Institute of Oceanology, Chinese Academy of Sciences
Article Information
- MA Zengling, ZHANG Xiaoqiao, LI Renhui, WANG Min, QIN Wenli, ZHANG He, LI Gang, YU Henguo, DAI Chuanjun, ZHAO Min
- Competitiveness of alga Microcystis aeruginosa co-cultivated with cyanobacterium Raphidiopsis raciborskii confirms its dominating position
- Journal of Oceanology and Limnology, 40(5): 1804-1818
- http://dx.doi.org/10.1007/s00343-022-1393-x
Article History
- Received Nov. 18, 2021
- accepted in principle Jan. 7, 2022
- accepted for publication Mar. 7, 2022
2 National and Local Joint Engineering Research Center of Ecological Treatment Technology for Urban Water Pollution, Wenzhou University, Wenzhou 325035, China;
3 Key Laboratory of Tropical Marine Bio-resources and Ecology, South China Sea Institute of Oceanology, Chinese Academy of Sciences, Guangzhou 510301, China
Cyanobacterial harmful algal blooms (CyanoHABs) are almost a ubiquitous phenomenon in stagnant water worldwide (Huisman et al., 2018; Paerl, 2018). These algal blooms have presented a great environmental challenge because of their severe ecological impacts and associated health threats not only to humans but also to the many aquatic animals that inhabit the water (Olokotum et al., 2020; Amorim and Moura, 2021; Plaas and Paerl, 2021). Microcystis aeruginosa, Raphidiopsis raciborskii, Dolichospermum flos-aquae, Aphanizomenon flos-aquae, and Planktothrix sp. are the most successful CyanoHAB-forming species in many freshwater ecosystems (Padisák and Reynolds, 1998; Nixdorf et al., 2003; Hoeger et al., 2004; Paerl et al., 2011). Among them, M. aeruginosa is the most important and the blooms it causes are the most frequent and widely distributed algal blooms as well as being the largest scale worldwide (Harke et al., 2016). In addition, during the proliferation of M. aeruginosa, the odor compounds and microcystins (MCs) that it produces can lead to serious environmental disaster (Dokulil and Teubner, 2000; Dittmann and Wiegand, 2006; Zhang et al., 2010; Kim et al., 2020).
In recent years, R. raciborskii has become the second most concerning species in terms of the causation of CyanoHABs. Raphidiopsis raciborskii has a particularly broad growth temperature range (Briand et al., 2004; Chonudomkul et al., 2004; Soares et al., 2013), well suited to different light habitats (Pierangelini et al., 2014a, 2015b; Kovács et al., 2016) and inorganic carbon centration (Pierangelini et al., 2014b, 2015a). In addition, it is more competitive when phosphorus and nitrogen availability is low and/or variable availability (Amaral et al., 2014; Burford et al., 2014, 2018; Willis et al., 2015). Therefore, R. raciborskii also causes algal blooms globally, and there seem to be signs of R. raciborskii replacing M. aeruginosa as the main culprit of algal blooms in some tropical and subtropical shallow lakes (Chislock et al., 2014; Burford et al., 2016; Lei et al., 2020; Xiao et al., 2020). Therefore, R. raciborskii and M. aeruginosa are of particular concern and these two species of cyanobacteria have received widespread attention (Nixdorf et al., 2003; Tomioka et al., 2011).
Currently, R. raciborskii is well regarded as an invasive cyanobacterial species in natural waters (Hamilton et al., 2005; Stüken et al., 2006; Moreira et al., 2015), and has gradually become the dominant species in some tropical and subtropical lakes where M. aeruginosa used to be the dominant species (Saker and Griffiths, 2001; Moisander et al., 2012). According to the results of field investigation, R. raciborskii and M. aeruginosa usually coexist in the same bodies of natural water as dominant species (Rzymski et al., 2014; Jia et al., 2020). However, to our knowledge, there has been a lack of studies focusing on the interactions between these two species using a bi-algal cultivation system in the lab, and only non-MCs-producing strains of M. aeruginosa were used in the previous studies that investigated the competition for dominance between R. raciborskii and M. aeruginosa (Rzymski et al., 2014; Bai et al., 2020; Jia et al., 2020). In addition, it has been reported that the competition between R. raciborskii and M. aeruginosa can be affected by environmental factors such as temperature, light, and nutrients (Marinho et al., 2013; Soares et al., 2013; da Silva Brito et al., 2018). Furthermore, the biological invasion is a dynamic process from low-density cell introduction to high-density cell replacement of the dominant species, and the biomass of R. raciborskii and that of M. aeruginosa are almost unequal in every natural body of water that has been tested (Mallon et al., 2015; Sukenik et al., 2015). Therefore, the competition for dominance between R. raciborskii and M. aeruginosa as initiated by different biomass ratios are more suitable for simulating their competition in natural waters.
Compared with the non-MCs-producing M. aeruginosa strain, the MCs-producing strain exerts a higher inhibitory effect on other co-existing microalgae because of its MCs-producing ability, and therefore, it usually acquires a greater advantage in such competition for dominance (Li and Li, 2012; Lei et al., 2015; Ma et al., 2015). MCs are well-known toxicants that are primarily responsible for the inhibitory effects of M. aeruginosa strains, which can induce oxidative stress and eventually inhibit the photosynthesis and growth of their competitors (Campos et al., 2013; Yang et al., 2014; Kaur et al., 2019). Besides MCs, other compounds released by M. aeruginosa strains are found to be inhibitory to its competitors, and together with MCs, they can exert a synergistic effect (Campos et al., 2013; Yang et al., 2014). A few studies have demonstrated that R. raciborskii strains with the ability to synthesize cylindrospermopsin (CYN), and can rely on its contribution to enhance their competitiveness and extend their population size (Bar-Yosef et al., 2010; Sukenik et al., 2012). In addition, the CYN pool size of R. raciborskii is constitutive and not affected by light and CO2 conditions, thus toxicity of blooms formed by R. raciborskii is determined by the absolute abundance of R. raciborskii cells within the water column and the relative abundance of toxic and nontoxic strains (Pierangelini et al., 2015b), although different CYN-producing strains possess different constituent CYN cell quotas (Willis et al., 2015). As a hepatotoxic alkaloid, the main targets of CYN included green algae (Bar-Yosef et al., 2010; Campos et al., 2013, Pinheiro et al., 2013) and cyanobacterial species (Rzymski and Poniedzialek, 2014; Rzymski et al., 2014). For example, it was reported that 10- and 50-μg/L CYN (both of which are environmentally-relevant concentrations) can induce growth inhibition and cell necrosis of M. aeruginosa, respectively (Rzymski et al., 2014). Both MCs and CYN can exert similar effects on the cyanobacteria with respect to the process of their specific competition for dominance, but the effects of direct cell-to-cell contact between CYN-producing R. raciborskii and MCs-producing M. aeruginosa remain unclarified.
The consequences of competition between two different species depend on the niche difference and relative fitness difference between the two species, as well as the spatial and resource constraints (MacDougall et al., 2009; Narwani et al., 2013). These consequences can be the replacement of one of the species by the other, the failure of one of the species to maintain a steady population growth, or the coexistence of both species in a more balanced stage. Coexistence is defined as the ability of each of the two species to invade an established population of the other species from rarity (Chesson, 2000). Both R. raciborskii and M. aeruginosa are CyanoHABs-forming species with similar environmental adaptation strategies, such as high growth rate, high genetic diversity, and the possession of gas vesicles to attain buoyancy, indicating that they may occupy the same niche (Gugger et al., 2005; Duan et al., 2009). However, as two distinct species, they also have their own biological characteristics besides the obvious differences in morphology. For example, R. raciborskii can fix nitrogen with its heterocysts to alleviate nitrogen deficiency (Plominsky et al., 2013; Yang et al., 2018b), whereas M. aeruginosa has no heterocyst but can form colonies to gain greater buoyancy and withstand higher tolerance to a stressful environment (Mantzouki et al., 2016; Xiao et al., 2018). These biological similarities and differences determine their different competitive potentials for dominance in the same water body.
Based on the above-mentioned field results and the biological similarities and differences, it is reasonable to speculate that R. raciborskii can coexist with M. aeruginosa, but the consequences of such competition would depend on whether the M. aeruginosa strain is capable or incapable of producing MCs. To verify the hypothesis, a CYN-producing strain of R. raciborskii was co-cultivated with either an MCs-producing or non-MCs-producing strain of M. aeruginosa at different initial biomass ratios, and the contribution of biomass from each species to the total algal biomass in the culture and the morphological changes of the two species were determined to assess their competitive potentials as well as to predict the success of Raphidiopsis and Microcystis blooms in natural waters.
