
Dignum M, Hoogveld H L, Floris V, et al. 2004a. Flow cytometric detection of phosphatase activity combined with 13C-CO2 tracer-based growth rate assessment in phytoplankton populations from a shallow lake. Aquatic Microbial Ecology, 37(2): 159-169.
DOI:10.3354/ame037159 |
Dignum M, Hoogveld H L, Matthijs H C P, et al. 2004b. Detecting the phosphate status of phytoplankton by enzyme-labelled fluorescence and flow cytometry. FEMS Microbiology Ecology, 48(1): 29-38.
DOI:10.1016/j.femsec.2003.12.007 |
Dobronoki D, B-Béres V, Vasas G, et al. 2019. Potential role of the cellular matrix of Aphanizomenon strains in the effects of cylindrospermopsin-an experimental study. Journal of Applied Phycology, 31(3): 1805-1817.
DOI:10.1007/s10811-018-1699-4 |
Doonan B B, Jensen T E. 1980. Physiological aspects of alkaline phosphatase in selected cyanobacteria. Microbios, 29(117-118): 185-207.
|
Dyhrman S T, Palenik B. 1999. Phosphate stress in cultures and field populations of the dinoflagellate Prorocentrum minimum detected by a single-cell alkaline phosphatase assay. Applied and Environmental Microbiology, 65(7): 3205-3212.
DOI:10.1128/AEM.65.7.3205-3212.1999 |
Dyhrman S T, Ruttenberg K C. 2006. Presence and regulation of alkaline phosphatase activity in eukaryotic phytoplankton from the coastal ocean: implications for dissolved organic phosphorus remineralization. Limnology and Oceanography, 51(3): 1381-1390.
DOI:10.4319/lo.2006.51.3.1381 |
Dyhrman S. 2005. Ectoenzymes in Prorocentrum minimum. Harmful Algae, 4(3): 619-627.
DOI:10.1016/j.hal.2004.08.011 |
Fang F, Gao Y, Gan L, et al. 2018. Effects of different initial pH and irradiance levels on cyanobacterial colonies from Lake Taihu, China. Journal of Applied Phycology, 30(3): 1777-1793.
DOI:10.1007/s10811-018-1394-5 |
Feng W Y, Yang F, Zhang C, et al. 2020. Composition characterization and biotransformation of dissolved, particulate and algae organic phosphorus in eutrophic lakes. Environmental Pollution, 265: 114838.
DOI:10.1016/j.envpol.2020.114838 |
Fernández-Juárez V, Bennasar-Figueras A, Tovar-Sanchez A, et al. 2019. The role of iron in the p-acquisition mechanisms of the unicellular N2-fixing cyanobacteria Halothece sp., found in association with the Mediterranean Seagrass Posidonia oceanica. Frontiers in Microbiology, 10: 1903.
DOI:10.3389/fmicb.2019.01903 |
Feuillade J, Feuillade M, Blanc P. 1990. Alkaline-phosphatase activity fluctuations and associated factors in a eutrophic lake dominated by Oscillatoria-rubescens. Hydrobiologia, 207: 233-240.
DOI:10.1007/BF00041461 |
Fitzgerald G P, Nelson T C. 1975. Extractive and enzymatic analyses for limiting or surplus phosphorus in algae. Journal of Phycology, 11(S1): 32-37.
DOI:10.1111/j.1529-8817.1975.tb04539.x |
Fonseca-De-Souza A L, Dick C F, Dos Santos A L A, et al. 2008. A Mg2+-dependent ecto-phosphatase activity on the external surface of Trypanosoma rangeli modulated by exogenous inorganic phosphate. Acta Tropica, 107(2): 153-158.
DOI:10.1016/j.actatropica.2008.05.017 |
Francko D A, Wetzel R G. 1982. The isolation of cyclic adenosine 3'-5'-monophosphate (camp) from lakes of differing trophic status: correlation with planktonic metabolic variables. Limnology and Oceanography, 27(1): 27-38.
DOI:10.4319/lo.1982.27.1.0027 |
Francko D A. 1984. Relationships between phosphorus functional classes and alkaline phosphatase activity in reservoir lakes. Journal of Freshwater Ecology, 2(6): 541-547.
DOI:10.1080/02705060.1984.9664636 |
Gao G, Zhu G W, Qin B Q, et al. 2006. Alkaline phosphatase activity and the phosphorus mineralization rate of Lake Taihu. Science in China Series D, 49(1): 176-185.
DOI:10.1007/s11430-006-8117-5 |
Gerloff G C, Fitzgerald G P, Skoog F. 1950. The isolation, purification, and culture of blue-green algae. American Journal of Botany, 37(3): 216-218.
DOI:10.1002/j.1537-2197.1950.tb12184.x |
Giraudet H, Abrial D, Berthon J L, et al. 1999. Seasonal variation of the alkaline phosphatase activity of phytoplankton in the hypereutrophic Grangent reservoir (Loire). Annales De Limnologie-International Journal of Limnology, 35(4): 213-221.
