Institute of Oceanology, Chinese Academy of Sciences
Article Information
- WANG Ying, KUANG Shaoping, ZHANG Guangtao
- Accelerated biogenic silica dissolution by marine invertebrate digestion: in comparison with phosphorus and iron
- Journal of Oceanology and Limnology, 40(3): 1110-1120
- http://dx.doi.org/10.1007/s00343-021-1117-7
Article History
- Received Apr. 14, 2021
- accepted in principle Jun. 3, 2021
- accepted for publication Jul. 1, 2021
2 Jiaozhou Bay Marine Ecosystem Research Station, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China;
3 Laboratory for Marine Ecology and Environmental Science, Pilot National Laboratory for Marine Science and Technology (Qingdao), Qingdao 266237, China
The role of herbivorous animals in biogeochemical processes has become central in understanding ecosystem function (Vanni, 2002; Schmitz et al., 2010). Dissolved inorganic nutrients released via excretion and egestion may be an equally or more important control of primary production compared with the feeding pressure (Prins et al., 1997; Van Broekhoven et al., 2014). Regarding the historical perspective, the importance of consumers for mediating nutrient dynamics has been recognized in many terrestrial (McNaughton et al., 1988) and freshwater ecosystems (Kitchell et al., 1979), but in many marine ecosystems, animals are considered unimportant relative to microbes for biogeochemical cycling (Pomeroy, 1974). This is especially true for silicate recycling. It was suggested that bacteria-mediated silicon regeneration could explain most of the reported upper-ocean silicon regeneration (Bidle and Azam, 1999, 2001). Meanwhile, metazoa-mediated dissolution was not observed in the only such report from copepod (Tande and Slagstad, 1985).
However, the acidic and suboxic-anoxic environments in animal guts may serve as biogeochemical hotspots (Tang et al., 2011). Acidic digestion of zooplankton mobilizes the attached iron (Fe) on lithogenic particles (Schmidt et al., 2016), and that of common lugworm Arenicola marina enhances weathering and early diagenetic processes of basalt (Needham et al., 2004). Meanwhile, a substantial number of studies have reported the presence of gut microbes in aquatic invertebrates, some of which further reveal the association of bacteria to digestion (King et al., 2012; Chen et al., 2021). As bacteria assemblages in natural seawater can hasten biogenic silica (BSi) dissolution by degrading the organic matrix which protects diatom frustules (Bidle and Azam, 1999, 2001), it is hypothesized that the digestion process may function in this manner as well.
Digestion-associated nutrient mobilization may play an important role in recycling of nutrients regenerated exclusively by egestion. Concerning phosphorus (P) that is regenerated via both excretion and egestion, egestion may involve nutrient repackaging rather than recycling in aquatic ecosystems (Halvorson et al., 2017). Animal grazing may increase Fe cycling in surface waters, but also produce Fe-bearing particulate fecal matter, which is unavailable to phytoplankton until remineralization (Le Mézo and Galbraith, 2021). Even though most diatom frustules break once ingested, some remain intact even when the internal carbon has been digested, while some diatoms can even pass through zooplankton guts alive (Platt et al., 1983). Bacteria-mediated BSi dissolution can account for the efficient regeneration of diatom Si (Bidle and Azam, 1999), as fast as those reported for acid cleaning (Roubeix et al., 2008). Meanwhile, silicate dissolution is higher for feces after full-digestion than pseudofeces containing unconsumed foods in the presence of natural bacteria (Van Broekhoven et al., 2015).
While importance of fecal pellets in biogeochemical cycle has been well recognized, digestion effects can hardly be distinguished during the fecal decomposing processes in water column. Digestion of cultivated bivalves produces substantial quantities of feces and pseudofeces, called biodeposits (Tsuchiya, 1980; Smaal et al., 1986), which decomposes more rapidly than phytoplankton or macroalgae (Giles and Pilditch, 2006). This decomposition forms an important pathway of nutrient feedback, and the difference of regeneration rate among various nutrient components may induce deviation in nutrient structure from stoichiometric balance (Giles and Pilditch, 2006; Jansen et al., 2012). Many factors may explain the different regeneration rates among nutrient components and studies, including the variation in hydrodynamic regimes, suspended particulate matter quality and quantity (McKindsey et al., 2011). Until now, it is unclear whether the digestion process itself can induce variation in regeneration rate among nutrient components. Nonetheless, element and consumer specific differences are expected to play a role in digestion-associated nutrients mobilization, as BSi dissolution might be dependent on the digestion efficiency of its organic coats and Fe releasing would vary with gut pH values of consumers.
We hypothesized that rather than just functioning in repackaging, gut digestion might also be an accelerator of nutrient dissolution, especially for BSi, which is mostly dissolved after egestion. In our study, dissolution loss was compared between two food items and the fecal pellets of two marine invertebrates fed on them. The aim of the present study was (1) to specify rates and proportions of digestion-mediated nutrient regeneration and (2) to the compare digestion contribution among dissolution rates of the three nutrient elements. The comparison between two macroinvertebrate species may help to further our understanding of environmental impacts from bivalve mariculture.
