Institute of Oceanology, Chinese Academy of Sciences
Article Information
- GÓMEZ Fernando
- Dinophysoid dinoflagellates from subphotic depths: Amphisolenia sp. aff. brevicauda, Dinofurcula tricornuta sp. nov., and Dinophysis profunda sp. nov. (Dinophysales, Dinophyceae)
- Journal of Oceanology and Limnology, 40(2): 703-711
- http://dx.doi.org/10.1007/s00343-021-0463-9
Article History
- Received Dec. 1, 2020
- accepted in principle Feb. 15, 2021
- accepted for publication Apr. 1, 2021
The microbial world represents the last truly unexplored frontier in the diversity of life on Earth. Microbial-community amplicon-based sequence abundance distributions have a long 'tail' of low-abundance organisms, referred to as the rare biosphere, which often comprises the large majority of species (Sogin et al., 2006). Microscopy-based plankton studies are focused in coastal waters and the surface of the ocean, while the sampling effort associated with subphotic depths is much lower. At the beginning of the 19th century, Kofoid described numerous species of dinophysoid dinoflagellates from the eastern tropical Pacific Ocean based on plankton net vertical hauls from 1 500-m depth to the surface (Kofoid, 1907; Kofoid and Skogsberg, 1928). Kofoid described nearly all the known species of Triposolenia Kof. and several species of Amphisolenia F. Stein, whose records in many cases remain restricted to the original descriptions. Kofoid also described the two species of genus Dinofurcula Kof. & Skogsb.; records that remain restricted to the eastern tropical Pacific Ocean. While dinoflagellate studies in the Mediterranean Sea, where about 1/3 of the described species has been reported (Gómez, 2003, 2012) have largely been focused on coastal and surface waters, sporadic samplings in mesopelagic zone (200–1 000 m-depth) revealed the presence of species of dinophysoid genera such as Amphisolenia, and especially Triposolenia (Gómez et al., 2011; Dolan et al., 2019). In this study, several samples were analysed from subphotic depths of the Ionian and Marmara Seas. The aim was the isolation of individuals to obtain molecular data following the method described in Gómez et al.(2011, 2012). However, this study reports on three species that did not yield any molecular data, but whose distinctive morphologies deserve attention.
2 MATERIAL AND METHODThe concentration of large volume of seawater is required for the observations of plankton at subphotic depths due to their very low abundances. Net sampling with vertical hauls inflicts mechanical damage to the individuals due to the drag from deep water, and even with closing plankton nets, there is the potential for contamination of the sample with individuals from upper depths. The use of oceanographic bottles (i.e. Niskin) allows sampling at discrete depths without contamination and reduces damage, but the sample volume is smaller. In this study, samples were collected with Niskin bottles during the research cruise MARM10_02 onboard R/V Urania in October 2010 (http://ricerca.ismar.cnr.it/CRUISE_REPORTS/2010-2019/MARM10_02_REP/MARM10_02_REP/node5.html). A 12-L sample was collected at 500-m depth (bottom at 3 226-m depth) in the Ionian Sea, site KM3 (36°28′58.3″N, 15°39′00.3″E) on October 1, 2010. Another 18-L sample was collected at 70-m depth (bottom at 3 633-m depth) in the Ionian Sea (36°36′28.8″N, 17°58′15.6″E) on October 2, 2010. On October 5, 2010, a third sample of 120 L was collected at 154-m depth (bottom at 161-m depth) in the Marmara Sea, site SN4 (40°43′43.3″N, 29°23′11.9″E). Seawater from the Niskin bottles was gently poured through 200-μm pore size Nylon mesh to remove macrozooplankton, and then through 20-μm mesh to concentrate the sample. The material retained on the 20-μm mesh was washed and resuspended with 0.22-μm filtered seawater. The plankton concentrate was divided into a sample preserved with Lugol's solution at final concentration of 2% (volume/volume), and another sample fixed with absolute ethanol at final concentration of 80% (volume/volume). Samples were kept in darkness and at a temperature of 4 ℃ until analyses. Lugol's solution preserves the cell morphology, including most of the unarmoured forms, but PCR efficiency is reduced compared to ethanol-fixation. The ethanol lysed or distorted the unarmoured forms and discoloured the cells, and is sometimes associated with the formation of a mucilage or precipitation of salts that makes microscopy observation difficult. Lugol's preserved samples were treated with small amounts (150-200 μL) of 10% (weight/volume) sodium thiosulfate to remove iodine. The Lugol's samples were examined first, and the ethanol-fixed samples were assessed in order to further identify individuals. Subsamples were examined in Utermöhl chambers with an inverted microscope (Eclipse TE2000-S, Nikon, Tokyo, Japan) and photographed with digital camera (Nikon DS-2M). Micrographs of Amphisolenia brevicauda Kof. are included for a comparison with the Amphisolenia species. These individuals were collected in the surface waters of the Gulf of Lions at Marseilles and Banyuls-sur-Mer, France, following the method described in Gómez et al. (2011).
