Institute of Oceanology, Chinese Academy of Sciences
Article Information
- JIANG Ying, LÜ Lian-Gang, LIU Zongwei, YANG Chunmei, GUO Jingsong
- Identification of Antarctic minke and killer whales with passive acoustic monitoring in Prydz Bay, Antarctica
- Journal of Oceanology and Limnology, 40(2): 485-495
- http://dx.doi.org/10.1007/s00343-021-1017-x
Article History
- Received Jan. 15, 2021
- accepted in principle Apr. 6, 2021
- accepted for publication May. 6, 2021
2 Laboratory for Regional Oceanography and Numerical Modeling, Pilot National Laboratory for Marine Science and Technology (Qingdao), Qingdao 266237, China;
3 Key Laboratory of Marine Science and Numerical Modeling, Ministry of Natural Resources, Qingdao 266061, China
The highly generative waters around Antarctica attract or are home to many marine mammal species, such as pinnipeds and cetaceans. Because marine mammals produce various sounds for communication, prey and navigation, passive acoustic methods provide powerful tools to study their behavior, monitor their presence, and investigate their abundance and distribution.
Recently, passive acoustic monitoring (PAM) of aquatic mammals has been used widely to observe the presence, movement, and behavior of target species (reviewed by Mellinger et al., 2007). In the wild, PAM can be divided roughly into stationary and towed detection methods (Mellinger et al., 2007). Using stationary sensor, acoustic-based surveys can be used as a monitoring technique for surveying cetaceans in remote locations and adverse weather conditions. Towed hydrophone array on a shipboard line transect survey can be used to obtain locations of repeatedly vocalizing animals. PAM allows researchers to examine the variability in calling activities over a range of spatial and temporal scales. The ability to monitor throughout the night facilitates analyses of the acoustic activity of dolphins, which is not possible with visual monitoring and might allow the sampling of animals that cannot be observed in visual surveys (Soldevilla et al., 2010).
Identifying underwater sounds to the species level is an important step when processing passive acoustic recordings obtained in a marine environment. Various mysticete calls have been successfully identified and their signals have been detected automatically in passive acoustic recordings (e.g., Širović et al., 2004, 2009; Oleson et al., 2007; Munger et al., 2008), as well as a few odontocete calls (e.g., Mellinger et al., 2004; Verfuß et al., 2007; Soldevilla et al., 2010), but the discrimination of most odontocete calls remains difficult.
Prydz Bay is a deep embayment off Antarctica between the Lars Christensen Coast and Ingrid Christensen Coast. Prydz Bay is located at the downstream end of a giant glacial drainage system that originates in the East Antarctic interior. The Lambert Glacier flows from Lambert Graben into the Amery Ice Shelf on the southwest side of Prydz Bay. Other major glaciers drain into the southern end of the Amery Ice Shelf at 73°S where the marine part of the system starts at the modern grounding zone. Australia's Davis, Russia's Progress, Romania's Law-Racoviţă, and China's Zhongshan Stations are located on the southern side of Prydz Bay. Thus, Prydz Bay is a region of active oceanographic research.
The odontocete species detected in Prydz Bay include four species: beaked whales, sperm whales (Physeter macrocephalus), killer whales (Orcinus orca), and long-finned pilot whales (Globicephala melas) (Kasamatsu and Joyce, 1995). Beaked whales can produce stereotypical echolocation clicks with unique temporal and spectral characteristics (Baumann-Pickering et al., 2013). Sperm whales produce loud impulsive vocalizations and clicks (Douglas et al., 2005; Miller et al., 2008). The repertoire of long-finned pilot whales comprises a variety of clicks and buzzes, broadband nonharmonic calls, and different types of whistles, as well as different types of pulsed calls ranging from simple calls with single segment to complex calls with up to six segments (Nemiroff and Whitehead, 2009; Vester et al., 2017). Killer whales are known to produce three main sound categories: echolocation clicks used for foraging and navigation, and pulsed sounds and whistles used for social activities (Ford, 1989). In recent years, high-frequency modulated signals have been described in several populations (Samarra et al., 2010; Filatova et al., 2012; Simonis et al., 2012; Andriolo et al., 2015). In addition, it has been reported that low-frequency (below 300 Hz) signals with an inverted "u" shape are produced by northeast Atlantic killer whales in Icelandic waters (Samarra et al., 2016).
Among the 13 species of baleen whale that are currently recognized globally, six can be defined as true Antarctic whales: humpback (Megaptera novaeangeliae), blue (Balaenoptera musculus), minke (Balaenoptera bonaerensis), fin (Balaenoptera physalus), sei (Balaenoptera borealis), and southern right whales (Eubalaena australis) (Leaper and Miller, 2011). Humpback, blue, fin, and Antarctic minke whales have been recorded in Prydz Bay (Leaper and Miller, 2011).
