Institute of Oceanology, Chinese Academy of Sciences
Article Information
- LIN Huina, WU Weiwei, SUN Lin, WITKOWSKI Andrzej, LI Xiaoye, PATIL Vishal, LIANG Junrong, LI Xuesong, GAO Yahui, CHEN Changping
- Chinia gen. nov.—the second diatom genus simonsenioid raphe from mangroves in Fujian, China
- Journal of Oceanology and Limnology, 40(3): 1220-1232
- http://dx.doi.org/10.1007/s00343-021-1067-0
Article History
- Received Feb. 22, 2021
- accepted in principle Mar. 16, 2021
- accepted for publication Jun. 7, 2021
2 State Key Laboratory of Marine Environmental Science, Xiamen University, Xiamen 361102, China;
3 Institute of Marine and Environmental Sciences, University of Szczecin, Mickiewicza 16a, PL-70-383 Szczecin, Poland
Mangrove forests are plant communities occurring in the intertidal zone of tropical and subtropical estuaries and coastal areas. Due to their transitional location between the ocean, estuary, and land, they support high levels of primary productivity and a rich biodiversity (de Albuquerque Ribeiro et al., 2019). As primary producers, diatoms play a vital part in the food web of the mangrove forests (Siqueiros-Beltrones and Sánchez-Castrejón, 1999; Gao, 2001). Several unique and rare species/genera of diatoms have been found (Siqueiros-Beltrones and Sánchez-Castrejón, 1999; Siqueiros-Beltrones and López-Fuerte, 2006; Seddon et al., 2014; Bąk et al., 2019; Li et al., 2020c) among the habitats associated with mangrove forests, in the plankton or the benthos on the sediment or the mangroves themselves (Du and Jin, 1983; Maples, 1983; Chen et al., 2010; Sahoo and Ansari, 2018; Sahoo et al., 2018). However, only very few publications reported new diatom taxa from mangrove forests along the coast of southeastern China (Chen et al., 2010; Li et al., 2020c).
In the last decade, a wider effort by diatomologists characterized the diatoms of the Chinese Pacific coast, adding to a number of published papers on local and regional scales, using morphological and molecular approaches (Liu et al., 2012; Li et al., 2015, 2017, 2018a; Witkowski et al., 2016; Krzywda et al., 2019). This led to the description of several genera and dozens of species (new to science) and expanded the records of species occurring in various habitats of the Yellow Sea, the East China Sea, and the South China Sea, including epibiotic diatoms (Li et al., 2014, 2018b, 2020a, 2020b).
In this paper, a new diatom genus featuring the simonsenioid canal raphe type is described from mangroves in the Jiulong estuary on the Fujian coast in the South China Sea. The paper presents some cytological characteristics (plastid) and a detailed ultrastructure revealed by light microscopy (LM), scanning electron microscopy (SEM) and transmission electron microscopy (TEM) observations for this diatom genus, along with some basic information on ecology. At present, our attempts to obtain molecular data for this taxon were not successful, and its possible phylogenetic position is discussed based on its morphology.
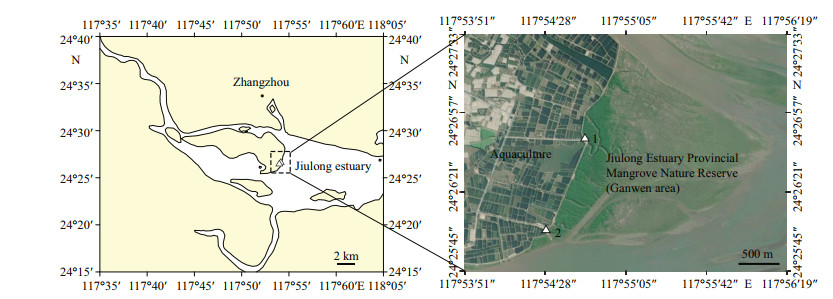
2 MATERIAL AND METHODThe study site is located in the mangrove forest in the Jiulong estuary, the Jiulong Estuary Provincial Mangrove Nature Reserve (Ganwen area), in Longhai district of Zhangzhou city in Fujian Province, Southeastern (SE) China (24°26'4"N-24°26'45"N, 117°54'29"E-117°54'46"E; Fig. 1). The mangrove ecoregion features a semidiurnal tide ranging from 1.0 to 5.5 m (Chen et al., 2017). The ecoregion was listed as one of the key protected wetlands in China, with an area covering about 420.2 hm2 and large aquaculture facilities located in the vicinity.
|
| Fig.1 Location of the Jiulong Estuary Provincial Mangrove Nature Reserve (Ganwen area), Zhangzhou, Fujian Province, SE China Triangles indicate the sampling sites in the mangrove area. |
Samples of mangrove leaves (Kandelia obovata Sheue, H. Y. Liu & J. Yong), fiddler crabs (Uca arcuata de Haan), seaweed (Ishige okamurai Yendo), mangrove prop roots (K. obovata), stones, shells, and sediment were collected with forceps in high tide zone during low tide in May 2018, May 2019, August 2019, and October 2019. Water salinity, pH, and temperature were measured in situ using a Horiba U-52 portable probe (Japan). The samples were kept in zip lock bags, cryopreserved and taken to the laboratory.
