Institute of Oceanology, Chinese Academy of Sciences
Article Information
- LIU Xudong, ZHANG Ran, FENG Jia, RINDI Fabio, XIE Shulian
- Neglectella glomeratasp. nov., a new species and implications for the systematics of the genus Neglectella (Oocystaceae, Trebouxiophyceae, Chlorophyta)
- Journal of Oceanology and Limnology, 39(6): 2370-2379
- http://dx.doi.org/10.1007/s00343-020-0356-3
Article History
- Received Sep. 20, 2020
- accepted in principle Nov. 14, 2020
- accepted for publication Dec. 2, 2020
2 Dipartimento di Scienze della Vita e dell'Ambiente, Università Politecnica delle Marche, Ancona 60131, Italy
The genus Neglectella Vodenicarov & Benderliev consists of small coccoid green algae and is commonly documented in freshwater environments in lakes, ponds, and swamps (Vodenicarov and Benderliev, 1971; Vodeničarov, 1989; Schagerl, 1993). Some species of this genus were also described from marine littoral environments, or even from Antarctica, and a few were reported to grow mixed with mosses or lichens (Komárek and Fott, 1983; Štenclová et al., 2017; Faluaburu et al., 2019).
Members of Neglectella are morphologically similar to the well-known genus Oocystis Nägeli A. Braun, except for the different arrangement of their chloroplasts. Based on the unique arrangement of the plastids placed radially and peripherically in the cell, Vodenicarov and Benderliev (1971) first described Neglectella as a distinct genus within the family Oocystaceae, with N. eremosphaerophila Vodenicarov & Benderliev as the type species. Then, in a comprehensive work focusing on the subfamily Oocystoideae, Fott (1976) transferred two species from Oocystis to Neglectella-Neglectella permagna (Behre) Fott and Neglectella asterifera (Skuja) Fott- and concluded that Oocystis gigas var. incrassata W. West & G. S. West sensu Skuja, was conspecific with the type species. However, Vodeničarov (1989), not taking into account the lack of pyrenoids, cell size, and ecology, did not accept this synonymy and renamed this alga Neglectellopsis skujae Vodenicarov. Furthermore, Vodeničarov (1989) removed Neglectella asterifera (Skuja) Fott from Neglectella because of its different plastid structure and renamed it Skujaster asterifera (Skuja) Vodenicarov. Two new combinations, Neglectella rotula (Playfair) Vodenicarov and Neglectella ovalis (W. B. Turner) Vodenicarov, were also proposed by Vodeničarov (1989), and Neglectella permagna (Behre) Fott was considered by this author a synonym of the latter (Vodeničarov, 1989). In 1993, a new species, Neglectella peisonis Schagerl, was described based on differences in ecology and cell size, pyrenoid position, and pole thickening from the similar Neglectella eremosphaerophila.
In a recent taxonomic reassessment of the Oocystaceae, Štenclová et al. (2017) transferred Oocystis solitaria Wittrock into the genus Negletella as N. solitaria (Wittrock) Štenclová & Kaštovský, and assessed the phylogenetic position of Neglectella in the subfamily Eremosphaeroideae (Oocystaceae) using molecular phylogenetic analyses. Different from the other members of Neglectella, N. solitaria is very common and has been widely studied (Komárek and Fott, 1983; Quader et al., 1983; Quader, 1986; Turmel et al., 2009; Liu et al., 2010). The close relationship of N. solitaria and Neglectella peisonis was proposed by Schagerl (1993), due to the similar larger cell size and higher number of chloroplasts than for other members of Oocystaceae (Hepperle et al., 2000; Štenclová et al., 2017). At the same time, however, Schagerl (1993) pointed out the different arrangement of the chloroplasts in N. solitaria, which are loosely distributed and not radially arranged. Therefore, the concept of Neglectella-like chloroplast characteristic was expanded to numerous chloroplasts stacked in the surface layer of the cell, which can be different from Eremosphaera, the other genus in the Eremosphaeroideae (Štenclová et al., 2017). Chloroplasts of Eremosphaera are irregularly scattered and radiating from the central nucleus to the plasma membrane (De Bary, 1858; Štenclová et al., 2017).
Currently, five taxonomically accepted species of Neglectella are recognized in AlgaeBase (Guiry, 2020). In this work, a new strain with Neglectella-like morphology was collected in China. Morphological and phylogenetic analyses identified this strain as a new species, Neglectella glomerata sp. nov., belonging to the Eremosphaeroideae (Oocystaceae, Trebouxiophyceae, Chlorophyta).
