Institute of Oceanology, Chinese Academy of Sciences
Article Information
- Gao Xinming, Yang Haiyan, Tang Daojun, Du Chen, Jin Shan, Hou Congcong, Zhang Chundan, Zhu Junquan, Wang Jianping
- Physiological and histological responses of Phascolosoma esculenta (Sipuncula: Phascolosomatidea) to acute heat stress
- Journal of Oceanology and Limnology, 40(2): 643-655
- http://dx.doi.org/10.1007/s00343-021-1013-1
Article History
- Received Jan. 11, 2021
- accepted in principle Mar. 22, 2021
- accepted for publication Apr. 19, 2021
2 Ningbo Academy of Oceanology and Fisheries, Ningbo, 315012, China
Temperature is an important factor affecting the physiology, energy expenditure, growth, development, and survival of organisms, especially aquatic organisms, which are mostly poikilotherms (Selong et al., 2001). In such organisms, water temperature fluctuations result in directly corresponding changes in body temperature, which result in they are easily exposed in temperature stress, and the effects of high temperature on these organisms have received increasing attention. To date, physiological responses and tissue structures are reported to be affected in aquatic organisms such as fish (Liu et al., 2014; Yan et al., 2017), shrimp (Madeira et al., 2015), crabs (Meng et al., 2014), mollusks (An and Choi, 2010; Jiang et al., 2016), and echinodermata (Xu et al., 2015).
It has been reported that aquatic organisms exposed to heat stress overproduce endogenous reactive oxygen species (ROS; including O2-, H2O2, and -OH; Meng et al., 2014; Cheng et al., 2015). Under normal physiological conditions, ROS are in dynamic equilibrium, their generation balanced by their elimination through antioxidant systems. However, under stress, including high temperature stress, this dynamic equilibrium fails as excess ROS is generated, causing oxidative stress. This is lethal for cells, as excess ROS can damage cell DNA, proteins, and lipids (Halliwell, 1999; Simon et al., 2000). Under these conditions, oxidized lipids decompose and finally generate malondialdehyde (MDA; Farmer and Muelle, 2013; Jiang et al., 2016). MDA was therefore deemed a suitable biomarker for the assessment of cellular and histological oxidative damage and organism-level stress. The key physiological response is the increase in antioxidant activities, including O2- removal by superoxide dismutase (SOD) and catalysis of H2O2 reduction by glutathione peroxidase (GPX). These activities, at the appropriate level, eliminate excess ROS, protecting cells and preventing damage to cellular functions (Kashiwagi et al., 1997; Verlecar et al., 2007). The expression of heat shock proteins (HSPs) is also upregulated, protecting other newly synthesized proteins, restoring impaired proteins, and regulating the degradation of unrecoverable damaged proteins (Mosser et al., 1997; Kiang and Tsokos, 1998; Chen et al., 2018). The levels of antioxidant activity and hsp gene expression can therefore reflect the adaptability of an organism to high temperature, acting as biomarkers of stress. Furthermore, the exposure of aquatic organisms to heat stress affects their immunological defense capacity, reflected by changes in immune-related enzyme activity, such as that of acidic phosphatase (ACP) and alkaline phosphatase (AKP; Liu et al., 2004; Chen et al., 2007a, b ).
Although animals have diverse physiological mechanisms enabling adaptation to heat stress, cellular and histological damage will occur when the stress exceeds the organism's coping ability. Examples of damage resulting from heat stress include: nuclear shrinkage and pyknosis in the columnar epithelial cells of the intestine in Apostichopus japonicus (Xu et al., 2015); damage to gill tissue involving lamellar fusion, lamellar aneurism, epithelial lifting, and hyperplasia in Paralichthys olivaceus (Liu et al., 2014); and swelling of muscle cells and increased space between muscle cells in Lophiosilurus alexandri (Martins et al., 2014). These pathological changes in the histological structure represent further detectable biomarkers for stress assessment. Further, they could provide pointers for the screening of genes for resistant forms that prevent histological damage.
The phylum Sipuncula contains worm-like marine invertebrates that are widely distributed globally, from polar to tropical oceans and from the intertidal zone to the deep sea (Lan et al., 2007). To our knowledge, the physiological and histological responses of sipunculids to high temperature stress are currently unknown. This limits our understanding of the effects of temperature stress on these organisms. The sipunculid Phascolosoma esculenta is commonly found in the intertidal zone around Southeast China and farmed in aquaculture operations around the Zhejiang, Fujian, and Guangxi regions for consumption as traditional seafood with high nutritional and medicinal value. However, suitable culture temperatures and an understanding of the physiological mechanisms underlying the response of P. esculenta to temperature stress remain unclear.
In this study, we selected P. esculenta as a model to investigate the effects of acute high temperature stress on sipunculids. Specifically, we measured its physiological responses in terms of MDA concentration, activities of antioxidant and immunityrelated enzymes, and hsp70 and hsp90 gene expression as well as its histological structure, with the aim of uncovering elements of its physiological mechanisms for heat stress adaptation. The results are novel in terms of the phylum studied and provide a series of usable indicators for practical heat stress testing for ecology and aquaculture purposes.
2 MATERIAL AND METHOD 2.1 Experimental animalPhascolosoma esculenta individuals were captured from the Xizhou of Ningbo (Zhejiang Provinces, China). They had a mean body weight of 4.3±0.9 g; they were acclimated for 1 week in filtered and aerated seawater at a temperature of 20 ℃ and salinity of 23.0.
