Institute of Oceanology, Chinese Academy of Sciences
Article Information
- HAN Jinfen, NAN Fangru, FENG Jia, LÜ Junping, LIU Qi, LIU Xudong, XIE Shulian
- Phylogenetic, evolutionary, and biogeographic origin of the genus Sheathia (Batrachospermales, Rhodophyta)
- Journal of Oceanology and Limnology, 40(2): 729-744
- http://dx.doi.org/10.1007/s00343-021-1075-0
Article History
- Received Mar. 10, 2021
- accepted in principle Apr. 25, 2021
- accepted for publication May. 17, 2021
Sheathia Salomaki & M. L. Vis 2014, belonging to Rhodophyta, Florideophyceae, Batrachospermales, and Batrachospermaceae, is a typical Rhodophyta algal genus that inhabits exclusively in streams or rivers (Salomaki et al., 2014). Most of the Florideophyceae taxa have a triphasic life history, including gametophyte, carposporophyte, and tetrasporophyte. However, in freshwater red algae, except for a few species alternating with three typical generations, most species, including Batrachospermales taxa, alternate between gametophyte and carposporophyte. Nevertheless, a diminutive diploid degenerated tetrasporophyte "Chantransia" stage exists between gametophyte and carposporophyte in the life history of the Batrachospermales order (van den Hoek et al., 1995; Han, 2012; Johnston et al., 2018). Like other taxa of the Batrachospermales order, the Sheathia genus prefers to live in low temperature, low light, high dissolved oxygen, and clean springs and streams. This genus is characterized as gelatinous gametophyte filaments, with a beaded appearance; the whorls are spherical, and either separated or adjacent; the undifferentiated carpogonial branches typically arise from periaxial cells, with a small number of unstalked carpogonia occurring on periaxial cells; the carposporophytes are spherical within or stretching out of the whorls (Salomaki et al., 2014). Furthermore, the most typical feature of this genus is heterocortication. However, this feature is absent in several species of this genus, including S. abscondita Stancheva, Sheath & M. L. Vis; S. arcuata (Kylin) Salomaki & M. L. Vis; S. assamica Necchi, J. A. West, E. K. Ganesan & Yasmin; S. californica Stancheva Sheath & M. L. Vis; S. dispersa Necchi, J. A. West, E. K. Ganesan & S. K. Rai; S. indonepalensis Necchi, J. A. West, E. K. Ganesan, S. K. Rai & F. Yasmin; S. longipedicellata (D. Hua & Z. X. Shi) J.-F. Han & al.; S. murpheyi A. L. Szinte, J. C. Taylor & M. L. Vis; S. plantuloides M. L. Vis; and S. transpacific M. L. Vis (Necchi et al., 2019c; Vis et al., 2020).
Batrachospermum longipedicellatum S. L. Xie & J. Feng (currently regarded as S. longipedicellata) was previously reported in China's Jiangsu Province. It is characterized by spherical and separated whorls of 600–1 000 μm in diameter, dense and irregular fascicles arising from internode, carpogonial branches of 5–9 cells arising from primary fascicles or cortical filaments, 23.0–35.0-μm long and 4.5–6.0 μm in diameter carpogonia, spherical carposporophytes stretching out of the whorls, and 10.0–11.0-μm long, 8.0–9.0 μm in diameter carposporoangia (Hua and Shi, 1996). Originally, B. longipedicellatum was classified into the section Batrachospermum of the genus Batrachospermum. Until 2014, this species was transferred into the sect. Helminthoidea of genus Batrachospermum based on cox 2-3 spacer sequences (Ji et al., 2014). Subsequently, Han et al. (2018) transferred this species to the genus Sheathia based on the large subunit of ribulose-1, 5-bisphosphate carboxylase/oxygenase (rbcL) and the photosystem Ⅱ reaction center protein D1 (psbA) genes. Sheathia longipedicellata is endemic to China. According to Sun et al. (2001), S. longipedicellata is more suitable for growing in springs with low and constant water temperature, pH 7.5, high calcium content, and low nutrient elements content.
Molecular phylogenetic analyses based on rbcL, psbA, and the 5' region of the mitochondrial cytochrome c oxidase I gene (COI-5P) have been widely used in phylogenetic inference and phylogenetic position examination of freshwater red algae. DNA sequence data for the rbcL and COI-5P have recently become available for solving the paraphyletic of genus Batrachospermum. Based on the molecular evidence, several sections, including Virescentia, Helminthoidea, Turfosa, Acarposporophytum, Aristata, and Macrospora sections have been raised to the genus level (Salomaki et al., 2014; Necchi et al., 2018, 2019a, b; Vis et al. 2020). The first freshwater coralline alga was reported based on molecular phylogenetic analysis of the psbA gene (Žuljević et al., 2016). Furthermore, rbcL and psbA have been analyzed to determine the affinities of four putative "Chantransia" isolates collected from China, and two new genus Sheathia species have been proposed (Han et al., 2020).
Sheathia is a typical genus of the Rhodophyta phylum. To date, species attributable to this genus have been reported from numerous locations in Asia, Europe, Oceania, North America, and Africa (Xie and Ling, 2004; Shi, 2006; Salomaki et al., 2014; Han et al., 2020). The research on the ancestral geographical origin and divergence time estimation of the genus Sheathia is fundamental in describing the evolutionary history of the Rhodophyta taxa. However, although various investigations on the divergence time and historical biogeography of Rhodophyta based on genome and individual genes have been reported recently (Feng et al., 2015; Yang et al., 2016; Nan, 2017), no related reports have covered members of the genus Sheathia.
Based on the morphological and molecular data, the phylogenetic position of the four Sheathia taxa, including both gametophyte and "Chantransia" stages, was determined. Furthermore, based on the modern geographical distribution of the Sheathia genus, a preliminary analysis of the origin and ancestral geographical distribution of the genus was performed. To further clarify the evolution of genus Sheathia, divergence times were estimated using Bayesian relaxed-clock methods.
2 MATERIAL AND METHOD 2.1 Sample collection and morphological observationFour Sheathia samples were collected from Shanxi and Henan provinces in China. Samples included gametophyte (SAS18052 and SAS18523) and "Chantransia" (YTS19161 and YTS19017) stages. Table 1 shows collection information. Materials were rinsed several times in ultrapure water, removing impurities and epiphytic algae.
After a preliminary morphological observation using a BX-51 Olympus microscope equipped with a charge-coupled device (DP72; Olympus, Tokyo, Japan), samples were divided for morphological and genetic analyses. For morphological analysis each sample was fixed in 4% formalin solution. For DNA extraction, samples were stored in silica gel. Voucher specimens were deposited in the herbarium of Shanxi University (SXU). Voucher information is detailed in Table 1.
Critical diagnostic morphological features (Necchi and Zucchi, 1995; Kumano, 2002; Salomaki et al., 2014) of each sample were observed via a BX-51 Olympus microscope equipped with a charge-coupled device.
2.2 DNA extraction, amplification, and sequencingTotal DNA was extracted following the protocol originally described by Saunders (1993) and revised by Vis and Sheath (1997). Three molecular markers, including rbcL, COI-5P, and psbA, were amplified using the primers and protocols described by Vis et al. (1998) and Saunders (2005). PCR products sequencing was performed as described previously in Han et al. (2018, 2020).