2 MATERIAL AND METHOD 2.1 Strain and culture conditionPure cultures of CYN-producing R. raciborskii FACHB-3438, MCs-producing M. aeruginosa FACHB-905, and non-MCs-producing M. aeruginosa FACHB-526 were obtained from the Institute of Hydrobiology, Chinese Academy of Sciences located in Wuhan City of China. These species were grown in 250-mL Erlenmeyer flasks, each containing 150 mL of sterilized CT liquid medium (Watanabe and Ichimura, 1977). The cultures were incubated at 25±0.5 ℃ and exposed to 40 μmol photons/(m2·s) in a 12-h: 12-h light-dark cycle, as it somewhat replicates the environmental conditions of night and day.
2.2 Co-cultivation experimentsTo explore the competitive potentials of R. raciborskii and the two M. aeruginosa strains under conditions of unequal biomass densities, different initial biomass ratios of R. raciborskii to either M. aeruginosa strain were used, and these were as follows: (1) 100% R. raciborskii (100R); (2) 95% R. raciborskii and 5% M. aeruginosa (95R: 5M); (3) 75% R. raciborskii and 25% M. aeruginosa (75R: 25M); (4) 50% R. raciborskii and 50% M. aeruginosa (50R: 50M); (5) 25% R. raciborskii and 75% M. aeruginosa (25R: 75M); (6) 5% R. raciborskii and 95% M. aeruginosa (5R: 95M); and (7) 100% M. aeruginosa (100M).
The cultures containing the 95R: 5M and 5R: 95M biomass ratios were regarded as the invasive groups, based on the 95:5 resident-to-invader ratios (Narwani et al., 2013; Gallego et al., 2019). M. aeruginosa was the invader and R. raciborskii was the defender in the culture with 95R: 5M ratio, while the roles were reversed in the culture with the 5R: 95M ratio. To obtain the above-mentioned co-cultures, all the strains at the logarithmic growth phase were harvested by centrifugation at 10 000×g and resuspended in fresh medium to acquire the designated biomass ratios. Both monocultures and co-cultures were prepared to have an equal initial total biomass density of 0.016 mg/mL, which correspond to 2×108, 7×108, and 6×108 cells/mL, respectively, in the monoculture of CYN-producing R. raciborskii, MCs-producing and non-MCs-producing M. aeruginosa. To get the biomass densities of the three microalgae used in this study, the cell concentrations were determined by cell counting with an inverted microscope (ECLIPSE Ts2, Nikon, Tokyo, Japan) and their morphologies were recorded using a digital camera system (Nikon DS-Ri2, Tokyo, Japan). The biovolume calculation was performed according the method of Hillebrand et al. (1999) and their biomasses were estimated by assuming an average density for phytoplankton was 1 g/mL. All the cultures were prepared in 250-mL flasks, each containing 150-mL cell suspensions. Triplicate samples were prepared for each treatment and all the cultures were grown under the same temperature and light intensity as stated above. During the cultivation period, all flasks were shaken three times per day and their positions in the incubator were randomly adjusted after shaking to eliminate the possible uneven irradiance levels caused by the arrangement of their positions in the incubator. The experiment lasted for 24 days, and samples of the cultures were taken every three days.
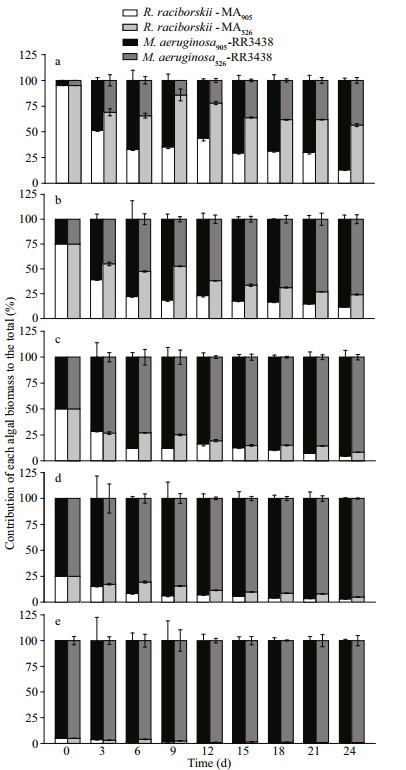
2.3 Determination of specific growth rateThe sampled cell suspensions were fixed with 1% Lugol's iodine solution and the numbers of cells in the samples were then counted under a microscope (ECLIPSE Ts2, Nikon, Tokyo, Japan). The biovolume of each species was calculated by multiplying its abundance with its measured cell size (Hillebrand et al., 1999). The specific growth rate (μ) of the species was calculated using the following formula (Orr and Jones, 1998):
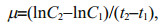 (1)
(1)where C1 and C2 represent the biomass concentrations (mg/mL) of CYN-producing R. raciborskii plus MCs-producing or non-MCs-producing M. aeruginosa at time t1 and t2.
2.4 Morphological examinationTo explore the final differences in morphologies of R. raciborskii filaments in the co-cultivation, the photographs of the filaments were recorded with a digital microscope camera system (Nikon DS-Ri2), and the widths of 100 filaments were randomly measured with the Nikon software NIS Element Ar 3.0 (Nikon, Tokyo, Japan) at the end of cultivation. The width of the vegetative cell was equal to that of the filament. Furthermore, the ratios of the number of filaments containing heterocysts to the total number of filaments in different treatments were determined as we accidentally discovered that the heterocysts in the filaments were disappearing during the co-cultivation process. In addition, at the end of cultivation, only a small quantity of R. raciborskii filaments was observed when the initial biomass of R. raciborskii relative to M. aeruginosa was less than 50%. Therefore, the ratios of R. raciborskii filaments containing the heterocysts to the total filaments were only determined for co-cultures where the initial biomass of R. raciborskii was more than 50%. About 1 000 filaments in each culture were examined for this purpose.
2.5 Determination of total dissolved nitrogen (TN) and phosphorus (TP) in the culturesTo address the changes of the extracellular microenvironment during the cultivation, the concentrations of total dissolved nitrogen (TN) and total dissolved phosphorus (TP) were measured every three days. After sampling, the cell suspension was immediately filtered through a 0.45- m cellulose acetate film, and the filtrate was used for determination of the concentrations of TN and TP by potassium persulfate oxidation and Mo-Sb antispectrophotometry. For each culture, the determination of TN and TP was carried out for three replicates.
2.6 Statistical analysisData were presented as mean ± standard deviation for each set of measurements. Shapiro-Wilk and Levene tests were used to assess the normality and homogeneity of variance. Differences between groups were analyzed by t-test or one-way ANOVA followed by Tukey's multiple comparison test, and statistical significances were considered at the P < 0.05 level. Statistical analysis was performed with SPSS V.16.0 (SPSS Inc., USA), and all figures were generated using Origin 9.0 (OriginLab, USA).
3 RESULT 3.1 Effects of biomass ratios and incubation time on the growth of R. raciborskii and M. aeruginosa in the co-culturesIn the monocultures (Control, 100R), the biomass densities of R. raciborskii increased with prolonged incubation (Fig. 1a–b). However, when R. raciborskii was co-cultivated with M. aeruginosa, its density significantly decreased at all tested initial R. raciborskii to M. aeruginosa biomass ratios. In addition, the inhibition of R. raciborskii growth was stronger when it was co-cultivated with the MCs-producing M. aeruginosa strain compared with the non-MCs-producing M. aeruginosa strain. At the end of the cultivation, the densities of R. raciborskii were 0.25, 0.23, 0.09, 0.07, and 0.01 mg/mL in the co-cultures where the initial biomass ratios of R. raciborskii to the MCs-producing M. aeruginosa strain were 95R: 5M, 75R: 25M, 50R: 50M, 25R: 75M, and 5R: 95M, respectively (Fig. 1a). The corresponding densities of R. raciborskii in the cases of the co-cultures containing the non-MCs-producing M. aeruginosa strain at the same initial biomass ratios were 0.73, 0.31, 0.16, 0.10, and 0.02 mg/mL (Fig. 1b).
