Institute of Oceanology, Chinese Academy of Sciences
Article Information
- ZOU Jian, LI Qun, LIU Hui, LIU Ying, HUANG Lifen, WU Haiyan, QIU Jiangbing, ZHANG Hua, LÜ Songhui
- Taxonomy and toxin profile of harmful benthic Prorocentrum (Dinophyceae) species from the Xisha Islands, South China Sea
- Journal of Oceanology and Limnology, 40(3): 1171-1190
- http://dx.doi.org/10.1007/s00343-021-1045-6
Article History
- Received Feb. 8, 2021
- accepted in principle Mar. 30, 2021
- accepted for publication May. 25, 2021
2 State Environmental Protection Key Laboratory of Drinking Water Source Management and Technology, Shenzhen Academy of Environmental Science, Shenzhen 510008, China;
3 Yellow Sea Fisheries Research Institute, Qingdao 266071, China;
4 College of Environmental Science and Engineering, Ocean University of China, Qingdao 266100, China;
5 Shenzhen Academy of Environmental Science, Shenzhen 510008, China;
6 Southern Marine Science and Engineering Guangdong Laboratory, Zhuhai 519000, China
Benthic dinoflagellates have drawn significant attention in recent decades (Berdalet et al., 2012). Some species could produce toxins and then cause harm to the health of aquaculture, benthic ecosystem, and humans (Berdalet et al., 2017). Even in the recent two decades, the blooms of benthic dinoflagellates occur more often and broadly, which further threaten public health (Accoroni and Totti, 2016; Zou et al., 2020). Among these dinoflagellates, species of Prorocentrum C.G. Ehrenberg is abundant and distributes globally, and these dinoflagellates could inhabit the planktonic and benthic environment (Boisnoir et al., 2019). So far, approximately 71 species of this genus were identified from the tropic to temperate regions (Murray et al., 2009; Luo et al., 2017; Chomérat et al., 2019). The planktonic Prorocentrum micans C.G. Ehrenberg was first identified by Ehrenberg in 1834 and was considered as a type species of Prorocentrum (Ehrenberg, 1834). Among Prorocentrum, about 35 species of Prorocentrum epiphytically inhabit the benthic environment including macrophytes, dead corals, sediments, and so on (Hoppenrath et al., 2013; Rodríguez et al., 2018; Lim et al., 2019).
The Prorocentrum morphologically possesses two large thecal plates separated by a sagittal suture, a topmost periflagellar area composed of several tiny platelets and two pores (Fensome et al., 1993; Hoppenrath et al., 2013). Cell shape and size, the relative location of the nucleus, thecal surface micromorphology, thecal pore and intercalary band ornamentations and, most importantly, the architecture of periflagellar area are used for the morphological identifications of benthic Prorocentrum species (Hoppenrath et al., 2013; Lim et al., 2019). However, the shape and size of cell and the numbers and patterns of pore vary among several species from different geographical regions, which cause difficulties for the taxonomy of Prorocentrum (Ten-Hage et al., 2000a; Hoppenrath et al., 2013). In general, the periflagellar area is located in the apical area of Prorocentrum and the ultrastructure analysis reveals that it is composed of a flagellar pore, an accessory pore, and several tiny platelets (Hoppenrath et al., 2013; Chomérat et al., 2019). The ultrastructure of periflagellar area is relatively conservative and then is used for the identification of Prorocentrum (Hoppenrath et al., 2013). However, to date, the details of the periflagellar area of certain species like P. borbonicum L. Ten-Hage, J. Turquet, J.-P. Quod, S. Puiseux-Dao & Couté and P. elegans Faust are very significant but remain unclear (Faust, 1993; Hoppenrath et al., 2013; David et al., 2014).
The morphology of Prorocentrum is relatively homologous and has apparent plasticity (Hoppenrath et al., 2013; Zhang et al., 2015). Consequently, the misidentification of Prorocentrum can be easily caused. Therefore, molecular phylogenetic analyses are necessary for the identifications of previously described species and descriptions of new species. For example, Mohammad-Noor et al. (2007) reexamined P. arabianum Morton et Faust isolated from original stations and suggested it was synonymized with P. concavum Fukuyo based on morphologies and phylogenies. P. maculosum M. A. Faust was synonymized with the toxic species P. hoffmannianum M. A. Faust (Rodríguez et al., 2018). The phylogenetic analysis is essential to well understand the genetic diversity in Prorocentrum. Recently, a taxonomical study in Lesser Antilles, eastern Caribbean Sea revealed phylogenies of benthic Prorocentrum mainly divided into six clades based on large subunit (LSU) (D1-D2) sequences. Among these clades, P. lima complex and P. fukuyoi complex, which showed high genetic diversity, had six and five subclades, respectively (Chomérat et al., 2019). In addition, a recent study in Japan revealed P. lima complex had high genetic diversity and existence of cryptic species (Nishimura et al., 2020a). Hence, it is necessary to research the phylogenies of benthic Prorocentrum in a wide area.
Interest in marine epibenthic Prorocentrum has grown in recent years because most of them are potential toxins producers (Zhou and Fritz, 1993; Lim et al., 2019) or harmful to marine invertebrates (Zou et al., 2020). Among the benthic Prorocentrum, nine species of them produce toxic polyether compounds like okadaic acid (OA) and/ or its related derivatives dinophysistoxins (DTXs), borbotoxins, and prorocentrolides (Ten-Hage et al., 2000a; Amar et al., 2018; Lim et al., 2019; www.marinespecies.org/). These toxic compounds can contaminate shellfish and subsequently cause shellfish poisoning in humans (Morton, 1998; Ten-Hage et al., 2000b). In addition, some species (e.g., P. concavum) have no detectable diarrheic shellfish poisoning (DSP) toxins but harm marine invertebrates (Zou et al., 2020). DSP toxins are lipophilic and frequently cause severe poisoning events. OA and its DTX derivatives can induce diarrhea, nausea, vomiting, and abdominal pain in humans (Toyofuku, 2006). OA was first extracted from an oceanic sponge. It is a polyether derived by a 38-carbon fatty acid and causes strong inhabitation of protein phosphatases-1 and -2A activities (Goris et al., 1989). So far, OA producers of Prorocentrum include P. lima complex (Ehrenberg) F. Stein, P. caipirignum Silvia M. Nascimento, P.hoffmannianum, P. rhathymum A. R. Loeblich Ⅲ, Sherley & Schmidt, P. concavum, P. faustiae S. L. Morton, P. leve M. A. Faust, Kibler, Vandersea, Tester & Litaker, and P. fukuyoi (Yasumoto et al., 1987; Dickey et al., 1990; Morton, 1998; Ten-Hage et al., 2000b; Marasigan et al., 2001; An et al., 2010; Luo et al., 2017; Nishimura et al., 2020b). Among them, the toxic P. lima complex are cosmopolitan species and extensively distributed from tropical to temperate areas (Yasumoto et al., 1987; Bouaïcha et al., 2001; Vila et al., 2001; Luo et al., 2017).
Considering the potential threat of benthic Prorocentrum to public health and fishery of Xisha Islands in China, the investigation on the biodiversity and toxicity of these species is urgently required in this area where relatively little information related to benthic dinoflagellates is available now. Here, we identified benthic Prorocentrum species based on light, epifluorescence and scanning electron microscopy coupled with molecular phylogeny. Besides, the toxin contents were detected using liquid chromatography-tandem mass spectrometry (LC-MS/ MS). The present study aims to provide an insight into the diversity of benthic Prorocentrum and to identify DSP toxins produced by these species in the Xisha Islands.
2 MATERIAL AND METHOD 2.1 Study region and samplingThe survey region was the Xisha Islands (15°46′N–17°08′N, 111°11′E–112°54′E), the largest archipelago in the South China Sea, and the islands are internationally called as Paracel Islands. This region has a tropical climate. The islands consist of Xuande Islands and Yongle Islands, with a total land area of 9.22 km2 and a total marine area of 500 000 km2. Sampling was carried out at four sites on the Xisha Islands between 2015 and 2017 (Fig. 1).
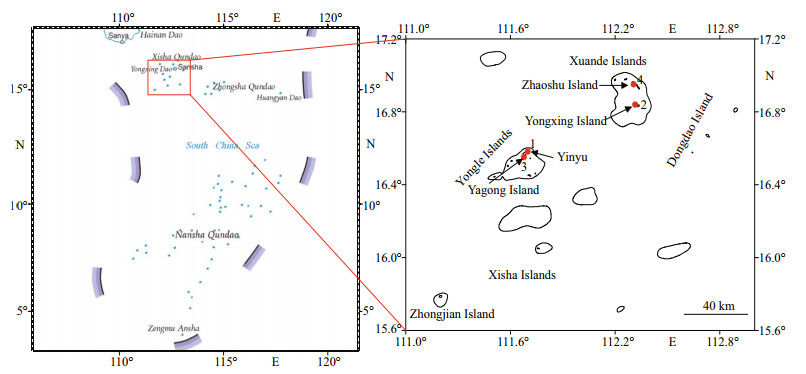
|
| Fig.1 Map of studying sites in the Xisha Islands (map review No. GS(2019)1671) |
Samples of macroalgae, seagrasses, dead coral reefs, and sediments were collected by the diver in shallow water (~2–5-m depth) and transferred into bottles underwater. They were filtered using a 120-μm screen to remove sands and substrates, and the microalgae were collected on 20-μm mesh. The microalgae retained on the latter filters were resuspended for cell isolation. Single-cell of Prorocentrum was isolated using a light microscope (CX31, Olympus, Japan) and cultivated in a 96-well cell culture cluster containing L1 medium without silicate (Guillard and Hargraves, 1993). Successfully established strains were transferred into glass tubes with L1 medium without silicate and used to research the biodiversity of benthic Prorocentrum in the Xisha Islands. All cultures were maintained at temperature of 25 ℃ (±0.5 ℃) and 30–31 in salinity, with a photoperiod of 12-h light꞉12-h dark and irradiation of 120 μmol photons/(m2·s).