Although underwater vocalizations of cetaceans have been investigated in some locations, such as Ross Sea, Weddell Sea, and areas off Antarctic Peninsula (e.g., Širović et al., 2004, 2009; Richlen and Thomas, 2008; Van Opzeeland, 2010; Pitman et al., 2011; Risch et al., 2014; Dominello and Širović, 2016; Reyes Reyes et al., 2017; Schall and Van Opzeeland, 2017), their vocalizations in Prydz Bay, a deep embayment off Antarctica between the Lars Christensen Coast and Ingrid Christensen Coast, to our knowledge were rare. Furthermore, low frequency inverted "u" signals also have not been previously described from Antarctic waters. Herein we report underwater marine mammal vocalizations recorded in Prydz Bay with a stationary sensor, and identify low-frequency signals with an inverted "u" shape, whistles, pulsed sounds, clicks, bio-duck sounds, and downsweeps. To investigate relationships between vocalizations and mammal species, especially Antarctic killer whales, statistical analyses of call acoustic parameters are given.
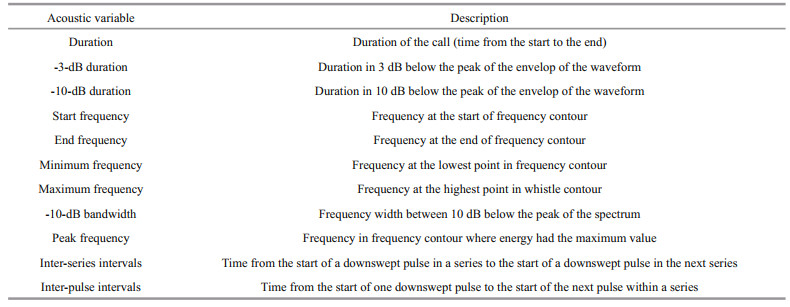
2 METHOD 2.1 Data collectionUnderwater sound recordings were acquired at two sites (S1 and S2) in Prydz Bay (Fig. 1). Observations were conducted at S1 (69°03'S, 76°13'E) on March 5, 2017 between 22:00 and 24:00 (local time). The sound data were collected at night so no visual observations of marine mammals were available at S1. Sound recordings were obtained at S2 (68°55'S, 75°58'E) only in the presence of Antarctic minke whales on March 6, 2017 between 06:00 and 07:30 (local time). During this period, researchers saw several Antarctic minke whales (6-8 individuals) swimming around the survey ship at a distance of no more than a few hundred meters.
|
| Fig.1 Locations where underwater sound observations were conducted in Prydz Bay Sound data were collected at night at S1 (69°03'S, 76°13'E) without visual information. At S2 (68°55'S, 75°58'E), sound recordings were acquired in the morning and only Antarctic minke whales were observed near the site. |
Recordings were acquired using a multi-channel recorder (LTT 186/16, Integrated System Ltd.) with a sampling frequency of 520.8 kHz and 16-bit resolution, and an omnidirectional hydrophone with a sensitivity of -170.1 dB re 1 V/μPa and a flat frequency response from 10 Hz to 100 kHz (±2 dB). Sound data were stored on a computer hard disk connected to the recorder. The hydrophone was deployed at a water depth of 10 m from the Chinese icebreaker, Xue Long, (Snow Dragon in Chinese).
2.2 Acoustic and statistical analysesAcoustic recordings were downsampled to 192 kHz and initially inspected using Adobe Audition 4.0 (Adobe Systems Inc., San Jose CA). Only recordings with a good signal-to-noise ratio (SNR) were included in the aural and visual inspections of the sounds and spectrogram (Rendell et al., 1999). The quality of the recordings was assessed based on two criteria: (1) clear contours with respect to the SNR; and (2) no overlap with more than one other call of the same type.
Vocalizations were divided into six acoustics categories: bio-duck calls, downsweep calls, inverted "u" shaped calls, whistles, pulsed calls, and click trains. Bio-duck calls are characterized by their repetitive nature, where most of the energy is located in the range of 50-300 Hz and with higher intensity harmonics up to 1 kHz (Van Opzeeland, 2010; Risch et al., 2014). A bio-duck call comprises several series where a series is defined as a cluster of downswept pulses separated by less than 1 s (Dominello and Širović, 2016). Inverted "u" shaped calls are characterized by an inverted "u" contour with an increase in frequency followed by a decrease, ranging below 500 Hz, and with one or more harmonics (Samarra et al., 2016). Clicks are pulses of sound, typically in a series, and they are generally used as echolocation signals by odontocetes (Ford, 1989). Whistles have a nonpulsed or continuous waveform, which appears as a single narrow-band tone in the spectrogram with little or no harmonic or side-band structure (Ford, 1989). Pulsed calls are sounds made up of pulses generated at a high rate, and they are resolved as a sideband equivalent to the pulse repetition rate (Ford, 1989). According to Filatova et al. (2009), the pulsed sounds were divided into monophonic call types without an overlapping high-frequency component (HFC) and biphonic call types containing an overlapping HFC.
Initially, the vocalizations were qualitatively categorized using a triple blind, independent observer method (Soto et al., 2014). The vocalizations were originally categorized by a primary observer, before the initial categorizations of the vocalizations were then tested and verified by two other independent observers. The sound files were randomly sorted and relabeled. The other two observers had no information about the vocalization types. The same acoustics software (Adobe Audition 4.0) and spectrogram parameters were employed by the other two observers. The classifications were compared to determine the number of final vocalization types determined by all observers (Rehn et al., 2011).