2 MATERIAL AND METHOD 2.1 Food preparationTwo types of food materials were prepared in our study. Diatom Phaeodactylum tricornutum (PT) is a commonly used food type in aquaculture, whereas rice husk powder containing similarly high BSi is introduced as a less favorable food type that potentially induces pseudofeces production. PT was obtained from Marine Algae Species Conservation Center of Institute of Oceanology, Chinese Academy of Sciences (IOCAS) (Qingdao, China). Batch cultures were grown in f/2 liquid medium under a 12-h/12-h photoperiod at 19 ℃ (the room temperature for the feeding and dissolution experiments is 18–20℃). Cultures were harvested from the late-exponential to early-stationary phase, determined approximately by color change from light to deep brown. Upon microscopic checks, the harvested density varied between 0.63×106 and 2.27×106 cells/mL. Along with each harvest for feeding experiments, a halved volume of PT was filtered on the pre-burned and acid-washed GF/F membrane. The membrane was then rinsed twice with deionized water, wrapped in tinfoil and frozen at -80 ℃.
Rice husks were purchased from the Binzhou high-tech Development Zone, Shandong Province. After manual removal of visible impurities, the rice husk was washed several times in tap water to remove floating dust, and then washed twice with distilled water. The washed rice husk, laid on the porcelain plate covered with tinfoil, was dried at 60℃. Rice husks were crushed with a high-speed pulverizer and screened through 70-μm mesh. Considering that rice husk was selected as an unfavorable food type, purified soybean protein was added to a final mass content of 20% to satisfy the nutrient demand of animals during the entire cultivation period. Similarly, along with each rice husk powder (RHP) feeding, a halved amount of rice husk powder was soaked in 1-L filtered seawater for 24 h, and filtered on burned GF/F membrane. This was then rinsed and flash frozen at -80 ℃ for chemical measurements.
2.2 Feeding and fecal pellet collectionOf the two macroinvertebrates investigated here, oyster Crassostrea gigas is a suspension feeder, cultivated by bottom seeding or on artificial reefs in coastal waters. Urechis unicinctus, a burrowing Echiuran worm in the intertidal zone, is recognized as a detrital feeder, which occasionally performs coprophagy. The endemic oyster C. gigas was obtained from the mariculture laboratory of IOCAS, and U. unicinctus from the Great Urechis Haichuang Biotechnology Company. C. gigas with similar shell length of 8.0 cm was selected, and U. unicinctus had an average wet weight of 0.36. Before the feeding experiments, all animals were cultured in treated seawater and fed the same artificial food (Spirulina powder) for two weeks.
Four treatments were set up in triplicate. After starvation for 24 h, three oysters or 20 Echiuran worms were placed in one 30-L incubator.After being deployed individually in petri dishes, oysters were cultivated in 20-L filtered seawater, covered with a 5-cm layer of beach sand (previously sieved through 50-μm meshes and washed repeatedly), the worm incubators were then filled with a further 20-L filtered seawater. After burrowing into the sand layer, the worms expelled fecal pellets above the sand layer and piled them up around the digging hole. Treatments of oyster and worm were fed with PT and RHP, respectively, with daily amount of about 5% wet tissue weight. Calculated with cell density, 30–80-mL algal liquid was diluted into each incubator. Rice husk powder was soaked in filtered seawater of the same volume and provided simultaneously in the same manner.
Fecal pellets were collected every day. The oyster incubator was emptied before each collection; after transferring the oysters to a new incubator with the same equipment, fecal pellets were gently picked out from the original petri dishes to artificial seawater. For the worm incubator, seawater was refreshed at each collection, but the bottom sand layer was retained throughout the feeding experiment. Fecal pellets were piped out with long plastic pipette into artificial seawater. All the fecal pellets were re-sorted in artificial seawater to remove debris and sand particles. Finally, the fecal particles were filtered on burned GF/F membrane. The membranes were rinsed twice with deionized water, wrapped in tinfoil and frozen at -80 ℃.
2.3 Dissolution experimentIn order to distinguish the digestive effects of bacteria degradation after egestion, the dissolution incubation was carried out in artificial seawater. All kinds of samples were freeze-dried at -80℃, and time-series samples from the same treatment was mixed and weighed. A total of 0.3-g fecal pellets at PT feeding and 0.9 g at RHP feeding, in parallel with food mixture of 0.6 g, were used for dissolution incubation, which was performed in triplicate.
Incubations were performed in cylindrical transparent polypropylene bottles containing 250-mL artificial seawater, which was prepared following standard ASTM D1141-1998. These bottles, along with three blank controls, containing only artificial seawater, were wrapped in tin foil and placed in a room with a constant temperature of 18–20 ℃. To homogenize the dissolution process, bottles were gently shocked for 30 min once a day before sampling.
The incubation bottles were sampled on days 1, 2, 3, 4, 5, 7, 9, 11, 14, 17, and 21, respectively. At each sampling, 50 mL of artificial seawater was taken through a hydrophilic polyethersulfone needle filter with a diameter of 25 mm and a pore size of 0.45 μm. With the same apparatus, 50 mL of artificial seawater was refilled into the incubation bottle to maintain the dissolution volume of 250 mL. The water samples were stored at -20 ℃ in centrifugal tubes.
2.4 Measurements and analysisElement content in water samples was determined by Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES). For food and fecal materials, P and Fe were measured by ICP-OES, while phase analysis by nondestructive X-Rays Diffraction (XRD) was carried out on BSi in advance, and total BSi content was determined with the same batch of samples.
BSi was extracted using the NaOH/HF digestion method (Ragueneau and Tréguer, 1994) and corrected to the Si/Al ratio method (Ragueneau et al., 2005). For each sample, 25 mg was put into a 15-mL plastic centrifuge tube, and digested in 4-mL 0.2-mol/L NaOH at 100 ℃ for 40 min. After cooling, 1 mL of 1.0-mol/L HCl was added to the centrifuge tube to neutralize the extract. The samples were then centrifuged for 10 min at 4 000 r/min, 2 mL of supernatant was diluted into 25-mL deionized water. The extraction process was repeated, and BSi concentration was calculated as Si concentration in the first extraction minus Al concentration corrected by Si/Al ration in the second extraction.