2.2 Morphological observationTo describe the morphologies of benthic Prorocentrum from Xisha Islands, light, fluorescence, and scanning electron microscopy were carried out by the descriptions of Zou et al. (2020). In general, cells fixed were used to take pictures using a light microscope (BX61, Olympus, Japan) equipped with a digital camera (QImaging Retiga 4000R, British Columbia, Canada). The cell sizes of each Prorocentrum species were calculated based on the length and width of at least 30 cells. Live cells and cells stained with Fluorescent Brightener 28 (Sigma, USA) and SYBR Safe DNA gel stain (Invitrogen, Thermo Fisher Scientific, USA) were taken micrograph at ×1 000 magnification for the observations of shape and location of nucleus and chloroplasts.
A 5-mL well-grown algal culture was collected and rinsed thrice with filtered seawater and ultrasonically cleaned for 40 min. The cells were fixed in a 2.5% v/v solution of glutaraldehyde dissolved in filtered seawater and maintained at 4 ℃ overnight. The fixed microalgae were rinsed with Milli-Q water and dehydrated using a series of graded ethanol (10%, 30%, 50%, 60%, 80%, and 95% v/v) for 5 min at each step and followed by 99.9% v/v twice for 15 min every time. Then, cells were dried to a critical point by supercritical drying (CPD 300, Leica, Wetzler, Germany) and coated with gold (Leica EM SCD 500, Leica, Wetzler, Germany). The prepared samples of Prorocentrum were identified and photographed using a ZEISS ULTRATM 55 scanning electron microscope. The platelet arrangements of the periflagellar areas of Prorocentrum were identified using the descriptions of Hoppenrath et al. (2013).
2.3 DNA extraction, PCR amplification and sequencingA 5-mL well-grown cells of Prorocentrum were centrifuged at 13 000×g at 4 ℃ for 2 min and extracted using a MiniBEST Universal DNA Extraction Kit (TaKaRa, Tokyo, Japan) based on manufacturer's instructions. The extracted total DNA was amplified using the primers D1R/D3B (Scholin et al., 1994) and ITS1F/ITS1R (Pin et al., 2001) for large ribosomal subunit (LSU) and ITS1-5.8S-ITS2 region, respectively. The 50-L reactions consisted of 22-μL sterile Milli-Q water, 1 μL of each primer with a concentration of 1 μmol/L, 1-μL total DNA (final concentration, 10–50 ng/μL), and 25-μL 2X Accurate Taq Master Mix (dye plus) (Accurate Biotechnology Co., Ltd.). The reactions were amplified based on previous methods of Zou et al. (2020). The products were sequenced using an ABI PRISM 3730XL (Applied Biosystems, CA, USA).
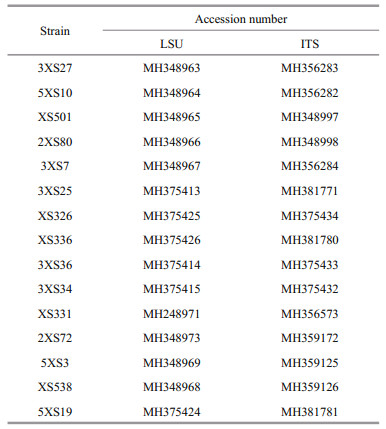
2.4 Sequence alignment and phylogenetic analysesThe sequenced products were jointed using pairwise alignment (ContigExpress V. 3) and the obtained new sequences were uploaded to the GenBank database (Table 1). The new sequences of partial LSU and ITS were aligned with those of Prorocentrum downloaded from the GenBank database using multiple sequence alignment (MUSCLE, www.ebi.ac.uk/Tools/msa/muscle/). Adenoides eludens (Herdman) Balech and Karena brevis (C. C. Davis) Gert Hansen & ?.Moestrup were used as outgroups of LSU and ITS phylogeny, respectively. The tool RAxML-HPC2 on XSEDE v. 8.2.12 (https://www.phylo.org/) was used to build the maximum-likelihood trees with default algorithm parameters and node support was run with 1 000 bootstrap repetitions. The best models GTR and SYM+G were selected using PAUP v 4.0 to analyze Bayesian Inference (BI) in LSU and ITS region, respectively. The BI trees were constructed with MrBayes V. 3.1.2 and four Markov chain Monte Claro chains calculated no less 10 000 000 replicates with sampling every 100 generations. The pairwise genetic distances were aligned using MUSCLE and calculated using MEGA-X (Kimura 2-parameter model).
A 0.5-L exponential algal culture (three triplicates) was harvested at 12 000×g at 4 ℃ for 10 min. Pellets were added with 3-mL methanol and ultrasonicated (3s꞉3s) for 40 min at an ice bath. The extractions were centrifuged at 3 000×g at 4 ℃ for 15 min. The pellets were extracted with 3-mL methanol twice and all supernatants were put together. The 0.5-mL supernatants were filtered with 0.22-μm spin-filters (Pall Corporation, USA). Three toxins (OA, DTX-1, and DTX-2) were determined and quantified as follows. Chromatographic separation was carried out using an HPLC system (Shimadzu prominence LC-20ADXR) coupled with a tandem mass spectrometer (5500 QTRAP LC-MS/MS system, AB Sciex Instruments, Foster City, CA). Toxins were separated using Phenomenex Kinetex XB-C18 (150 mm× 2.1 mm, 2.6 μm) column. The mobile phase consisted of acetonitrile (solvent A) and 0.15% formic acid in water (solvent B). The initial proportion of solvent A was 20%, increased to 90% within 7 min, kept constant for 3 min, and reduced to 20% in 0.1 min. The total run time for the analysis was 12 min. The injection volume was 5 μL and the flow rate was kept constant at 0.35 mL/min. The turbo ions-pray source settings were: Ions-pray voltage (negative polarity), -4 500 V; Curtain gas, 20 psi; GS1 and GS2, 50 psi; Ion Source Temperature, 550 ℃; CAD, medium. Nitrogen served as nebulizer gas and collision gas in both modes. Multiple reaction monitoring with two products were selected: OA and DTX-2, 803.5 > 563.4 (55 eV) /255.2 (61 eV); DTX-1, 817.6 > 563.4 (60 eV) /255.2 (65 eV).
3 RESULT 3.1 Morphological feature 3.1.1 Prorocentrum borbonicum Ten-HageProrocentrum borbonicum cells are oval to ovoid and almost symmetric. The posterior area of cell is broadly rounded (Fig. 2a & d). The cell length is 24.40–31.92 μm (average=26.18±3.43 μm, n=68) and its width is 18.45–26.69 μm (average=22.84± 1.99 μm, n=68). Their length/width (L/W) ratios are 1.05–1.25. The pyrenoid is located in the center of the cells and is encompassed by numerous golden-brown chloroplasts (Fig. 2b). The nucleus is small, ovoid and located in the posterior (Fig. 2c). The thecal plates are ornamented by numerous depressions and dozens of scattered pores. The thecal surfaces of the plate have two types of large, sparse pores. The diameters vary between 0.12 and 0.2 μm in large pores (average=0.15±0.02 μm, n=30) and between 0.08 and 0.09 μm in small pores (average=0.08± 0.03 μm, n=30). The center of cell is devoid of large pores but it is ornamented by scattered small pores and depressions (Fig. 2e–f). The wide, V-shaped periflagellar area is located in the anterior areas of cells and consists of eight or nine platelets with unequal size and shape (1, 2, 3, 4, 5, 6a, 6b, 7, and 8). The periflagellar area has one relatively large flagellar pore and a small accessory pore (Fig. 2h).
