Institute of Oceanology, Chinese Academy of Sciences
Article Information
- LIU Xingyu, HAN Xiaolu, HAN Zhiqiang
- Effects of climate change on the potential habitat distribution of swimming crab Portunus trituberculatus under the species distribution model
- Journal of Oceanology and Limnology, 40(4): 1556-1565
- http://dx.doi.org/10.1007/s00343-021-1082-1
Article History
- Received Mar. 26, 2021
- accepted in principle May 6, 2021
- accepted for publication Sep. 2, 2021
Surface water temperature along the eastern coast of China will exhibit a warming trend in the next 80 years, and temperature increase will be higher in summer than in winter (Zhang et al., 2020). These changes will markedly affect the marine ecology and fishery resources in eastern China. Moreover, one study has shown that climate change may lead to the migration of species to higher latitudes and the reduction of suitable habitats. Shifts in the distribution of various marine species have already been observed across all ocean regions (Poloczanska et al., 2016). In the Yellow Sea, the distribution range of Gadus macrocephalus has moved northward by 0.5° N since 1959 (Li et al., 2012). Alterations in the distribution will result in community restructuring and ecosystem biodiversity losses (Rilov, 2016). Such alterations are projected to have a serious impact on marine fisheries and coastal human communities (Silva et al., 2019). Apart from the shift in distribution, the life histories and life cycles of marine species have also been significantly influenced by climate change (Merino et al., 2012). Thus, projecting the spatiotemporal habitat distribution of marine species under future climate scenarios is necessary to explore the potential effects of climate change on coastal fisheries. Such projections will improve our knowledge of how marine species will respond to climate change.
Swimming crab Portunus trituberculatus is widely distributed in the coastal waters of China, Japan, and Korea. It is one of the most important commercial species in China, with a catch of 458 380 metric tons in 2019 (Fishery Administration of the Ministry of Agriculture and Rural Areas et al., 2020). This species exhibits the typical habit of seasonal migration, and its spatial distribution varies significantly across the seasons. Every spring, swimming crabs come to the shallow coastal waters of China (approximately 3–5-m deep) for growth and spawning. Then, they gradually migrate to the deep waters (10–30-m deep) in autumn (Dai et al., 1977). Previous studies have focused on the morphology, habits, reproduction, breeding, and migration of swimming crabs (Fu et al., 2019; Lu et al., 2019; Su et al., 2020). Temperature and salinity are important abiotic factors that affect the growth, survival, and molting of this species (Ren et al., 2017; Lu et al., 2019). Hu et al. (2011) reported that the suitable salinity for swimming crabs is 15–35, and the optimum salinity is 25–30. Dai et al. (2014) found that temperature increase could accelerate the molting of crabs and promote their development. Simultaneously, their energy expenditure and metabolism increase, and their survival rate decreases. The distribution and biomass of swimming crabs may be closely related to climate factors. However, the potential effects of climate change on the distribution of this species have not yet been determined. Therefore, assessing the impact of climate change on the distribution of swimming crabs is necessary and can provide important data for developing future climate adaptation management strategies.
The species distribution model (SDM), which is based on the relationship between species occurrence and environmental variables, is used to evaluate the potential distribution of a species and identify its large-scale habitat preferences (Becker et al., 2020). In recent years, researchers have proposed more than a dozen SDMs, but they differ in their scope of use and algorithms. Among them, maximum entropy (Maxent) has received a good evaluation. It calculates the optimal state of species distribution laws under the constraints of niche based on the principle of climate similarity and maximum entropy. A generalized linear model (GLM) is a simple and commonly used model that can predict the current species distribution ability, but it cannot handle complex response curves. A generalized additive model (GAM) is also optimized on this basis. An artificial neural network (ANN) can be conveniently used in information processing that is diffcult to express using conventional calculation methods (Zhao et al., 2016). For example, Xue et al. (2018) used Maxent to predict the potential distribution of Scomber japonicus in the northwest Pacific Ocean during peak fishing season (May to October) and found range shifts in other months. In addition, SDM can also assess the possible effects of climate change on the distribution of marine species, with good predictions of key habitat variables (Silva et al., 2019; Zhang et al., 2019).
