Institute of Oceanology, Chinese Academy of Sciences
Article Information
- WEN Qianzhi, XIAO Peng, LI Hua, LI Wenke, YU Gongliang, LI Renhui
- Succession of Aphanizomenon flos-aquae and Microcystis aeruginosa in direct co-culture experiments at different temperatures and biomasses
- Journal of Oceanology and Limnology, 40(5): 1819-1828
- http://dx.doi.org/10.1007/s00343-022-2041-1
Article History
- Received Jan. 27, 2022
- accepted in principle Mar. 8, 2022
- accepted for publication Jun. 6, 2022
2 University of Chinese Academy of Sciences, Beijing 100049, China;
3 College of Life and Environmental Sciences, Wenzhou University, Wenzhou 325035, China
Cyanobacteria, a category of earth's most primitive oxygenic photoautotrophs, are able to adapt to complicated and varied environments on the earth (Paerl and Otten, 2013). Aggravated water eutrophication due to modern anthropogenic activities promoted the proliferation of cyanobacteria, increased the frequency of cyanobacterial bloom outbreak in many freshwater ecosystems (Gehringer and Wannicke, 2014), and damage local aquatic ecosystems, which poses great threats to water quality and challenge to the management (Glibert et al., 2005; Best, 2019; Mushtaq et al., 2020). Microcystis, Dolichospermum, Planktothrix, Raphidiopsis, and Aphanizomenon are regarded as the most common bloom-forming cyanobacteria genera (Paerl and Otten, 2016). Aphanizomenon belongs to the order Nostocales, and contains several members causing harmful blooms (Cires and Ballot, 2016; Codd et al., 1999). It was reported that Aphanizomenon species could produce several types of toxins, including paralytic shellfish poisoning (PSP) toxins, cylindrospermopsins (CYNs), microcystins (MCs), and anatoxins (ATXs) (Rapala et al., 1993; Sabour et al., 2005; Pearson et al., 2010; Rzymski et al., 2011; Cirés and Ballot, 2016). Furthermore, it has been reported that these species can produce odor compounds (Suurnäkki et al., 2015).
The seasonal succession of dominant species is the typical characteristic of phytoplankton assemblages in freshwater ecosystems (Tsukada et al., 2006; Moustaka-Gouni et al., 2007; Messineo et al., 2010). Eukaryotic algae are usually dominant species of phytoplankton in spring, and shortly after is replaced by cyanobacteria in summer (Wang et al., 2021). In addition, cyanobacterial communities undergo successions among different genera (Wu et al., 2016; Shan et al., 2019). Succession of dominant species is controlled by multiple biotic and abiotic factors, including temperature, nutritional levels, light intensity, hydrological conditions, allelopathy, and grazing pressure (Bormans et al., 2005; Smayda, 2008; Paerl and Paul, 2012; Tan et al., 2019)
Several field studies have reported that the seasonal succession between A. flos-aquae and Microcystis species is a common phenomenon in lakes, such as Dianchi Lake, China (Liu et al., 2006b; Wu et al., 2016) and Ford Lake in the southeastern Michigan (McDonald and Lehman, 2013), showed that Microcystis could become a dominant over A. flos-aquae in a warming water. Microcystis has developed several traits to form its ecologically competitive advantages, such as colony formation (Yang et al., 2006), ability to regulate buoyancy (Brookes and Ganf, 2001), secondary metabolites production (Ma et al., 2015), luxury phosphorus uptake (Shen and Song, 2007), high affinity for dissolved inorganic nitrogen (Takamura et al., 1987), and fast growth at warmer temperatures (Carey et al., 2012; Paerl and Otten, 2013). However, A. flos-aquae also posses some of the above-stated features, such as presence of gas vesicles and the formation of fascicles as aggregates in waters (Liu et al., 2006a; Yang et al., 2006). These shared traits between A. flos-aquae and Microcystis indicate that their niches overlap to some extents. A. flos-aquae as a common type of filamentous heterocystous cyanobacteria, owns some unique traits such as nitrogen fixation ability and cellular differentiation into heterocysts and akinetes, which is distinct from unicellular Microcystis. Therefore, these unique traits that differentiate their niches and reflect relative fitness may provide the potential for the dominance of A. flos-aquae or coexistence between A. flos-aquae and Microcystis.
There have been many reports about blooms formed by A. flos-aquae in winter (Jones, 1979; Baker, 1981; Üveges et al., 2012). Wu et al. (2010) described that A. flos-aquae bloomed in the winter and early-spring in Dianchi Lake, showing its tolerance to lower temperature; they also studied the effect of environmental factors on the seasonal succession of Microcystis and A. flos-aquae in the lake, and suggested that temperature is the most influential factor on the initiation of rapid growth and succession between A. flos-aquae and Microcystis. Ma et al. (2015) demonstrated that Microcystis strains are able to suppress the growth of A. flos-aquae as shown in A. flos-aquae co-culture with Microcystis filtrate, and this allelopathic effects may partially explain the driving factor for the seasonal succession from A. flos-aquae to Microcystis species. Apparently, studies in field and laboratory experiments have revealed the mechanism for the succession between Microcystis and A. flos-aquae to some extents (Wu et al., 2010, 2016; Ma et al., 2015), but did not set an experimental system on nor explore the direct co-culture of them. Since the indoor co-culture can simulate more precisely the mechanism of the competition between the two species, and help understanding the details of bloom succession for better control of eutrophic water bodies and cyanobacteria blooms. Furthermore, the influence of biomass shall be considered in study of the succession of different cyanobacterial species since the biomass is not equal in the natural waters.