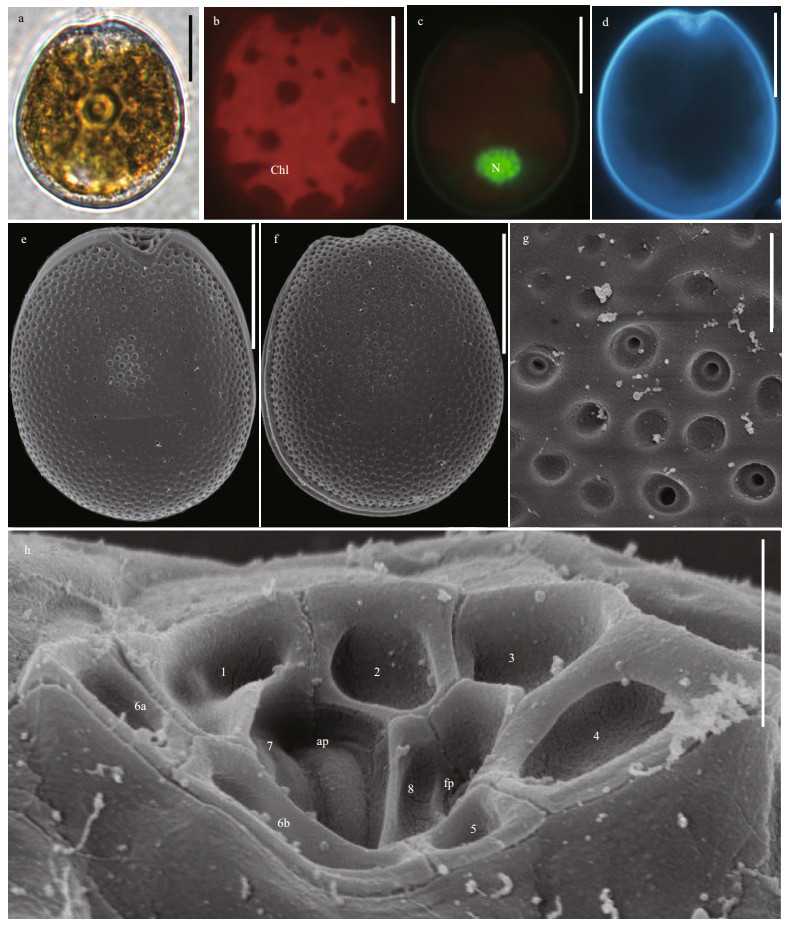
|
| Fig.2 The morphological characters of Prorocentrum borbonicum based on light microscopy (LM) and scanning electron microscopy (SEM) a. the LM of cell shape; b & c. fluorescence LM of the arrangement of chloroplasts (Chl) and the shape and location of nucleus (N); d. fluorescence of the shape of cell and periflagellar area; e. SEM of the right large lateral plate; f. SEM of left large lateral plate; g. SEM of thecal ornamentations (pores, depressions, and so on); h. SEM of the shape of periflagellar area, flaegllar pore (fp), accessory pore (ap), and arrangement pattern of platelets. Scale bars in a–f, g, and h are 10 μm, 2 μm, and 1 μm, respectively. |
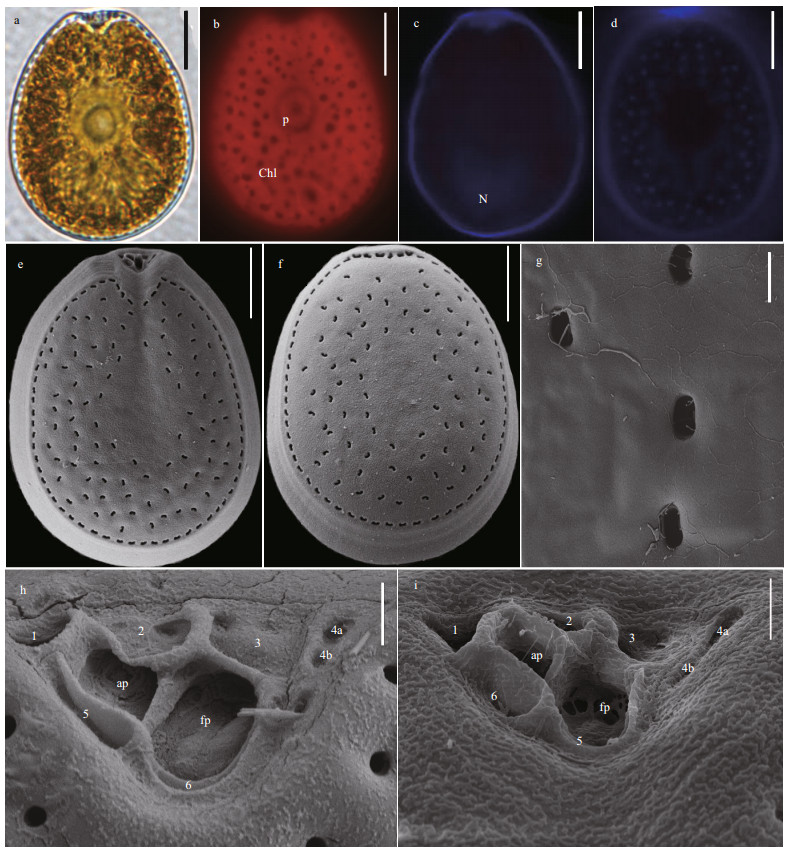
Morphologies of P. caipirignum cells are symmetrically oval to ovoid (Fig. 3a & d). The length and width are 35.1–45.0 μm (average=40.57±2.44 μm, n=31) and 28.0–33.4 μm (average=31.59±1.98 μm, n=31), respectively, with L/W ratios of 1.13–1.41. The pyrenoid has starch ring and is located in the center of cell. Many chloroplasts radially arrange from the center to the margin (Fig. 3b). The heart-shaped nucleus is located in the posterior of cell (Fig. 3c). The large lateral plates are ornamented with many kidney-shaped pores, but the center of cells is devoid of these structures (Fig. 3e–f). The pores are 0.69–0.98-μm long (mean 0.84±0.09 μm, n=30) and 0.27–0.49-μm wide (mean 0.38±0.06 μm, n=30) (Fig. 3g). The periflagellar area is V-shaped and platelet pattern is 1, 2, 3, 4a, 4b, 5 and 6 (Fig. 3h–i).
|
| Fig.3 The morphological characters of Prorocentrum caipirignum based on light microscopy (LM) and scanning electron microscopy (SEM) a. the LM of cell shape and pyrenoid (p); b & c. fluorescence LM of the arrangement of chloroplasts (Chl) and the shape and location of nucleus (N); d. fluorescence of the shape of cell and periflagellar area; e. SEM of the right large lateral plate; f. SEM of left large lateral plate; g. SEM of thecal ornamentations (pores, depressions and so on); h & i. SEM of the shape of periflagellar area, flaegllar pore (fp), accessory pore (ap), and arrangement pattern of platelets. Scale bars in a–f, g, and h–i are 10 μm, 2 μm, and 1 μm, respectively. |
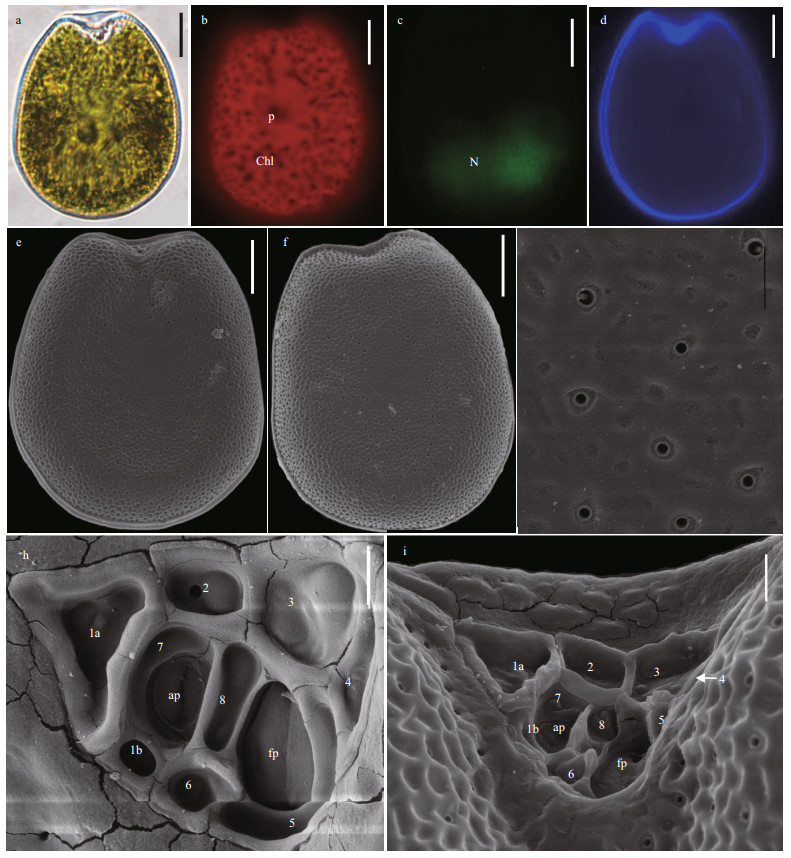
Broad oval cells of P. concavum are symmetrical, and the cell length and width are 40.6–52.6 μm (average=48.1±2.7 μm, n=47) and 35.1–43.0 μm (average=39.9±2.0 μm, n=47), respectively (Fig. 4a & d). Pyrenoid is located in the center of cells and chloroplasts position peripherally and radiate toward the cell center (Fig. 4b). The cells have L/W ratios of 1.07–1.33 and have oval nucleus at the posterior (Fig. 4c). The thecal cell surface possesses numerous round to oval reticulate-foveate depressions and scatters pores encircled by ring-like structures (Fig. 4e–g). Thecal pores are 0.14–0.29 μm (mean 0.18±0.14 μm, n=26) and are loosely scattered on the cell surface except in central area (Fig. 4e–g). Figure 4h–i shows the wide V-shaped periflagellar area and the arrangement of periflagellar platelets (1a, 1b, 2, 3, 4, 5, 6, 7, and 8).
|
| Fig.4 The morphological characters of Prorocentrum concavum based on light microscopy (LM) and scanning electron microscopy (SEM) a. the LM of cell shape and pyrenoid (p); b & c. fluorescence LM of the arrangement of chloroplasts (Chl) and the shape and location of nucleus (N); d. fluorescence of the shape of cell and periflagellar area; e. SEM of the right large lateral plate; f. SEM of left large lateral plate; g. SEM of thecal ornamentations (pores, depressions, and so on); h & i. SEM of the shape of periflagellar area, flaegllar pore (fp), accessory pore (ap), and arrangement pattern of platelets. Scale bars in a–f, g, and h–i are 10 μm, 2 μm, and 1 μm, respectively. |
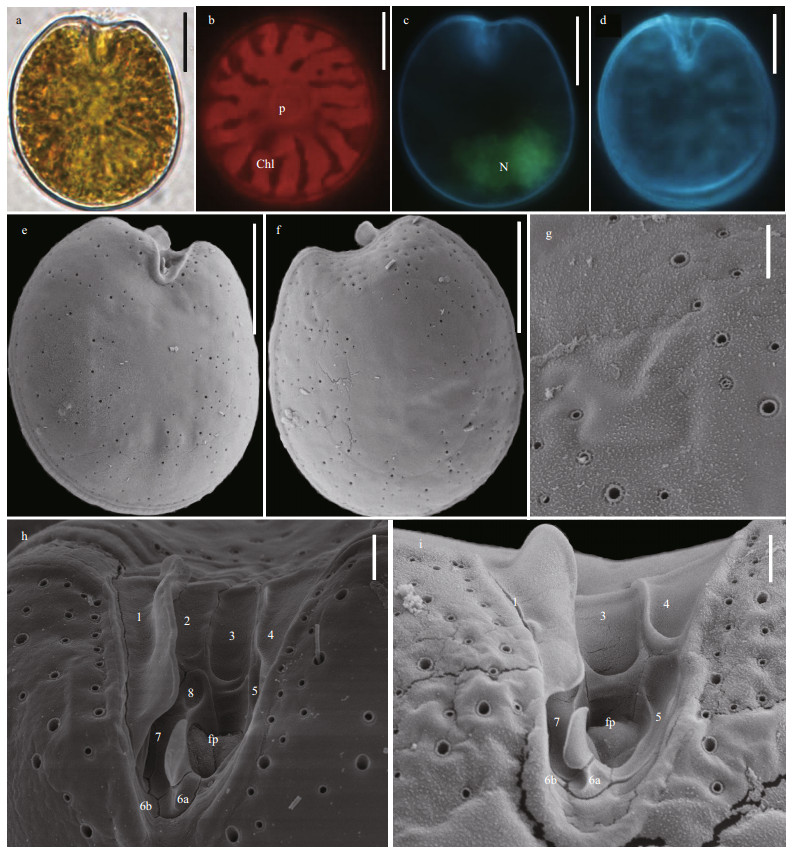
Prorocentrum elegans cells are oval and slightly asymmetric (Fig. 5a & d). They are 18.76–22.04-μm long (average=20.31±0.9 μm, n=65) and 14.61–17.82-μm wide (average=15.84±1.14 μm, n=65), with L/W ratios of 1.17–1.49. There is no pyrenoid but numerous chloroplasts are observed (Fig. 5b). The teardrop-shaped nucleus is located between the posterior and the center of cell (Fig. 5c). The thecal view of each cell is flattened. Except the center, the theca is ornamented with pores in two different sizes. The diameters of larger pores are 0.25–0.37 μm (average=0.30±0.03 μm, n=33) and those of smaller pores are 0.07–0.12 μm (average=0.1±0.01 μm, n=41). The larger pores near the periflagellar area generally form radial rows. There are no pores in the center of each cell (Fig. 5e–f). The wide, V-shaped periflagellar area is located in the cell apex and consist of seven or eight platelets (1, 2, 3, 4, 5, 6, 7, and 8). Platelet 1 possesses a distinct wing and the flagellar pore larger than the accessory pore (Fig. 5h–i).