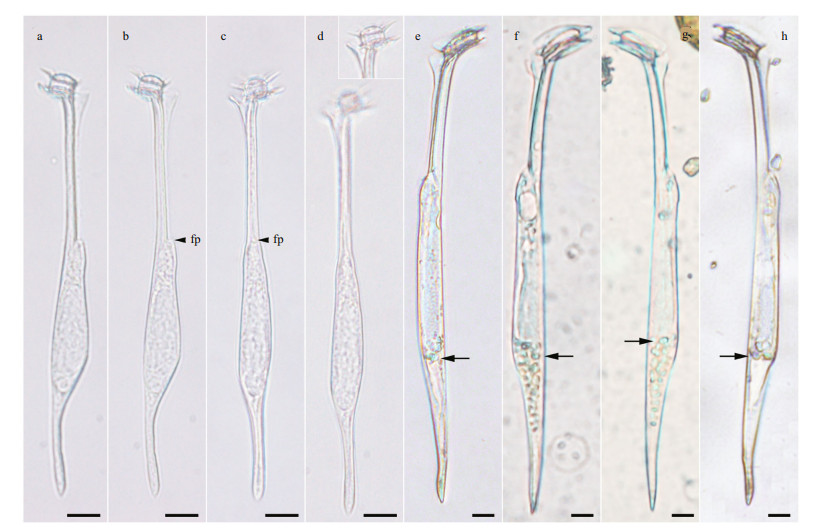
3 RESULT 3.1 Amphisolenia sp. aff. brevicaudaA single individual of a small amphisolenoid cell with a slight sigmoidal curvature of the midbody and antapical process was observed in the Ionian Sea at 70-m depth (36°36′28.8″N, 17°58′15.6″E). The total cell length was 133 μm, midbody of 50 μm, neck of 45 μm, and antapical process of 30 μm. The epitheca was tiny and dome-shaped, slightly inclined anteriorly. The cingulum was about ~7 μm in diameter. The cingular lists were 1.5 times as wide as the cingulum. The neck was straight, about 2.5 μm in diameter, with a triangular elongated left lateral sulcal list without ribs. The midbody was slightly laterally flattened [12- μm depth (dorso-ventral distance) and 8-μm wide (transdiameter)], widened posteriorly, slightly convex in the dorsal side with a hump in the posterior ventral side, and an anterior ventral small hump associated with the flagellar pore. The midbody tapered posteriorly to an antapical process (3 μm in diameter), unbranched, with an acute end devoid of spinules, and slightly oriented towards the ventral side. In lateral view, the outline of the midbody and antapical process was slightly sigmoidal. The theca was apparently structureless (Fig. 1a–b).