In this study, to address the dominant environmental factor on the succession and dominance priority between A. flos-aquae and M. aeruginosa, we performed an A. fl os-aquae-M. aeruginosa co-culture at 15 ℃ and 25 ℃ in different initial biomass gradients. In the co-culture experiment, the characteristics of growth and succession of the two species were studied and the morphological change of A. flos-aquae was observed.
2 MATERIAL AND METHOD 2.1 Strain and culture conditionTwo strains of A. flos-aquae CHAB7452 and M. aeruginosa CHAB7427 were isolated for this study from Meiliang Bay, Taihu Lake, China in May 2019 (Fig. 1). Strain CHAB7452 was originally in large fascicle-like colonies and composed of several straight trichomes, and the bundle filaments could irreversibly transform into solitary trichomes under laboratory conditions. Strain CHAB7427 was unicellular.
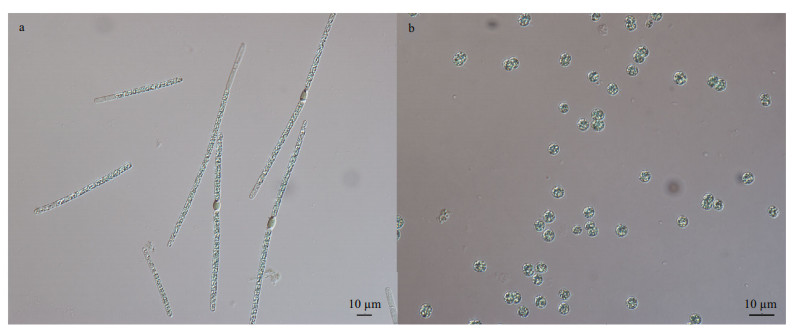
|
| Fig.1 Photomicrographs of A. flos-aquae strain CHAB7452 (a) and Microcystis aeruginosa strain CHAB7427 (b) |
The cultures of the both cyanobacterial strains were maintained in BG11 medium in 250-mL flasks containing 120 mL of the medium, at 25 ℃ with a 12-h: 12-h light: dark cycle under a constant white light intensity of 30 μmol/(m2·s). The cultures were manually shaken thrice daily during incubation until the exponential phase.
2.2 Design of the experimentThe co-cultured experiment included 7 groups, and each group consisted of triplicate cultures at 15 ℃ and 25 ℃. The first two groups, Groups A and B, were set for controls corresponding to monocultures of A. flos-aquae (CHAB7452) and M. aeruginosa (CHAB7427), respectively. The remaining groups, from C to G, were set for test groups of co-culture of A. flos-aquae (CHAB7452) and M. aeruginosa (CHAB7427) in different initial ratios of biomass with a total initial biomass of 24.3 mg/mL in 250-mL flasks as listed below:
A: 100% Aphanizomenon (A 100%) (A: M=100:0);
B: 100% Microcystis (M 100%) (A: M=0:100);
C: 95% Aphanizomenon (A 95%) and 5% Microcystis (M 5%) (A: M=95:5);
D: 75% Aphanizomenon (A 75%) and 25% Microcystis (M 25%) (A: M=75:25);
E: 50% Aphanizomenon (A 50%) and 50% Microcystis (M 50%) (A: M=50:50);
F: 25% Aphanizomenon (A 25%) and 75% Microcystis (M 75%) (A: M=25:75);
G: 5% Aphanizomenon (A 5%) and 95% Microcystis (M 95%) (A: M=5:95).
All the flasks were placed in an incubator under the same cultivation conditions except for temperature and manually shaken three times a day and their positions in the incubator were randomly adjusted during incubation. For each group, 10-mL subsamples were collected from the flasks during the experiment, from which 5 mL was fixed with Lugol's iodine solution (1% final concentration) for cell counting and the rest 5 mL was used for the morphological observation. Fresh sterile BG11 media were supplemented after sampling to maintain constant 120-mL culture volumes.
2.3 Growth measurementThe subsamples were taken for cell counting under a microscope (Olympus CX21, 400× magnification, Olympus, Tokyo, Japan), and specific growth rate (μ) was calculated by the equation:

where C1 and C2 are the biomass (mg/L) at time t for A. flos-aquae and M. aeruginosa.
Analyses of the morphology were based on photomicrographs taken with a Nikon eclipse 80i microscope (Japan) equipped with a DS-Ri1 digital camera (Nikon, Japan) photomicrographic system at 400× magnification. The cell width, length, and filament length of A. flos-aquae were measured and analyzed using NIS-Elements D 3.2, in which 360 vegetative cells and 360 trichomes were randomly chosen for the morphological analysis.
2.4 Statistical analysisAll experiments were conducted in triplicate; data were presented in mean±standard deviation. Statistical differences were evaluated by Kruskal-Wallis test in SPSS v19.0 software for windows. Differences with P values less than 0.05 were considered significant. Figures were generated using Microsoft Excel and R v3.5.1.
