Institute of Oceanology, Chinese Academy of Sciences
Article Information
- LIU Kaixuan, JIANG Lei, YANG Jinsheng, MA Shuzhan, CHEN Kaining, ZHANG Yufeng, SHI Xiaoli
- Comparison of three flocculants for heavy cyanobacterial bloom mitigation and subsequent environmental impact
- Journal of Oceanology and Limnology, 40(5): 1764-1773
- http://dx.doi.org/10.1007/s00343-022-1351-7
Article History
- Received Oct. 25, 2021
- accepted in principle Jan. 28, 2022
- accepted for publication May 12, 2022
2 State Key Laboratory of Lake Science and Environment, Nanjing Institute of Geography and Limnology, Chinese Academy of Sciences, Nanjing, 210008, China;
3 University of Chinese Academy of Sciences, Beijing, 100049, China;
4 Jiangsu Collaborative Innovation Center of Regional Modern Agriculture & Environmental Protection, Huaiyin Normal University, Huaiyin, 223001, China
Eutrophication is characterized by excess nutrients in aquatic ecosystems, often leading to a turbid, phytoplankton-dominated state with a predominance of cyanobacteria (Ulrich et al., 2016; Waajen et al., 2016; Watson et al., 2016). Long-term in-situ observations in the field have shown that the daily growth rate of cyanobacteria is high during an outbreak of cyanobacteria, and the current approaches to mitigate and treat a cyanobacterial bloom do not effectively control its growth, resulting in a large accumulation of cyanobacteria on the leeward side of eutrophic water bodies. In some large eutrophic lakes, a high biomass of cyanobacteria can accumulate in lakeside areas. Cyanobacterial blooms can greatly harm aquatic ecosystems, especially when bloom-forming species release toxins that can cause fish kills, are potentially toxic to humans, dogs, waterfowl, and other animals, reduce biodiversity, and cause unpleasant surface scum and malodors (Yang et al., 2020; Zhang et al., 2021). Thus, the prevention, control, and emergency management of cyanobacteria in lakeside zones of large eutrophic lakes are important measures for alleviating the ecological disasters caused by cyanobacterial blooms and reducing the impact of cyanobacterial blooms on human homeostasis and life, which are primary objectives for lake managers.
In general, cyanobacterial bloom mitigation measures can be divided into biological, physical, and chemical methods (Stroom and Kardinaal, 2016). Biological methods are intended to change the ecosystem toward less favorable conditions for cyanobacteria but often fail due to high nutrient loading, high cyanobacterial biomass, and inappropriate growth conditions (Lürling and Mucci, 2020). Physical methods include ultrasonic methods, adsorption methods, and separation membranes (Park et al., 2019; Jiang et al., 2020; Truttmann et al., 2020). However, these methods have high operating costs and laborious operations; the energy costs are high for full-scale operations in the field, and the effective range may be limited (Visser et al., 2016). Chemical methods are used to directly reduce the cyanobacterial biomass in water bodies via algaecides. Copper-based algaecide is commonly used but has been mostly abandoned due to its nonspecificity and potential toxicity to other aquatic biotas (Li et al., 2011; Bishop et al., 2018). Hydrogen peroxide is considered an environmentally friendly algaecide because it can selectively suppress cyanobacterial growth without releasing any residual chemicals. However, a high dosage of H2O2 is required to suppress cyanobacteria with high algal biomass and large colony size, which could cause the release of toxins (Matthijs et al., 2012; Liu et al., 2017).
In comparison with the methods mentioned above, flocculation and settling techniques have been successfully used to control phosphorus and algal biomass in many freshwater bodies worldwide. This method consists of applying a low dose of coagulants and clays, naturally or chemically modified, to promote the removal of phosphorus and algal biomass from the water column through flocculation and sedimentation. Coagulants based on metal, polyaluminum chloride (PAC), ferric chloride (FeCl3), and the natural coagulant cationic starch with chitosan have been widely used in small-sized stagnant water bodies, with chlorophyll-a (Chl-a) concentration normally being lower than 300 μg/L (Li et al., 2011; Aktas et al., 2013; Ma et al., 2015; Wan et al., 2015; Jin et al., 2019). However, cyanobacteria could heavily concentrate along the downwind shore in the large eutrophic lake, resulting to much larger cyanobacterial biomass than that in small lake. Our pre-experimental results showed that flocculation and sedimentation was the only method that can mitigate cyanobacterial blooms with high algal biomass, and this is an essential prerequisite for dealing with the large accumulations of cyanobacteria in the coastal areas of eutrophic water bodies. However, the precipitation efficiency of the surface cyanobacterial scums on the sediment depends on the type and concentration of coagulants and clays applied, as well as on the concentration of algal biomass. Furthermore, the addition of various coagulants and the accumulation of high cyanobacterial biomass in the sediments would change the physicochemical parameters of the water column and sediments. And this could have a potential influence on the water quality and the implementation of subsequent lake restoration strategies.