Various acoustic variables were measured for different vocalization categories (Table 1). A custom code written in MATLAB R2016a (Mathworks, Natick, MA) was also used to obtain acoustic parameters. The mean and standard deviation of each parameter were calculated. Four primary acoustic variables were measured for each bio-duck and downsweep: (1) start frequency (Hz); (2) end frequency (Hz); (3) peak frequency (Hz); and (4) duration (s). Two additional values were determined from each bio-duck call: (1) inter-series interval and (2) inter-pulse interval. Five acoustic variables were measured for inverted "u" shaped calls, whistles, and pulsed calls: (1) start frequency (kHz); (2) end frequency (kHz); (3) minimum frequency (kHz); (4) maximum frequency (kHz); and (5) duration (s). Clicks with a SNR higher than 20 dB were selected to measure acoustic parameters, where the SNR was calculated by computing the root mean squared sound pressure level in a window that covered 95% of the relative energy (Madsen and Wahlberg, 2007). Four variables were obtained for each click: -3-dB and -10-dB durations, peak frequency, and -10-dB bandwidth.
Whistles, pulsed sounds, clicks, inverted "u" shaped calls, bio-duck sounds, and downsweeps were detected in the recordings obtained from S1, but only bio-duck and downsweep sounds were identified in the recordings acquired from S2.
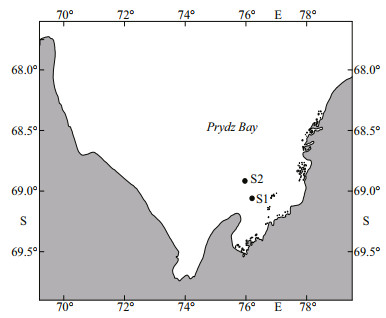
3.1 Bio-duck calls and downsweepsThe bio-duck calls comprised a series of 2-5 downswept pulses with a mean peak frequency at 138 Hz (Fig. 2a-d; Table 2). The individual downswept pulses had a mean duration of 0.25 s. This calls had similarities in terms of the frequency range and pulse number with previously described calls (Risch et al., 2014; Dominello and Širović, 2016). Only eight bio-duck calls were detected in the recordings obtained from S1 and one in the recordings acquired from S2. One call had a consistent number (four) of pulses in a series (Fig. 2a), whereas the others comprised series with a variable number (2-5) of pulses (Fig. 2b-d). The inter-series interval had a mean±standard deviation of 4.9±2.5 s, and the inter-pulse interval had a mean±standard deviation of 0.46±0.12 s. All of the bio-duck calls belonged to type A, which is a common Antarctic minke whale call (Dominello and Širović, 2016).
|
| Fig.2 Representative spectrograms of bio-duck sounds (a-d) and four concatenated downsweeps (e) (512 point fast Fourier transform (FFT); 90% overlap; Hann window) Note one recording from Site S2 (d), and the others from Site S1. |

|
Nine individually occurring downsweeps (Fig. 2e) were identified in our recordings (eight from S1 and one from S2). Their spectrograms were similar to those obtained previously for the downsweeps from Antarctic minke whales (see Fig. 2d in Risch et al., 2014; and Fig. 2f in Dominello and Širović, 2016). The frequency and temporal parameters measured in the present study (Table 2) were also similar to those reported previously (Risch et al., 2014; Dominello and Širović, 2016).
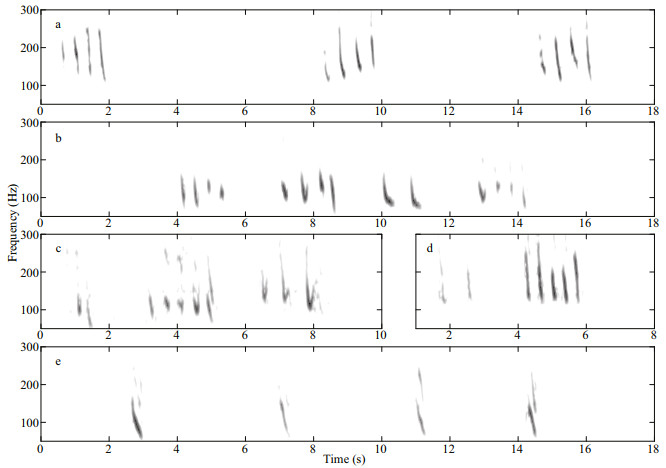
3.2 Inverted "u" shaped calls, whistles, pulsed sounds, and click trainsLow frequency calls were characterized by an inverted "u" shape, an increase followed by a decrease in frequency, with at least one partially visible harmonic (Fig. 3). The inverted "u" shape calls had an average maximum frequency of 128.8±14.7 Hz, an average minimum frequency of 64.3±8.8 Hz, and an average duration of 0.33±0.03 s (n=22). These calls were characterized by a start frequency of 113.1±20.5 Hz and an end frequency of 62.6±5.4 Hz. Calls were generally asymmetrical, i.e., higher start frequency and relatively lower end frequency (Samarra et al., 2016), and very similar (in terms of the spectrogram shape, frequency range, and oscillogram) to the low frequency signals measured in Icelandic waters, described as an inverted "u" shape and related to killer whales (see Fig. 1 in Samarra et al., 2016).