DOI:10.1051/limn/1999030 |
Giraudet H, Berthon J L, Buisson B. 1997. A comparison of the daily alkaline phosphatase activity of a cyanobacterium (Microcystis aeruginosa) and a diatom (Synedra capitata). Comptes Rendus De L'académie Des Sciences-Series Ⅲ-Sciences De La Vie, 320(6): 451-458.
DOI:10.1016/S0764-4469(97)81972-5 |
Girault M, Arakawa H, Hashihama F. 2013. Phosphorus stress of microphytoplankton community in the western subtropical North Pacific. Journal of Plankton Research, 35(1): 146-157.
DOI:10.1093/plankt/fbs076 |
González-Gil S, Keafer B A, Jovine R V M, et al. 1998. Detection and quantification of alkaline phosphatase in single cells of phosphorus-starved marine phytoplankton. Marine Ecology Progress Series, 164: 21-35.
DOI:10.3354/meps164021 |
Hadas O, Pinkas R, Delphine E, et al. 1999. Limnological and ecophysiological aspects of Aphanizomenon ovalisporum bloom in Lake Kinneret, Israel. Journal of Plankton Research, 21(8): 1439-1453.
DOI:10.1093/plankt/21.8.1439 |
Harke M J, Berry D L, Ammerman J W, et al. 2012. Molecular response of the bloom-forming cyanobacterium, Microcystis aeruginosa, to phosphorus limitation. Microbial Ecology, 63(1): 188-198.
DOI:10.1007/s00248-011-9894-8 |
Harke M J, Davis T W, Watson S B, et al. 2016. Nutrient-controlled niche differentiation of western lake Erie Cyanobacterial populations revealed via metatranscriptomic surveys. Environmental Science & Technology, 50(2): 604-615.
DOI:10.1021/acs.est.5b03931 |
Healey F P, Hendzel L L. 1979a. Indicators of phosphorus and nitrogen deficiency in five algae in culture. Journal of the Fisheries Research Board of Canada, 36(11): 1364-1369.
DOI:10.1139/f79-195 |
Healey F P, Hendzel L L. 1979b. Fluorometric measurement of alkaline phosphatase activity in algae. Freshwater Biology, 9(5): 429-439.
DOI:10.1111/j.1365-2427.1979.tb01527.x |
Healey F P, Hendzel L L. 1980. Physiological indicators of nutrient deficiency in lake phytoplankton. Canadian Journal of Fisheries and Aquatic Sciences, 37(3): 442-453.
DOI:10.1139/f80-058 |
Healey F P. 1978. Physiological indicators of nutrient deficiency in algae. SIL Communications, 1953-1996, 21(1): 34-41.
DOI:10.1080/05384680.1978.11903948 |
Hernández I, Hwang S J, Heath R T. 1996. Measurement of phosphomonoesterase activity with a radiolabelled glucose-6-phosphate. Role in the phosphorus requirement of phytoplankton and bacterioplankton in a temperate mesotrophic lake. Archiv für Hydrobiologie, 137(2): 265-280.
DOI:10.1127/archiv-hydrobiol/137/1996/265 |
Hernández I, Whitton B A. 1996. Retention of p-nitrophenol and 4-methylumbelliferone by marine macroalgae and implications for measurement of alkaline phosphatase activity. Journal of Phycology, 32(5): 819-825.
DOI:10.1111/j.0022-3646.1996.00819.x |
Hong S J, Lee C G. 2008. Statistical optimization of culture media for production of phycobiliprotein by Synechocystis sp. PCC 6701. Biotechnology and Bioprocess Engineering, 13(4): 491-498.
DOI:10.1007/s12257-008-0154-9 |
Hong S J, Pan Q H, Chen S Y, et al. 2021. Identification and characterization of alkaline phosphatase gene phoX in Microcystis aeruginosa PCC7806. 3 Biotech, 11(5): 218.
DOI:10.1007/s13205-021-02774-z |
Hoppe H G. 2003. Phosphatase activity in the sea. Hydrobiologia, 493(1-3): 187-200.
DOI:10.1023/A:1025453918247 |
Hsieh Y J, Wanner B L. 2010. Global regulation by the seven-component Pi signaling system. Current Opinion in Microbiology, 13(2): 198-203.
DOI:10.1016/j.mib.2010.01.014 |
Hynes A M, Chappell P D, Dyhrman S T, et al. 2009. Cross-basin comparison of phosphorus stress and nitrogen fixation in Trichodesmium. Limnology and Oceanography, 54(5): 1438-1448.
DOI:10.4319/lo.2009.54.5.1438 |
Ihlenfeldt M J A, Gibson J. 1975. Phosphate utilization and alkaline phosphatase activity in Anacystis nidulans (Synechococcus). Archives of Microbiology, 102(1): 23-28.