In this study, we determined the doses of coagulant and ballast agents required for different cyanobacterial biomasses through preliminary experiments. The Chl-a concentration of the maximum cyanobacterial biomass was up to 1 500 μg/L, representing the heavy accumulation of cyanobacteria on the leeward side of a large eutrophic lake. Then, the efficiency of the agents in mitigating cyanobacteria and their impacts on the major physicochemical features of the water column and sediments were evaluated, which showed potential risks for water quality deterioration that could potentially influence the implementation of subsequent lake restoration strategies. Our results can help lake managers develop strategies for emergencies to rapidly remove large amounts of cyanobacterial biomass from the water column on the leeward side of eutrophic water bodies.
2 MATERIAL AND METHOD 2.1 Field sampling in Chaohu LakeWith a surface area of 760 km2 (Huang et al., 2018; Wu et al., 2021), Chaohu Lake is the fifth largest freshwater lake in China and is typically eutrophic, with a location downstream of the Changjiang (Yangtze) River. Colonial Microcystis was collected from a surface bloom in September 2020 in Chaohu Lake. Water was collected by a 5-L Plexiglass water collector, and the sediments were collected by a column sediment sampler (inner diameter of sampling tube Φ=84 mm) from the bottom of Chaohu Lake. All samples were stored at a low temperature, protected from light, and brought back to the laboratory on the same day they were sampled.
2.2 Laboratory experiment 2.2.1 Experiment setupThe sediments were pooled together and then homogenized with a dough mixer, and 200 mL of the sediment was transferred into plastic beakers (2 L). Lake water (filtered with a 20-μm filter) was slowly poured over the sediment surface in each beaker, and then cyanobacterial scum was added to simulate cyanobacterial blooms of different biomasses. The average concentrations of Chl a in different experimental groups from low to high were 413.30 μg/L, 637.10 μg/L, 1 001.63 μg/L, and 1 727.36 μg/L, respectively.
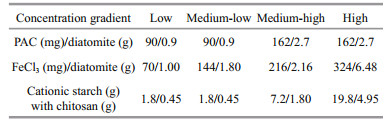
Flocculants, including polyaluminum chloride (PAC), ferric chloride (FeCl3), and cationic starch with chitosan, were slowly added to the beakers at triplicates per concentration, and the flocculant dosages were determined as the concentration at which the cyanobacteria flocculated and sank within a short period. Additionally, diatomite was used as a "ballast" to facilitate the settling of the flocculated complexes to the bottom for PAC and FeCl3. The final dosages for each treatment with the different flocculants and for each group with different cyanobacterial biomasses are shown in Table 1. The detailed laboratory experimental setup is shown in Fig. 1.
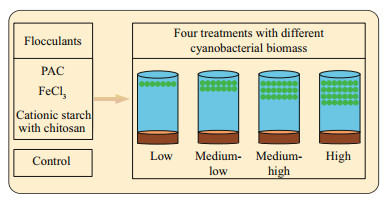
|
| Fig.1 Diagram of the experimental setup |
Water samples were taken to analyze Chl-a concentration, dissolved oxygen (DO), pH, and specific nutrients concentrations, including total dissolved nitrogen (TDN) and total dissolved phosphorus (TDP). Sediment samples collected in September 2020, were freeze-dried to measure the contents of Chl a, total nitrogen (TN), total phosphorus (TP), and total oxygen carbon (TOC).
Water samples were filtered by GF/C membranes to collect Chl a, which was determined by a fluorescence spectrophotometer (RF-5301PC, Shimadzu Corporation, Japan) after acetone extraction. DO and pH were measured using a multiparameter water quality analyzer (YSI 6600, Yellow Spring Instruments, USA). TDN and TDP were determined after filtration through a 0.45-μm membrane filter (Millipore Corp) after persulfate oxidation. The TN and TP contents of the sediments were analyzed by peroxodisulfate oxidation and spectrophotometry. TOC was measured by a TOC meter (Shimadzu TOC 5000A) after filtration through a GF/F (Waterman) membrane.