|
| Fig.1 Light micrographs of Amphisolenia spp. a–d. Amphisolenia sp. aff. brevicauda from the Ionian Sea; e–h. individuals of A. brevicauda from the Gulf of Lions, NW Mediterranean Sea. The arrow points tentative endosymbiotic microalgae. fp: flagellar pore. Scale bars: 10 μm. |
The close relative, Amphisolenia brevicauda, is shown in Fig. 1e–h for comparison. Amphisolenia sp. aff. brevicauda was 133-μm long, while A. brevicauda was 200–210-μm long. The epitheca of Amphisolenia sp. aff. brevicauda was dome-shaped and slightly protruded over the upper cingular list (Fig. 1a–d), while the epitheca of A. brevicauda was flat, and it did not protrude over the upper cingular list (Fig. 1e–h). The cingulum of A. brevicauda was 16 μm in diameter, while 7 μm in Amphisolenia sp. aff. brevicauda. The neck Amphisolenia sp. aff. brevicauda was straight and the length was almost similar to that of the midbody, while the neck of A. brevicauda was slightly dorsally deflected and one half of the midbody length. The ventral margin of the midbody of A. brevicauda was almost straight (Fig. 1e–h), while more asymmetric with a dorsal hump in Amphisolenia sp. aff. brevicauda (Fig. 1a–d). The midbody of A. brevicauda gradually merged into the antapical process, while the antapical process of Amphisolenia sp. aff. brevicauda was well set off from the midbody. The individuals of A. brevicauda were collected in surface waters, and they harboured endosymbiont microalgae (Fig. 1e–h). There was no evidence for the presence of endosymbiotic microalgae in Amphisolenia sp. aff. brevicauda despite the fact that the collection depth of 70 m corresponded to the deep chlorophyll maximum. Nothing is known about the life cycle of the species of Amphisolenia, so the potential that Amphisolenia sp. aff. brevicauda (Fig. 2a) is a part of the life cycle of A. brevicauda (Fig. 2b–d) cannot be discarded.

|
| Fig.2 Line drawings of Amphisolenia spp. a. Amphisolenia sp. aff. brevicauda; b. A. brevicauda from Kofoid (1907); c. A. brevicauda redrawn from Wood (1963a); d. A. brevicauda var. curvata redrawn from Wood (1963b). |

|
| Fig.3 Light micrographs of Dinophysis profunda sp. nov. from the Ionian Sea a–e. left lateral view; f–h. right lateral view. Scale bars: 10 μm. |
|
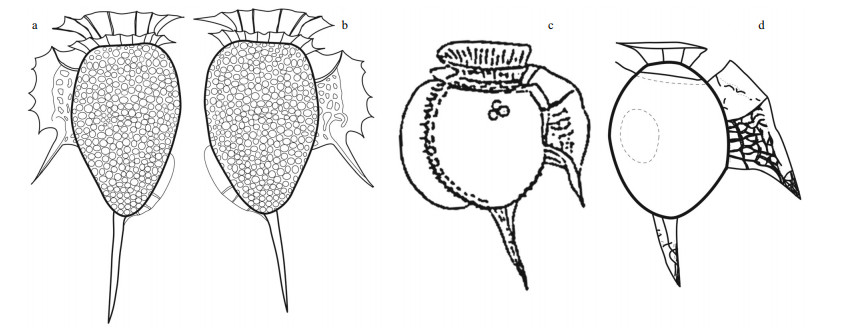
| Fig.4 Line drawings of Dinophysis spp. a–b. left and right lateral views of Dinophysis profunda sp. nov.; c. Dinophysis alata from Jørgensen (1923); d. Dinophysis balechii redrawn from Norris and Berner (1970). |
Diagnosis: A heterotrophic dinophysoid cell with ovoid hypotheca, narrower towards the antapex. The length of the cell body was 46-μm long, and it possessed a prominent antapical spine of 23-μm long. The greatest depth (dorso-ventral distance) was 27 μm. The epitheca was low, 18-μm depth and the cingulum was wide and excavated. The left sulcal list showed a serrate crest-like margin with a long third rib as a prominent spine. A scarcely developed sail emerged from the dorsal margin of the lower half of the hypotheca, with several posterior ribs. Theca with poroids.
Holotype: Fig. 3e.
Iconotype: Fig. 4a.