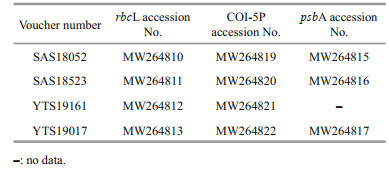
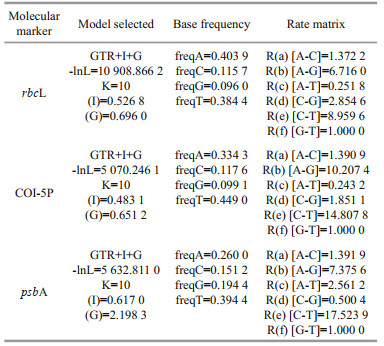
2.3 Phylogenetic analysesSequences generated in this study were submitted to the GenBank databases (Table 2). Additional related sequence data of the Batrachospermales order and outgroup taxa Bangia were downloaded from GenBank (http://www.ncbi.nlm.nih.gov/) (listed in Supplementary Table S1). The 65 rbcL, 47 COI-5P, and 42 psbA sequences were aligned by Clustal-X 2.0 (Thompson et al., 1997) and MEGA 5.0 (Tamura et al., 2011). Pairwise distance and the number of nucleotide variances for the taxa's molecular markers were calculated via MEGA 5.0. For phylogenetic analyses, the appropriate models for the sequence evolution were determined by Modeltest 3.7 (Posada and Buckley, 2004) (Table 3). PHYML software (Felsenstein, 1981; Guindon and Gascuel, 2003) was utilized to construct the Maximum Likelihood (ML) trees with 1 000 replicates of bootstrap analysis. Bayesian Inferences (BI) were performed in MrBayes version 3.1.2 (Ronquist and Huelsenbeck, 2003) and runs 5 000 000 generations sampling every 1 000 generations until the standard error was lower than 0.01. The resulting phylogenetic trees were edited using Figtree1.3.1 (http://tree.bio.ed.ac.uk/software/figtree/).
|
|
We estimated the divergence time of clades on combined sequences (rbcL, COI-5P, and psbA) matrix using Beast v1.7.5 (Drummond and Rambaut, 2007). An uncorrelated lognormal relaxed clock model was used to account for the uncertainty in the divergence time estimation (Drummond et al., 2006). To estimate the divergence time for each node, eight fossil calibrations were used to constraint time. Four fossil calibrations were used for divergence time estimation of Rhodophyta. The first calibration was the 1 047 (+13/-17) million years ago (Ma) Bangiomorpha pubescens fossil in the Bylot Supergroup of Baffin Island, which was used to constraint the divergence between rhodophytes and viridiplantae (Gibson et al., 2018). The second calibration was the Corellinalean algae from the Doushantuo Formation, which can be traced back to 635–551 Ma and belonged to the modern red algal subclass Corallinophycidae (Xiao et al., 1998; Condon et al., 2005). Two calibration points in Corallinophycidae were the 136–130 Ma for the Sporolithales split and 120–114 Ma for Corallinales and Hapalidiales split (Aguirre et al., 2000, 2010). Additionally, four calibration nodes were used to constraint green algae and plants based on three fossil records, including 480–471 Ma for the age of land plants, 422–401 Ma for the age of the Euphyllophytes, 351–313 Ma for the age of the seed plants, and 163–138 Ma for the split of Eudicotyledoneae (Magallón et al., 2013). The program Tracer v 1.6 (Rambaut et al., 2014) was used to analyze the results. When the adequate sample size (ESS) for all relevant parameters was above 200, it was assumed that stationarity had been reached. Finally, the final tree was viewed and edited in FigTree 1.3.1 (Rambaut, 2009).
2.5 Ancestral geographical origin of the genusrbcL and COI-5P gene sequences of Sheathia taxa and other species distributed in different areas were used to reconstruct the ancestral area (Supplementary Fig.S1, Supplementary Table S2). Ancestral area reconstructions of Sheathia were inferred using Reconstruct Ancestral State in Phylogenies (RASP) based on the tree data and the final tree generated in Beast. Ancestral areas at the internal nodes within the phylogenetic tree were inferred using the Bayesian binary method (BBM) implemented in the RASP software (Nylander et al., 2008). Distribution ranges for Sheathia and other taxa of orders Batrachospermales and Thoreales were divided into six areas: A, Asia; B, Europe; C, Oceania; D, North America; E, South America; and F, Africa. During the Carboniferous to Triassic period, the continental plates collided with each other, causing all the land in the world to be closed together, forming the Pangaea supercontinent (Scotese et al., 1979). Therefore, the maximum areas of each node were set to 6, and the geographical combinations were set following this definition. All other options remained as default.
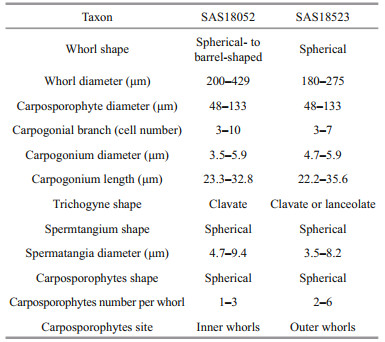
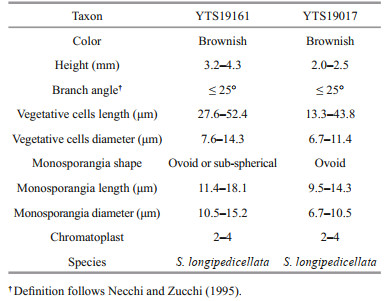
3 RESULT 3.1 Morphological characterizationThe morphological characteristics of the four specimens in this study were observed and measured as shown in Fig. 1 and Tables 4–5.