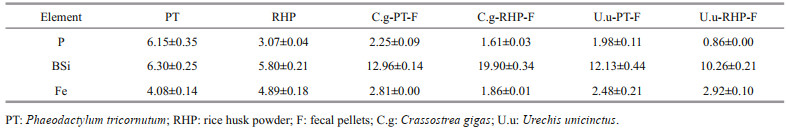
3 RESULT 3.1 Fecal pellet productionFor both macroinvertebrates, fecal pellet production was higher at rice husk powder than Phaeodactylum tricornutum feeding (Fig. 1). Daily fecal pellet production of C. gigas varied between 149 and 338 mg at RHP feeding and 42–71 mg at PT feeding, while that of U. unicinctus was in the range of 263– 840 mg and 96–200 mg, respectively. Meanwhile, fecal pellets at different diets can also be distinguished by visual appearances. When fed with PT, C. gigas produced solid rod-like feces, while RHP feeding resulted in fecal pellets resembling loose flakes, which appeared more similar to pseudofeces. For U. unicinctus, fecal pellets at PT feeding were cylindrical in appearance, while those at RHP feeding were similar in shape but larger and/or looser.
|
| Fig.1 Fecal pellet production of C. gigas(C.g) (a) and U. unicinctus (U.u) (b) feeding on Phaeodactylum tricornutum (PT) and rice husk powder (RHP) |
The inter-specific difference on fecal production was also detected on total production and variation in daily production. Daily fecal production of C. gigas increased over the first four days and decreased thereafter, whereas for U. unicinctus incubation it peaked at the 5th day. Total fecal production of C. gigas was 0.476 g at PT feeding and 1.833 g at RHP feeding, while that of U. unicinctus was 1.573 and 4.368 g, respectively. Based on the watercolor at feces collection, higher fecal production rate of U. unicinctus was more likely a result of higher filter feeding efficiency than lower digestion rate.
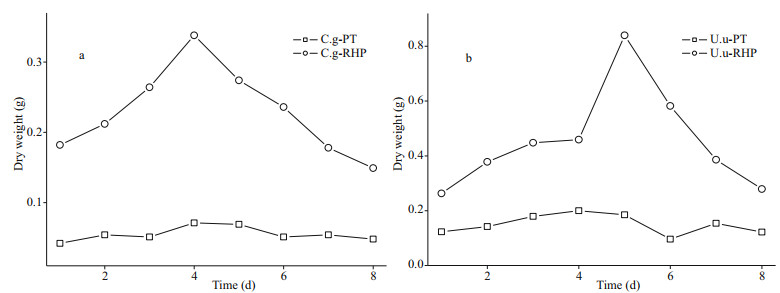
3.2 Element loss after digestionIn XRD diffractograms (Fig. 2), Phaeodactylum tricornutum showed wide curve broad peaks at 2θ angle of 10°, 34.78°, and 59.66°, while the peak in rice husk powder presented a 2θ angle of 22°, indicating that silicon existed in the amorphous form (opal). However, crystal content was detected in all fecal materials. Diffraction peaks presented at various angles in the diffractograms of U. unicinctus fecal pellets. The highest peak was identified as quartz. Comparatively, fewer peaks presented in diffractograms of C. gigas fecal pellets, representing the existence of mainly calcium carbonate and calcite. Even though these mineral components, involved into fecal pellets by non-selective feeding or physical attachment after egestion, are indissoluble, BSi content was used for dissolution rate calculation instead of total Si content.
|
| Fig.2 XRD scan of food materials and fecal pellets The abbreviations in each panel are the same as in Table 1. |
While BSi increased in percentage mass proportion during the digestion process, P and Fe content were lower in fecal pellets than the corresponding food items (Table 1), indicating active assimilation. Comparing to RHP, P content was two-fold higher in PT, BSi content was also higher, and Fe content was lower. When fed on the same food items, U. unicinctus removed P more efficiently during the digestion process than C. gigas. According to the mass proportion change after digestion, U. unicinctus assimilated Fe more efficiently at PT feeding, but less efficiently at RHP feeding. At PT feeding, BSi content in fecal pellets nearly doubled in both macroinvertebrates, whereas at RHP feeding BSi content in fecal pellets of C. gigas was two-fold higher.
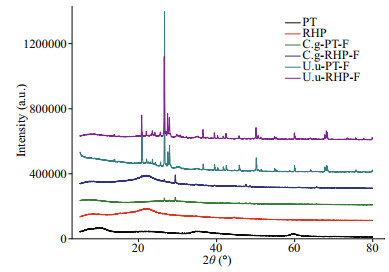
During dissolution incubation, digestion-promoted dissolubility was most significant in BSi, and to a lesser extent in P and Fe (Fig. 3). Total dissolved silicate in artificial seawater released from fecal pellets was 13.9–36.0 times of that released from the corresponding food materials. In the two subsamples collected before C. gigas and U. unicinctus feeding, PT lysed by freeze-thaw released dissolved silicate at rates of 0.17 and 0.10 mg/g, while the dissolution rate of RHP was the same at 0.09 mg/g. In C. gigas, total BSi dissolution rate of fecal pellets was 2.65 mg/g at PT feeding and 3.05 mg/g at RHP feeding. Comparatively lower BSi dissolution rates of 1.58 and 1.18 mg/g were recorded in fecal pellets produced by U. unicinctus at PT and RHP feeding, respectively. In all samples, the daily dissolution rates of BSi decreased during the incubation period and dissolution could hardly be detected in the last week (Table 2).