2 MATERIAL AND METHODNeglectella glomerata samples were obtained from a pond in the Luoping County in the Yunnan Province of China in April 2019 (24°51'53"N, 104°18'25"E, alt. 1 475.3 m, water temperature=18.6 ℃, pH=8.05). The sample was fixed in Formaldehyde and subsequently incorporated into the Herbarium of Shanxi University (SXU). Water samples were examined under an inverted microscope and isolated into single cells using the serial dilution pipetting technique (Hoshaw and Rosowski, 1973). Liquid BG11 medium was used to culture the strains for a better observation of mucilage covers (Stanier et al., 1971). The standard culture conditions were: constant temperature of 25 ℃ and cool-white fluorescent light source of 30–50 μmol photons/(m2·s) on a light?dark cycle as 12 h: 12 h.
Morphological observation was conducted using differential interference contrast by an Olympus BX53 light microscope (Olympus Corp., Tokyo, Japan). Micrographs were captured using an Olympus DP80 camera with software. Negative staining by India ink was used to show the mucilage envelope. For transmission electronic microscopy (TEM), algal samples were collected in exponential growth phase and fixed in 2.5% glutaraldehyde in phosphate buffer overnight at 4 ℃. Then, following washing in 0.05-mol/L phosphate buffer, algal cells were then fixed in 0.1-mol/L cacodylate buffer with 1% aqueous OsO4 for 2 h. After repeated washing by phosphate buffer saline (PBS) and stepwise dehydration with isopropanol, samples were embedded in Spurr's resin (Spurr, 1969). Uranyl acetate and lead citrate were used to stain the final ultrathin sections (Reynolds, 1963).
A Universal DNA Isolation Kit (AxyPrep) was used to extract the DNA following the manufacturer's instructions. The 18S rDNA and rbcL cpDNA genes were selected for phylogenetic inference for comparability with the results of previous studies (Štenclová et al., 2017). Primers selection and procedures for PCR amplification followed Xia et al. (2013).
All informative 18S rDNA and rbcL sequences of Oocystaceae were downloaded from GenBank (https://www.ncbi.nlm.nih.gov/genbank/) after BLAST searches. The sequences for concatenated analyses were selected based on previous studies by Štenclová et al. (2017) and Liu et al.(2017, 2018, 2020). The accession numbers of the new species are MT752941 for 18S rDNA and MT757669 for rbcL. The concatenated 18S rRNA and rbcL data set included sequences of Oocystaceae, and three Chlorellaceae were selected as outgroups to root the tree according to Štenclová et al. (2017). Ankistrodesmus fusiformis was selected as outgroup for the 18S rRNA analyses and members of Chlorellaceae (including Chlorella variabilis and a Chlorella sp., Micractinium pusillum and Auxenochlorella protothecoides) were chosen for rbcL (Liu et al., 2020). All data sets were aligned using ClustalW (Larkin et al., 2007) and, after visual inspection, edited manually in Mega 5.2.2 (Tamura et al., 2011). Phylogenetic trees were inferred for three datasets (18S rRNA, rbcL, and concatenated 18S rRNA + rbcL) by Randomized Axelerated Maximum Likelihood using RAxML v.8.0 (Stamatakis, 2014), applying the GTRGAMMA model. Nonparametric bootstrap support (1 000 replicates) was calculated to determine the maximum likelihood (ML) branch support. Bayesian analyses (BI) were conducted in MrBayes 3.1.2 (Huelsenbeck and Ronquist, 2001). Four Markov chains (three heated, one cold) were run for 3×106 generations for Markov chain Monte Carlo (MCMC) analyses. Trees were sampled every 1 000 generations and the first 25% was discarded as burn-in.