3 RESULT 3.1 Effect of A: M biomass ratios on the growth of the two cyanobacterial strains at two different temperaturesAt 15 ℃, biomasses of A. flos-aquae and M. aeruginosa all increased during the whole experiment period (Fig. 2). After co-culture in different biomass ratios of M. aeruginosa, all the biomass of A. flos-aquae in each group increased from initial values of 24.300, 23.085, 18.225, 12.150, 6.075, and 1.215 mg/L in Groups A (A: M=100:0), C (A: M=95:5), D (A: M=75:25), E (A: M=50:50), F (A: M=25:75), and G (A: M=5:95), respectively, to final biomass values of 174.489, 156.529, 139.758, 121.345, 70.754, and 15.941 mg/L, respectively (Fig. 2). A. flos-aquae in Groups G (A: M=5:95), with minimal initial biomass, showed the greatest increase in biomass of 13.12 times. The growth pattern of M. aeruginosa was similar to that of A. flos-aquae. The biomass of M. aeruginosa in all of the groups reached the peak value at the end of the experiment, reaching 75.973, 4.913, 23.995, 43.366, 33.507, and 66.332 mg/L in Groups B (A: M=0:100), C (A: M=95:5), D (A: M=75:25), E (A: M=50:50), F (A: M=25:75), and G (A: M=5:95), respectively (Fig. 2). M. aeruginosa in Groups C (A: M=95:5) also had the lowest initial biomass and the greatest increase of 4.04 times in the experiment.
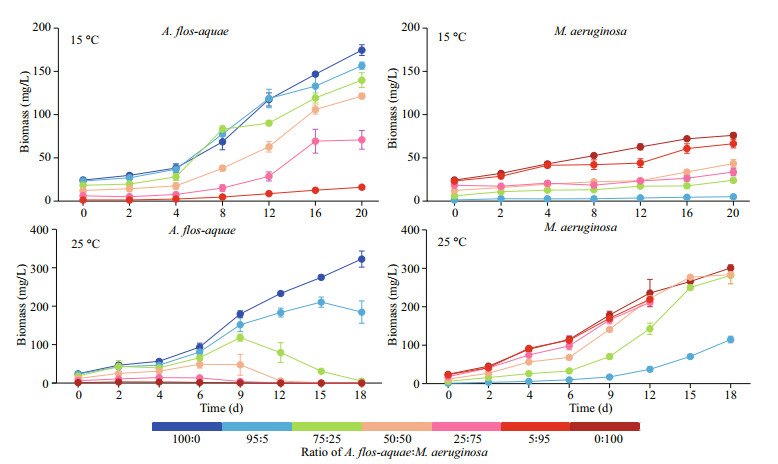
|
| Fig.2 The growth curves of the A. flos-aquae and M. aeruginosa at 15 ℃ and 25 ℃ |
The specific growth rates (μ) of A. flos-aquae in Groups E, F, and G were significantly higher than that of Group A (P < 0.05) (Table 1). However, there were no significant differences in Groups A from Groups C and D (P > 0.05) (Table 1). In the test groups, the μ value of A. flos-aquae in Group C was significantly lower than other groups (P < 0.05) (Table 1), and those of M. aeruginosa in Groups C and D were significantly higher than that of Group B, whereas Group F was significantly lower than Group B (P < 0.05) (Table 1). In the test groups, there were no significant differences among Groups C, D, and E (P > 0.05) (Table 1). However, the μ value of M. aeruginosa in the three groups were significantly higher than those of Groups F and G (P < 0.05) (Table 1).
At 25 ℃, the results are different from those at 15 ℃. The biomass of M. aeruginosa in all groups was sustained to grow over the experiment period, whereas the biomass of A. flos-aquae presented a trend of earlier increase to later decrease except for Group A (100% A) (Fig. 2). The whole experiment lasted 18 days; however, the filaments of A. flos-aquae were hardly seen in the Groups F and G until Day 12. The biomass of A. flos-aquae dropped to 5.070 (on Day 18), 0.280 (on Day 18), 0.282 (on Day 12), 0.019 mg/L (on Day 12) from the initial values of 18.225, 12.150, 6.075, and 1.215 mg/L in Groups D, E, F, and G, respectively (Fig. 2). The highest biomass of M. aeruginosa reached 300.931 (on Day 18), 114.811 (on Day 18), 281.483 (on Day 18), 283.501 (on Day 18), 211.178 (on Day 12), and 219.325 mg/L (on Day 12) in Groups B, C, D, E, F, and G, respectively (Fig. 2).
3.2 Effect of A: M biomass ratios on the morphological features of A. flos-aquaeThe length and width of the vegetative cell were significantly different among groups at 15 ℃ (Fig. 3b–c) (P < 0.05). The lengths were 4.76±1.85, 3.65±1.24, 5.16±1.97, 5.00±1.57, 3.54±0.86, and 4.20±1.19 μm in Groups A, C, D, E, F, and G, respectively (Fig. 3b); and the widths were 3.70±0.61, 3.09±0.79, 3.21±0.56, 3.25±0.50, 3.12±0.64, and 3.41±0.82 μm for Groups A, C, D, E, F, and G, respectively (Fig. 3c). The vegetative cell widths in the test groups were significantly lower than that of control group. In addition, there is no significantly difference between control group and test group in the filament length except for Group D (Fig. 3a).

|
| Fig.3 The morphological characteristics of A. flos-aquae in each group on Day 20 at 15 ℃ a, b, and c represent the filament length, vegetative cell length and width respectively; * stands for significant difference between test group and control group (Group A) (P < 0.05). |
The vegetative cell length and width were significantly different among groups at 25 ℃ (Fig. 4b–c) (P < 0.05). Comparing with the control group (Group A), the filament length in Groups E and F was significantly lower, whereas there are no significantly differences with other test groups (Fig. 4a) (P > 0.05). The vegetative cell lengths were 3.23±0.83, 4.13±1.16, 3.74±1.17, 3.01±0.78, 3.02±0.57, and 3.11±1.15 μm for Groups A, C, D, E, F, and G, respectively (Fig. 4b).