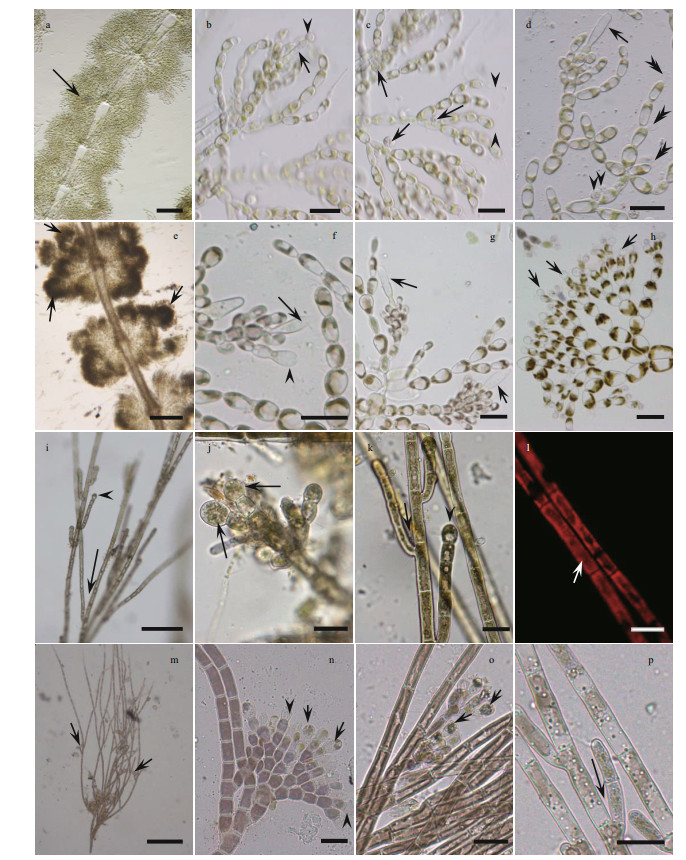
|
| Fig.1 Morphological characters of samples of both gametophyte stage samples and "Chantransia" stage samples investigated in this study a–d. sample SAS18052. a. spherical- to barrel-shaped, adjacent and well-developed whorls with a small spherical carposporophytes within the whorls (black arrow); b. carpogonium with clavate trichogyne (black arrow) with attached spermatium (black arrowhead); c. morphological characters of the vegetative fascicles with spermatangia (black arrows) and terminal hairs (black arrowheads); d. carpogonium with clavate trichogyne (black arrow) and vegetative fascicles with spermatangia (black arrowheads) and empty, discharged spermatangia (black double arrowheads). e–h. sample SAS18523. e. separated and spherical whorls with carposporophytes (black arrows) extending out of the whorls; f. carpogonium with lanceolate trichogyne (black arrow) on a branch composed of undifferentiated cells with second carpogonium (black arrowhead) sessile on an involucral filament; g. carpogonia with clavate and lanceolate trichogynes (black arrows); h. spermatangia (black arrows) on the tips of fascicle cells. i–l. sample YTS19161. i. filament showing branch angle ≤ 25° (black arrow) and monosporangium (black arrowhead); j. monosporangial branch with ovoid and sub-spherical monosporangia (black arrows); k. filament showing branch angle ≤ 25° (black arrow) and monosporangium (black arrowhead); l. cells have parietal laminate or irregularly lobed chromatoplast (white arrow). m–p. sample YTS19017. m. morphological observation of the erect filament and monosporangia (black arrows) grow on top of the spore branch; n. monosporangial branch with mature monosporangia (black arrows) and empty, discharged monosporangia (black arrowheads); o. monosporangial branch with ovoid monosporangia (black arrows); p. vegetative fascicles showing branch angle ≤25° (black arrow). Scale bars: 100 μm in (a, e, i), 20 μm in (b, c, d, f, g, h, j, k, l, n, o, p), 200 μm in (m). |
The two gametophyte stage specimens (SAS18052 and SAS18523) are in good agreement with the dimension circumscription description of S. longipedicellata. The SAS18052 specimen displayed a lighter colored thallus with spherical- to barrel-shape, adjacent whorls, while the SAS18523 specimen displayed a darker colored thallus with separated and spherical whorls. Furthermore, the carposporophytes of the SAS18052 specimen were within the whorls, while those of the SAS18523 specimen were out of the whorls.
The two "Chantransia" isolates (YTS19161 and YTS19017), were morphologically similar to Audouinella pygmaea (Kützing) Weber Bosse. These two isolates had the following characteristics: microscopic (2.0–4.3 mm) Tuft-shaped with a brownish thallus, composed of creeping and erect filaments, narrow branching angles (≤ 25°), cylindrical vegetative cells, and small abundant ovoid or sub-spherical monosporangia (9.5–18.1 μm in length and 6.7–15.2 μm in diameter).
3.2 Molecular analysisThe rbcL data matrix consisted of 44 Sheathia specimens (including 20 species), 1 106 characters, of which 452 (40.87%) were variable and 381 (34.45%) were parsimony informative. The COI-5P alignment included 33 Sheathia sequences (including 17 species) and 571 characters, of which 258 (45.36%) were variable and 223 (39.05%) were parsimony informative. The psbA alignment included 21 Sheathia sequences (including 9 species) and 834 characters, of which 285 (34.17%) were variable and 253 (30.34%) were parsimony informative sites. Supplementary Tables S3–S5 list the pairwise distance based on the three sequences between all specimens in this study and other species of the order Batrachospermales.
The relationship among Sheathia species and the genus Sheathia with other genera of Batrachospermales based on rbcL, COI-5P, and psbA alignment, with supporting values calculated from BI and ML methods, are presented in Supplementary Figs.S2–S4. Because we mainly focus on the phylogenetic relationship of genus Sheathia, here, only BI trees depicting the relationship among Sheathia species based on rbcL, COI-5P, and psbA gene sequences are shown with supporting values calculated from the two methods in Figs. 2–4. The rbcL and COI-5P data sets include all four Sheathia specimens utilized in this study, while the psbA data set had only three of them (except for YTS19161). Phylogenetic trees of the three markers showed the genus Sheathia forming a distinct, strongly supported clade (Fig. 2: 1.00/93.7; Fig. 3: 1.00/85.4; Fig. 4: 1.00/100).
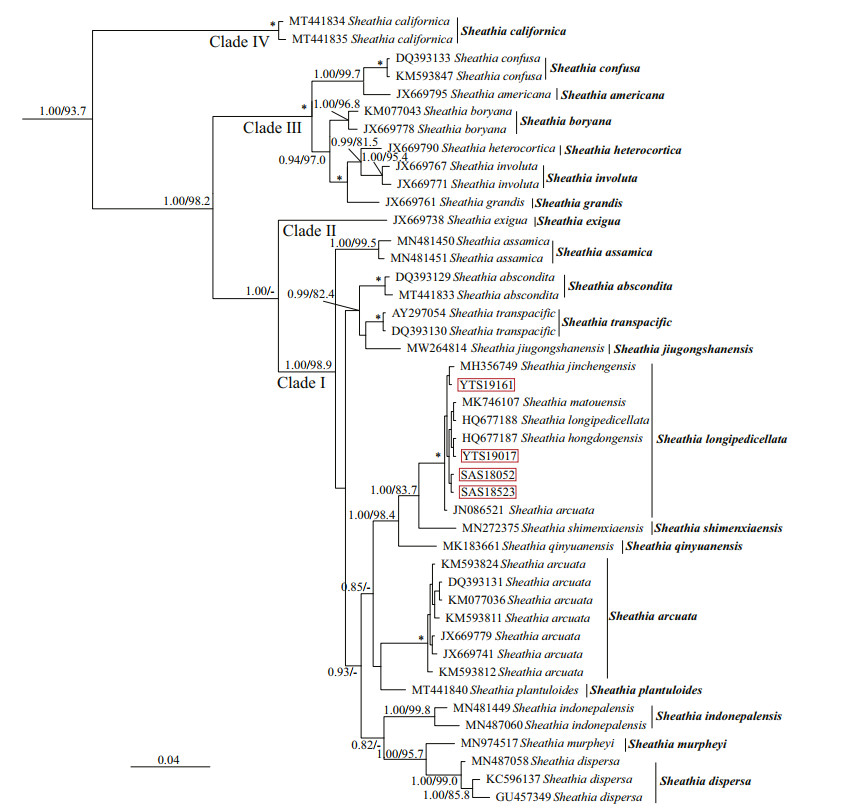
|
| Fig.2 Bayesian inference phylogeny depicting the relationship of among Sheathia species based on rbcL gene sequences Bayesian posterior probabilities (> 0.80) and ML bootstrap values (> 80%) are shown at branches. All newly generated sequences in this study are indicated in red boxes. Asterisks on the nodes represent that the support values of Bayesian inference and Maximum Likelihood are both 100%. |