In laboratory, samples were rinsed twice with filtered seawater through 0.45-μm filters to wash off planktonic species. Then, diatoms were removed from different biotic substrates in ultrasound bath (300 W for 30 s) and washed four times with the filtered seawater. Diatom samples were then boiled at 100 ℃ for 20 min in concentrated HCl to remove organic matter of the cells, and washed with distilled water 10 times until neutral pH was reached. Permanent microscope slides of the processed samples were mounted with Naphrax for LM observations under the Olympus BX51 microscope with a DP71 digital camera system (Olympus, Japan) and a 100× oil immersion objective. Cleaned diatom frustules for SEM observations were mounted onto small pieces of coverslip or 0.22-μm pore size filters, air-dried and fixed on aluminum stubs, then coated for 60 s with 4-5 nm of gold palladium (working distance: 60 mm, current: 30 mA) in the Leica EM CPD300 (Germany). SEM observations were conducted on the JEOL JSM-6390A (Japan) and FEI Quanta 650 FEG Scanning Electron Microscopy (USA) at 20 kV. Frustules were mounted onto a copper grid and air-dried for TEM observations using the Hitachi HT-7800 (Japan) at 80 kV. Three-dimensional (3D) model images of Chinia longhaiensis gen. et sp. nov. were illustrated with the SolidWorks. Samples and permanent slides were deposited at the School of Life Sciences, Xiamen University, China. Diatom terminology followed Round et al. (1990) and Witkowski et al. (2015).
3 RESULT 3.1 TaxonomyThe new genus was observed from each type of sampled substrates. The taxon was conspicuous due to its characteristic elliptical frustules and large fenestrae (cf. Figs. 2-7).

|
| Fig.2 Light micrographs of Chinia longhaiensis gen. et sp. nov. showing the cell and valve size diminution series with the distinct fenestrae features a-c. living cells showing the plate-like plastid; a. the cell during division; b-c. different sizes of cells illustrating the undulate valve surface; d-k. light micrographs of cleaned Chinia longhaiensis gen. et sp. nov. valves varying in size in girdle view; d-e. girdle view illustrated in bright field optics; f-k. girdle view imaged with phase contrast optics; j. whole frustule with detached valves; k. valve in girdle view with eight fenestrae. Scale bar: 10 μm (a-k). |
|
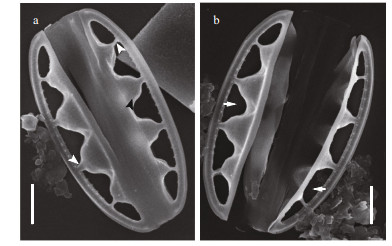
| Fig.3 SEM images of Chinia longhaiensis gen. et sp. nov., showing the outline of whole frustule exterior with large fenestrae and fenestral bars a. near complete frustule showing large fenestrae, fenestral bars (white arrowheads), and infundibulum-like portulae (black arrowhead); b. cell with displaced valves showing large fenestrae (white arrows). Scale bars: 2 μm (a, b). |
|
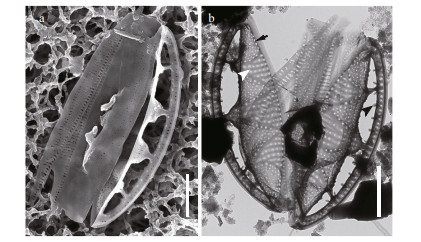
| Fig.4 SEM and TEM images of Chinia longhaiensis gen. et sp. nov. showing the girdle bands of cell a. girdle bands adhering to a single valve as illustrated in SEM; b. Girdle bands of two detached valves as illustrated in TEM. Note the difference in ornamentation of transapical striae in the elevated valve part (white arrowhead) and depressed part (black arrow); also the spinules (black arrowhead). Scale bars: 2 μm (a, b). |

|
| Fig.5 SEM images of Chinia longhaiensis gen. et sp. nov. a. valve internal view with undulate surface, illustrating the position of fibulae (white arrow); b. close-up of the specimen illustrated in (a); note the presence of tiny spinules on infundibulum-like surface (white arrowhead) and the undulate valve; c. external view of the whole valve with the slightly displaced and elevated canal raphe; d-e. close-up of the valve apical part illustrating the apical raphe fissure termination (black arrowheads); f. raphe canal apical end clearly illustrating the presence of helictoglossa (black arrow) and portula (white arrow). Scale bars: 5 μm (a, c, d, e); 3 μm (b, f). |

|