2.2.3 Statistical analysisAll the data in this paper were processed and graphed using Microsoft Excel and Origin 8.5 software, respectively. The differences in the various characteristics between the different treatment groups were evaluated using IBM SPSS Statistics 20 software through one-way ANOVA.
3 RESULT 3.1 Physicochemical parameters of the water columnWhen flocculants and diatomite were added, the concentration of Chl a dropped rapidly. Of the treatments, the FeCl3 treatments had the fastest rate of Chl-a decline, followed by the PAC treatment. In all the cyanobacterial biomass groups, the Chl-a concentration dropped to almost 0 μg/L after 1 h for FeCl3 treatment and after 1.5 h for PAC treatment. However, in the cationic starch with chitosan treatments, the concentration of Chl a was still as high as 159.54 and 68.89 μg/L in the high and medium-high groups, respectively in the end of the experiment (Fig. 2).
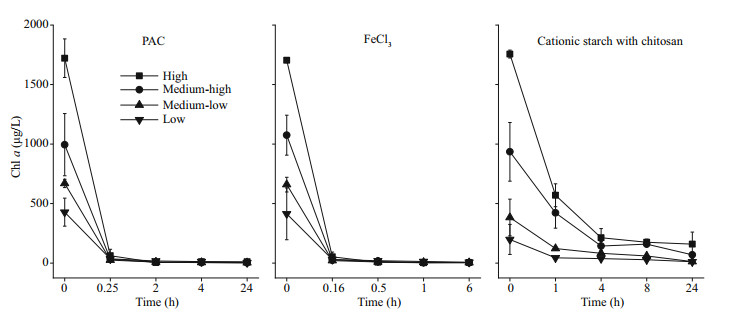
|
| Fig.2 Chl-a content in the water column after the addition of flocculants |
The DO content declined with a rise in cyanobacterial biomass, which was 7.0 and 4.7 mg/L in the PAC treatment groups and 4.1 and 3.7 mg/L in the FeCl3 treatment groups after 24 h in the medium-high and high groups, respectively (P < 0.05). In contrast, the DO contents in the cationic starch with chitosan treatment groups were 2.5 and 2.2 mg/L in the medium-high and high groups, respectively. The DO curve still showed a downward trend at the end of the experiment, which had a high probability of further developing into an anaerobic state, thus threatening the ecological balance of the aquatic environment (Fig. 3).
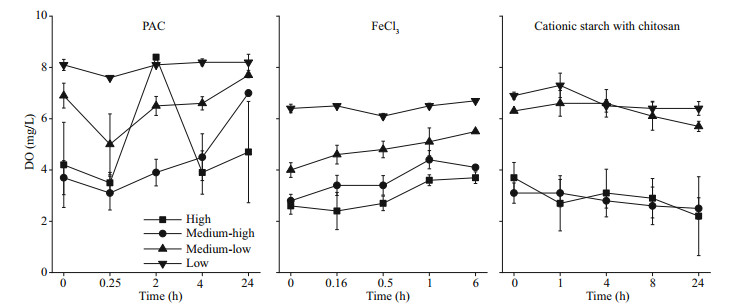
|
| Fig.3 DO in the water column after the addition of flocculants |
During the experiment, the pH in the water column of cyanobacteria with different biomasses was maintained between 6.9 and 7.8 after the addition of PAC and cationic starch with chitosan. However, the pH of the water column showed a sharp decrease after the addition of FeCl3 (P < 0.05), and the lowest pH values were 6.6, 6.4, 6.0, and 5.6 in the low, medium-low, medium-high, and high groups, respectively (Fig. 4).
