Institute of Oceanology, Chinese Academy of Sciences
Article Information
- MA Shuzhan, WU Yue, CHEN Siwen, CHEN Bingfa, LIU Cheng, GU Xiaozhi, SHI Xiaoli, CHEN Kaining
- The impact of the accumulation of algal blooms on reed wetlands in the littoral zones of Chaohu Lake
- Journal of Oceanology and Limnology, 40(5): 1750-1763
- http://dx.doi.org/10.1007/s00343-021-1258-8
Article History
- Received Aug. 7, 2021
- accepted in principle Sep. 22, 2021
- accepted for publication Nov. 4, 2021
2 University of Chinese Academy of Sciences, Beijing 100049, China;
3 Institute of Agricultural Resources and Environmental Sciences, Jiangsu Academy of Agricultural Sciences, Nanjing 210014, China;
4 Jiangsu Collaborative Innovation Center of Technology and Material of Water Treatment, Suzhou University of Science and Technology, Suzhou 215009, China
Eutrophication and associated algal blooms have been increasing in frequency and intensity in lakes and reservoirs worldwide in recent decades due mainly to human-induced nutrient (nitrogen and phosphorus) over-enrichment (Xu et al., 2010; Ho et al., 2019; Wurtsbaugh et al., 2019) and partly because of climate change (e.g., increased temperature) (Paerl and Huisman, 2008). The overproliferation of algae causes serious ecological and socioeconomic problems, including oxygen deficiency, bad odors, degradation of aquatic ecosystems, and losses of biodiversity (Kudari and Kanamadi, 2008; Paerl and Huisman, 2008; Von Sperling et al., 2008; Paerl and Otten, 2013). Thus, the removal and control of algal blooms is key to the restoration of the health and the functioning of lake ecosystems (Wu et al., 2019).
The littoral zone of a lake is the nearshore region, which can support large stands of aquatic macrophyte and phytoplankton growth (Xiang et al., 2014; Zhu et al., 2020; Wong et al., 2021). The intensity of algal accumulation varies with the characteristics of landforms and vegetation in littoral zones (Cai et al., 2018). Common reed (Phragmites australis) is a cane-like perennial grass with a worldwide distribution in littoral zones (Hao et al., 2013). Reed is considered an effective macrophyte for removing nutrients from lakes (Fukuhara et al., 2007; Sollie et al., 2008; Wang et al., 2011). However, in eutrophic lakes, the presence of reeds causes the accumulation of algae in littoral zones (Wang et al., 2006a), negatively affecting water quality (Wang et al., 2006b; Xiang et al., 2014; Zhu et al., 2020).
Chaohu Lake is the fifth-largest freshwater lake in China and has suffered from increasingly severe algal blooms in recent decades due to rapid growth of the local human population as well as economic development (Xu et al., 2003; Kong et al., 2013; Zhang and Kong, 2015). Massive algal bloom outbreaks have occurred in this lake almost every year since the 1980s (Jiang et al., 2010, 2014; Cai and Kong, 2013; Kong et al., 2013; Yu et al., 2014). In Chaohu Lake, floating-leaved and submerged macrophytes are rarely present, and reeds are the dominant macrophytes in the littoral zones. The littoral zones are the region most frequently affected by human activities (Cai et al., 2018), and algae in these littoral zones may serve as a seed stock for subsequent blooms (Gu, 2012; Chen et al., 2016). Thus, the accumulation of algal blooms in the littoral zones of shallow lakes have received much attention (Xiang et al., 2014; Qi et al., 2017). However, most studies have only focused on the effects of algal blooms on water quality (Sollie et al., 2008; Li et al., 2010) but have underestimated the impact of algal accumulation on the emergent macrophyte wetlands. Thus, knowledge related to the pattern of algal accumulation in reed wetlands is important for understanding the biogeochemical cycle of algae in Chaohu Lake.
In this study, the physicochemical features of water and sediment were investigated seasonally in reed wetlands and surrounding unvegetated lake regions. The purpose of this study is to clarify the factors influencing the accumulation of algal blooms in reed wetlands and to evaluate how the accumulation of algal biomass affects the physicochemical environmental parameters of reed wetlands. Finally, management recommendations for the prevention and control of algal blooms in the littoral zones of Chaohu Lake are proposed.
2 MATERIAL AND METHOD 2.1 Study areaChaohu Lake, which is located in the lower Changjiang (Yangtze) River Basin (31°25'28"N– 31°43'28"N, 117°16'54"E–117°51'46"E), features a 774-km2 surface area and a 184.66-km-long shoreline (Yu et al., 2011; Wang et al., 2018). Lake depth is usually less than 4 m but depends on hydrological conditions, with a maximum depth of 6.78 m and an average value of 3 m (Zhang et al., 2016). The volume of Chaohu Lake is 3.23×109 m3 in the rainy season, with a residence time of approximately 150 days. In contrast, the volume is only 1.72×109 m3 in the dry season, with a residence time of approximately 210 days (Duan et al., 2017). Chaohu Lake has a basin area of 13 350 km2 and is characterized by a subtropical monsoon climate with an average annual rainfall of 1 100 mm. The annual water temperature averages approximately 15 ℃, and the lowest and highest temperatures are 2–3 ℃ in January and 28– 30 ℃ in July, respectively. The dominant wind directions are southeast in summer and northwest in winter (Yu et al., 2011). This lake performs multiple functions, including aquaculture, agricultural irrigation, flood control, tourism, and drinking water supply. This lake has undergone rapid eutrophication and frequent cyanobacterial blooms with nutrient inputs from urban sewerage and agricultural nonpoint sources over the past three decades (Shang et al., 2015), and cyanobacterial blooms are primarily dominated by Microcystis and Anabaena (Jiang et al., 2010, 2014).
2.2 Field samplingIn this study, samples were collected monthly from fifteen sites (1#–15#) in open water areas in Chaohu Lake from April 2018 to November 2019 (Fig. 1). Three littoral zones (C1–C3), representing different downwind directions, were sampled seasonally in April 2018, July 2018, January 2019, and November 2019. Every littoral zone included the reed-covered littoral zone (RCLZ) and its surrounding unvegetated littoral zone (ULZ). The reed densities in the three RCLZ averaged 120 plants per square meter, with a standard deviation of 15 plants per square meter. Surface water samples, including samples from open water areas and three littoral zones, were collected and stored under low-temperature conditions (4 ℃) for analysis in the laboratory. Sediment cores were also collected from zones (C1–C3) using a gravity corer sampling apparatus (90 mm in diameter × 500 mm in length; Rigo Co., Ltd., Saitama, Japan). Each sediment core was immediately sealed with a rubber stopper and sealing film.
