Institute of Oceanology, Chinese Academy of Sciences
Article Information
- SONG Dade, XIONG Ying, JIANG Tao, YANG Jian, ZHONG Xiaming, TANG TANG
- Early life migration and population discrimination of the small yellow croaker Larimichthys polyactis from the Yellow Sea: inferences from otolith Sr/Ca ratios
- Journal of Oceanology and Limnology, 40(2): 818-829
- http://dx.doi.org/10.1007/s00343-021-1041-x
Article History
- Received Feb. 10, 2021
- accepted in principle Mar. 30, 2021
- accepted for publication May. 17, 2021
2 Jiangsu Marine Fisheries Research Institute, Nantong 226007, China;
3 Key Laboratory of Fishery Ecological Environment Assessment and Resource Conservation in Middle and Lower Reaches of Yangtze River, Freshwater Fisheries Research Center, Chinese Academy of Fishery Sciences, Wuxi 214081, China
The benthic small yellow croaker Larimichthys polyactis (family: Sciaenidae) is endemic to the northwestern Pacific Ocean, including the Bohai, Yellow, and East China Seas (Fishbase, 2019). This species is a multiple spawner with persistent and directional movement annually (Lee et al., 2020). Inshore or coastal waters are major spawning and nursery areas, and overwintering grounds are located in offshore areas (Lim et al., 2010). Previous studies have focused on the population division and migration of L. polyactis owing to its high ecological and commercial importance (Wang et al., 2013, 2016a; Zhang et al., 2014, 2020; Xiao et al., 2015; Xiong et al., 2016; ). Although progress has been made regarding the whole life migration (Lin, 1987; Liu, 1990; Lee et al., 2020), the larval-dispersal component of connectivity remains unclear, limiting our understanding of population dynamics. Previous studies revealed that the spawning grounds of L. polyactis have expanded from nearshore to offshore waters based on environmental DNA (Wang et al., 2020) and catch data (Lin et al., 2008). Previous studies based on catch data, otolith morphology, and population genetics have generated inconsistent results for population division, especially regarding the L. polyactis in the Yellow Sea (Xiong et al., 2021). In addition, the natural resources of L. polyactis have severely decreased since the 1970s due to increasing fishing efforts (Xiong et al., 2017b; Ma et al., 2020). Comparing its historical biological characteristics, distinct trends in biological parameter changes have been observed, such as miniaturization, younger age, and earlier maturation, but with fishing effort remaining at a high level (Zhang et al., 2016; Lee et al., 2020; Li et al., 2020). Therefore, there is an urgent need to further our understanding of the early life migration and population discrimination of L. polyactis to improve the sustainable utilization of this economically important fishery resource.
Connectivity, the exchange of larvae, juveniles, or adults across a species' range, plays a fundamental role in metapopulation dynamics, genetic diversity, and the resiliency of populations to human exploitation (Botsford et al., 2001; Cowen et al., 2007). It is necessary to obtain basic life history information (including migration, habitat selections, foraging and reproductive locations, home-range characteristics, and spatial-temporal dispersal) to understand the relationship between fish and the environment they inhabit (Cooke et al., 2013). It is critical to identify the movement patterns among habitats during the lifespan of species, which determine the appropriate spatial scale for fishery management (Gillanders, 2005; Carlson et al., 2017). Accurately characterizing fish migration and population structure is a longstanding goal for the management of sustainable fisheries because it is based on the understanding offi sh movement across complex oceanographic and inshore environments over large spatial scales (King and McFarlane, 2003; Ovenden, 2013). Furthermore, in many cases, it is impractical to directly observe their movements to estimate population connectivity in marine environments (Lowe and Allendorf, 2010).
The classification of different migrant individuals or populations can be based on a number of techniques, including visual censuses of tagged individuals (Walker et al., 2011), electric tagging (acoustic sounding and satellite-tracking tags) (Sedberry and Loefer, 2001), and the autologous chemical markers, such as calcified otoliths (Jiang et al., 2017; Delerue-Ricard et al., 2019; Schulz-Mirbach et al., 2019). Otolith microchemistry is superior to the other approaches used to determine fish migration patterns because every fish is innately tagged. In addition, the traditional tagging methods only offer information on the location and time of capture and recapture, whereas otolith microchemistry can provide habitat environmental information over a lifetime.
The potential for using otoliths as a tool to identify and track different populations is reliant on the elements, such as Sr/Ca, being incorporated into discrete layers of otolith material that form daily (Arai et al., 2019). Therefore, the otoliths provide a precise elemental log over the lifetime of a fish. In previous studies, otolith ratios of Sr/Ca have been used as habitat environmental markers for wild diadromous fish, even being successful in corresponding studies that used limited (e.g. < 10) otolith samples (Chatterjee et al., 2015; Jiang et al., 2016; Chino et al., 2018). The otolith Sr/Ca ratio is influenced by environmental factors, including ambient water chemistry, salinity, and temperature (Elsdon and Gillanders, 2003; Taddese et al., 2019), with minor influence from diet (Milton and Chenery, 2001). The relationship can be predicted between ambient environmental variables and otolith Sr/Ca (Jiang et al., 2017), which can be used to reconstruct fish movement patterns through environmental gradients based on otolith Sr/Ca ratios (Liu et al., 2011). Trace elements are not decomposed after daily deposition (Elsdon et al., 2008); hence, the chemistry of the otolith core represents the early life history and the otolith edge reflects the water conditions during the most recent period of the fishing area. Indeed, reconstructing migratory patterns (Dou et al., 2012; Tran et al., 2019), discriminating natal or nursery origins (Jiang et al., 2017; Rogers et al., 2019), and assessing the contributions of different larval areas to adult populations (Zlokovitz et al., 2003; Delerue-Ricard et al., 2019) have been accomplished with otolith Sr/Ca ratios. Furthermore, Xiong et al. (2021) revealed temporal stability in the otolith Sr/Ca ratio of L. polyactis in the southern Yellow Sea. Therefore, otolith Sr/Ca can be a useful indicator for tracking the migratory paths of fish based on the reconstruction of environmental histories as recorded in the layers of the otolith (Reis-Santos et al., 2015; Avigliano et al., 2017).
We hypothesised that the early life migration of L. polyactis was from inshore to nearshore waters in the Yellow Sea. To test this hypothesis, the otolith Sr/Ca ratio of L. polyactis was compared between different growth zones to confirm the shift in habitat and characterize early life migration. In the present study, we investigated three groups of L. polyactis from spawning and overwintering grounds of the Yellow Sea, and attempted to do the following: (1) investigate the characteristics of early life migration using the otolith Sr/Ca ratios of different growth zones, and (2) use the Sr/Ca ratio profiles to discriminate populations further.