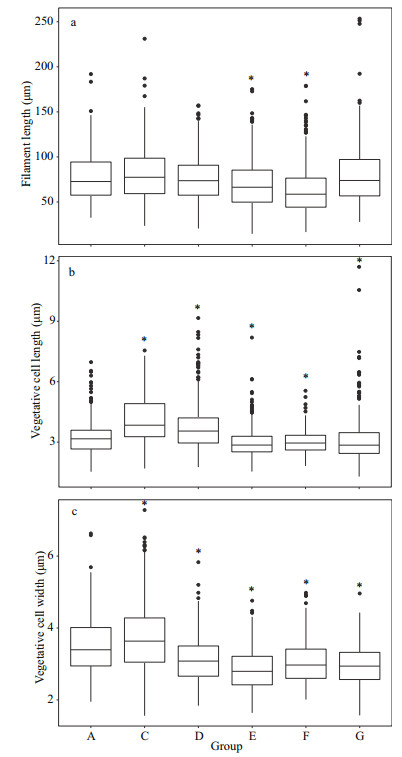
|
| Fig.4 The morphological characteristics of A. flos-aquae in each group on Day 6 at 25 ℃ * stands for significant difference between test group and control group (Group A) (P < 0.05). |
The vegetative cell widths in the test groups were significantly lower than that of control group (3.49±0.75 μm) except Group C (3.73±0.98 μm), similar to the result at 15 ℃ (P < 0.05) (Fig. 4c).
3.3 Effect of initial A: M biomass ratios on final biomass percentages of the two cyanobacterial strainsAt 15 ℃, when the experiment ended, the percentage of M. aeruginosa as invader dropped to 3.04% from 5.00% in Group C (A: M=95:5), compared to the percentage of A. flos-aquae as invader up to 19.38% in Group G (A: M=5:95) (Fig. 5). In Groups D (A: M=75:25), E (A: M=50:50), and F (A: M=25:75), the percentages of M. aeruginosa went up first and then fell to 14.65%, 26.33%, and 32.14%, respectively, whereas the percentages of A. flos-aquae increased up to 85.35%, 73.67%, and 67.86%, respectively on Day 20 (Fig. 5). The direct competition experiments between A. flos-aquae and M. aeruginosa at 15 ℃ indicated that both two species were able to coexist and A. flos-aquae completely dominated at the end of the experiment period in the competitive groups. Thus, A. flos-aquae showed a stronger competitive ability at 15 ℃ over M. aeruginosa.
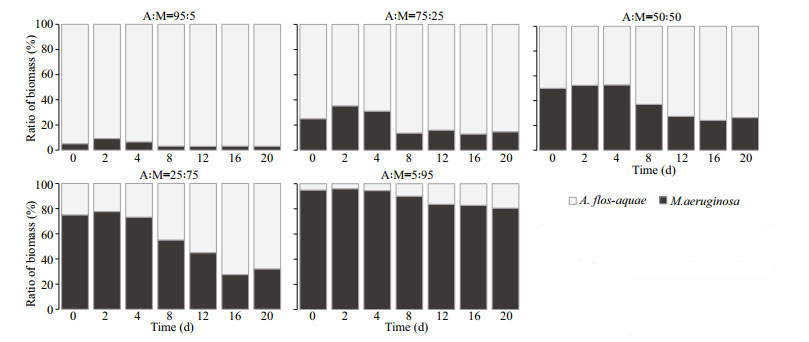
|
| Fig.5 Competition experiments under different biomass ratios of A. flos-aquae and M. aeruginosa at 15 ℃ Columns represent the percentage of biomass contribution of each strain to total biomass. |
At 25 ℃, the relative contribution of M. aeruginosa in total biomass as invader reached 38.34% on Day 18 from initial 5.00% in Group C (A: M=95:5), while the percentage of A. flos-aquae as invader dropped to nearly zero in Group G (A: M=5:95) at the end of experiment (Fig. 6). Similarly, in the Groups D (A: M=75:25), E (A: M=50:50), and F (A: M=25:75), the percentages of M. aeruginosa in total biomass increased from 25.00%, 50.00%, 75.00% (Day 0) to 98.23% (Day 18), 99.90% (Day 18), 99.87% (Day 12), completely dominated at the end of the experiment (Fig. 6). The competitive outcomes indicated that A. flos-aquae was unable to coexist with M. aeruginosa in the competitive groups, and M. aeruginosa showed a strong competitive ability at 25 ℃.
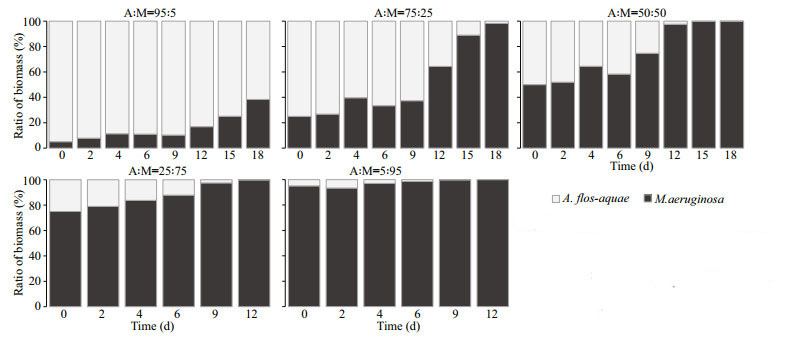
|
| Fig.6 Competition experiments under different biomass ratios of A. flos-aquae and M. aeruginosa at 25℃ Columns represent the percentage of biomass contribution of each strain to total biomass. |
In the competition experiments between A. flos-aquae and M. aeruginosa under two temperatures, the competitive outcomes were different, and the relative dominance of A. flos-aquae and M. aeruginosa was significantly affected by water temperature (Figs. 5–6).