|
| Fig.3 Accumulated dissolved nutrient quantities throughout the incubation period in days Element mass standardized to dry mass of the start material in the chamber; error bars=±SD; n=3 for each treatment. Abbreviation in each panel as the same as in Table 1. |

|
Digestion accelerated total Fe dissolution by 13.0%–79.8% and total P dissolution by 29.8%– 162.9%. Compared with BSi, lower acceleration rates of Fe dissolution arose firstly from the high dissolubility of raw food materials. Subsamples of PT and RHP released Fe at rates of 0.47–0.71 mg/g. Second, the Fe dissolution rate of fecal pellets (0.53–1.10 mg/g) was also lower than that of silicate. In all samples, Fe release was recorded only in the first week. Different to Fe, P release was not observed in the initial days. While 0.33 and 0.36 mg/g P was released from fecal pellets of two macroinvertebrates at PT feeding, P release from freeze-thaw PT varied between 0.22–0.25 mg/g. Owing to the low P content, RHP released P at a rate of 0.03–0.05 mg/g, and releasing rate of fecal pellets at RHP feeding varied between 0.08–0.12 mg/g.
In both macroinvertebrate species, nutrient dissolubility in fecal pellets was independent to that in food materials. Compared to RHP, PT lysed by freeze-thaw had higher dissolubility in BSi and P, but similarly solubilities to Fe. However, upon digestion, C. gigas exhibited higher Fe and BSi mobilization potential on RHP diets than PT, while U. unicinctus showed the opposite. Although their performance on P mobilization was similarly efficient on both diets, P release from fecal pellets of U. unicinctus started much earlier than that of C. gigas, which was especially true for PT feeding. Through comparison of percentage loss during the incubation period between food materials and fecal pellets produced accordingly, C. gigas digestion promoted BSi dissolution by 7.59- and 11.5-fold upon PT and RHP feeding, with differences of 4.47- and 5.01-fold for P, and 2.24- and 3.06-fold for Fe. Comparatively, U. unicinctus digestion was more efficient on promotion of BSi dissolution on the PT diet (8.48- fold) and P dissolution on RHP diet (9.33-fold), but less efficient on the others.
4 DISCUSSIONOur results show direct evidence of consumer-mediated silicate regeneration. The hypothesis of element and consumer specific differences in digestion-associated nutrient mobilization is also supported. Astonishingly, to some extent, BSi mobilization showed no food dependence but exhibited significant inter-specific difference. Thus, it can be suggested that, in addition to top-down and bottom-up effects, herbivores may exert influence on stoichiometric nutrient balance through species replacement.
4.1 Element specific mobilizationOur results show that consumer-mediated nutrient recycling is element specific, and special attention should be paid to digestion-associated BSi mobilization. First, BSi in raw food materials was nearly indissoluble in the absence of seawater bacteria assemblages. When incubated in artificial seawater, the BSi dissolution rate of food materials was even lower than that for diatoms incubated in autoclaved seawater (Sun et al., 2014) and grass exposed to rain water (Vandevenne et al., 2013). For iron, while a higher dissolution rate was observed in fecal pellets, direct release from raw materials was also evident. Second, BSi dissolution started after egestion, while P and Fe assimilation also indicated excretion regeneration. For Fe, the leaching process may start as early as sloppy feeding, by which Fe can be lost before entering the digestive tract (Le Mézo and Galbraith, 2021). According to percentage change of three elements during the digestion process, iron and phosphorus were assimilated in considerable amounts during the digestion process, whereas biogenic silica was most likely repackaged. The assimilated part of these two elements can then be recycle in the dissolved forms through excretion. Third, BSi dissolution rate was highest in the first day and dissolution lasts for a longer time once mobilized. This indicates that, even though bacterial colonization is prevented by the compact physical structure of fecal pellets, dissolution is expected if only the silica skeleton establishes contact with seawater. Conversely, P regeneration happened in the later days, showing less digestive effects.
While dissolution acceleration through digestion process was fastest in BSi, total dissolution loss rate of the measured fecal elements was highest for Fe. High Fe regeneration rates from fecal pellets ensures its availability in the water column, as only a small fraction of animal absorbed Fe is excreted back into the water by dissolution (Schmidt et al., 2016). In contrast to Fe, phosphate release from fecal pellets lagged behind, suggesting that phosphorus in animal ingesta is simply repackaged and the recycling occurs mostly as excretion or mediated by bacterial degradation (Valdés et al., 2017). Even though the total release rate was higher in fecal pellets, it might be attributed to higher phosphorus oxidation rate after digestive decomposition of complex organic compounds.