2.2 Experimental design and sample collectionFour experimental groups and a control group consisted of 50 P. esculenta individuals each contained in separate 12.5-L temperature-controlled tanks filled with filtered seawater. Control tanks were maintained at 20 ℃, and experimental tanks were maintained at 25 ℃, 30 ℃, 35 ℃, or 40 ℃; each condition was simultaneously maintained in triplicate. Only P. esculenta exhibiting normal behavior were used. During the experiments, the seawater in the tank was exchanged daily with isothermal fresh seawater. Six randomly-selected individuals were collected from each tank after 3, 6, 12, 24, 48, 72, and 96 h. The coelom fluid, intestine, body wall, retractor muscle, and nephridium were extracted by dissection and immediately transferred into RNAse-free centrifuge tubes for rapid freezing in liquid nitrogen and storage at -80 ℃ until later detection of enzyme activity and gene expression. The intestine, body wall, retractor muscle, and nephridium from P. esculenta in the control and 40-℃ groups were dissected from individuals collected after 96 h, cut into cubes, and fixed for 24 h in Bouin's fixative (containing 75 mL of saturated picric acid buffer solution, 25 mL of 40% formaldehyde, and 5 mL of 100% acetic acid). Thereafter, the samples were rinsed in 70% ethanol and stored until use.
2.3 Determination of MDA content and enzyme activitiesCoelom fluid was homogenized using an Ultra Turrax Homogenizer (IKA, Staufen, Germany), combining 1 g of tissue with 9 mL of 0.9% normal saline in an ice bath, and then centrifuged at 956 ×g for 10 min at 4 ℃. The supernatant was collected and used to measure the content of MDA and the activity levels of SOD, GPX, ACP, and AKP. MDA concentrations and enzyme activity levels were measured using assay kits from the Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
2.4 Histological analysisThe fixed and rinsed samples described in Section 2.2 were embedded in paraffin and cut into 7-μm sections. These sections were stained with standard hematoxylin-eosin and then observed and photographed under a BX51 light microscope (Olympus, Tokyo, Japan) to analyze the cellular and histological damage.
2.5 Real-time quantitative PCR (qPCR) analysisTotal RNA was extracted from the frozen samples described in Section 2.2 using TRIzol reagent (Invitrogen, Carlsbad, USA) and reverse-transcribed using the Prime Script™ RT reagent kit with gDNA Eraser (TaKaRa, Japan) to obtain first-strand cDNA. The primers used in this study are listed in Table 1. The gapdh gene was used as the internal control (Su et al., 2010). The hsp70-F/R primers were synthesized based on Su et al. (2010) and the hsp90-F/R primers were designed based on the hsp90 cDNA sequence (GenBank accession No: GQ503177.1). The Expression levels of hsp70 and hsp90 mRNA were quantified by qPCR using SYBR Green Master I (Roche, Basel, Switzerland) and analyzed on the Roche LightCycler480 System. The thermal cycling program was as follows: activation at 95 ℃ for 3 min, followed by 40 cycles of 95 ℃ for 20 s, 60 ℃ for 30 s, and 72 ℃ for 20 s. Three biological replicates were measured for each group, and all samples were examined in triplicate on the same plate. The expression levels of hsp70 and hsp90 were calculated by the 2-ΔΔCt method. mRNA expression levels were calculated relative to the control group samples treated with 20 ℃.
All data are expressed as the mean±standard deviation of three replicates. Statistical analysis was performed using SPSS v19.0 software (IBM Corp., Armonk, USA). The differences were analyzed using one-way analysis of variance (ANOVA). P < 0.05 were considered statistically significant.
3 RESULT 3.1 Coelom fluid MDA concentrationAs shown in Fig. 1, the coelom fluid MDA concentration initially increased and then decreased in all four experimental groups; at 48 h, the MDA concentration was 2.51-, 4.41-, 5.34-, and 6.06-fold greater than that in the control group for the 25-℃, 30-℃, 35-℃, and 40-℃ stress groups, respectively (P < 0.01). The MDA concentrations in all of the experimental groups remained significantly higher than that in the control group after 96 h of heat stress (P < 0.05). This result indicates that acute heat stress results in oxidative stress in P. esculenta.

|
| Fig.1 Malondialdehyde (MDA) content of the coelom fluid of P. esculenta under different temperature conditions Data presented are the mean±SD of three replicates. Asterisks represent statistically significant differences from the control group (*P < 0.05, **P < 0.01) obtained using one-way analysis of variance. |
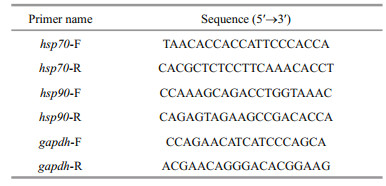
As shown in Fig. 2, the coelom fluid SOD activity increased first and then decreased in the experimental groups. In the 40-℃ group, it peaked after 6 h (1.43- fold greater than that in the control, P < 0.01) and decreased thereafter. In the other three experimental groups, peak activity levels of SOD were observed after 12 h (1.32-, 1.48-, and 1.52-fold greater than that in the control for the 25-℃, 30-℃, and 35-℃ experimental groups, respectively; P < 0.01). Similarly, GPX activity also first increased and then decreased in all the acute heat stress groups. Peak activity levels of GPX were observed after 12 h (1.33-, 1.41-, 1.38-, and 1.45-fold greater than that in the control for the 25-℃, 30-℃, 35-℃, and 40-℃ experimental groups, respectively; P < 0.01). Antioxidant enzymes were thus demonstrated to have increased; they may play a role during acute heat stress in P. esculenta.