However, a single SDM typically exhibits greater uncertainty and non-universality (Thuiller, 2003; Zhang et al., 2011). Ensemble SDMs provided by Biomod2 software (Thuiller et al., 2014) can solve these shortcomings. This software can map the primary trend of a model (i.e., average, median, or other percentiles) to the overall change or uncertainty of all models. In addition, Aguirre-Gutiérrez et al. (2013) compared the synthesis effects of five models, namely, GAM, GLM, generalized boosted model (GBM), random forest (RF), and ANN, in Biomod2 with the accuracy of Maxent. They found that the ensemble model exhibited a better effect. Therefore, an ensemble SDM that integrated nine single SDM algorithms was applied to predict the potential distribution of swimming crabs in the future by using environmental and biological data in the current study. We also attempted to identify the key environmental variables that will determine the distribution of this species.
2 MATERIAL AND METHOD 2.1 Swimming crab dataThe coordinate data of species occurrence point were obtained through fishery resource surveys. From 1998 to 2000, the "Beidou" fishery science survey vessel conducted four fishery resource surveys in the East China Sea, Yellow Sea, and Bohai Sea (Fig. 1). The surveys used bottom trawl sampling to obtain species distribution data. The bottom trawl had a mesh size of 836 mesh × 20 cm, and towing speed was approximately 3.0 knots. A total of 356 stations were sampled per season, but not all station sampling was successful. Subsequently, 306 occurrence records of swimming crabs in spring, 296 records in summer, 275 records in autumn, and 290 records in winter were included (Fig. 1; Jin et al., 2006).
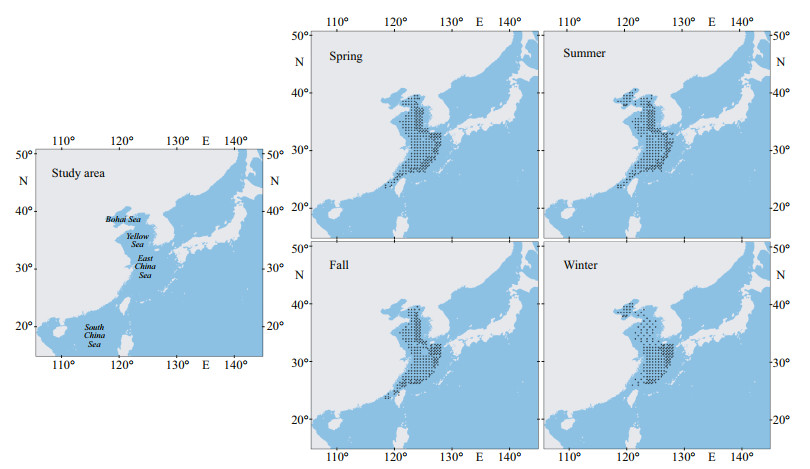
|
| Fig.1 Study area and swimming crab occurrence data for the four seasons |
Although the distribution and life cycle of marine species are influenced by many environmental factors, previous studies have found that species distribution can be accurately predicted by a few key variables (Belanger et al., 2012; Assis et al., 2018; Bosch et al., 2018; Goldsmit et al., 2018). Considering the availability, match ability, high resolution, and biological relevance of environmental data, we used five predictor environmental variables in SDM. Sea surface temperature, surface salinity, current velocity, and offshore distance were extracted from the Bio-ORACLE database (http://www.bio-oracle.org) (Morley and Heusser, 1977; Basher et al., 2014). Meanwhile, variable depth was extracted from the Global Marine Environment Dataset (http://gmed.auckland.ac.nz).
Temperature directly affects the physiological habit of swimming crabs, and it varies considerably across seasons (Doney et al., 2012). The mean sea surface temperature was used to represent the mean season surface temperature in spring and autumn, and the average temperatures of the warmest and coldest months were used for the environmental layers in summer and winter, respectively. We adopted a spatial resolution of 5×5 arcmin or higher and a geographic coverage of approximately 9.2×9.2 km2 to match the five environmental data layers. The all-pairwise Pearson correlation coeffcient of the environmental data was below |0.7| to remove prohibitive levels of redundancy among layers (Zhang et al., 2019).