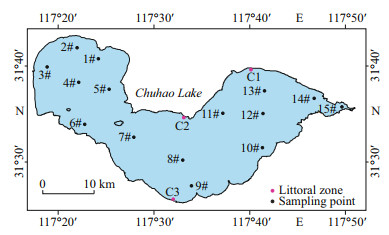
|
| Fig.1 The distribution of sampling sites |
Dissolved oxygen (DO) and temperature of surface water (WT) were measured in situ with a Yellow Springs Instruments (YSI) 6600 V2 multisensor sonde. Environmental parameters, including total nitrogen (TN), total phosphorus (TP), and chlorophyll a (Chl a), were determined in the laboratory-based on standard methods. TN was determined using the alkaline potassium persulfate digestion method in unfiltered water (State Environmental Protection Administration and Editorial Board of Water and Wastewater Monitoring and Analysis Methods, 2002). TP was determined using the molybdenum blue colorimetric method in unfiltered water (State Environmental Protection Administration and Editorial Board of Water and Wastewater Monitoring and Analysis Methods, 2002). Chl a was determined photometrically at wavelengths of 630, 645, 665, and 750 nm after filtration on Whatman GF/F glass filters and 24 h extraction in hot 90% acetone (Chen et al., 2006). In this study, the concentration of Chl a was used as the indicator to characterize the intensity of algal accumulation.
2.4 Physicochemical properties of the surface sedimentAll sediment cores were segmented at 2-cm intervals. The segmented sediment samples were then freeze-dried in a vacuum freeze drier (Biosafer, Nanjing, China). The dried sediment samples were ground in an agate mortar and sieved through a 100-mesh screen (0.15 mm) before analysis. TN in the sediment was extracted using alkaline potassium persulfate and then analyzed with an UVeVis spectrophotometer (State Environmental Protection Administration and Editorial Board of Water and Wastewater Monitoring and Analysis Methods, 2002). TP in the sediment was analyzed by inductively coupled plasma atomic emission spectrometry (ICP-AES, Perkin-Elmer DV4300, USA) (Yin et al., 2018). The detailed pretreatment procedure and method for determining Chl-a concentration in the sediment were described by Yan et al. (2004). The water content of the sediment was determined by weight loss after drying at 105 ℃ in an oven (Senxin DGG-9240A, Shanghai, China) for 24 h, and sediment porosity was calculated from the water content (Cermelj et al., 1997). Organic matter in the sediment was measured by loss on ignition (LOI), which was determined after ignition at 550 ℃ for 5 h (Dean, 1974). All the analytical methods described above were used consistently during the long-term investigation.
2.5 Pore water diffusive flux calculationsThe pore water in the sediment cores was sampled using a series of mini-peepers (Ding et al., 2010). Each peeper contained 30 vertically disposed dialysis cells at a vertical resolution of 4 mm. The capacity of each cell was approximately 300 μL. Before each peeper was used, a nitric fiber filter membrane with an aperture of 0.45 μm was fixed on both sides of the peeper, and the peeper was placed in deionized water and filled with nitrogen for 24 h to discharge the oxygen from the peeper. All of the peepers were inserted into the sediment with approximately 3–5 cm exposed above the sediment-water interface (SWI) to obtain an intact interstitial water-overlying water profile and allowed to stabilize for three days, after which the peepers were pulled gently from the sediment and flushed with oxygen-free deionized water before analysis for ammonia nitrogen (NH4+-N) and soluble reactive phosphorus (SRP). The balanced pore water in each peeper was removed with an enzyme marker plate. The concentrations of NH4+-N and SRP were analyzed using a microtiter plate reader (Biotek Epoch, Winooski, VT, USA) by a miniaturized photometrical method (Laskov et al., 2007). Based on the analyzed pore water profiles, the diffusive fluxes of NH4+-N and SRP across the SWI were calculated using Fick's first law of diffusion (Ullman and Aller, 1982). The detailed method for calculating the diffusive fluxes was described by Liu et al. (2016).
2.6 Meteorological dataMeteorological data, including wind speed and wind direction in April 2018, July 2018, January 2019, and November 2019, were obtained from the National Meteorological Data Service Center website, (http://data.cma.cn/en), using daily records from meteorological station #58326 of the China Meteorological Administration.
2.7 Statistical analysisVisualization and spatial interpolation were performed using Origin 9.0 software (OriginLab, Northampton, MA, USA) and ArcGIS (Kriging method, ESRI, Redlands), respectively. Analysis of covariance (ANCOVA) was carried out using SPSS 23.0 (IBM, New York, NY, USA) statistical software. The significance levels are reported as not significant (P > 0.05) or significant (P < 0.05) for all tests.
3 RESULT 3.1 Spatiotemporal patterns of nutrients and chlorophyll-a concentrations and wind in Chaohu LakeThe concentrations of TN varied from 2.08 to 3.10 mg/L at the 15 sampling sites in Chaohu Lake from 2018–2019 (Fig. 2a). The average TN concentrations in spring, summer, autumn, and winter were 2.46±0.29, 2.16±0.09, 2.81±0.28, and 2.58± 0.35 mg/L, respectively. The value of TP ranged from 0.03 to 0.22 mg/L (Fig. 2b). The mean values of TP in spring, summer, autumn, and winter were 0.09±0.06, 0.21±0.01, 0.19±0.01, and 0.08±0.06 mg/L, respectively. Chl-a concentration varied from 8.44 to 136.82 μg/L, with mean values of 26.21±7.74, 112.53±25.38, 64.50±22.53, and 23.29±12.87 μg/L in spring, summer, autumn, and winter, respectively (Fig. 2c). There were two distinct peaks: the first was in June, and the second was in August.
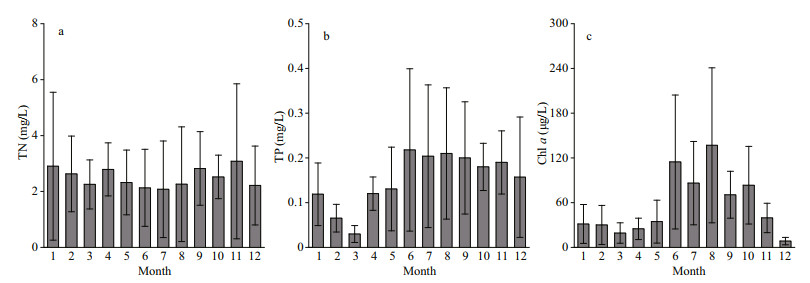
|
| Fig.2 The temporal variation in total nitrogen (TN), total phosphorus (TP), and chlorophyll-a (Chl-a) concentrations in Chaohu Lake Error bars indicate the standard deviations for 15 sampling sites. |
The TN and TP concentrations gradually decreased from the western to the eastern lake region (Fig. 3a–b). In spring, relatively high values of Chl a were observed in the northeastern region, with the highest concentration of 101.03 μg/L at site 11# (Fig. 3c). In contrast, the concentrations of Chl a in summer decreased slowly from the western to the eastern lake region, with the highest value detected at site 2# (Fig. 3d). The highest value of Chl a was observed at site 2# in autumn, with higher values detected in the southeastern region (Fig. 3e). In winter, the values of Chl a were higher in the southern region than in the northern region (Fig. 3f).
