Institute of Oceanology, Chinese Academy of Sciences
Article Information
- YANG Yujia, LI Xiaoni, SUN Lina
- Triterpenoid saponin biosynthesis genes and their expression patterns during the development of sea cucumber Apostichopus japonicus
- Journal of Oceanology and Limnology, 39(6): 2295-2308
- http://dx.doi.org/10.1007/s00343-020-0209-0
Article History
- Received May. 31, 2020
- accepted in principle Jul. 25, 2020
- accepted for publication Nov. 24, 2020
2 CAS Key Laboratory of Marine Ecology and Environmental Sciences, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China
Saponins are a class of chemical compounds, which are abundant in various plant species, including the soapwort plant, maple, and ginseng. They are characterized by a hydrophobic triterpene or sterol backbone and a hydrophilic carbohydrate chain (Mugford and Osbourn, 2012). Saponins are broadly classified into steroid glycosides, triterpenoids, spirostanols, and furostanol saponins (Vincken et al., 2007). It is known that saponins and their biosynthetic intermediates exhibit different biological activities and play particularly important roles in the pharmaceutical, cosmetic, and food industries (Moses et al., 2014; Xiao et al., 2019). Saponins are the main bioactive compounds in numerous plants and folk medicines, especially traditional Chinese remedies (Bahrami and Franco, 2015). As bioactive molecules, saponins have been used as vaccine adjuvants (Kensil, 1996), dietary supplements, and nutritional food ingredients (Zhao et al., 2018). While at certain concentrations some saponins are toxic to cold-blooded organisms, they can enhance nutrient absorption (e.g., oat and spinach) and aid digestion (Forester and Hartmut, 2006). These natural products are understood to be plant derived. Only a few marine organisms, such as echinoderms, marine sponges, and octocorals, have been shown to produce saponins (Stonik, 2001; Francis et al., 2002; Skropeta, 2008; Mitu et al., 2017). Sea cucumber Apostichopus japonicus, also called marine ginseng in China, is a species of echinoderm. It has been demonstrated that sea cucumber can generate numerous agricultural, nutraceutical, pharmaceutical, and cosmeceutical products (Bahrami et al., 2014). Sea cucumbers are characterized by slow motion and the absence of prominent structural defenses (Moghimipour and Handali, 2015). The abundance of saponins in the body wall and viscera was considered as a physical barrier of sea cucumber (Van Dyck et al., 2010). Highly diverse saponins, including 59 triterpene glycosides, have been previously detected in the organism (Caulier et al., 2011). The structural architecture of the saponins is associated with intra-individual variability, which leads to specific functions (Caulier et al., 2011). The majority of saponins isolated from sea cucumber are triterpene glycosides, which exhibit various biological effects (e.g., antifungal, cytotoxic, hemolytic, and cytostatic immunomodulatory activities) (Maier et al., 2001). In addition to playing important roles in the defense mechanisms of sea cucumber, it has been speculated that saponins also inhibit oocyte maturation during spawning and control gametogenesis (Bahrami et al., 2014).