|
| Fig.2 Changes of superoxide dismutase (SOD) (a) and glutathione peroxidase (GPX) (b) activity in the coelom fluid of P. esculenta under different temperature conditions Data presented are the mean±SD of three replicates. Asterisks represent statistically significant differences from the control group (*P < 0.05, **P < 0.01) obtained using one-way analysis of variance. |
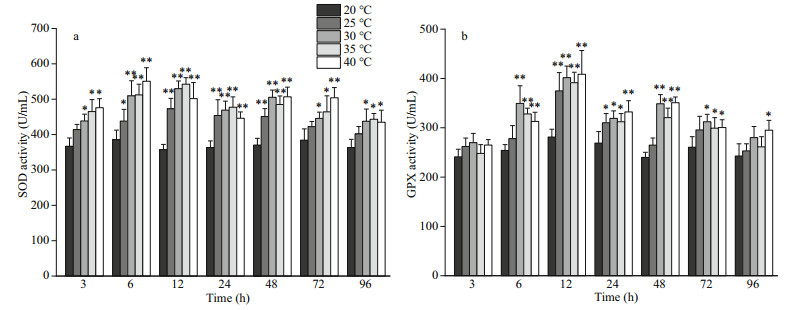
The qPCR results showed that hsp70 and hsp90 mRNA were present in the coelom fluid, intestine, retractor muscle, nephridium, and body wall (Fig. 3). The expression level of hsp70 mRNA in the coelom fluid was the highest, followed in order by those of the intestine, retractor muscle, nephridium, and body wall (Fig. 3a). The expression of hsp90 mRNA in the coelom fluid was the highest; it was lower in the intestine and retractor muscle and the lowest in the body wall and nephridium (Fig. 3b).
|
| Fig.3 Expression of hsp70 (a) and hsp90 (b) mRNA in different tissues of P. esculenta Relative expression values used coelom fluid levels as the baseline. Data presented are the mean±SD of three replicates. Abbreviations: CF: coelom fluid; I: intestine; RM: retractor muscle; N: nephridium; BW: body wall. |
As shown in Fig. 4, upon exposure to high temperature, the hsp70 and hsp90 mRNA expression in all the experimental groups was significantly upregulated in the coelom fluid and intestine. Compared to that in the control, the expression of hsp70 mRNA in the coelom was 1.59-fold higher after 6 h at 25 ℃, 3.09-fold higher after 48 h at 30 ℃, 3.56-fold higher after 48 h at 35 ℃, and 4.87-fold higher after 48 h at 40 ℃ (Fig. 4a). Compared to that in the control, the expression of hsp70 mRNA in the intestine was 1.62-fold higher after 6 h at 25 ℃, 2.37- fold higher after 6 h at 30 ℃, 2.96-fold higher after 24 h at 35 ℃, and 3.16-fold higher after 24 h at 40 ℃ (Fig. 4c). In the 35-℃ and 40-℃ groups, the expression of hsp70 mRNA was still significantly higher than that in the control group in both the coelom fluid and intestine after 96 h of stress (Fig. 4a & c; P < 0.01). Similarly, the coelom fluid and intestinal hsp90 mRNA expression in all the experimental groups was significantly upregulated following exposure to acute heat stress. Compared to that in the control, the hsp90 mRNA expression in the coelom was 1.69-fold higher after 6 h at 25 ℃, 2.68-fold higher after 6 h at 30 ℃, 2.45-fold higher after 24 h at 35 ℃, and 2.78-fold higher after 24 h at 40 ℃ (Fig. 4b). Compared to that in the control, the hsp90 mRNA expression in the intestine was 1.37-fold higher after 48 h at 25 ℃, 1.81-fold higher after 48 h at 30 ℃, 3.33-fold higher after 48 h at 35 ℃, and 3.90-fold higher after 48 h at 40 ℃ (Fig. 4d). In the 35-℃ and 40-℃ groups, the coelom fluid and intestinal expression of hsp90 mRNA was still significantly higher than that in the control group after 96 h of stress (Fig. 4b & d; P < 0.01). These increases suggest that heat shock protein (HSP) have an important role in the response to acute heat stress in P. esculenta.

|
| Fig.4 Expression of hsp70 (a & c) and hsp90 (b & d) mRNA in the coelom fluid and intestine of P. esculenta under different temperature conditions Data presented are the mean±SD of three replicates. Relative expression values used the control group at 3 h as a baseline. Asterisks represent statistically significant differences from the control group (*P < 0.05, **P < 0.01) obtained using one-way analysis of variance. |
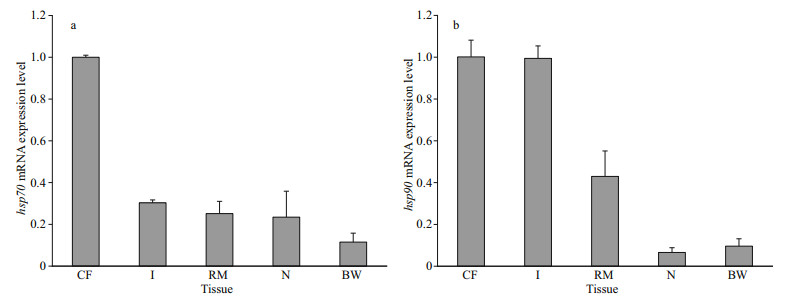
As shown in Fig. 5, the ACP and AKP activity levels in coelom fluid first increased and then decreased in all the experimental groups. In the 40-℃ experimental group, ACP and AKP activity peaked after 24 h (2.41- and 3.49-fold greater values than that in the control, respectively (P < 0.01)). In the other three treatment groups, peak activities of ACP and AKP were observed after 48 h. ACP activity peaks were 1.77-, 1.91-, and 2.2-fold greater than that in the control in the 25-℃, 30-℃, and 35-℃ groups, respectively (P < 0.01). For AKP, these were 2.29-, 2.89-, and 3.17-fold greater than that in the control for the 25-℃, 30-℃, and 35-℃ experimental groups, respectively (P < 0.01). This result indicates that acute heat stress affects the nonspecific immunity of P. esculenta.
|
| Fig.5 Changes in acidic phosphatase (ACP) (a) and alkaline phosphatase (AKP) (b) activity in the coelom fluid of P. esculenta under different temperature conditions Data presented are the mean±SD of three replicates. Asterisks represent statistically significant differences from the control group (*P < 0.05, **P < 0.01) obtained using one-way analysis of variance. |
The body wall of P. esculenta is composed of the cuticle, epithelial layer, and muscularis (Fig. 6a & b). Its retractor muscle consists of smooth muscle fibers (Fig. 6e & f). In the control group, the muscle cells were bundled tightly in the muscularis of the body wall and retractor muscle (Fig. 6a & e); the nuclei of the columnar cells in the body wall epithelium and the muscle cells in the retractor muscle were oval, with the chromatin in the form of small, scattered plaques in the nucleus (Fig. 6c & g). However, after 96 h of stress at 40 ℃, crevices were observed between the muscle cells in the body wall and retractor muscle (Fig. 6b & f). In addition, the chromatin was condensed around the nuclear membrane, and a round basophilic granule was present in the nuclear core of the columnar cells in the body wall epithelium and the retractor muscle cells (Fig. 6d & h).