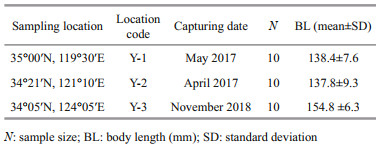
2 MATERIAL AND METHOD 2.1 Sample collection and otolith preparationA total of 30 adult L. polyactis were collected from spawning grounds along the coastal waters of the Yellow Sea and overwintering ground off the Yellow Sea, including 10 from Y-1, 10 from Y-2, and 10 from Y-3 (Table 1; Fig. 1a) in 2017 and 2018. All fish were randomly sampled from catches by commercial vessels operating the gill net (mesh size 50 mm). All samples were stored at -4 ℃ until further analysis in the laboratory. In all cases, all specimens were measured (body length in mm) and weighed (body weight in g) before being dissected. The sex and maturity of fish gonads were determined by visual examination. Sagittal otoliths were extracted from L. polyactis, dried, weighed, and stored in plastic tubes. Two experienced readers counted the number of annuli in the otolith to confirm the age. By analyzing the gonad maturity and sampling location, the L. polyactis from Y-1, Y-2, and Y-3 were found to be in their pre-spawning, spawning, and wintering periods, respectively. L. polyactis can reach sexual maturity at 1 year old (Lin et al., 2008), and thus, the life history of these adults covers one complete migration. Detailed information of samples for otolith microchemistry analysis is shown in Table 1.
|
|
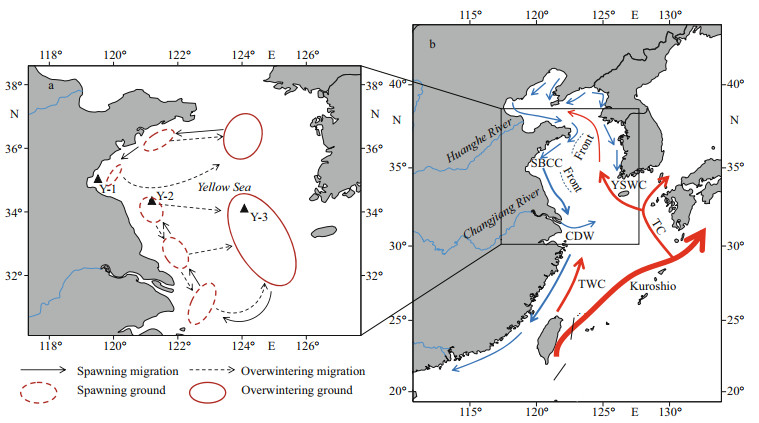
| Fig.1 Sampling locations (▲) and migratory routes of Larimichthys polyactis in the Yellow Sea and major surface currents in winter a. sampling locations and migratory routes for L. polyactis in the Yellow Sea (Liu, 1990); b. major currents: Taiwan Warm Current (TWC), Yellow Sea Warm Current (YSWC), Subei Costal Current (SBCC), and Changjiang Diluted Water (CDW) (Liu, 2013; Huang et al., 2018). Red arrows denote the warm currents and blue arrows denote the cold currents. The dashed lines represent the hydrological front in the Yellow Sea. |
The procedures for analyzing the otolith Sr/Ca ratios of L. polyactis for use in electron probe microanalysis (EPMA) measurement followed those described by Jiang et al. (2017) and Xiong et al. (2021). All pre-prepared sagittal otoliths were mounted in clear epoxy resin (EpoFix; Struers, Copenhagen, Denmark) and thin-sectioned with a diamond cup wheel (Discoplan-TS; Struers, Copenhagen, Denmark) in the sagittal plane. Thin sections were adhered to glass microscope slides with AB glue and sanded to the core encircled by clear daily rings on both sides with 1 200–2 000 grit SiC paper and further polished with 0.3-μm alumina on an automated polishing wheel to remove major scratches (Labopol-35, struers, Copenhagen, Denmark). Thereafter, the otoliths were sonicated in an ultrasonic cleaner for 5 min and cleaned with deionized water. Finally, all samples were dried and carbon coated with a high vacuum evaporator (JEE-420, JEOL Ltd., Tokyo, Japan) for further analysis.
Otolith sections for EPMA analysis were prepared following the procedure described by Jiang et al. (2017), but with a slight modification. Otolith Sr and Ca were measured along the longest straight line from the core to the edge (i.e. otolith radius) using an electron probe microanalyzer (JXA-8100; JEOL Ltd.). Quantitative analyses were performed under the following beam conditions: 20 nA for the beam current, 15 kV for accelerating voltage, and a 3-μm diameter circular scanning beam, with measurements spaced at 20-μm intervals. Commercial standards of tausonite (SrTiO3) and calcite (CaCO3) (Institute of Mineral Resources, Chinese Academy of Geological Sciences, Beijing, China) were used to calibrate the Sr and Ca concentrations in the otoliths.
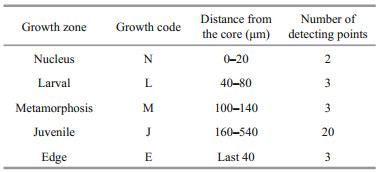
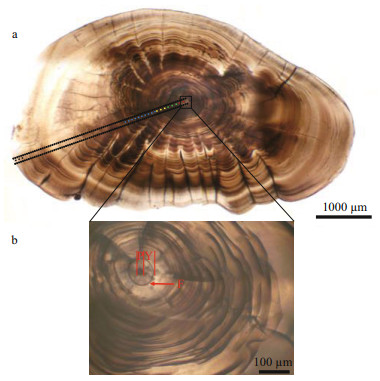
2.3 Selection of different developmental stages and signal processingPrimary increments were validated as being daily in L. polyactis, and characteristic marks represent incubation, first feeding, and metamorphosis during the larval and juvenile stages (Li et al., 2013; Zhan et al., 2016; Xiong et al., 2017a; Zhang et al., 2019b). Based on the above-cited studies and focusing on the relationship between otolith microstructure and growth increment of L. polyactis, five zones along the transect analyzed were isolated to measure the Sr/ Ca ratios (Table 2; Fig. 2a): (1) the "nucleus" (N) zone is the area within hatching increments that are associated with the embryonic stage comprising the primordium and yolk sack (Zhan et al., 2016). As shown in the Fig. 2b, the first feeding check appeared on the 4th increment with a distance of 20.67±2.28 μm to the central nucleus, displaying larger width, deeper color, and high clarity (Li et al., 2013; Zhang et al., 2019b); (2) the "larval" (L) zone is associated with the larval phase, during which L. polyactis began to feed on the zooplanktivorous Copepoda, Euphausiacea, and Mysidacea (Xue et al. 2005; Xiong et al., 2017a); (3) the "metamorphosis" (M) zone corresponds to the metamorphosis period characterized by the presence of sub-daily increments (Zhang et al., 2019b) and transition from larval to juvenile (Li et al., 2013). The sub-daily otolith increments of L. polyactis during the early life history display irregularity and incompleteness compared with daily increments, particularly from the L zone to J zone, which caused difficulties in the integration of daily increments to investigate the early life migration; (4) the "juvenile" (J) zone just after metamorphosis reflects the signature of the nursery ground colonized at demersal settlement with a change in the feeding strategy to mainly feeding on demersal shrimp and fish (Xue et al., 2005; Xiong et al., 2017a); (5) the "edge" (E) zone corresponds to the last weeks before being captured, representing the signature of the sampling location.
|
|
| Fig.2 Larimichthys polyactis sagittal otoliths showing spots of otolith chemistry analysis and microstructure characteristics a. the red, green, yellow, blue, and black dots represent the N, L, M, J, and E zones, respectively; b. sagittal microstructure characteristics. P, Y, and F represent the primordium and yolk sack, and the first feeding ring of the sagittal otolith, respectively. |
In conventional otolith research, otoliths were analyzed for Sr and Ca and reported as ratios of Sr to Ca amplified by 1 000 by a conversion based on the molecular mass of CaO and SrO. Statistical analyses were performed using Origin 2021 (Originlab, Northampton, MA, USA). In addition, a sequential regime shift algorithm was used to determine significant changes in the mean Sr/Ca ratio during the lifespan according to Rodionov and Overland (2005). The parameters of the regime shift algorithm were set as follows: cut-off length: 5, Huber's weight parameter: 1, and probability level: 0.5.