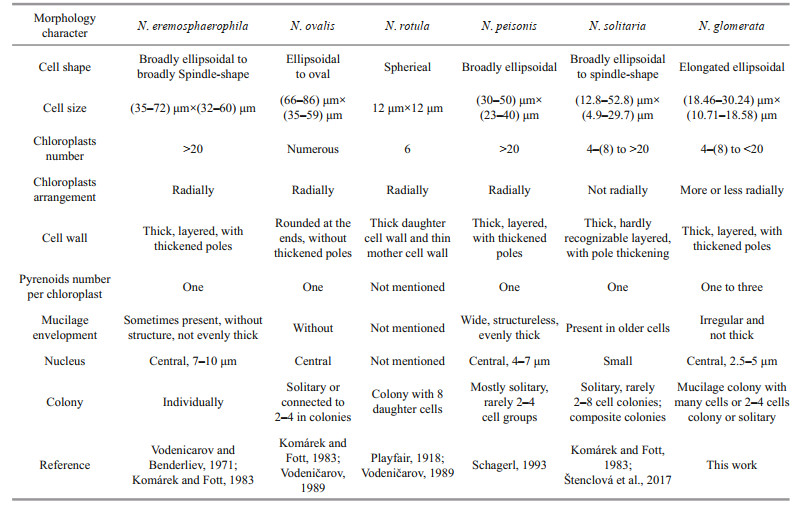
3 RESULT 3.1 Morphological observationsThe alga consisted of single cells (Fig. 1a–b), small colonies formed by 2–4 cells (Fig. 1c–d), and large mucilaginous colonies formed by dozens to hundreds of cells (Fig. 1e–i). The cells in the colonies were embedded in mucilage, which was released from the mother cell wall; the external mucilage envelope surrounding the cell was mostly not thick (Fig. 1h). The mother cell wall was not expanded while the daughter cells gradually matured, but eventually it ruptured and released them from one pole (Fig. 1e). The inner daughter cells that were not in proximity of the wall opening often were retained within the wall, and the released daughter cells were often still adhering to the inner ones or stuck to the mother cell wall remnant, then they divided forming new generations (Fig. 1e–f). This made the small colonies more or less irregularly dendroid (Fig. 1h). The opening direction was variable, and sometimes the daughter cells were released back into the old mother cell wall (Fig. 1c), which made the morphology of the mucilaginous colonies amorphous (Fig. 1i). Cells were elongated, elliptical in shape, 18.46–30.24-μm long and 10.71–18.58-μm wide, with round ends (Fig. 1a). Their walls formed polar thickenings when they became old (Fig. 1b). Multiple parietal chloroplasts occurred peripherally below the plasma membrane and were arranged more or less radially (Fig. 1a). Each chloroplast contained 1–3 pyrenoids, which were often indistinct and not easily observed in light microscopy (Fig. 1d & g). Numerous oil droplets and other brown particles occurred commonly in fully developed cells (Fig. 1b–c). Propagation took place by formation of 2–4–8 autospores (Fig. 1g). The autospores were tightly surrounded by the mother cell wall before to be released (Fig. 1g). The mother cell wall remnants expanded after the rupture occurred, and could be easily observed in the mucilaginous colonies before the final gelatinization (Fig. 1e & h). Sexual reproduction and flagellated stages were not observed.
|