4 DISCUSSIONUnderstanding the mechanisms of cyanobacterial bloom formation and seasonal succession will help to harness cyanobacterial blooms. Succession of dominant bloom-forming species with seasonal change has frequently occurred in many eutrophic waters worldwide. A large number of studies based on field survey have reported the succession from blooms of A. flos-aquae to Microcystis as a typical and common case in the whole bloom period. Researches into the mechanism of the succession have been also conducted based on mainly the monoculture of A. flos-aquae or Microcystis, and co-culture of A. flos-aquae and of Microcystis filtrates. It was shown that both Microcystis and A. flos-aquae were able to reach optimal growth at 25 ℃, but A. flos-aquae showed was a stronger competitor against Microcystis at 15 ℃. On the other hand, the growth of A. flos-aquae was significantly inhibited by Microcystis culture at 25 ℃ in a culture-filtrate assay system (Ma et al., 2015). Apparently, the results from this study could partially explain the reason for the seasonal succession from blooms of A. flos-aquae to those of Microcystis, and the mechanisms need to be verified in more experiments. Moreover, competition experiments between the two cyanobacterial species shall consider the initial biomass contribution from each of them.
Therefore, we designed an experiment of co-culture system in which A. flos-aquae with M. aeruginosa were mixed at 15 ℃ and 25 ℃ in different biomasses, to clarify the competition and dominance of the two strains. A. flos-aquae and M. aeruginosa showed different behaviors in competitiveness at 15 ℃ and 25 ℃. At 15 ℃, the two species were more than coexistence, and A. flos-aquae had a higher specific growth rate when its growth was stimulated by the presence of M. aeruginosa. However, at 25 ℃, the growth of A. flos-aquae was inhibited by the invasion of M. aeruginosa, and M. aeruginosa could suppress A. flos-aquae in the competition experiments at last.
Our findings in the present study showed that the variation of temperature can lead to the succession between A. flos-aquae and M. aeruginosa, and these laboratory results are similar to the seasonal succession of A. flos-aquae and M. aeruginosa in Dianchi Lake (Wu et al., 2010, 2016). Tsujimura et al. (2001) demonstrated that the optimal temperature for A. flos-aquae ranged 23–29 ℃ and the lowest temperature for A. flos-aquae growth was 8 ℃ and could survive at 5 ℃ for several days at dim light conditions. Üveges et al. (2012) showed that A. flos-aquae could grow over a wide range of temperature, and it could grow slowly in a low-temperature condition and contribute to its succession and dominance in cold ecosystems.
Aphanizomenon flos-aquae blooms were reported to occur in winter even under the ice cover (Üveges et al., 2012). In this study, A. flos-aquae showed quicker growth at 25 ℃ than at 15 ℃. However, A. flos-aquae was depressed by M. aeruginosa in co-culture at 25 ℃. On the contrary, A. flos-aquae did not show down the optimal growth at 15 ℃, but it still had the competitive advantage over M. aeruginosa at 15 ℃ in the co-culture. Compared to the previous studies of monoculture and co-culture, this study provided more insight into the low-temperature tolerance of A. flos-aquae and its low-temperature intolerance and preference in high temperature with M. aeruginosa (Konopka and Brock, 1978; Robarts and Zohary, 1987; Wu et al., 2010; Üveges et al., 2012).
Previous studies have reported that many filamentous cyanobacteria have strong phenotypic plasticity, and their morphological characteristics may be influenced when exogenous factors changed (Soares et al., 2013; Khan et al., 2017). The competition between different species can also influence their morphological traits (Zhu et al., 2015). It has been reported that interactions between Raphidiopsis raciborskii and M. aeruginosa could lead to significant changes in filament length, cell length, and width of R. raciborskii (Jia et al., 2020). In this study, A. flos-aquae displayed certain morphological plasticity in response to the co-culture with M. aeruginosa of a different biomass. All the length and width of vegetative cell were sensitive to the change in M. aeruginosa biomass at two temperatures. At 15 ℃, the width of all test groups was significantly shorter than that of control group. At 25 ℃, the vegetative cell width in all test groups was significantly smaller than that of control group except for the Group C. Therefore, the competition between M. aeruginosa and A. flos-aquae showed a tendency of decrease in the width of vegetative cell of A. flos-aquae, which contrasts to the result by Jia et al. (2020) showing that the width of vegetative cells of R. raciborskii in the test groups were significantly greater than that of control group in a competition experiment with M. aeruginosa. Previous studies reported that cell size is a main characteristic that influenced the ecological niches of phytoplankton (Litchman et al., 2007; Guan et al., 2020). Nutrient utilization, light utilization, as well as grazer resistance of phytoplankton were proved has a significantly correlation with cell size (Bohannan et al., 2002; Finkel et al., 2010; Litchman, 2003). To phytoplankton, the trade-offs between these functional traits can improve resource utilization efficiency and adaptability to environmental changes, thereby facilitating population growth (Violle et al., 2012; Guan et al., 2020). There was also a study reported that small cells had greater competitive advantages than large cells (Grover, 1989), thus we speculated that phenotypic variation maybe was a trade-off strategy adopted by A. flos-aquae. At 15 ℃, the significant change in filament length occurred only in Group F (A: M=25:75). Similarly, at 25 ℃, the filament length of A. flos-aquae was significantly shorter than that of control group in Groups E and F only. Such results suggested that filament length of A. flos-aquae was less sensitive to the changes in biomass of M. aeruginosa than vegetative cells, which is consistent with the study by Jia et al. (2020). Moreover, several previous studies indicated that the changes in filament length of cyanobacteria could be affected by many factors, for example, turbulence as a main factor could lead to the decrease in filament length of Anabaena cylindrica and A. flos-aquae (Thomas and Gibson, 1990; Xiao et al., 2016). However, the present study did not test the other factors on the morphological changes of filaments in A. flos-aquae during the co-culture competition experiments, which will be studied in the future.