One-way analysis of variance (ANOVA) was applied to determine the differences in otolith Sr/Ca ratios of (1) the same growth zone of L. polyactis among the three sampling groups, and (2) different growth zones in the same sampling group. In order to meet the assumptions for normality and homogeneity of variance for data analysis, the averaged data for each otolith were computed and subsequently log10 transformed. To evaluate the use of Sr/Ca ratios of different growth zones as a predictive tracer for fish groups, canonical discriminant analysis (CDA) was carried out. Cross-validated classification accuracy was analyzed for each group. Considering that the capture date of L. polyactis from the three sampling locations was not consistent, the Sr/Ca ratios of the E zone were not applied to CDA.
3 RESULT 3.1 Temporal and spatial variation in otolith Sr/Ca ratiosThe 30 specimens of L. polyactis from Y-1, Y-2, and Y-3 shared a common feature: the Sr/Ca ratios were higher in the N zone, and then decreased sharply to the E zone (Fig. 3). Values in the N zone were significantly higher than those in the other zones (P < 0.05). No significant differences were observed in the Sr/Ca ratios between the L and M zones in Y-1, Y-2, and Y-3 (P>0.05); nevertheless, there was a significant difference between the M and J zones in Y-1, Y-2, and Y-3 (P < 0.05), suggesting that the variables of the environment inhabited by L. polyactis remained unchanged from the L zone to M zone but changed from the M zone to J zone.
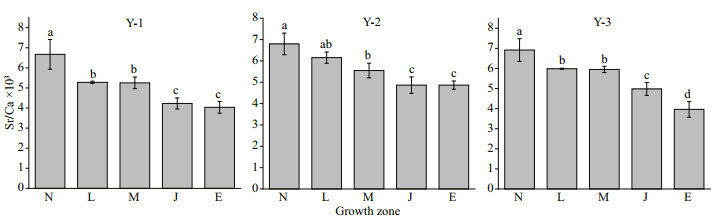
|
| Fig.3 Sr/Ca ratio variation in different growth zones for Larimichthys polyactis from Y-1, Y-2, and Y-3 |
By analyzing the spatial differences between Y-1, Y-2, and Y-3, the Sr/Ca ratios of the L, J, and E zones in Y-2 were found to be significantly higher than those of Y-1 (Fig. 4, P < 0.05). Although the Sr/Ca ratios of the L zone showed no significant differences between Y-3 and Y-1, the Sr/Ca ratios of Y-3 were evidently higher than those of Y-1. The Sr/Ca ratios of the J zone in Y-3 were significantly higher than those of Y-1 (Fig. 4, P < 0.05). The Sr/Ca ratios of the N, L, M, and J zones showed no significant differences between Y-2 and Y-3 (Fig. 4, P < 0.05).

|
| Fig.4 Sr/Ca ratio variation in the same growth zones for Larimichthys polyactis from Y-1, Y-2, and Y-3 Different letters indicate significant differences between the same growth zones (P<0.05). |
The Sr/Ca ratio (mean±SD) of Y-1, Y-2, and Y-3 during the pre-developmental stage, including N, L, M, and J zones, was 4.33±0.58, 5.04±0.52, and 5.03±0.52, respectively. There was no significant difference between Y-2 and Y-3 (P>0.05), whereas significant differences were observed between Y-1 and Y-3, and between Y-1 and Y-2 (P < 0.05). By analyzing the Sr/Ca ratios based on the aforementioned sequential regime shift algorithm, two distinct Sr/Ca shifts were observed between the N and L zones, and between the M and J zones, suggesting that the environmental variable or ontogenetic stage had changed (Fig. 5).
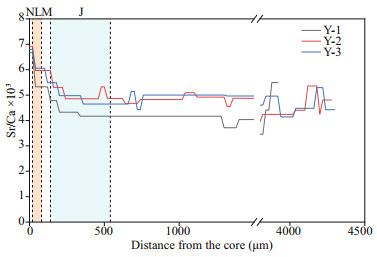
|
| Fig.5 Fluctuations in weighed otolith Sr/Ca ratios along the line transects from the core (0 μm) to the edge in the sagittal plane of Larimichthys polyactis Colored columns are used to distinguish the N, L, M, and J zones only. |
Overall, spatial differences were reflected in the assignment to individual groups. The percentage of good assignment of these samples to their actual group with CDA (jackknife cross-validation) was 80% for Y-1, 50% for Y-3, and 70% for Y-2. The overall accuracy of discrimination was 66.67%. Furthermore, 40% L. polyactis of Y-3 and 30% of Y-2 were misjudged as belonging to the opposite groups (Table 3), and a strong overlap in Sr/Ca ratios was observed between Y-3 and Y-2 (Fig. 6). The discriminant analysis provided a visual separation among Y-1, Y-3, and Y-2 (Fig. 6), in which the first canonical variable explained 94.31% of the variance, and the second canonical variable explained 5.69% of the variance. A total of 30 individuals from three locations were clearly divided into two groups, namely, the northern Yellow Sea group represented by the Y-1 group and the southern Yellow Sea group represented by the Y-3 and Y-2 groups.
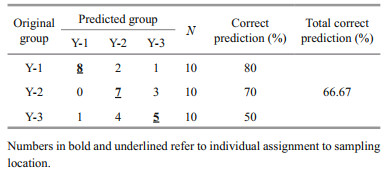
|
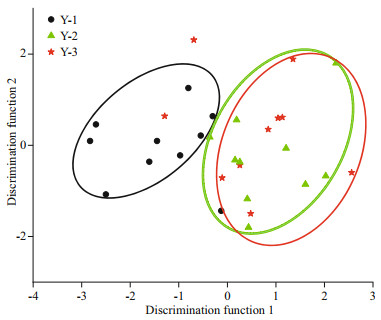
|
| Fig.6 Scatter plot of canonical discriminant analysis showing the separation of Larimichthys polyactis from Y-1, Y-2, and Y-3 Color-coding and shape identify sampling locations. Ellipses are for visualization purposes only. |
To fully understand population connectivity, it is vital to develop a process-based understanding of dispersal, which explores how hydrodynamic and biological processes interact to disperse the larvae at different spatiotemporal scales (Cowen et al., 2007). Nevertheless, the combination of otolith chemistry and process-oriented approaches promises a truly predictive understanding of larval dispersal and connectivity (Cowen et al., 2007). Our results revealed how the Sr/Ca ratios vary from the N zone to J zone in the otoliths and the differences in the same growth zone among Y-1, Y-3, and Y-2, combined with environmental variables (salinity, temperature, and currents) to explore how this phenomenon has arisen. Clustering the Sr/Ca ratios of the N, L, M, and J zones pointed to two groups, verifying the existence of largescale spatial connectivity (>350 km) between the spawning and overwintering grounds of L. polyactis.