|
| Fig.1 Changes in algal biomass for cultures with different initial R. raciborskii to M. aeruginosa biomass ratios Biomass of R. raciborskii co-cultured with MCs-producing (a) and non-MCs-producing (b) M. aeruginosa strains. Biomass of MCs-producing (c) and non-MCs-producing (d) M. aeruginosa strains co-cultured with R. raciborskii. Data are the means standard errors from triplicate determinations. |
For M. aeruginosa, the biomass density increased with prolonged cultivation, and when higher initial biomass relative to R. raciborskii was used at the time of inoculation, higher biomass was also achieved at the end of the co-cultivation (Fig. 1c–d). Furthermore, at the same R. raciborskii to M. aeruginosa biomass ratios, the MCs-producing M. aeruginosa strain grew better than the non-MCs-producing one (Fig. 1c–d). At the end of the cultivation, the densities of the MCs-producing M. aeruginosa strain co-cultivated with R. raciborskii at initial biomass ratios of 95R: 5M, 75R: 25M, 50R: 50M, 25R: 75M, and 5R: 95M increased to 1.68, 1.75, 2.00, 2.20 and 2.38 mg/mL, respectively, from an initial density of 0.016 mg/mL (Fig. 1c). For the same R. raciborskii to M. aeruginosa biomass ratios, the corresponding densities of the non-MCs-producing M. aeruginosa strain reached 0.56, 0.96, 1.75, 1.84, and 1.88 mg/mL at the end of the co-cultivation (Fig. 1d). Thus, regardless of the initial biomass ratios of R. raciborskii to M. aeruginosa (both MCs-producing and non-MCs-producing strains), the biomass of M. aeruginosa relative to that of R. raciborskii exhibited an increasing trend at the end of the co-cultivation. This suggested the ability of M. aeruginosa to proliferate out-competed R. raciborskii in the co-culture system.
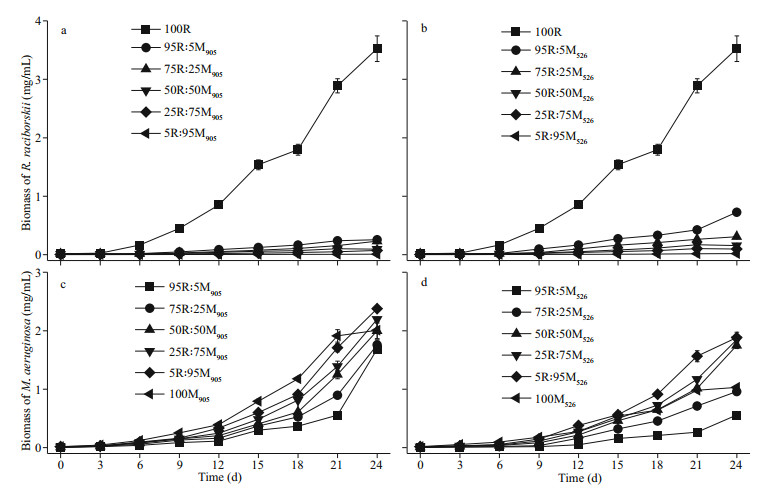
The average specific growth rate of R. raciborskii in the co-cultures was suppressed by both strains of M. aeruginosa (Fig. 2a–b). The inhibition of R. raciborskii growth exerted by the MCs-producing M. aeruginosa strain was stronger than that exerted by the non-MCs-producing strain at all initial biomass ratios tested (Fig. 2a). At the end of the cultivation, the average specific growth rate of R. raciborskii in the monoculture was 0.22/d, but the specific growth rates of R. raciborskii in the co-cultures with initial R. raciborskii to MCs-producing M. aeruginosa biomass ratios of 95R: 5M, 75R: 25M, 50R: 50M, 25R: 75M and 5R: 95M decreased to 0.12, 0.12, 0.10, 0.12, and 0.10/d, respectively (Fig. 2a). Similarly, a decrease in the specific growth rate of R. raciborskii was also evident when it was co-cultured with the non-MCs-producing M. aeruginosa strain, reaching 0.16, 0.13, 0.12, 0.13, and 0.13/d, respectively when the biomass ratios were 95R: 5M, 75R: 25M, 50R: 50M, 25R: 75M, and 5R: 95M (Fig. 2a). However, the average specific growth rates of the MCs-producing and non-MCs-producing M. aeruginosa strains in the co-cultures were significantly (P < 0.01) higher than those in the monocultures (Fig. 2b). The specific growth rates of M. aeruginosa strain at initial biomass ratios of 95R: 5M, 75R: 25M, 50R: 50M, 25R: 75M and 5R: 95M were 0.32, 0.25, 0.23, 0.22, and 0.21/d, respectively, in the case of the MCs-producing strain, and were 0.27, 0.23, 0.22, 0.21, and 0.20/d, respectively, for the non-MCs-producing strain (Fig. 2b).
|
| Fig.2 Specific growth rates of R. raciborskii and M. aeruginosa co-cultivated at different initial biomass ratios a. specific growth rates of R. raciborskii co-cultivated with MCs-producing or non-MCs-producing M. aeruginosa at different initial biomass ratios; b. specific growth rates of MCs-producing and non-MCs-producing M. aeruginosa co-cultivated with R. raciborskii at different initial biomass ratios. Data are the means standard errors from triplicate determinations. The lower case letters a and b indicate significant differences at the P < 0.01 level. MA905, MA526, and RR3438 stand for M. aeruginosa905, M. aeruginosa526, and R. raciborskii 3438. |
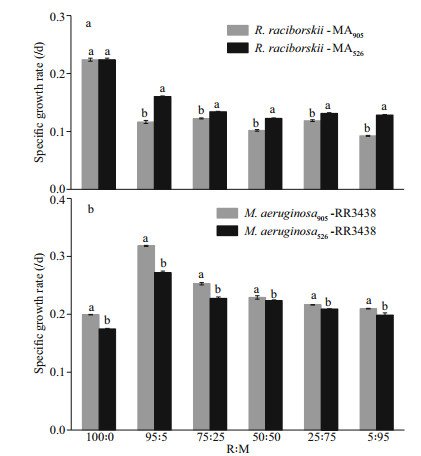
To visually show the dominating competitiveness of the microalgae during the co-cultivation, the contribution of each algal biomass to the total biomass during the entire co-cultivation period was determined. Overall, the contribution of R. raciborskii to the total biomass was positively correlated with its initial biomass in the culture, but such contribution decreased with prolonged cultivation time, with the decrease occurring at a faster rate when the co-cultivated M. aeruginosa strain was the MCs-producing strain (Fig. 3a–e). Correspondingly, the contribution of M. aeruginosa to the total biomass also increased with prolonged cultivation time and the contribution of the MCs-producing strain exceeded that of the non-MCs-producing strain. At the end of the cultivation, the biomass contribution of either M. aeruginosa strain was significantly higher (P < 0.01) than that of R. raciborskii, except in the culture where the initial biomass of R. raciborskii was 95% of the total initial biomass and that R. raciborskii was co-cultured with the non-MCs-producing strain of M. aeruginosa (Fig. 3a). For the co-cultures with initial biomass ratios of 95R꞉5M, 75R꞉25M, 50R꞉50M, 25R꞉75M, and 5R꞉95M, the biomass contribution of R. raciborskii decreased by 84.94%, 88.36 %, 95.49 %, 96.90 %, and 99.97 %, respectively, when MCs-producing M. aeruginosa was the cocultured strain, or by 43.39 %, 75.76 %, 91.85 %, 95.09 %, and 99.91 %, respectively, when non-MCs-producing M. aeruginosa was the cocultured strain (Fig. 3a–e). The results suggested that loss in biomass contribution by R. raciborskii was less severe when it was co-cultured with the non-MCs-producing M. aeruginosa strain, and in cultures with higher initial R. raciboskii biomass than those with higher initial M. aeruginosa biomass.