Type locality: Ionian Sea (36°28′58.3″N, 15°39′00.3″E) at 500-m depth.
Etymology: profundus, Latin from prō+fundus ("bottom"), meaning deep, profound. The individual was collected at 500-m depth.
Description: The most distinctive features of this dinophysoid cell are the cell shape, the prominent antapical spine, the dorsal sail, and especially the morphology of the left sulcal list (Fig. 3). The straight antapical spine emerged from the ventral side of the antapex and extended slightly oriented towards the ventral side. The posterior half of the dorsal hypotheca showed a scarcely developed sail with a smooth margin and several ribs in the most posterior half (Fig. 3a–b). The sulcal list and the antapical spine were placed in distinct focal planes, and the left sulcal list was a serrate crest with five peaks located between the second and third rib (Fig. 3d–f). The first sulcal rib was short, partially hidden by the lower cingular list. The second rib was conspicuous and anteriorly curved. The third rib was a long spine of 15 μm that protruded over the sulcal list with an angle of 45° with respect to the longitudinal axis of the cell. The proximal half of the left sulcal list was reticulated, while the distal half was smooth (Fig. 3d–f). The theca showed poroids (Fig. 3e & h).
Closely related to D. profunda sp. nov. (Fig. 4a–b) are Dinophysis alata Jørg. (Fig. 4c) and D. balechii D. R. Norris & L. D. Berner (Fig. 4d). The cingulum of D. profunda sp. nov. was wider than in D. alata and D. balechii. The shapes of the hypothecae of D. alata and D. balechii were ellipsoidal and rotund, respectively, while the hypotheca of the new species was ovoid. The anterior cingular list of D. balechii was narrow, while wider in D. profunda sp. nov. The margin of the left sulcal list of D. alata and D. balechii was smooth, while serrate in the new species. When compared to the other two species, the third rib of D. profunda sp. nov. largely protruded over the left sulcal list like an spine. The first rib of the left sulcal list of D. alata and D. balechii was visible, while inconspicuous in the new species. Dinophysis alata is characterized by a large dorsal sail that extended from the cingulum to the antapex (Fig. 4c), while absent in D. balechii (Fig. 4d). The small dorsal sail of D. profunda sp. nov. extended along the posterior half of the hypotheca. The antapical spine of the new species was longer than in the two other species. Dinophysis profunda sp. nov. is described from mesopelagic depths, while Dinophysis alata and D. balechii are only known from the euphotic zone.
3.3 Dinofurcula tricornuta F. Gómez sp. nov. (Figs. 5a–p, 6a–b)

|
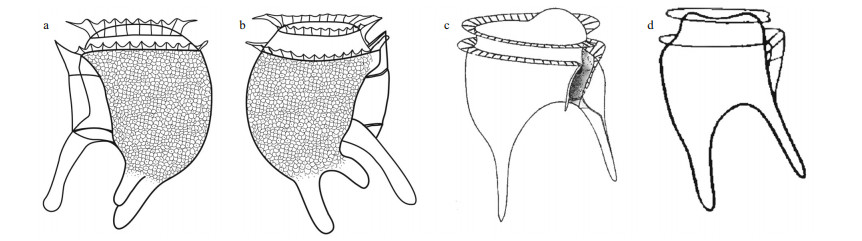
| Fig.5 Light micrographs of three individuals of Dinofurcula tricornuta sp. nov. from the Marmara Sea a–h. one individual; i–m. other individual; n–p. another individual; a–e, k, n–p. left lateral view; i–j. right lateral view; f–h, l–m. ventral view. LPP: long posterior process; r1: first rib of the left sulcal list; r2: second rid; r3: third rib; spi: spinule; SPP: short posterior process; su: sulcus; VPP: ventro-posterior process. Scale bars: 10 μm. |
|
| Fig.6 Line drawings of Dinofurcula spp. a–b. left and right views of Dinofurcula tricornuta sp. nov.; c. Dinofurcula ultima from Kofoid (1907); d. Dinofurcula ventralis from Kofoid and Skogsberg (1928). |
Diagnosis: Phalacromoid heterotrophic cell, bilaterally compressed of 53–57 μm in length. The epitheca was low and it did not protrude over the upper cingular list. The cingulum was wide. The hypotheca occupied most of the cell body and was characterized by three posterior-oriented process with round ends. The longer process emerged from the middle of the margin of the ventral hypotheca, and the two shorter processes emerged like a bifurcated antapex. The more dorsal antapical process was longer and oriented towards the right side, while the other one was shorter and oriented towards the left side.