4.1 Variation in Sr/Ca ratios and the possible factorsNatal homing, or philopatry, is a life history characteristic shared by many fishes (Cowen et al., 2007). Natal fidelity has important ramifications for population connectivity, population persistence, and the potential management of fish populations (Gahagan et al., 2012). Hence, attention should be paid to the phenomenon that eggs, yolk-sac, and preflexion larval L. polyactis were mainly distributed in Chinese inshore waters at depths >30 m, whereas post-flexion larvae and juveniles were mainly distributed in the relatively shallow waters at depths of approximately 10 m (Lin et al., 2018). The pelagic larval developmental stage is a vital phase in marine fish during ontogenetic migration and can influence population recruitment, including connectivity, dispersal, and survival (Cowen et al., 2007). Owing to their lack of sufficient swimming ability, pre-flexion larvae usually passively drift with ocean currents (Miller and Kendall, 2009). Usually, pre-flexion larvae are located "down-stream" of ocean currents from natal sites (Lin et al., 2018). The inshore abundance of L. polyactis could be recruited by planktonic larvae from distant places by coastal currents such as the Lubei Coast Current (LBCC), the Subei Coastal Current (SBCC), and the Yellow Sea Warm Current (YSWC) (Xiong et al., 2017a; Zhang et al., 2020). Post-larvae and juveniles are known to be able to actively migrate to nurseries (Zhan et al., 2016). The shallower waters used as nursery grounds could provide sufficient nutriment for foraging until the fish develop sufficient swimming ability to avoid predation (Lin et al., 2008).
In the present study, the otolith microstructure comprised the N, L, M, and J zones during the early developmental stages. The Sr/Ca ratios of early developmental stages were generally characterized by a downward trend from the N zone to J zone, which is consistent with the results of Xiong et al. (2017a). Elements that are incorporated into otoliths are likely derived from either water or diet (Milton and Chenery, 2001; Elsdon and Gillanders, 2003; Taddese et al., 2019). Sr/Ca ratios are believed to be negatively correlated with ambient temperature and positively correlated with ambient water salinity (Arkhipkin et al., 2004; Liu et al., 2011). Taking the Changjiang (Yangtze) River estuary and adjacent waters as an example, before the occurrence of juveniles (i.e. early April to June), the SST gradually increases from low (9–12 ℃; Lin et al., 2018) to high (21–25 ℃; Dai et al., 2018), and from deeper waters at depths >30 m to the shallower waters at depths of about 10 m, the sea surface salinity (SSS) gradually decreased from high (25–30; Lin et al., 2018) to low (5–15; Lin et al., 2018). The decreasing Sr/Ca ratios from the embryonic stage to juveniles may be explained by an increase in SST and a decrease in SSS. Moreover, a significant difference was observed between the N, M, and J zones among three sampling locations (P < 0.05). The time of metamorphism from larvae to juvenile L. polyactis, corresponding to the M zone in the present study, occurred at the mean age of 37 days in the Lvsi spawning ground (Li et al., 2013) when the first otolith secondary primordium had formed. The small yellow croaker experiences a shift in hydrodynamic habitat from the larval to juvenile stages (Xiong et al., 2017a), and the diet changes at a body length of 20–29 mm, which is equal to the length during the juvenile stage (Guo et al., 2010). The Sr/Ca ratios of the E zone for L. polyactis in Y-2 were higher than those in Y-1 (P < 0.05), which can be further verified by the capture time and location of Y-2 (i.e. April, inshore) and Y-1 (i.e. May, nearshore) (Fig. 1a; Table 1). In summary, the early migratory route of L. polyactis might be from inshore to nearshore waters. Although the migratory route from the embryonic stage to juvenile of L. polyactis is clear, the detailed migratory route from overwintering to spawning grounds remains unclear. Combined with the maturity of fish gonads and sampling location of L. polyactis from Y-1 (pre-spawning period) and Y-2 (spawning period), we inferred that its spawning migration might be from the overwintering grounds to the nearest nearshore waters of the Shandong Peninsula, then southwards along the Lubei Coast Current (LBCC), ending in inshore waters for spawning.
It is worth noting that the Sr/Ca ratios of the embryonic stage were generally higher than those of larvae and juveniles, which is consistent with the phenomenon found in Collichthys lucidus (Liu et al., 2015), Anguilla japonica (Tzeng, 1996; Tsukamoto and Arai, 2001), and Miichthys miiuy (Xiong et al., 2015). Three reasons likely led to such a high Sr concentration in the N zone. First, the high element concentration within otoliths is thought to be due to the ontogenetic stage. Changes in the life history of fish result in changes to both the morphology and physiology of individuals, for example, ingestion from endogenous to exogenous nutrition and growth from egg to larvae (Elsdon and Gillanders, 2003). Yatsu et al. (1998) revealed that the element concentrations of Sr and Ca in otolith cores of Ommastrephes bartramii were probably differently affected during the embryonic stage compared with other ontogenetic stages. L. polyactis consumed the yolk sac completely on the 6th day after hatching, and subsequently, initially fed on the exogenous nutrition with a weak feeding ability (Zhan et al., 2016). Second, these specimens were caught from wild groups, and therefore, environmental variables of Sr/ Ca ratios that could cause this change (i.e. salinity and temperature) cannot be ruled out. L. polyactis mainly spawn in inshore seas at depths >30 m where the SSS is 25–30, and the SST is 9–12 ℃ (Dai et al., 2018; Lin et al., 2018). The interaction of lower temperature and higher salinity may lead to a high Sr/Ca ratio in the N zone. Third, a previous study suggested that the otolith core may require large amounts of protein during the calcification process (Murayama et al., 2004), and another study suggested that the otolith core has a lower Ca concentration than the edge (Dove et al., 1996). Higher concentrations of protein and lower concentrations of Ca could lead to high concentrations of other elements related to Ca. Elements that replace Ca in the calcium carbonate matrix as their primary inclusion method in otoliths, such as Sr (Campana, 1999), may lead to a high Sr/Ca ratio in the N zone. The third reason is likely to result in changes of the elemental composition of the otolith influenced by the biomineralization process. Although the habitat environment is often assumed to be the most important factor affecting the otolith elemental signatures, certain extrinsic and intrinsic factors can confuse the simple correlations between them (Walther, 2019).