|
| Fig.3 Representative spectrograms of inverted "u" shaped calls with the waveform for one example shown at the top (1 024 point FFT; 90% overlap; Hann window) Four spectrograms are examples of the inverted "u" shaped calls at the bottom, and the waveform of the first example is given at the top. |
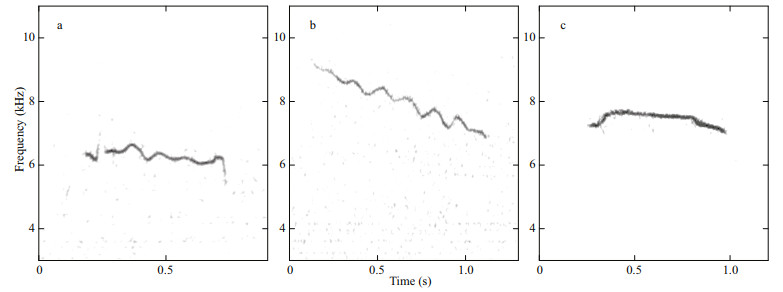
Whistles displayed much higher frequencies than inverted "u" shaped calls, with 94 signals detected and 85 of whose parameters were calculated (Table 3). Their frequencies ranged about from 5.56 kHz to 7.53 kHz. The mean of duration was usually 0.54 s. 81.2% of whistles were with no harmonic. The basic fundamental contour shapes of these signals were shown as constant frequency (Fig. 4a), downsweep (Fig. 4b), and scarce as convex (Fig. 4c). The most common shape was downsweep, occupying 70.6%. Whistles with no harmonic usually had higher frequencies than those with harmonics.
|
| Fig.4 Examples of the spectrograms obtained for whistles lumped according to the basic fundamental contour shapes (4 096 point FFT; 90% overlap; Hann window) a. constant frequency; b. downsweep; c. convex. |
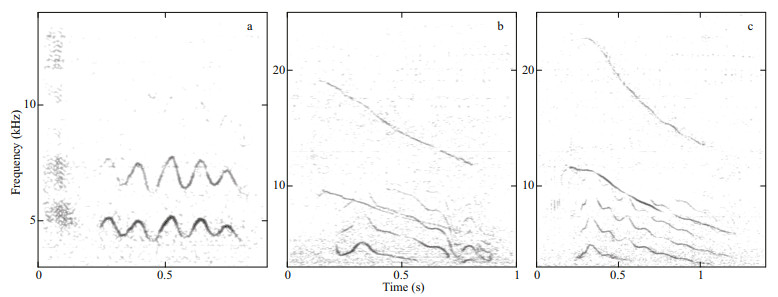
With 38 calls detected, pulsed sounds were common in our recording. Such calls were complex and composed of several call parts. The pulsed sounds included monophonic call types (Fig. 5a), and biphonic call types (Fig. 5b-c). Only one monophonic call was detected. The biphonic call types comprising overlapping lower-frequency component (LFC) and HFC were rich in harmonics, but highly variable in the extent of frequency modulation; for example, despite similarities in the biphonic calls depicted in Fig. 5b-c, the LFC was highly frequency-modulated (indicated by several inflection points) and the HFC frequency greater in the latter than former.
|
| Fig.5 Representative spectrograms of pulsed sounds (4 096 point FFT; 90% overlap; Hann window) a. monophonic call; b-c. biphonic calls. |
Biphonic calls with clear spectrogram signatures that did not overlap with other calls (n=37) were selected for measuring the acoustic parameters. The acoustic parameters were measured separately for both LFC and HFC structures in biphonic calls (Table 4). The HFC duration was slightly longer than that of the LFC. The LFC frequency was typically below 6 kHz, and the HFC frequency was typically centered above 6 kHz. It seemed like a frequency boundary existed between LFCs and HFCs.

|
With 281 click trains detected, clicks were the prevalent call type in our recording. A total of 717 clicks with SNR higher than 20 dB were selected to parameter measurement. The clicks were broadband pulses (-10-dB bandwidth of 16 kHz) with a -3-dB duration of 58 μs and a -10-dB duration of 125 μs. The peak frequency ranged from 12 to 44 kHz with a mean±standard deviation of 17±4 kHz. Measurable energy usually extended to 50 kHz and occasionally to 80 kHz.
4 DISCUSSION 4.1 Presence of Antarctic minke whalesThe bio-duck and downsweep sounds recorded at S2 in the presence of Antarctic minke whales which were identified in visual supported the attribution of these sounds to Antarctic minke whales. Thus, although no concurrent visual observations were available at S1 (our recordings were made at night), the occurrence of bio-duck calls and downsweeps suggested that Antarctic minke whales were also around site S1.