We retrieved the future environmental data layers under the typical concentration path emission scenarios of Representative Concentration Pathway (RCP)4.5 and RCP8.5 from Bio-ORACLE to predict the spatial distribution of swimming crabs under climate change. Future layers were produced by averaging data from distinct atmosphere-ocean coupled general circulation models (i.e., submodels of the Community Climate System Model 4, the Hadley Center Global Environmental Model 2, and the Model for Interdisciplinary Research on Climate 5) provided by the Coupled Model Intercomparison Project Phase 5 (CMIP5) (Assis et al., 2018). This database provides two time series, namely, 2050s (2040–2050) and 2100s (2090–2100), for the prediction of environment variables (Tyberghein et al., 2012). RCP4.5 is a medium emission scenario, and RCP8.5 is a pessimistic scenario with higher concentrations (Moss et al., 2010). Therefore, we disregarded RCP2.6 and RCP6.0 and considered RCP4.5 and RCP8.5 in investigating potential future distributional shifts of swimming crabs.
2.3 Ensemble prediction model 2.3.1 Construction and evaluation of ensemble modelIn this study, we used nine algorithms in the Biomod2 software package to build an ensemble model for prediction. The nine algorithms were GAM, GLM, GBM, RF, classification tree analysis (CTA), ANN, surface range envelope (SRE), flexible discriminant analysis (FDA), and Maxent. The Biomod2 model requires presence and pseudo-absence data (non-distributed data). After inputting the presence point data, the model randomly generates three sets of pseudo-absence data based on the presence and background point data (the non-distributed data in the research area), with 500 pseudo-existence points in each group. Then, the sample point data (presence and pseudo-absence points) participating in the modeling was divided into two parts: 80% as the training set and 20% as the test set. Each model algorithm was repeated 10 times, and each time can produce 270 single-model operation results (Zhu et al., 2019).
The kappa coeffcient, true skill statistics (TSS), and receiver operating characteristic (ROC) were used to evaluate the accuracy of the model (Lobo et al., 2008). The value range of TSS was [-1, 1]. When TSS was greater than 0.8, the simulation effect was excellent, 0.6–0.8 was good, and 0.4–0.6 was average. The value range of the area under the curve (AUC) was [0.5, 1]. When the value range of AUC was 0.5– 0.6, the simulation effect failed, 0.6–0.7 was poor, 0.7–0.8 was fair, 0.8–0.9 was good, and 0.9–1 was excellent (Swets, 1988). We screened single models with TSS > 0.85 and AUC > 0.9 and used the weighted average method to construct a combined forecasting model. The weight of the calculation results of a single model was determined by its TSS value. The normalized results of a single model were multiplied by the corresponding weights, and the combined model was constructed to calculate the potential suitable habitat index of swimming crabs in the study area. The relative importance of each variable in predicting the distribution of the species was measured using a randomization approach.
2.3.2 Prediction of potential habitat range and related changesTo produce a presence/absence map of swimming crabs, we selected the fixed threshold method to binarize the image. A habitat greater than 0.3 was identified as a suitable habitat; otherwise, it was an unsuitable habitat. The results of the ensemble model were visualized via the Biomod range size function in Biomod2 to show the loss, stability, and acquisition of suitable habitats under future scenarios. From the binary layer, we can calculate the rate of change of habitats.
In addition, centroid calculation was implemented in the SDMTools software package. The distribution centroid of species was the weighted average of each grid data coordinate. NA (missing value) data were automatically removed from the analysis. We used 0.2 ℃ as the temperature increase to simulate the reduction rate of the species' suitable habitat when the temperature parameter increased by 0.2–2 ℃. We obtained 10 data and plotted the reduction trend line of summer species.
3 RESULT 3.1 Model assessment and environmental variable factor contributionThe results of the contribution of environmental variables indicated that the contribution of temperature was higher, while the contribution of flow velocity was the weakest. The response curves of temperature showed that the suitable temperature range for this species varied considerably across seasons (Fig. 2). The result indicated that the optimum temperature in spring and autumn was approximately 13–25 ℃; it was approximately 24–29 ℃ in summer, and the crabs can survive if it was above 0℃ in winter. The accuracy of the developed model was assessed through TSS and ROC values. The TSS values for every single SDM varied from 0.73 (SRE) to 0.86 (GAM) (Fig. 3). The ROC values varied from 0.88 (SRE) to 0.94 (RF) (Fig. 4). Considering the values of TSS and AUC, the GAM model provided the best predictive performance. Considering the cutoff values of TSS and ROC, all the nine model algorithms were applied for further ensemble modeling. The values of TSS and AUC in the ensemble model were 0.90±0.02 and 0.97±0.20, respectively. The predictive performance of the ensemble model exceeded the performance of each single model, indicating a high level of accuracy in the ensemble model.