|
| Fig.5 The morphological characters of Prorocentrum elegans based on light microscopy (LM) and scanning electron microscopy (SEM) a. the LM of cell shape; b & c. fluorescence LM of the arrangement of chloroplasts (Chl) and the shape and location of nucleus (N); d. fluorescence of the shape of cell and periflagellar area; e. SEM of the right large lateral plate; f. SEM of left large lateral plate; g. SEM of thecal ornamentations (pores, depressions, and so on); h & i. SEM of the shape of periflagellar area, flaegllar pore (fp), accessory pore (ap), and arrangement pattern of platelets. Scale bars in a–f, g, and h–i are 10 μm, 2 μm, and 1 μm, respectively. |
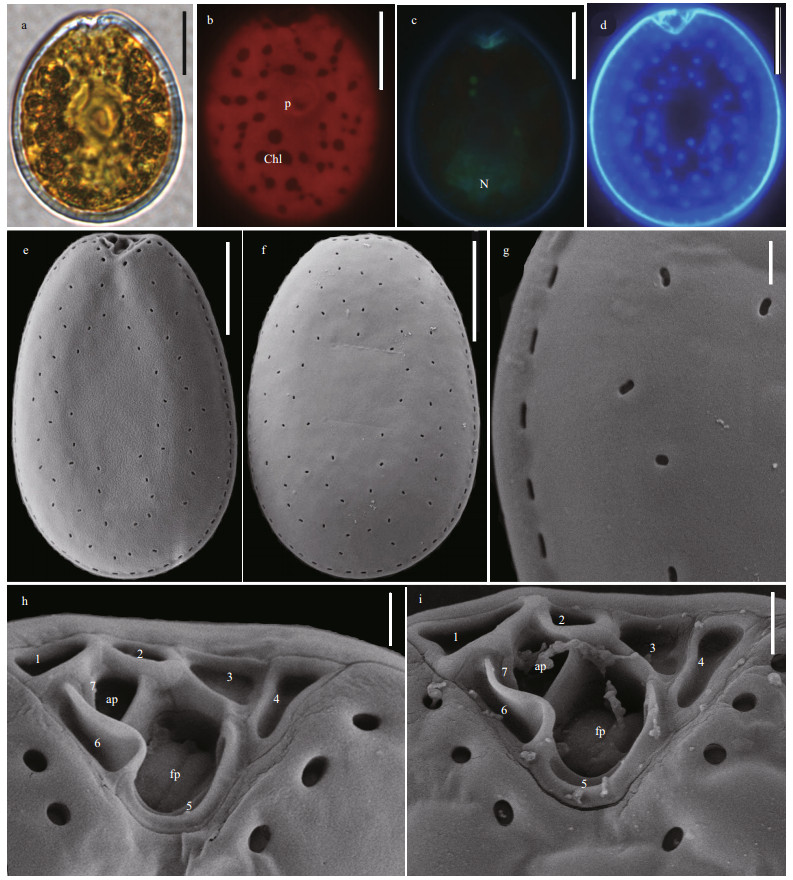
Round to oval P. cf. emarginatum cells are asymmetric and 29.95–37.65-μm long (average= 33.98±2.04, n=32) and 23.22–33.76-μm wide (average=28.89±3.11 μm, n=32), with L/W ratios of 1.06–1.50 (Fig. 6a & d). The elongated nucleus and pyrenoid encircled by numerous chloroplasts from center to periphery are located in the posterior and in the center area of the cell, respectively (Fig. 6b–c). The thecal surfaces are foveate and ornamented with pores of two different sizes. The mean diameter of the larger pores is 0.18 μm and that of the smaller pores is 0.1 μm. The centre of each cell lacks pores (Fig. 6e–f). Pores of both sizes are located in deep depressions and arrange in stripes (Fig. 6g). The anterior narrow V-shaped periflagellar area is composed of nine platelets (1, 2, 3, 4, 5, 6a, 6b, 7, and 8). A wing-shaped spine is observed between platelet 1 and platelet 2 in each cell (Fig. 6h & i).
|
| Fig.6 The morphological characters of Prorocentrum cf. emarginatum based on light microscopy (LM) and scanning electron microscopy (SEM) a. the LM of cell shape and pyrenoid (p); b & c. fluorescence LM of the arrangement of chloroplasts (Chl) and the shape and location of nucleus (N); d. fluorescence of the shape of cell and periflagellar area; e. SEM of the right large lateral plate; f. SEM of left large lateral plate; g. SEM of thecal ornamentations (pores, depressions and so on); h & i. SEM of the shape of periflagellar area, flaegllar pore (fp), accessory pore (ap), and arrangement pattern of platelets. Scale bars in a–f, g, and h–i are 10 μm, 2 μm, and 1 μm, respectively. |
Prorocentrum lima complex cells are oval to oblong-oval and symmetric (Fig. 7a & d). The length, width, and ratios of L/W are 34.8–40.7 μm (average= 38.09±1.59 μm, n=33), 22.9–27.4 μm (average 25.47±1.26, n=33), and 1.36–1.61, respectively. Pyrenoid is located in the center of cell and is surrounded by numerous chloroplasts (Fig. 7b). The oval nucleus is located in the posterior area of cell (Fig. 7c). The thecal plates are smooth and ornamented by many scattered and oblong to kidney-shaped pores with 0.25–0.50 μm in length and 0.17–0.39 μm in width. The central area of cell is devoid of pores. The marginal pores arrange in ring (Fig. 7e–g). Seven platelets present in a wide, V-shaped periflagellar area (1, 2, 3, 4, 5, 6, and 7). A flagellar pore is much larger than an accessory pore (Fig. 7h–i).
|
| Fig.7 The morphological characters of Prorocentrum lima complex based on light microscopy (LM) and scanning electron microscopy (SEM) a. the LM of cell shape and pyrenoid (p); b & c. fluorescence LM of the arrangement of chloroplasts (Chl) and the shape and location of nucleus (N); d. fluorescence of the shape of cell and periflagellar area; e. SEM of the right large lateral plate; f. SEM of left large lateral plate; g. SEM of thecal ornamentations (pores, depressions, and so on); h & i. SEM of the shape of periflagellar area, flaegllar pore (fp), accessory pore (ap), and arrangement pattern of platelets. Scale bars in a–f, g, and h–i are 10 μm, 2 μm, and 1 μm, respectively. |
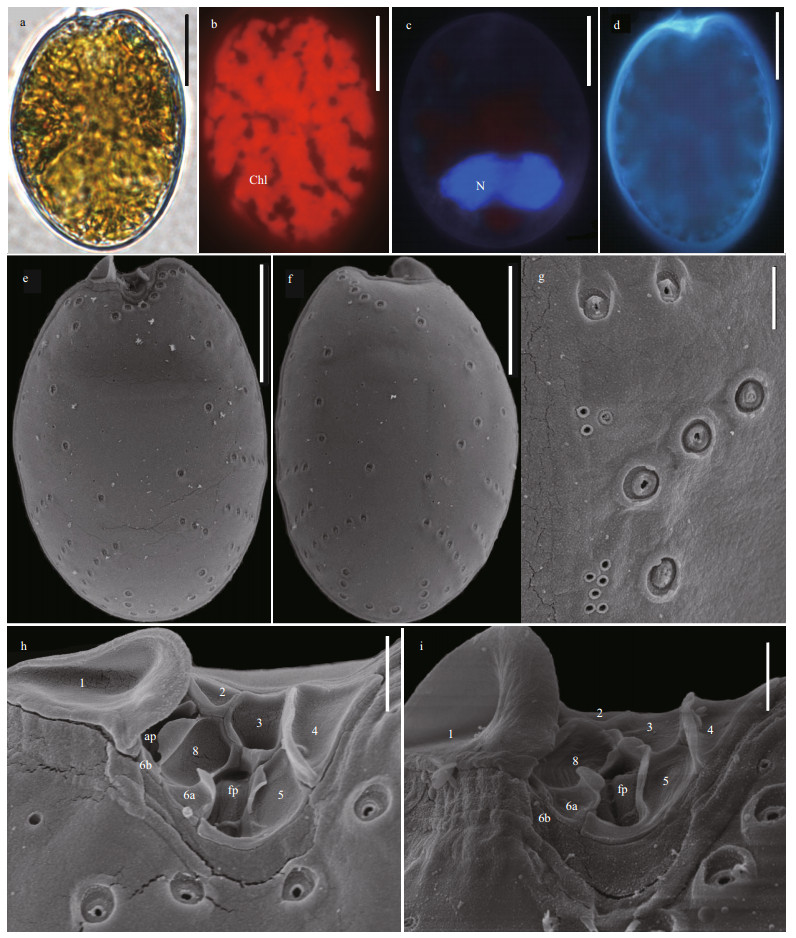
The shapes of P.rhathymum cell are asymmetrically oval, and sizes are 27.5–34.7-μm long (average=32.8±1.5 μm, n=32) and 20.7–25.5-μm wide (average=23.6±1.14 μm, n=32), with L/W ratios of 1.2–1.56 (Fig. 8a & d). The cell lacks pyrenoid but has numerous chloroplasts (Fig. 8b). The posterior of cell possesses kidney-shaped nucleus (Fig. 8c). The large lateral plates are ornamented with pores in two size. The diameters of larger pores are 0.41–0.71 μm (average=0.57± 0.09 μm, n=33) in the depressions, and the smaller pores are only 0.07–0.24 μm (average=0.15±0.07, n=33). The pores radiate from the smooth center to the margin (Fig. 8e & f). The wide V-shaped periflagellar area is composed of nine platelets (1, 2, 3, 4, 5, 6a, 6b, 7, and 8) and has an ear-shaped wing supported by platelet 1 (Fig. 8h–i).