| Fig.6 TEM (a-b, d-e) and SEM (c) of Chinia longhaiensis gen. et sp. nov. a. girdle view illustrating large fenestrae and the central part of the raphe slit, without central nodule (black arrow); b. valve view, showing two rows of puncta in the outer walls of the raphe canal perforating into the other side of the canal (white arrow); c. internal valve surface and the internal surface of the raphe canal; note spinules and lace-curtain-like siliceous deposits (white arrow); two spinules at the poles (white arrowheads) of the canal raphe; d. close-up of distal raphe end (black arrow); e. close-up showing areolation details of the valve surface (the white arrowhead shows the hymenate areolae) and of the raphe canal outer wall (black arrowhead). Scale bars: 2 μm (a-c); 1 μm (d, e). |
|
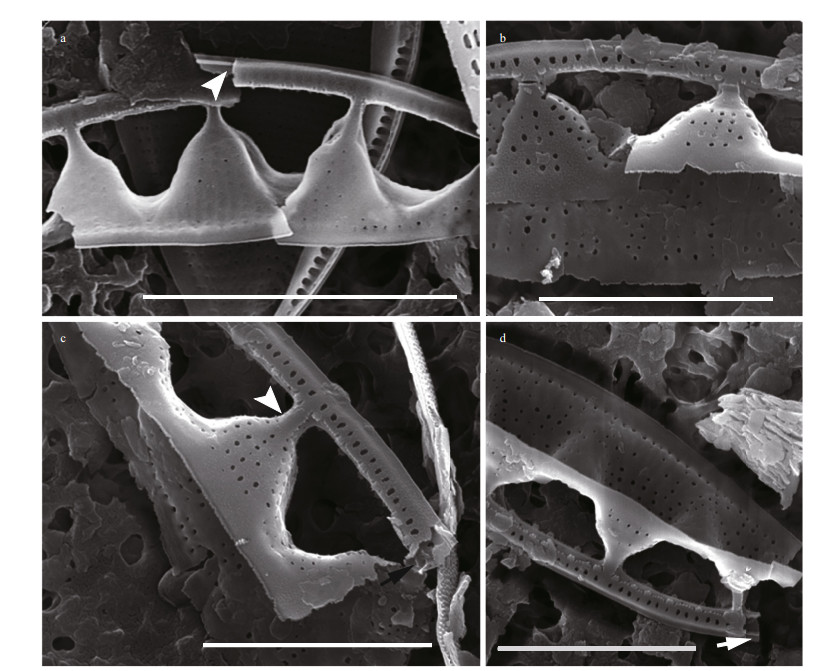
| Fig.7 SEM images of broken Chinia longhaiensis gen. et sp. nov. valves a. broken canal raphe illustrating an internal view of a raphe-bearing tube (white arrowhead); b. broken infundibulum-like structure; c. broken parts showing two rows of puncta on the canal raphe side, tube-like portula (black arrow) and fenestral bar adhering to external surface of portula tube (white arrowhead); note the two depressed valve parts (fibulae) connecting the neighboring infundibulum-like portulae; d. broken valve showing the tube-like raphe canal and the internal raphe position (white arrow). Scale bars: 5 μm (a); 3 μm (b, c, d). |
Systematics:
Class: Bacillariophyceae Haeckel
Subclass: Bacillariophycideae D. G. Mann
Order: Bacillariales Hendey
Family: Bacillariaceae Ehrenberg
Chinia Y. Gao, H. Lin, C. Chen et A. Witkowski gen. nov. (Figs. 2-3)
Description: Frustules elliptical in girdle view with a single plate-like plastid (Fig. 2). Valves lanceolate with acutely rounded apices, valve face distinctly undulate (Fig. 3). Girdle relatively broad, composed of densely perforated copulae with apically oriented rows of pores (Fig. 4). Raphe-bearing canal perforated, positioned on the elevated keel, moderately eccentric (Figs. 5a & c, 6b & c). Raphe slit continuous, without central nodule (Fig. 6a). External apical raphe endings strongly bent in one direction (Fig. 5d-e), internally ending in somewhat elevated helictoglossae (Fig. 5f). Raphe canal connected to cell interior through 3-8 infundibulum-like portulae (Figs. 2-7). Parallel transapical striae composed of irregularly distributed small, circular areolae, observable by TEM and SEM (Figs. 4-6). When observed by LM, cells with a single plate-like plastid.
Etymology: The genus is dedicated to the esteemed biologist and diatomologist Professor Dexiang JIN (CHIN T. G., 1910-1997) from Xiamen University, China, for his contributions to the establishment of the Diatom Laboratory at Xiamen University in the 1930s and in recognition of his achievements in both the marine diatom and biological sciences.
Generitype: Chinia longhaiensis Y. Gao, H. Lin, C. Chen et A. Witkowski sp. nov.
Taxonomic note: The new genus resembles Simonsenia with the simonsenioid type of canal raphe elevated and supported on portulae; the raphe slit without a central brake (central nodule) and perforated fibulae. However, they differ in terms of the perforated raphe canal, reduced fenestral braces and densely perforated copulae in Chinia gen. nov. The two genera also differ in terms of plastids, with two apically positioned plastids in Simonsenia (Witkowski et al., 2015; Kim et al., 2019) and one plate-like plastid in Chinia (this study).
Chinia longhaiensis Y. Gao, H. Lin, C. Chen et A.
Witkowski sp. nov.
Description: Frustules solitary, small, elliptical in girdle view with distinct fenestrae (Fig. 2), and one plate-like axial plastid (Fig. 2b-c). Valves (n=25) lanceolate with acutely rounded apices 8.0-17.0-μm long, 2.1-3.6-μm wide. Valve surface undulate with distinct portulae connecting the raphe-bearing canal to the inner cell. Transapical striae not resolvable by LM.