| Fig.1 Light microscopy of Neglectella glomerata sp. nov. a. characteristic morphology of matured cell showing chloroplast arrangement; b. characteristic morphology of old cell showing oil droplets and brown particles; c. daughter cells released back to old mother cell wall; d. morphology of simply scattered colony with daughter cells tightly enclosed by mother cell wall; e. characteristic morphology of mucilage colony with more generations; f. mother cell wall expands after rupture at one pole to release daughter cells; g. characteristic morphology of autospores and released cells adhered each other; h. negative staining with India ink showing mucilaginous envelopes and irregularly dendroid morphology in small colony; i. negative staining with India ink showing amorphous morphology in big colony with numerous cells. Scale bar: a, b, d, g: 5 μm; c, e, f, h: 10 μm; i: 20 μm. |
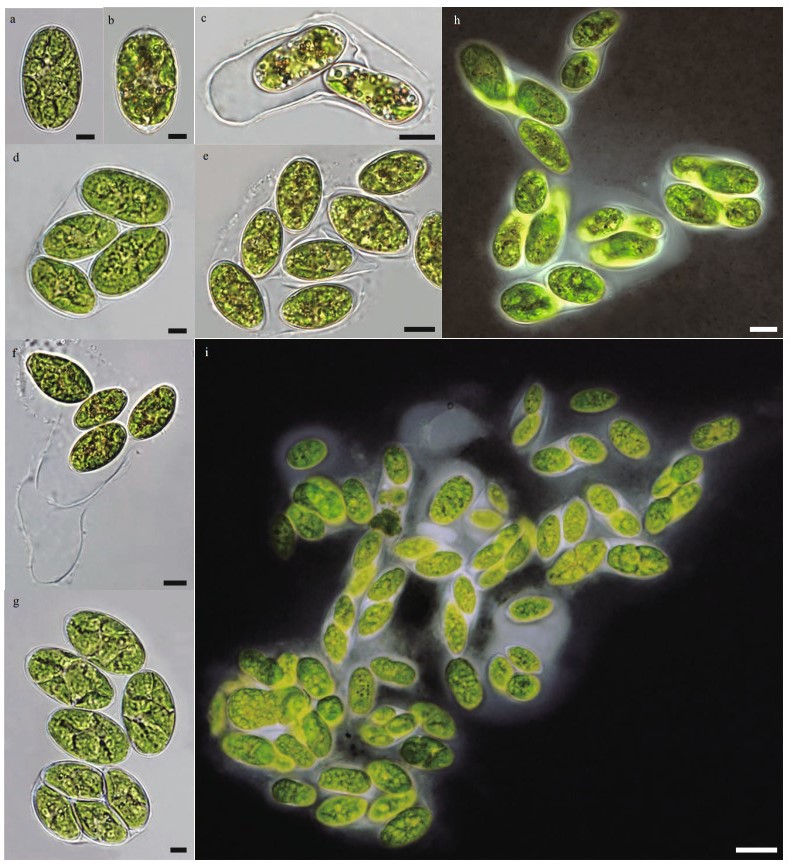
In TEM observation, multiple wedge-shaped chloroplasts surrounding the central nucleus were obvious (Fig. 2a & d). Several pyrenoids and numerous starch grains occurred within each chloroplast (Fig. 2a). Each globular pyrenoid was surrounded by a thick starch sheath (Fig. 2c & e). Many tubular thylakoids were tightly stacked and some penetrated the pyrenoid (Fig. 2c & e). The ultrastructure of the cell wall showed the typical Oocystis-like multilayered cellulose arrangement with perpendicularly oriented adjacent layers (Fig. 2f). Cellulose layers broke at the thickened pole of cells (Fig. 2b).
|
| Fig.2 Transmission electronic microscopy (TEM) of Neglectella glomerata sp. nov. a. mature cell showing multiple peripheral pyrenoids more or less radial and numerous starch grains inserted in; b. details of thickening end of cell wall; c. details of pyrenoid; d. details of central nucleus; e. details of chloroplasts consist of closed stacked tubular thylakoids; f. details of typical Oocystis-like cell wall ultrastructure and mucilage envelopment outside the mother cell wall. T: thylakoids; N: nucleus; P: pyrenoid; St: starch sheath; S: starch grains; Pa: particles; CW: cell wall; MCW: mother cell wall; ME: mucilage envelopment. White and black arrows in c and e all are showing the tubular thylakoids which penetrated the pyrenoid, Scale bar: a: 2 μm; b–f: 1 μm. |
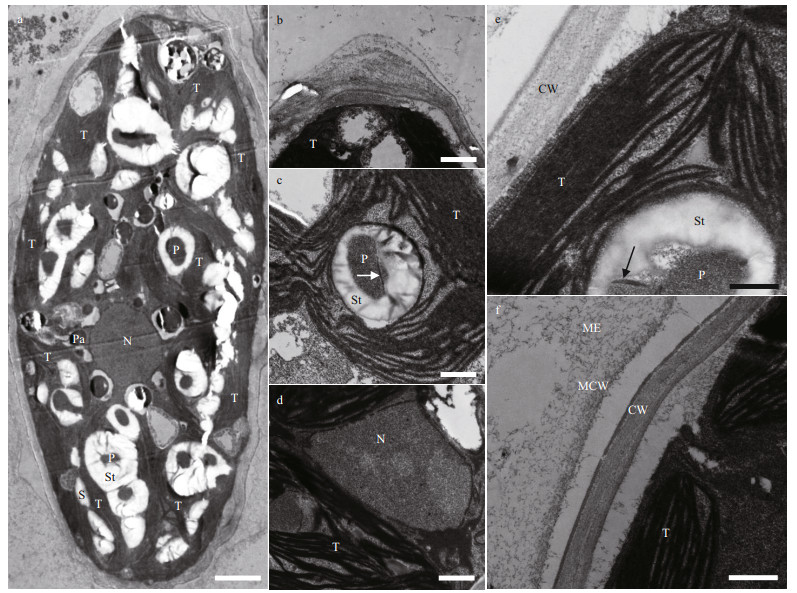
The 18S rDNA and rbcL sequences were obtained for the strain. Introns were not found in the sequences of either marker. The final dataset contained 1 463 characters for 18S rDNA, 1 037 characters for rbcL cpDNA, and 2 514 characters for the concatenated dataset. ML and BI yielded similar topologies; the ML tree for the concatenated dataset is presented in Fig. 3.