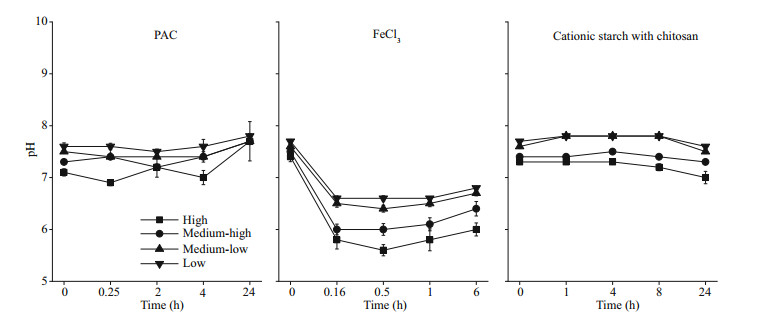
|
| Fig.4 pH of the water column after the addition of flocculants |
Our experimental results show that treatment with PAC and FeCl3 resulted in a marked decrease in TDN and TDP in the water. TDN decreased to approximately 0.20 mg/L and 0.28 mg/L at the end of the experiment under the PAC and FeCl3 treatments, respectively, regardless of cyanobacterial concentration. After 24 h, TDN in the cationic starch with chitosan treatment decreased to 0.48 mg/L in the medium-low and low-concentration algae groups but increased to 1.86 and 2.71 mg/L in the medium-high and high algae groups, respectively (P < 0.05). Similarly, in the PAC and FeCl3 treatment groups, TDP dropped rapidly to approximately 0.01 mg/L. In the cationic starch with chitosan treatment group, TDP dropped to 0.011 and 0.014 mg/L at low and medium-low concentrations of cyanobacteria, while TDP dropped to 0.04 and 0.03 mg/L at medium-high and high concentrations of cyanobacteria, respectively (P < 0.05), after 24 h of treatment (Fig. 5).
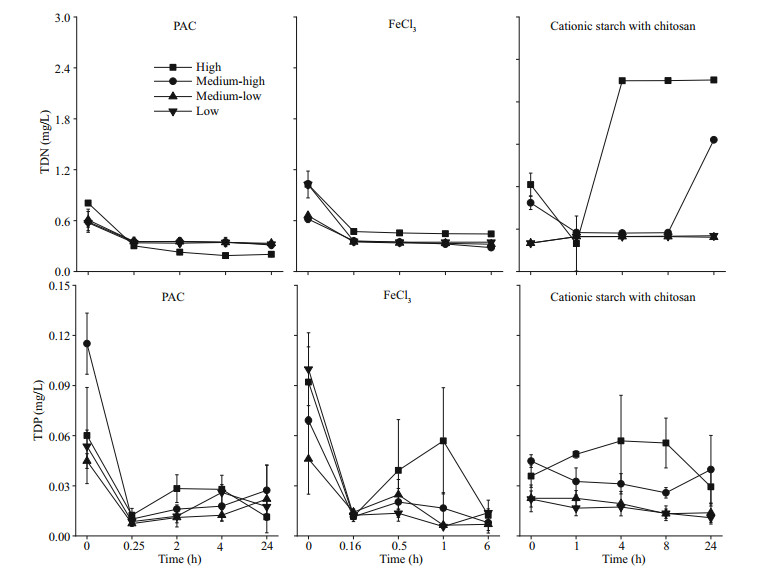
|
| Fig.5 TDN and TDP in the water column after the addition of flocculants |
The average Chl-a content in the sediment was 1 406.64 ng/g in the control group, which increased with cyanobacterial concentrations. After PAC addition, the Chl-a content of the sediment was 35 433.88, 59 133.19, 94 536.92, and 109 012.22 ng/g in the four cyanobacterial groups, respectively. The Chl-a values in the FeCl3 treatment were generally lower than those in the PAC treatment, which were 26 753.75, 59 260.05, 77 233.99, and 103 897.79 ng/g in the four groups, respectively. In comparison, the Chl-a content in the sediments was lower for the cationic starch with chitosan treatments, with 14 408.64, 21 215.64, 4 755.62, and 63 232.74 ng/g in the four groups (P < 0.05), respectively (Fig. 6b).

|
| Fig.6 Variation in Chl a, TN, TP, and TOC in the sediments in the treatments with different cyanobacterial biomasses |
TN and TP in the sediments increased with a rise in cyanobacterial biomass after using the flocculants. Consistent with the Chl-a pattern, TN and TP in the sediments were higher in the PAC treatments than in the FeCl3 treatments, followed by the cationic starch with chitosan treatments. Specifically, TN in the sediments was 6 133.84 mg/kg, 10 200.45 mg/kg, and 13 553.11 mg/kg, and TP in sediments was 1 350.19 mg/kg, 1 693.06 mg/kg, and 2 075.98 mg/kg in the PAC groups in the low, medium-high, and high concentration groups, respectively (P < 0.05) (Fig. 6b & c).
The sedimentation of cyanobacteria after the addition of flocculants led to an increase in the TOC content of the sediments. The TOC content of the sediments changed slightly in the low and medium-low groups. In the medium-high and high groups, the TOC content of the sediments increased with increasing cyanobacterial biomass. Notably, in the high cyanobacterial biomass group, the TOC content in the sediment was highest for the cationic starch with chitosan treatment at 19.57%, and it was 9.84% and 9.73% in the PAC group and FeCl3 group, respectively (Fig. 6d).