5 CONCLUSIONBased on direct co-culture experiments, we provide a new insight into the succession between A. flos-aquae and M. aeruginosa during a whole bloom process, and confirm that temperature is the dominating factor on the alternant succession of A. flos-aquae and M. aeruginosa considering the initial biomass ratios of the both strains under specific conditions of this study. However, in the field, the succession and competition between A. flos-aquae and Microcystis were controlled by more environmental factors or combination of these factors, not only by the temperature. Thus, in order to better understand the mechanism of succession between A. flos-aquae and Microcystis species, the nutrition level, especially the nitrogen concentration or total nitrogen : total phosphorus ration should be taken into consideration in terms of N2-fixing ability for A. flos-aquae and non-N 2-fixing ability for Microcystis in the future.
6 DATA AVAILABILITY STATEMENTAll data generated and/or analyzed during this study are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENTWe thank the Wuhan Branch, Supercomputing Center, Chinese Academy of Sciences, for providing computing facilities, and all staff from the Analysis and Testing Center at Institute of Hydrobiology, CAS, for technical supports.
Baker K K. 1981. Ecology and taxonomy of five natural populations of the genus Aphanizomenon Morren (Cyanophyceae). Archiv fur Hydrobiologie, 92: 222-251.
DOI:10.1046/j.1529-8817.2000.99146.x |
Best J. 2019. Anthropogenic stresses on the world's big rivers. Nature Geoscience, 12(1): 7-21.
DOI:10.1038/s41561-018-0262-x |
Bohannan B. J. M., Kerr B., Jessup C. M., Hughes J. B., Sandvik G.. 2002. Trade-offs and coexistence in microbial microcosms. Antonie Van Leeuwenhoek International Journal of General and Molecular Microbiology, 81: 107-115.
DOI:10.1023/a:1020585711378 |
Bormans M, Ford P W, Fabbro L. 2005. Spatial and temporal variability in cyanobacterial populations controlled by physical processes. Journal of Plankton Research, 27(1): 61-70.
DOI:10.1093/plankt/fbh150 |
Brookes J D, Ganf G G. 2001. Variations in the buoyancy response of Microcystis aeruginosa to nitrogen, phosphorus and light. Journal of Plankton Research, 23(12): 1399-1411.
DOI:10.1093/plankt/23.12.1399 |
Carey C C, Ibelings B W, Hoffmann E P, et al. 2012. Ecophysiological adaptations that favour freshwater cyanobacteria in a changing climate. Water Research, 46(5): 1394-1407.
DOI:10.1016/j.watres.2011.12.016 |
Cirés S, Ballot A. 2016. A review of the phylogeny, ecology and toxin production of bloom-forming Aphanizomenon spp. and related species within the Nostocales (cyanobacteria). Harmful Algae, 54: 21-43.
DOI:10.1016/j.hal.2015.09.007 |
Codd G, Bell S, Kaya K, et al. 1999. Cyanobacterial toxins, exposure routes and human health. European Journal of Phycology, 34(4): 405-415.
DOI:10.1017/s0967026299002255 |
Finkel Z. V., Beardall J., Flynn K. J., Quigg A., Rees T. A. V., Raven J. A.. 2010. Phytoplankton in a changing world: cell size and elemental stoichiometry. Journal of Plankton Research, 32(1): 119-137.
DOI:10.1093/plankt/fbp098 |
Gehringer M M, Wannicke N. 2014. Climate change and regulation of hepatotoxin production in Cyanobacteria. FEMS Microbiology Ecology, 88(1): 1-25.
DOI:10.1111/1574-6941.12291 |
Glibert P M, Anderson D M, Gentien P, et al. 2005. The global, complex phenomena of harmful algal blooms. Oceanography, 18(2): 136-147.
DOI:10.5670/oceanog.2005.49 |
Grover J P. 1989. Influence of cell shape and size on algal competitive ability. Journal of Phycology, 25(2): 402-405.
DOI:10.1111/j.1529-8817.1989.tb00138.x |
Guan Y, Zhang M, Yang Z, Shi X, Zhao X. 2020. Intraannual variation and correlations of functional traits in Microcystis and Dolichospermum in Lake Chaohu. Ecological Indicators, 111: 106052.
DOI:10.1016/j.ecolind.2019.106052 |
Jia N N, Yang Y M, Yu G L, et al. 2020. Interspecific competition reveals Raphidiopsis raciborskii as a more successful invader than Microcystis aeruginosa. Harmful Algae, 97: 101858.