|
| Fig.8 The morphological characters of Prorocentrum rhathymum based on light microscopy (LM) and scanning electron microscopy (SEM) a. the LM of cell shape; b & c. fluorescence LM of the arrangement of chloroplasts (Chl) and the shape and location of nucleus (N); d. fluorescence of the shape of cell and periflagellar area; e. SEM of the right large lateral plate; f. SEM of left large lateral plate; g. SEM of thecal ornamentations (pores, depressions, and so on); h & i. SEM of the shape of periflagellar area, flaegllar pore (fp), accessory pore (ap), and arrangement pattern of platelets. Scale bars in a–f, g, and h–i are 10 μm, 2 μm, and 1 μm, respectively. |
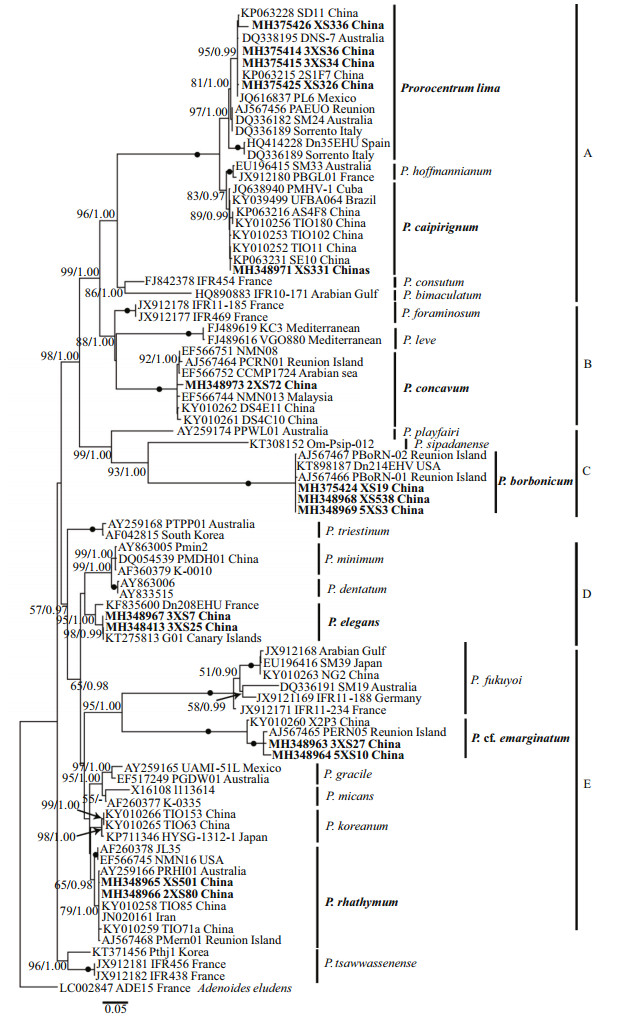
The maximum likelihood and Bayesian inference differed only in a few topologies based on LSU region, and they also had a few topological differences in ITS analysis. Phylogenies inferred from LSU rDNA and ITS region are illustrated by ML trees (Fig. 9 & Supplementary Fig.S1).
|
| Fig.9 Molecular phylogenetic tree (maximum likelihood) of Prorocentrum inferred from D1–D3 LSU rDNA The species Adenoides eludens is selected as the outgroup. The bootstrap of ML analysis (left) and the probabilities of BI analysis (right) were represented at nodes. Filled circles explicating support values of 100 and 1 for ML and BI analysis, respectively. And the values below 80 and 0.5 for ML and BI respectively are not shown. Newly obtained sequences in present study are highlighted in bold. |
In the ML tree inferred from LSU rDNA (Fig. 9), Adenoides eludens was an outgroup. Considering the whole tree, the phylogenies of Prorocentrum divided into two large clades. The first clade, including clade A, clade B, clade C, P. cf. norrisianum, and P. glenanicum, mainly consisted of benthic Prorocentrum. The second clade, including clade D and clade E, was composed of planktonic and epibenthic species (Fig. 9). In the first clade, clade A consisted of P. hoffmannianum, P. caipirignum, P. lima complex, P. consutum Chomérat & Nézan, and P. bimaculatum Chomérat & Saburova with a high support value (95/1.00). The new obtained strains of P. lima complex from Xisha Islands belonged to P. lima complex, with a support value of 84/1.00. Moreover, P. lima complex divided into two subclade and four strains from Xisha Islands were located in a same clade (100/1.00). P. caipirignum, which consisted of XS331 and other strains, and P. hoffmannianum were the sister clade of P. lima complex, with the highest support value (100/1.00). Besides, the newly characterized strain 2XS72 and other sequences of P. concavum downloaded from GenBank formed a clade with a full support value (100/1.00). The clade A and clade B were a sister clade with clade C, which consisted of P. playfairi, P. sipadanense Mohammad-Noor, Daugbjerg et Moestrup, and P. borbonicum. P. borbonicum consisted of Xisha strains (XS538, 5XS3, 5XS19) and other strains showed the highest support (100/1.00). In the clade mainly composed of planktonic species, clade D consisted of P. fukuyoi complex, P. cf. sculptile, and P. cf. emarginatum, with a high support value (98/1.00). The new characterized strains and two previous strains were composed of P. cf. emarginatum clade (100/1.00). In addition, a clade E, comprising Xisha strains of two benthic species (P. elegans and P. rhathymum) and other strains of planktonic species, including P. dentatum Stein, P. minimum (Pavillard) J. Schiller, P. triestinum J. Schiller, P. gracile F. Schütt, P. micans, and P. koreanum M.-S. Han, S. Y. Cho & P. Wang in M.-S. Hana, was sister of clade D. Among them, P. elegans was basal to P. dentatum Stein and divided into three subclades. In addition, the new obtained strains (X501 and 2XS80) belonged to P. rhathymum, which consisted of two subclades, with a support value of 93/1.00. In the analysis using ITS sequences, Karena brevis was an outgroup. In general, the tree topologies were similar to the phylogenies based on LSU sequences and exhibited analogous results (Supplementary Fig.S1).
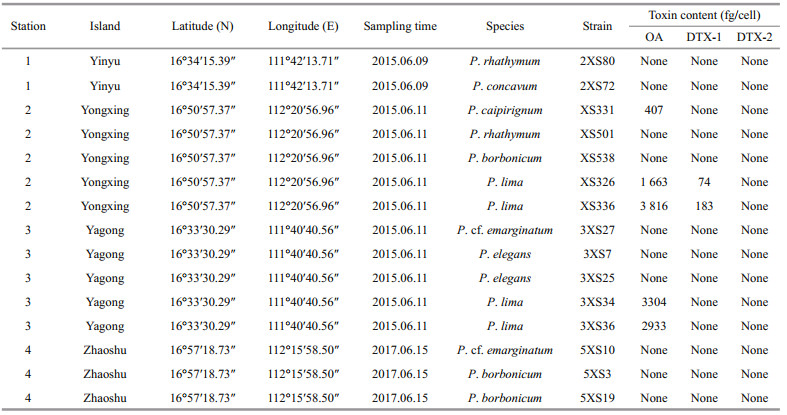
3.3 Detection of toxinsThe contents of OA, DTX-1, and DTX-2 in all fifteen strains (one P. concavum strain, one P. caipirignum strain, two P. rhathymum strains, three P. borbonicum strains, two P. elegans strains, two P. cf. emarginatum strain, and four P. lima complex strains; Table 2) from Xisha Islands were analyzed using LC-MS/MS. All strains of P. lima produced OA, with 1 663-3 816 fg/cell (Table 2). Two P. lima complex strains (XS336 and XS326) from Yongxing Island generated DTX-1 ranging from 74 to 183 fg/cell (Table 2). In addition, P. caipirignum strain (XS331) from Yongxing Island produced OA (407 fg/cell) but no DTXs. Other five species, including P. rhathymum, P. concavum, P. borbonicum, P. elegans, and P. cf. emarginatum, from Xisha Islands had no detectable toxins (Table 2).