4.2 Populations of Larimichthys polyactis in the Yellow SeaThe significant finding in the present study was the compelling support for the spawning group of Y-2 and the overwintering group of Y-3 being the same group representing the southern Yellow Sea and the spawning group of Y-1 being another group representing the northern Yellow Sea. These results were also demonstrated by the findings of Lin (1987), Liu (1990), and Zhang et al. (2014). According to the above studies, 34°00′N has traditionally been regarded as the geographic boundary separating the northern Yellow Sea group and the southern Yellow Sea group of L. polyactis (Liu, 1990; Zhang et al., 2019a; Zhu et al., 2020). In the present study, Y-1 (35°00′N, 119°30′E) located in Haizhou Bay exhibited significant differences in the Sr/Ca ratios of early developmental stages compared with Y-2 (34°21′N, 121°10′E) and Y-3 (34°05′N, 124°05′E), and the Y-2 and Y-3 groups were clustered as one group. Consequently, compared with the results of L. polyactis reported in the 1990s (Liu, 1990), the northern geographical distribution boundary of the southern Yellow Sea group might have potentially moved northward. This phenomenon is also supported by the finding that the northern and southern Yellow Sea groups co-exist in Haizhou Bay (Y-1) (Jiang et al., 2019; Zhang et al., 2014).
The differences in geographical and water environments in the Yellow Sea may lead to the division of L. polyactis. First, the Yellow Sea is a typical semi-enclosed shallow sea located between the eastern part of Chinese mainland and the Korean Peninsula (31°40′–39°50′N, 119°35′–126°50′E). The northern, and southern Yellow Sea covers an area of approximately 80 000 km2 at an average depth of 38 m and 300 000 km2 at an average depth of 45.3 m, respectively (Liu, 2013). The deepest areas of the northern and southern Yellow Sea are off Bailingdao Island and Cheju Island, respectively (Liu, 2013). Second, water masses vertically mixed under the influence of strong wind and formed a mass of lowtemperature cold water in winter in the central, deeper part of the Yellow Sea, and the mass of lowtemperature cold water remains under a thermocline at 15–30-m depth in summer (Liu, 2013). The lowtemperature properties of the YSCWM in summer protect the cold-adapted fauna (Sun et al., 2010), such as Calanus sinicus and Euphausia pacifica, serving as food for different growth stages of L. polyactis toward the over-wintering grounds (Xue et al., 2005). Third, the hydrodynamic conditions of the Yellow Sea are mainly influenced by continental runoff and costal current, particularly in the western coastal waters, where the seasonal variation in salinity, temperature, and currents is distinct. In winter and early spring, the YSWC deriving from the northward Kuroshio and Taiwan Warm Current invades into the central Yellow Sea (Lin et al., 2011) (Fig. 1b). Two frontal waves emerged in offshore areas, one off Shandong Peninsula and the other off northern Jiangsu Province (Huang et al., 2018) (Fig. 1b). The frontal wave was sharply contrasted between the warm saline YSWC and cold fresh LBCC and SBCC, which may result in the distribution of southern and northern Yellow Sea groups of L. polyactis (Huang et al., 2018). In the present study, clear otolith Sr/Ca differences were found between the southern Yellow Sea group (Y-2 and Y-3) and the northern Yellow Sea group (Y-1), which might be attributed to the geographical environment, water mass, and hydrological front.
In recent decades, genetic data from putatively neutral markers and genome scan approaches have been extensively used to understand the population genetics of L. polyactis. However, these applications have demonstrated the main limitations in charting the population structure. These subpopulations may have relatively recently expanded but require sufficient time to differentiate. Alternatively, this species may be able to overcome the effects of genetic drift due to its high migration capability (Wang et al., 2013; Zhang, 2019). Recent otolith morphometric studies, discriminating the L. polyactis along the Chinese coast, showed an overall classification success rate of 80.0% and 82.0% obtained using the elliptic Fourier transform and discrete wavelet transform, respectively (Song et al., 2018). In a recent otolith chemistry study, Wang et al. (2016b) compared the stable isotopes of δ18O and δ13C in otoliths of L. polyactis from the Yellow Sea and the Bohai Sea, and successfully discriminated the population group with 85.7% classification accuracy. The results of the present study provide further evidence that otoliths could be a valuable tool for delineating population structure. In conclusion, the results of the present study verified the assumption that otolith Sr/Ca ratio probed by EPMA is a credible scalar for charting the life migration and discriminating populations of L. polyactis. The present study preliminarily revealed the characteristics of population discrimination of L. polyactis in the Yellow Sea. In following work, we intend to investigate the elemental composition of the nucleus using laser ablation inductively coupled plasma mass spectrometry (LA-ICPMS) for population identification of L. polyactis similar to work carried out in previous studies (Wang et al., 2009; Avigliano et al., 2017).
5 CONCLUSIONThe Sr/Ca ratios of early developmental stages were generally characterized by a downward trend, which suggests that L. polyactis spawn in inshore waters and feed in nearshore waters, and the three groups analyzed were divided into two populations, namely, the northern Yellow Sea group (Y-1) and the southern Yellow Sea group (Y-2 and Y-3), which verifies the existence of large-scale spatial connectivity (>350 km) between spawning and overwintering grounds. The application of the Sr/Ca ratio based on ontogenetic stage is recommended for early life migration analysis and population discrimination.
6 DATA AVAILABILITY STATEMENTThe data generated and/or analyzed in this study are available from the corresponding author upon reasonable request.
7 ACKNOWLEDGMENTThe authors are grateful to all scientific staff and crew for their assistance with sample collection and experimental implementation.
Arai T, Taha H, Amalina R, Iizuka Y, Chang C W. 2019. Anadromy and heterogenous population of a tropical shad Tenualosa ilisha in Malaysia, as revealed by otolith microchemistry and molecular evidence. Journal of Fish Biology, 95(6): 1506-1511.
DOI:10.1111/jfb.14154 |
Arkhipkin A I, Campana S E, FitzGerald J, Thorrold S R. 2004. Spatial and temporal variation in elemental signatures of statoliths from the Patagonian longfin squid (Loligo gahi). Canadian Journal of Fisheries and Aquatic Sciences, 61(7): 1212-1224.
DOI:10.1139/f04-075 |
Avigliano E, de Carvalho B M, Leisen M, Romero R, Velasco G, Vianna M, Barra F, Volpedo A V. 2017. Otolith edge fingerprints as approach for stock identification of Genidens barbus. Estuarine, Coastal and Shelf Science, 194: 92-96.
DOI:10.1016/j.ecss.2017.06.008 |
Botsford L W, Hastings A, Gaines S D. 2001. Dependence of sustainability on the configuration of marine reserves and larval dispersal distance. Ecology Letters, 4(2): 144-150.
DOI:10.1046/j.1461-0248.2001.00208.x |
Campana S E. 1999. Chemistry and composition of fish otoliths: pathways, mechanisms and applications. Marine Ecology Progress Series, 188: 263-297.