|
| Fig.3 Phylogenetic tree inferred using concatenated genes of 18S rDNA and rbcL cpDNA sequences from Oocystaceae species Bootstrap of ML and posterior probability of BI are presented on the nodes in order. Values above 50 for ML and 0.9 for BI are shown. Black triangle marked the phylogenetic position of the new species. |
All three phylogenies consistently recovered the newly isolated strain in the lineage corresponding to the genus Neglectella (Eremosphaeroidea, Oocystaceae) with high statistical support (Fig. 3, Supplementary Figs.S1 & S2). Two well-supported clades were formed within Neglectella. One included strains of Neglectella solitaria; in the other, Neglectella glomerata was sister to Neglectella peisonis with robust support.
4 DISCUSSIONRecently, a high diversity of Oocystaceae has been discovered in the Yunnan Province (China), which has led to the description of many new taxa (Liu et al., 2017, 2018, 2020). In this work, Neglectella glomerata, a new species of the genus Neglectella (Eremosphaeroidea, Oocystaceae), is presented.
Neglectella was originally erected by Vodenicarov and Benderliev (1971) to include Oocystis-like algae of relatively large size, with numerous radially arranged chloroplasts (Štenclová et al., 2017). Subsequently, Fott (1976) emphasized the unique radial chloroplast arrangement as a distinguishing feature and transferred two Oocystis species to this genus. While some members of Neglectella was moved out to establish two new genera, Neglectellopsis and Skujaster, by Vodeničarov (1989) and some new species were subsequently added (Vodeničarov, 1989; Schagerl, 1993), all members of the genus share the same chloroplast arrangement. Štenclová et al. (2017) expanded the circumscription of the genus based on molecular data and proposed the new combination Neglectella solitaria, transferring from Oocystis solitaria. This alga differs from the traditional Neglectella in its smaller cells and arrangement of peripheral chloroplasts; in Neglectella solitaria, the chloroplasts are irregularly arranged and not radially organized (Štenclová et al., 2017). The new species presented here, Neglectella glomerata, shows an intermediate chloroplast morphology between Neglectella solitaria and other Neglectella species with chloroplasts smaller in number but regularly organized around the cell and more or less radially from the middle of the cell (Fig. 1a). Also, the new species shares similarly thick cell walls, numerous assimilation products, and a central nucleus with the Neglectella species. Most importantly, however, the phylogenetic analyses robustly support its placement in the Neglectella clade (Fig. 3).
Within Neglectella, Neglectella glomerata can be distinguished from other species by its unique colony organization. All other species are usually unicellular and rarely form 2–8-celled colonies (Schagerl, 1993). Composite colonies formed by 2–3 generations of cells were occasionally documented in Neglectella solitaria (Komárek and Fott, 1983). In Neglectella glomerata, however, dozens to hundreds of cells in mucilaginous colonies are common. The colony organization of Neglectella glomerata is mainly due to the mucilage, differing from other species of Neglectella for the expanded mother cell wall that encloses the daughter cells. When observed, Neglectella glomerata shows a more or less dendroid organization, especially in small colonies; this arrangement cannot be found in other Neglectella species and resembles the type of colony morphology of the genus Ecballocystis Bohlin. However, as described by Iyengar (1932), the cells of Ecballocystis display a marked degree of polarity, which leads to a more obvious thickening in the basal cell wall, invariable rupture in apical position for the mother cell wall, a mucilage pad formed only from the basal part, and finally successive colony branching. In Neglectella glomerata, however, this polarity was not observed. The ruptured pole of the mother cell wall is not defined; therefore, some daughter cells are released back into the old mother cell wall (Fig. 1c). The mucilage pad at the base of the colony, which allows the Ecballocystis epiphytic or epilithic attachment (Iyengar, 1932; Komárek and Fott, 1983; Xia et al., 2013), is absent in Neglectella glomerata. This species lives in freshwater in stationary water pools. Moreover, its more or less dendroid morphology is due to the random adhesion of a part of the daughter cells which are released together (Fig. 1f & h). Unlike Ecballocystis, the remnant mother cell wall does not play an important role in the formation of colonies of Neglectella glomerata. When a larger mucilaginous colony is formed, the cell arrangement is often irregular.