4 DISCUSSION 4.1 Effect of different flocculants on cyanobacterial biomass reductionDue to the presence of negatively charged groups, such as hydroxyl groups, on the surface of microalga cells, they can be electrically neutralized with positively charged cationic electrolytes, thus reducing the repulsive effect between algal cells and enabling algal cells to aggregate and form flocs that can be easily separated from water (Knuckey et al., 2006). Chl a is often used as an index to evaluate mitigation efficiency since it not only determines the content of plankton but is also one of the main indicators to measure the degree of eutrophication of a water body (Tang et al., 2011).
PAC, FeCl3, and cationic starch with chitosan are reported to be efficient flocculants that can effectively remove cyanobacteria from water treatment systems (Waajen et al., 2016). Our results indicate that FeCl3 flocculates the fastest and has the best effect on cyanobacteria reduction, followed by PAC, which was not very different from FeCl3 in reducing cyanobacteria. Both of these flocculants can rapidly mitigate the high biomass of cyanobacteria, with the Chl-a concentration becoming higher than 1 500 μg/L within 15 min. It is worth noting that although FeCl3 has a favorable and fast flocculation effect, the dosage requirement is very strict. If the dosage of FeCl3 is slightly in excess, the color of the water body will change because Fe3+ appears yellowish or reddish brown after dissolving in water, thus having a negative impact on natural water bodies. In contrast, cationic starch with chitosan has the slowest flocculation rate, with flocculation being evident after approximately one hour. Meanwhile, when cyanobacterial biomass was high, with a Chl-a concentration of 200 μg/L, cyanobacteria could not be fully flocculated, and some cyanobacterial biomass remained in the water column at the end of the experiment. Chitosan is regarded as an eco-friendly and nontoxic coagulant that can rapidly compromise cell membrane integrity and kill certain cyanobacteria (Li and Pan, 2015). This cell-damaging effect can cause cyanobacterial cell lysis and reduce the possibility of flocculation and sinking. Differences in sensitivity to chitosan exist among different cyanobacterial species, and Microcystis was the least sensitive among the nine cyanobacteria species tested (Barko et al., 1991).
4.2 Effect of different flocculants on the physicochemical properties of water bodiesWhen cyanobacteria settled to the bottom of the water column, the physicochemical characteristics, such as DO and pH, changed with time in the four different treatments. In particular, DO in the water column dropped to 2.2 mg/L in cationic starch with chitosan treatments in the high-concentration cyanobacterial group. In fact, at high biomass, algal respiration can lead to a sharp decline in the DO of water bodies, which may make the water column biologically anaerobic and lead to the development of poor sediment and water quality (Huo et al., 2015). A low DO concentration can cause fish and shrimp death or even black odors from the water body in serious cases and affect the geochemical cycle (Yang et al., 2008). Therefore, when treating high concentrations of cyanobacterial blooms for emergency purposes, a combination of flocculants and oxygenators should be considered. The addition of FeCl3 can generate a positively charged cationic electrolyte that can electrically neutralize negatively charged groups, such as hydroxyl groups, present on the surface of microalgal cells, resulting in a decrease in the pH of the water column (Li et al., 2015). For medium-high and high cyanobacterial biomass groups, the anaerobic decay of algae produces carboxylic or dicarboxylic acids, which cause the pH of the water column to fall between 5.5 and 6.5, lower than the normal pH level of the water column (Li et al., 2015).
4.3 Effect of different flocculants on substrateIn our experiments for the emergency treatment of high biomass cyanobacteria, diatomaceous earth was used to settle the flocculated cyanobacterial complexes to the bottom of the water column (Li and Pan, 2015). The bottom sediments, however, are usually a collection point for pollutants, such as nutrients and heavy metals, and pollutants can become a source of endogenous pollutants in natural lakes after long-term accumulation (Jin et al., 2005). In the process of algae control, the better the effect of the flocculant is, the greater the algal cells settle to the bottom sediments; thus, the algae control effect of PAC is superior in the experimental group of cyanobacterial water bodies with different concentration gradients. Although temporarily effective in controlling high cyanobacterial blooms, if nutrients continue to accumulate in the sediments, the flocculent may eventually transform the contaminated sediment into endogenous nutrient load to the lake water body. Nutrients can be released into the overlying water column by absorption, diffusion, convection, and resuspension and become the "source" of lake water pollution (Zhong et al., 2008). The results from 18 European lakes have indicated that a TP content in surficial sediment of 1 000 mg/kg may represent an approximate threshold above which it will perpetuate internal loading for flow diversion (Cooke et al., 1994). Our results indicate that the background TP content in the sediments was already approximately 1 000 mg/kg. This value increased after the cyanobacterial scum was flocculated and sedimented, reaching more than 2 000 mg/kg. Considering the long-term management of eutrophic lakes, endogenous pollution can be reduced by physical methods such as substrate dredging and underwater aeration (Caille et al., 2003; Liu et al., 2015).