|
| Fig.3 Bayesian inference phylogeny depicting the relationship of among Sheathia species based on COI-5P gene sequences Bayesian posterior probabilities (> 0.80) and ML bootstrap values (> 80%) are shown at branches. All newly generated sequences in this study are indicated in red boxes. Asterisks on the nodes represent that the support values of Bayesian inference and Maximum Likelihood are both 100%. |

|
| Fig.4 Bayesian inference phylogeny depicting the relationship of among Sheathia species based on psbA gene sequences Bayesian posterior probabilities (> 0.80) and ML bootstrap values (> 80%) are shown at branches. All newly generated sequences in this study are indicated in red boxes. Asterisks on the nodes represent that the support values of Bayesian inference and Maximum Likelihood are both 100%. |
In the rbcL and COI-5P phylogenetic trees, the genus Sheathia was divided into four main clades (Clades Ⅰ, Ⅱ, Ⅲ, and Ⅳ; Figs. 2–3). However, due to the lack of sequence information in the GenBank database, the psbA phylogenetic tree was only divided into three clades (Clades Ⅰ, Ⅱ, and Ⅲ; Fig. 4). In the rbcL phylogenetic tree, Clade Ⅰ included twelve distinct lineages. From these S. jiugongshanensis J.-F. Han, F.-R. Nan & S. L. Xie, S. shimenxiaensis J.-F. Han, F.-R. Nan & S. L. Xie, and S. qinyuanensis J.-F. Han, F.-R. Nan & S. L. Xie were only observed in the "Chantransia" sporophyte generations. The remaining nine lineages lacked heterocortication. However, in the COI-5P and psbA trees, Clade Ⅰ contained nine and four distinct lineages, respectively. Clade Ⅱ only contained S. exigua Salomaki & M. L.Vis, a species that was observed to have heterocortication only on the thickest axis of the thallus near the base of the plant. Clade Ⅲ was composed of specimens where heterocortication is easy to observe. In the rbcL and COI-5P trees, Clade Ⅲ consists of six closely-related species, S. confusa (Bory) Salomaki & M. L. Vis, S. americana Salomaki & M. L. Vis, S. boryana (Sirodot) Salomaki & M. L. Vis, S. heterocortica (Sheath & K. M. Cole) Salomaki, S. grandis Salomaki & M. L. Vis, and S. involuta (M. L. Vis & Sheath) Salomaki & M. L. Vis. In contrast, only four of them were included in the psbA tree. Furthermore, in the rbcL and COI-5P trees, Clade Ⅳ was consistent with specimens attributed to a new species S. californica. The four specimens (both gametophyte stages and "Chantransia" stages included) utilized in this study were within Clade Ⅰ and clustered with several sequences from China to formed a large clade named S. longipedicellata, which had high support values except for the Bayesian posterior probabilities (PP) support value in the psbA tree (Fig. 2: 1.00/100; Fig. 3: 100/99.4; Fig. 4: -/96.7).
3.3 Divergence time estimationRhodophyta divergence time estimation based on combined sequences (rbcL, COI-5P, and psbA) matrix (Fig. 5) showed that Cyanidiophyceae, Rhoclellophyceae, Compsopogonophyceae, Stylonematophyceae, and Porphyridiophyceae diverged first, followed by the divergence of Florideophyceae and Bangiophyceae. Within Florideophyceae, Nemaliophycidae diverged at approximately 662.01 Ma (95% highest posterior density (HPD): 779.83–580.51 Ma) and Batrachospermales and Thoreales orders split occurred at 456.10 Ma (95% HPD: 552.80–367.88 Ma). The divergence time of genus Sheathia can be traced back to 251.49 Ma (95% HPD: 328.07–184.73 Ma).
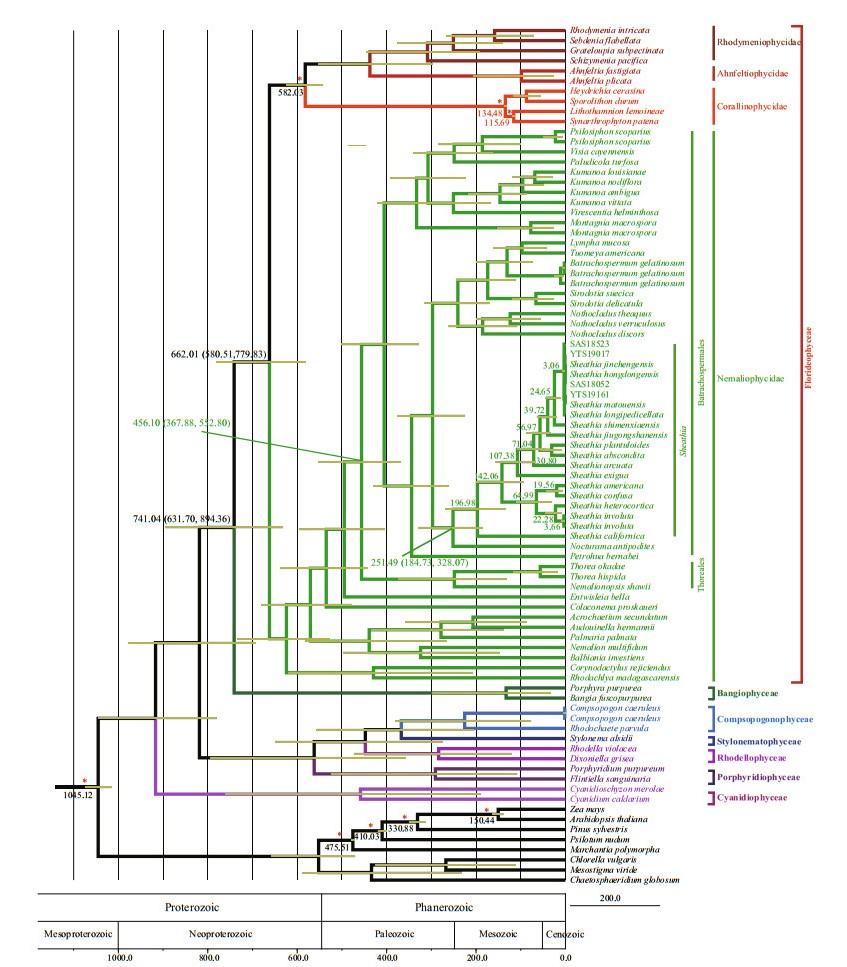
|
| Fig.5 Divergence time estimation phylogram inferred from combined rbcL, COI-5P, and psbA data using BEAST Horizontal bars indicate 95% highest posterior density of the important nodes. Clade constraints are indicated with red asterisks. The geological timescale is given in million years ago. |
Since we focus primarily on the ancestral geographic origin of the genus Sheathia, other unrelated clades were not shown in the results (Fig. 6). The ancestral area reconstruction based on rbcL and COI-5P gene data yielded similar results. The biogeographic inference map generated using BBM based on the rbcL gene data showed that the most recent common ancestor of the genus Sheathia was in North America, as shown in node 176 (North America, relative probability=0.872 0) (Fig. 6a). All specimens in Clade Ⅰ, including the four samples utilized in this study, showed an Asian distribution at their basal node (node 164, Fig. 6a). The Clade Ⅰ clustering together with S. exigua from France has an inferred Asian, European, or North American origin. For species in Clade Ⅲ with marked heterocortication, a Europe, North America, or Europe + North America distribution at their basal node is shown.