Bio-duck calls have been recorded at various locations in the Southern Ocean, but the origin of these signals remained unknown for many years (Van Opzeeland, 2010). Evidence that this sound is produced by Antarctic minke whales was only obtained recently by tag deployment on an individual in Wilhelmina Bay off the western Antarctic Peninsula (Risch et al., 2014). Antarctic minke whales also produce low frequency downsweeps (Risch et al., 2014). Therefore, bio-duck calls and downsweeps can be used to investigate the seasonal trends and diel patterns in Antarctic minke whale call production, and their relationships with sea ice cover (Dominello and Širović, 2016). The bio-duck and downsweep call rates should be low during this season because of the extensive sea ice over (Dominello and Širović, 2016), which is consistent with our results.
4.2 Presence of killer whalesThe relative time of occurrence for each signal type during our entire recording at S1 is depicted in Fig. 6. The signals containing clicks, pulsed calls, whistles, and inverted "u" shaped calls had strong temporal correlations. Among the odontocete species reported in Prydz Bay, only killer whales and long finned pilot whales have been shown to produce all of the recorded sound types comprising whistles, echolocation clicks and pulsed sounds (Reyes Reyes et al., 2017).

|
| Fig.6 Relative timings of call types during acoustic recordings obtained at site S1 The top four filled symbols indicate the presence of killer whales, and the bottom two unfilled symbols denote the presence of Antarctic minke whales. |
Only killer whales, false killer whales, and both species of pilot whales have been reported to produce calls as a lower component of biphonic sounds (Filatova et al., 2016), and the frequency features of biphonic calls in our recordings, such as the apparent frequency boundary between the LFCs and HFCs, linked our pulsed calls with those made by killer whales (Filatova et al., 2016).
Only killer whales and long finned pilot whales produce whistles in Prydz Bay. The sound structures of the whistles determined in the present study (Fig. 4) were similar to those made by killer whales (Richlen and Thomas, 2008; Schall and Van Opzeeland, 2017).
Odontocetes are known to produce echolocation click signals, thereby making killer whales, beaked whales, sperm whales, and long-finned whales possible candidates for the production of the clicks in our recordings. The echolocation clicks produced by beaked whales comprise 250-μs transients with a peak energy in the 30-40-kHz band (Madsen et al., 2005). Sperm whale clicks have been described as multipulsed, long duration signals with a moderate intensity, and a spectrum peaking below 13 kHz (Møhl et al., 2003). The echolocation clicks from long-finned pilot whales have higher peak frequencies than those produced by killer whales, with a mean peak frequency at 50 kHz (Eskesen et al., 2011). In addition, the echolocation clicks with a peak frequency around 17 kHz and duration of 125 μs in our recordings were similar to the clicks described for populations of killer whales in the northwestern Antarctic Peninsula (Reyes Reyes et al., 2017) and southern California Bight (Gassmann et al., 2013). These similarities suggest that our click signals were produced by Antarctic killer whales.
Inverted "u" shaped calls were first reported in Iceland and conclusively (by tagging) attributed to killer whales (Samarra et al., 2016). Similarities in oscillogram, spectrogram shape, and frequency range of our Antarctic sounds and the Icelandic inverted "u" shaped calls (Samarra et al., 2016) suggest a conspecific origin. However, it is possible that the inverted "u" shaped calls could have been produced by another species, e.g., humpback whale (Helble et al., 2015), and thus visual confirmation of the presence of killer whales during sound production will be needed in the future.
These reasons lead us believe that our clicks, pulsed sounds, whistles, and inverted "u" shape signals were produced by Antarctic killer whales. These signals are also appropriate targets for PAM methods for killer whales.
The underwater vocalizations of Antarctic killer whales have been investigated in locations such as Ross Sea, Weddell Sea, and areas off the Antarctic Peninsula, but to the best of our knowledge, the vocalizations of killer whales have not been reported previously in Prydz Bay. Furthermore, there are few descriptions of the acoustic repertoires of Antarctic killer whales. Clicks, whistles, pulsed signals, and buzzes have been recorded in Ross Sea (possibly type C), but the clicks have not been analyzed due to the frequency limits of the recordings (Richlen and Thomas, 2008). Additional signals produced by Antarctic killer whales have been described recently, especially high-frequency modulated signals from killer whales (possibly type A) in the northwestern Antarctic Peninsula (Reyes Reyes et al., 2017), and calls produced by type C killer whales in the eastern Weddell Sea coast (Schall and Van Opzeeland, 2017). Low frequency inverted "u" shaped calls have not been previously described from Antarctic waters. Thus, our descriptions of inverted "u" shaped calls, whistles, pulsed sounds, and clicks could help to understand the call repertoires of Antarctic killer whales.
4.3 Type C killer whalesFish-eating killer whales in the Northern Hemisphere are known to frequently produce sounds (Filatova et al., 2013), whereas mammal-eating killer whales generally restrict sound production to avoid alerting their acoustically sensitive prey (Deecke et al., 2005). Additionally, Icelandic killer whales that produce low-frequency inverted "u" signals are also fish-eaters (Samarra et al., 2016). Accordingly, we believe that the high whistle and click call rate, and low-frequency inverted "u" signals in our recording are indicative of the occurrence of fish-eating killer whales around our recording site.