DOI:10.1007/BF00428340 |
Istánovics V, Pettersson K, Pierson D, et al. 1992. Evaluation of phosphorus deficiency indicators for summer phytoplankton in Lake Erken. Limnology and Oceanography, 37(4): 890-900.
DOI:10.4319/lo.1992.37.4.0890 |
Ivančić I, Kraus R, Najdek M, et al. 2021. Ecological importance of alkaline phosphatase activity in changing marine environmental conditions. Water, 13(19): 2750.
DOI:10.3390/w13192750 |
Jamet D, Amblard C, Devaux J. 2001. Size-fractionated alkaline phosphatase activity in the hypereutrophic Villerest Reservoir (Roanne, France). Water Environment Research, 73(2): 132-141.
DOI:10.2175/106143001X138787 |
Jansson M, Olsson H, Pettersson K. 1988. Phosphatases: origin, characteristics and function in lakes. Hydrobiologia, 170: 157-175.
DOI:10.1007/BF00024903 |
Jansson M. 1976. Phosphatases in lake water: characterization of enzymes from phytoplankton and zooplankton by gel filtration. Science, 194(4262): 320-321.
DOI:10.1126/science.184531 |
Jansson M. 1981. Induction of high phosphatase activity by aluminum in acid lakes. Archiv für Hydrobiologie, 93(1): 32-44.
|
Juntarajumnong W, Hirani T A, Simpson J M, et al. 2007. Phosphate sensing in Synechocystis sp. PCC 6803: SphU and the SphS-SphR two-component regulatory system. Archives of Microbiology, 188(4): 389-402.
DOI:10.1007/s00203-007-0259-0 |
Kageyama H, Tripathi K, Rai A K, et al. 2011. An alkaline phosphatase/phosphodiesterase, PhoD, induced by salt stress and secreted out of the cells of Aphanothece halophytica, a halotolerant cyanobacterium. Applied and Environmental Microbiology, 77(15): 5178-5183.
DOI:10.1128/AEM.00667-11 |
Kathuria S, Martiny A C. 2011. Prevalence of a calcium-based alkaline phosphatase associated with the marine cyanobacterium Prochlorococcus and other ocean bacteria. Environmental Microbiology, 13(1): 74-83.
DOI:10.1111/j.1462-2920.2010.02310.x |
Kelly L T, Ryan K G, Wood S A. 2019. Differential strain response in alkaline phosphatase activity to available phosphorus in Microcoleus autumnalis. Harmful Algae, 89: 101664.
DOI:10.1016/j.hal.2019.101664 |
Koch M S, Kletou D C, Tursi R. 2009. Alkaline phosphatase activity of water column fractions and seagrass in a tropical carbonate estuary, Florida Bay. Estuarine, Coastal and Shelf Science, 83(4): 403-413.
DOI:10.1016/j.ecss.2009.04.007 |
Kolowith L C, Ingall E D, Benner R. 2001. Composition and cycling of marine organic phosphorus. Limnology and Oceanography, 46(2): 309-320.
DOI:10.4319/lo.2001.46.2.0309 |
Kuenzler E J, Perras J P. 1965. Phosphatases of marine algae. The Biological Bulletin, 128(2): 271-284.
DOI:10.2307/1539555 |
Kumar D, Singh J B, Kumar H D. 1992. Effects of low temperature on two cyanobacteria. The Journal of General and Applied Microbiology, 38(3): 263-270.
DOI:10.2323/jgam.38.263 |
Lehman J T, Doubek J P, Jackson E W. 2013. Effect of reducing allochthonous P load on biomass and alkaline phosphatase activity of phytoplankton in an urbanized watershed, Michigan. Lake and Reservoir Management, 29(2): 116-125.
DOI:10.1080/10402381.2013.800173 |
Li J H, Wang Z W, Cao X, et al. 2015a. Effect of orthophosphate and bioavailability of dissolved organic phosphorous compounds to typically harmful cyanobacterium Microcystis aeruginosa. Marine Pollution Bulletin, 92(1-2): 52-58.
DOI:10.1016/j.marpolbul.2015.01.001 |
Li T L, Zheng L L, Zhang Q, et al. 2015b. Progress of aseptic purification technique of cyanobacteria. Current Biotechnology, 5(5): 329-334.
(in Chinese with English abstract) |
Lim J H, Lee C W, Bong C W, et al. 2018. Distributions of particulate and dissolved phosphorus in aquatic habitats of Peninsular Malaysia. Marine Pollution Bulletin, 128: 415-427.
DOI:10.1016/j.marpolbul.2018.01.037 |
Lin S J, Litaker R W, Sunda W G. 2016. Phosphorus physiological ecology and molecular mechanisms in marine phytoplankton. Journal of Phycology, 52(1): 10-36.