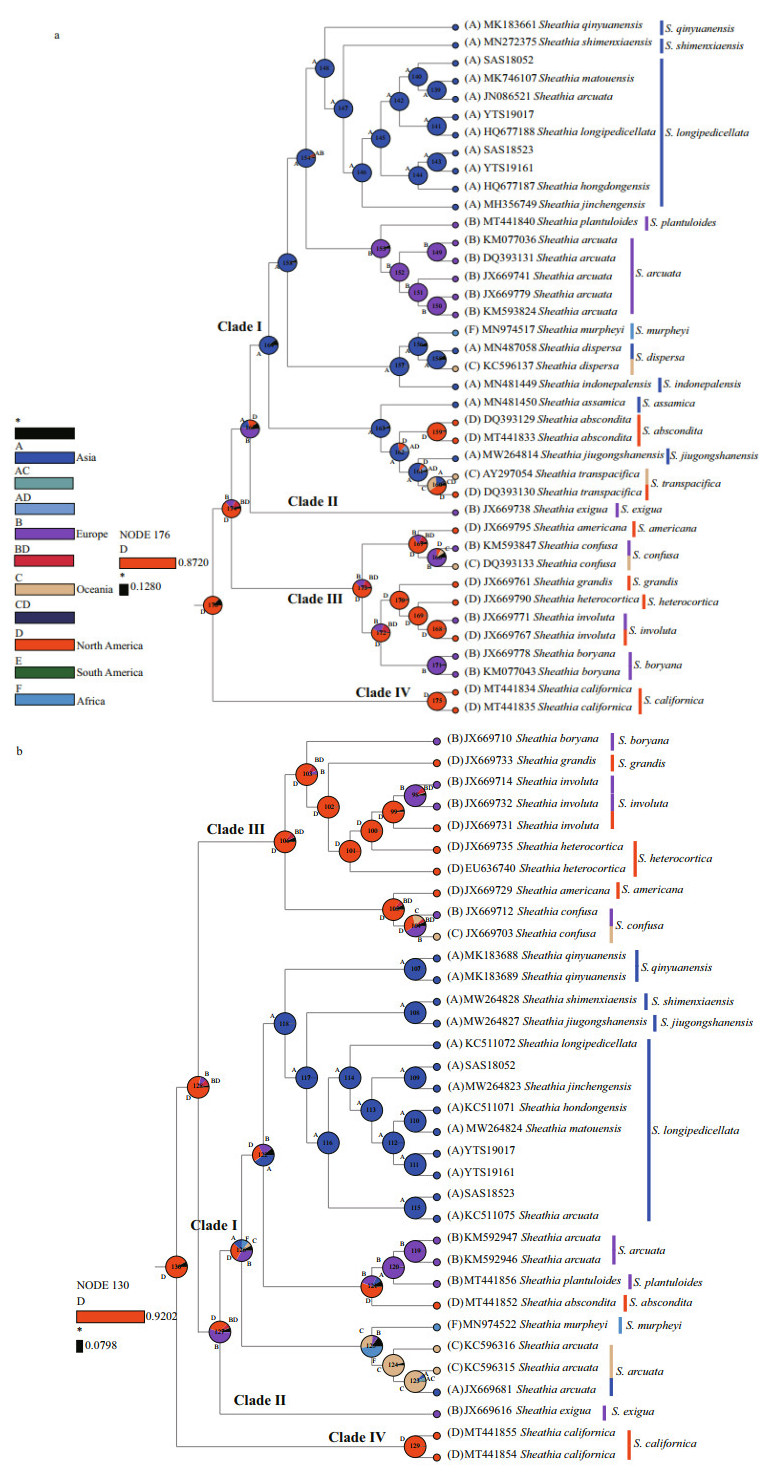
|
| Fig.6 Reconstruction of ancestral distribution area of genus Sheathia based on BBM a. the result of ancestral geographical origin inference based on the rbcL sequence. b. the result of ancestral geographical origin inference based on the COI-5P sequence. Possible ancestral ranges: *: other ancestral ranges; A: Asia; B: Europe; C: Oceania; D: North America; E: South America; F: Africa. |
Based on the COI-5P gene, the geographical origin of the genus Sheathia was also traced back to North America, as shown in node 130 (North America, relative probability=0.920 2) (Fig. 6b). All samples lacking heterocortication, including the four specimens in this study, were grouped with S. exigua from Bulgaria to yield node 127, implying a Europe, North America, or Europe + North America origin. The species that show heterocortication more likely had their origin in North American, as shown in node 106 (North America, relative probability=0.770 4).
According to Condie (1982), from the Carboniferous to Jurassic, North America was in the northwest of the Pangaea continent; the Eurasian and the African continents adjoin North America by their west and north, respectively. After its origin in North America, the genus Sheathia could first spread to Eurasia and Africa. However, Sheathia taxa in the Oceania spread from African species through India-Antarctica.
4 DISCUSSIONBatrachospermum genus was considered the genus with the most abundant species in the order Batrachospermales (Sheath, 1984; Kumano, 2002). At present, all species of this order are reported in freshwater. The genus Batrachospermum was classified into several sections according to traditional taxonomic assignments and was shown to be paraphyletic since the first phylogenetic study of the order Batrachospermales. Recently, sections of this genus have been methodically investigated and raised to separated genera using DNA sequences and morphology (Entwisle et al., 2009; Salomaki et al., 2014; Rossignolo and Necchi, 2016; Necchi et al., 2019a, c; Vis et al., 2020). The genus Sheathia was elevated from the sect. Helminthoidea and was distinguished from other genera by heterocortication (Salomaki et al., 2014). More than twenty species of this genus have been reported worldwide, of which S. longipedicellata is endemic to China. The morphological observations suggested that most of the Sheathia genus species were difficult to distinguish by unique morphological characters. Only specimens of S. confusa are distinguished from other species by spermatangia on the involucral filaments of the carpogonium (Vis and Sheath, 1996; Salomaki et al., 2014). In this study, phylogenetic trees showed that the branch of the Sheathia species contained four main clades. Interestingly, species in these four clades contain different cortical cell morphologies: heterocortication can be seen in all species of Clade Ⅲ, but in the Clade Ⅰ, Ⅱ, and Ⅳ, heterocortication was either only present on the thickest axis of the thallus near the base or completely absent (Salomaki et al., 2014; Necchi et al., 2019c). Molecular analyses of the three gene regions combined with morphological information confirmed that SAS18052 and SAS18523 belong to S. longipedicellata while the two "pygmaea" isolates YTS19161 and YTS19017 represent the "Chantransia" of S. longipedicellata and not the Audouinella genus. Therefore, no correlation was found between the molecular and morphological analysis of the "Chantransia" stage, making this stage an obstacle to species identification. Furthermore, the close relationship among S. longipedicellata, S. hongdongensis (S. L. Xie and J. Feng) J.-F. Han & al., S. jinchengensis J.-F. Han & al. and S. matouensis J.-F. Han, F.-R. Nan, J. Feng, J.-P. Lv, Q. Liu, X.-D. Liu & S. L. Xie has been suggested by previous phylogenetic analyses (Necchi et al., 2019c). Based on the three gene trees, we agree that they are a single species. Therefore, the number of S. longipedicellata distribution sites in China expands to two.