The sound structures of the whistles (Fig. 4) were similar to the sounds from type C killer whales (Richlen and Thomas, 2008; Schall and Van Opzeeland, 2017). The similarity between the calls depicted in Fig. 5 of this study and those in call type AM1 and AM7 (Richlen and Thomas, 2008) suggest the potential presence of type C killer whales. Furthermore, 76.3% of the pulsed calls in our recording had similar sound structures to the call segments of type C killer whales (Schall and Van Opzeeland, 2017).
Type C and D killer whales consume fish in the waters around Antarctica (Pitman and Ensor, 2003; Pitman et al., 2011). Type C occurs mostly off East Antarctica, whereas type D has a distribution in sub-Antarctic waters. Given the locations of our study sites in Prydz Bay, the killer whales around the recording sites were possibly type C. Furthermore, the vocal activities of the killer whales also supported this conclusion (Barrett-Lennard et al., 1996; Van Opzeeland et al., 2005; Filatova et al., 2013).
Therefore, although no visual observations were made in the area, we suggest that the clicks, pulsed sounds, whistles, and inverted "u" shaped calls in our recording were probably produced by Antarctic type C killer whales.
The occurrence times shown in Fig. 6 suggest that Antarctic minke and killer whales probably co-existed around site S1, thereby indicating that Antarctic minke whales were aware that they could vocalize while killer whales were also present in the area. That implies Antarctic minke whales may be able to detect differences between the sounds of mammal-eating versus fish-eating killer whales.
5 CONCLUSIONWe reported minke whale sounds (bio-duck and downsweep calls) and the calls of killer whales (clicks, pulsed calls, whistles, and inverted "u" shape calls) in Prydz Bay. The whistles and pulsed calls resembled those described previously for type C killer whales, thereby suggesting the presence of this type of killer whale, even without visual confirmation. The co-occurrence of calls made by Antarctic minke and killer whales may imply that minke whales can detect differences between the sounds of mammal-eating and fish-eating killer whales.
Inverted "u" shape calls were first found in Antarctic waters. Their similar sound structures to the previous reported signals of killer whales and co-occurrence with other killer whale sounds suggest that these signals were produced by type C killer whales. Further research is needed to confirm whether Antarctic type C killer whales produce inverted "u" shape calls, and what the social function(s) of these sounds may be. From the perspective of killer whale evolution, the bipolar occurrence of inverted "u" shape signals warrants additional study.
Our descriptions and interpretations of underwater vocalizations contribute to the limited body of information regarding the distribution and acoustic behavior of cetaceans in Prydz Bay, and they might be important for PAM studies in Antarctic waters.
6 DATA AVAILABILITY STATEMENTThe data that support the findings of this study are available from the corresponding author upon reasonable request.
7 ACKNOWLEDGMENTThe authors thank the crew of the Chinese icebreaker Xue Long for support in data collection.
Andriolo A, Reis S S, Amorim T O S, Sucunza F, de Castro F R. 2015. Killer whale (Orcinus orca) whistles from the western South Atlantic Ocean include high frequency signals. Journal of the Acoustical Society of America, 138(3): 1696-1701.
DOI:10.1121/1.4928308 |
Barrett-Lennard L G, Ford J K B, Heise K A. 1996. The mixed blessing of echolocation: differences in sonar use by fisheating and mammal-eating killer whales. Animal Behaviour, 51(3): 553-565.
DOI:10.1006/anbe.1996.0059 |
Baumann-Pickering S, McDonald M A, Simonis A E, Berga A S, Merkens K P B, Oleson E M, Roch M A, Wiggins S M, Rankin S, Hildebrand J A. 2013. Species-specific beaked whale echolocation signals. Journal of the Acoustical Society of America, 134(3): 2293-2301.
DOI:10.1121/1.4817832 |
Deecke V B, Ford J K B, Slater P J B. 2005. The vocal behaviour of mammal-eating killer whales: communicating with costly calls. Animal Behaviour, 69(2): 395-405.
DOI:10.1016/j.anbehav.2004.04.014 |
Dominello T, Širović A. 2016. Seasonality of Antarctic minke whale (Balaenoptera bonaerensis) calls off the western Antarctic Peninsula. Marine Mammal Science, 32(3): 826-838.
DOI:10.1111/mms.12302 |
Douglas L A, Dawson S M, Jaquet N. 2005. Click rates and silences of sperm whales at Kaikoura, New Zealand. Journal of the Acoustical Society of America, 118(1): 523-529.
DOI:10.1121/1.1937283 |
Eskesen I G, Wahlberg M, Simon M, Larsen O E. 2011. Comparison of echolocation clicks from geographically sympatric killer whales and long-finned pilot whales (L). Journal of the Acoustical Society of America, 130(1): 9-12.
DOI:10.1121/1.3583499 |
Filatova O A, Fedutin I D, Nagaylik M M, Burdin A M, Hoyt E. 2009. Usage of monophonic and biphonic calls by freeranging resident killer whales (Orcinus orca) in Kamchatka, Russian Far East. Acta Ethologica, 12(1): 37-44.