DOI:10.3354/meps188263 |
Carlson A K, Phelps Q E, Graeb B D S. 2017. Chemistry to conservation: using otoliths to advance recreational and commercial fisheries management. Journal of Fish Biology, 90(2): 505-527.
DOI:10.1111/jfb.13155 |
Chatterjee M, Ghosh P, Ramdas L, Chakrabarti R. 2015. Isotopic and geochemical characterization of invader tilapia fishes from water bodies of West Bengal and Karnataka, India. Environmental Monitoring and Assessment, 187(11): 712.
DOI:10.1007/s10661-015-4929-0 |
Chino N, McCarthy T K, Arai T. 2018. Analysis of fluvial migration of the Irish pollan Coregonus autumnalis, using Sr: Ca ratios of otolith. Journal of Applied Animal Research, 46(1): 609-612.
DOI:10.1080/09712119.2017.1369089 |
Cooke S J, Midwood J D, Thiem J D, Klimley P, Lucas M C, Thorstad E B, Eiler J, Holbrook C, Ebner B C. 2013. Tracking animals in freshwater with electronic tags: past, present and future. Animal Biotelemetry, 1(1): 5.
DOI:10.1186/2050-3385-1-5 |
Cowen R K, Gawarkiewicz G, Pineda J, Thorrold S R, Werner F E. 2007. Population connectivity in marine systems: an overview. Oceanography, 20(3): 14-21.
DOI:10.5670/oceanog.2007.26 |
Dai L B, Tian S Q, Peng X, Gao C X, Ye S, Du X X, Liu P. 2018. Distribution of Larimichthys polyactis and its relationship with environmental factors in offshore water of southern Zhejiang. Chinese Journal of Applied Ecology, 29(4): 1352-1358.
(in Chinese with English abstract) DOI:10.13287/j.1001-9332.201804.033 |
Delerue-Ricard S, Darnaude A M, Raeymaekers J A M, Dundas S H, Skadal J, Volckaert F A M, Geffen A J. 2019. Extensive larval dispersal and restricted movement of juveniles on the nursery grounds of sole in the Southern North Sea. Journal of Sea Research, 155: 101822.
DOI:10.1016/j.seares.2019.101822 |
Dou S Z, Yokouchi K, Yu X, Cao L, Kuroki M, Otake T, Tsukamoto K. 2012. The migratory history of anadromous and non-anadromous tapertail anchovy Coilia nasus in the Yangtze River Estuary revealed by the otolith Sr/Ca ratio. Environmental Biology of Fishes, 95(4): 481-490.
DOI:10.1007/s10641-012-0042-1 |
Dove S G, Gillanders B M, Kingsford M J. 1996. An investigation of chronological differences in the deposition of trace metals in the otoliths of two temperate reef fishes. Journal of Experimental Marine Biology and Ecology, 205(1-2): 15-33.
DOI:10.1016/S0022-0981(96)02610-X |
Elsdon T S, Gillanders B M. 2003. Reconstructing migratory patterns of fish based on environmental influences on otolith chemistry. Reviews in Fish Biology and Fisheries, 13(3): 217-235.
DOI:10.1023/b:rfbf.0000033071.73952.40 |
Elsdon T S, Wells B K, Campana S E, Gillanders B M, Jones C M, Limburg K E, Secor D H, Thorrold S R, Walther B D. 2008. Otolith chemistry to describe movements and lifehistory parameters of fishes: hypotheses, assumptions, limitations and inferences. Oceanography and Marine Biology, 46: 297-330.
DOI:10.1201/9781420065756.ch7 |
Fishbase. 2019. https://fishbase.in/summary/Larimichthyspolyactis.html. Accessed on 2020-10-10.
|
Gahagan B I, Vokoun J C, Whitledge G W, Schultz E T. 2012. Evaluation of otolith microchemistry for identifying natal origin of anadromous river herring in Connecticut. Marine and Coastal Fisheries, 4(1): 358-372.
DOI:10.1080/19425120.2012.675967 |
Gillanders B M. 2005. Otolith chemistry to determine movements of diadromous and freshwater fish. Aquatic Living Resources, 18(3): 291-300.
DOI:10.1051/alr:2005033 |
Guo B, Zhang B, Jin X S. 2010. Diet composition and ontogenetic variation in feeding habits of juvenile small yellow croaker Pseudosciaena polyactis Bleeker in the Yellow Sea. Journal of Fishery Sciences of China, 17(2): 289-297.
(in Chinese with English abstract) |
Huang M H, Liang X S, Wu H, Wang Y H. 2018. Different generating mechanisms for the summer surface cold patches in the Yellow Sea. Atmosphere-Ocean, 56(4): 1-13.
DOI:10.1080/07055900.2017.1371580 |
Jiang T, Liu H B, Lu M J, Chen T T, Yang J. 2016. A possible connectivity among estuarine tapertail anchovy (Coilia nasus) populations in the Yangtze River, Yellow Sea, and Poyang Lake. Estuaries and Coasts, 39(6): 1762-1768.
DOI:10.1007/s12237-016-0107-z |
Jiang T, Yang J, Lu M J, Liu H B, Chen T T, Gao Y W. 2017. Discovery of a spawning area for anadromous Coilia nasus Temminck et Schlegel, 1846 in Poyang Lake, China. Journal of Applied Ichthyology, 33(2): 189-192.
DOI:10.1111/jai.13293 |
Jiang Y Q, Zhang C, Ye Z J, Tian Y J. 2019. Analyses of egg size, otolith shape, and growth revealed two components of small yellow croaker in Haizhou Bay spawning stock. Journal of Oceanology and Limnology, 37(4): 1423-1429.
DOI:10.1007/s00343-019-8105-1 |
King J R, McFarlane G A. 2003. Marine fish life history strategies: applications to fishery management. Fisheries Management and Ecology, 10(4): 249-264.
DOI:10.1046/j.1365-2400.2003.00359.x |
Lee Q, Lee A, Liu Z L, Szuwalski C S. 2020. Life history changes and fisheries assessment performance: a case study for small yellow croaker. Journal of Marine Science, 77(2): 645-654.
DOI:10.1093/icesjms/fsz232 |
Li Y X, Tang J H, Xu X M, Xu J, Liu Z Y, Xu H, Cheng J H. 2013. Comparison of otolith microstructures in small yellow croaker larvae and juveniles from Sanmen Bay and Lvsi. Marine Fisheries, 35(4): 423-431.
(in Chinese with English abstract) DOI:10.13233/j.cnki.mar.fish.2013.04.008 |
Li Y Z, Sun M, Zhang C L, Zhang Y L, Xu B D, Ren Y P, Chen Y. 2020. Evaluating fisheries conservation strategies in the socio-ecological system: a grid-based dynamic model to link spatial conservation prioritization tools with tactical fisheries management. PLoS One, 15(4): e0230946.
DOI:10.1371/journal.pone.0230946 |
Lim H K, Le M H, An C M, Kim S Y, Park M S, Chang Y J. 2010. Reproductive cycle of yellow croaker Larimichthys polyactis in southern waters off Korea. Fisheries Science, 76(6): 971-980.