Variation in dissolution loss among elements can be explained by different recycling pathways. As phosphorus was regenerated efficiently through excretion, digestion promoted dissolution can hardly be detected. Increasing BSi dissolution is generally attributed to the creation of BSi hotspots directly exposed to seawater after digestive removal of organic matter covering the silica skeleton or physical damage of silica structures (Vandevenne et al., 2013). In our study, increased BSi content in fecal pellets indicated organic matter removal. Crystal silicate (quartz) was detected in fecal pellets, but its contribution to dissolution was unlikely, as silicate dissolution was lower in U. unicinctus with quartz ingestion rather than gut passage macrobiologically, while enhanced weathering was observed over a time duration of months (Needham et al., 2006). While all organisms are capable of breaking down complexes and extracting Fe from their food using digestion enzymes produced by the animals or the gut microbiome (Freese et al., 2012), some animals also possess gastric glands and can perform acid lysis of the food cells for Fe release (Štrus et al., 2019). In addition, mechanical release of Fe presents upon ingestion of some animals, which crush or triturate their prey in the guts (Grosell et al., 2011). In our results, considerable dissolution loss was observed in Fe from food materials lysed simply by freeze-thaw, whereas this effect was negligible in BSi. After egestion, the dissolution rate was also higher in Fe than BSi, which might be mobilized in multiple ways.
However, silicate dissolution showed no significant difference between fecal pellets produced from the two food types that were presumed to be different in consumption efficiency. When fed on the proposed unfavorable RHP, oyster produced pseudofeces that were identifiable by their bulky and loose appearance, whereas feces after diatom ingestion were slim and tight. However, decreased silicate regeneration was not observed on the so-called pseudofeces relative to feces. In previous reports, accelerated mineralisation in feces relative to pseudofeces was attributed to bacteria succession after egestion (Harris, 1993; Fabiano et al., 1994), but this proposition cannot be applied into the bacteria-free incubation in our study. Instead, silicate dissolution from pseudofeces may be promoted for two reasons. First, based on food and fecal P content comparison, RHP was also considerably digested by two invertebrates, even though the consumption efficiency was lower than that of PT. Second, relative to PT, RHP was less nutritious but easier for gut mobilization. Results from an earlier dissolution experiment with forage in the rumen of a cow showed that more BSi dissolves from old grass already impacted by some decomposition as compared with fresh grass (Blackman and Bailey, 1971). Microscopic evidence also suggested that old grass leaves show more signs of internal mechanical damage, which can explain the higher reactivity of BSi in hay (Vandevenne et al., 2013). It has been suggested that, even though it is difficult to digest, the fragmental structure in rice husk powder would benefit the exposure of active BSi sites.
4.2 Species-specific regeneration rateInstead of food dependence, consumer specific difference was observed in our study on herbivore-mediated BSi dissolution. This is consistent with the previous results that digestion promoted BSi mobilization was observed in bivalves but absent in copepod (Tande and Slagstad, 1985; Van Broekhoven et al., 2015). However, based on the lower P content in fecal pellets, of was more efficient during U. unicinctus digestion than C. gigas, whereas BSi mobilization was less efficient during U. unicinctus digestion. It is thus suggested that, other than organic matrix removal and fragmentation, some other unspecified factors might be involved in BSi mobilization.
First, differences in digestive systems is capable of influencing BSi dissolution. Digestion efficiency increases with mean retention time of food particles in the animal gut. In terrestrial ecosystems, food retention is influenced by digestion type and is higher in ruminants compared with hindgut fermenters (Vandevenne et al., 2013). Ruminants are characterized by a long retention time, low food intake and a relatively high digestibility of organic matter and fiber, whereas opposite strategies are observed in hindgut fermenters rendering lower digestibility rates (Vandevenne et al., 2013). Differences in digestive systems may also explain why accelerated BSi dissolution was not observed in copepod feces (Tande and Slagstad, 1985). These zooplankton, which have short gut residence times < 1 h (Tirelli and Mayzaud, 2005), have developed a gut lining and digestive strategy that provides for assimilation of only soluble material (Reinfelder and Fisher, 1991). Based on previous reports, the gut residence time of oyster was nearly one day (Van Broekhoven et al., 2014). Although the gut residence time was not reported on Urechis unicinctus, it is known that its gut is tortuous and several times of body length (Huang et al., 2020).
Second, direct silica demand is proposed for animal growth, as various siliceous structures have been described. Mollusks require silicon during the formation of shells, radula teeth, spicules and penial spines, while crustaceans have opal and willemite-based teeth. The growth demands indicate the silicon assimilation and BSi digestion abilities of these animals. Of the two animals tested in our study, oyster had high silica content in body mass, whereas no siliceous structure was reported in Urechis unicinctus. It is unclear how silicon is absorbed by mollusks, but for some trace elements, a number of field and laboratory studies have shown that food ingestion is the predominant route for diverse benthic organisms to absorb metals which deposited in the shell together with silicon (Luoma et al., 1992; Wang et al., 1999; Wang and Fisher, 1999; Lee et al., 2000).
As for Fe recycling, the inter-species difference presents before egestion, because different taxa may tend to have different Fe absorption efficiencies. In previous reports, zooplankton absorbed Fe more efficiently than fish (Le Mézo and Galbraith 2021), and for same fish species Fe absorption was significantly different between larva and adults (Wang and Wang, 2016).
4.3 Fecal silicate mobilizationDigestion-promoted BSi mobilization helps to add silicate recycling into the consumer-mediated nutrient dynamics, which is suggested to be an important avenue of research in ecology (Allgeier et al., 2017). According to our results, herbivore digestion elevated BSi dissolubility by 13.9–36.0 times, while the dissolution rate in the first day was similar to that of diatom debris induced by seawater bacteria assemblages (Bidle and Azam, 1999, 2001). Meanwhile, the digested materials, when exposed to bacteria assemblages in natural seawater, may release silicate faster than those that remain undigested (Van Broekhoven et al., 2015). Relative to living diatom cells, fecal pellets benefit bacteria colonization, at least on the surface. In a modeling study, the lack of herbivores may promote diatom bloom and thus intensify silicate removal (Grégoire et al., 2008). Furthermore, inter-species differences in digestion-associated BSi mobilization means that different food web structure may result in varying silicate regeneration rates. As showed in our study and previous reports, BSi dissolution can hardly be detected in feces of copepods, but is quantifiable in those of salps (Tande and Slagstad, 1985; Cabanes et al., 2017), and shellfish might accelerate BSi dissolution more efficiently than non-siliceous feeders.