Apart from the colony morphology, Neglectella glomerata can also be distinguished from other species of Neglectella for the higher number of pyrenoids per chloroplast, smaller cell size, and more elongated cell shape (Table 1). Neglectella glomerata has more than one pyrenoid in each chloroplast whereas the other species have only one. The pyrenoid number is considered an important taxonomic feature, especially in the Oocystaceae (Printz, 1913; Korsikov, 1939). In this family, multiple pyrenoids in each chloroplast are documented in Eremosphaera (Komárek and Fott, 1983), another genus of the Eremosphaeroideae, which further indicates the close phylogenetic relationship between Neglectella and Eremosphaera. Another genus with a similar pyrenoid structure is Euchlorocystis, a recently described genus also found in China (Liu et al., 2018). These three genera are all positioned at the base of the family Oocystaceae (Krienitz and Bock, 2011; Štenclová et al., 2017), which might support a basal origin of multiple pyrenoids in the Oocystaceae as mentioned by Liu et al. (2018). Apart from the characteristic pyrenoid, Neglectella glomerata is morphologically different from congeners in terms of cell size and cell shape. The cell size of the other Neglectella species (except the Neglectella solitaria) is almost twice as this new species (Schagerl, 1993). Considering the cell shape, the broadly ellipsoid cells with broad round end of Neglectella eremosphaerophila, Neglectella ovalis, and Neglectella peisonis, as well as the round shape of Neglectella rotula, are all different from the elongated elliptical shape of Neglectella glomerata (Playfair, 1918; Komárek and Fott, 1983; Schagerl, 1993).
Among the six species of genus Neglectella, Neglectella rotula is obviously different in morphology for having round cell shape and central pyrenoid. Its taxonomic position needs further verification. The remaining members can be subdivided into three types based on cell and colony morphology. The first and biggest types, including Neglectella eremosphaerophila, Neglectella ovalis, and Neglectella peisonis, represent the traditional Neglectella morphology, with numerous radial chloroplasts, clearly bigger cell size, and broadly ellipsoid cell shape (Schagerl, 1993). The second type only includes Neglectella solitaria with relatively smaller cell size and different chloroplast organization and number (Štenclová et al., 2017). Compared to the two types mentioned, the third type represented by our new species shows different colony morphology. Currently, three representative species of the three types in Neglectella have been sequenced. Based on the molecular phylogenetic analyses, Neglectella glomerata is resolved as sister to Neglectella peisonis (Fig. 3). However, the new species shows a more similar cell morphology with Neglectella solitaria, especially obvious on the smaller cell size and not broad round cell poles. The chloroplast, with more or less radially arrangement which resembles the first type and the lower number which resembles the second type, may further imply the Neglectella glomerata is in intermediate taxonomic position between the other two morphology types. The diverse morphology types in genus Neglectella may also imply more hidden species that have not been discovered yet. More species collections and sequencing will be needed in the future for this genus.
Taxonomic assessment
Neglectella glomerata LIU, ZHANG et XIE sp. nov. (Fig. 1a-i)
Diagnosis: Dozens or hundreds of cells were embedded in the mucilage colony, sometimes it was the scattered colony with 2–4 cells or solitary cells observed. The cells in the colony connected via the mucilage and organized more or less via dendroid or amorphous. The cell mucilage envelopment was irregular and not thick. The cell was elongated elliptical, with the ends thickening when old, at 18.46–30.24-μm long and 10.71–18.58-μm wide. Four-multiple chloroplasts extended more or less peripherally and radially from the center of the cell, each with 1–3 pyrenoids. Assimilate particles and oil droplets were numerous. Propagation occurred via 2–4–8 autospores. The mother cell wall tightly enclosed the daughter cells initially, then released the latter at one pole. This species differed from other members of the Neglectella genus due to its unique mucilage colony with a large number of cells, multiple pyrenoids in one chloroplast, smaller cell size, more elongated cell shape and the nucleotide sequence for 18S rDNA and rbcL cpDNA.
Type: Luoping County, Yunnan Province, China: sample collected in a pond, 24°51′53″N, 104°18′25″E, 18/4/2019, Xudong LIU, (Holotype SXU-ZR83!). Population partially illustrated here in light microscopy (Fig. 1).