DOI:10.1016/j.hal.2020.101858 |
Jones R I. 1979. Notes on the growth and sporulation of a natural population of Aphanizomenon flos-aquae. Hydrobiologia, 62(1): 55-58.
DOI:10.1007/bf00012562 |
Khan Z, Wan Omar W M, Merican F M M S, Azizan A A, Foong C P, Convey P, Najimudin N, Smykla J, Alias S A. 2017. Identification and phenotypic plasticity of Pseudanabaena catenata from the Svalbard archipelago. Polish Polar Research, 38(4): 445-458.
DOI:10.1515/popore-2017-0022 |
Konopka A, Brock T D. 1978. Effect of temperature on blue-green algae (Cyanobacteria) in lake Mendota. Applied and Environmental Microbiology, 36(4): 572-576.
DOI:10.1128/aem.36.4.572-576.1978 |
Litchman E, Klausmeier C A, Schofield O M, Falkowski P G. 2007. The role of functional traits and trade-offs in structuring phytoplankton communities: scaling from cellular to ecosystem level. Ecology Letters, 10(12): 1170-1181.
DOI:10.1111/j.1461-0248.2007.01117.x |
Litchman E.. 2003. Competition and coexistence of phytoplankton under fluctuating light: experiments with two cyanobacteria. Aquatic Microbial Ecology, 31(3): 241-248.
DOI:10.3354/ame031241 |
Liu X Y, Shi M, Liao Y H, et al. 2006a. Feeding characteristics of an amoeba (Lobosea: Naegleria) grazing upon cyanobacteria: food selection, ingestion and digestion progress. Microbial Ecology, 51(3): 315-325.
DOI:10.1007/s00248-006-9031-2 |
Liu Y M, Chen W, Li D H, et al. 2006b. First report of aphantoxins in China—waterblooms of toxigenic Aphanizomenon flos-aquae in Lake Dianchi. Ecotoxicology and Environmental Safety, 65(1): 84-92.
DOI:10.1016/j.ecoenv.2005.06.012 |
Ma H Y, Wu Y L, Gan N Q, et al. 2015. Growth inhibitory effect of Microcystis on Aphanizomenon flos-aquae isolated from cyanobacteria bloom in Lake Dianchi, China. Harmful Algae, 42: 43-51.
DOI:10.1016/j.hal.2014.12.009 |
McDonald K E, Lehman J T. 2013. Dynamics of Aphanizomenon and Microcystis (cyanobacteria) during experimental manipulation of an urban impoundment. Lake and Reservoir Management, 29(2): 103-115.
DOI:10.1080/10402381.2013.800172 |
Messineo V, Melchiorre S, Di Corcia A, et al. 2010. Seasonal succession of Cylindrospermopsis raciborskii and Aphanizomenon ovalisporum blooms with cylindrospermopsin occurrence in the volcanic Lake Albano, central Italy. Environmental Toxicology, 25(1): 18-27.
DOI:10.1002/tox.20469 |
Moustaka-Gouni M, Vardaka E, Tryfon E. 2007. Phytoplankton species succession in a shallow Mediterranean lake (L. Kastoria, Greece): steady-state dominance of Limnothrix redekei, Microcystis aeruginosa and Cylindrospermopsis raciborskii. Hydrobiologia, 575(1): 129-140.
DOI:10.1007/s10750-006-0360-4 |
Mushtaq N, Singh D V, Bhat R A et al. 2020. Freshwater contamination: sources and hazards to aquatic biota. In: Qadri H, Bhat R A, Mehmood M A et al. eds. Fresh Water Pollution Dynamics and Remediation. Springer, Singapore. p. 27–50, https://doi.org/10.1007/978-981-13-8277-2_3.
|
Paerl H W, Otten T G. 2013. Harmful cyanobacterial blooms: causes, consequences, and controls. Microbial Ecology, 65(4): 995-1010.
DOI:10.1007/s00248-012-0159-y |
Paerl H W, Otten T G. 2016. Duelling 'CyanoHABs': unravelling the environmental drivers controlling dominance and succession among diazotrophic and non-N2-fixing harmful cyanobacteria. Environmental Microbiology, 18(2): 316-324.
DOI:10.1111/1462-2920.13035 |
Paerl H W, Paul V J. 2012. Climate change: links to global expansion of harmful cyanobacteria. Water Research, 46(5): 1349-1363.
DOI:10.1016/j.watres.2011.08.002 |
Pearson L, Mihali T, Moffitt M, et al. 2010. On the chemistry, toxicology and genetics of the cyanobacterial toxins, microcystin, nodularin, saxitoxin and cylindrospermopsin. Marine Drugs, 8(5): 1650-1680.
DOI:10.3390/md8051650 |
Rapala J, Sivonen K, Luukkainen R, et al. 1993. Anatoxin-a concentration in Anabaena and Aphanizomenon under different environmental conditions and comparison of growth by toxic and non-toxic Anabaena-strains — a laboratory study. Journal of Applied Phycology, 5(6): 581-591.
DOI:10.1007/bf02184637 |
Robarts R D, Zohary T. 1987. Temperature effects on photosynthetic capacity, respiration, and growth rates of bloom-forming cyanobacteria. New Zealand Journal of Marine and Freshwater Research, 21(3): 391-399.