Holotype: Fig. 5c.
Iconotype: Fig. 6a.
Type locality: Marmara Sea (40°43′43.3″N, 29°23′11.9″E) at 154-m depth.
Etymology: tri-, derived from both Latin and Greek roots, means three. Latin adjective cornūtum, from cornū ("horn"), means horned, having horns.
Morphology: Three individuals were examined. They were similar in size and shape, with slight differences in the thickness and orientation of the ventro-posterior process, and the presence of corpuscles in the hyposome, probably food vacuoles (Fig. 5). The length of the cells, including the antapical process ranged from 53–57 μm. The depth (dorso-ventral distance) varied due to the irregular outline of the ventral margin of the hypotheca. The maximum depth excluding the ventral process was 30 μm. The epitheca was very low, dome-shaped, 17–20 μm in lateral view, and it did not protrude over the anterior cingular list. The cingulum was wide and excavated (5-μm wide), with a list at both the anterior and posterior edges and ribs almost perpendicular to the cell body. The left sulcal list was poorly developed, smooth with the margin parallel to the longitudinal axis of the cell. The first sulcal rib was straight and oriented anteriorly. The second rib was short and closer to the first rib than to the third rib. The third rib was straight and perpendicular to the hypotheca and emerged at the basis of the ventro-posterior process (Fig. 5a, c, & i). The dorsal margin of the hypotheca was convex being wider at the middle. The ventral margin between the cingulum and the ventro-posterior process was sigmoidal, concave at the upper basis of the process. There were three posterior-oriented processes in the hypotheca. The longest process (25-μm long, named ventro-posterior process) was ventral and located in the right valve of the hypotheca, and it was slightly oriented towards the left side. This ventro-posterior process emerged with an angle of ~45° with respect to the longitudinal axis of the cell, and decreased to ~20°. The distal end was rotund or spatulate depending on the individual, and it showed, at least, a spinule (Fig. 5d). The other two posterior processes were shorter and located in the left hypotheca (Fig. 5j & p). They can be interpreted as result of the bifurcation of the antapex. One process was shorter (named short posterior process), 10–11-μm long, emerged from a more ventral position and was oriented towards the left side of the cell (Fig. 5f–h). The other process was longer (15-μm long, named long posterior process), located in the antapex and it was oriented towards the left side with more angle than the ventro-posterior process. The distal end of the long posterior process showed, at least, a spinule (Fig. 5h). The cell theca was covered by poroids, apparently absent from the posterior processes (Fig. 5b–c). The inner hypotheca showed corpuscles in distinct number and size, suggesting a heterotrophic nutrition (Fig. 5k).
Currently, the genus Dinofurcula contains two species: D. ultima (Kof.) Kof. & Skogsb. (Fig. 6c) and D. ventralis Kof. & Skogsb. (Fig. 6d). The former showed a large, rounded hump on the ventral side of the epitheca and the sulcus in a lateral position (Fig. 6c). These species possessed two processes, one is ventro-posterior and other one is antapical. In contrast, D. tricornuta showed two antapical process (Fig. 6a–b). The records of D. ultima and D. ventralis are restricted to the eastern tropical Pacific. This is the first record of the genus Dinofurcula in another ocean region.