Although many pieces of research have been carried out on Sheathia in recent years, many aspects of the geographical origin and divergence time of this genus remain unresolved. However, several studies on the evolutionary history of Florideophyceae taxa have important reference value for illuminating the origin and evolution of the genus Sheathia. Molecular-clock estimates of lineage divergence times in the tree of life depend primarily on the oldest recognized fossils reported. However, the age of a fossil only gives a minimum age for the lineage, leading to the uncertainty of the dating of nodes in molecular phylogenetic trees. According to Bengtson et al. (2017), Rafatazmia chitrakootensis and Ramathallus lobatus belong to crown-group rhodophytes and were present in the ~1.6 billion years ago (Ga) Vindhyan stromatolitic microbialites. Nevertheless, other studies argued that the morphology of Rafatazmia chitrakootensis and Ramathallus lobatus do not allow for an unequivocal assignment to crown rhodophytes and that they are hundreds of millions of years older than the currently accepted divergence time between rhodophytes and viridiplantae (Betts et al., 2018; Gibson et al., 2018). So far, the fossil red alga Bangiomorpha pubescens is the oldest known taxonomically resolved eukaryotic. It was first described in chert from peritidal carbonate facies of the Hunting Formation on Somerset Island and has long been presumed to arise 1.2 Ga (Butterfield et al., 1990, 2000).
Nevertheless, several researchers have rejected using the 1.2 Ga Bangiomorpha as a calibration point for the appearance of bangiophycean or even rhodophyte, because it had seemed too old to make sense (Berney and Pawlowski, 2006; Parfrey et al., 2011; Sharpe et al., 2015). The eukaryote tree of Berney and Pawlowski (2006) showed that when Bangiomorpha was excluded, the origination date of the last ancestor of the eukaryote crown group (LECA) shift from 4 182–1 830 Ma to 1 357–947 Ma. Parfrey et al. (2011) placed Bangiomorpha at the base of the crown-group rhodophytes and placed the origin of rhodophytes at 959–625 Ma when Proterozoic fossils were included in the calibration, and 1 285–1 180 Ma when excluded. Similarly, they found that according to whether the Proterozoic fossils were included in the calibration, the divergence time of florideophyte and Bangiales was different: when Proterozoic fossils constraints were excluded, they split at ca. 620 Ma (~700–495 Ma), whereas, when those fossils included, their divergence time returns to ca. 765 Ma (915–630 Ma). Sharpe et al. (2015) excluded Bangiomorpha as a calibration point and arrived at a rhodophyte/chlorophyte divergence date of 996 Ma (1 186–801 Ma). Yang et al. (2016) and Nan (2017) accepted Bangiomorpha as a rhodophyte; however, they used Bangiomorpha as a calibration point for the early rhodophyte stem group instead of Bangiophyceae and Florideophyceae. Based on a seven-gene concatenated dataset and a Bayesian relaxed-clock model, Yang et al. (2016) suggested that the class Florideophyceae diverged approximately 943 (1 049–817) Ma and Nemaliophycidae diverged approximately 661 (736–597) Ma. Subsequently, divergence time estimation based on chloroplast and mitochondrial genomes indicated that the typical freshwater orders Thoreales and Batrachospermales diverged approximately 484–415 Ma (Nan, 2017).
Bangiomorpha pubescens was also reported in the Angmaat Formation of the Bylot Supergroup on Baffin Island (Knoll et al., 2013). Based on the Re-Os geochronology analysis, Gibson et al. (2018) revised the first appearance of B. pubescens to 1 047 (+13 /-17) Ma and suggested that this date permits more precise estimates of early eukaryotic evolution. Using relaxed molecular clock analysis with the new fossil age as the calibration for rhodophyte and chlorophyte, we suggest that Florideophyceae and Nemaliophycidae originated at Neoproterozoic. Moreover, this study showed that the divergence between orders Batrachospermales and Thoreales occurred during the Early Paleozoic Era to early Late Paleozoic Era (95% HPD: 552.80–367.88 Ma), nearly a million years later than previous studies.
The reconstruction of the geographical area of origin is of great significance to understand the evolution and development of red algae. Several studies have been conducted on the biogeography of red algae (Xie, 2001; Feng et al., 2015; Nan et al., 2016). With the help of the distribution of primitive taxa and sister groups, Xie (2001) speculated that the Batrachospermales order might originate in the area connecting the southern edge of Laurentia and the northern edge of Gondwana. Based on geographic information and molecular phylogenetic evidence, Feng et al. (2015) suggested that the genus Thorea most probably originated in Asia. According to Nan et al. (2016), the genus Compsopogon originated in North America, which was part of the Laurentia landmass located in the Rodinia supercontinent during the Proterozoic era. Based on the above, we speculate that the genus Sheathia should also originate in the Northern Hemisphere. Based on the comprehensive analysis of the geographic reconstruction and divergence time, we proposed that genus Sheathia originated in North America, a portion of the Pangaea supercontinent from the Carboniferous to the Jurassic period (95% HPD: 328.07–184.73 Ma).
In the late Carboniferous, the collision between the Euramerica continent and the Gondwana paleocontinent formed the western half of the Pangaea supercontinent. At the end of Permian, the formation of the Pangaea supercontinent caused most of the oceans formed during the Pannotia supercontinent division to disappear. With the equator as the center, Pangaea extended from the Antarctic to the Arctic. The veritable Pangaea was finally formed in the Late Triassic (Scotese et al., 1979).
The species of the genus Sheathia are widely distributed in Asia, Europe, North America, Africa, and Oceania, suggesting that the genus Sheathia may have appeared before the Pangaea separated. Divergent time estimates based on a relaxed molecular clock suggested that the genus Sheathia originated between the Carboniferous and the Jurassic period. However, according to Condie (1982), by the mid-to-late Jurassic, Africa began to drift away from North America and India-Antarctica. Therefore, the genus Sheathia should appear before this period. Since North America was connected to Eurasia and Africa continent in Pangaea (Scotese et al., 1979), Sheathia taxa could directly expand to these two continents after their origin in North America. Nevertheless, Oceania does not directly border North America in Pangaea (Scotese et al., 1979). Hence we inferred that the Sheathia species of Oceania were spread from Africa.
5 CONCLUSIONUsing morphological characteristics and genetic data (rbcL, COI-5P, and psbA), we analyzed Sheathia genus samples from the Shanxi (gametophyte stages) and Henan ("Chantransia" stages) provinces. Morphological data show that the "Chantransia" stage samples (YTS19161 and YTS19017) were similar to A. pygmaea, while the gametophyte stage samples (SAS18052 and SAS18523) were highly consistent with the description of S. longipedicellata. Conforming to the morphological identification results, all three molecular markers supported SAS18052 and SAS18523 as S. longipedicellata. Nevertheless, phylogenetic trees indicated that YTS19161 and YTS19017 are the "Chantransia" of S. longipedicellata rather than the genus Audouinella. Moreover, the evolutionary history of Sheathia showed that it had originated about 328.07–184.73 Ma in the portion of Pangea that was to become North America, then spread to other localities.
6 DATA AVAILABILITY STATEMENTThe data that support the findings of this study are available on request from the corresponding author.
7 ACKNOWLEDGMENTDr. N. R. C. Eva (University of Puerto Rico, USA) is acknowledged for the English editing.
Electronic supplementary materialSupplementary material (Supplementary Tables S1–S5 and Figs. S1–S4) is available in the online version of this article at https://doi.org/10.1007/s00343-021-1075-0.
Aguirre J, Perfectti F, Braga J C. 2010. Integrating phylogeny, molecular clocks, and the fossil record in the evolution of coralline algae (Corallinales and Sporolithales, Rhodophyta). Paleobiology, 36(4): 519-533.