However, the acceleration rate of BSi dissolution observed in laboratory might be unachievable under natural conditions, possibly owing to effects of repackaging or aggregating. During a 21-day incubation in natural seawater, 22% of BSi dissolved from the mussel feces, and 17% dissolved from the pseudofeces (Van Broekhoven et al., 2015). These values are comparable to oyster results in our study, but much lower than that from bacteria decomposed diatom debris (Bidle and Azam, 1999). The freeze-thaw process in our study and the bacteria decomposing experiments may facilitate BSi dissolution through full exposure to seawater interface. It has been demonstrated that BSi dissolution decreased inside the fecal pellets and with diatom aggregation (Jansen, 2001; Passow et al., 2003).
Our results support the idea of shellfish introduction as a biological silicate pump, functioning through digestion (Van Broekhoven et al., 2015) and biodeposition (Ragueneau et al., 2002). In addition to the accelerated BSi dissolution, a higher mobilization efficiency is also expected in shellfish digestion in comparison with non-siliceous suspension feeders and planktonic feeders, such as copepods. Thus, it is critical to investigate the impacts of coprophagy. Two key issues should thus be addressed in the future: (1) whether BSi dissolution rate after coprophagy can reach as high as that from bacteria decomposed diatom debris (Bidle and Azam, 1999) or chemically cleaned diatom frustules (Saad et al., 2020); (2) to what extent the fecal silicate pump may contribute to the higher BSi dissolution/production ratio in some coastal waters (Nelson et al., 1995).
5 CONCLUSIONWithout considering the participation of bacteria and other microorganisms, the process of digestion can accelerate silicon regeneration; the release effect of different elements is different through digestion, but the effect of species-specific digestion on silicon regeneration is the most significant. It is suggested that animal digestion, with significant element and consumer specific differences, plays an important role in the regulation of nutrient regeneration and limitation.
6 DATA AVAILABILITY STATEMENTThe datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENTWe thank the staff of the Jiaozhou Bay Marine Ecosystem Research Station for their assistance in relevant research. We are grateful to S. Y. PENG for making the facilities and equipment available for this work.
Allgeier J E, Burkepile D E, Layman C A. 2017. Animal pee in the sea: consumer-mediated nutrient dynamics in the world's changing oceans. Global Change Biology, 23(6): 2166-2178.
DOI:10.1111/gcb.13625 |
Bidle K D, Azam F. 1999. Accelerated dissolution of diatom silica by marine bacterial assemblages. Nature, 397(6719): 508-512.
DOI:10.1038/17351 |
Bidle K D, Azam F. 2001. Bacterial control of silicon regeneration from diatom detritus: significance of bacterial ectohydrolases and species identity. Limnology and Oceanography, 46(7): 1606-1623.
|
Blackman E, Bailey C B. 1971. Dissolution of silica from dried grass in nylon bags placed in the rumen of a cow. Canadian Journal of Animal Science, 51(2): 327-332.
DOI:10.4141/cjas71-045 |
Cabanes D J E, Norman L, Santos-Echeandía J, Iversen M H, Trimborn S, Laglera L M, Hassler C S. 2017. First evaluation of the role of salp fecal pellets on iron biogeochemistry. Frontiers in Marine Science, 3: 289.
DOI:10.3389/fmars.2016.00289 |
Chen W B, Zhang S S, Sun Y, Tian B, Song L J, Xu Y, Liu T. 2021. Effects of substrate on the physiological characteristics and intestinal microbiota of Echiura worm (Urechis unicinctus) juveniles. Aquaculture, 530: 735710.
DOI:10.1016/j.aquaculture.2020.735710 |
Fabiano M, Danovaro R, Olivari E, Misic C. 1994. Decomposition of faecal matter and somatic tissue of Mytilus galloprovincialis: changes in organic matter composition and microbial succession. Marine Biology, 119(3): 375-384.
DOI:10.1007/BF00347534 |
Freese D, Kreibich T, Niehoff B. 2012. Characteristics of digestive enzymes of calanoid copepod species from different latitudes in relation to temperature, pH and food. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 162(4): 66-72.
DOI:10.1016/j.cbpb.2012.04.007 |
Giles H, Pilditch C A. 2006. Effects of mussel (Perna canaliculus) biodeposit decomposition on benthic respiration and nutrient fluxes. Marine Biology, 150(2): 261-271.
DOI:10.1007/s00227-006-0348-7 |
Grégoire M, Raick C, Soetaert K. 2008. Numerical modeling of the central Black Sea ecosystem functioning during the eutrophication phase. Progress in Oceanography, 76(3): 286-333.
DOI:10.1016/j.pocean.2008.01.002 |
Grosell M, Farrell A P, Brauner C J. 2011. The Multifunctional Gut of Fish. Academic Press, Boston.
|
Halvorson H M, Hall D J, Evans-White M A. 2017. Long-term stoichiometry and fates highlight animal egestion as nutrient repackaging, not recycling, in aquatic ecosystems. Functional Ecology, 31(9): 1802-1812.