4 DISCUSSIONMore than one century ago, plankton observations from open ocean research cruises were associated with the descriptions of new dinoflagellate species. For example, Kofoid and collaborators described more than 300 species of thecate dinoflagellates from several cruises in the eastern tropical Pacific Ocean (Kofoid, 1907; Kofoid and Michener, 1911; Kofoid and Skogsberg, 1928). In contrast, few new species of dinoflagellates have been described from open ocean research cruises in the last two decades (Gómez, 2007; Hernández-Becerril et al., 2008; Parra-Toriz et al., 2014; Esqueda-Lara and Hernández-Becerril, 2017). This should not be interpreted as all the existing dinoflagellates already having been described. In the last two decades, there has been an increase in the description of new species of neritic photosynthetic dinoflagellates collected near specialized laboratories and cultivated in standard culture media. The abundant material from the cultures allows detailed studies of the morphology and molecular phylogeny, but most of these new species lack distinctive features and cannot be easily recognized in routine plankton microscopy observations. In contrast, oceanic species inhabiting in the aphotic zone are heterotrophic, with unknown nutritional and environmental requirements, and are uncultivable with standard protocols. Due to low abundances, observations are often restricted to single or few individuals, making detailed morphological and molecular studies extremely difficult. Despite their distinctive morphologies, these species suffer a disadvantage compared to abundant and/or cultured organisms with respect to the process of new species descriptions. The consequence is that our knowledge of the biodiversity of dinoflagellates below the euphotic zone remains largely restricted to the observations by Kofoid and collaborators (Kofoid, 1907; Kofoid and Michener, 1911; Kofoid and Skogsberg, 1928).
Dinoflagellates typical of subphotic depths are the dinophysoid genera Triposolenia and Dinofurcula, with highly distinctive generic diagnostic characters and easily recognizable in routine plankton observations. The records of most of the species of Triposolenia are Kofoid's original descriptions, and there are only two records of Dinofurcula species after Kofoid. In this study, three distinctive species of dinophysoid dinoflagellates are described after the observation of plankton concentrates from large volumes of seawater. This suggests a reservoir of undocumented biodiversity that remains in subphotic depths.
The dinophysoid dinoflagellates have received considerable attention in neritic waters because several chloroplast-containing species of the genus Dinophysis Ehrenb. are responsible of toxic events. Dinophysis profunda sp. nov. belongs to the D. hastata-group that contains heterotrophic species with an antapical spine, often with a high developed left sulcal list (Gómez et al., 2011; Esqueda-Lara et al., 2013). These species inhabit in the oligotrophic open ocean. The closest morphologic relatives of Dinophysis profunda sp. nov. are D. alata (Fig. 4c; Jørgensen, 1923) and D. balechii (Fig. 4d; Norris and Berner, 1970). The description of Dinophysis profunda collected at 500-m depth is the deepest recorded in the last century.
The records of the genus Dinofurcula were restricted to the eastern tropical Pacific, especially the productive Peruvian Current (Kofoid and Skogsberg, 1928). More recent records of Dinofurcula from surface samples are associated with the upwelling of deep waters (Hernández-Becerril and Bravo-Sierra, 2004; Ochoa and Baylón, 2005). Hernández-Becerril and Bravo-Sierra (2004) found several individuals in a single sample from a eutrophic site associated with deep water upwelling. In this study, three individuals were collected in a single sample collected at 154-m depth, near the bottom, in the eutrophic waters of the Marmara Sea. This suggests that the species of Dinofurcula may reach a relatively important local abundance below eutrophic waters. Taxonomic studies of dinoflagellates in the Marmara Sea are frequent, but mostly restricted to surface waters (Balkis, 2000).
The new species of Dinofurcula showed three posterior-oriented processes, while the other species of this genus have only two processes. The "tripartite" outline of D. tricornuta sp. nov. converges with Triposolenia, but the genera Metaphalacroma L. S. Tai and Skogsb. and Pseudophalacroma Jørg. are characterized by a crest-like structure in the epitheca. Scanning electron microscope observations of Dinofurcula cf. ultima revealed the presence of a crest on the epitheca, already cited in the original species description (Hernández-Becerril and Bravo-Sierra, 2004). This suggests that Dinofurcula could be related with the clade of Pseudophalacroma/Metaphalacroma rather than to the clade of Phalacroma F. Stein within the main clade of the Dinophysales. Species of Pseudophalacroma or Metaphalacroma do not have processes or body extensions; however, the presence of processes are not always associated with genetic differences. For example, the species Dinophysis caudata Kent, D. tripos Gourret and D. miles Cleve are characterized by distinct degrees of development of a dorsal process, while these species share identical SSU rRNA gene sequences.