3.2 Morphology observed by EMWhen observed in a girdle view, the valves show a series of elevated conical parts (infundibulum-like) supporting the portulae alternating with depressed ones (Figs. 2-7) and with a distinct hyaline mantle (Fig. 4). The elevated valve parts constitute the striated valve surface, whereas the depressed, finely perforated parts correspond to fibulae (Fig. 5a). The raphe canal is elevated above the valve surface, supported by portulae and moderately eccentric. Careful examination of SEM images reveals reduced fenestral bars (braces) that adhere tightly to the external surfaces of portula tubes (Fig. 3a). The raphe canal external surface is finely perforated on each side (40- 62 in 10 μm; n=15) and somewhat displaced toward the proximal mantle, with a raphe slit continuous from pole to pole (Figs. 5-6) and external apical ends strongly bent on the same side, ending in small and distinct grooves at the poles (Figs. 5d-e, 6d). Internally, the raphe terminates in small but distinct helictoglossae (Fig. 5f). The raphe canal is separated from the valve surface by distinct fenestrae (4-5 in 10 μm; n=15) (Fig. 3). The raphe canal is ornamented with spinules and lace-curtain-like siliceous deposits, and equipped with two or three spinules at the poles (Figs. 5f & 6c). Tiny spinules positioned over the outer wall of the canal point toward the valve face (Figs. 4b, 5c & f, 6c). Transapical striae are composed of small hymenate, irregularly distributed areolae (Fig. 6e). External areolae are positioned in shallow parallel groves (Figs. 4b, 5, 6) (42–56 in 10 μm; n=10). Striae forming areolae are distinctly larger than those of perforated plate-like fibulae (Fig. 4b).
We were unable to observe the portulae and fibulae from the valve interior in a position perpendicular to the electron beam due to the geometry of the valve forming a relatively high cone with an elevated raphe canal that is unstable in SEM/TEM preparations. For this reason, we have illustrated these structures using 3D images with the SolidWorks (Fig. 8) compiled from numerous SEM and TEM images obtained during this study. The peculiar fenestral bars (braces) adhere to the external surface of the portulae (Fig. 8a; see also Fig. 3a). Internally, plate-like fibulae are perforated near the portula tube-passage (Fig. 8c; see also Fig. 5a–e). Portulae turn into passages connecting the valve interior to the canal raphe (Fig. 8d–f; see also Fig. 5f).

|
| Fig.8 Three-dimensional models of Chinia longhaiensis gen. et sp. nov. illustrated by the SolidWorks a–b. external valve views with fenestral bars adhering to the external surface of portulae (black arrowhead in a). Spinules and lace-curtain-like siliceous deposits on the raphe canal (white arrows in a and b), and two or three spinules at the poles (white arrowheads in a and b); c. internal valve view along the apical axis showing portulae (black arrowhead) and plate-like fibulae (black arrow); d. whole valve view along the apical axis; e. location of portulae connecting the inner valve lumen and the raphe canal (black arrow) on the cross section along the transapical axis; f. location of the raphe canal (black arrowhead) on the cross section along the transapical axis. |
Holotype: Slide No. GWH18050204 (holotype designated here on Fig. 2h) housed at the School of Life Sciences, Xiamen University, Xiamen, China (see Figs. 2–7).
Isotype: Slide No. SZCZ27378 housed in the Diatomological Collection of Andrzej Witkowski at the Institute of Marine and Environmental Sciences, University of Szczecin, Szczecin, Poland.
Type habitat: Jiulong estuary mangrove area in Longhai district, the city of Zhangzhou, Fujian Province, China (24°26′4″N–24°26′45″N; 117°54′29″E– 117°54′46″E), collector: Huina LIN, May 2, 2018.
Etymology: The specific name longhaiensis refers to the sampling site located in Longhai district, the city of Zhangzhou, Fujian Province, Southeastern China.
Ecology: Currently, Chinia longhaiensis was only observed at this particular sampling site. The new taxon was found in four benthic habitats (mangrove leaves, fiddler crabs, seaweeds, and mangrove prop roots) collected from the study area. The taxon inhabits a brackish-water environment with salinities ranging from 8 to 20, in the neutral pH range (7.12 to 7.84), with dissolved oxygen (DO) ranging from 4.59 to 7.68 mg/L and water temperature ranging from 26 to 30 ℃. The associated diatom flora included: Simonsenia aveniformis A. Witkowski, A. Gomes & E. Gusev, Psammodictyon panduriforme var. continuum (Grunow) P. Snoeijs, Tryblionella granulata (Grunow) D. G. Mann, Tryblionella punctata W. Smith, Surirella atomus F. Hustedt, Diploneis bombus C. G. Ehrenberg, Pseudofallacia tenera (Hustedt) Y. Liu, J. P. Kociolek & Q. Wang, Luticola sp., Navicula sp., Amphora sp., and Pleurosigma sp.; they are dominant diatoms in the examined samples.
Taxonomic note: At a first glance, Chinia longhaiensis sp. nov. slightly resembles Simonsenia cf. paucistriata and S. paucistriata (Kim et al., 2019), marine species from the Korean part of the Yellow Sea coast and the Texas coast of the Gulf of Mexico. They have a similar shape, canal raphe position, and portulae. Simonsenia paucistriata and S. cf. paucistriata in fact show morphological features of the valve face that resemble C. longhaiensis as they have an undulate valve surface and solitary rows of areolae in the transapical striae (Kim et al., 2019). However, the main differences between these species are the size of the fenestrae and the width of the girdle; the size and the length are much larger in C. longhaiensis than in any Simonsenia species (Lange-Bertalot, 1979; Witkowski et al., 2014, 2015; You et al., 2016; Kim et al., 2019), including S. cf. paucistriata and S. paucistriata, whereas C. longhaiensis lacks the free-standing, rib-like, supporting braces for the raphe canal which are known only in Simonsenia spp. (Kim et al., 2019; this paper).