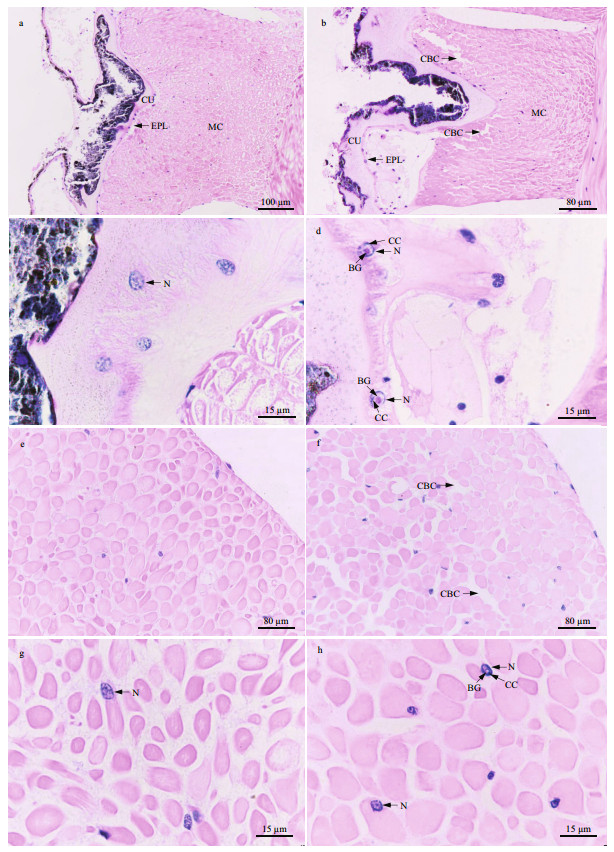
|
| Fig.6 Light micrograph showing the histological structure of the body wall and retractor muscle a, c. body wall in control group; b, d. body wall in 40-℃ group; e, g. retractor muscle in control group; f, h. retractor muscle in 40-℃ group. CU: cuticle; EPL: epithelial layer; MC: muscularis; N: nucleus; CBC: crevice between cells; CC: condensed chromatin around nuclear membrane; BG: basophilic granule. |
The intestinal wall of P. esculenta is composed of serosa, muscularis, submucosa, and mucosa. In the control group, the columnar epithelial cells in the mucosa were columnar; their nuclei were round or oval and located near the base of the cells, with distributed small plaques of chromatin (Fig. 7a, c, & e). However, after 96 h of stress at 40 ℃, the shape of the columnar epithelial cells in the mucosa was irregular, and some apoptotic body-like components were observed (Fig. 7b, d, & f). Furthermore, the chromatin was condensed around the border of the nuclear membranes, and a round basophilic granule was present in the nuclear core of the columnar epithelial cells (Fig. 7f). This was similar to the pattern observed in the body wall and muscle cells described in Section 3.6.1.
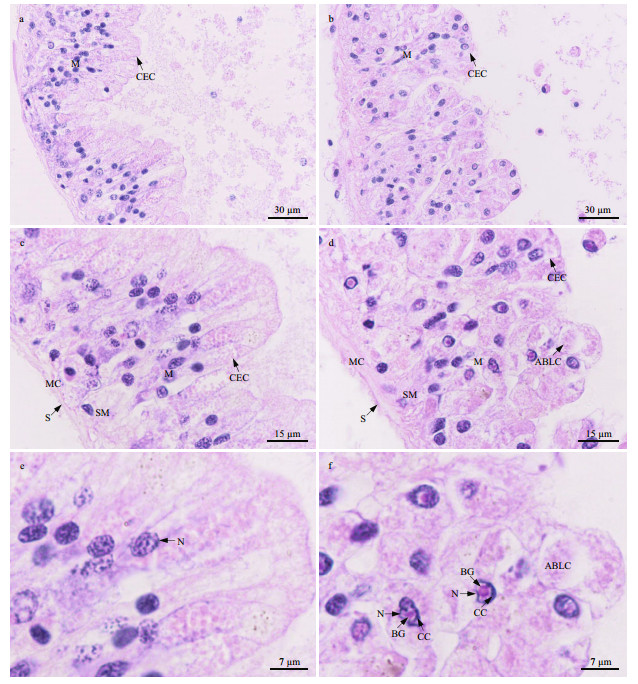
|
| Fig.7 Light micrograph showing intestinal histological structure a, c, e. intestine in control group; b, d, f: intestine in 40-℃ group. CEC: columnar epithelial cells; S: serosa; MC: muscularis; SM: submucosa; M: mucosa; N: nucleus; CC: condensed chromatin around nuclear membrane; BG: basophilic granule; ABLC: apoptotic body-like components. |
The nephridial wall of P. esculenta is composed of flask-shaped infoldings, muscularis, and epithelium. In the control group, the columnar epithelial cells in the epithelium and the cuboidal epithelial cells in the flaskshaped infoldings were fascicled, with substantial amounts of particulate matter in the cytoplasm (Fig. 8a, c, & e); their nuclei were ovate with chromatin plaques (Fig. 8c & e). However, after 96 h of stress at 40 ℃, the boundary between some epithelial cells was blurred (Fig. 8b, d, & f); damage to the cell membrane resulted in the outflow of granular matter with vacuolization at the top of some columnar epithelial cells (Fig. 8b & f). In addition, some cells had irregularly shaped nuclei in which condensed chromatin was gathered around the nuclear membrane; a basophilic granule was also observed in the nuclear core (Fig. 8d & f).