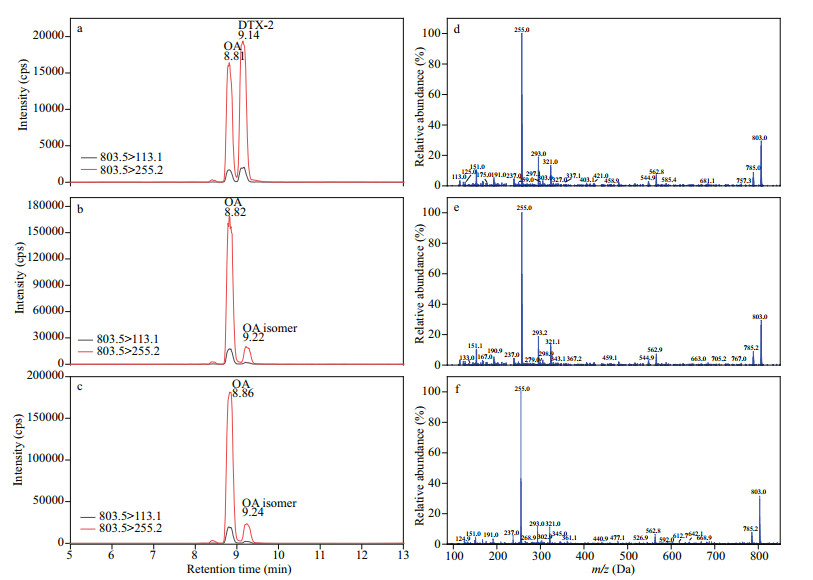
Interestingly, we found a suspected DTX-2 in two P. lima strains (3XS34 and 3XS36). The peak area ratios between different transitions of OA and suspected DTX-2 in samples were compared with the standard OA and DTX-2 (Supplementary Table S1), which demonstrated that the qualitative of OA could be satisfactorily supported by these results, but the suspected DTX-2 peak could not be testified completely because there was about 10% variation between samples and standard (Fig. 10). The mass spectrums of standard DTX-2 and the suspected DTX-2 in samples at collision energy 80 EV showed that the fragment ions of pseudo-molecular ions of both standard DTX-2 and the suspected compounds were basically same and their relative abundance were also similar (Fig. 10). However, the retention time of OA did not differ more than 0.04 min between standard and samples, and this difference in retention time reached up to 0.08–0.1 min between the DTX-2 standard and the corresponding peak in both samples. Therefore, we considered this suspected DTX-2 was an isomer of OA and DTX-2.
|
| Fig.10 Multiple reaction monitoring (MRM) chromatograms of: OA and DTX-2 reference standards (a), and OA and putative DTX-2 in strain 3XS34 and 3XS36 extracts (b and c, respectively); mass spectrum of DTX-2 in standard (d), and putative OA isomer in 3XS34 (e) and 3XS36 (f) with a collision energy of 80 eV |
Prorocentrum borbonicum and P. elegans were first reported in Chinese water, allowing us to compare the morphological and phylogenetic characteristics of Xisha Islands strains with other previous strains. Except the periflagellar area, the morphologies of P. borbonicum from Xisha Islands are consistent with the original descriptions (Ten-Hage et al., 2000a). They described the species with only eight platelets, but the periflagellar area of Xisha Islands strains consisted of nine platelets as platelet 6 divided into 6a and 6b (Fig. 2h). Our findings are in agreement with the descriptions by Hoppenrath et al. (2013), who showed the periflagellar area of P. borbonicum consisted of eight or nine platelets. Besides, P. borbonicum could morphologically differ from the similar species P. sipadanense. The latter is smaller (17.9–23.9-μm long and 15.0–19.8-μm wide) and devoid of pores in the cell center, but P. borbonicum from Xisha Islands was larger (24.40–31.92-μm long and 18.45–26.69-μm wide) and scattered small pores in the center of cell (Fig. 2). Also, they could differ from the characteristics of phylogeny (Fig. 9). P. borbonicum, comprising Xisha Islands strains and other sequences from GenBank with highest support value, was basal to P. sipadanense from France and P. playfairi Croome from Australian freshwater in ML tree inferred from LSU rDNA, with a high support value (99/1.00, Fig. 9). In addition, P. borbonicum was less reported to date and was found in France, the southeast Indian Ocean (Ten-Hage et al., 2000a), Greece, Mediterranean (Aligizaki et al., 2009), and Xisha Island, China (present study).
The morphologies of P. elegans from Xisha Islands were consistent with the original descriptions from Twins Cay, Belize (Faust, 1993). This species was characterized by a large, wide V-shaped periflagellar area, a protuberant spine at platelet 1 and 2 different sizes of pores (David et al., 2014). They isolated P. elegans strain Dn208EHU and firstly obtained molecular sequences with high support values, which revealed that this species obviously differed from other Prorocentrum species (David et al., 2014). In the present study, the phylogenies showed that P. elegans, comprising two Xisha Islands stains and two strains from Canary Islands and France, divided into three subclades, which indicates this species has high genetic diversity. To date, P. elegans was only reported in Biscay, French (David et al., 2014), Twin Cays, Belize (Faust, 1993), and Chinese waters (present study).
4.1.2 Prorocentrum lima complex and Prorocentrum caipirignumWe found DSP toxins were detected only in P. lima complex and P. caipirignum. A study suggested that P. lima complex could divide into five morphotypes collected from Hainan Island closed to our study area (Zhang et al., 2015), while P. caipirignum was erected by morphotypes 4 of P. lima complex based on the descriptions of Nascimento et al. (2017). The Xisha Islands strains of P. lima were basically in accord with original descriptions of specimens collected in French Polynesia, New Caledonia and Ryukyu Islands (Fukuyo, 1981). The exception was that Platelets 8 of periflagellar area in Xisha Islands strains was absent and platelet 7 was not obvious (Fig. 7h–i). Besides, P. lima complex was morphologically analogous with P. caipirignum. They could only be morphologically distinguished by their cell shape and thecal plate ornamentations (pore shape and size; absence or presence of a flange) (Nascimento et al., 2017). The distinct differences between P. caipirignum and P. lima complex in morphology presented in the present study. For instance, P. caipirignum cells are oval (L/W ratios: 1.13–1.41) while P. lima complex is ovoid (L/W ratios: 1.36–1.61). The marginal pores of P. lima complex were elongated, but P. caipirignum has kidney-shaped marginal pores, which twice to thrice larger than the former one (Nascimento et al., 2017). Moreover, the periflagellar area (1, 2, 3, 4a, 4b, 5, and 6) of P. caipirignum were consistent with the original descriptions that platelet 2 extended the right lateral plate and separated the flagellar pore and accessory pore (Nascimento et al., 2017). Interestingly, the platelet 4 potentially divided into 4a and 4b in Xisha Islands stains (Fig. 3h–i), which differed from the original descriptions (Nascimento et al., 2017). It is necessary to further clarify the morphological characteristics in P. caipirignum in the future.
Based on the ML trees inferred from LSU and ITS rDNA, the two toxic species of benthic Prorocentrum from the Xisha Islands could differ from each other (Fig. 9 & Supplementary Fig.S1). Many surveys reveal P. lima complex has high genetic diversity and may exist cryptic species. Aligizaki et al. (2009) stated P. lima had a high genetic diversity and replaced the term by 'P. lima complex'. Recently, a study in Lesser Antilles, eastern Caribbean Sea revealed that P. lima complex divided into five subclades and genetically related to P. caipirignum, P. consutum, P. bimaculatum, and P. hoffmannianum (Chomérat et al., 2019). Nishimura et al. (2020a) collected 244 strains of benthic Prorocentrum from Japan and showed P. lima complex had four species complex/ phylotypes. In the present study, the ML tree inferred from LSU rDNA showed P. lima complex consisted of two clades and four stains from Xisha Islands were located in a same clade (Fig. 9), which was the clade 1a by Nishimura et al. (2020a). However, the ML tree based on ITS region showed four strains from Xisha Islands divided into two clades (Supplementary Fig.S1). The strains (3XS34 and 3XS36) from Yongxing Island formed a clade, and Yagong Island strains (XS336 and XS334) were located in the other clade (Supplementary Fig.S1 & Table 2). Our findings confirm P. lima complex from Xisha Islands also is a complex and genetically related to clade 1a from Japan (Nishimura et al., 2020a). In addition, these findings coupled with the differences in toxin production provide a new proof for Nishimura et al. (2020a) stated there might be the existence of relationship between phylogenetic features and DSP toxins productions (Table 2).
4.1.3 Prorocentrum concavum, Prorocentrum cf. emarginatum, and Prorocentrum rhathymumProrocentrum concavum and P. rhathymum from Xisha Islands could differ from other benthic Prorocentrum species in both morphology and phylogeny, and the taxonomic characteristics of them were consistent with the original descriptions and recent findings (Fukuyo, 1981; Gómez et al., 2017; Zou et al., 2020). From a morphological view, P. cf. emarginatum differed from P. fukuyoi complex in cell size and thecal ornamentation. The former was 29.95–37.65-μm long and 23.22–33.76-μm wide (L/W, 1.06–1.50) with foveate thecae, and P. fukuyoi was 26.2–37.9-μm long and 18.0–26.5-μm wide from Beihai, Guangxi, with a smooth ornamentation (Luo et al., 2017). However, P. cf. emarginatum from Xisha Islands was similar to P. cf. sculptile from eastern Caribbean in morphology (Chomérat et al., 2019). From a molecular point, the ML tree inferred from LSU region showed P. cf. emarginatum from Xisha Islands was well divergent from P. cf. sculptile from eastern Caribbean, with a high support (98/1.00, Fig. 9). As suggested by Chomérat et al. (2019), the genetic distance between Xisha Islands strains and strains of Martinique were calculated based on LSU region and was 0.26. Considering morphology and phylogeny, we described this species as P. cf. emarginatum.