|
| Fig.3 Contribution of R. raciborskii and the two M. aeruginosa strains to the total biomass in the co-cultures at different initial biomass ratios and incubation times a. 95R: 5M; b. 75R: 25M; c. 50R: 50M; d. 25R: 75M; e. 5R: 95M. Data are the means standard errors from triplicate determinations. |
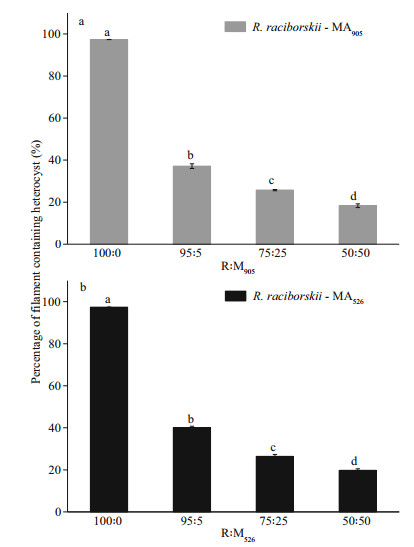
The morphological changes in R. raciborskii and M. aeruginosa that have been co-cultivated for 24 days are shown in Fig. 4. Changes in the morphological parameters of M. aeruginosa were ignored in the present study because no visible morphological changes were found. For the monoculture of R. raciborskii, the filaments contained heterocysts (Fig. 4a & g), but an obvious loss of heterocysts in the filaments occurred when the strain was co-cultured with the MCs-producing strain of M. aeruginosa (Fig. 4b–f), and the percent of heterocyst-containing filaments also decreased with increased initial biomass ratios of MCs-producing M. aeruginosa to R. raciborskii (Fig. 5a). The loss of heterocyst in R. raciborskii filaments also occurred when the strain was co-cultured with the non-MCs-producing M. aeruginosa strain (Fig. 4h–l), but the loss occurred at a lower rate (Fig. 5b). For the mono-cultured R. raciborskii, the heterocyst-containing filaments accounted for 97.36% of the total number of filaments. However, when R. raciborskii was co-cultivated with the MCs-producing M. aeruginosa strain at initial biomass ratios of 95R: 5M, 75R: 25M, and 50R: 50M, the percentage of heterocyst-containing filaments decreased to 37.13 %, 25.75% and 18.30%, respectively (Fig. 5a). As for R. raciborskii co-cultivated with the non-MCs-producing M. aeruginosa, this percentage decreased to 40.17 %, 26.40 %, and 19.77 %, respectively (Fig. 5b).

|
| Fig.4 Morphological characteristics of the filaments of R. raciborskii co-cultivated with M. aeruginosa Raphidiopsis raciborskii was co-cultivated with MCs-producing (a–f) and non-MCs-producing (g–l) M. aeruginosa strains for 24 days at different initial biomass ratios. a and g: monocultured R. raciborskii filaments; b–f: R. raciborskii filaments co-cultivated with MCs-producing M. aeruginosa strain at initial biomass ratios of 95R: 5M (b), 75R: 25M (c), 50R: 50M (d), 25R: 75M (e), and 5R: 95M (f); h–l: R. raciborskii filaments co-cultivated with non-MCs-producing M. aeruginosa strain at the corresponding biomass density ratios as in b–f. Scale bars: 10 μm. |
|
| Fig.5 Percentage of heterocyst-containing R. raciborskii filaments co-cultivated with M. aeruginosa Raphidiopsis raciborskii was co-cultivated with MCs-producing (a) and non-MCs-producing (b) M. aeruginosa strains at different initial biomass ratios for 24 days. Data are the means±standard errors based on 1 000 randomly chosen filaments. The lower case letters indicate significant differences at the P < 0.05 level. |
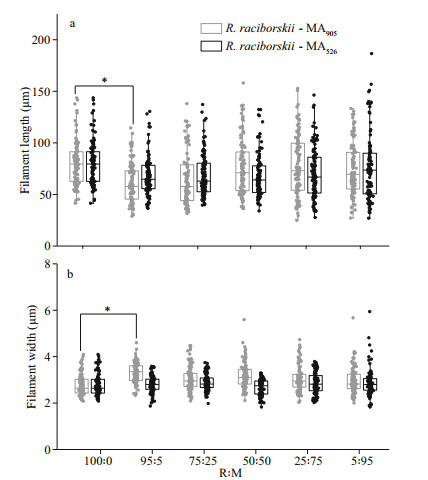
In addition to the changes in the heterocysts of the filaments, the average length and width of the filaments of R. raciborskii co-cultivated with the MCs-producing M. aeruginosa strain also decreased significantly (P < 0.05) when the initial biomass ratio was 95R: 5M (Fig. 6a–b). In contrast, no significant difference in the average length and width of the filaments were detected when R. raciborskii was co-cultivated with the non-MCs-producing M. aeruginosa strain regardless of the initial biomass ratios of the cultures and incubation time.
|
| Fig.6 Changes in filament length (a) and width (b) of R. raciborskii co-cultivated with M. aeruginosa at different initial biomass ratios for 24 days Data are the means±standard errors based on 1 000 randomly chosen filaments. Asterisk indicates a significant difference at the P < 0.05 level. |
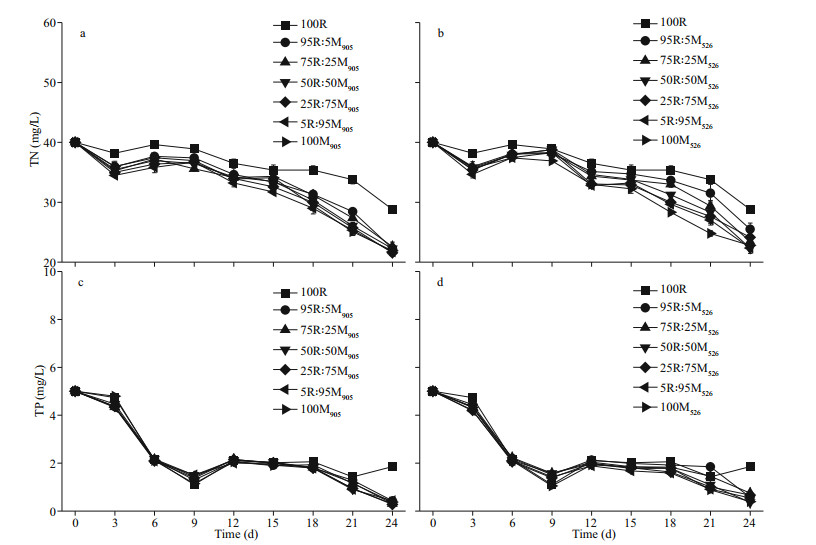
Changes in TN and TP in the media of both R. raciborskii monoculture and R. raciborskii-M. aeruginosa co-culture showed a gradually decreasing trend with prolonged incubation (Fig. 7), but the rate of decrease for TN was slower in the case of the monoculture (Fig. 7a–b). In addition, the rates of decrease for TN were similar in the co-cultures containing either the MCs-producing or non-MCs-producing M. aeruginosa strain with no significant difference between the two cultures regardless of incubation time. As for TP, its concentration in both the monoculture and co-culture decreased sharply from the point of inoculation to Day 9, followed by a more gradual decrease that also fluctuated slightly among the different set of cultures (Fig. 7c–d). Unlike the change in TN, the TP concentration in both R. raciborskii monoculture and R. raciborskii-M. aeruginosa co-culture displayed almost identical trends, with no significant difference between the two cultures regardless of incubation time and the initial biomass ratios in the case of the co-culture (Fig. 7c–d).
|
| Fig.7 Changes in total soluble nitrogen (TN) and total soluble phosphorus (TP) concentrations in R. raciborskii-M. aeruginosa co-cultures at different initial biomass ratios and incubation times a and c: R. raciborskii co-cultured with MCs-producing M. aeruginosa strain; b and d: R. raciborskii co-cultivated with non-MCs-producing M. aeruginosa strain. Data are the means standard errors from triplicate determinations. |
Co-cultivation is considered to be a good method for studying the allelopathic interaction between two different phytoplankton species because direct evidence for allelopathic interactions under natural aquatic conditions is difficult to provide owing to the interferences from other processes (Sukenik et al., 2002; Legrand et al., 2003; Ma et al., 2015; Dunker et al., 2017; Chia et al., 2018). Through co-cultivation, we have shown in the present study that the growth rates of R. raciborskii could be inhibited by both MCs-producing and non-MCs-producing M. aeruginosa strains (Figs. 1a–b & 2a). In addition, the inhibition of R. raciborskii growth exerted by the MCs-producing M. aeruginosa strain was greater than that exerted by the non-MCs-producing one (Figs. 1a, b, & 2a). This is in accordance with the reported allelopathic effects of M. aeruginosa on several other species of green algae and dinoflagellates (Sukenik et al., 2002; Bittencourt-Oliveira et al., 2015; Ma et al., 2015; Yang et al., 2018a; Omidi et al., 2019). MCs-producing M. aeruginosa strains can suppress the growth and photosynthesis of their competitors by producing MCs as well as other odor substances (Pietsch et al., 2001; Singh et al., 2001; Pflugmacher, 2002; Hu et al., 2004; Babica et al., 2007; Wang et al., 2017), which give them an advantage in such competition (Ma et al., 2015; Wang et al., 2017).