4 DISCUSSIONNo species resembling C. longhaiensis have been recorded so far. In this context, Chinia gen. nov. is a surprising discovery for having its novel combination of cytological and morphological characteristics.
4.1 Comparison with other canal raphe-bearing generaCompared to other canal raphe-bearing diatoms, Chinia has similar simonsenioid raphe of Simonsenia, but differs from the genus Nitzschia in the raphe system, which applies in particular to the marginal (diagonal) position of the raphe canal within the frustule. The girdle that in both genera is composed of several copulae each bearing one to two rows of pores. To a certain extent, the fibulae of Chinia and Simonsenia are slightly similar. The fibulae in the former genus are perforated and spacious with a slightly conical surface but are well separated from the valve surface, perforated and horizontally positioned in the latter genus (Lange-Bertalot, 1979; Witkowski et al., 2014). Although both genera have an elevated raphe canal, the raphe canal in Chinia is perforated with rows of solitary pores, whereas that in Simonsenia is closed and forms an unperforated tube. These two genera differ in the development of fenestral bars (braces) that are clearly visible in Simonsenia. The fenestral bars play apparently an important role in supporting the raphe-bearing tube, and the reduced fenestral bars adhering to the external portulae surface in Chinia are merely visible by careful examination (Witkowski et al., 2015; this study). They also differ in terms of plastid morphology: two apically positioned plastids in Simonsenia (Witkowski et al., 2015; Kim et al., 2019) and one plate-like plastid in Chinia (this paper).
As shown by Witkowski et al. (2015) and You et al. (2016), the simonsenioid raphe system in Simonsenia and Chinia (this study) bears some similarity to the surirelloid type in the apical part of the valve; however, this similarity only applies to the external view, as the surirelloid raphe-bearing tube is connected to the valve surface, while simonsenioid raphe-bearing tubes are suspended. The surirelloid raphe system also differs from that of Chinia in the wing of the raphe, which is supported by portulae (=alar canals) at distinct locations in Chinia but connected to the rest of the valve in the surirelloid system (Krammer and Lange-Bertalot, 1987; Round et al., 1990; Ruck and Kociolek, 2004; Ruck and Theriot, 2011). In contrast to the nitzschioid morphology, where the raphe canal is more or less open toward the valve interior, the separation of the raphe canal by large fenestrae makes Chinia easy to distinguish from most Nitzschia species (Krammer and Lange-Bertalot, 1987; Round et al., 1990; Ruck and Theriot, 2011; Trobajo et al., 2013; Mann and Trobajo, 2014). Table 1 presents a summary of the morphological features of Chinia gen. nov. and a comparison of the new genus with the established genera including, among others, Simonsenia, Nitzschia, and Surirella.

|
The longitudinal slit called a "raphe" across the valve component of the siliceous exoskeleton of diatoms serves as a diagnostic character for a monophyletic group of diatoms referred to as "raphid pennates". It is this raphe that allows raphid pennate diatoms to actively glide over various substrates. This raphe system takes several morphological forms across the raphid diatoms, including the "canal" raphe, where the raphe is associated with a canal-like structure supported by transverse siliceous bridges (fibulae). The canal raphe was considered to be the result of a single evolutionary event for decades (Simonsen, 1979; Sims and Paddock, 1982; Round et al., 1990), though more recently, molecular data derived from nuclear and chloroplast genes (Ruck and Theriot, 2011) suggested that the canal raphe system developed independently in Bacillariales, Rhopalodiales, and Surirellales.
Three distinct morphological categories of canal raphe were defined by Ruck and Kociolek (2004) and Witkowski et al. (2015). The nitzschioid type shows a simple canal and fibulae on a relatively simple keel, positioned on the valve at least somewhat eccentrically and open to the valve interior. This type of canal raphe can be found in the genera Bacillaria, Hantzschia, Nitzschia, Pseudonitzschia, Fragilariopsis, and Denticula. The surirelloid type presents a wing elevated on a shallow or deep keel, with the raphe canal connected to the valve interior by narrow tubes (portulae) and is found in the genera Surirella, Stenopterobia, Petrodictyon, and Plagiodiscus. The simonsenioid type displays an elevated raphe canal, which is either strongly or moderately eccentric and runs along the apical axis of the frustule on a wing (ala), supported by portulae (alar canals) and two rows of fenestral bars (braces). This canal raphe type has so far been found in Simonsenia only (Lange-Bertalot, 1979; Witkowski et al., 2015; Kim et al., 2019).
While the surirelloid canal raphe type appears to be homogenous among the taxa belonging to Surirellales (Ruck and Kociolek, 2004), Bacillariales show a wide variation in the raphe canal position and ultrastructure, representing two types of raphe systems: nitzschioid and simonsenioid (Witkowski et al., 2015; Kim et al., 2019; Mann et al., 2021). Despite the differences in the structure of raphe canal systems outlined above, Simonsenia is well positioned within Bacillariceae. In one of the largest published DNA sequence datasets of the family (Mann et al., 2021), Simonsenia clade is derived within Bacillariaceae. This relationship is congruent with other molecular phylogenies of the family constructed by the use of plastid-encoded markers (Witkowski et al., 2015; Kim et al., 2019).