DOI:10.1007/s10211-009-0056-7 |
Filatova O A, Ford J K B, Matkin C O, Barrett-Lennard L G, Burdin A M, Hoyt E. 2012. Ultrasonic whistles of killer whales (Orcinus orca) recorded in the North Pacific (L). Journal of the Acoustical Society of America, 132(6): 3618-3621.
DOI:10.1121/1.4764874 |
Filatova O A, Guzeev M A, Fedutin I D, Burdin A M, Hoyt E, Bulletin B. 2013. Dependence of killer whale (Orcinus orca) acoustic signals on the type of activity and social context. Biology Bulletin, 40(9): 790-796.
DOI:10.1134/S1062359013090045 |
Filatova O A, Samarra F I P, Barrett-Lennard L G, Miller P J O, Ford J K B, Yurk H, Matlin C O, Hoyt E. 2016. Physical constraints of cultural evolution of dialects in killer whales. Journal of the Acoustical Society of America, 140(5): 3755-3764.
DOI:10.1121/1.4967369 |
Ford J K B. 1989. Acoustic behaviour of resident killer whales (Orcinus orca) off Vancouver Island, British Columbia. Canadian Journal of Zoology, 67(3): 727-745.
DOI:10.1139/z89-105 |
Gassmann M, Henderson E E, Wiggins S M, Roch M A, Hildebrand J A. 2013. Offshore killer whale tracking using multiple hydrophone arrays. Journal of the Acoustical Society of America, 134(5): 3513-3521.
DOI:10.1121/1.4824162 |
Helble T A, Ierley G R, D'Spain G L, Martin S W. 2015. Automated acoustic localization and call association for vocalizing humpback whales on the navy's pacific missile range facility. Journal of the Acoustical Society of America, 137(1): 11-21.
DOI:10.1121/1.4904505 |
Kasamatsu F, Joyce G G. 1995. Current status of Odontocetes in the Antarctic. Antarctic Science, 7(4): 365-379.
DOI:10.1017/S0954102095000514 |
Leaper R, Miller C. 2011. Management of Antarctic baleen whales amid past exploitation, current threats and complex marine ecosystems. Antarctic Science, 23(6): 503-529.
DOI:10.1017/S0954102011000708 |
Madsen P T, Johnson M, de Soto N A, Zimmer W M X, Tyack P. 2005. Biosonar performance of foraging beaked whales (mesoplodon densirostris). Journal of Experimental Biology, 208(Pt2): 181-194.
|
Madsen P T, Wahlberg M. 2007. Recording and quantification of ultrasonic echolocation clicks from free-ranging toothed whales. DeepSea Research Part I: Oceanographic Research Papers, 54(8): 1421-1444.
DOI:10.1016/j.dsr.2007.04.020 |
Mellinger D K, Stafford K M, Fox C G. 2004. Seasonal occurrence of sperm whale (Physeter macrocephalus) sounds in the Gulf of Alaska, 1999-2001. Marine Mammal Science, 20(1): 48-62.
DOI:10.1111/j.1748-7692.2004.tb01140.x |
Mellinger D K, Stafford K M, Moore S E, Dziak R P, Matsumoto H. 2007. An overview of fixed passive acoustic observation methods for cetacean. Oceanography, 20(4): 36-45.
DOI:10.5670/oceanog.2007.03 |
Miller P J O, Aoki K, Rendell L E, Amano M. 2008. Stereotypical resting behavior of the sperm whale. Current Biology, 18(1): R21-R23.
DOI:10.1016/j.cub.2007.11.003 |
Møhl B, Wahlberg M, Madsen P T, Heerfordt A, Lund A. 2003. The monopulsed nature of sperm whale clicks. Journal of the Acoustical Society of America, 114(2): 1143-1154.
DOI:10.1121/1.1586258 |
Munger L M, Wiggins S M, Moore S E, Hildebrand J A. 2008. North Pacific right whale (Eubalaena japonica) seasonal and diel calling patterns from long-term acoustic recordings in the southeastern Bering Sea, 2000-2006. Marine Mammal Science, 24(4): 795-814.
DOI:10.1111/j.1748-7692.2008.00219.x |
Nemiroff L, Whitehead H. 2009. Structural characteristics of pulsed calls of long-finned pilot whales (Globicephala melas). Bioacoustics, 19(1-2): 67-92.
DOI:10.1080/09524622.2009.9753615 |
Oleson E M, Wiggins S M, Hildebrand J A. 2007. Temporal separation of blue whale call types on a southern California feeding ground. Animal Behaviour, 74(4): 881-894.
DOI:10.1016/j.anbehav.2007.01.022 |
Pitman R L, Durban J W, Greenfelder M, Guinet C, Jorgensen M, Olson P A, Plana J, Tixier P, Towers J R. 2011. Observations of a distinctive morphotype of killer whale (Orcinus orca), type D, from subantarctic waters. Polar Biology, 34(2): 303-306.
DOI:10.1007/s00300-010-0871-3 |
Pitman R L, Ensor P. 2003. Three forms of killer whales (Orcinus orca) in Antarctic waters. Journal of Cetacean Research and Management, 5(2): 131-139.
|
Rehn N, Filatova O A, Durban J W, Foote A D. 2011. Cross-cultural and cross-ecotype production of a killer whale "excitement" call suggests universality. Naturwissenschaften, 98(1): 1-6.