MCs are allelochemicals that can induce suppression of the growth of cyanobacteria and eukaryotic algae (Sukenik et al., 2002; Qian et al., 2009; Tan et al., 2019). The mechanism of growth suppression is rather diverse, and it includes reducing carbon dioxide fixation and nitrogen fixation activities (Singh et al., 2001), obstructing electron transport by specifically binding to relevant sites in photosystem II (PSII) (Keating, 1999), hindering adenosine triphosphate (ATP) synthesis (Zhu et al., 2010), abolishing intracellular carbonic anhydrase activity (Sukenik et al., 2002), and causing oxidative damage (Qian et al., 2009). Besides the negative allelopathy, a recent study has found that M. aeruginosa can inhibit the growth of Chlorella vulgaris via the release of linoleic acid (Song et al., 2017).
However, for M. aeruginosa strains, co-cultivation with R. raciborskii stimulated their growth rates and the stimulating effects decreased with increased proportions of initial biomass of R. raciborskii in the co-cultures (Figs. 1c–d & 2b). In addition, under such co-cultivation, the toxic M. aeruginosa strain achieved a higher growth rate than the non-toxic one (Fig. 2b). This is also supported by other previous experimental studies. For example, environmentally relevant concentrations of CYN were found to have no effect on the growth of the cyanobacteria Nannochloropsis sp. and Chlamydomonas reinhardti (Pinheiro et al., 2013), whereas CYN was found to stimulate the growth of the green alga C. vulgaris despite its relatively high concentration, up to 179 μg/L (Campos et al., 2013). Taken together, these results may suggest that M. aeruginosa, similar to other microalgae, can cope with CYN-induced oxidative stress through increasing the activity of antioxidant enzymes (Campos et al., 2013). Furthermore, the biomass contribution of the MCs-producing or non-MCs-producing M. aeruginosa strain to the total biomass increased regardless of the initial R. raciborskii to M. aeruginosa biomass ratios, and a ratio of 5R: 95M resulted in the complete elimination of R. raciborskii from the co-culture on the 9th day and 21th day for the MCs-producing and non-MCs-producing M. aeruginosa strains, respectively (Fig. 3e). According to the trend of biomass contribution by each algal strain to the total biomass during the entire co-cultivation period (Fig. 3a–e), it could be inferred that with further incubation time, both the MCs-producing and non-MCs-producing M. aeruginosa strains could eventually exclude R. raciborskii completely regardless of the initial biomass ratios, but the non-MCs-producing strain would need a longer incubation to completely replace R. raciborskii.
Raphidiopsis species have been reported to exert stronger inhibitory effects than pure CYN on the growth of green algae and cyanobacteria (Campos et al., 2013; Rzymski et al., 2014). Raphidiopsis raciborskii FACHB-3438 is a CYN-producing species (Jiang et al., 2014). However, the higher growth rates of both MCs-producing and non-MCs-producing M. aeruginosa strains observed when they were co-cultivated with R. raciborskii FACHB-3438 (Fig. 2b) suggested that CYN could stimulate the growth of M. aeruginosa. Furthermore, it could also mean that the MCs-producing species are more likely to gain a competitive advantage over their competitors than the CYN-producing species.
Under natural conditions, most of the R. raciborskii filaments contain heterocysts needed for nitrogen fixation, a function that gives this alga an advantage in the competition with non-nitrogen-fixing algae, especially in N-deficient environments (Moisander et al., 2012; Yang et al., 2017). In the present study, the formation of heterocysts in R. raciborskii filaments was inhibited by M. aeruginosa (Figs. 4–5), and the inhibitory effect increased with an increased proportion of M. aeruginosa biomass in the co-culture. In addition, co-cultivation with the MCs-producing M. aeruginosa strain led to a greater loss of heterocysts than co-cultivation with the non-MCs-producing one (Fig. 5a–b). To our knowledge, this is the first report to mention the disappearance of heterocysts in cyanobacterial filaments caused by allelopathy although the physiological mechanism is unclear. A previous study has reported that M. aeruginosa can exert an inhibitory effect on the differentiation of heterocysts in the filamentous cyanobacterium Trichormus variabilis through an unknown mechanism (Bártová et al., 2011). In addition, co-culture with M. aeruginosa can also result in the growth reduction of Planktothrix (Briand et al., 2019). In either case, the molecular mechanism responsible for the effect has not been clarified. However, the disappearance of heterocysts in the R. raciborskii filaments and the morphological changes observed for the filaments could be an adaptation feature resulting from the pressure brought by the presence of M. aeruginosa in the culture. This could increase the biotic stress for R. raciborskii, although the direct effects on the competition for dominance between R. raciborskii and M. aeruginosa remain unclear. Therefore, a further study involving the use of multiple environmental factors and multiple technical methods should be carried out to reveal the precise effects of the extracellular microenvironment on the morphological changes of R. raciborskii filaments and the underlying molecular mechanisms.
The competition for dominance between the two algae is affected by many factors, such as temperature (Ma et al., 2015; Yang et al., 2018a), nutrient levels (Marinho et al., 2013; Chia et al., 2018), algae species even strains of the competitors (Sukenik et al., 2002; Ma et al., 2015; Wang et al., 2017). For example, in the co-cultivation of Scendesmus obliquus with M. aeruginosa, S. obliquus is more competitive at 15 ℃, the two species are equally competitive at 20–30 ℃, but M. aeruginosa is more competitive at 35 ℃ (Yang et al., 2018a). Furthermore, studies have shown that R. raciborskii is able to utilize a range of nitrogen sources such as ammonium, nitrate, and urea with a clear preference for ammonium (Burford et al., 2006; Stucken et al., 2014), thus appears to
gain competitive advantage under low and fluctuating dissolved nitrogen concentrations, in line with the term "facultative diazotroph" coined by Moisander et al. (2012). In addition, R. raciborskii has high dissolved inorganic phosphorus uptake rates and storage capacity relative to a range of other cyanobacterial species (Isvánovics et al., 2000; Prentice et al., 2015). Consistent with this, R. raciborskii can dominate in a range of phosphorus levels (Chislock et al., 2014). In this study, the changes in the concentrations of TN and TP in the co-cultures displayed a similar trend without any significant differences among the different sets of cultures regardless of the initial biomass ratios and incubation time (Fig. 7a–d). This may be due to the demand for nitrogen and phosphorus by the two algae being completely satisfied throughout the incubation period. Therefore, the different effects that the MCs-producing and non-MCs-producing M. aeruginosa strains had on R. raciborskii as shown in the present study, were unlikely to be caused by variations in nutrients in co-cultivations.
5 CONCLUSIONIn the co-cultivation of R. raciborskii with M. aeruginosa at different biomass ratios, the growth of R. raciborskii was inhibited by both MCs-producing and non-MCs-producing M. aeruginosa strains, with the former exerting a stronger inhibition than the latter. On the other hand, co-cultivation of M. aeruginosa with R. raciborskii stimulated the growth of the M. aeruginosa. Therefore, in the co-cultivation, R. raciborskii could be completely eliminated by M. aeruginosa even when R. raciborskii was present in dominating proportion at the start of the cultivation (R: M=95:5 in biomass ratio), and during the cultivation, the MCs-producing M. aeruginosa strain took less time to replace R. raciborskii than the non-MCs-producing strain. Furthermore, the co-cultivation of R. raciborskii with M. aeruginosa led to the disappearance of heterocysts containing in filaments of R. raciborskii. Compared with the non-MCs-producing M. aeruginosa strain, the MCs-producing strain exerted a more potent inhibitory effect on R. raciborskii, thereby, enabling it to acquire stronger competitiveness for dominance These results indicated that it is difficult for R. raciborskii to replace the dominant position of M. aeruginosa in the same natural water if based purely on the allelopathy between the two species. This study has provided important evidence pertaining to the competition for dominance between R. raciborskii and M. aeruginosa, and essentially ruled out the possibility that R. raciborskii could replace the dominant position of M. aeruginosa in natural waters.
6 DATA AVAILABILITY STATEMENTThe datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENTThe authors highly appreciate Dr Alan K. Chang (Wenzhou University) for his effort in the revision of the manuscript's language, and the anonymous reviewers for their very useful and insightful comments.
Amaral V, Bonilla S, Aubriot L. 2014. Growth optimization of the invasive cyanobacterium Cylindrospermopsis raciborskii in response to phosphate fluctuations. European Journal of Phycology, 49(1): 134-141.