|
| Fig.2 Response curves of the predicted occurrence probability of swimming crabs against temperature based on the GAM model Redlines represent the results of the second (middle) run of the response curve algorithm, and blue lines show the first and third runs. Vertical lines represent temperatures at species distribution sites. |
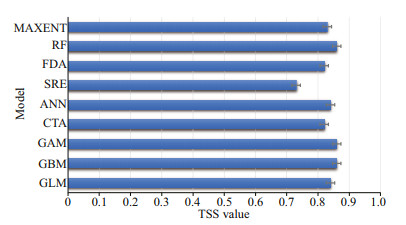
|
| Fig.3 TSS values for single models |

|
| Fig.4 Prediction accuracy of different models based on ROC values |
The current habitat predicted by the ensemble model is basically consistent with the occurrence records of swimming crabs in our field survey (Figs. 1 & 5). Figure 6 predicts the change in the distribution area of swimming crabs, including the increased, decreased, and stable areas. Under future climate scenarios, the suitable habitat for swimming crabs will decrease in spatial extent, particularly in summer (Fig. 6). Table 1 shows the calculation of the total habitat change rates for the species. Under RCP4.5 climate change scenario, a decrease of 4.47% (winter) to 35.29% (summer) was predicted for suitable habitats in the 2050s. Meanwhile, the loss of suitable habitats in the 2100s was predicted from 3.37% (winter) to 45.23% (summer). The loss of potential distribution will be more aggravated under RCP8.5 than under RCP4.5. The loss of future potential distribution under RCP8.5 will mostly occur in summer, with 37.87% (2050s) to 88.26% (2100s). Under climate change, the loss of potential distribution will largely occur in the South China Sea (Fig. 6). The South China Sea is predicted to become less suitable for this species by 2100s. By contrast, suitable habitats in the Bohai Sea and the northern part of the Sea of Japan will increase.

|
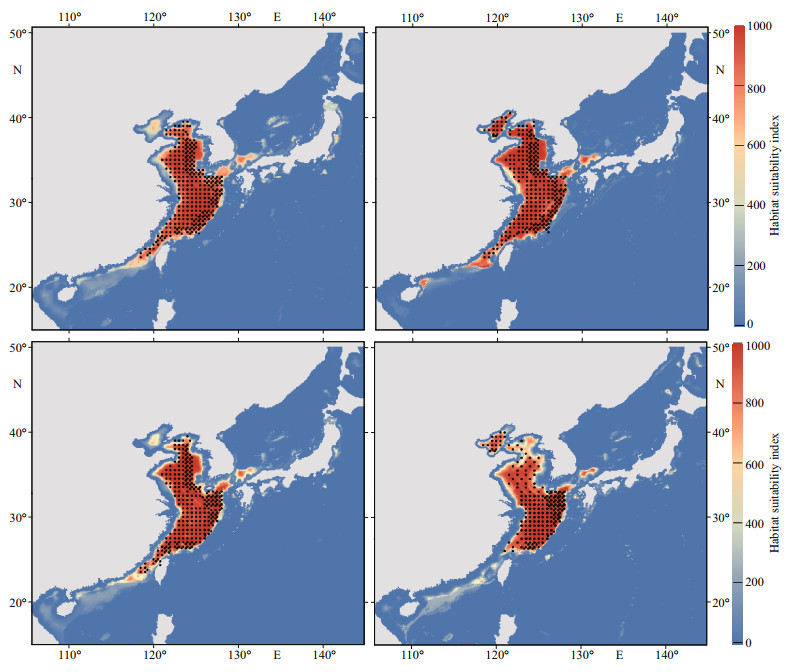
|
| Fig.5 Predicted current potential distribution of swimming crabs The color gradient indicates variation in habitat suitability (red: highest, blue: lowest). |

|
| Fig.6 Changes in the suitable habitat of swimming crabs in different seasons in 2050s and 2100s under RCP4.5 and RCP8.5 Gain: new predicted habitats in the future; stable: an area where habitats are stable; loss: habitats predicted to be lost in the future. |
Future predictions under RCP4.5 and RCP8.5 showed that suitable habitats for this species will shift northward (higher latitude). The distribution centroid of swimming crab will shift northward under future climate scenarios, and the trend will become more obvious with the passage of climate scenarios and years (Table 2). Notably, the latitude shift of centroid in summer is more obvious than that in other seasons. The current latitudinal centroid of the potential habitat of swimming crabs in summer is 31.92°N. Under RCP4.5, the centroid in summer will shift 2.31° northward by the mid 21st century and then 0.86° northward by the late 21st century. Under RCP8.5, the northward shift of the centroid in summer will be 2.49° (2050s) and 13.31° (2100s).