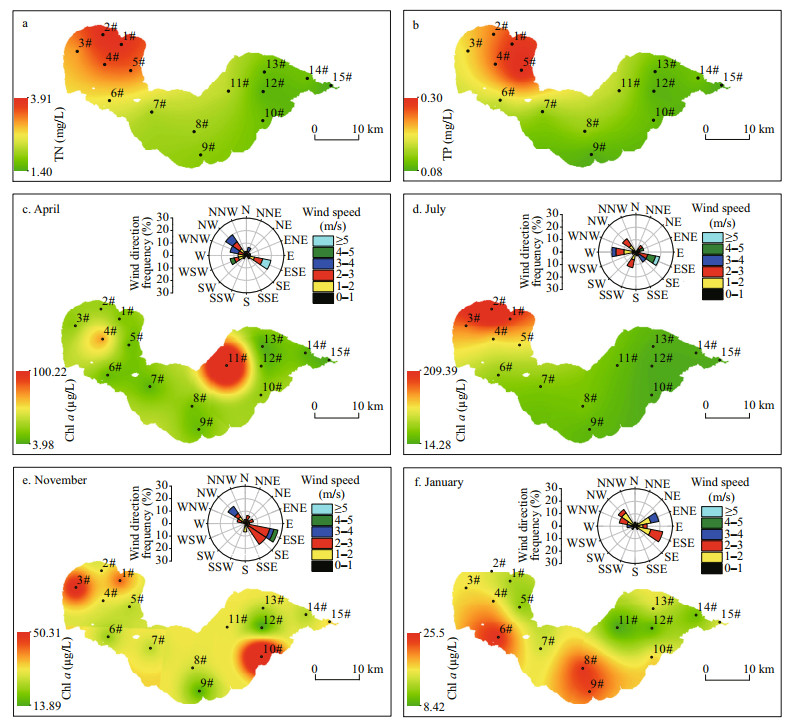
|
| Fig.3 The spatial distribution of total nitrogen (TN), total phosphorus (TP) and Chl-a concentrations, and wind roses in Chaohu Lake TN and TP: annual mean value; Chl a: April represents spring, July represents summer, November represents the autumn, and January represents the winter. |
Wind roses displaying seasonal patterns of wind at Chaohu Lake were produced for April 2018, July 2018, January 2019, and November 2019 (Fig. 3c–f). In spring, the prevailing winds were from the northwest or east-southeast. Wind speeds of 1–3 m/s occurred at a frequency greater than 60%, and wind speeds > 3 m/s occurred at a frequency less than 30%. In summer, the prevailing winds were from the west or east-southeast. Wind speeds were typically < 3 m/s, with this range representing approximately 80% of wind speeds recorded. The wind rose charts showed a typical pattern of dominant southeast or east-southeast winds in autumn. Wind speeds were typically < 3 m/s, at approximately 20% frequency for each of the prevailing wind direction. The prevailing winds during winter were primarily from the east-southeast or east-northeast. Wind speeds of 1–3 m/s constituted approximately 90% of recorded wind speeds; wind speeds > 3 m/s accounted for approximately 10%.
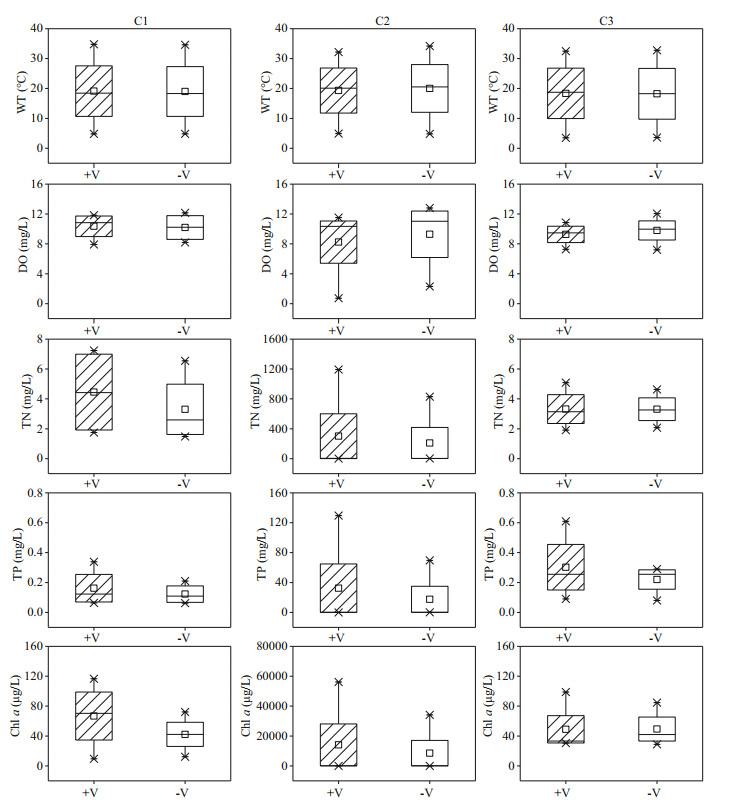
3.2 Differences in physicochemical parameters and algal biomass in the water column in the littoral zonesThe littoral zone included the RCLZ (V+) and its surrounding ULZ (V-). Water temperatures ranged between 3.5 ℃ and 34.6 ℃ over the entire year in the littoral zones (Fig. 4). The DO concentrations varied from 0.73 to 12.15 mg/L, with relatively high values (> 10.86 mg/L) in winter. In addition, the concentrations of TN, TP, and Chl a in the littoral zones were generally higher during summer than during spring, autumn, and winter. The mean concentration of TN was 340.71 mg/L in summer, which was 62.57, 163.59, and 134.74 times higher than the values in spring, autumn, and winter, respectively. The mean value of TP was 33.33 mg/L in summer, which was 118.04, 415.63, and 157.71 times higher than that in spring, autumn, and winter, respectively. The average concentration of Chl a was 15 086.72 μg/L in summer, followed by lower contents in spring, autumn, and winter.

|
| Fig.4 Seasonal variation in dissolved oxygen (DO), water temperature (WT), total nitrogen (TN), total phosphorus (TP), and Chl-a concentrations in the littoral zones +V: in the RCLZ; -V: in the ULZ; ×: the value of percentage 1% and 99%; □: the mean value; the box connecting different symbols quartiles represents the range of concentration; April represents spring, July represents summer, November represents the autumn, and January represents the winter. |
The lowest concentration of DO (0.73 mg/L) was observed in summer in the RCLZ. Moreover, the concentrations of Chl a were much higher in the RCLZ than in the ULZ, particularly in spring and summer, indicating that algal blooms could be trapped and accumulate dramatically in the RCLZ compared with more restrained growth in the ULZ. However, the Chl-a concentrations in the RCLZ were close to those in the ULZ in autumn and winter. Likewise, the concentrations of TN and TP were also higher in the RCLZ than in the ULZ during spring and summer.