DOI:10.1080/09670262.2014.897760 |
Amorim C A, Moura A D N. 2021. Ecological impacts of freshwater algal blooms on water quality, plankton biodiversity, structure, and ecosystem functioning. Science of the Total Environment, 758: 143605.
DOI:10.1016/j.scitotenv.2020.143605 |
Babica P, Hilscherová K, Bártová K, et al. 2007. Effects of dissolved microcystins on growth of planktonic photoautotrophs. Phycologia, 46(2): 137-142.
DOI:10.2216/06-24.1 |
Bai F, Shi J Q, Yang S Q, et al. 2020. Interspecific competition between Cylindrospermopsis raciborskii and Microcystis aeruginosa on different phosphorus substrates. Environmental Science and Pollution Research, 27: 42264-42275.
DOI:10.1007/s11356-020-08652-0 |
Bártová K, Hilscherová K, Babica P, et al. 2011. Extract of Microcystis water bloom affects cellular differentiation in filamentous cyanobacterium Trichormus variabilis (Nostocales, Cyanobacteria). Journal of Applied Phycology, 23(6): 967-973.
DOI:10.1007/s10811-010-9624-5 |
Bar-Yosef Y, Sukenik A, Hadas O, et al. 2010. Enslavement in the water body by toxic Aphanizomenon ovalisporum, inducing alkaline phosphatase in Phytoplanktons. Current Biology, 20(17): 1557-1561.
DOI:10.1016/j.cub.2010.07.032 |
Bittencourt-Oliveira M D C, Chia M A, de Oliveira H S B, et al. 2015. Allelopathic interactions between microcystin-producing and non-microcystin-producing cyanobacteria and green microalgae: implications for microcystins production. Journal of Applied Phycology, 27(1): 275-284.
DOI:10.1007/s10811-014-0326-2 |
Briand E, Reubrecht S, Mondeguer F, et al. 2019. Chemically mediated interactions between Microcystis and Planktothrix: impact on their growth, morphology and metabolic profiles. Environmental Microbiology, 21(5): 1552-1566.
DOI:10.1111/1462-2920.14490 |
Briand J F, Leboulanger C, Humbert J F, et al. 2004. Cylindrospermopsis raciborskii (Cyanobacteria) invasion at mid-latitudes: selection, wide physiological tolerance, orglobalwarming?. Journal of Phycology, 40(2): 231-238.
DOI:10.1111/j.1529-8817.2004.03118.x |
Burford M A, Beardall J, Willis A, et al. 2016. Understanding the winning strategies used by the bloom-forming cyanobacterium Cylindrospermopsis raciborskii. Harmful Algae, 54: 44-53.
DOI:10.1016/j.hal.2015.10.012 |
Burford M A, Davis T W, Orr P T, et al. 2014. Nutrient-related changes in the toxicity of field blooms of the cyanobacterium Cylindrospermopsis raciborskii. FEMS Microbiology Ecology, 89(1): 135-148.
DOI:10.1111/1574-6941.12341 |
Burford M A, McNeale K L, McKenzie-Smith F J. 2006. The role of nitrogen in promoting the toxic cyanophyte Cylindrospermopsis raciborskii in a subtropical water reservoir. Freshwater Biology, 51(11): 2143-2153.
DOI:10.1111/j.1365-2427.2006.01630.x |
Burford M A, Willis A, Chuang A, et al. 2018. Recent insights into physiological responses to nutrients by the cylindrospermopsin producing cyanobacterium, Cylindrospermopsis raciborskii. Journal of Oceanology and Limnology, 36(4): 1032-1039.
DOI:10.1007/s00343-018-7179-5 |
Campos A, Araújo P, Pinheiro C, et al. 2013. Effects on growth, antioxidant enzyme activity and levels of extracellular proteins in the green alga Chlorella vulgaris exposed to crude cyanobacterial extracts and pure microcystin and cylindrospermopsin. Ecotoxicology and Environmental Safety, 94: 45-53.
DOI:10.1016/j.ecoenv.2013.04.019 |
Chesson P. 2000. Mechanisms of maintenance of species diversity. Annual Review of Ecology and Systematics, 31: 343-366.
DOI:10.1146/annurev.ecolsys.31.1.343 |
Chia M A, Jankowiak J G, Kramer B J, et al. 2018. Succession and toxicity of Microcystis and Anabaena (Dolichospermum) blooms are controlled by nutrient-dependent allelopathic interactions. Harmful Algae, 74: 67-77.
DOI:10.1016/j.hal.2018.03.002 |
Chislock M F, Sharp K L, Wilson A E. 2014. Cylindrospermopsis raciborskii dominates under very low and high nitrogen-to-phosphorus ratios. Water Research, 49: 207-214.
DOI:10.1016/j.watres.2013.11.022 |
Chonudomkul D, Yongmanitchai W, Theeragool G, et al. 2004. Morphology, genetic diversity, temperature tolerance and toxicity of Cylindrospermopsis raciborskii (Nostocales, Cyanobacteria) strains from Thailand and Japan. FEMS Microbiology Ecology, 48(3): 345-355.
DOI:10.1016/j.femsec.2004.02.014 |
da Silva Brito M T, Duarte-Neto P J, Molica R J R. 2018. Cylindrospermopsis raciborskii and Microcystis aeruginosa competing under different conditions of pH and inorganic carbon. Hydrobiologia, 815(1): 253-266.
DOI:10.1007/s10750-018-3567-2 |
Dittmann E, Wiegand C. 2006. Cyanobacterial toxins—occurrence, biosynthesis and impact on human affairs. Molecular Nutrition & Food Research, 50(1): 7-17.
DOI:10.1002/mnfr.200500162 |
Dokulil M T, Teubner K. 2000. Cyanobacterial dominance in lakes. Hydrobiologia, 438(1–3): 1-12.
DOI:10.1023/A:1004155810302 |
Duan H T, Ma R H, Xu X F, et al. 2009. Two-decade reconstruction of algal blooms in China's Lake Taihu. Environmental Science & Technology, 43(10): 3522-3528.
DOI:10.1021/es8031852 |
Dunker S, Althammer J, Pohnert G, et al. 2017. A fateful meeting of two phytoplankton species—chemical vs. cell-cell-interactions in co-cultures of the green algae Oocystis marsonii and the cyanobacterium Microcystis aeruginosa. Microbial Ecology, 74(1): 22-32.
DOI:10.1007/s00248-016-0927-1 |
Gallego I, Venail P, Ibelings B W. 2019. Size differences predict niche and relative fitness differences between phytoplankton species but not their coexistence. The ISME Journal, 13(5): 1133-1143.
DOI:10.1038/s41396-018-0330-7 |
Gugger M, Molica R, Le Berre B, et al. 2005. Genetic diversity of Cylindrospermopsis strains (cyanobacteria) isolated from four continents. Applied and Environmental Microbiology, 71(2): 1097-1100.
DOI:10.1128/AEM.71.2.1097-1100.2005 |
Hamilton P B, Ley L M, Dean S, et al. 2005. The occurrence of the cyanobacterium Cylindrospermopsis raciborskii in Constance Lake: an exotic cyanoprokaryote new to Canada. Phycologia, 44(1): 17-25.
|
Harke M J, Steffen M M, Gobler C J, et al. 2016. A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp. Harmful Algae, 54: 4-20.
DOI:10.1016/j.hal.2015.12.007 |
Hillebrand H, Dürselen C D, Kirschtel D, et al. 1999. Biovolume calculation for pelagic and benthic microalgae. Journal of Phycology, 35(2): 403-424.
DOI:10.1046/j.1529-8817.1999.3520403.x |
Hoeger S J, Shaw G, Hitzfeld B C, et al. 2004. Occurrence and elimination of cyanobacterial toxins in two Australian drinking water treatment plants. Toxicon, 43(6): 639-649.
DOI:10.1016/j.toxicon.2004.02.019 |
Hu Z Q, Liu Y D, Li D H. 2004. Physiological and biochemical analyses of microcystin-RR toxicity to the cyanobacterium Synechococcus elongatus. Environmental Toxicology, 19(6): 571-577.
DOI:10.1002/tox.20064 |
Huisman J, Codd G A, Paerl H W, et al. 2018. Cyanobacterial blooms. Nature Reviews Microbiology, 16(8): 471-483.