DOI:10.1111/jpy.12365 |
Lin W T, Zhao D D, Luo J F. 2018. Distribution of alkaline phosphatase genes in cyanobacteria and the role of alkaline phosphatase on the acquisition of phosphorus from dissolved organic phosphorus for cyanobacterial growth. Journal of Applied Phycology, 30(2): 839-850.
DOI:10.1007/s10811-017-1267-3 |
Liu Z Y, Wu C D. 2012. Response of alkaline phosphatases in the cyanobacterium Anabaena sp. FACHB 709 to inorganic phosphate starvation. Current Microbiology, 64(6): 524-529.
DOI:10.1007/s00284-012-0101-z |
Livingstone D, Whitton B A. 1984. Water chemistry and phosphatase activity of the blue-green alga Rivularia in upper Teesdale Streams. The Journal of Ecology, 72(2): 405-421.
DOI:10.2307/2260055 |
Lu Z, Lei L M, Lu Y, et al. 2021. Phosphorus deficiency stimulates dominance of Cylindrospermopsis through facilitating cylindrospermopsin-induced alkaline phosphatase secretion: integrating field and laboratory-based evidences. Environmental Pollution, 290: 117946.
DOI:10.1016/j.envpol.2021.117946 |
Luo H W, Benner R, Long R A, et al. 2009. Subcellular localization of marine bacterial alkaline phosphatases. Proceedings of the National Academy of Sciences of the United States of America, 106(50): 21219-21223.
DOI:10.1073/pnas.0907586106 |
Luo M, Guo Y C, Deng J Y, et al. 2010. Characterization of a monomeric heat-labile classical alkaline phosphatase from Anabaena sp. PCC7120. Biochemistry (Moscow), 75(5): 655-664.
DOI:10.1134/S0006297910050172 |
Majumdar A, Ghatak A, Ghosh R K. 2005. Identification of the gene for the monomeric alkaline phosphatase of Vibrio cholerae serogroup O1 strain. Gene, 344: 251-258.
DOI:10.1016/j.gene.2004.11.005 |
Malherbe G, Humphreys D P, Dave E. 2019. A robust fractionation method for protein subcellular localization studies in Escherichia coli. Biotechniques, 66(4): 171-178.
DOI:10.2144/btn-2018-0135 |
Martland M, Robison R. 1929. The preparation and use of the bone phosphatase. Biochemical Journal, 23(2): 237-242.
DOI:10.1042/bj0230237 |
McComb R B, Bowers Jr G N, Posen S. 1979. Purification. In: McComb R B, Bowers G N, Posen S eds. Alkaline Phosphatase. Springer, Boston. p. 153-188, https://doi.org/10.1007/978-1-4613-2970-1_4.
|
McLaughlin K, Sohm J A, Cutter G A, et al. 2013. Phosphorus cycling in the Sargasso Sea: investigation using the oxygen isotopic composition of phosphate, enzyme-labeled fluorescence, and turnover times. Global Biogeochemical Cycles, 27(2): 375-387.
DOI:10.1002/gbc.20037 |
Meseck S L, Alix J H, Wikfors G H, et al. 2009. Differences in the soluble, residual phosphate concentrations at which coastal phytoplankton species up-regulate alkaline-phosphatase expression, as measured by flow-cytometric detection of ELF-97Ⓡ fluorescence. Estuaries and Coasts, 32(6): 1195-1204.
DOI:10.1007/s12237-009-9211-7 |
Modi N, Bárcena-Uribarri I, Bains M, et al. 2013. Role of the central arginine R133 toward the ion selectivity of the phosphate specific channel OprP: effects of charge and solvation. Biochemistry, 52(33): 5522-5532.
DOI:10.1021/bi400522b |
Moegenburg S M, Vanni M J. 1991. Nutrient regeneration by zooplankton: effects on nutrient limitation of phytoplankton in a eutrophic lake. Journal of Plankton Research, 13(3): 573-588.
DOI:10.1093/plankt/13.3.573 |
Moisander P H, Daley M C, Shoemaker K M, et al. 2022. Nitrogen fixation influenced by phosphorus and nitrogen availability in the benthic bloom-forming cyanobacterium Hydrocoleum sp. identified in a temperate Marine Lagoon. Journal of Phycology, 58(3): 377-391.
DOI:10.1111/jpy.13244 |
Monds R D, Newell P D, Schwartzman J A, et al. 2006. Conservation of the Pho regulon in Pseudomonas fluorescens Pf0-1. Applied and Environmental Microbiology, 72(3): 1910-1924.
DOI:10.1128/AEM.72.3.1910-1924.2006 |
Moore L R, Ostrowski M, Scanlan D J, et al. 2005. Ecotypic variation in phosphorus-acquisition mechanisms within marine picocyanobacteria. Aquatic Microbial Ecology, 39(3): 257-269.