While we were unable to obtain any molecular data from Chinia, we could propose a classification for Chinia based on the simonsenioid canal raphe morphology it exhibits, which leads us to tentatively include the new genus Chinia in the Bacillariaceae, with Simonsenia. It does not escape our notice that Chinia is unique in the Bacillariaceae in having a single plate-like plastid, which is typical to Surirella and Entomoneis (Round et al., 1990). Simonsenia itself, like most of the Bacillariaceae, is characterized by anterior and posterior plastids (Witkowski et al., 2015; Kim et al., 2019).
4.3 Distribution and ecologyAt present, Chinia longhaiensis was observed in our mangrove zone collections only, though it was present in all sampled habitat types. The taxon did not reach a high relative abundance (approximately 1%) in any of the samples. It is not clear how water salinity affects its distribution as C. longhaiensis was sampled in the salinity ranging from 8 to 20; however, the taxon developed well under our laboratory culture conditions at salinity up to ca. 35. This can be interpreted as an indication of euryhaline autecology. Chinia longhaiensis was only present in sonification samples from biological substrates e.g. mangrove prop roots, mangrove leaves, fiddler crabs, and seaweeds, but not present in other samples such as stones, shells, or sediments. Although no images of Chinia frustules attached to the substrates studied were made, we consider that, in terms of functional ecology, it may represent a marine epibiontic diatom. To the best of our knowledge, a similar diatom has never been published even as an unidentified taxon. The discovery of C. longhaiensis is a contribution to the variation in the canal-raphe structure and adds a new genus with simonsenioid canal raphe.
5 CONCLUSIONA novel epibiontic diatom genus, Chinia gen. nov., is described with a new species, Chinia longhaiensis sp. nov., from estuarine mangrove habitats in Fujian, China. The new genus is distinguished by its single plate-like plastid, and disctinct morphological characters showing simonsenioid type raphe canal with sparse infundibulum-like portulae and large fenestrae. These distinctive features of Chinia suggest its affinity to the Bacillariaceae.
6 DATA AVAILABILITY STATEMENTAll data generated and/or analyzed in this study are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENTWe greatly appreciate the useful advice on this new genus provided by Prof. Tamotsu NAGUMO at the Nippon Dental University in Japan; Dr. Xueliang HOU at the School of Life Sciences, Xiamen University for his helpful suggestions on the nomenclature of this new genus and species, and Dr. Caiming WU, Dr. Luming YAO, and Ms. Jie LIU from Xiamen University for their assistance with EM observations. We are grateful to anonymous reviewers for their comments and suggestions which greatly improved this manuscript. The authors greatly appreciate English language edits and critical comments by Dr. Matt ASHWORTH, Texas State University at Austin.
Bąk M, Kryk A, Peszek Ł, Kociolek J P, Bemiasa J, Bemanaja E. 2019. New and interesting Luticola species (Bacillariophyta) from the mangroves of Nosy Be Island, NW Madagascar. Oceanological and Hydrobiological Studies, 48(1): 13-22.
DOI:10.1515/ohs-2019-0002 |
Chen C P, Gao Y H, Lin P. 2010. Geographical and seasonal patterns of epiphytic diatoms on a subtropical mangrove (Kandelia candel) in southern China. Ecological Indicators, 10(2): 143-147.
DOI:10.1016/j.ecolind.2009.04.003 |
Chen C P, Sun J D, Zhao L, Sun L, Li X S, Liang J R, Gao Y H. 2017. Navicula amoyensis sp. nov. (Bacillariophyceae), a new benthic brackish diatom species from the Jiulong River estuary, Southern China. Phytotaxa, 291(4): 253-263.
DOI:10.11646/phytotaxa.291.4.2 |
Cvetkoska A, Levkov Z, Hamilton P B. 2014. Surirella subrotunda sp. nov. and Surirella parahelvetica sp. nov., two new diatom (Bacillariophyta) species from Lake Prespa, Macedonia. Phytotaxa, 156(3): 145-155.
DOI:10.11646/phytotaxa.156.3.5 |
de Albuquerque Ribeiro R, Rovai A S, Twilley R R, Castañeda-Moya E. 2019. Spatial variability of mangrove primary productivity in the neotropics. Ecosphere, 10(8): e02841.
DOI:10.1002/ecs2.2841 |
Du Q, Jin D X. 1983. Studies on the epiphytic diatoms in the intertidal zones of the Jiulong river estuary, Fujian, China. Taiwan Strait, 2(2): 76-98.
(in Chinese with English abstract) |
Gao Y H. 2001. Studies on taxonomy, ecology and bioactive products of marine microalgae. Journal of Xiamen University (Natural Science), 40(2): 566-573.
(in Chinese with English abstract) |
Hamilton P B, Laird K R. 2001. Nitzschia pseudosinuata sp. nov., a new holocene diatom from the sediment of Moon Lake, North Dakota, U.S.A. Diatom Research, 16(2): 317-324.
DOI:10.1080/0269249X.2001.9705523 |
Kim B S, Witkowski A, Park J G, Li C L, Trobajo R, Mann D G, Kim S Y, Ashworth M, Bąk M, Gastineau R. 2019. Taxonomy and diversity of a little-known diatom genus Simonsenia (Bacillariaceae) in the marine littoral: novel taxa from the Yellow Sea and the Gulf of Mexico. Plant Ecology and Evolution, 152(2): 248-261.