DOI:10.1666/09041.1 |
Aguirre J, Riding R, Braga J C. 2000. Diversity of coralline red algae: origination and extinction patterns from the early Cretaceous to the Pleistocene. Paleobiology, 26(4): 651-667.
DOI:10.1666/0094-8373(2000)026<0651:docrao>2.0.co;2 |
Bengtson S, Sallstedt T, Belivanova V, Whitehouse M. 2017. Three-dimensional preservation of cellular and subcellular structures suggests 1.6 billion-year-old crown-group red algae. PLoS Biology, 15(3): e2000735.
DOI:10.1371/journal.pbio.2000735 |
Berney C, Pawlowski J. 2006. A molecular time-scale for eukaryote evolution recalibrated with the continuous microfossil record. Proceedings of the Royal Society B. Biological Sciences, 273(1596): 1867-1872.
DOI:10.1098/rspb.2006.3537 |
Betts H C, Puttick M N, Clark J W, Williams T A, Donoghue P C J, Pisani D. 2018. Integrated genomic and fossil evidence illuminates life's early evolution and eukaryote origin. Nature ecology & evolution, 2(10): 1556-1562.
DOI:10.1038/s41559-018-0644-x |
Butterfield N J, Knoll A H, Swett K. 1990. A bangiophyte red alga from the Proterozoic of arctic Canada. Science, 250(4977): 104-107.
DOI:10.1126/science.11538072 |
Butterfield N J. 2000. Bangiomorpha pubescens n. gen., n. sp. : implications for the evolution of sex, multicellularity, and the Mesoproterozoic/Neoproterozoic radiation of eukaryotes. Paleobiology, 26(3): 386-404.
DOI:10.1666/0094-8373(2000)026<0386:bpngns>2.0.co;2 |
Condie K C. 1982. Plate Tectonics & Crustal Evolution. Pergamon Press, New York. 492p.
|
Condon D, Zhu M Y, Bowring S, Wang W, Yang A H, Jin Y G. 2005. U-Pb ages from the neoproterozoic doushantuo formation, China. Science, 308(5718): 95-98.
DOI:10.1126/science.1107765 |
Drummond A J, Ho S Y W, Phillips M J, Rambaut A. 2006. Relaxed phylogenetics and dating with confidence. PLoS Biology, 4(5): e88.
DOI:10.1371/journal.pbio.0040088 |
Drummond A J, Rambaut A. 2007. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evolutionary Biology, 7(1): 214.
DOI:10.1186/1471-2148-7-214 |
Entwisle T J, Vis M L, Chiasson W B, Necchi O Jr, Sherwood A R. 2009. Systematics of the Batrachospermales (Rhodophyta)—a synthesis. Journal of Phycology, 45(3): 704-715.
DOI:10.1111/j.1529-8817.2009.00686.x |
Felsenstein J. 1981. Evolutionary trees from DNA sequences: a maximum likelihood approach. Journal of Molecular Evolution, 17(6): 368-376.
DOI:10.1007/bf01734359 |
Feng J, Chen L, Wang Y N, Xie S L. 2015. Molecular systematics and biogeography of Thorea (Thoreales, Rhodophyta) from Shanxi, China. Systematic Botany, 40(2): 376-385.
DOI:10.1600/036364415x688763 |
Gibson T M, Shih P M, Cumming V M, Fischer W W, Crockford P W, Hodgskiss M S W, Wörndle S, Creaser R A, Rainbird R H, Skulski T M, Halverson G P. 2018. Precise age of Bangiomorpha pubescens dates the origin of eukaryotic photosynthesis. Geology, 46(2): 135-138.
DOI:10.1130/g39829.1 |
Guindon S, Gascuel O. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology, 52(5): 696-704.
DOI:10.1080/10635150390235520 |
Han J F, Nan F R, Feng J, Lv J P, Liu Q, Kociolek J P, Xie S L. 2018. Sheathia jinchengensis (Batrachospermales, Rhodophyta), a new freshwater red algal species described from North China. Phytotaxa, 367(1): 63-70.
DOI:10.11646/phytotaxa.367.1.7 |
Han J F, Nan F R, Feng J, Lv J P, Liu Q, Liu X D, Xie S L. 2020. Affinities of four freshwater putative "Chantransia" stages (Rhodophyta) in Southern China from molecular and morphological data. Phytotaxa, 441(1): 47-59.
DOI:10.11646/phytotaxa.441.1.4 |
Han X J. 2012. Phylogenetic Relationship of Audouinella based on Gene Sequences. Shanxi University, Taiyuan. 65p.
(in Chinese with English abstract)
|
Hua D, Shi Z X. 1996. A new species of Batrachospermum from Jiangsu, China. Acta Phytotaxonomica Sinica, 34(3): 324-326.
(in Chinese with English abstract) |
Ji L, Xie S L, Feng J, Chen L, Wang J. 2014. Molecular systematics of four endemic Batrachospermaceae (Rhodophyta) species in China with multilocus data. Journal of Systematics and Evolution, 52(1): 92-100.
DOI:10.1111/jse.12058 |
Johnston E T, Dixon K R, West J A, Buhari N, Vis M L. 2018. Three gene phylogeny of the Thoreales (Rhodophyta) reveals high species diversity. Journal of Phycology, 54(2): 159-170.
DOI:10.1111/jpy.12618 |
Knoll A H, Wörndle S, Kah L C. 2013. Covariance of microfossil assemblages and microbialite textures across an upper Mesoproterozoic carbonate platform. Palaios, 28(7): 453-470.
DOI:10.2110/palo.2013.p13-005r |
Kumano S. 2002. Freshwater Red Algae of the World. Biopress Ltd, Bristol. 375p.
|
Magallón S, Hilu K W, Quandt D. 2013. Land plant evolutionary timeline: gene effects are secondary to fossil constraints in relaxed clock estimation of age and substitution rates. American Journal of Botany, 100(3): 556-573.
DOI:10.3732/ajb.1200416 |
Nan F R, Feng J, Lv J P, Liu Q, Xie S L. 2016. Evolutionary history of the monospecific Compsopogon genus (Compsopogonales, Rhodophyta). Algae, 31(4): 303-315.
DOI:10.4490/algae.2016.31.10.22 |
Nan F R. 2017. Investigation on the Phylogenetics and Evolution History of Different Lineages in Freshwater Rhodophyta Based on Phylogenomic Analysis. Shanxi University, Taiyuan. 142p. (in Chinese with English abstract)
|
Necchi O Jr, Agostinho D C, Vis M L. 2018. Revision of Batrachospermum section Virescentia (Batrachospermales, Rhodophyta) with the establishment of the new genus, Virescentia stat. nov. Cryptogamie, Algologie, 39(3): 313-338.
DOI:10.7872/crya/v39.iss3.2018.313 |
Necchi O Jr, Filho A G, Paiano M O. 2019a. Revision of Batrachospermum sections Acarposporophytum and Aristata (Batrachospermales, Rhodophyta) with the establishment of the new genera Acarposporophycos and Visia. Phytotaxa, 395(2): 51-65.
DOI:10.11646/phytotaxa.395.2.1 |
Necchi O Jr, Garcia Fo A S, Paiano M O, Vis M L. 2019b. Revision of Batrachospermum section Macrospora (Batrachospermales, Rhodophyta) with the establishment of the new genus Montagnia. Phycologia, 58(6): 582-591.