DOI:10.1007/s00114-010-0732-5 |
Rendell L E, Matthews J N, Gill A, Gordon J C D, Macdonald D W. 1999. Quantitative analysis of tonal calls from five odontocete species, examining interspecific and intraspecific variation. Journal of Zoology, 249(4): 403-410.
DOI:10.1111/j.1469-7998.1999.tb01209.x |
Reyes Reyes M V, Baumann-Pickering S, Simonis A, Melcón M L, Trickey J, Hildebrand J, Iñíguez M. 2017. High-frequency modulated signals recorded off the Antarctic Peninsula area: are killer whales emitting them?. Acoustics Australia, 45(2): 253-260.
DOI:10.1007/s40857-017-0103-x |
Richlen M F, Thomas J A. 2008. Acoustic behavior of Antarctic Killer whales (Orcinus orca) recorded near the ice edge of McMurdo Sound, Antarctica. Aquatic Mammals, 34(4): 448-457.
DOI:10.1578/AM.34.4.2008.448 |
Risch D, Gales N J, Gedamke J, Kindermann L, Nowacek D P, Read A J, Siebert U, Van Opzeeland L C, Van Parijs S M, Friedlaender A S. 2014. Mysterious bio-duck sound attributed to the Antarctic minke whale (Balaenoptera bonaerensis). Biology Letters, 10(4): 20140175.
DOI:10.1098/rsbl.2014.0175 |
Samarra F I P, Deecke V B, Miller P J O. 2016. Low-frequency signals produced by Northeast Atlantic killer whales (Orcinus orca). Journal of the Acoustical Society of America, 139(3): 1149-1157.
DOI:10.1121/1.4943555 |
Samarra F I P, Deecke V B, Vinding K, Rasmussen M H, Swift R J, Miller P J O. 2010. Killer whales (Orcinus orca) produce ultrasonic whistles. Journal of the Acoustical Society of America, 128(5): 205-210.
DOI:10.1121/1.3462235 |
Schall E, van Opzeeland I. 2017. Calls produced by ecotype C Killer whales (Orcinus orca) off the Eskstr?m Iceshelf, Antarctica. Aquatic Mammals, 43(2): 117-126.
DOI:10.1578/AM.43.2.2017.117 |
Simonis A E, Baumann-Pickering S, Oleson E, Melcón L, Gassmann M, Wiggins S M, Hildebrand J A. 2012. High-frequency modulated signals of killer whales (Orcinus orca) in the North Pacific. Journal of the Acoustical Society of America, 131(4): 295-301.
DOI:10.1121/1.3690963 |
Sirovic A, Hildebrand J A, Wiggins S M, McDonald M A, Moore S E, Thiele D. 2004. Seasonality of blue and fin whale calls and the influence of sea ice in the Western Antarctic Peninsula. Deep Sea Research Part R. Topical Studies in Oceanography, 51(17-19): 2327-2344.
DOI:10.1016/j.dsr2.2004.08.005 |
Širović A, Hildebrand J A, Wiggins S M, Thiele D. 2009. Blue and fin whale acoustic presence around Antarctica during 2003 and 2004. Marine Mammal Science, 25(1): 125-136.
DOI:10.1111/j.1748-7692.2008.00239.x |
Soldevilla M S, Wiggins S M, Hildebrand J A. 2010. Spatial and temporal patterns of Risso's dolphin echolocation in the Southern California Bight. Journal of the Acoustical Society of America, 127(1): 124-132.
DOI:10.1121/1.3257586 |
Soto A B, Marsh H, Everingham Y, Smith J N, Parra G J, Noad M. 2014. Discriminating between the vocalizations of Indo-Pacific humpback and Australian snubfin dolphins in Queensland, Australia. Journal of the Acoustical Society of America, 136(2): 930-938.
DOI:10.1121/1.4884772 |
Van Opzeeland I C, Corkeron P J, Leyssen T, Similä T, Van Parijs S M. 2005. Acoustic behaviour of Norwegian killer whales, Orcinus orca, during carousel and seiner foraging on spring-spawning herring. Aquatic Mammals, 31(1): 110-119.
DOI:10.1578/AM.31.1.2005.110 |
Van Opzeeland I C. 2010. Acoustic ecology of marine mammals in polar oceans. Reports on Polar and Marine Research, Bremerhaven.
|
Verfuß U K, Honnef C G, Meding A, Dähne M, Mundry R, Benke H. 2007. Geographical and seasonal variation of harbour porpoise (Phocoena phocoena) presence in the German Baltic Sea revealed by passive acoustic monitoring. Journal of the Marine Biological Association of the United Kingdom, 87(1): 165-176.
DOI:10.1017/S0025315407054938 |
Vester H, Hallerberg S, Timme M, Hammerschmidt K. 2017. Vocal repertoire of long-finned pilot whales (Globicephala melas) in northern Norway. Journal of the Acoustical Society of America, 141(6): 4289-4299.
DOI:10.1121/1.4983685 |
 2022, Vol. 40
2022, Vol. 40