DOI:10.5091/plecevo.2019.1614 |
Krammer K, Lange-Bertalot H. 1978. Bacillariophyceae, 2. teil: bacillariaceae, epithemiaceae, surirellaceae. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D eds. Süsswasserflora von Mitteleuropa. Gustav Fischer Verlag, Stuttgart.
|
Krzywda M, Gastineau R, Bąk M, Dąbek P, Górecka E, Zhou C X, Lange-Bertalot H, Li C L, Witkowski A. 2019. Morphology and molecular phylogeny of Gomphonemopsis sieminskae sp. nov. isolated from brackish waters of the East China Sea coast. Plant and Fungal Systematics, 64(1): 17-24.
DOI:10.2478/pfs-2019-0003 |
Lange-Bertalot H. 1979. Simonsenia, a new genus with morphology intermediate between Nitzschia and Surirella. Bacillaria, 2: 127-136.
|
Lange-Bertalot H, Witkowski A, Kulikovskiy M, Seddon A W R, Kociolek J P. 2015. Taxonomy, frustular morphology and systematics of Platichthys, a new genus of canal raphe bearing diatoms within the Entomoneidaceae. Phytotaxa, 236(2): 135-149.
DOI:10.11646/phytotaxa.236.2.3 |
Li C L, Ashworth M P, Witkowski A, Dabek P, Medlin L K, Kooistra W H C F, Sato S, Zglobicka I, Kurzydlowski K J, Theriot E C, Sabir J S M, Khiyami M A, Mutwakil M H Z, Sabir M J, Alharbi N S, Hajarah N H, Qing S, Jansen R K. 2015. New insights into Plagiogrammaceae (Bacillariophyta) based on multigene phylogenies and morphological characteristics with the description of a new genus and three new species. PLoS One, 10(10): e0139300.
DOI:10.1371/journal.pone.0139300 |
Li C L, Witkowski A, Ashworth M P, DĄBEK P, Sato S, ZGŁOBICKA I, Witak M, Khim J S, Kwon C J. 2018a. The morphology and molecular phylogenetics of some marine diatom taxa within the Fragilariaceae, including twenty undescribed species and their relationship to Nanofrustulum, Opephora and Pseudostaurosira. Phytotaxa, 355(1): 1-104.
DOI:10.11646/phytotaxa.355.1.1 |
Li L, Chen C P, Zhang J W, Liang J R, Gao Y H. 2020a. Morphology and occurrence of two epibiotic marine gomphonemoid diatoms in China. Nova Hedwigia, 111(3-4): 271-285.
DOI:10.1127/nova_hedwigia/2020/0604 |
Li L, Chen C P, Sun L, Zhang J W, Liang J R, Gao Y H. 2020b. Protoraphis Simonsen, a newly recorded marine epizoic diatom genus for China. Acta Oceanologica Sinica, 39(4): 120-126.
DOI:10.1007/s13131-019-1467-z |
Li L, Sun L, Chen C P, Li X S, Liang J R, Gao Y H. 2018b. Epitypification and emendation of Olifantiella pseudobiremis, an epizoic diatom from the East China Sea Okinawa Trough. Phytotaxa, 362(3): 292-296.
DOI:10.11646/phytotaxa.362.3.6 |
Li X S, Chen C P, Liang J R, Wu W Z, Gao Y H. 2014. Morphology and occurrence of a marine epizoic diatom Falcula hyalina Takano (Bacillariophyta) in China. Algological Studies, 145-146: 169-179.
DOI:10.1127/1864-1318/2014/0158 |
Li Y H, Chen X M, Sun Z M, Xu K D. 2017. Taxonomy and molecular phylogeny of three marine benthic species of Haslea (Bacillariophyceae), with transfer of two species to Navicula. Diatom Research, 32(4): 451-463.
DOI:10.1080/0269249X.2017.1401008 |
Li Y H, Nagumo T, Sun Z M, Xu K D. 2020c. Morphology of three benthic diatoms from a tropical mangrove in Hainan Island, China: Gyrosigma centropunctatum sp. nov., G. dongzhaiense sp. nov. and G. orbitum Thaler et Kaczmarska. Diatom Research, 35(3): 255-267.
DOI:10.1080/0269249X.2020.1800518 |
Liu Y, Kociolek J P, Fan Y W, Wang Q X. 2012. Pseudofallacia gen. nov., a new freshwater diatom (Bacillariophyceae) genus based on Navicula occulta Krasske. Phycologia, 51(6): 620-626.
DOI:10.2216/11-098.1 |
Mann D G, Trobajo R. 2014. Symmetry and sex in Bacillariaceae (Bacillariophyta), with descriptions of three new Nitzschia species. European Journal of Phycology, 49(3): 276-297.
DOI:10.1080/09670262.2014.915063 |
Mann D G, Trobajo R, Sato S, Li C L, Witkowski A, Rimet F, Ashworth M P, Hollands R M, Theriot E C. 2021. Ripe for reassessment: a synthesis of available molecular data for the speciose diatom family Bacillariaceae. Molecular Phylogenetics and Evolution, 158: 106985.