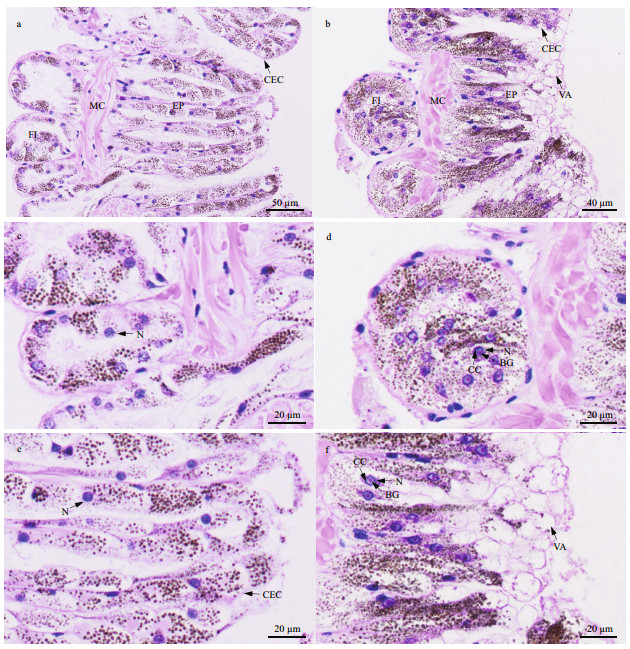
|
| Fig.8 Light micrograph showing nephridial histological structure a, c, e. nephridium in control group; b, d, f. nephridium in 40-℃ group. FI; flask-shaped infoldings; MC: muscularis; EP: epithelium; CEC: columnar epithelial cells; N: nucleus; CC: condensed chromatin around nuclear membrane; BG: basophilic granule; VA: vacuolization at the top of some columnar epithelial cells. |
Heat stress can lead to excess ROS generation, causing oxidative stress and damage to biological molecules, including the polyunsaturated fatty acids in the cell membranes (Park et al., 2009). The final product of lipid oxidation is MDA, which has therefore become a commonly used indicator to evaluate lipid peroxidation and oxidative stress. The antioxidant defense system plays an important role in scavenging ROS to reduce such oxidative stress (Kashiwagi et al., 1997; Verlecar et al., 2007). The data in our study showed that MDA concentrations were universally higher in the coelom fluid after 24 h of heat stress, compared to the control, demonstrating that lipid peroxidation and oxidative stress occurred under these conditions in P. esculenta. Similar results have been reported in crabs, mollusks, and fish, namely Eriocheir sinensis (Hong et al., 2007), Crassostrea gigas (Park et al., 2009), and Oreochromis niloticus (Qiang et al., 2012), following exposure to high temperature stress. The cascade of events is considered to start with a raised body temperature, caused by higher water temperatures, which, in turn, increases oxygen consumption. This then triggers mitochondrial damage and disturbances to the oxidation-reduction processes, resulting in overproduction and accumulation of ROS (Verlecar et al., 2007). It is noteworthy that the raised MDA concentrations were not sustained; the levels decreased following prolonged stress in P. esculenta. This phenomenon has also been reported in Scapharca broughtonii (An and Choi, 2010). This reduction in oxidative stress may indicate the involvement of the antioxidant system.
In the present study, we observed that the activities of the antioxidant enzymes SOD and GPX were significantly increased in coelom fluid under acute heat stress (Fig. 2). SOD functions during the first step of ROS elimination, converting intracellular O2- to H2O2 and O2 (Downs et al., 2001). H2O2 is then broken down by GPX, thus reducing its toxic effect (Verlecar et al., 2007). Our results therefore reflect the important roles of SOD and GPX in heat stress: scavenging the ROS (in the forms of O2- and H2O2) to protect P. esculenta by reducing the cellular damage caused by heat stress. In fact, in many aquatic organisms in both vertebrate and invertebrate taxa, such as Chlamys farreri (Chen et al., 2007a, b ), Takifugu obscurus (Cheng et al., 2015), and S. broughtonii (An and Choi, 2010), similar increased activities of SOD and GPX induced by heat stress have been detected. Antioxidant enzymes could therefore be universally involved in protecting against heat stress in animals. The genes for such enzymes have potential as heat-resistance genes and are worth investigating in this regard in future research.
4.2 Expression of hsp70 and hsp90Heat shock proteins are important molecular chaperones that play critical roles in preventing protein denaturation, refolding denatured protein, and removing irreversibly damaged proteins (Peng et al., 2016). Among the HSP families, HSP70 and HSP90 are generally reported to be significantly upregulated under heat stress. For example, the upregulated expression of hsp70 or hsp90 or both was reported in Misgurnus anguillicaudatus (Yan et al., 2017), Huso dauricus (Peng et al., 2016), T. obscurus (Cheng et al., 2015), and Megalobrama amblycephala (Zhang et al., 2014) under high temperature stress. In the present study, we detected the upregulated expression of hsp70 and hsp90 in the coelom fluid and intestine of heatstressed P. esculenta (Fig. 4). The widespread reporting of this observation indicates that it plays an important role in the adaptation of animals to heat stress.
Given that heat stress causes excess ROS generation and oxidative stress, which damage proteins, and considering the chaperone functions of HSP70 and HSP90, we infer that high expression levels of HSPs could enable the repair of denatured proteins and the removal of irreversibly damaged proteins. These functions are undoubtedly important for cell homeostasis and the protection of cells from damage. The HSP70 and HSP90, therefore, complement the antioxidant system to make the organism adapting to the oxidative stress. Furthermore, HSP70 is also reported to be a negative regulator of apoptosis, acting to restrain the signaling cascade initiating the apoptotic process (Beere and Green, 2001; Ravagnan et al., 2001). Cellular apoptosis in response to heat stress was reported in aquatic animals (Cheng et al., 2015); thus, this could be the mechanism by which high HSP70 expression levels are associated with heat tolerance in animals. Future testing of the heat tolerance of hsp70- and hsp90-knockout animals will reveal the exact function of these molecular chaperones under heat stress.