Relatively low concentrations of DO were observed in the northern (C2) region, with the lowest value (0.73 mg/L) observed in the littoral zones during the investigation period occurring in this region (Fig. 5). Additionally, the concentrations of TN, TP, and Chl a in the littoral zones of the C2 region were much higher than values in the northeastern (C1) and southern regions (C3). For instance, the mean concentrations of TN, TP, and Chl a were 255.79 mg/L, 25.01 mg/L, and 11 324.19 μg/L in the C2 region, which were 76.05, 95.19, and 229.07 times higher than those in the C3 region, respectively. In addition, the water temperatures and DO concentrations of the RCLZ were close to those of the ULZ. Furthermore, the concentrations of Chl a in the RCLZ were 58.1% and 62.3% higher than those in the ULZ in the C1 and C2 regions, respectively. The concentrations of TN and TP were also higher in the RCLZ than in the ULZ. However, the concentrations of TN, TP, and Chl a were similar between the RCLZ and ULZ in the C3 region.
|
| Fig.5 Spatial variation in dissolved oxygen (DO), water temperature (WT), total nitrogen (TN), total phosphorus (TP), and Chl-a concentrations in the littoral zones +V: in the RCLZ; -V: in the ULZ; ×: the value of percentage 1% and 99%; □: the mean value; the box connecting different symbols quartiles represents the range of concentration. |
TN, TP, Chl a, and LOI for the surface sediment were much higher during autumn than during spring, summer, and winter in the littoral zones (Fig. 6), and the differences were significant (P < 0.05), except for that for the content of LOI. For example, the mean values of TN, TP, Chl a, and LOI in autumn were 1 085.64 mg/kg, 259.68 mg/kg, 2453.95 ng/g, and 1.44%, which were 1.81, 1.87, 4.28, and 0.97 times higher than those in winter, respectively. Moreover, seasonal scale analysis showed that the values of TN, TP, Chl a, and LOI in the surface sediments were higher in the RCLZ than in the ULZ. For instance, the values of TN, TP, Chl a, and LOI in the surface sediments of the RCLZ were 30.5%, 31.4%, 15.4%, and 2.8%, respectively, higher than those in the ULZ in autumn. In addition, the water content and porosity of the surface sediment were very similar between the RCLZ and ULZ during the research period.
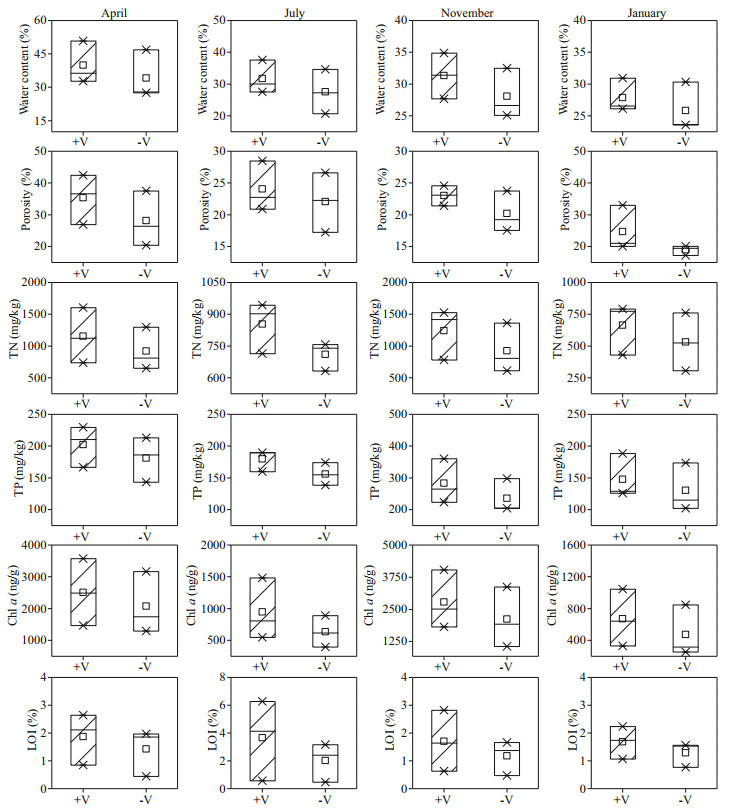
|
| Fig.6 Seasonal variation in physicochemical properties of the surface sediments in the littoral zones TN: total nitrogen; TP: total phosphorus; Chl a: chlorophyll a; LOI: loss on ignition; +V: in the RCLZ; -V: in the ULZ; ×: the value of percentage 1% and 99%; □: the mean value; the box connecting different symbols quartiles represents the range of concentration. April represents spring, July represents summer, November represents the autumn, and January represents the winter. |
The diffusive flux of NH4+-N in the littoral zones was higher during summer and autumn than during spring and winter (Fig. 7a). Furthermore, the diffusive flux of NH4+-N was higher in the RCLZ than in the ULZ at the seasonal scale. Specifically, the values for the diffusive flux of NH4+-N in the RCLZ in spring, summer, autumn, and winter were 42.6%, 45.8%, 71.0%, and 90%, respectively, higher than those in the ULZ. However, the diffusive flux of SRP in the littoral zones was higher during spring and summer than during autumn and winter (Fig. 7b). Moreover, the seasonal-scale results showed that the diffusive flux of SRP was also higher in the RCLZ than in the ULZ.

|
| Fig.7 Diffusive fluxes of NH4+-N (a) and SRP (b) across the SWI in the littoral zones Error bars represent the standard deviations of sampling points from three littoral zones. +V=in the RCLZ, -V=in the ULZ. |
There was a seasonal pattern in the Chl-a concentration in the littoral zone water columns (Fig. 4). Our results indicate that Chl-a concentration was higher in summer than in other seasons (Figs. 2–4). Thus, the level of algal accumulation in the littoral zones is a more serious concern during summer than during spring, autumn, or winter. Previous studies have confirmed that the formation of a surface water bloom is determined by sufficient biomass of buoyant algae (Paerl and Otten, 2013). Furthermore, the initial algal biomass in the water column can influence the thickness of the algal patch that forms and its viscosity at the water surface (Deng et al., 2016). Water temperature has been found to be an important factor affecting algal blooms (Trombetta et al., 2019), with warm temperatures normally favoring algae (Jöhnk et al., 2008). Moreover, our data also showed a clear trend of higher algal biomasses in the warmer seasons (Fig. 4). In addition, nitrogen and phosphorus are important nutrients for lake algal blooms. In Chaohu Lake, nutrient concentrations were sufficiently high to meet algal growth requirements (Fig. 2), which provided favorable conditions for massive algal proliferation (Xu et al., 2003).