DOI:10.3354/ame039257 |
Nausch M. 1998. Alkaline phosphatase activities and the relationship to inorganic phosphate in the Pomeranian Bight (southern Baltic Sea). Aquatic Microbial Ecology, 16(1): 87-94.
DOI:10.3354/ame016087 |
Nedoma J, Van Wambeke F, Štrojsová, et al. 2007. Affinity of extracellular phosphatases for ELF97 phosphate in aquatic environments. Marine and Freshwater Research, 58(5): 454-460.
DOI:10.1071/MF06211 |
Newman S, McCormick P V, Backus J G. 2003. Phosphatase activity as an early warning indicator of wetland eutrophication: problems and prospects. Journal of Applied Phycology, 15(1): 45-59.
DOI:10.1023/A:1022971204435 |
Olsen Y, Vadstein O, Andersen T, et al. 1989. Competition between Staurastrum Luetkemuellerii (Chlorophyceae) and Microcystis aeruginosa (Cyanophyceae) under varying modes of phosphate supply. Journal of Phycology, 25(3): 499-508.
DOI:10.1111/j.1529-8817.1989.tb00255.x |
Olsson H. 1983. Origin and production of phosphatases in the acid Lake Gårdsjön. Hydrobiologia, 101(1-2): 49-58.
DOI:10.1007/BF00008656 |
Ou L J, Huang B Q, Lin L Z, et al. 2006. Phosphorus stress of phytoplankton in the Taiwan Strait determined by bulk and single-cell alkaline phosphatase activity assays. Marine Ecology Progress Series, 327: 95-106.
DOI:10.3354/meps327095 |
Perry M J. 1972. Alkaline phosphatase activity in subtropical central North Pacific waters using a sensitive fluorometric method. Marine Biology, 15(2): 113-119.
DOI:10.1007/BF00353639 |
Pettersson K. 1985. The availability of phosphorus and the species composition of the spring phytoplankton in Lake Erken. Internationale Revue Der Gesamten Hydrobiologie und Hydrographie, 70(4): 527-546.
DOI:10.1002/iroh.19850700407 |
Pick F R. 1987. Interpretations of alkaline phosphatase activity in Lake Ontario. Canadian Journal of Fisheries and Aquatic Sciences, 44(12): 2087-2094.
DOI:10.1139/f87-258 |
Prentice M J, Hamilton D P, Willis A, et al. 2019. Quantifying the role of organic phosphorus mineralisation on phytoplankton communities in a warm-monomictic lake. Inland Waters, 9(1): 10-24.
DOI:10.1080/20442041.2018.1538717 |
Rabouille S, Tournier L, Duhamel S, et al. 2022. Organic phosphorus scavenging supports efficient growth of diazotrophic cyanobacteria under phosphate depletion. Frontiers in Microbiology, 13: 848647.
DOI:10.3389/fmicb.2022.848647 |
Rao N N, Torriani A. 1990. Molecular aspects of phosphate-transport in Escherichiacoli. MolecularMicrobiology, 4(7): 1083-1090.
DOI:10.1111/j.1365-2958.1990.tb00682.x |
Raoui S M, Rachiq S, Chadli N, et al. 2002. Seasonal variations of the alkaline phosphatase activity from the bacterial and algal planktonic communities in the mesotrophic reservoir lake Allal El Fassi, Morocco. Annales de Limnologie-International Journal of Limnology, 38(4): 287-295.
DOI:10.1051/limn/2002023 |
Ray J M, Bhaya D, Block M A, et al. 1991. Isolation, transcription, and inactivation of the gene for an atypical alkaline phosphatase of Synechococcus sp. strain PCC 7942. Journal of Bacteriology, 173(14): 4297-4309.
DOI:10.1128/jb.173.14.4297-4309.1991 |
Rejmánková E, Komárková J. 2000. A function of cyanobacterial mats in phosphorus-limited tropical wetlands. Hydrobiologia, 431(2-3): 135-153.
DOI:10.1023/A:1004011318643 |
Rejmánková E, Komárková J. 2005. Response of cyanobacterial mats to nutrient and salinity changes. Aquatic Botany, 83(2): 87-107.
DOI:10.1016/j.aquabot.2005.05.011 |
Rengefors K, Pettersson K, Blenckner T, et al. 2001. Species-specific alkaline phosphatase activity in freshwater spring phytoplankton: application of a novel method. Journal of Plankton Research, 23(4): 435-443.
DOI:10.1093/plankt/23.4.435 |
Rengefors K, Ruttenberg K C, Haupert C L, et al. 2003. Experimental investigation of taxon-specific response of alkaline phosphatase activity in natural freshwater phytoplankton. Limnology and Oceanography, 48(3): 1167-1175.
DOI:10.4319/lo.2003.48.3.1167 |
Rose C, Axler R P. 1997. Uses of alkaline phosphatase activity in evaluating phytoplankton community phosphorus deficiency. Hydrobiologia, 361(1-3): 145-156.