Flocculation and sedimentation are effective in removing large cyanobacterial biomass from the water column in a relatively short time when an emergency situation occurs in certain lake areas. However, long-term prevention and control strategies are essential for lake managers. In fact, cyanobacterial biomass mitigation is only the first step; lake restoration should be subsequently implemented to achieve macrophyte-dominated clear water ecosystems, particularly in coastal lake regions. The physiochemical characteristics of sediment exert an important influence on macrophyte productivity and species composition. Lake sediment is a recognized site for the accumulation of organic carbon. Its TOC content can not only indicate the input of organic matter to the lake but can also reflect the storage status of organic matter and the status of lake productivity characteristics (Meyers, 1994). In addition, TOC content is related to bulk density, nutrient content, and nutrient availability. Macrophyte nutrition in highly organic sediments may also be disrupted by the presence of phytotoxic compounds produced during anaerobic decomposition. Adding low levels of labile organic matter to the sediments resulting from the precipitation of algal detritus may provide nutritional benefits to submerged macrophytes, particularly if on coarse-textured sediments (Kiørboe, 1980). However, the accumulation of large amounts of refractory organic matter with potentially inhibitory properties in sediments can generally be expected to diminish sediment nutrient availability and the associated growth of rooted submerged macrophytes (Barko et al., 1991). In a previous study, the growth of Hydrilla verticillata and Myriophyllum spicatum decreased almost linearly with increasing sediment organic matter up to a concentration of approximately 20% (Barko et al., 1986). The maximum TOC content in sediments can reach approximately 20% during cationic starch with chitosan treatment, which would be difficult to follow with lake restoration measures.
5 CONCLUSIONThe flocculants PAC, FeCl3, and cationic starch with chitosan were tested for their effectiveness in controlling cyanobacteria blooms at four different concentrations, and the subsequent environmental impacts on the water column and sediments were also evaluated. Our results indicate that PAC and FeCl3 are efficient flocculants that rapidly mitigated cyanobacterial blooms with Chl-a concentrations higher than 1 500 μg/L within 15 min. In comparison, cationic starch with chitosan could treat only cyanobacterial blooms with Chl-a concentrations less than 200 μg/L. However, treatment with FeCl3 and cationic starch with chitosan could increase risks for water quality deterioration. For example, the addition of FeCl3 caused a decline in the pH value. DO in the water column dropped to 2 mg/L with cationic starch of chitosan treatment in the high cyanobacterial biomass group. Additionally, the cell lysis of cyanobacteria caused by cationic starch with chitosan could result in an increase in DTP and DTN. Thus, PAC is the optimum flocculant for mitigating heavy cyanobacteria blooms under emergency in comparison with FeCl3 and cationic starch with chitosan. However, the high TOC content in the sediments due to the settling of cyanobacterial biomass could threaten the success of lake restoration achieved by planting submerged macrophytes. Hence, cyanobacteria could still recover over time in eutrophic water bodies, requiring repeated use of PAC. This would bring other environmental and ecological problems, which requires further study.
6 DATA AVAILABILITY STATEMENTThe datasets generated and/or analyzed during the current study are available from the corresponding author on request.
Aktas T S, Takeda F, Maruo C, et al. 2013. Comparison of four kinds of coagulants for the removal of picophytoplankton. Desalination and Water Treatment, 51(16–18): 3547-3557.
DOI:10.1080/19443994.2012.750777 |
Barko J W, Adams M S, Clesceri N L. 1986. Environmental factors and their consideration in the management of submersed aquatic vegetation: a review. Journal of Aquatic Plant Management, 24: 1-10.
|
Barko J W, Gunnison D, Carpenter S R. 1991. Sediment interactions with submersed macrophyte growth and community dynamics. Aquatic Botany, 41(1-3): 41-65.