4.2 Toxin profileIn our research, seven benthic Prorocentrum species were collected from Xisha islands and only two (P. caipirignum and P. lima complex) produced DSP toxins. There were contradictory conclusions whether P. concavum produced OA and/or DTXs. Previous studies found P. concavum collected from the Caribbean Sea could produce OA using high-performance liquid chromatography and mouse assay (Dickey et al., 1990; Juranovic et al., 1997). However, no OA or DTXs were detected in P. concavum isolated from regions such as Okinawa, Japan, and Hainan, China (Yasumoto et al., 1987; Luo et al., 2017). Although no DSP toxins were detected, strains of P. concavum from Okinawa, Japan (Yasumoto et al., 1987) and Hainan Island, China (Zou et al., 2020) was potently toxic to fish, mice, and invertebrates, respectively. Also, no DSP toxins were detected in P. concavum strains from Xisha Islands (present study). The production of DSP toxins in P. concavum might show a strain-specific variation. Caillaud et al. (2010) stated that LC-MS/MS revealed low quantities of OA in P. rhathymum from Malaysia. In addition, An et al. (2010) found that one of five strains of P. rhathymum from Florida Bay produced OA. However, our results showed that no detectable OA was found in P. rhathymum from Xisha Islands, which is similar to previous findings (Yasumoto et al., 1987; Aligizaki et al., 2009). Recently, Lim et al. (2019) and Luo et al. (2017) reported all strains of P. mexicanum (was synonymized with P. rhathymum by Gómez et al. (2017)) and P. rhathymum produced no detectable DSP toxins, respectively. The findings that only one of six strains of P. rhathymum collected from Florida Bay produced OA (0.153 μg/L in the culture medium) (An et al., 2010). It suggested strain-specific variations also existed in this species. Previous studies showed that P. borbonicum produced borbotoxin (Ten-Hage et al., 2002) and its methanolic extract was lethal to mice and Artemia shrimp (Ten-Hage et al., 2000a, 2002; Aligizaki et al., 2009). However, this species did not generate any measurable OA, DTX-1, or DTX-2 (Aligizaki et al., 2009), which was consistent with our results of LC-MS/MS analysis. These results also were corroborated by the descriptions of Aligizaki et al. (2009) who used the protein phosphatase type 2A inhibition assay for their analyses. In addition, we found P. cf. emarginatum is a nontoxic species, which was suggested by previous reports (Escoffier et al., 2007; Aligizaki et al., 2009). We also determined that P. elegans did not produce OA, DTX-1, or DTX-2, which was analogous to the results of David et al. (2014). In the present work, only OA was detected in the Xisha Islands strain of P. caipirignum. This observation was supported by the results of the Belize study (Nascimento et al., 2017). The OA content of Xisha Islands P. caipirignum was 407 fg/cell, which was much lower than that reported in the Belizean strain (20 000 fg/cell) (Nascimento et al., 2017). The difference of OA content between strains of Xisha Islands and Belizean strain might be caused by culture conditions (temperature and light intensity) or strain-specific variations (Aquino-Cruz et al., 2018). In this study, all P. lima complex strains collected from Xisha Islands contained OA and DTX. It demonstrated that P. lima complex was a toxic species worldwide (Bouaïcha et al., 2001; Foden et al., 2005; Vale et al., 2009; Luo et al., 2017; Pan et al., 2017). Notably, the types of DSP toxins generated by P. lima complex showed strain-specific. Both P. lima complex strains (XS326 and XS336) from Yongxing Island produced OA and DTX-1. The strains of Mexico, the UK, Spain, and Portugal also contained both toxins in different concentrations (Heredia-Tapia et al., 2002; Foden et al., 2005; Vale et al., 2009). However, P. lima complex strains 3XS34 and 3XS36 from Yagong Island produced an isomer of OA and DTX-2 along with OA but no DTX-1 was detected. Our findings supported the concept that geographically distinct strains of P. lima complex not only can produce DSP toxins at different concentrations, but also they can generate various kinds of toxins.
5 CONCLUSIONIn the present study, we demonstrated seven species of benthic Prorocentrum, including P. borbonicum, P. caipirignum, P. concavum, P. elegans, P. cf. emarginatum, P. lima complex, and P. rhathymum using morphology and phylogenetic analysis in Xisha Islands, South China Sea. All of them inhabited benthic environments. Among them, P. elegans and P. borbonicum were the first records in Chinese waters. According to the results of LC-MS/MS analysis, no DSP toxins were produced by P. concavum, P. rhathymum, P. elegans, P. cf. emarginatum, P. borbonicum. But the other two species (P. caipirignum and P. lima complex) could produce OA. P. lima complex could generate DTX-1 or an isomer of OA and DTX-2. Our results demonstrated the risk of diarrheic shellfish poisoning in this area and it should be paid attention to.
6 DATA AVAILABILITY STATEMENTThe datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENTWe sincerely appreciate the three anonymous reviewers' comments and suggestions, which are helpful for the manuscript. Prof. Yubo LIANG from the National Marine Environmental Monitoring Center is deeply appreciated for providing data about toxins. We also thank Prof. Aifeng LI for providing valuable suggestions for the paper revision.
Electronic supplementary materialSupplementary material (Supplementary Fig.S1 and Table S1) is available in the online version of this article at https://doi.org/10.1007/s00343-021-1045-6.
Accoroni S, Totti C. 2016. The toxic benthic dinoflagellates of the genus Ostreopsis in temperate areas: a review. Advances in Oceanography and Limnology, 7(1): 1-15.
DOI:10.4081/aiol.2016.5591 |
Aligizaki K, Nikolaidis G, Katikou P, Baxevanis A D, Abatzopoulos T J. 2009. Potentially toxic epiphytic Prorocentrum (Dinophyceae) species in Greek coastal waters. Harmful Algae, 8(2): 299-311.
DOI:10.1016/j.hal.2008.07.002 |
Amar M, Aráoz R, Iorga B I, Yasumoto T, Servent D, Molgó J. 2018. Prorocentrolide-A from cultured Prorocentrum lima dinoflagellates collected in Japan blocks sub-types of nicotinic acetylcholine receptors. Toxins, 10(3): 97.
DOI:10.3390/toxins10030097 |
An T Y, Winshell J, Scorzetti G, Fell J W, Rein K S. 2010. Identification of okadaic acid production in the marine dinoflagellate Prorocentrum rhathymum from Florida Bay. Toxicon, 55(2-3): 653-657.
DOI:10.1016/j.toxicon.2009.08.018 |
Aquino-Cruz A, Purdie D A, Morris S. 2018. Effect of increasing sea water temperature on the growth and toxin production of the benthic dinoflagellate Prorocentrum lima. Hydrobiologia, 813(1): 103-122.
DOI:10.1007/s10750-018-3512-4 |
Berdalet E, Bravo I, Evans J, Fraga S, Kibler S, Kudela R, Larsen J, Litaker W, Penna A, Tester P A, Vila M, Zingone A. 2012. Global Ecology and Oceanography of Harmful Algal Blooms, GEOHAB Core Research Project: HABs in Benthic Systems. GEOHAB, Paris.
|
Berdalet E, Tester P A, Chinain M, Fraga S, Lemée R, Litaker W, Penna A, Usup G, Vila M, Zingone A. 2017. Harmful algal blooms in benthic systems: recent progress and future research. Oceanography, 30(1): 36-45.
DOI:10.5670/oceanog.2017.108 |
Boisnoir A, Pascal P Y, Cordonnier S, Lemée R. 2019. Spatiotemporal dynamics and biotic substrate preferences of benthic dinoflagellates in the Lesser Antilles, Caribbean sea. Harmful Algae, 81: 18-29.
DOI:10.1016/j.hal.2018.11.012 |
Bouaïcha N, Chézeau A, Turquet J, Quod J P, Puiseux-Dao S. 2001. Morphological and toxicological variability of Prorocentrum lima clones isolated from four locations in the south-west Indian Ocean. Toxicon, 39(8): 1195-1202.
DOI:10.1016/S0041-0101(00)00258-0 |
Caillaud A, de la Iglesia P, Campàs M, Elandaloussi L, Fernández M, Mohammad-Noor N, Andree K, Diogène J. 2010. Evidence of okadaic acid production in a cultured strain of the marine dinoflagellate Prorocentrum rhathymum from Malaysia. Toxicon, 55(2-3): 633-637.
DOI:10.1016/toxicon.2009.07.016 |
Chomérat N, Bilien G, Zentz F. 2019. A taxonomical study of benthic Prorocentrum species (Prorocentrales, Dinophyceae) from Anse Dufour (Martinique Island, eastern Caribbean Sea). Marine Biodiversity, 49(3): 1299-1319.
DOI:10.1007/s12526-018-0913-6 |
David H, Laza-Martínez A, García-Etxebarria K, Riobó P, Orive E. 2014. Characterization of Prorocentrum elegans and Prorocentrum levis (Dinophyceae) from the southeastern Bay of Biscay by morphology and molecular phylogeny. Journal of Phycology, 50(4): 718-726.
DOI:10.1111/jpy.12200 |
Dickey R W, Bobzin S C, Faulkner D J, Bencsath F A, Andrzejewski D. 1990. Identification of okadaic acid from a Caribbean dinoflagellate, Prorocentrum concavum. Toxicon, 28(4): 371-377.
DOI:10.1016/0041-0101(90)90074-H |
Ehrenberg C G. 1834. Dritter Beitrag zur Erkenntnis grosser organisationen in der Richtung des kleinsten raumes. Abhandlungen der Königlichen. Akademie der Wissenschaften, Berlin, Germany, 1833: 145-336.
|
Escoffier N, Gaudin J, Mezhoud K, Huet H, Chateau-Joubert S, Turquet J, Crespeau F, Edery M. 2007. Toxicity to medaka fish embryo development of okadaic acid and crude extracts of Prorocentrum dinoflagellates. Toxicon, 49(8): 1182-1192.
DOI:10.1016/j.toxicon.2007.02.008 |
Faust M A. 1993. Prorocentrum belizeanum, Prorocentrum elegans, and Prorocentrum caribbaeum, three new benthic species (Dinophyceae) from a mangrove island, Twin Cays, Belize. Journal of Phycology, 29(1): 100-107.
DOI:10.1111/j.1529-8817.1993.tb00287.x |
Fensome R, Taylor F, Norris G, Sarjeant W A S., 1993. A Classification of Living and Fossil Dinoflagellates: Geological Survey of Canada Contribution, Micropaleontology. American Museum of Natural History, Salem.
|
Foden J, Purdie D A, Morris S, Nascimento S. 2005. Epiphytic abundance and toxicity of Prorocentrum lima populations in the Fleet Lagoon, UK. Harmful Algae, 4(6): 1063-1074.