|
We also investigated the temperature increase sensitivity of swimming crabs in summer. The rate of habitat loss increased exponentially with increasing temperature. The habitat loss rate increased to 80.5% when associated with a temperature increase of 2 ℃ in the surface waters of the coastal zone off eastern China (Fig. 7). Notably, the loss rate dropped sharply by 24% when temperature rose by 1.2 ℃ compared with the loss rate when temperature increased by 1 ℃. Our study predicted the loss of suitable habitats for swimming crabs due to increases in surface water temperatures associated with climate change in the study area. Notably, we only considered the overall temperature increase in Fig. 7.

|
| Fig.7 Habitat loss rate of swimming crabs in summer under temperature increase |
In this study, we first developed an ensemble SDM for swimming crabs to predict the seasonal potential suitable habitats for this species under current and future climate conditions. This study may be the first to be conducted in the Northwestern Pacific that considered seasonal distribution variation on the impact of climate change. Our ensemble SDM not only predicted seasonally suitable habitats (i.e., survival probability) for swimming crabs, but also exhibited high performance. Its high predictive performance cannot only be observed from several commonly used statistical parameters, such as AUC, TSS, and kappa, but can also be confirmed by accurately predicting the current known distribution of the species. The present study indicates that the current potential habitat of swimming crabs is widely distributed across the Bohai Sea, Yellow Sea, and East China Sea, with the highest suitability found in the East China Sea (Fig. 5). This result agrees well with fishing data (Fishery Administration of the Ministry of Agriculture and Rural Areas et al., 2020). The East China Sea is a major fishing ground for swimming crabs. Reliable reports of this species being found in the South China Sea are available (Liao et al., 2008). No catch statistics about swimming crabs have been published for the South China Sea.
4.2 Effects of climate change on suitable habitatsThe spatial variability of suitable habitats for swimming crabs confirmed previous empirical studies that showed the effects of climate change on marine nekton distributional ranges and abundance (Yáñez et al., 2008; Cheung et al., 2010). The present SDM provides a decrease in swimming crab habitat suitability in the East China Sea, indicating that climate change will result in a spatial redistribution of this species and a serious decline in catch by the 2050s and 2100s. However, the Bohai Sea will be more suitable than the Yellow Sea and the East China Sea for swimming crabs under the two RCP scenarios. Although the Bohai Sea and Yellow Sea are both located on the continental shelf, the warming seawater will compensate for the inherent lack of high latitude and low temperature in the Bohai Sea, attracting the migration of the species' population. The predicted shift in range is biologically plausible under climate change. In accordance with our results, the high water temperature will force the northward distribution of swimming crabs in the East China Sea. Sea temperature can affect various physiological processes of swimming crabs involved in growth, survival, and molting. A high water temperature exceeding 30 ℃ can lead to the arrested larval development of swimming crabs (Liao et al., 2008). This thermal tolerance limit will certainly influence the geographical distribution of this species. In addition, the migratory route of swimming crabs will be shortened, changing their life history.
Under the future climate scenario, summer sea temperature will rise significantly. Simultaneously, it will increase with the passage of climate scenarios and time (Moss et al., 2010; Zhang et al., 2020). In addition, nearshore warming will be more evident than that in the deep sea, and warming rate will be faster in high latitudes than in low latitudes, but the temperature base in low latitudes is larger. Therefore, the suitable habitat will first decrease during the initial stage in the coastal and South China Sea, and it will increase slightly in the northern Bohai Sea. Under the pessimistic situation in the later period, the spatial range in which the temperature exceeds the tolerance value of swimming crabs will gradually increase (Fig. 6). In summer, the habitat reduction of this species exhibits a dynamic trend from low latitude to high latitude and from nearshore to deep sea. Notably, summer is the spawning period of swimming crabs, which is the most vulnerable life stage of the species (Dahlke et al., 2020). At this moment, the shift of the centroid of summer suitable habitats toward the north may be one of the reproductive strategies of this species to cope with climate change. However, inevitable losses will occur. Simply changing the timing and location of spawning may result in a mismatch between feeding grounds and areas of high phytoplankton density. Moreover, predicting whether new spawning locations can provide the necessary sediments and corresponding hydrological characteristics for egg deposition is diffcult. Therefore, the change in suitable habitats during summer requires further research.