DOI:10.1016/0304-3770(91)90038-7 |
Bishop W M, Richardson R J, Willis B E. 2018. Comparison of partitioning and efficacy between copper algaecide formulations: refining the critical burden concept. Water, Air, & Soil Pollution, 229(9): 300.
DOI:10.1007/s11270-018-3958-z |
Caille N, Tiffreau C, Leyval C, et al. 2003. Solubility of metals in an anoxic sediment during prolonged aeration. Science of the Total Environment, 301(1–3): 239-250.
DOI:10.1016/S0048-9697(02)00289-9 |
G. Dennis Cooke, Eugene B. Welch, Spencer A. Peterson, et al. 1994. Restoration and management of lakes and reservoirs (2nd edn). 9(2): 131–132, https://doi.org/10.1002/rrr.3450090207.
|
Huang J C, Zhang Y J, Huang Q, et al. 2018. When and where to reduce nutrient for controlling harmful algal blooms in large eutrophic Lake Chaohu, China?. Ecological Indicators, 89: 808-817.
DOI:10.1016/j.ecolind.2018.01.056 |
Huo X C, Chang D W, Tseng J H, et al. 2015. Exposure of Microcystis aeruginosa to hydrogen peroxide under light: kinetic modeling of cell rupture and simultaneous microcystin degradation. Environmental Science & Technology, 49(9): 5502-5510.
DOI:10.1021/acs.est.5b00170 |
Jiang Y, Liu Y, Shi D T, et al. 2020. Membrane fouling in a powdered activated carbon-membrane bioreactor (PAC-MBR) for micro-polluted water purification: fouling characteristics and the roles of PAC. Journal of Cleaner Production, 277: 122341.
DOI:10.1016/j.jclepro.2020.122341 |
Jin X C, Xu Q J, Huang C Z. 2005. Current status and future tendency of lake eutrophication in China. Science in China Series C: Life Sciences, 48(2): 948-954.
DOI:10.1007/BF03187133 |
Jin X G, Bi L, Lyu T, et al. 2019. Amphoteric starch-based bicomponent modified soil for mitigation of harmful algal blooms (HABs) with broad salinity tolerance: flocculation, algal regrowth, and ecological safety. Water Research, 165: 115005.
DOI:10.1016/j.watres.2019.115005 |
Kiørboe T. 1980. Distribution and production of submerged macrophytes in tipper grund (ringkobing fjord, Denmark), and the impact of waterfowl grazing. Journal of Applied Ecology, 17(3): 675-687.
DOI:10.2307/2402646 |
Knuckey R M, Brown M R, Robert R, et al. 2006. Production of microalgal concentrates by flocculation and their assessment as aquaculture feeds. Aquacultural Engineering, 35(3): 300-313.
DOI:10.1016/j.aquaeng.2006.04.001 |
Li H, Pan G. 2015. Simultaneous removal of harmful algal blooms and microcystins using microorganism- and chitosan-modified local soil. Environmental Science & Technology, 49(10): 6249-6256.
DOI:10.1021/acs.est.5b00840 |
Li P N, Zhang L Z, Wang W W, et al. 2011. Rapid catalytic microwave method to damage Microcystis aeruginosa with FeCl3-loaded active carbon. Environmental Science & Technology, 45(10): 4521-4526.
|
Li X Q, Pei H Y, Hu W R, et al. 2015. The fate of Microcystis aeruginosa cells during the ferric chloride coagulation and flocs storage processes. Environmental Technology, 36(7): 920-928.
DOI:10.1080/09593330.2014.966768 |
Liu C, Shao S G, Shen Q S, et al. 2015. Use of multi-objective dredging for remediation of contaminated sediments: a case study of a typical heavily polluted confluence area in China. Environmental Science and Pollution Research, 22(22): 17839-17849.
DOI:10.1007/s11356-015-4978-5 |
Liu M X, Shi X L, Chen C, et al. 2017. Responses of Microcystis colonies of different sizes to hydrogen peroxide stress. Toxins, 9(10): 306.
DOI:10.3390/toxins9100306 |
Lürling M, Mucci M. 2020. Mitigating eutrophication nuisance: in-lake measures are becoming inevitable in eutrophic waters in the Netherlands. Hydrobiologia, 847(21): 4447-4467.