We observed spatial variation in Chl-a concentration in the water among the littoral zone regions, with much higher concentrations of Chl a in the C2 region than in the C1 and C3 regions (Fig. 5). Our results showed that more algal biomass was trapped and accumulated in the C2 region than in the C1 and C3 regions. Most cyanobacterial species have gas vacuoles, organelles of low density that provide buoyancy (Oliver and Walsby, 1984; O'Brien et al., 2004). When the wind blows in one direction, these positively buoyant cyanobacteria easily concentrate along the downwind shore (Cyr, 2017). Moreover, wind speed and wind direction can affect the horizontal distribution of algae (Chen et al., 2003; Deng et al., 2016; Xu and Chen, 2020). Cao et al. (2006) found that algal blooms in Taihu Lake were formed at wind speeds under a critical value of 3.1 m/s. Wu et al. (2015) reported that a decline in wind speed promoted the extension of algal bloom areas. In addition, previous field studies have demonstrated that algae are horizontally transported to downwind zones by wind-driven currents under critical wind speeds (Bai et al., 2005; Moreno-Ostos et al., 2009; Deng et al., 2016). In this study, the wind speed was predominantly less than 3.0 m/s, and wind direction was mainly from the east to southeast at Chaohu Lake (Fig. 3). The wind pattern was capable of transporting algae to downwind zones. Thus, compared with the C1 and C3 regions, the C2 region was conducive to high algae accumulation.
We observed an obvious capture effect on algal blooms by reed wetlands (Figs. 4–5). The reasons for the capture by reed wetlands of algal blooms are as follows. 1) Affected by the barrier and adsorption properties of reed wetlands in the littoral zones, algae can easily adsorb onto reeds once they enter reed wetlands. 2) Reed wetlands can weaken stormy waves in the littoral zones; thus, algae that already appear within these regions are less likely to be transported out. Reed wetlands continue to exert a capture effect in autumn and winter. The reason may be that withered reeds can still weaken wind and waves when algal biomass is low. Therefore, reed wetlands in the littoral zones can undoubtedly play a significant role in the mechanics of algal accumulation in eutrophic lakes.
The accumulation of algae in the littoral zones, directly and indirectly, affects a variety of water quality parameters. The growth and decay of algae could in turn lead to changes in DO and nutrient concentrations (Zhu et al., 2013). However, our data show that the DO level was influenced only by algal accumulation in summer, since the RCLZ of the C2 region formed an anaerobic environment (DO < 2 mg/L, Figs. 4–5). A possible explanation is that photosynthetic rates and DO strongly increased during the accumulation phase, but oxygen was rapidly consumed during decomposition, especially at night. The accumulation of algae may be responsible for elevated TN and TP levels. Zhu et al. (2013) reported that the trends for both TN and TP tracked those of Chl-a concentration in the littoral zones of Taihu Lake, consistent with our results. A previous study indicated that algal blooms might also contribute to increased levels of particulate phosphorus (Zhu et al., 2008). In addition, we observed higher concentrations of TN and TP in the RCLZ than in the ULZ (Figs. 4–5). Thus, we suggest that reed wetlands could aggravate the enriched levels of nitrogen and phosphorus in the water column.
4.2 Influence of algal accumulation on surface sediment in the littoral zonesDifferent seasons show different responses to algal accumulation in the littoral zones in terms of overall nutrient levels and organic matter content in surface sediment. We found that the values of TN, TP, Chl a, and LOI for surface sediment were higher in autumn than in other seasons (Fig. 6). Algae that accumulated dramatically in summer did not settle on the sediment surface at that time; in contrast, they settled rapidly on the sediment surface and decomposed in autumn. In addition, compared with the ULZ, we observed higher values of TN, TP, Chl a, and LOI for the RCLZ. This phenomenon may be due to a high rate of primary production and a reduced rate of decomposition under anaerobic conditions (Aerts et al., 1999). Thus, reed wetlands could aggravate the deposition of pollutants in surface sediments.
Compared with rates in spring and winter, we observed higher rates of NH4+-N diffusion in summer and autumn during the investigation period (Fig. 7a). The high content of organic matter in the surface sediment (Fig. 6) would be expected to lead to consumption of oxygen across the SWI in summer and autumn due to microbial decomposition of the organic matter (Kristensen, 2000). The consumption of oxygen may accelerate the formation of anoxic conditions across the SWI and thereby accelerate the dissolution of NH4+-N from the sediment (Brandes and Devol, 1997) and increase diffusion rates. However, we found that the rates of SRP diffusion were higher during spring and summer than during autumn and winter (Fig. 7b). A possible explanation is that the sedimentary P release was induced by low levels or depletion of DO during algal decomposition (Gao et al., 2013; Zhu et al., 2013). Furthermore, we also found that the diffusive fluxes of NH4+-N and SRP were lower in the littoral zones than in the open regions of many eutrophic lakes (Lewandowski et al., 2007; Liu et al., 2016). The reason may be the lower water content and porosity of the sediments in the littoral zones of Chaohu Lake (Fig. 6). Liu et al. (2019) also found that reduced water content and porosity of sediment might also suppress the diffusion of NH4+-N and SRP from sediment. In addition, we observed that the accumulation of algae had a more serious impact on the diffusive fluxes of NH4+-N and SRP in the RCLZ than in the ULZ. The RCLZ would be favorable for the formation of anaerobic environments due to their higher levels of algal accumulation.
4.3 Management insightsThe current study demonstrated that reed wetlands were able to capture and accumulate algae in the littoral zones of Chaohu Lake. These results are important for ecological restoration methodology, such as planting reeds in the littoral zones can more effectively retain accumulated algae to mitigate algal nuisance in the open regions. Considering the limited ability of reed wetlands to degrade algae as well as the partial deterioration of the ecological environment in the littoral zones, physical measures such as mechanical salvage are suggested to remove massive algal blooms trapped by reed wetlands in the warm seasons or the downwind region of Chaohu Lake. Fundamentally speaking, the main methods for preventing and controlling algal blooms involve reductions of nutrient levels in Chaohu Lake (e.g., controlling pollution sources in upstream basins, treating the water in the river, treatment of heavily polluted sediments and removal of algal sources from sediment).