4.3 Effect on the nonspecific immune systemIt has been reported that heat stress can influence immunity-related enzyme activity; this is commonly tested in aquatic organisms using ACP and AKP (Liu et al., 2004; Hu et al., 2015). ACP is a typical lysosomal enzyme, involved in killing and digesting microbial pathogens as a component of innate immunity; AKP, usually located in the membrane system, participates in carbohydrate metabolism, growth, and differentiation (Qiang et al., 2012). Both can catalyze the hydrolysis of various phosphatecontaining compounds and participate in the degradation of foreign proteins, carbohydrates, and lipids (Liu et al., 2004; Qiang et al., 2012). They are important indices for the assessment of nonspecific immune system function in animals. Typically, high activity represents high function. In our study, increased activities of AKP and ACP were detected under high temperature stress, especially after 24 h and 48 h (Fig. 5). A similar phenomenon was reported in both Mytilus coruscus (Hu et al., 2015) and Haliotis discus hannai (Jiang et al., 2017). However, in Procambarus clarkii, heat stress resulted in reduced activity (Wang et al., 2012). This variation may be related to the stress intensity and duration or reflect interspecific differences. Regardless, these results reflect the sensitivity of ACP and AKP to temperature variations; in P. esculenta, high temperature affects the nonspecific immune system. Furthermore, it is worth noting that SOD, GPX, HSP70, and HSP90 were also reported to participate in nonspecific immunity (Spallholz, 1990; Fu et al., 2011; Lu et al., 2015; Yan et al., 2017). The observed high activities and abundances of these proteins were likely associated with the nonspecific immune response. Our results certainly suggest that this was the case in P. esculenta. However, to our knowledge, no studies have yet shown that organisms show enhanced resistance to pathogens after high temperature stress. An investigation of the changes in the actual immune capacity under these conditions may be an interesting and fruitful future study.
4.4 Tissue damageHigh temperatures, exceeding the coping ability of animals, will cause cell and tissue damage. Studying the resulting pathological changes can help us understand the failure of animals to adapt successfully to high temperatures at a histological level. Theoretically, the genes that prevent such tissue damage are potential heat-resistance genes, offering a route to identify these through screening. Furthermore, tissue damage can function as a quantitative indicator of the degree of damage. In aquatic organisms, it has been reported that heat stress causes oncotic problems in muscle fibers in the form of distension of the spaces between muscle cells in L. alexandri (Martins et al., 2014), and leads to epithelial cytoplasmic shrinkage, organelle reduction, and condensed chromatin in the intestinal cells of A. japonicus and others (Xu et al., 2015). These results reveal that heat stress can affect the functions of organs involved in movement, food absorption, and digestion. In the present study, we detected tissue damage in the body wall (Fig. 6), retractor muscle (Fig. 6), intestine (Fig. 7), and nephridium (Fig. 8) under 40-℃ stress. The body wall and retractor muscle are related to movement; the intestine functions in food absorption and digestion (Lei et al., 2013); the nephridium functions in excretion and osmotic pressure regulation (Long et al., 2014). The observed damage to these structures confirms such effects in P. esculenta. Notably, a significant feature of damaged cells is condensed chromatin around the nuclear membrane (Figs. 6–8). This is also reported to be a morphological feature of apoptosis (Van Cruchten and Van den Broeck, 2002). Its presence suggests that apoptosis had taken place. However, we did not observe the ultrastructure of the damaged cells, and molecular biological indicators that reveal apoptosis were not evaluated. Therefore, we cannot conclusively state whether apoptosis occurred.
As described above, we detected increased MDA levels, reflecting excess ROS generation. The ROS was, in turn, the primary factor causing lipid peroxidation, resulting in protein denaturation, DNA damage, and finally apoptosis (Simon et al., 2000). It is therefore likely that the probable apoptosis detected in tissues may be related to the ROS. In the future, we will analyze the relationship between ROS level and apoptosis to explore the molecular mechanism underlying tissue damage in depth and thus provide reference information for the screening of heat-stress resistance genes in P. esculenta.
5 CONCLUSIONWe found that exposure to high temperature stress caused oxidative stress, evidenced by the increased activity of the antioxidant enzymes SOD and GPX and the upregulated expression of hsp70 and hsp90 mRNA. These results suggest that the antioxidant system could play an important role in eliminating the ROS generated owing to heat stress. Protein protection could be provided by HSP70 and HSP90 to enhance the tolerance of P. esculenta to high temperatures, forming part of its physiological mechanism of adaptation to such conditions. In addition, the high temperature affected the activity of the immunerelated enzymes ACP and AKP, indicating that the nonspecific immunity of P. esculenta was affected. Furthermore, acute high temperature stress caused tissue damage to its body wall, retractor muscle, intestine, and nephridium, indicating that heat stress could affect the movement, absorption and digestion of food, and excretion process. This study thus contributes to our understanding of the physiological responses of other sipunculids when adapting to high temperatures, using P. esculenta as a model for this phylum. As the biochemical indicators and hsp expression levels used in this study were shown to be sensitive to high temperatures and the cellular damage, such as condensed chromatin around the nuclear membrane, was easy to observe, these could function as useful indicators for evaluating heat stress status and screening optimal culture temperature of P. esculenta in the future.
6 DATA AVAILABILITY STATEMENTThe data of this study are available from the corresponding author upon reasonable request.
An M I, Choi C Y. 2010. Activity of antioxidant enzymes and physiological responses in ark shell, Scapharca broughtonii, exposed to thermal and osmotic stress: effects on hemolymph and biochemical parameters. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 155(1): 34-42.
DOI:10.1016/j.cbpb.2009.09.008 |
Beere H M, Green D R. 2001. Stress management-heat shock protein-70 and the regulation of apoptosis. Trends in Cell Biology, 11(1): 6-10.