Reference strain: the cultured strain was deposited as No. FACHB-2424 in Freshwater Algae Culture Collection at the Institute of Hydrobiology, Wuhan, China (FACHB).
Habitat: pond; planktonic; altitude 1 475.3 m; water temperature=18.6 ℃; pH=8.05.
Etymology: the species was named for a mucilage colony with numerous embedded cells.
5 CONCLUSIONA new freshwater green algal species, Neglectella glomerata sp. nov., is erected here based on morphological and ultrastructure comparison and molecular phylogenetic analysis. It is morphologically characterized by the difference in unique mucilage colony with a large number of cells, multiple pyrenoids in one chloroplast, smaller cell size and more elongated cell shape. Further morphology analysis is completed including all the species in genus Neglectella and three types are summarized. Our new species may represent a new transitional morphology type and taxonomic position between the other two types.
6 DATA AVAILABILITY STATEMENTThe authors declare that the datasets in this study are available on reasonable request from the corresponding author.
Electronic supplementary material
Supplementary material (Supplementary Figs.S1–S8 and Tables S1–S8) is available in the online version of this article at https://doi.org/10.1007/s00343-021-0164-4.
De Bary A. 1858. Untersuchungen über die familie der conjugaten (Zygnemeen und Desmidieen). In: Felix A ed. Ein Beitrag Zur Physiologischen und Beschreibenden Botanik. Förstnersche Buchhandlung, Leipzig. p. 1-91.
|
Faluaburu M S, Nakai R, Imura S, Naganuma T. 2019. Phylotypic characterization of mycobionts and photobionts of rock tripe lichen in East Antarctica. Microorganisms, 7(7): 203.
DOI:10.3390/microorganisms7070203 |
Fott B. 1976. Oocystis und verwandte Gattungen aus der Unterfamilie der Oocystoideae; Namensänderungen, taxonomische Notizen und Bestimmungsschlüssel. Preslia, Praha, 48: 193-206.
|
Guiry M D. 2020. AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org. Accessed on 2020-07-13.
|
Hepperle D, Hegewald E, Krienitz L. 2000. Phylogenetic position of the oocystaceae (Chlorophyta). Journal of Phycology, 36(3): 590-595.
DOI:10.1046/j.1529-8817.2000.99184.x |
Hoshaw R W, Rosowski J R. 1973. Methods for microscopic algae. In: Stein J R ed. Handbook of Phycological Methods. Cambridge University Press, New York. p. 53-68.
|
Huelsenbeck J P, Ronquist F. 2001. MRBAYES: bayesian inference of phylogenetic trees. Bioinformatics, 17(8): 754-755.
DOI:10.1093/bioinformatics/17.8.754 |
Iyengar M O P. 1932. Two little-known genera of green algae(Tetrasporidium and Ecballocystis). Annals of Botany, 46: 191-192.
|
Komárek J, Fott B. 1983. Chlorophyceae (grünalgen), ordnung: chlorococcales. In: Huber-Petalozzi G, Heynig H, Mollenhauer D eds. Das Phytoplankton des Sübwassers. Systematic und Biologie. E. Schweizerbart'sche Verlagsbuchhandlung, Stuttgart. p. 1-1 044.
|
Korsikov A A. 1939. Contribution to the algal flora of the Gorky District Phytoplankton of the Oka River in August 1932. Zapiski Gorkovskogo Universiteta, 9: 101-128.
|
Krienitz L, Bock C. 2011. Elongatocystis ecballocystiformis gen. et comb. nov., and some reflections on systematics of Oocystaceae (Trebouxiophyceae, Chlorophyta). Fottea, 11(2): 271-278.
DOI:10.5507/fot.2011.026 |
Larkin M A, Blackshields G, Brown N P, Chenna R, McGettigan P A, McWilliam H, Valentin F, Wallace I M, Wilm A, Lopez R, Thompson J D, Gibson T J, Higgins D G. 2007. Clustal W and clustal X version 2.0. Bioinformatics, 23(21): 2947-2948.
DOI:10.1093/bioinformatics/btm404 |
Liu X D, Wang Q H, Zhu H, Liu B W, Rindi F, Liu G X, Xie S L, Hu Z Y. 2020. Reticulocystis yunnanense gen. et sp. nov., a new member of freshwater Oocystaceae algae(Trebouxiophyceae, Chlorophyta). European Journal of Phycology, 55(4): 507-516.