5 CONCLUSIONAfter studying the impact of the accumulation of algal blooms on reed wetlands in the littoral zones of Chaohu Lake, we conclude that more algal biomass was trapped in the RCLZ than in the ULZ, indicating that reed wetlands could obviously retain accumulated algae in the littoral zones. The accumulation of algae in the littoral zones exhibited obvious spatiotemporal characteristics. Algal accumulation levels were highest in summer due to high water temperatures and algal biomasses. Likewise, the northern littoral zones were conducive to the development of large algal blooms because of the wind pattern. The nutrient levels and organic matter content in surface sediment in autumn showed significantly responses to algal accumulation in the littoral zones. The values of TN, TP, Chl a, and LOI in surface sediments were higher in the RCLZ than in the ULZ due to a high rate of primary production and a reduced rate of decomposition under anaerobic conditions. Moreover, different seasons showed different responses to algal accumulation in the littoral zones in terms of the rates of NH4+-N and SRP diffusion. The diffusive fluxes of NH4+-N and SRP were lower in the littoral zones than in the open regions due to the lower water content and porosity of the sediments. The accumulation of algae had a more serious impact on the diffusive fluxes of NH4+-N and SRP in the RCLZ than in the ULZ due to the higher levels of algal accumulation. Thus, considering the limited ability of reed wetlands to degrade algae as well as the partial deterioration of the ecological environment in the littoral zones, we need to combine mechanical salvage with other physical methods to eliminate blooms when algae accumulate massively in the RCLZ. Our study may provide a scientific basis for the prevention and control of algal blooms in the littoral zones of Chaohu Lake and other similar large shallow eutrophic lakes.
6 DATA AVAILABILITY STATEMENTThe datasets generated and/or analyzed during the current study are available from the corresponding author on request.
7 ACKNOWLEDGMENTWe thank Lei JIANG and Chengfei TONG from Nanjing Institute of Geography and Limnology, Chinese Academy of Sciences for their assistance in the sampling and parameters setting.
Aerts R, Verhoeven J T A, Whigham D F. 1999. Plant-mediated controls on nutrient cycling in temperate fens and bogs. Ecology, 80(7): 2170-2181.
DOI:10.1890/0012-9658(1999)080[2170:PMCONC]2.0.CO;2 |
Bai X H, Hu W P, Hu Z X, Li X H. 2005. Importation of wind-driven drift of mat-like algae bloom into Meiliang Bay of Taihu Lake in 2004 summer. Environmental Science, 26(6): 57-60.
(in Chinese with English abstract) DOI:10.13227/j.hjkx.2005.06.011 |
Brandes J A, Devol A H. 1997. Isotopic fractionation of oxygen and nitrogen in coastal marine sediments. Geochimica et Cosmochimica Acta, 61(9): 1793-1801.
DOI:10.1016/S0016-7037(97)00041-0 |
Cai L L, Zhu G W, Liu J W, Xiang S L, Liu J, Chang B H, Dai X, Guo Y. 2018. Characteristics and effects on nutrients of algal blooms accumulation and dissipation in littoral zone. China Environmental Science, 38(8): 3087-3093.
(in Chinese) DOI:10.19674/j.cnki.issn1000-6923.2018.0329.(inChinese) |
Cai Y F, Kong F X. 2013. Diversity and dynamics of picocyanobacteria and the bloom-forming cyanobacteria in a large shallow eutrophic lake (lake Chaohu, China). Journal of Limnology, 72(3): 473-484.
DOI:10.4081/jlimnol.2013.e38 |
Cao H S, Kong F X, Luo L C, Shi X L, Yang Z, Zhang X F, Tao Y. 2006. Effects of wind and wind-induced waves on vertical phytoplankton distribution and surface blooms of Microcystis aeruginosa in Lake Taihu. Journal of Freshwater Ecology, 21(2): 231-238.
DOI:10.1080/02705060.2006.9664991 |
Cermelj B, Bertuzzi A, Faganeli J. 1997. Modelling of pore water nutrient distribution and benthic fluxes in shallow coastal waters (Gulf of Trieste, Northern Adriatic). Water, Air, and Soil Pollution, 99(1-4): 435-443.
DOI:10.1007/BF02406883 |
Chen B F, Feng M H, Shang L X, Ke F, Wu X D, Li Y. 2016. Effects on cyanobacterial growth and water quality after harvesting accumulated cyanobacteria in autumn: an in-situ experiment in Lake Chaohu. Journal of Lake Sciences, 28(2): 253-262.
(in Chinese with English abstract) DOI:10.18307/2016.0203 |
Chen Y W, Chen K N, Hu Y H. 2006. Discussion on possible error for phytoplankton chlorophyll-a concentration analysis using hot-ethanol extraction method. Journal of Lake Sciences, 18(5): 550-552.
(in Chinese with English abstract) DOI:10.18307/2006.0519 |
Chen Y W, Qin B Q, Teubner K, Dokulil M T. 2003. Long-term dynamics of phytoplankton assemblages: Microcystis-domination in Lake Taihu, a large shallow lake in China. Journal of Plankton Research, 25(4): 445-453.
DOI:10.1093/plankt/25.4.445 |
Cyr H. 2017. Winds and the distribution of nearshore phytoplankton in a stratified lake. Water Research, 122: 114-127.
DOI:10.1016/j.watres.2017.05.066 |
Dean W E. 1974. Determination of carbonate and organic matter in calcareous sediments and sedimentary rocks by loss on ignition; comparison with other methods. Journal of Sedimentary Research, 44(1): 242-248.
DOI:10.1306/74D729D2-2B21-11D7-8648000102C1865D |
Deng J C, Chen F, Liu X, Peng J X, Hu W P. 2016. Horizontal migration of algal patches associated with cyanobacterial blooms in an eutrophic shallow lake. Ecological Engineering, 87: 185-193.
DOI:10.1016/j.ecoleng.2015.12.017 |
Ding S M, Sun Q, Xu D. 2010. Development of the DET technique for high-resolution determination of soluble reactive phosphate profiles in sediment pore waters. International Journal of Environmental Analytical Chemistry, 90(14-15): 1130-1138.
DOI:10.1080/03067310903434733 |
Duan H T, Tao M, Loiselle S A, Zhao W, Cao Z G, Ma R H, Tang X X. 2017. MODIS observations of cyanobacterial risks in a eutrophic lake: implications for long-term safety evaluation in drinking-water source. Water Research, 122: 455-470.
DOI:10.1016/j.watres.2017.06.022 |
Fukuhara H, Nemoto F, Takeuchi Y, Toda N. 2007. Nitrate dynamics in a reed belt of a shallow sand dune lake in Japan: analysis of nitrate retention using stable nitrogen isotope ratios. Hydrobiologia, 584(1): 49-58.
DOI:10.1007/s10750-007-0589-6 |
Gao L, Zhang L H, Hou J Z, Wei Q, Fu F, Shao H B. 2013. Decomposition of macroalgal blooms influences phosphorus release from the sediments and implications for coastal restoration in Swan Lake, Shandong, China. Ecological Engineering, 60: 19-28.
DOI:10.1016/j.ecoleng.2013.07.055 |
Gu Z D. 2012. Life cycle of bloom-forming cyanobacteria and its influencing factors. Applied Mechanics and Materials, 209-211: 1227-1230.