DOI:10.1080/00318884.2019.1624143 |
Necchi O Jr, West J A, Ganesan E K, Yasmin F, Rai S K, Rossignolo N L. 2019c. Diversity of the genus Sheathia (Batrachospermales, Rhodophyta) in northeast India and east Nepal. Algae, 34(4): 277-288.
DOI:10.4490/algae.2019.34.10.30 |
Necchi O Jr, Zucchi M R. 1995. Systematics and distribution of freshwater Audouinella (Acrochaetiaceae, Rhodophyta) in Brazil. European Journal of Phycology, 30(3): 209-218.
DOI:10.1080/09670269500650991 |
Nylander J A, Olsson U, Alstrom P, Sanmartin I. 2008. Accounting for phylogenetic uncertainty in biogeography: a Bayesian approach to dispersal vicariance analysis of the thrushes (Aves: Turdus). Systematic Biology, 57(2): 257-268.
DOI:10.1080/10635150802044003 |
Parfrey L W, Lahr D J G, Knoll A H, Katz L A. 2011. Estimating the timing of early eukaryotic diversification with multigene molecular clocks. Proceedings of the National Academy of Sciences of the United States of America, 108(33): 13624-13629.
DOI:10.1073/pnas.1110633108 |
Posada D, Buckley T R. 2004. Model selection and model averaging in phylogenetics: advantages of Akaike information criterion and Bayesian approaches over likelihood ratio tests. Systematic Biology, 53(5): 793-808.
DOI:10.1080/10635150490522304 |
Rambaut A, Suchard M A, Xie D, Drummond A J. 2014. Tracer v1.6. Computer program and documentation distributed by the author. http://beast.community/tracer. Accessed on 2015-01-15.
|
Rambaut A. 2009. FigTree, version 1.3.1. Computer program distributed by the author. http://tree.bio.ed.ac.uk/software/figtree/. Accessed on 2011-01-04.
|
Ronquist F, Huelsenbeck J P. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19(12): 1572-1574.
DOI:10.1093/bioinformatics/btg180 |
Rossignolo N L, Necchi O Jr. 2016. Revision of section Setacea of the genus Batrachospermum (Batrachospermales, Rhodophyta) with emphasis on specimens from Brazil. Phycologia, 55(4): 337-346.
DOI:10.2216/15-144.1 |
Salomaki E D, Kwandrans J, Eloranta P, Vis M L. 2014. Molecular and morphological evidence for Sheathia gen. nov. (Batrachospermales, Rhodophyta) and three new species. Journal of Phycology, 50(3): 526-542.
DOI:10.1111/jpy.12179 |
Saunders G W. 1993. Gel purification of red algal genomic DNA: an inexpensive and rapid method for the isolation of polymerase chain reaction-friendly DNA. Journal of Phycology, 29(2): 251-254.
DOI:10.1111/j.0022-3646.1993.00251.x |
Saunders G W. 2005. Applying DNA barcoding to red macroalgae: a preliminary appraisal holds promise for future applications. Philosophical Transactions of the Royal Society B: Biological sciences, 360(1462): 1879-1888.
DOI:10.1098/rstb.2005.1719 |
Scotese C R, Bambach R K, Barton C, van der Voo R, Ziegler A M. 1979. Paleozoic base maps. The Journal of Geology, 87(3): 217-277.
DOI:10.1086/628416 |
Sharpe S C, Eme L, Brown M W, Roger A J. 2015. Timing the origins of multicellular eukaryotes through phylogenomics and relaxed molecular clock analyses. In: Ruiz-Trillo I, Nedelcu AM eds. Evolutionary Transitions to Multicellular Life. Springer, Dordrecht. p. 3–29.
|
Sheath R G. 1984. The biology of freshwater red algae. In: Round F E, Chapman D J eds. Progress in Phycological Research. BioPress Ltd, Bristol. p. 89–157.
|
Shi Z X. 2006. Flora Algarum Sinicarum Aquae Dulcis. Tomus XⅢ, Rhodophyta and Phaeophyta. Science Press, Beijing, China. p. 1–77. (in Chinese)
|
Sun C H, Liu X F, Liu X D, Guo X W, Guo D C, Xu J P, Liu X Y, Hua D. 2001. Studies on physiological and ecological speciality of Batrachospermum longipedicellatum. Resources Science, 23(3): 93-96.
(in Chinese with English abstract) |
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, 28(10): 2731-2739.
DOI:10.1093/molbev/msr121 |
Thompson J D, Gibson T J, Plewniak F, Jeanmougin F, Higgins D G. 1997. The Clustal-X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research, 25(24): 4876-4882.
DOI:10.1093/nar/25.24.4876 |
van den Hoek C, Mann D, Jahns H M, Jahns M. 1995. Algae: An Introduction to Phycology. Cambridge University Press, Cambridge. 614pp.
|
Vis M L, Lee J, Eloranta P, Chapuis I S, Lam D W, Necchi Jr O. 2020. Paludicola gen. nov. and revision of the species formerly in Batrachospermum section Turfosa (Batrachospermales, Rhodophyta). Journal of Phycology, 56(4): 844-861.
DOI:10.1111/jpy.13001 |
Vis M L, Saunders G W, Sheath R G, Dunse K, Entwisle T J. 1998. Phylogeny of the Batrachospermales (Rhodophyta) inferred from rbcL and 18S ribosomal DNA gene sequences. Journal of Phycology, 34(2): 341-350.
DOI:10.1046/j.1529-8817.1998.340341.x |
Vis M L, Sheath R G. 1996. Distribution and systematics of Batrachospermum (Batrachospermales, Rhodophyta) in North America. 9. Section Batrachospermum: description of five new species. Phycologia, 35(2): 124-134.
DOI:10.2216/i0031-8884-35-2-124.1 |
Vis M L, Sheath R G. 1997. Biogeography of Batrachospermum gelatinosum (Batrachospermales, Rhodophyta) in North America based on molecular and morphological data. Journal of Phycology, 33(3): 520-526.
DOI:10.1111/j.0022-3646.1997.00520.x |
Xiao S H, Zhang Y, Knoll A H. 1998. Tree-dimensional preservation of algae and animal embryos in a Neoproterozoic phosphorite. Nature, 391(6667): 553-558.
DOI:10.1038/35318 |
Xie S L, Ling Y J. 2004. A taxonomic study of freshwater red algae from Shanxi province, North China. Acta Botanica Boreali-Occidentalia Sinica, 24(8): 1489-1492.
(in Chinese with English abstract) |
Xie S L. 2001. Studies on Batrachospermales (Rhodophyta) from China. Doctoral Dissertation, Institute of Hydrobiology, Chinese Academy of Sciences. (in Chinese with English abstract)
|
Yang E C, Boo S M, Bhattacharya D, Saunders G W, Knoll A H, Fredericq S, Graf L, Yoon H S. 2016. Divergence time estimates and the evolution of major lineages in the florideophyte red algae. Scientific Reports, 6(1): 21361.
DOI:10.1038/srep21361 |
Žuljević A, Kaleb S, Peña V, Despalatović M, Cvitković I, De Clerck O, Le Gall L, Falace A, Vita F, Braga J C, Antolić B. 2016. First freshwater coralline alga and the role of local features in a major biome transition. Scientific Reports, 6(1): 19642.
DOI:10.1038/srep19642 |
 2022, Vol. 40
2022, Vol. 40