DOI:10.1007/s12562-010-0288-5 |
Lin L S, Cheng J H, Jiang Y Z, Yuan X W, Li J S, Gao T X. 2008. Spatial distribution and environmental characteristics of the spawning grounds of small yellow croaker in the southern Yellow Sea and the East China Sea. Acta Ecologica Sinica, 28(8): 3485-3492.
(in Chinese with English abstract) |
Lin N, Chen Y G, Jin Y, Yuan X W, Ling J Z, Jiang Y Z. 2018. Distribution of the early life stages of small yellow croaker in the Yangtze River estuary and adjacent waters. Fisheries Science, 84(2): 357-363.
DOI:10.1007/s12562-018-1177-6 |
Lin X P, Yang J Y, Guo J S, Zhang Z X, Yin Y Q, Song X Z, Zhang X H. 2011. An asymmetric upwind flow, Yellow Sea Warm Current: 1. new observations in the western Yellow Sea. Journal of Geophysical Research: Oceans, 116(C4): C04026.
DOI:10.1029/2010jc006513 |
Lin X Z. 1987. Biological characteristics and resources status of three main commercial fishes in offshore waters of China. Journal of Fisheries of China, 11(3): 187-194.
(in Chinese with English abstract) |
Liu B L, Chen X J, Ch en, Y, Lu H J, Qian W G. 2011. Trace elements in the statoliths of jumbo flying squid off the Exclusive Economic Zones of Chile and Peru. Marine Ecology Progress Series, 429: 93-101.
DOI:10.3354/meps09106 |
Liu H B, Jiang T, Huang H H, Shen X Q, Zhu J B, Yang J. 2015. Estuarine dependency in Collichthys lucidus of the Yangtze River Estuary as revealed by the environmental signature of otolith strontium and calcium. Environmental Biology of Fishes, 98(1): 165-172.
DOI:10.1007/s10641-014-0246-7 |
Liu J Y. 2013. Status of marine biodiversity of the China seas. PLoS One, 8(1): e50719.
DOI:10.1371/journal.pone.0050719 |
Liu X S. 1990. Fisheries Resources Survey and Zoning in the Yellow Sea and Bohai Sea Area. Ocean Press, Beijing, China. 191-200.
(in Chinese)
|
Lowe W H, Allendorf F W. 2010. What can genetics tell us about population connectivity?. Molecular Ecology, 19(15): 3038-3051.
DOI:10.1111/j.1365-294x.2010.04688.x |
Ma Q Y, Jiao Y, Ren Y P, Xue Y. 2020. Population dynamics modelling with spatial heterogeneity for yellow croaker(Larimichthys polyactis) along the coast of China. Acta Oceanologica Sinica, 39(10): 107-119.
DOI:10.1007/s13131-020-1602-4 |
Miller B S, Kendall A W Jr. 2009. Early Life History of Marine Fishes. University of California Press, Berkeley. 376.
|
Milton D A, Chenery S R. 2001. Sources and uptake of trace metals in otoliths of juvenile barramundi (Lates calcarifer). Journal of Experimental Marine Biology and Ecology, 264(1): 47-65.
DOI:10.1016/s0022-0981(01)00301-x |
Murayama E, Takagi Y, Nagasawa H. 2004. Immunohistochemical localization of two otolith matrix proteins in the otolith and inner ear of the rainbow trout, Oncorhynchus mykiss: comparative aspects between the adult inner ear and embryonic otocysts. Histochemistry and Cell Biology, 121(2): 155-166.
DOI:10.1007/s00418-003-0605-5 |
Ovenden J R. 2013. Crinkles in connectivity: combining genetics and other types of biological data to estimate movement and interbreeding between populations. Marine and Freshwater Research, 64(3): 201-207.
DOI:10.1071/mf12314 |
Reis-Santos P, Tanner S E, França S, Vasconcelos R P, Gillanders B M, Cabral H N. 2015. Connectivity within estuaries: An otolith chemistry and muscle stable isotope approach. Ocean & Coastal Management, 118: 51-59.
DOI:10.1016/j.ocecoaman.2015.04.012 |
Rodionov S, Overland J E. 2005. Application of a sequential regime shift detection method to the Bering Sea ecosystem. Journal of Marine Science, 62(3): 328-332.
DOI:10.1016/j.icesjms.2005.01.013 |
Rogers T A, Fowler A J, Steer M A, Gillanders B M. 2019. Discriminating natal source populations of a temperate marine fish using larval otolith chemistry. Frontiers in Marine Science, 6: 711.
DOI:10.3389/fmars.2019.00711 |
Schulz-Mirbach T, Ladich F, Plath M, Heß M. 2019. Enigmatic ear stones: what we know about the functional role and evolution of fish otoliths. Biological Reviews, 94(2): 457-482.
DOI:10.1111/brv.12463 |
Sedberry G R, Loefer J K. 2001. Satellite telemetry tracking of swordfish, Xiphias gladius, off the eastern United States. Marine Biology, 139(2): 355-360.
DOI:10.1007/s002270100593 |
Song J J, Zhao B, Liu J H, Cao L, Dou S Z. 2018. Comparison of otolith shape descriptors and morphometrics for stock discrimination of yellow croaker along the Chinese coast. Journal of Oceanology and Limnology, 36(5): 1870-1879.
DOI:10.1007/s00343-018-7228-0 |
Sun S, Huo Y Z, Yang B. 2010. Zooplankton functional groups on the continental shelf of the Yellow Sea. Deep Sea Research Part Ⅱ: Topical Studies in Oceanography, 57(11-12): 1006-1016.
DOI:10.1016/j.dsr2.2010.02.002 |
Taddese F, Reid M R, Closs G P. 2019. Direct relationship between water and otolith chemistry in juvenile estuarine triplefin Forsterygion nigripenne. Fisheries Research, 211: 32-39.
DOI:10.1016/j.fishres.2018.11.002 |
Tran N T, Labonne M, Hoang H D, Panfili J. 2019. Changes in environmental salinity during the life of Pangasius krempfi in the Mekong Delta (Vietnam) estimated from otolith Sr: Ca ratios. Marine and Freshwater Research, 70(12): 1734-1746.
DOI:10.1071/mf18269 |
Tsukamoto K, Arai T. 2001. Facultative catadromy of the eel Anguilla japonica between freshwater and seawater habitats. Marine Ecology Progress Series, 220: 265-276.
DOI:10.3354/meps220265 |
Tzeng W N. 1996. Effects of salinity and ontogenetic movements on strontium: calcium ratios in the otoliths of the Japanese eel, Anguilla japonica Temminck and Schlegel. Journal of Experimental Marine Biology and Ecology, 199(1): 111-122.
DOI:10.1016/0022-0981(95)00185-9 |
Walker K A, Trites A W, Haulena M, Weary D M. 2011. A review of the effects of different marking and tagging techniques on marine mammals. Wildlife Research, 39(1): 15-30.
DOI:10.1071/wr10177 |
Walther B D. 2019. The art of otolith chemistry: interpreting patterns by integrating perspectives. Marine and Freshwater Research, 70(12): 1643-1658.