DOI:10.1038/s41579-018-0040-1 |
Isvánovics V, Shafik H M, Presing M, et al. 2000. Growth and phosphate uptake kinetics of the cyanobacterium, Cylindrospermopsis raciborskii (Cyanophyceae) in throughflow cultures. Freshwater Biology, 43(2): 257-275.
DOI:10.1046/j.1365-2427.2000.00549.x |
Jia N N, Yang Y M, Yu G L, et al. 2020. Interspecific competition reveals Raphidiopsis raciborskii as a more successful invader than Microcystis aeruginosa. Harmful Algae, 97: 101858.
DOI:10.1016/j.hal.2020.101858 |
Jiang Y G, Xiao P, Yu G L, et al. 2014. Sporadic distribution and distinctive variations of cylindrospermopsin genes in cyanobacterial strains and environmental samples from Chinese freshwater bodies. Applied and Environmental Microbiology, 80(17): 5219-5230.
DOI:10.1128/aem.00551-14 |
Kaur S, Srivastava A, Kumar S, et al. 2019. Biochemical and proteomic analysis reveals oxidative stress tolerance strategies of Scenedesmus abundans against allelochemicals released by Microcystis aeruginosa. Algal Research, 41: 101525.
DOI:10.1016/j.algal.2019.101525 |
Keating K I. 1999. Allelochemistry in plankton communities. In: Inderjit, Dakshibi K M M, Foy C L eds. Principles and Practices in Plant Ecology: Allelochemical Interactions. CRC, Boca Raton. p. 165–178.
|
Kim M, Lee J, Yang D, et al. 2020. Seasonal dynamics of the bacterial communities associated with cyanobacterial blooms in the Han River. Environmental Pollution, 266: 115198.
DOI:10.1016/j.envpol.2020.115198 |
Kovács A W, Présing M, Vörös L. 2016. Thermal-dependent growth characteristics for Cylindrospermopsis raciborskii (Cyanoprokaryota) at different light availabilities: methodological considerations. Aquatic Ecology, 50(4): 623-638.
DOI:10.1007/s10452-016-9582-3 |
Legrand C, Rengefors K, Fistarol G O, et al. 2003. Allelopathy in phytoplankton — biochemical, ecological and evolutionary aspects. Phycologia, 42(4): 406-419.
DOI:10.2216/i0031-8884-42-4-406.1 |
Lei L M, Huang H T, Peng L, et al. 2020. Life-history responses of Daphnia sinensis simultaneously exposed to Microcystis aeruginosa and Cylindrospermopsis raciborskii. Ecotoxicology, 29(6): 771-779.
DOI:10.1007/s10646-020-02220-5 |
Lei L M, Li C L, Peng L, et al. 2015. Competition between toxic and non-toxic Microcystis aeruginosa and its ecological implication. Ecotoxicology, 24(7): 1411-1418.
DOI:10.1007/s10646-015-1456-2 |
Li Y X, Li D H. 2012. Competition between toxic Microcystis aeruginosa and nontoxic Microcystis wesenbergii with Anabaena PCC7120. Journal of Applied Phycology, 24(1): 69-78.
DOI:10.1007/s10811-010-9648-x |
Ma Z L, Fang T X, Thring R W, et al. 2015. Toxic and non-toxic strains of Microcystis aeruginosa induce temperature dependent allelopathy toward growth and photosynthesis of Chlorella vulgaris. Harmful Algae, 48: 21-29.
DOI:10.1016/j.hal.2015.07.002 |
MacDougall A S, Gilbert B, Levine J M. 2009. Plant invasions and the niche. Journal of Ecology, 97(4): 609-615.
DOI:10.1111/j.1365-2745.2009.01514.x |
Mallon C A, van Elsas J D, Salles J F. 2015. Microbial invasions: the process, patterns, and mechanisms. Trends in Microbiology, 23(11): 719-729.
DOI:10.1016/j.tim.2015.07.013 |
Mantzouki E, Visser P M, Bormans M, et al. 2016. Understanding the key ecological traits of cyanobacteria as a basis for their management and control in changing lakes. Aquatic Ecology, 50(3): 333-350.
DOI:10.1007/s10452-015-9526-3 |
Marinho M M, Souza M B G, Lürling M. 2013. Light and phosphate competition between Cylindrospermopsis raciborskii and Microcystis aeruginosa is strain dependent. Microbial Ecology, 66(3): 479-488.
DOI:10.1007/s00248-013-0232-1 |
Moisander P H, Cheshire L A, Braddy J, et al. 2012. Facultative diazotrophy increases Cylindrospermopsis raciborskii competitiveness under fluctuating nitrogen availability. FEMS Microbiology Ecology, 79(3): 800-811.
DOI:10.1111/j.1574-6941.2011.01264.x |
Moreira C, Fathalli A, Vasconcelos V, et al. 2015. Phylogeny and biogeography of the invasive cyanobacterium Cylindrospermopsis raciborskii. Archives of Microbiology, 197(1): 47-52.
DOI:10.1007/s00203-014-1052-5 |
Narwani A, Alexandrou M A, Oakley T H, et al. 2013. Experimental evidence that evolutionary relatedness does not affect the ecological mechanisms of coexistence in freshwater green algae. Ecology Letters, 16(11): 1373-1381.
DOI:10.1111/ele.12182 |
Nixdorf B, Mischke U, Rücker J. 2003. Phytoplankton assemblages and steady state in deep and shallow eutrophic lakes — an approach to differentiate the habitat properties of Oscillatoriales. Hydrobiologia, 502(1–3): 111-121.
DOI:10.1023/B:HYDR.0000004274.65831.e5 |
Olokotum M, Mitroi V, Troussellier M, et al. 2020. A review of the socioecological causes and consequences of cyanobacterial blooms in Lake Victoria. Harmful Algae, 96: 101829.
DOI:10.1016/j.hal.2020.101829 |
Omidi A, Esterhuizen-Londt M, Pflugmacher P. 2019. Desmodesmus subspicatus co-cultured with microcystin producing (PCC 7806) and the non-producing (PCC 7005) strains of Microcystis aeruginosa. Ecotoxicology, 28(7): 834-842.
DOI:10.1007/s10646-019-02082-6 |
Orr P T, Jones G J. 1998. Relationship between microcystin production and cell division rates in nitrogen-limited Microcystis aeruginosa cultures. Limnology and Oceanography, 43(7): 1604-1614.
DOI:10.4319/lo.1998.43.7.1604 |
Padisák J, Reynolds C S. 1998. Selection of phytoplankton associations in Lake Balaton, Hungary, in response to eutrophication and restoration measures, with special reference to the cyanoprokaryotes. Hydrobiologia, 384(1–3): 41-53.
DOI:10.1023/A:1003255529403 |
Paerl H W, Hall N S, Calandrino E S. 2011. Controlling harmful cyanobacterial blooms in a world experiencing anthropogenic and climatic-induced change. Science of the Total Environment, 409(10): 1739-1745.
DOI:10.1016/j.scitotenv.2011.02.001 |
Paerl H W. 2018. Mitigating toxic planktonic cyanobacterial blooms in aquatic ecosystems facing increasing anthropogenic and climatic pressures. Toxins, 10(2): 76.
DOI:10.3390/toxins10020076 |
Pflugmacher S. 2002. Possible allelopathic effects of cyanotoxins, with reference to microcystin-LR, in aquatic ecosystems. Environmental Toxicology, 17(4): 407-413.
DOI:10.1002/tox.10071 |
Pierangelini M, Sinha R, Willis A, et al. 2015a. Constitutive cylindrospermopsin pool size in Cylindrospermopsis raciborskii under different light and CO2 partial pressure conditions. Applied and Environmental Microbiology, 81(9): 3069-3076.
DOI:10.1128/AEM.03556-14 |
Pierangelini M, Stojkovic S, Orr P T, et al. 2014a. Photosynthetic characteristics of two Cylindrospermopsis raciborskii strains differing in their toxicity. Journal of Phycology, 50(2): 292-302.
DOI:10.1111/jpy.12157 |
Pierangelini M, Stojkovic S, Orr P T, et al. 2014b. Elevated CO2 causes changes in the photosynthetic apparatus of a toxic cyanobacterium, Cylindrospermopsis raciborskii. Journal of Plant Physiology, 171(12): 1091-1098.
DOI:10.1016/j.jplph.2014.04.003 |
Pierangelini M, Stojkovic S, Orr P T, et al. 2015b. Photo, acclimation to low light—Changes from growth to antenna size in the cyanobacterium Cylindrospermopsis raciborskii. Harmful Algae, 46: 11-17.