DOI:10.1080/00288330.1987.9516235 |
Rzymski P, Poniedzialek B, Karczewski J. 2011. Gastroenteritis and liver carcinogenesis induced by cyanobacterial toxins. Gastroenterologia Polska, 18(4): 159-162.
|
Sabour B, Loudiki M, Oudra B, et al. 2005. Dynamics and toxicity of Anabaena aphanizomenoides (Cyanobacteria) waterblooms in the shallow brackish Oued Mellah Lake (Morocco). Aquatic Ecosystem Health & Management, 8(1): 95-104.
DOI:10.1080/14634980590914944 |
Shan K, Song L R, Chen W, et al. 2019. Analysis of environmental drivers influencing interspecific variations and associations among bloom-forming cyanobacteria in large, shallow eutrophic lakes. Harmful Algae, 84: 84-94.
DOI:10.1016/j.hal.2019.02.002 |
Shen H, Song L R. 2007. Comparative studies on physiological responses to phosphorus in two phenotypes of bloom-forming Microcystis. Hydrobiologia, 592(1): 475-486.
DOI:10.1007/s10750-007-0794-3 |
Smayda T J. 2008. Complexity in the eutrophication-harmful algal bloom relationship, with comment on the importance of grazing. Harmful Algae, 8(1): 140-151.
DOI:10.1016/j.hal.2008.08.018 |
Soares M C S, Luerling M, Huszar V L M. 2013. Growth and temperature-related phenotypic plasticity in the cyanobacterium Cylindrospermopsis raciborskii. Phycological Research, 61(1): 61-67.
DOI:10.1111/pre.12001 |
Suurnäkki S, Gomez-Saez G V, Rantala-Ylinen A, et al. 2015. Identification of geosmin and 2-methylisoborneol in cyanobacteria and molecular detection methods for the producers of these compounds. Water Research, 68: 56-66.
DOI:10.1016/j.watres.2014.09.037 |
Takamura N, Iwakuma T, Yasuno M. 1987. Uptake of 13C and 15N (ammonium, nitrate and urea) by Microcystis in Lake Kasumigaura. Journal of Plankton Research, 9(1): 151-165.
DOI:10.1093/plankt/9.1.151 |
Tan X, Gu H H, Ruan Y L, et al. 2019. Effects of nitrogen on interspecific competition between two cell-size cyanobacteria: Microcystisaeruginosa and Synechococcus sp. Harmful Algae, 89: 101661.
DOI:10.1016/j.hal.2019.101661 |
Thomas W H, Gibson C H. 1990. Effects of small-scale turbulence on microalgae. Journal of Applied Phycology, 2(1): 71-77.
DOI:10.1007/bf02179771 |
Tsujimura S, Ishikawa K, Tsukada H. 2001. Effect of temperature on growth of the cyanobacterium Aphanizomenon flos-aquae in Lake Biwa and Lake Yogo. Phycological Research, 49(4): 275-280.
DOI:10.1046/j.1440-1835.2001.00255.x |
Tsukada H, Tsujimura S, Nakahara H. 2006. Seasonal succession of phytoplankton in Lake Yogo over 2 years: effect of artificial manipulation. Limnology, 7(1): 3-14.
DOI:10.1007/s10201-005-0159-4 |
Üveges V, Tapolczai K, Krienitz L, et al. 2012. Photosynthetic characteristics and physiological plasticity of an Aphanizomenon flos-aquae (Cyanobacteria, Nostocaceae) winter bloom in a deep oligo-mesotrophic lake (Lake Stechlin, Germany). Hydrobiologia, 698(1): 263-272.
DOI:10.1007/s10750-012-1103-3 |
Violle C, Enquist B J, McGill B J, Jiang L, Albert C H, Hulshof C, Jung V, Messier J. 2012. The return of the variance: intraspecific variability in community ecology. Trends in Ecology & Evolution, 27(4): 244-252.
DOI:10.1016/j.tree.2011.11.014 |
Wang Z H, Akbar S, Sun Y F, et al. 2021. Cyanobacterial dominance and succession: factors, mechanisms, predictions, and managements. Journal of Environmental Management, 297: 113281.
DOI:10.1016/j.jenvman.2021.113281 |
Wu W J, Li G B, Li D H, et al. 2010. Temperature may be the dominating factor on the alternant succession of Aphanizomenon flos-aquae and Microcystis aeruginosa in Dianchi Lake. Fresenius Environmental Bulletin, 19(5): 846-853.
|
Wu YL, Li L, Zheng L L, et al. 2016. Patterns of succession between bloom-forming cyanobacteria Aphanizomenon flos-aquae and Microcystis and related environmental factors in large, shallow Dianchi Lake, China. Hydrobiologia, 765(1): 1-13.
DOI:10.1007/s10750-015-2392-0 |
Xiao Y, Li Z, Li C, et al. 2016. Effect of small-scale turbulence on the physiology and morphology of two bloom-forming cyanobacteria. PLoS One, 11(12): e0168925.
DOI:10.1371/journal.pone.0168925 |
Yang Z, Kong F X, Shi X L, et al. 2006. Morphological response of Microcystis aeruginosa to grazing by different sorts of zooplankton. Hydrobiologia, 563(1): 225-230.
DOI:10.1007/s10750-005-0008-9 |
Zhu X, Wang J, Lu Y, Chen Q, Yang Z. 2015. Grazer-induced morphological defense in Scenedesmus obliquus is affected by competition against Microcystis aeruginosa. Scientific Reports, 5: 12743.
DOI:10.1038/srep12743 |
 2022, Vol. 40
2022, Vol. 40