The records of Amphisolenia are more common than those of Triposolenia and Dinofurcula. Amphisolenia shows a large vertical distribution, with species containing endosymbiotic microalgae. Amphisolenia sp. aff. brevicauda was collected at 70-m depth, but this single record does not allow comment on potential trends regarding the vertical distribution. The closely related species A. brevicauda is found in surface waters (Figs. 1e–h & 2b–d; Wood 1963a, b). The most common species of Amphisolenia (i.e., A. bidentata Schröd.) are strongly antero-posteriorly elongated. These species have little differentiation between the outlines of midbody, neck and antapical process, but the end of the antapical process showed a distinct angle, named the foot, and the end had distal spinules or other ornamentations (Kofoid and Skogsberg, 1928). In contrast, Amphisolenia sp. aff. brevicauda belongs to a less common group of species with a clearly differentiated midbody, and a short antapical process with a simple morphology, lacking bifurcations or spines. The thicker midbody of A. brevicauda, A. inflata, and Amphisolenia sp. aff. brevicauda suggest that these species are basal in the clade of Amphisolenia because they are closer to the morphology of Triposolenia. Unfortunately, molecular data are not available to test that hypothesis.
5 CONCLUSIONThe subphotic depths are the unexplored frontier in the diversity of life on Earth. Despite the low volume of sample, this study suggests a rich undocumented biodiversity. There are environmental sequencing surveys in subphotic depths, but they are rarely accompanied with microscope observations by qualified observers. The dinoflagellates inhabiting subphotic depths are at low abundance, but these species are not necessarily rare. What is rare is the opportunity to collect and examine samples where these species are located.
6 DATA AVAILABILITY STATEMENTAll data generated and/or analyzed during this study are included in this published article.
7 ACKNOWLEDGMENTI thank P. López-García for sampling, and L. Gasperini and G. Bortoluzzi of the Istituto di Geologia Marina (ISMAR), CNR, Bolonia (Italy) for allowing to participate in the MARM10_02 R/V Urania cruise. I thank R. J. Gast for her improvements in the text.
Balkis N. 2000. Five dinoflagellate species new to Turkish Seas. Oebalia, 26: 97-108.
|
Dolan J R, Ciobanu M, Marro S, Coppola L. 2019. An exploratory study of heterotrophic protists of the mesopelagic Mediterranean Sea. ICES Journal of Marine Science, 76(3): 616-625.
DOI:10.1093/icesjms/fsx218 |
Esqueda-Lara K, Parra-Toriz D, Hernández-Becerril D U. 2013. Morphology and taxonomy of Dinophysis species of the section Hastata (Dinoflagellata), including the description of Dinophysis conjuncta sp. nov., from the Mexican marine waters. Journal of the Marine Biological Association of United Kingdom, 93(5): 1 187-1 202.
DOI:10.1017/S0025315412001750 |
Esqueda-Lara K, Hernández-Becerril D U. 2017. Two new species of the dinoflagellate genus Phalacroma Stein (Dinophyceae) from the tropical Mexican Pacific. Nova Hedwigia, 105(3-4): 301-312.
DOI:10.1127/nova_hedwigia/2017/0411 |
Gómez F. 2003. Checklist of Mediterranean free-living dinoflagellates. Botanica Marina, 46(3): 215-242.
DOI:10.1515/BOT.2003.021 |
Gómez F. 2007. Gynogonadinium aequatoriale gen. et sp. nov., a new dinoflagellate from the open western equatorial Pacific. Algae, 22(1): 11-15.
DOI:10.4490/algae.2007.22.1.011 |
Gómez F. 2012. A checklist and classification of living dinoflagellates (Dinoflagellata, Alveolata). CICIMAR Oceánides, 27(1): 65-140.