DOI:10.1016/j.hal.2015.04.004 |
Pietsch C, Wiegand C, Amé M V, et al. 2001. The effects of a cyanobacterial crude extract on different aquatic organisms: evidence for cyanobacterial toxin modulating factors. Environmental Toxicology, 16(6): 535-542.
DOI:10.1002/tox.10014 |
Pinheiro C, Azevedo J, Campos A, et al. 2013. Absence of negative allelopathic effects of cylindrospermopsin and microcystin-LR on selected marine and freshwater phytoplankton species. Hydrobiologia, 705(1): 27-42.
DOI:10.1007/s10750-012-1372-x |
Plaas H E, Paerl H W. 2021. Toxic cyanobacteria: a growing threat to water and air quality. Environmental Science & Technology, 55(1): 44-64.
DOI:10.1021/acs.est.0c06653 |
Plominsky Á M, Larsson J, Bergman B, et al. 2013. Dinitrogen fixation is restricted to the terminal heterocysts in the invasive cyanobacterium Cylindrospermopsis raciborskii CS-505. PLoS One, 8(2): e51682.
DOI:10.1371/journal.pone.0051682 |
Prentice M J, O'Brien K R, Hamilton D P, et al. 2015. Highland low-affinity phosphate uptake and its effect on phytoplankton dominance in a phosphate-depauperate lake. Aquatic Microbial Ecology, 75(2): 139-153.
DOI:10.3354/ame01751 |
Qian H F, Xu X Y, Chen W, et al. 2009. Allelochemical stress causes oxidative damage and inhibition of photosynthesis in Chlorella vulgaris. Chemosphere, 75(3): 368-375.
DOI:10.1016/jxhemosphere.2008.12.040 |
Rzymski P, Poniedzialek B, Kokociński M, et al. 2014. Interspecific allelopathy in cyanobacteria: Cylindrospermopsin and Cylindrospermopsis raciborskii effect on the growth and metabolism of Microcystis aeruginosa. Harmful Algae, 35: 1-8.
DOI:10.1016/j.hal.2014.03.002 |
Rzymski P, Poniedzialek B. 2014. In search of environmental role of cylindrospermopsin: a review on global distribution and ecology of its producers. Water Research, 66: 320-337.
DOI:10.1016/j.watres.2014.08.029 |
Saker M L, Griffiths D J. 2001. Occurrence of blooms of the cyanobacterium Cylindrospermopsis raciborskii (Woloszyñska) Seenayya and Subba Raju in a north Queensland domestic water supply. Marine and Freshwater Research, 52(6): 907-915.
DOI:10.1071/MF00110 |
Singh D P, Tyagi M B, Kumar A, et al. 2001. Antialgal activity of a hepatotoxin-producing cyanobacterium, Microcystis aeruginosa. World Journal of Microbiology and Biotechnology, 17(1): 15-22.
DOI:10.1023/A:1016622414140 |
Soares M C S, Lürling M, Huszar V L M. 2013. Growth and temperature-related phenotypic plasticity in the cyanobacterium Cylindrospermopsis raciborskii. Phycological Research, 61(1): 61-67.
DOI:10.1111/pre.12001 |
Song H, Lavoie M, Fan X J, et al. 2017. Allelopathic interactions of linoleic acid and nitric oxide increase the competitive ability of Microcystis aeruginosa. The ISME Journal, 11(8): 1865-1876.
DOI:10.1038/ismej.2017.45 |
Stucken K, John U, Cembella A, Soto-Liebe K, Vasquez M. 2014. Impact of nitrogen sources on gene expression and toxin production in the diazotroph Cylindrospermopsis raciborskii CS-505 and non-diazotroph Raphidiopsis brookii D9. Toxins, 6(6): 1896-1915.
DOI:10.3390/toxins6061896 |
Stüken A, Rücker J, Endrulat T, et al. 2006. Distribution of three alien cyanobacterial species (Nostocales) in northeast Germany: Cylindrospermopsis raciborskii, Anabaena bergii and Aphanizomenon aphanizomenoides. Phycologia, 45(6): 696-703.
DOI:10.2216/05-58.1 |
Sukenik A, Eshkol R, Livne A, et al. 2002. Inhibition of growth and photosynthesis of the dinoflagellate Peridinium gatunense by Microcystis sp. (cyanobacteria): a novel allelopathic mechanism. Limnology and Oceanography, 47(6): 1656-1663.
DOI:10.4319/lo.2002.47.6.1656 |
Sukenik A, Hadas O, Kaplan A, et al. 2012. Invasion of Nostocales (cyanobacteria) to subtropical and temperate freshwater lakes—physiological, regional, and global driving forces. Frontiers in Microbiology, 3: 86.
DOI:10.3389/fmicb.2012.00086 |
Sukenik A, Quesada A, Salmaso N. 2015. Global expansion of toxic and non-toxic cyanobacteria: effect on ecosystem functioning. Biodiversity and Conservation, 24(4): 889-908.
DOI:10.1007/s10531-015-0905-9 |
Tan K T, Huang Z Q, Ji R B, et al. 2019. A review of allelopathy on microalgae. Microbiology, 165(6): 587-592.
DOI:10.1099/mic.0.000776 |
Tomioka N, Imai A, Komatsu K. 2011. Effect of light availability on Microcystis aeruginosa blooms in shallow hypereutrophic Lake Kasumigaura. Journal of Plankton Research, 33(8): 1263-1273.
DOI:10.1093/plankt/fbr020 |
Wang L C, Zi J M, Xu R B, et al. 2017. Allelopathic effects of Microcystis aeruginosa on green algae and a diatom: Evidence from exudates addition and co-culturing. Harmful Algae, 61: 56-62.
DOI:10.1016/j.hal.2016.11.010 |
Watanabe M M, Ichimura T. 1977. Fresh, and salt-water forms of Spirulina platensis in axenic cultures. Bulletin of the Japanese Society of Phycology, 25 Suppl: 371-377.
|
Willis A, Adams M P, Chuang A W, et al. 2015. Constitutive toxin production under various nitrogen and phosphorus regimes of three ecotypes of Cylindrospermopsis raciborskii ((Wołoszyńska) Seenayya et Subba Raju). Harmful Algae, 47: 27-34.
DOI:10.1016/j.hal.2015.05.011 |
Xiao M, Hamilton D P, O'Brien K R, et al. 2020. Are laboratory growth rate experiments relevant to explaining bloom-forming cyanobacteria distributions at global scale?. Harmful Algae, 92: 101732.
DOI:10.1016/j.hal.2019.101732 |
Xiao M, Li M, Reynolds C S. 2018. Colony formation in the cyanobacterium Microcystis. Biological Reviews, 93(3): 1399-1420.
DOI:10.1111/brv.12401 |
Yang J W, Tang H X, Zhang X X, et al. 2018a. High temperature and pH favor Microcystis aeruginosa to outcompete Scenedesmus obliquus. Environmental Science and Pollution Research, 25(5): 4794-4802.
DOI:10.1007/s11356-017-0887-0 |
Yang J, Deng X R, Xian Q M, et al. 2014. Allelopathic effect of Microcystis aeruginosa on Microcystis wesenbergii: microcystin-LR as a potential allelochemical. Hydrobiologia, 727(1): 65-73.
DOI:10.1007/s10750-013-1787-z |
Yang Y M, Chen Y X, Cai F F, et al. 2018b. Toxicity-associated changes in the invasive cyanobacterium Cylindrospermopsis raciborskii in response to nitrogen fluctuations. Environmental Pollution, 237: 1041-1049.
DOI:10.1016/j.envpol.2017.11.024 |
Yang Y M, Jiang Y G, Li X C, et al. 2017. Variations of growth and toxin yield in Cylindrospermopsis raciborskii under different phosphorus concentrations. Toxins, 9(1): 13.
DOI:10.3390/toxins9010013 |
Zhang T T, Zheng C Y, Hu W, et al. 2010. The allelopathy and allelopathic mechanism of phenolic acids on toxic Microcystis aeruginosa. Journal of Applied Phycology, 22(1): 71-77.
DOI:10.1007/s10811-009-9429-6 |
Zhu J Y, Liu B Y, Wang J, et al. 2010. Study on the mechanism of allelopathic influence on cyanobacteria and chlorophytes by submerged macrophyte (Myriophyllum spicatum) and its secretion. Aquatic Toxicology, 98(2): 196-203.
DOI:10.1016/j.aquatox.2010.02.011 |
 2022, Vol. 40
2022, Vol. 40