DOI:10.4028/www.scientific.net/AMM.209-211.1227 |
Hao B B, Wu H P, Liu W Z, Yu W P, Xing W. 2013. Vegetation features and degradation causes in Chaohu lakeshore zone. Environmental Science and Management, 38(6): 59-65.
(in Chinese with English abstract) DOI:10.3969/j.issn.1673-1212.2013.06.013 |
Ho J C, Michalak A M, Pahlevan N. 2019. Widespread global increase in intense lake phytoplankton blooms since the 1980s. Nature, 574(7780): 667-670.
DOI:10.1038/s41586-019-1648-7 |
Jiang X, Wang S H, Zhong L X, Jin X C, Sun S Q. 2010. Seasonal variation characteristics of algae biomass in Chaohu Lake. Environmental Science, 31(9): 2056-2062.
(in Chinese with English abstract) DOI:10.13227/j.hjkx.2010.09.013 |
Jiang Y J, He W, Liu W X, Qin N, Ouyang H L, Wang Q M, Kong X Z, He Q S, Yang C, Yang B, Xu F L. 2014. The seasonal and spatial variations of phytoplankton community and their correlation with environmental factors in a large eutrophic Chinese lake (Lake Chaohu). Ecological Indicators, 40: 58-67.
DOI:10.1016/j.ecolind.2014.01.006 |
Jöhnk K D, Huisman J, Sharples J, Sommeijer B, Visser P M, Stroom J M. 2008. Summer heatwaves promote blooms of harmful cyanobacteria. Global Change Biology, 14(3): 495-512.
DOI:10.1111/j.1365-2486.2007.01510.x |
Kong X Z, Jørgensen S E, He W, Qin N, Xu F L. 2013. Predicting the restoration effects by a structural dynamic approach in Lake Chaohu, China. Ecological Modelling, 266: 73-85.
DOI:10.1016/j.ecolmodel.2013.07.001 |
Kristensen E. 2000. Organic matter diagenesis at the oxic/anoxic interface in coastal marine sediments, with emphasis on the role of burrowing animals. Hydrobiologia, 426(1): 1-24.
DOI:10.1023/A:1003980226194 |
Kudari V A, Kanamadi R D. 2008. Impact of changed trophic status on the zooplankton composition in six water bodies of Dharwad district, Karnataka state (South India). Environmental Monitoring and Assessment, 144(1-3): 301-313.
DOI:10.1007/s10661-007-9993-7 |
Laskov C, Herzog C, Lewandowski J, Hupfer M. 2007. Miniaturized photometrical methods for the rapid analysis of phosphate, ammonium, ferrous iron, and sulfate in pore water of freshwater sediments. Limnology and Oceanography: Methods, 5(1): 63-71.
DOI:10.4319/lom.2007.5.63 |
Lewandowski J, Laskov C, Hupfer M. 2007. The relationship between Chironomus plumosus burrows and the spatial distribution of pore-water phosphate, iron and ammonium in lake sediments. Freshwater Biology, 52(2): 331-343.
DOI:10.1111/j.1365-2427.2006.01702.x |
Li K Y, Liu Z W, Gu B H. 2010. The fate of cyanobacterial blooms in vegetated and unvegetated sediments of a shallow eutrophic lake: a stable isotope tracer study. Water Research, 44(5): 1591-1597.
DOI:10.1016/j.watres.2009.11.007 |
Liu C, Du Y H, Chen K N, Ma S Z, Chen B F, Lan Y M. 2019. Contrasting exchanges of nitrogen and phosphorus across the sediment-water interface during the drying and re-inundation of littoral eutrophic sediment. Environmental Pollution, 255: 113356.
DOI:10.1016/j.envpol.2019.113356 |
Liu C, Zhong J C, Wang J J, Zhang L, Fan C X. 2016. Fifteen-year study of environmental dredging effect on variation of nitrogen and phosphorus exchange across the sediment-water interface of an urban lake. Environmental Pollution, 219: 639-648.
DOI:10.1016/j.envpol.2016.06.040 |
Moreno-Ostos E, Cruz-Pizarro L, Basanta A, George D G. 2009. Spatial heterogeneity of cyanobacteria and diatoms in a thermally stratified canyon-shaped reservoir. International Review of Hydrobiology, 94(3): 245-257.
DOI:10.1002/iroh.200811123 |
O'Brien K R, Meyer D L, Waite A M, Ivey G N, Hamilton D P. 2004. Disaggregation of Microcystis aeruginosa colonies under turbulent mixing: laboratory experiments in a grid-stirred tank. Hydrobiologia, 519(1-3): 143-152.
DOI:10.1023/B:HYDR.0000026501.02125.cf |
Oliver R L, Walsby A E. 1984. Direct evidence for the role of light-mediated gas vesicle collapse in the buoyancy regulation of Anabaena flos-aquae (Cyanobacteria). Limnology and Oceanography, 29(4): 879-886.
DOI:10.4319/lo.1984.29.4.0879 |
Paerl H W, Huisman J. 2008. Blooms like it hot. Science, 320(5872): 57-58.
DOI:10.1126/science.1155398 |
Paerl H W, Otten T G. 2013. Harmful cyanobacterial blooms: causes, consequences, and controls. Microbial Ecology, 65(4): 995-1010.
DOI:10.1007/s00248-012-0159-y |
Qi C, Wang G X, Wu X T, Xu X G, Han R M, Wu S J. 2017. Deposition characteristics of suspended solids and the response of dissolved nutrients in Spring in the Western Lakeside of Taihu Lake. Environmental Science, 38(1): 95-103.
(in Chinese with English abstract) DOI:10.13227/j.hjkx.201607102 |
Shang L X, Feng M H, Liu F F, Xu X G, Ke F, Chen X C, Li W C. 2015. The establishment of preliminary safety threshold values for cyanobacteria based on periodic variations in different microcystin congeners in Lake Chaohu, China. Environmental Science: Processes & Impacts, 17(4): 728-739.
DOI:10.1039/c5em00002e |
Sollie S, Coops H, Verhoeven J T A. 2008. Natural and constructed littoral zones as nutrient traps in eutrophicated shallow lakes. Hydrobiologia, 605(1): 219-233.
DOI:10.1007/s10750-008-9356-6 |
State Environmental Protection Administration, Editorial Board of Water and Wastewater Monitoring and Analysis Methods. 2002. Methods for the Examination of Water and Wastewater. 4th edn. China Environmental Science Press, Beijing, China. p. 425-426. (in Chinese)
|
Trombetta T, Vidussi F, Mas S, Parin D, Simier M, Mostajir B. 2019. Water temperature drives phytoplankton blooms in coastal waters. PLoS One, 14(4): e0214933.
DOI:10.1371/journal.pone.0214933 |
Ullman W J, Aller R C. 1982. Diffusion coefficients in nearshore marine sediments. Limnology and Oceanography, 27(3): 552-556.