DOI:10.1023/A:1003178502883 |
Ruiz R G, Hernández I, Lucena J, et al. 1997. Preliminary studies on the significance of alkaline phosphatase activity in the diatom Phaeodactylum tricornutum Bohlin. Scientia Marina, 61(4): 517-525.
|
Rychtecký P, Řeháková K, Kozlíková E, et al. 2015. Light availability may control extracellular phosphatase production in turbid environments. Microbial Ecology, 69(1): 37-44.
DOI:10.1007/s00248-014-0483-5 |
Schindler D W. 1977. Evolution of phosphorus limitation in lakes. Science, 195(4275): 260-262.
DOI:10.1126/science.195.4275.260 |
Sebastian M, Ammerman J W. 2009. The alkaline phosphatase PhoX is more widely distributed in marine bacteria than the classical PhoA. The ISME Journal, 3(5): 563-572.
DOI:10.1038/ismej.2009.10 |
Sebastián M, Arístegui J, Montero M F, et al. 2004. Alkaline phosphatase activity and its relationship to inorganic phosphorus in the transition zone of the North-western African upwelling system. Progress in Oceanography, 62(2-4): 131-150.
DOI:10.1016/j.pocean.2004.07.007 |
Sharma U, Pal D, Prasad R. 2014. Alkaline phosphatase: an overview. Indian Journal of Clinical Biochemistry, 29(3): 269-278.
DOI:10.1007/s12291-013-0408-y |
Singh P K, Shrivastava A K, Chatterjee A, et al. 2015. Cadmium toxicity in diazotrophic Anabaena spp. adjudged by hasty up-accumulation of transporter and signaling and severe down-accumulation of nitrogen metabolism proteins. Journal of Proteomics, 127: 134-146.
DOI:10.1016/j.jprot.2015.05.019 |
Singh S K, Singh S S, Pandey V D, et al. 2006. Factors modulating alkaline phosphatase activity in the diazotrophic rice-field cyanobacterium, Anabaena oryzae. World Journal of Microbiology and Biotechnology, 22(9): 927-935.
DOI:10.1007/s11274-006-9137-1 |
Sirová D, Rejmánková E, Carlson E, et al. 2013. Current standard assays using artificial substrates overestimate phosphodiesterase activity. Soil Biology and Biochemistry, 56: 75-79.
DOI:10.1016/j.soilbio.2012.02.008 |
Sirová D, Vrba J, Rejmánková E. 2006. Extracellular enzyme activities in benthic cyanobacterial mats: comparison between nutrient-enriched and control sites in marshes of northern Belize. Aquatic Microbial Ecology, 44(1): 11-20.
DOI:10.3354/ame044011 |
Siuda W. 1984. Phosphatases and their role in organic phosphorus transformation in natural waters: a review. Polskie Archiwum Hydrobiologii, 31(3): 207-233.
|
Smith R E H, Kalff J. 1981. The Effect of phosphorus limitation on algal growth rates: evidence from alkaline phosphatase. Canadian Journal of Fisheries and Aquatic Sciences, 38(11): 1421-1427.
DOI:10.1139/f81-188 |
Srivastava A, Saavedra D E M, Thomson B, et al. 2021. Enzyme promiscuity in natural environments: alkaline phosphatase in the ocean. The ISME Journal, 15(11): 3375-3383.
DOI:10.1038/s41396-021-01013-w |
Steffen M M, Dearth S P, Dill B D, et al. 2014. Erratum: nutrients drive transcriptional changes that maintain metabolic homeostasis but alter genome architecture in Microcystis. The ISEM Journal, 8(10): 2152.
DOI:10.1038/ismej.2014.133 |
Steiner M. 1938. Zur kenntnis des phosphatkreislaufes in seen. Naturwissenschaften, 26(44): 723-724.
DOI:10.1007/BF01772992 |
Stevens R J, Parr M P. 1977. The significance of alkaline phosphatase activity in Lough Neagh. Freshwater Biology, 7(4): 351-355.
DOI:10.1111/j.1365-2427.1977.tb01683.x |
Stihl A, Sommer U, Post A F. 2001. Alkaline phosphatase activities among populations of the colony-forming diazotrophic cyanobacterium Trichodesmium spp. (cyanobacteria) in the Red Sea. Journal of Phycology, 37(2): 310-317.
DOI:10.1046/j.1529-8817.2001.037002310.x |
Stoecker D, Autio R, Rintala J M, et al. 2005. Ecto-cellular enzyme activity associated with filamentous cyanobacteria. Aquatic Microbial Ecology, 40(2): 151-161.
DOI:10.3354/ame040151 |
Štrojsová, Vrba J, Nedoma J, et al. 2005. Extracellular phosphatase activity of freshwater phytoplankton exposed to different in situ phosphorus concentrations. Marine and Freshwater Research, 56(4): 417-424.