4.3 Implication for fishery managementThis study can provide important insights into the threat of climate change to suitable habitats of P. trituberculus, which is one of the current concerns of climate change researchers and fisheries managers. Other species in the study area are also speculated to change their geographic distributions in response to climate change, affecting the distribution and catch of existing fisheries. Moreover, fluctuations in the climate during the spawning season can leave species without good recruitment. Therefore, this study can provide a scientific basis for the formulation of a fishery management policy, such as changing the time and location of fishing. We should protect habitats that may be added, monitor environmental changes of habitats that may be lost, and moderately utilize resources in stable habitats.
5 CONCLUSIONPredictions regarding the future habitats of swimming crabs for the period of 2050–2100 will become less favorable due to the negative effects of global warming. Range shift and loss of habitat distribution will reduce the catch of fishery species and ultimately influence local fishing activities. Further studies are necessary to investigate the effects of climate change on fishery resources in the East China Sea and Yellow Sea. Conservation and management actions will be required to mitigate the negative consequences of the predicted climate change in the coastal areas of China. The socioecological system must consider adaptation measures to address the impact of future climate scenarios along China's coast and other parts of the world.
6 DATA AVAILABILITY STATEMENTThe datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Aguirre-GutiȦrrez J, Carvalheiro L G, Polce C, van Loon E E, Raes N, Reemer M, Biesmeijer J C. 2013. Fit-for-Purpose: species distribution model performance depends on evaluation criteria-Dutch hoverflies as a case study. PLoS One, 8(5): e63708.
DOI:10.1371/journal.pone.0063708 |
Assis J, Tyberghein L, Bosch S, Verbruggen H, Serrão E A, De Clerck O. 2018. Bio-ORACLE v2.0: extending marine data layers for bioclimatic modelling. Global Ecology and Biogeography, 27(3): 277-284.
DOI:10.1111/geb.12693 |
Basher Z, Bowden D A, Costello M J. 2014. Global marine environment datasets (GMED). Version 1.0 (Rev. 01. 2014). http://gmed.auckland.ac.nz. Accessed on 2018-10-30.
|
Becker E A, Carretta J V, Forney K A, Barlow J, Brodie S, Hoopes R, Jacox M G, Maxwell S M, Redfern J V, Sisson N B, Welch H, Hazen E L. 2020. Performance evaluation of cetacean species distribution models developed using generalized additive models and boosted regression trees. Ecology and Evolution, 10(12): 5759-5784.
DOI:10.1002/ece3.6316 |
Belanger C L, Jablonski D, Roy K, Berke S K, Krug A Z, Valentine J W. 2012. Global environmental predictors of benthic marine biogeographic structure. Proceedings of the National Academy of Sciences of the United States of America, 109(35): 14046-14051.
DOI:10.1073/pnas.1212381109 |
Bosch S, Tyberghein L, Deneudt K, Hernandez F, De Clerck O. 2018. In search of relevant predictors for marine species distribution modelling using the MarineSPEED benchmark dataset. Diversity and Distributions, 24(2): 144-157.
DOI:10.1111/ddi.12668 |
Cheung W W L, Lam V W Y, Sarmiento J L, Kearney K, Watson R, Zeller D, Pauly D. 2010. Large-scale redistribution of maximum fisheries catch potential in the global ocean under climate change. Global Change Biology, 16(1): 24-35.
DOI:10.1111/j.1365-2486.2009.01995.x |
Dahlke F T, Wohlrab S, Butzin M, Pörtner H O. 2020. Thermal bottlenecks in the life cycle define climate vulnerability of fish. Science, 369(6499): 65-70.
DOI:10.1126/science.aaz3658 |
Dai A Y, Feng Z G, Song Y Z, Huang Z X, Wu H C. 1977. Preliminary investigation of fishery biology of Portunus trituberculatus. Journal of Zoology, 12(2): 30-33.