DOI:10.4319/lo.1982.27.3.0552 |
Von Sperling E, da Silva Ferreira A C, Gomes L N L. 2008. Comparative eutrophication development in two Brazilian water supply reservoirs with respect to nutrient concentrations and bacteria growth. Desalination, 226(1-3): 169-174.
DOI:10.1016/j.desal.2007.02.105 |
Wang H J, Wang W D, Lu J W, Yin C Q. 2006a. Algae trapping in Macrophyte-covered littoral zone and water sources protection. China Water & Wastewater, 22(7): 1-3, 8.
(in Chinese with English abstract) DOI:10.3321/j.issn:1000-4602.2006.07.001 |
Wang H J, Wang W D, Yin C Q, Wang Y C, Lu J W. 2006b. Littoral zones as the photspotsq of nitrous oxide (N2O) emission in a hyper-eutrophic lake in China. Atmospheric Environment, 40(28): 5522-5527.
DOI:10.1016/j.atmosenv.2006.05.032 |
Wang L, Li D L, Ding J J, Liang Z H. 2011. Relationship between N and P contents in sediments and two emerged plants in Taihu lakeside wetland. Ecology and Environmental Sciences, 20(10): 1523-1529.
(in Chinese with English abstract) DOI:10.16258/j.cnki.1674-5906.2011.10.009 |
Wang X L, Zhang M, Yin J. 2018. Composition and influential factors of phytoplankton function groups in Lake Chaohu. Journal of Lake Sciences, 30(2): 431-440.
(in Chinese with English abstract) DOI:10.18307/2018.0214 |
Wong W W, Greening C, Shelley G, Lappan R, Leung P M, Kessler A, Winfrey B, Poh S C, Cook P. 2021. Effects of drift algae accumulation and nitrate loading on nitrogen cycling in a eutrophic coastal sediment. Science of the Total Environment, 790: 147749.
DOI:10.1016/j.scitotenv.2021.147749 |
Wu T F, Qin B Q, Brookes J D, Shi K, Zhu G W, Zhu M Y, Yan W M, Wang Z. 2015. The influence of changes in wind patterns on the areal extension of surface cyanobacterial blooms in a large shallow lake in China. Science of the Total Environment, 518-519: 24-30.
DOI:10.1016/j.scitotenv.2015.02.090 |
Wu Y, Huang L C, Wang Y L, Li L, Li G B, Xiao B D, Song L R. 2019. Reducing the phytoplankton biomass to promote the growth of submerged macrophytes by introducing artificial aquatic plants in shallow eutrophic waters. Water, 11(7): 1370.
DOI:10.3390/w11071370 |
Wurtsbaugh W A, Paerl H W, Dodds W K. 2019. Nutrients, eutrophication and harmful algal blooms along the freshwater to marine continuum. Wiley Interdisciplinary Reviews: Water, 6(5): e1373.
DOI:10.1002/wat2.1373 |
Xiang S L, Zhu M Y, Zhu G W, Xu H. 2014. Water quality responses to accumulation of Microcystis during cyanobacterial bloom event in different littoral vegetation zones of Lake Taihu, China. Asian Journal of Chemistry, 26(14): 4427-4434.
DOI:10.14233/ajchem.2014.16787 |
Xu D, Chen H M. 2020. Randomly directed and light winds exacerbate the emergence of large-scale cyanobacterial bloom areas in Lake Taihu, China. Applied Ecology and Environmental Research, 18(3): 4695-4708.
DOI:10.15666/aeer/1803_46954708 |
Xu F L, Tao S, Dawson R W, Xu Z R. 2003. The distributions and effects of nutrients in the sediments of a shallow eutrophic Chinese Lake. Hydrobiologia, 492(1-3): 85-93.
DOI:10.1023/A:1024861727693 |
Xu H, Paerl H W, Qin B Q, Zhu G W, Gaoa G. 2010. Nitrogen and phosphorus inputs control phytoplankton growth in eutrophic Lake Taihu, China. Limnology and Oceanography, 55(1): 420-432.
DOI:10.4319/lo.2010.55.1.0420 |
Yan R, Kong F X, Han X B. 2004. Analysis of the recruitment of the winter survival algae on the sediments of Lake Taihu by fluorometry. Journal of Lake Sciences, 16(2): 163-168.
(in Chinese with English abstract) DOI:10.18307/2004.0210 |
Yin H B, Yang C H, Jia Y X, Chen H, Gu X H. 2018. Dual removal of phosphate and ammonium from high concentrations of aquaculture wastewaters using an efficient two-stage infiltration system. Science of the Total Environment, 635: 936-946.
DOI:10.1016/j.scitotenv.2018.04.218 |
Yu H B, Xi B D, Jiang J Y, Heaphy M J, Wang H L, Li D L. 2011. Environmental heterogeneity analysis, assessment of trophic state and source identification in Chaohu Lake, China. Environmental Science and Pollution Research, 18(8): 1333-1342.
DOI:10.1007/s11356-011-0490-8 |
Yu L, Kong F X, Zhang M, Yang Z, Shi X L, Du M Y. 2014. The dynamics of Microcystis genotypes and microcystin production and associations with environmental factors during blooms in Lake Chaohu, China. Toxins, 6(12): 3238-3257.
DOI:10.3390/toxins6123238 |
Zhang M, Kong F X. 2015. The process, spatial and temporal distributions and mitigation strategies of the eutrophi-cation of Lake Chaohu (1984-2013). Journal of Lake Sciences, 27(5): 791-798.
(in Chinese with English abstract) DOI:10.18307/2015.0505 |
Zhang M, Zhang Y C, Yang Z, Wei L J, Yang W B, Chen C, Kong F X. 2016. Spatial and seasonal shifts in bloom-forming cyanobacteria in Lake Chaohu: patterns and driving factors. Phycological Research, 64(1): 44-55.
DOI:10.1111/pre.12112 |
Zhu G W, Wang F, Gao G, Zhang Y L. 2008. Variability of phosphorus concentration in large, shallow and eutrophic Lake Taihu, China. Water Environment Research, 80(9): 832-839.
DOI:10.2175/106143008x304749 |
Zhu L, Shi W Q, Dam B V, Kong L W, Yu J H, Qin B Q. 2020. Algal accumulation decreases sediment nitrogen removal by uncoupling nitrification-denitrification in shallow eutrophic lakes. Environmental Science & Technology, 54(10): 6194-6201.
DOI:10.1021/acs.est.9b05549 |
Zhu M Y, Zhu G W, Zhao L L, Yao X, Zhang Y L, Gao G, Qin B Q. 2013. Influence of algal bloom degradation on nutrient release at the sediment-water interface in Lake Taihu, China. Environmental Science and Pollution Research, 20(3): 1803-1811.
DOI:10.1007/s11356-012-1084-9 |
 2022, Vol. 40
2022, Vol. 40