DOI:10.1111/1365-2435.12875 |
Harris J M. 1993. The presence, nature, and role of gut microflora in aquatic invertebrates: a synthesis. Microbial Ecology, 25(3): 195-231.
DOI:10.1007/BF00171889 |
Huang D, Qin S, PU Y, Jiao X D. 2020. Advances in studies of artificial breeding and culturing techniques and the comprehensive utilization of Urechis unicinctus. Marine Sciences, 44(12): 123-131.
(in Chinese with English abstract) |
Jansen H M, Verdegem M C J, Strand Ø, Smaal A C. 2012. Seasonal variation in mineralization rates (C-N-P-Si) of mussel Mytilus edulis biodeposits. Marine Biology, 159(7): 1567-1580.
DOI:10.1007/s00227-012-1944-3 |
Jansen H, Wolf-Gladrow D A. 2001. Carbonate dissolution in copepod guts: a numerical model. Marine Ecology Progress Series, 221: 199-207.
DOI:10.3354/meps221199 |
King G M, Judd C, Kuske C R, Smith C. 2012. Analysis of stomach and gut microbiomes of the eastern oyster (Crassostrea virginica) from coastal Louisiana, USA. PLoS One, 7(12): e51475.
DOI:10.1371/journal.pone.0051475 |
Kitchell J F, O'Neill R V, Webb D, Gallepp G W, Bartell S M, Koonce J F, Ausmus B S. 1979. Consumer regulation of nutrient cycling. BioScience, 29(1): 28-34.
DOI:10.2307/1307570 |
Le Mézo P K, Galbraith E D. 2021. The fecal iron pump: global impact of animals on the iron stoichiometry of marine sinking particles. Limnology and Oceanography, 66(1): 201-213.
DOI:10.1002/lno.11597 |
Lee B G, Lee J S, Luoma S N, Choi H J, Koh C H. 2000. Influence of acid volatile sulfide and metal concentrations on metal bioavailability to marine invertebrates in contaminated sediments. Environmental Science & Technology, 34(21): 4517-4523.
DOI:10.1021/es001033h |
Luoma S N, Johns C, Fisher N S, Steinberg N A, Oremland R S, Reinfelder J R. 1992. Determination of selenium bioavailability to a benthic bivalve from particulate and solute pathways. Environmental Science & Technology, 26(3): 485-491.
DOI:10.1021/es00027a005 |
McKindsey C W, Archambault P, Callier M D, Olivier F. 2011. Influence of suspended and off-bottom mussel culture on the sea bottom and benthic habitats: a review. Canadian Journal of Zoology, 89(7): 622-646.
DOI:10.1139/z11-037 |
McNaughton S J, Ruess R W, Seagle S W. 1988. Large mammals and process dynamics in African ecosystems. BioScience, 38(11): 794-800.
DOI:10.2307/1310789 |
Needham S J, Worden R H, Cuadros J. 2006. Sediment ingestion by worms and the production of bio-clays: a study of macrobiologically enhanced weathering and early diagenetic processes. Sedimentology, 53(3): 567-579.
DOI:10.1111/j.1365-3091.2006.00781.x |
Needham S J, Worden R H, Mcilroy D. 2004. Animal-sediment interactions: the effect of ingestion and excretion by worms on mineralogy. Biogeosciences, 1(2): 113-121.
DOI:10.5194/bg-1-113-2004 |
Nelson D M, Tréguer P, Brzezinski M A, Leynaert A, Quéguiner B. 1995. Production and dissolution of biogenic silica in the ocean: revised global estimates, comparison with regional data and relationship to biogenic sedimentation. Global Biogeochemical Cycles, 9(3): 359-372.
DOI:10.1029/95GB01070 |
Passow U, Engel A, Ploug H. 2003. The role of aggregation for the dissolution of diatom frustules. Fems Microbiology Ecology, 46(3): 247-255.
DOI:10.1016/S0168-6496(03)00199-5 |
Platt T, Rao D V S, Smith J C, Li W K, Irwin B, Horne E P W, Sameoto D D. 1983. Photosynthetically-competent phytoplankton from the aphotic zone of the deep ocean. Marine Ecology Progress Series, 10(2): 105-110.
DOI:10.3354/meps010105 |
Pomeroy L R. 1974. The ocean's food web, a changing paradigm. BioScience, 24(9): 499-504.
DOI:10.2307/1296885 |
Prins T C, Smaal A C, Dame R F. 1997. A review of the feedbacks between bivalve grazing and ecosystem processes. Aquatic Ecology, 31(4): 349-359.
DOI:10.1023/A:1009924624259 |
Ragueneau O, Dittert N, Pondaven P, Tréguer P, Corrin L. 2002. Si/C decoupling in the world ocean: is the Southern Ocean different?. Deep Sea Research Part Ⅱ: Topical Studies in Oceanography, 49(16): 3127-3154.
DOI:10.1016/S0967-0645(02)00075-9 |
Ragueneau O, Savoye N, Amo Y D, Cotten J, Tardiveau B, Leynaert A. 2005. A new method for the measurement of biogenic silica in suspended matter of coastal waters: using Si: Al ratios to correct for the mineral interference. Continental Shelf Research, 25(5-6): 697-710.
DOI:10.1016/j.csr.2004.09.017 |
Ragueneau O, Tréguer P. 1994. Determination of biogenic silica in coastal waters: applicability and limits of the alkaline digestion method. Marine Chemistry, 45(1-2): 43-51.