DOI:10.1071/MF04283 |
Štrojsová, Vrba J, Nedoma N, et al. 2003. Seasonal study of extracellular phosphatase expression in the phytoplankton of a eutrophic reservoir. European Journal of Phycology, 38(4): 295-306.
DOI:10.1080/09670260310001612628 |
Su Z C, Dam P, Chen X, et al. 2003. Computational inference of regulatory pathways in microbes: an application to phosphorus assimilation pathways in Synechococcus sp. WH8102. Genome Informatics, 14: 3-13.
DOI:10.11234/gi1990.14.3 |
Su Z C, Olman V, Xu Y. 2007. Computational prediction of Pho regulons in cyanobacteria. BMC Genomics, 8: 156.
DOI:10.1186/1471-2164-8-156 |
Subramanian G, Sekar S, Sampoornam S. 1994. Biodegradation and utilization of organophosphorus pesticides by cyanobacteria. International Biodeterioration & Biodegradation, 33(2): 129-143.
DOI:10.1016/0964-8305(94)90032-9 |
Tetu S G, Brahamsha B, Johnson D A, et al. 2009. Microarray analysis of phosphate regulation in the marine cyanobacterium Synechococcus sp. WH8102. The ISME Journal, 3(7): 835-849.
DOI:10.1038/ismej.2009.31 |
Thingstad T F, Zweifel U L, Rassoulzadegan F. 1998. P limitation of heterotrophic bacteria and phytoplankton in the northwest Mediterranean. Limnology and Oceanography, 43(1): 88-94.
DOI:10.4319/lo.1998.43.1.0088 |
Tiwari B, Singh S, Kaushik M S, et al. 2015. Regulation of organophosphate metabolism in cyanobacteria. A review. Microbiology, 84(3): 291-302.
DOI:10.1134/S0026261715030200 |
Vaitomaa J, Repka S, Saari L, et al. 2002. Aminopeptidase and phosphatase activities in basins of Lake Hiidenvesi dominated by cyanobacteria and in laboratory grown Anabaena. Freshwater Biology, 47(9): 1582-1593.
DOI:10.1046/j.1365-2427.2002.00901.x |
Vershinina O A, Znamenskaya L V. 2002. The Pho regulons of bacteria. Microbiology, 71(5): 497-511.
DOI:10.1023/A:1020547616096 |
Vrba J, Komárkova J, Vyhnálek V. 1993. Enhanced activity of alkaline phosphatases - Phytoplankton response to epilimnetic phosphorus depletion. Water Science and Technology, 28(6): 15-24.
DOI:10.2166/wst.1993.0124 |
Wagner K U, Masepohl B, Pistorius E K. 1995. The cyanobacterium Synechococcus sp. strain PCC 7942 contains a second alkaline phosphatase encoded by phoV. Microbiology, 141(12): 3049-3058.
DOI:10.1099/13500872-141-12-3049 |
Wan L L, Chen X Y, Deng Q H, et al. 2019. Phosphorus strategy in bloom-forming cyanobacteria (Dolichospermum and Microcystis) and its role in their succession. Harmful Algae, 84: 46-55.
DOI:10.1016/j.hal.2019.02.007 |
Wang C Y, Yang Y Y, Yang B, et al. 2021. Causal relationship between alkaline phosphatase activities and phosphorus dynamics in a eutrophic coastal lagoon in Lake Michigan. Science of the Total Environment, 787: 147681.
DOI:10.1016/j.scitotenv.2021.147681 |
Wei S J, Zhuang G J, Cheng L R J, et al. 2022. The proliferation rule of Microcystis aeruginosa under different initial pH conditions and its influence on the pH value of the environment. Environmental Science and Pollution Research, 29(10): 13835-13844.
DOI:10.1007/s11356-021-16719-9 |
White S H, Fabbro L D, Duivenvoorden L J. 2003. Changes in cyanoprokaryote populations, Microcystis morphology, and microcystin concentrations in Lake Elphinstone (Central Queensland, Australia). Environmental Toxicology, 18(6): 403-412.
DOI:10.1002/tox.10142 |
Whitton B A, Grainger S L J, Hawley G R W, et al. 1991. Cell-bound and extracellular phosphatase activities of cyanobacterial isolates. Microbial Ecology, 21: 85-98.
DOI:10.1007/BF02539146 |
Whitton B A, Potts M, Simon J W, et al. 1990. Phosphatase activity of the blue-green alga (cyanobacterium) Nostoc commune UTEX 584. Phycologia, 29(2): 139-145.
DOI:10.2216/i0031-8884-29-2-139.1 |
Wilkins A S. 1972. Physiological factors in the regulation of alkaline phosphatase synthesis in Escherichia coli. Journal of Bacteriology, 110(2): 616-623.