DOI:10.1080/09670262.2020.1751303 |
Liu X D, Zhu H, Song H Y, Liu B W, Wang Q H, Liu G X, Hu Z Y. 2018. Quadricoccopsis gen. nov., a new genus of Quadricoccus-like algae in Oocystaceae from China(Trebouxiophyceae, Chlorophyta). Fottea, 18(2): 189-199.
DOI:10.5507/fot.2018.005 |
Liu X S, Zhu H, Liu B W, Liu G X, Hu Z Y. 2017. Classification of planctonema-like algae, including a new genus Planctonemopsis gen. nov., a new species Planctonema gelatinosum sp. nov. and a reinstated genus Psephonema(Trebouxiophyceae, Chlorophyta). Journal of Phycology, 53(4): 869-879.
DOI:10.1111/jpy.12551 |
Liu Y, Liu Z H, Zhang J L, Hu Y Y, Sun H L. 2010. Experimental study on the utilization of DIC by Oocystis solitaria Wittr and its influence on the precipitation of calcium carbonate in karst and non-karst waters. Carbonates and Evaporites, 25(1): 21-26.
DOI:10.1007/s13146-009-0002-9 |
Playfair G I. 1918. New and rare freshwater algae. Proceedings of the Linnean Society of New South Wales, 43: 497-543.
|
Printz H. 1913. Eine systematische übersicht der gattung Oocystis nägeli. Nyt Magasin for Naturvidenskapene, 51: 165-203.
|
Quader H, Robinson D G, van Kempen R. 1983. Cell wall development in Oocystis solitaria in the presence of polysaccharide binding dyes. Planta, 157(4): 317-323.
DOI:10.1007/BF00397402 |
Quader H. 1986. Cellulose microfibril orientation in Oocystis solitaria: proof that microtubules control the alignment of the terminal complexes. Journal of Cell Science, 83: 223-234.
DOI:10.1242/jcs.83.1.223 |
Reynolds E S. 1963. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. The Journal of Cell Biology, 17(1): 208-212.
DOI:10.1083/jcb.17.1.208 |
Schagerl M. 1993. Lichtmikroskopische und ultrastrukturelle Untersuchungen an Neglectella peisonis spec. nov. - einer planktischen Oocystaceae aus dem Neusiedler See(Österreich). Nova Hedwigia, 56: 61-74.
|
Spurr A R. 1969. A low-viscosity epoxy resin embedding medium for electron microscopy. Journal of Ultrastructure Research, 26(1-2): 31-43.
DOI:10.1016/S0022-5320(69)90033-1 |
Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics, 30(9): 1312-1313.
DOI:10.1093/bioinformatics/btu033 |
Stanier R Y, Kunisawa R, Mandel M, Cohen-Bazire G. 1971. Purification and properties of unicellular blue-green algae(Order Chroococcales). Bacteriological Reviews, 35(2): 171-205.
DOI:10.1128/MMBR.35.2.171-205.1971 |
Štenclová L, Fučíková K, Kaštovský J, Pažoutová M. 2017. Molecular and morphological delimitation and generic classification of the family Oocystaceae(Trebouxiophyceae, Chlorophyta). Journal of Phycology, 53(6): 1263-1282.
DOI:10.1111/jpy.12581 |
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, 28(10): 2731-2739.
DOI:10.1093/molbev/msr121 |
Turmel M, Otis C, Lemieux C. 2009. The chloroplast genomes of the green algae Pedinomonas minor, Parachlorella kessleri, and Oocystis solitaria reveal a shared ancestry between the pedinomonadales and chlorellales. Molecular Biology and Evolution, 26(10): 2317-2331.
DOI:10.1093/molbev/msp138 |
Vodenicarov D, Benderliev K. 1971. Neglectella gen. nov. (Chlorococcales). Nova Hedwigia, 21: 801-812.
|
Vodeničarov D. 1989. Die gattung Neglectella vodenic. et benderl. und zwei neue gattungen: neglectellopsis gen. nov. und Skujaster gen. nov. (Chlorophyta, Chlorococcales). Algological Studies, 57: 409-424.
|
Xia S, Zhu H, Cheng Y Y, Liu G X, Zheng Y H. 2013. Phylogenetic position of Ecballocystis and Ecballocystopsis (Chlorophyta). Fottea, 13(1): 65-75.
DOI:10.5507/fot.2013.006 |
 2021, Vol. 39
2021, Vol. 39