DOI:10.1071/mf18270 |
Wang C H, Lin Y T, Shiao J C, You C F, Tzeng W N. 2009. Spatio-temporal variation in the elemental compositions of otoliths of southern bluefin tuna Thunnus maccoyii in the Indian Ocean and its ecological implication. Journal of Fish Biology, 75(6): 1173-1193.
DOI:10.1111/j.1095-8649.2009.02336.x |
Wang L, Liu S F, Zhuang Z M, Guo L, Meng Z N, Lin H R. 2013. Population genetic studies revealed local adaptation in a high gene-flow marine fish, the small yellow croaker(Larimichthys polyactis). PLoS One, 8(12): e83493.
DOI:10.1371/journal.pone.0083493 |
Wang X Y, Lu G Q, Zhao L L, Yang Q, Gao T X. 2020. Assessment of fishery resources using environmental DNA: small yellow croaker (Larimichthys polyactis) in East China Sea. PLoS One, 15(12): e0044495.
DOI:10.1371/journal.pone.0244495 |
Wang Y K, Huang J S, Dai Q F, Tang Y X, Sun Y, Jin X S. 2016a. Insights into population structure of juvenile small yellow croaker (Larimichthys polyactis) in the Yellow Sea and the Bohai Sea from otolith elemental fingerprints. Haiyang Xuebao, 38(6): 32-40.
(in Chinese with English abstract) DOI:10.3969/j.issn.0253-4193.2016.06.004 |
Wang Y K, Huang J S, Tang X X, Jin X S, Sun Y. 2016b. Stable isotopic composition of otoliths in identification of stock structure of small yellow croaker (Larimichthys polyactis) in China. Acta Oceanologica Sinica, 35(6): 29-33.
DOI:10.1007/s13131-016-0868-z |
Xiao Y S, Song N, Li J, Xiao Z Z, Gao T X. 2015. Significant population genetic structure detected in the small yellow croaker Larimichthys polyactis inferred from mitochondrial control region. Mitochondria DNA, 26(3): 409-419.
DOI:10.3109/19401736.2013.843076 |
Xiong Y, Liu H B, Jiang T, Liu P T, Tang J H, Zhong X M, Yang J, Wu L, Gao Y S. 2015. Investigation on otolith microchemistry of wild Pampus argenteus and Miichthys miiuy in the Southern Yellow Sea, China. Haiyang Xuebao, 37(2): 36-43.
(in Chinese with English abstract) DOI:10.3969/j.issn.0253-4193.2015.02.004 |
Xiong Y, Yang J, Jiang T, Liu H B, Zhong X M, Tang J H. 2017a. Early life history of the small yellow croaker(Larimichthys polyactis) in sandy ridges of the South Yellow Sea. Marine Biology Research, 13(9): 993-1002.
DOI:10.1080/17451000.2017.1319067 |
Xiong Y, Yang J, Jiang T, Liu H B, Zhong X M. 2021. Temporal stability in the otolith Sr: Ca ratio of the yellow croaker, Larimichthys polyactis (Actinopterygii, Perciformes, Sciaenidae), from the southern Yellow Sea. Acta Ichthyologica et Piscatoria, 51(1): 59-65.
DOI:10.3897/aiep.51.63245 |
Xiong Y, Zhong X M, Tang J H, Yang J, Li L Z. 2017b. Gillnet selectivity on the small yellow croaker Larimichthys polyactis in the Southern Yellow Sea. Turkish Journal of Fisheries and Aquatic Sciences, 17(6): 1287-1296.
DOI:10.4194/1303-2712-v17_6_22 |
Xiong Y, Zhong X M, Tang J H, Yang J. 2016. Migration and population structure characteristics of the small yellow croaker Larimichthys polyactis in the southern Yellow Sea. Acta Oceanologica Sinica, 35(6): 34-41.
DOI:10.1007/s13131-016-0844-7 |
Xue Y, Jin X, Zhang B, Liang Z. 2005. Seasonal, diel and ontogenetic variation in feeding patterns of small yellow croaker in the central Yellow Sea. Journal of Fish Biology, 67(1): 33-50.
DOI:10.1111/j.0022-1112.2005.00677.x |
Yatsu A, Mochioka N, Morishita K, Toh H. 1998. Strontium/Calcium ratios in statoliths of the neon flying squid, Ommastrephes bartrami (Cephalopoda), in the North Pacific Ocean. Marine Biology, 131(2): 275-282.
DOI:10.1007/s002270050320 |
Zhan W, Lou B, Chen R Y, Mao G M, Liu F, Xu D D, Wang L G, Ma T, Xu Q X. 2016. Observation of embryonic, larva and juvenile development of small yellow croaker, Larimichthys polyactis. Oceanologia et Limnologia Sinica, 47(5): 1033-1039.
(in Chinese with English abstract) DOI:10.11693/hyhz20160500114 |
Zhang B D, Li Y L, Xue D X, Liu J X. 2020. Population genomic evidence for high genetic connectivity among populations of small yellow croaker (Larimichthys polyactis) in inshore waters of China. Fisheries Research, 225: 105505.
DOI:10.1016/j.fishres.2020.105505 |
Zhang B D, Xue D X, Li Y L, Liu J X. 2019a. RAD genotyping reveals fine-scale population structure and provides evidence for adaptive divergence in a commercially important fish from the northwestern Pacific Ocean. PeerJ, 7: e7242.
DOI:10.7717/peerj.7242 |
Zhang C, Ye Z J, Wan R, Ma Q Y, Li Z G. 2014. Investigating the population structure of small yellow croaker(Larimichthys polyactis) using internal and external features of otoliths. Fisheries Research, 153: 41-47.
DOI:10.1016/j.fishres.2013.12.012 |
Zhang S M, Jin S F, Zhang H, Fan W, Tang F H, Yang S L. 2016. Distribution of bottom trawling effort in the Yellow Sea and East China Sea. PLoS One, 11(11): e0166640.
DOI:10.1371/journal.pone.0166640 |
Zhang T T, Wang Y K, Yuan W, Jin X S, Chen C, Sun Y. 2019b. Research on sagitta microstructure characteristics of Young of the Year (YOY) Larimichthys polyactis in the Bohai Sea. Progress in Fishery Sciences, 41(2): 35-40.
(in Chinese with English abstract) DOI:10.19663/j.issn2095-9869.20190221001 |
Zhu L X, Liang Z L, Ge C Z, Li C L. 2020. An application of the Bayesian Hierarchical Approach to refining the information on main life history parameters for small yellow croaker, Larimichthys polyactis, off the coast of China. Ocean Science Journal, 55(1): 143-155.
DOI:10.1007/s12601-020-0010-1 |
Zlokovitz E R, Secor D H, Piccoli P M. 2003. Patterns of migration in Hudson River striped bass as determined by otolith microchemistry. Fisheries Research, 63(2): 245-259.
DOI:10.1016/s0165-7836(03)00069-9 |
 2022, Vol. 40
2022, Vol. 40