DOI:10.1016/0304-4203(94)90090-6 |
Reinfelder J R, Fisher N S. 1991. The assimilation of elements ingested by marine copepods. Science, 251(4995): 794-796.
DOI:10.1126/science.251.4995.794 |
Roubeix V, Becquevort S, Lancelot C. 2008. Influence of bacteria and salinity on diatom biogenic silica dissolution in estuarine systems. Biogeochemistry, 88(1): 47-62.
DOI:10.1007/s10533-008-9193-8 |
Saad E M, Pickering R A, Shoji K, Hossain M I, Glover T G, Krause J W, Tang Y Z. 2020. Effect of cleaning methods on the dissolution of diatom frustules. Marine Chemistry, 224: 103826.
DOI:10.1016/j.marchem.2020.103826 |
Schmidt K, Schlosser C, Atkinson A, Fielding S, Venables H J, Waluda C M, Achterberg E P. 2016. Zooplankton gut passage mobilizes lithogenic iron for ocean productivity. Current Biology, 26(19): 2667-2673.
DOI:10.1016/j.cub.2016.07.058 |
Schmitz O J, Hawlena D, Trussell G C. 2010. Predator control of ecosystem nutrient dynamics. Ecology Letters, 13(10): 1199-1209.
DOI:10.1111/j.1461-0248.2010.01511.x |
Smaal A C, Verbagen J H G, Coosen J, Haas H A. 1986. Interaction between seston quantity and quality and benthic suspension feeders in the Oosterschelde, the Netherlands. Ophelia, 26(1): 385-399.
DOI:10.1080/00785326.1986.10422002 |
Štrus J, Žnidaršič N, Mrak P, Bogataj U, Vogt G. 2019. Structure, function and development of the digestive system in malacostracan crustaceans and adaptation to different lifestyles. Cell and Tissue Research, 377(3): 415-443.
DOI:10.1007/s00441-019-03056-0 |
Sun X L, Olofsson M, Andersson P S, Fry B, Legrand C, Humborg C, Mörth C M. 2014. Effects of growth and dissolution on the fractionation of silicon isotopes by estuarine diatoms. Geochimica et Cosmochimica Acta, 130: 156-166.
DOI:10.1016/j.gca.2014.01.024 |
Tande K S, Slagstad D. 1985. Assimilation efficiency in herbivorous aquatic organisms—The potential of the ratio method using 14C and biogenic silica as markers. Limnology and Oceanography, 30(5): 1093-1099.
DOI:10.4319/lo.1985.30.5.1093 |
Tang K W, Glud R N, Glud A, Rysgaard S, Nielsen T G. 2011. Copepod guts as biogeochemical hotspots in the sea: evidence from microelectrode profiling of Calanus spp. Limnology and Oceanography, 56(2): 666-672.
DOI:10.4319/lo.2011.56.2.0666 |
Tirelli V, Mayzaud P. 2005. Relationship between functional response and gut transit time in the calanoid copepod Acartia clausi: role of food quantity and quality. Journal of Plankton Research, 27(6): 557-568.
DOI:10.1093/plankt/fbi031 |
Tsuchiya M. 1980. Biodeposit production by the mussel Mytilus edulis L. on rocky shores. Journal of Experimental Marine Biology and Ecology, 47(3): 203-222.
DOI:10.1016/0022-0981(80)90039-8 |
Valdés V P, Fernandez C, Molina V, Escribano R, Joux F. 2017. Dissolved compounds excreted by copepods reshape the active marine bacterioplankton community composition. Frontiers in Marine Science, 4: 343.
DOI:10.3389/fmars.2017.00343 |
Van Broekhoven W, Jansen H, Verdegem M, Struyf E, Troost K, Lindeboom H, Smaal A. 2015. Nutrient regeneration from feces and pseudofeces of mussel Mytilus edulis spat. Marine Ecology Progress Series, 534: 107-120.
DOI:10.3354/meps11402 |
Van Broekhoven W, Troost K, Jansen H, Smaal A. 2014. Nutrient regeneration by mussel Mytilus edulis spat assemblages in a macrotidal system. Journal of Sea Research, 88: 36-46.
DOI:10.1016/j.seares.2013.12.007 |
Vandevenne F I, Barão A L, Schoelynck J, Smis A, Pyken N, Van Damme S, Meire P, Struyf E. 2013. Grazers: biocatalysts of terrestrial silica cycling. Proceedings of the Royal Society B Biological Sciences, 280(1772): 2013-2083.
DOI:10.1098/rspb.2013.2083 |
Vanni M J. 2002. Nutrient cycling by animals in freshwater ecosystems. Annual Review of Ecology and Systematics, 33: 341-370.
DOI:10.1146/annurev.ecolsys.33.010802.150519 |
Wang J, Wang W X. 2016. Novel insights into iron regulation and requirement in marine medaka Oryzias melastigma. Scientific Reports, 6(1): 26615.
DOI:10.1038/srep26615 |
Wang W X, Fisher N S. 1999. Delineating metal accumulation pathways for marine invertebrates. Science of the Total Environment, 237-238: 459-472.
DOI:10.1016/S0048-9697(99)00158-8 |
Wang W X, Qiu J W, Qian P Y. 1999. The trophic transfer of Cd, Cr, and Se in the barnacle Balanus amphitrite from planktonic food. Marine Ecology Progress Series, 187: 191-201.
DOI:10.3354/meps187191 |
 2022, Vol. 40
2022, Vol. 40