DOI:10.1128/jb.110.2.616-623.1972 |
Willis A, Chuang A W, Dyhrman S, et al. 2019. Differential expression of phosphorus acquisition genes in response to phosphorus stress in two Raphidiopsis raciborskii strains. Harmful Algae, 82: 19-25.
DOI:10.1016/j.hal.2018.12.003 |
Yadav S, Prajapati R, Atri N. 2016. Effects of UV-B and heavy metals on nitrogen and phosphorus metabolism in three cyanobacteria. Journal of Basic Microbiology, 56(1): 2-13.
DOI:10.1002/jobm.201500504 |
Yamaguchi H, Yamaguchi M, Fukami K, et al. 2005. Utilization of phosphate diester by the marine diatom Chaetoceros ceratosporus. Journal of Plankton Research, 27(6): 603-606.
DOI:10.1093/plankt/fbi027 |
Yates C A, Johnes P J, Owen A T, et al. 2019. Variation in dissolved organic matter (DOM) stoichiometry in U.K. freshwaters: assessing the influence of land cover and soil C: N ratio on DOM composition. Limnology and Oceanography, 64(6): 2328-2340.
DOI:10.1002/lno.11186 |
Yelloly J M, Whitton B A. 1996. Seasonal changes in ambient phosphate and phosphatase activities of the cyanobacterium Rivularia atra in intertidal pools at Tyne Sands, Scotland. Hydrobiologia, 325(3): 201-212.
DOI:10.1007/BF00014985 |
Yong S C, Roversi P, Lillington J, et al. 2014. A complex iron-calcium cofactor catalyzing phosphotransfer chemistry. Science, 345(6201): 1170-1173.
DOI:10.1126/science.1254237 |
Young C L, Ingall E D. 2010. Marine dissolved organic phosphorus composition: insights from samples recovered using combined electrodialysis/reverse osmosis. Aquatic Geochemistry, 16(4): 563-574.
DOI:10.1007/s10498-009-9087-y |
Yuan Y J, Bi Y H, Hu Z Y. 2016. Dynamics of alkaline phosphatase activity during the period of cyanobacterial bloom in a tributary of the Three Gorges Reservoir. Fresenius Environmental Bulletin, 25(11): 4900-4907.
|
Zaheer R, Morton R, Proudfoot M, et al. 2009. Genetic and biochemical properties of an alkaline phosphatase PhoX family protein found in many bacteria. Environmental Microbiology, 11(6): 1572-1587.
DOI:10.1111/j.1462-2920.2009.01885.x |
Zhang T X, Lu X R, Yu R D, et al. 2020a. Response of extracellular and intracellular alkaline phosphatase in Microcystis aeruginosa to organic phosphorus. Environmental Science and Pollution Research, 27(34): 42304-42312.
DOI:10.1007/s11356-020-09736-7 |
Zhang X H, Lin S J, Liu D Y. 2020b. Transcriptomic and physiological responses of Skeletonema costatum to ATP utilization. Environmental Microbiology, 22(5): 1861-1869.
DOI:10.1111/1462-2920.14944 |
Zhang X L, Deng J J, Xue Y M, et al. 2016. Stimulus response of Au-NPs@GMP-Tb core-shell nanoparticles: toward colorimetric and fluorescent dual-mode sensing of alkaline phosphatase activity in algal blooms of a freshwater lake. Environmental Science & Technology, 50(2): 847-855.
DOI:10.1021/acs.est.5b04600 |
Zhang X Y, Wang Z H, Luo Z X, et al. 2022. Insights into the conversion of dissolved organic phosphorus favors algal bloom, arsenate biotransformation and microcystins release of Microcystis aeruginosa. Journal of Environmental Sciences, 125: 205-214.
DOI:10.1016/j.jes.2021.11.025 |
Zhang X, Zhang J P, Yuan H M, et al. 2021. Seasonal dynamics of phytoplankton phosphorus stress in temperate Jiaozhou Bay, North China. Continental Shelf Research, 231: 104602.
DOI:10.1016/j.csr.2021.104602 |
Zhao G Y, Du J J, Jia Y, et al. 2012. The importance of bacteria in promoting algal growth in eutrophic lakes with limited available phosphorus. Ecological Engineering, 42: 107-111.
DOI:10.1016/j.ecoleng.2012.02.007 |
Zhou J, Han X X, Qin B Q, et al. 2021. Responses of alkaline phosphatase activity to wind-driven waves in a large, shallow lake: implications for phosphorus availability and algal blooms. Journal of Environmental Sciences, 99: 143-150.
DOI:10.1016/j.jes.2020.06.022 |
Zhou Y Y, Zhou X Y. 1997. Seasonal variation in kinetic parameters of alkaline phosphatase activity in a shallow Chinese freshwater lake (Donghu Lake). Water Research, 31(5): 1232-1235.
DOI:10.1016/S0043-1354(96)00366-1 |
 2022, Vol. 40
2022, Vol. 40