DOI:10.1016/S0962-8924(00)01874-2 |
Chen B, Feder M E, Kang L. 2018. Evolution of heat-shock protein expression underlying adaptive responses to environmental stress. Molecular Ecology, 27(15): 3040-3054.
DOI:10.1111/mec.14769 |
Chen M Y, Yang H S, Delaporte M, Zhao S J, Xing K. 2007a. Immune responses of the scallop Chlamys farreri after air exposure to different temperatures. Journal of Experimental Marine Biology and Ecology, 345(1): 52-60.
DOI:10.1016/j.jembe.2007.01.007 |
Chen M Y, Yang H S, Delaporte M, Zhao S J. 2007b. Immune condition of Chlamys farreri in response to acute temperature challenge. Aquaculture, 271(1-4): 479-487.
DOI:10.1016/j.aquaculture.2007.04.051 |
Cheng C H, Yang F F, Liao S A, Miao Y T, Ye C X, Wang A L, Tan J W, Chen X Y. 2015. High temperature induces apoptosis and oxidative stress in pufferfish (Takifugu obscurus) blood cells. Journal of Thermal B ology, 53: 172-179.
DOI:10.1016/j.jtherbio.2015.08.002 |
Downs C A, Fauth J E, Woodley C M. 2001. Assessing the health of grass shrimp (Palaemonetes pugio) exposed to natural and anthropogenic stressors: a molecular biomarker system. Marine Biotechnology, 3(4): 380-397.
DOI:10.1007/s10126-001-0008-3 |
Farmer E E, Muelle M J. 2013. ROS-mediated lipid peroxidation and RES-activated signaling. Annual Review of Plant Biology, 64: 429-450.
DOI:10.1146/annurev-arplant-050312-120132 |
Fu D K, Chen J H, Zhang Y, Yu Z N. 2011. Cloning and expression of a heat shock protein (HSP) 90 gene in the haemocytes of Crassostrea hongkongensis under osmotic stress and bacterial challenge. Fish & Shellfish Immunology, 31(1): 118-125.
DOI:10.1016/j.fsi.2011.04.011 |
Halliwell B. 1999. Antioxidant defence mechanisms: from the beginning to the end (of the beginning). Free Radical Research, 31(4): 261-272.
DOI:10.1080/10715769900300841 |
Hong M L, Chen L Q, Gu S Y, Liu C, Zhang L, Li E C. 2007. Effect of temperature change on immunochemical indexes of Eriocheir sinensis. Chinese Journal of Applied and Environmental Biology, 13(6): 818-822.
(in Chinese with English abstract) |
Hu M H, Li L S, Sui Y M, Li Y M, Wang Y J, Lu W Q, Dupont S. 2015. Effect of pH and temperature on antioxidant responses of the thick shell mussel Mytilus coruscus. Fish & Shellfish Immunology, 46(2): 573-583.
DOI:10.1016/j.fsi.2015.07.025 |
Jiang W W, Fang J G, Li J Q, Jiang Z J, Mao Y Z, Du M R, Gao Z K, Chen Q L. 2017. Effects of temperature change on physiological and biochemical activities of Haliotis discus hannai Ino. Journal of Fishery Sciences of China, 24(2): 220-230.
(in Chinese with English abstract) DOI:10.3724/SP.J.1118.2017.16133 |
Jiang W W, Li J Q, Gao Y P, Mao Y Z, Jiang Z J, Du M R, Zhang Y, Fang J G. 2016. Effects of temperature change on physiological and biochemical responses of Yesso scallop, Patinopecten yessoensis. Aquaculture, 451: 463-472.
DOI:10.1016/j.aquaculture.2015.10.012 |
Kashiwagi A, Kashiwagi K, Takase M, Hanada H, Nakamura M. 1997. Comparison of catalase in diploid and haploid Rana rugosa using heat and chemical inactivation techniques. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 118(3): 499-503.
DOI:10.1016/S0305-0491(97)00216-2 |
Kiang J G, Tsokos G C. 1998. Heat shock protein 70 kDa: molecular biology, biochemistry, and physiology. Pharmacology & Therapeutics, 80(2): 183-201.
DOI:10.1016/S0163-7258(98)00028-X |
Lan G B, Yang S F, Xie T S, Shi D S. 2007. Advance in phyletic studies of the phylum Sipuncula. Guangxi Sciences, 14(2): 186-192.
(in Chinese with English abstract) |
Lei S Y, Lu M M, Ding L F, Zhu J Q. 2013. The morphology of the digestive tract of Phascoloma esculenta. Journal of Biology, 30(2): 33-36.
(in Chinese with English abstract) |
Liu S L, Jiang X L, Hu X K, Gong J, Hwang H, Mai K S. 2004. Effects of temperature on non-specific immune parameters in two scallop species: Argopecten irradians (Lamarck 1819) and Chlamys farreri (Jones & Preston 1904). Aquaculture Research, 35(7): 678-682.
DOI:10.1111/j.1365-2109.2004.01065.x |
Liu Y F, Ma D Y, Zhao C Y, Wang W Q, Zhang X L, Liu X, Liu Y, Xiao Z Z, Xu S H, Xiao Y S, Liu Q H. 2014. Histological and enzymatic responses of Japanese flounder (Paralichthys olivaceus) and its hybrids (P. olivaceus♀ × P. dentatus♂) to chronic heat stress. Fish Physiology and Biochemistry, 40(4): 1031-1041.
DOI:10.1007/s10695-013-9903-6 |
Long L L, Sheng Z, Lu M M, Ding L F, Zhu J Q. 2014. Structural features of musculature in the nephridium of Phascolosoma esculenta. Journal of Biology, 31(2): 9-12.
(in Chinese with English abstract) |
Lu X, Wang C, Liu B Z. 2015. The role of Cu/Zn-SOD and Mn-SOD in the immune response to oxidative stress and pathogen challenge in the clam Meretrix meretrix. Fish & Shellfish Immunology, 42(1): 58-65.