DOI:10.1007/s10750-020-04297-9 |
Ma X X, Wang Y N, Feng S Q, et al. 2015. Comparison of four flocculants for removing algae in Dianchi Lake. Environmental Earth Sciences, 74(5): 3795-3804.
DOI:10.1007/s12665-015-4903-4 |
Matthijs H C P, Visser P M, Reeze B, et al. 2012. Selective suppression of harmful cyanobacteria in an entire lake with hydrogen peroxide. Water Research, 46(5): 1460-1472.
DOI:10.1016/j.watres.2011.11.016 |
Meyers P A. 1994. Preservation of elemental and isotopic source identification of sedimentary organic matter. Chemical Geology, 114(3–4): 289-302.
DOI:10.1016/0009-2541(94)90059-0 |
Park J, Son Y, Lee W H. 2019. Variation of efficiencies and limits of ultrasonication for practical algal bloom control in fields. Ultrasonics Sonochemistry, 55: 8-17.
DOI:10.1016/j.ultsonch.2019.03.007 |
Stroom J M, Kardinaal W E A. 2016. How to combat cyanobacterial blooms: strategy toward preventive lake restoration and reactive control measures. Aquatic Ecology, 50(3): 541-576.
DOI:10.1007/s10452-016-9593-0 |
Tang Y, Zhang H, Liu X N, et al. 2011. Flocculation of harmful algal blooms by modified attapulgite and its safety evaluation. Water Research, 45(9): 2855-2862.
DOI:10.1016/j.watres.2011.03.003 |
Truttmann L, Su Y X, Lee S, et al. 2020. Gravity-driven membrane (GDM) filtration of algae-polluted surface water. Journal of Water Process Engineering, 36: 101257.
DOI:10.1016/j.jwpe.2020.101257 |
Ulrich A E, Malley D F, Watts P D. 2016. Lake Winnipeg basin: advocacy, challenges and progress for sustainable phosphorus and eutrophication control. Science of the Total Environment, 542: 1030-1039.
DOI:10.1016/j.scitotenv.2015.09.106 |
Visser P M, Ibelings B W, Bormans M, et al. 2016. Artificial mixing to control cyanobacterial blooms: a review. Aquatic Ecology, 50(3): 423-441.
DOI:10.1007/s10452-015-9537-0 |
Waajen G, van Oosterhout F, Douglas G, et al. 2016. Geoengineering experiments in two urban ponds to control eutrophication. Water Research, 97: 69-82.
DOI:10.1016/j.watres.2015.11.070 |
Waajen G., van Oosterhout F., Douglas G.. 2016. Management of eutrophication in Lake De Kuil (The Netherlands) using combined flocculant: lanthanum modified bentonite treatment. Water Research, 97: 83-95.
DOI:10.1016/j.watres.2015.11.034 |
Wan C, Alam A, Zhao X Q, et al. 2015. Current progress and future prospect of microalgal biomass harvest using various flocculation technologies. Bioresource Technology, 184: 251-257.
DOI:10.1016/j.biortech.2014.11.081 |
Watson S B, Miller C, Arhonditsis G, et al. 2016. The reeutrophication of Lake Erie: harmful algal blooms and hypoxia. Harmful Algae, 56: 44-66.
DOI:10.1016/j.hal.2016.04.010 |
Wu Z S, Lai X J, Li K Y. 2021. Water quality assessment of rivers in Lake Chaohu basin (China) using water quality index. Ecological Indicators, 121: 107021.
DOI:10.1016/j.ecolind.2020.107021 |
Yang C Y, Hou X P, Wu D H, et al. 2020. The characteristics and algicidal mechanisms of cyanobactericidal bacteria, a review. World Journal of Microbiology and Biotechnology, 36(12): 188.
DOI:10.1007/s11274-020-02965-5 |
Yang M, Yu JW, Li ZL, et al. 2008. Taihu Lake not to blame for Wuxi's Woes. Science, 319: 157-159.
DOI:10.1126/science.319.5860.158a |
Zhang Z Y, Fan X J, Peijnenburg W J G M, et al. 2021. Alteration of dominant cyanobacteria in different bloom periods caused by abiotic factors and species interactions. Journal of Environmental Sciences, 99: 1-9.
DOI:10.1016/j.jes.2020.06.001 |
Zhong J C, You B S, Fan C X, et al. 2008. Influence of sediment dredging on chemical forms and release of phosphorus. Pedosphere, 18(1): 34-44.
DOI:10.1016/S1002-0160(07)60100-3 |
 2022, Vol. 40
2022, Vol. 40