(in Chinese) DOI:10.13859/j.cjz.1977.02.015 |
Dai C, Wang F, Fang Z H, Dong S L. 2014. Effects of temperature on the respiratory metabolism and activities of related enzymes of swimming crab Portunustrituberculatusenzyme activity in Portunus trituberculatus. Progress in Fishery Sciences, 235(2): 90-96.
(in Chinese with English abstract) |
Doney S C, Ruckelshaus M, Duffy J E, Barry J P, Chan F, English C A, Galindo H M, Grebmeier J M, Hollowed A B, Knowlton N, Polovina J, Rabalais N N, Sydeman W J, Talley L D. 2012. Climate change impacts on marine ecosystems. Annual Review of Marine Science, 4: 11-37.
DOI:10.1146/annurev-marine-041911-111611 |
Fu S L, Ding M M, Liang Q J, Yang Y J, Chen M, Wei X F, Wang A L, Liao S A, Ye J M. 2019. The key differentially expressed genes and proteins related to immune response in the spleen of pufferfish (Takifugu obscurus) infected by Aeromonas hydrophila. Fish & Shellfish Immunology, 91: 1-11.
DOI:10.1016/j.fsi.2019.05.016 |
Goldsmit J, Archambault P, Chust G, Villarino E, Liu G, Lukovich J V, Barber D V, Howland K L. 2018. Projecting present and future habitat suitability of ship-mediated aquatic invasive species in the Canadian Arctic. Biological Invasions, 20(2): 501-517.
DOI:10.1007/s10530-017-1553-7 |
Hu Z H, Xu J Z, Shi J G. 2011. Farming modes of swimming crab (Portunus trituberculatus) along the coast of Zhejiang Province. Modern Fisheries Information, 26(3): 3-5.
(in Chinese) |
Jin X S, Cheng J S, Qiu S Y, Li P J, Cui Y, Dong J. 2006. Integrated Research and Evaluation on Fisheries Resources in Yellow Sea and Bohai Sea. Ocean Press, Beijing, China. p. 350-357. (in Chinese)
|
Li Z L, Jin X S, Zhang B, Zhou Z P, Shan X J, Dai F Q. 2012. Interannual variations in the population characteristics of the Pacific cod Gadus macrocephalus in the Yellow Sea. Oceanologia et Limnologia Sinica, 43(5): 924-931.
(in Chinese) |
Liao Y Y, Xiao Z P, Yuan Y Y. 2008. Temperature tolerance of larva and juvenile of Portunus trituberculatus. Acta Hydrobiologica Sinica, 32(4): 534-543.
(in Chinese with English abstract) DOI:10.3321/j.issn:1000-3207.2008.04.014 |
Lobo J M, JimȦnez-Valverde A, Real R. 2008. AUC: a misleading measure of the performance of predictive distribution models. Global Ecology and Biogeography, 17(2): 145-151.
DOI:10.1111/j.1466-8238.2007.00358.x |
Lu S K, Li R H, Zheng W B, Chen L, Ren Z M, Mu C K, Song W W, Wang C L. 2019. Long-term low-salinity stress affects growth, survival and osmotic related gamma-aminobutyric acid regulation in the swimming crab Portunus trituberculatus. Aquaculture Research, 50(10): 2888-2895.
DOI:10.1111/are.14242 |
Merino G, Barange M, Blanchard J L, Harle J, Holmes R, Allen I, Allison E H, Badjeck M C, Dulvy N K, Holt J, Jennings S, Mullon C, Rodwell L D. 2012. Can marine fisheries and aquaculture meet fish demand from a growing human population in a changing climate?. Global Environmental Change, 22(4): 795-806.
DOI:10.1016/j.gloenvcha.2012.03.003 |
Morley J J, Heusser L E. 1997. Role of orbital forcing in East Asian monsoon climates during the last 350 kyr: evidence from terrestrial and marine climate proxies from core RC14-99. Geochemistry, Geophysics, Geosystems, 12(3): 483-493.
DOI:10.1029/97PA00213 |
Moss R H, Edmonds J A, Hibbard K A, Manning M R, Rose S K, van Vuuren D P, Carter T R, Emori S, Kainuma M, Kram T, Meehl G A, Mitchell J F B, Nakicenovic N, Riahi K, Smith S J, Stouffer R J, Thomson A M, Weyant J P, Wilbanks T J. 2010. The next generation of scenarios for climate change research and assessment. Nature, 463(7282): 747-756.