DOI:10.1016/j.fsi.2014.10.027 |
Madeira D, Mendonça V, Dias M, Roma J, Costa P M, Larguinho M, Vinagre C, Diniz M S. 2015. Physiological, cellular and biochemical thermal stress response of intertidal shrimps with different vertical distributions: Palaemon elegans and Palaemon serratus. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 183: 107-115.
DOI:10.1016/j.cbpa.2014.12.039 |
Martins Y S, Melo R M C, Campos-Junior P H A, Santos J C E, Luz R K, Rizzo E, Bazzoli N. 2014. Salinity and temperature variations reflecting on cellular PCNA, IGF-I and II expressions, body growth and muscle cellularity of a freshwater fish larvae. General and Comparative Endocrinology, 202: 50-58.
DOI:10.1016/j.ygcen.2014.03.047 |
Meng X L, Liu P, Li J, Gao B Q, Chen P. 2014. Physiological responses of swimming crab Portunus trituberculatus under cold acclimation: antioxidant defense and heat shock proteins. Aquaculture, 434: 11-17.
DOI:10.1016/j.aquaculture.2014.07.021 |
Mosser D D, Caron A W, Bourget L, Denis-Larose C, Massie B. 1997. Role of the human heat shock protein hsp70 in protection against stress-induced apoptosis. Molecular and Cellular Biology, 17(9): 5317-5327.
DOI:10.1128/MCB.17.9.5317 |
Park M S, Jo P G, Choi Y K, An K W, Choi C Y. 2009. Characterization and mRNA expression of Mn-SOD and physiological responses to stresses in the Pacific oyster Crassostrea gigas. Marine Biology Research, 5(5): 453-463.
DOI:10.1080/17451000802626554 |
Peng G G, Zhao W, Shi Z G, Chen H R, Liu Y, Wei J, Gao F Y. 2016. Cloning HSP70 and HSP90 genes of kaluga (Huso dauricus) and the effects of temperature and salinity stress on their gene expression. Cell Stress & Chaperones, 21(2): 349-359.
DOI:10.1007/s12192-015-0665-1 |
Qiang J, Yang H, Wang H, Xu P, He J. 2012. The effect of acute temperature stress on biochemical indices and expression of liver Hsp70 mRNA in gift nile tilapia juveniles (Oreochromis niloticus). Oceanologia et Limnologia Sinica, 43(5): 943-953.
(in Chinese with English abstract) |
Ravagnan L, Gurbuxani S, Susin S A, Maisse C, Daugas E, Zamzami N, Mak T, Jäättelä M, Penninger J M, Garrido C, Kroemer G. 2001. Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nature Cell Biology, 3(9): 839-843.
DOI:10.1038/ncb0901-839 |
Selong J H, McMahon T E, Zale A V, Barrows F T. 2001. Effect of temperature on growth and survival of bull trout, with application of an improved method for determining thermal tolerance in fishes. Transactions of the American Fisheries Society, 130(6): 1026-1037.
DOI:10.1577/1548-8659(2001)130<1026:EOTOGA>2.0.CO;2 |
Simon H U, Haj-Yehia A, Levi-Schaffer F. 2000. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis, 5(5): 415-418.
DOI:10.1023/A:1009616228304 |
Spallholz J E. 1990. Selenium and glutathione peroxidase: essential nutrient and antioxidant component of the immune system. In: Bendich A, Phillips M, Tengerdy R P eds. Advances in Experimental Medicine and Biology. Springer, Boston. p. 145–158, https://doi.org/10.1007/978-1-4613-0553-8_12.
|
Su X R, Du L L, Li Y Y, Li Y, Zhou J, Li T W. 2010. Cloning and expression of HSP70 gene of sipuncula Phascolosoma esculenta. Fish & Shellfish Immunology, 28(3): 461-466.
DOI:10.1016/j.fsi.2009.12.014 |
Van Cruchten S, Van den Broeck W. 2002. Morphological and biochemical aspects of apoptosis, oncosis and necrosis. Anatomia Histologia Embryologia, 31(4): 214-223.
DOI:10.1046/j.1439-0264.2002.00398.x |
Verlecar X N, Jena K B, Chainy G B N. 2007. Biochemical markers of oxidative stress in Perna viridis exposed to mercury and temperature. Chemico-Biological Interactions, 167(3): 219-226.
DOI:10.1016/j.cbi.2007.01.018 |
Wang T S, Zhou X, Zhao Z Y, Xu Z H, Shui Y. 2012. Activity of immunity-related enzymes under different temperature in Procambarus clarkii. Jiangsu Agricultural Sciences, 40(12): 239-241.
(in Chinese) |
Xu D X, Sun L N, Liu S L, Zhang L B, Yang H S. 2015. Histological, ultrastructural and heat shock protein 70 (HSP70) responses to heat stress in the sea cucumber Apostichopus japonicus. Fish & Shellfish Immunology, 45(2): 321-326.
DOI:10.1016/j.fsi.2015.04.015 |
Yan J, Liang X, Zhang Y, Li Y, Cao X J, Gao J. 2017. Cloning of three heat shock protein genes (HSP70, HSP90α and HSP90β) and their expressions in response to thermal stress in loach (Misgurnus anguillicaudatus) fed with different levels of vitamin C. Fish & Shellfish Immunology, 66: 103-111.
DOI:10.1016/j.fsi.2017.05.023 |
Zhang C N, Tian H Y, Li X F, Zhu J, Cai D S, Xu C, Wang F, Zhang D D, Liu W B. 2014. The effects of fructooligosaccharide on the immune response, antioxidant capability and HSP70 and HSP90 expressions in blunt snout bream (Megalobrama amblycephala Yih) under high heat stress. Aquaculture, 433: 458-466.
DOI:10.1016/j.aquaculture.2014.07.007 |
 2022, Vol. 40
2022, Vol. 40