DOI:10.37543/oceanides.v27i1.111 |
Gómez F, López-García P, Moreira D. 2011. Molecular phylogeny of dinophysoid dinoflagellates: the systematic position of Oxyphysis oxytoxoides and the Dinophysis hastata group (Dinophysales, Dinophyceae). Journal of Phycology, 47(2): 393-406.
DOI:10.1111/j.1529-8817.2011.00964.x |
Gómez F, Moreira D, López-García P. 2012. Sinophysis and Pseudophalacroma are distantly related to typical dinophysoid dinoflagellates (Dinophysales, Dinophyceae). Journal of Eukaryotic Microbiology, 59(2): 188-190.
DOI:10.1111/j.1550-7408.2011.00598.x |
Hernández-Becerril D U, Bravo-Sierra E. 2004. Observations on a rare planktonic dinoflagellate, Dinofurcula cf. ultima (Dinophyceae), from the Mexican Pacific. Phycologia, 43(4): 341-345.
DOI:10.2216/i0031-8884-43-4-341.1 |
Hernández-Becerril D U, Ceballos-Corona J G A, Esqueda-Lara K, Tovar-Salazar M A, León-Álvarez D. 2008. Marine planktonic dinoflagellates of the Order Dinophysiales (Dinophyta) from coasts of the tropical Mexican Pacific, including two new species of the genus Amphisolenia. Journal of the Marine Biological Association of the United Kingdom, 88(1): 1-15.
DOI:10.1017/S0025315408000143 |
Jørgensen E. 1923. Mediterranean Dinophysiaceae. In: Schmidt J ed. Ⅱ Biology J. 2. Report on the Danish Oceanographical Expeditions 1908–10 to the Mediterranean and Adjacent Seas. A. F. Høst, Copenhagen. p. 1–48.
|
Kofoid C A. 1907. New species of Dinoflagellates. Bulletin of the Museum of Comparative Zoology at Harvard College, 50(1): 161-207.
|
Kofoid C A, Michener J R. 1911. New genera and species of dinoflagellates. Bulletin of the Museum of Comparative Zoology at Harvard College, 54(7): 265-302.
|
Kofoid C A, Skogsberg T. 1928. The Dinoflagellata: the Dinophysoidae. Memoires of the Museum of Comparative Zoology at Harvard College, 51(1): 1-766.
|
Norris D R, Berner L D Jr. 1970. Thecal morphology of selected species of Dinophysis (Dinoflagellata) from the Gulf of Mexico. Contributions in Marine Science, 15: 145-192.
|
Ochoa N, Baylón M. 2005. Dinofurcula cf. ventralis en la costa central del Perú y primeros registros de dos especies de Protoperidinium. Revista Peruana de Biología, 12(3): 377-382.
DOI:10.15381/rpb.v12i3.2413 |
Parra-Toriz D, Hernández-Becerril D U, Esqueda-Lara K. 2014. Phalacroma gibbosum sp. nov. (Dinophyceae) from the southern Gulf of Mexico. Nova Hedwigia, 99(1-2): 83-96.
DOI:10.1127/0029-5035/2014/0189 |
Sogin M L, Morrison H G, Huber J A, Welch D M, Huse S M, Neal P R, Arrieta J M, Herndl G J. 2006. Microbial diversity in the deep sea and the underexplored "rare biosphere". Proceedings of the National Academy of Sciences of the United States of America, 103(32): 12 115-12 120.
DOI:10.1073/pnas.0605127103 |
Wood E J F. 1963a. Dinoflagellates in the Australian region. Ⅱ. Recent collections. Technical Paper Division of Fisheries and Oceanography CSIRO Australia, 14: 1-55.
|
Wood E J F. 1963b. Dinoflagellates in the Australian region. Ⅲ. Further collections. Technical Paper Division of Fisheries and Oceanography CSIRO Australia, 17: 1-20.
|
 2022, Vol. 40
2022, Vol. 40