DOI:10.1038/nature08823 |
Poloczanska E S, Burrows M T, Brown C J, GarcȪa Molinos J, Halpern B S, Hoegh-Guldberg O, Kappel C V, Moore P J, Richardson A J, Schoeman D S, Sydeman W J. 2016. Responses of marine organisms to climate change across oceans. Frontiers in Marine Science, 3: 62.
DOI:10.3389/fmars.2016.00062 |
Ren X Y, Yu X, Gao B Q, Li J, Liu P. 2017. iTRAQ-based identification of differentially expressed proteins related to growth in the swimming crab, Portunus trituberculatus. Aquaculture Research, 48(6): 3257-3267.
DOI:10.1111/are.13155 |
Rilov G. 2016. Multi-species collapses at the warm edge of a warming sea. Scientific Reports, 6: 36897.
DOI:10.1038/srep36897 |
Silva C, Leiva F, Lastra J. 2019. Predicting the current and future suitable habitat distributions of the anchovy (Engraulis ringens) using the Maxent model in the coastal areas off central-northern Chile. Fisheries Oceanography, 28(2): 171-182.
DOI:10.1111/fog.12400 |
Su X P, Liu J J, Wang F, Wang Q H, Zhang D, Zhu B S, Liu D P. 2020. Effect of temperature on agonistic behavior and energy metabolism of the swimming crab (Portunus trituberculatus). Aquaculture, 516: 734573.
DOI:10.1016/j.aquaculture.2019.734573 |
Swets J A. 1988. Measuring the accuracy of diagnostic systems. Science, 240(4857): 1285-1293.
DOI:10.1126/science.3287615 |
Thuiller W, Georges D, Engler R. 2014. Biomod2: ensemble platform for species distribution modelling. R package version 3.3-7.1.
|
Thuiller W. 2003. BIOMOD-optimizing predictions of species distributions and projecting potential future shifts under global change. Global Change Biology, 9(10): 1353-1362.
DOI:10.1046/j.1365-2486.2003.00666.x |
Tyberghein L, Verbruggen H, Pauly K, Troupin C, Mineur F, De Clerck O. 2012. Bio-ORACLE: a global environmental dataset for marine species distribution modelling. Global Ecology and Biogeography, 21(2): 272-28.
DOI:10.1111/j.1466-8238.2011.00656.x |
Xue J L, Fan W, Tang F H, Guo G G, Tang W, Zhang S M. 2018. Analysis of potential habitat distribution of Scomber japonicus in northwest Pacific Ocean using maximum entropy model. South China Fisheries Science, 14(1): 92-98.
(in Chinese with English abstract) DOI:10.3969/j.issn.2095-0780.2018.01.012 |
Yáñez E, Hormazábal S, Silva C, Montecinos A, Barbieri M A, Valdenegro A, Órdenes A, GȮmez F. 2008. Coupling between the environment and the pelagic resources exploited off Northern Chile: ecosystem indicators and a conceptual model. Latin American Journal of Aquatic Research, 36(2): 159-181.
DOI:10.3856/vol36-issue2-fulltext-3 |
Zhang C C, Wei H, Song G S, Xie C. 2020. IPCC-CMIP5 Based projection and analysis of future sea surface temperature changes in coastal Seas East of China. Oceanologia et Limnologia Sinica, 51(6): 1288-1300.
(in Chinese with English abstract) |
Zhang L, Liu S, Sun P S, Wang T L. 2011. Segmentation and mapping of uncertain components in the simulation of the impact of climate change on species distribution: a case study of Pinus tabulaeformis. Acta Ecologica Sinica, 19: 5749-5761.
(in Chinese) |
Zhang Z X, Xu S Y, Capinha C, Weterings R, Gao T X. 2019. Using species distribution model to predict the impact of climate change on the potential distribution of Japanese whiting Sillago japonica. Ecological Indicators, 104: 333-340.
DOI:10.1016/j.ecolind.2019.05.023 |
Zhao Z F, Wei H Y, Guo Y L, Gu W. 2016. Potential distribution of Panax ginseng and its predicted responses to climate change. Chinese Journal of Applied Ecology, 27(11): 3607-3615.
(in Chinese with English abstract) |
Zhu N. 2019. Simulation of suitable habitat distribution of Magnolia officinalis based on Ensemble Model. Journal of Sichuan Agricultural University, 37(04): 481-489.
|
 2022, Vol. 40
2022, Vol. 40