DOI:10.1016/j.hal.2005.03.004 |
Fukuyo Y. 1981. Taxonomical study on benthic dinoflagellates collected in coral reefs. Nippon Suisan Gakkaishi, 47(8): 967-978.
DOI:10.2331/suisan.47.967 |
Gómez F, Qiu D J, Lin S J. 2017. The synonymy of the toxic dinoflagellates Prorocentrum mexicanum and P. rhathymum and the description of P. steidingerae sp. nov. (Prorocentrales, Dinophyceae). Journal of Eukaryotic Microbiology, 64(5): 668-677.
DOI:10.1111/jeu.12403 |
Goris J, Hermann J, Hendrix P, Ozon R, Merlevede W. 1989. Okadaic acid, a specific protein phosphatase inhibitor, induces maturation and MPF formation in Xenopus laevis oocytes. FEBS Letters, 245(1-2): 91-94.
DOI:10.1016/0014-5793(89)80198-X |
Guillard R R L, Hargraves P E. 1993. Stichochrysis immobilis is a diatom, not a chrysophyte. Phycologia, 32(3): 234-236.
DOI:10.2216/i0031-8884-32-3-234.1 |
Heredia-Tapia A, Arredondo-Vega B O, Nuñez-Vázquez E J, Yasumoto T, Yasuda M, Ochoa J L. 2002. Isolation of Prorocentrum lima (Syn. Exuviaella lima) and diarrhetic shellfish poisoning (DSP) risk assessment in the Gulf of California, Mexico. Toxicon, 40(8): 1121-1127.
DOI:10.1016/S0041-0101(02)00111-3 |
Hoppenrath M, Chomérat N, Horiguchi T, Schweikert M, Nagahama Y, Murray S. 2013. Taxonomy and phylogeny of the benthic Prorocentrum species (Dinophyceae)—a proposal and review. Harmful Algae, 27: 1-28.
DOI:10.1016/j.hal.2013.03.006 |
Juranovic L R, Park D L, Fremy J M, Nielsen C A, Ah S S, Ayala C E. 1997. Isolation and separation of toxins produced by Gambierdiscus toxicus and Prorocentrum concavum. Journal of Aquatic Food Product Technology, 6(4): 5-25.
DOI:10.1300/J030v06n04_02 |
Lim Z F, Luo Z H, Lee L K, Hii K S, Teng S T, Chan L L, Chomérat N, Krock B, Gu H F, Lim P T, Leaw C P. 2019. Taxonomy and toxicity of Prorocentrum from Perhentian Islands (Malaysia), with a description of a non-toxigenic species Prorocentrum malayense sp.nov. (Dinophyceae). Harmful Algae, 83: 95-108.
DOI:10.1016/j.hal.2019.01.007 |
Luo Z H, Zhang H, Krock B, Lu S H, Yang W D, Gu H F. 2017. Morphology, molecular phylogeny and okadaic acid production of epibenthic Prorocentrum (Dinophyceae) species from the northern South China Sea. Algal Research, 22: 14-30.
DOI:10.1016/j.algal.2016.11.020 |
Marasigan AN, Sato S, Fukuyo Y, Kodama M. 2001. Accumulation of a high level of diarrhetic shellfish toxins in the green mussel Perna viridis during a bloom of Dinophysis caudata and Dinophysis miles in Sapian Bay, Panay Island, the Philippines. Fisheries Science, 67(5): 994-996.
DOI:10.1046/j.1444-2906.2001.00353.x |
Mohammad-Noor N, Moestrup Ø, Daugbjerg N. 2007. Light, electron microscopy and DNA sequences of the dinoflagellate Prorocentrum concavum (syn. P. arabianum) with special emphasis on the periflagellar area. Phycologia, 46(5): 549-564.
|
Morton S L. 1998. Morphology and toxicology of Prorocentrum faustiae sp.nov., a toxic species of non-planktonic dinoflagellate from Heron Island, Australia. Botanica Marina, 41(1-6): 565-569.
DOI:10.1515/botm.1998.41.1-6.565 |
Murray S, Ip C L C, Moore R, Nagahama Y, Fukuyo Y. 2009. Are prorocentroid dinoflagellates monophyletic? A study of 25 species based on nuclear and mitochondrial genes. Protist, 160(2): 245-264.
DOI:10.1016/j.protis.2008.12.004 |
Nascimento S M, Mendes M C Q, Menezes M, Rodríguez F, Alves-De-souza C, Branco S, Riobó P, Franco J, Nunes J M C, Huk M, Morris S, Fraga S. 2017. Morphology and phylogeny of Prorocentrum caipirignum sp. nov. (Dinophyceae), a new tropical toxic benthic dinoflagellate. Harmful Algae, 70: 73-89.
DOI:10.1016/j.hal.2017.11.001 |
Nishimura T, Uchida H, Noguchi R, Oikawa H, Suzuki T, Funaki H, Ihara C, Hagino K, Arimitsu S, Tanii Y, Abe S, Hashimoto K, Mimura K, Tanaka K, Yanagida I, Adachi M. 2020a. Abundance of the benthic dinoflagellate Prorocentrum and the diversity, distribution, and diarrhetic shellfish toxin production of Prorocentrum lima complex and P. caipirignum in Japan. Harmful Algae, 96: 101687.
DOI:10.1016/j.hal.2019.101687 |
Nishimura T, Uchida H, Suzuki T, Tawong W, Abe S, Arimitsu S, Adachi M. 2020b. First report on okadaic acid production of a benthic dinoflagellate Prorocentrum cf. fukuyoi from Japan. Phycological Research, 68(1): 30-40.
DOI:10.1111/pre.12401 |
Pan L, Chen J H, Shen H H, He X P, Li G J, Song X C, Zhou D S, Sun C J. 2017. Profiling of extracellular toxins associated with diarrhetic shellfish poison in Prorocentrum lima culture medium by high-performance liquid chromatography coupled with mass spectrometry. Toxins, 9(10): 308.
DOI:10.3390/toxins9100308 |
Pin L C, Teen L P, Ahmad A, Usup G. 2001. Genetic diversity of Ostreopsis ovata (Dinophyceae) from Malaysia. Marine Biotechnology, 3(3): 246-255.
DOI:10.1007/s101260000073 |
Rodríguez F, Riobó P, Crespín G D, Daranas A H, De Vera C R, Norte M, Fernández J J, Fraga S. 2018. The toxic benthic dinoflagellate Prorocentrum maculosum Faust is a synonym of Prorocentrum hoffmannianum Faust. Harmful Algae, 78: 1-8.
DOI:10.1016/j.hal.2018.06.009 |
Scholin C A, Herzog M, Sogin M, Anderson D M. 1994. Identification of group-and strain-specific genetic markers for globally distributed Alexandrium (Dinophyceae). Ⅱ. sequence analysis of a fragment of the LSU rRNA gene. Journal of Phycology, 30(6): 999-1011.
DOI:10.1111/j.0022-3646.1994.00999.x |
Ten-Hage L C, Delaunay N, Pichon V, Couté A, Puiseux-Dao S, Turquet J. 2000b. Okadaic acid production from the marine benthic dinoflagellate Prorocentrum arenarium Faust (Dinophyceae) isolated from Europa Island coral reef ecosystem (SW Indian Ocean). Toxicon, 38(8): 1043-1054.
DOI:10.1016/S0041-0101(99)00216-0 |
Ten-Hage L, Robillot C, Turquet J, Le Gall F, Le Caer J P, Bultel V, Guyot M, Molgó J. 2002. Effects of toxic extracts and purified borbotoxins from Prorocentrum borbonicum (Dinophyceae) on vertebrate neuromuscular junctions. Toxicon, 40(2): 137-148.
DOI:10.1016/S0041-0101(01)00200-8 |
Ten-Hage L, Turquet J, Quod J P, Puiseux-Dao S, Coute A. 2000a. Prorocentrum borbonicum sp. nov. (Dinophyceae), a new toxic benthic dinoflagellate from the southwestern Indian Ocean. Phycologia, 39(4): 296-301.
DOI:10.2216/i0031-8884-39-4-296.1 |
Toyofuku H. 2006. Joint FAO/WHO/IOC activities to provide scientific advice on marine biotoxins (research report). Marine Pollution Bulletin, 52(12): 1735-1745.
DOI:10.1016/j.marpolbul.2006.07.007 |
Vale P, Veloso V, Amorim A. 2009. Toxin composition of a Prorocentrum lima strain isolated from the Portuguese coast. Toxicon, 54(2): 145-152.
DOI:10.1016/j.toxicon.2009.03.026 |
Vila M, Garcés E, Masó M. 2001. Potentially toxic epiphytic dinoflagellate assemblages on macroalgae in the NW Mediterranean. Aquatic Microbial Ecology, 26(1): 51-60.
DOI:10.3354/ame026051 |
Yasumoto T, Seino N, Murakami Y, Murata M. 1987. Toxins produced by benthic dinoflagellates. The Biological Bulletin, 172(1): 128-131.
DOI:10.2307/1541612 |
Zhang H, Li Y, Cen J Y, Wang H L, Cui L, Dong Y L, Lu S H. 2015. Morphotypes of Prorocentrum lima (Dinophyceae) from Hainan Island, South China Sea: morphological and molecular characterization. Pycologia, 54(5): 503-516.
DOI:10.2216/15-8.1 |
Zhou J, Fritz L. 1993. Ultrastructure of two toxic marine dinoflagellates, Prorocentrum lima and Prorocentrum maculosum. Phycologia, 32(6): 444-450.
DOI:10.2216/i0031-8884-32-6-444.1 |
Zou J, Li Q, Lu S H, Dong Y L, Chen H, Zheng C Z, Cui L. 2020. The first benthic harmful dinoflagellate bloom in China: Morphology and toxicology of Prorocentrum concavum. Marine Pollution Bulletin, 158: 111313.
DOI:10.1016/j.marpolbul.2020.111313 |
 2022, Vol. 40
2022, Vol. 40