DOI:10.1016/j.ympev.2020.106985 |
Maples R S. 1983. Community structure of diatoms epiphytic on pneumatophores of the black mangrove, Avicennia germinans, in a Louisiana salt marsh. Gulf and Caribbean Research, 7(3): 255-259.
|
Round F E, Crawford R M, Mann D G. 1990. The Diatoms, Biology and Morphology of the Genera. Cambridge University Press, Cambridge. 760p.
|
Ruck E C, Kociolek J P. 2004. Preliminary Phylogeny of the Family Surirellaceae (Bacillariophyta). Bibliotheca Diatomologica, 50: 1-236.
|
Ruck E C, Theriot E C. 2011. Origin and evolution of the canal raphe system in diatoms. Protist, 162(5): 723-737.
DOI:10.1016/j.protis.2011.02.003 |
Sahoo G, Ansari Z A. 2018. Seasonality and vertical distribution of epiphytic diatoms on the pneumatophore surface of mangrove Avicennia officinalis L. Marine Biodiversity, 48(4): 2033-2041.
DOI:10.1007/s12526-017-0723-2 |
Sahoo G, Ansari Z A, Shaikh J B, Varik S U, Gauns M. 2018. Epibiotic communities (microalgae and meiofauna) on the pneumatophores of Avicennia officinalis (L. ). Estuarine. Coastal and Shelf Science, 207: 391-401.
DOI:10.1016/j.ecss.2017.08.018 |
Seddon A W R, Witkowski A, Froyd C A, KurzydłOwsk K J, Grzonka J, Willis K J. 2014. Diatoms from isolated islands II: Pseudostaurosira diablarum, a new species from a mangrove ecosystem in the Galápagos Islands. Diatom Research, 29(2): 201-211.
DOI:10.1080/0269249X.2013.877084 |
Simonsen R. 1979. The diatom system: ideas on phylogeny. Bacillaria, 2: 9-71.
|
Sims P, Paddock T B B. 1982. The fenestral fibula: a new structure in the diatoms. Bacillaria, 5: 7-42.
|
Siqueiros-Beltrones D A, López-Fuerte F O. 2006. Epiphytic diatoms associated with red mangrove (Rhizophora mangle) prop roots in BahíA Magdalena, Baja California Sur, Mexico. Revista De Biología Tropical, 54(2): 287-297.
DOI:10.15517/RBT.V54I2.13869 |
Siqueiros-Beltrones D A S, Sánchez-Castrejón E S. 1999. Structure of benthic diatom assemblages from a mangrove environment in a Mexican subtropical lagoon. Biotropica, 31(1): 48-70.
DOI:10.1111/j.1744-7429.1999.tb00116.x |
Trobajo R, Rovira L, Ector L, Wetzel C E, Kelly M, Mann D G. 2013. Morphology and identity of some ecologically important small Nitzschia species. Diatom Research, 28(1): 37-59.
DOI:10.1080/0269249X.2012.734531 |
Van de Vijver B, Kopalová K, Kociolek J P, Ector L. 2015. Denticula jamesrossensis, a new freshwater diatom (Bacillariophyta) species from the Maritime Antarctic Region. Fottea, 15(1): 105-111.
DOI:10.5507/fot.2015.009 |
Veselá J, Johansen J R, Potapova M. 2013. Surirella terryi and S. cruciata: two rare diatoms from North America. Diatom Research, 28(4): 503-516.
DOI:10.1080/0269249X.2013.853697 |
Witkowski A, Gomes A, Mann D G, Trobajo R, Li C L, Barka F, Gusev E, Dąbek P, Grzonka J, Kurzydłowski K J, Zgłobicka I, Harrison M, Boski T. 2015. Simonsenia aveniformis sp. Nov. (Bacillariophyceae), molecular phylogeny and systematics of the genus and a new type of canal raphe system. Scientific Reports, 5(1): 17115.
DOI:10.1038/srep17115 |
Witkowski A, Li C L, Zgłobicka I, Yu S X, Ashworth M, Dąbek P, Qin S, Tang C, Krzywda M, Ruppel M, Theriot E C, Jansen R K, Car A, Płociński T, Wang Y C, Sabir J S M, Daniszewska-Kowalczyk G, Kierzek A, Hajrah N H. 2016. Multigene assessment of biodiversity of diatom (Bacillariophyceae) assemblages from the littoral zone of the Bohai and Yellow Seas in Yantai region of Northeast China with some remarks on ubiquitous taxa. Journal of Coastal Research, 74(S1): 166-195.
DOI:10.2112/SI74-016.1 |
Witkowski A, Żelazna-Wieczorek J, Solak C, Kulikovskiy M. 2014. Morphology, ecology and distribution of the diatom (Bacillariophyceae) species Simonsenia delognei (Grunow) Lange-Bertalot. Oceanological and Hydrobiological Studies, 43(4): 393-401.
DOI:10.2478/s13545-014-0151-x |
You Q M, Kociolek J P, Yu P, Cai M J, Lowe R, Wang Q X. 2016. A new species of Simonsenia from a karst landform, Maolan Nature Reserve, Guizhou Province, China. Diatom Research, 31(3): 269-275.
DOI:10.1080/0269249X.2016.1227377 |
You Q M, Liu Y, Wang Y F, Wang Q X. 2009. Taxonomy and distribution of diatoms in the genera Epithemia and Rhopalodia from the Xinjiang Uygur Autonomous Region, China. Nova Hedwigia, 89(3-4): 397-430.
DOI:10.1127/0029-5035/2009/0089-0397 |
 2022, Vol. 40
2022, Vol. 40