Based on the characteristic reactions, the saponin biosynthesis enzymes could be categorized into four types, i.e., oxidosqualene cyclases (OSCs), cytochrome P450 monooxygenases (P450s), UDP-glycosyltransferases (UGTs), and other tailoring enzymes (Moses et al., 2014). In recent years, saponin biosynthesis enzymes have been studied in several plant species, including oat (Mugford et al., 2013), Lotus japonicus (Krokida et al., 2013), tomato, and potato (Adzhubei et al., 2013; Itkin et al., 2013). Identification of biosynthetic genes involved in new pathways is essential to synthesize specialized saponin metabolites in echinoderms such as sea cucumber. A recent study found that the OSC1 and OSC2 genes in A. japonicus exhibited high sequence divergence and contained plant-like motifs (Li et al., 2018). Moreover, a transition from lanosterol production to parkeol production was determined, which indicated convergent evolution in sea cucumber (Li et al., 2018). Another transcriptome study revealed the secondary metabolite biosynthesis pathways, including saponin biosynthesis, in the body wall of sea cucumber Holothuria scabra (Mitu et al., 2017). Core saponin biosynthesis genes (e.g., hydroxymethylglutaryl-CoA synthase, farnesyl diphosphate synthase, squalene synthase, and squalene epoxidase) in the body wall of H. scabra shared homology with those of plants, suggesting that the saponin biosynthesis pathways were highly conserved (Mitu et al., 2017). Nevertheless, even though the saponin biosynthesis genes were previously identified in another sea cucumber species, i.e., H. scabra, the complete set of saponin biosynthesis enzymes and their expression patterns during critical developmental stages have never been investigated in the genome of A. japonicus. In this study, we comprehensively studied the biosynthesis enzymes responsible for the generation of diverse saponin metabolites, including acetyl-CoA acetyltransferase (AACT), farnesyl diphosphate synthase (FPS), hydroxymethylglutaryl-CoA synthase (HMGS), oxidosqualene cyclase (OSC), squalene synthase (SS), squalene epoxidase (SE), and UDP-glycosyltransferase (UGT). Additionally, their putative mechanisms in A. japonicus were investigated. The findings of the current study will facilitate further research into the saponin biosynthesis in echinoderms.
2 MATERIAL AND METHOD 2.1 Gene identification and sequence analysisThe saponin biosynthesis enzymes were identified based on the A. japonicus genome assembly (http://www.genedatabase.cn/aja_genome_20161129.html) from a previous publication (Zhang et al., 2017). Sequence alignments were carried out using the ClustalW algorithm in DNAMAN software (Lynnon Biosoft, USA). The identification of domains was carried out by SMART (Schultz et al., 2000) and the Batch CD-search tool in the Conserved Domain Database (Marchler-Bauer et al., 2010) of NCBI. The copy numbers of saponin biosynthesis enzymes from different species were gained from the NCBI database.
2.2 Phylogenetic analysis and structural and function prediction analysisPhylogenetic analysis was conducted using amino acid sequences of enzymes responsible for saponin biosynthesis from A. japonicus and selected species retrieved from NCBI, Uniprot, and Ensembl, including sea urchin, sea star, and ginseng. Multiple alignments of protein sequences were conducted using the MUSCLE method (Edgar, 2004). The phylogenetic trees of the protein sequences were built using the maximum likelihood method and JTT+I+G model in MEGA 6 (Darriba et al., 2011; Tamura et al., 2013).
The 3D structural models were built based on the templates matching the target sequences in Swiss-Model (Guex and Peitsch, 1997). The models were visualized using the UCSF Chimera program (Pettersen et al., 2004). Sequence analysis and protein structure prediction were analyzed by PredictProtein (http://www.predictprotein.org) (Rost et al., 2004). The Gene Ontology (GO) terms were also predicted using PredictProtein (Rost et al., 2004).
2.3 Gene expression analysisThe expression patterns of crucial saponin biosynthesis enzymes during the developmental stages of A. japonicus were analyzed with our unpublished data. The RNA-seq datasets were obtained from five developmental stages of A. japonicus (fertilized oocytes, blastula, gastrula, doliolaria, and penractula). Accurate transcript quantification was analyzed with RSEM (Li and Dewey, 2011) using default parameters (http://deweylab.github.io/RSEM/). A Heatmap of expression patterns was built using GraphPad Prism 8. The putative triterpene saponins biosynthetic pathway was plotted. It was based on literature searches on the biosynthesis of triterpenoid saponins in other species of sea cucumber, including Stichopus horrens, Eupentacta fraudatrix, and Holothuria scabra (Makarieva et al., 1993; Silchenko et al., 2012; Mitu et al., 2017; Liu et al., 2018; Claereboudt et al., 2019).
3 RESULT 3.1 Phylogenetic and sequence analysis of saponin biosynthesis enzymes in A. japonicusIn the A. japonicus genome, 30 genes responsible for saponin biosynthesis were found (Table 1):
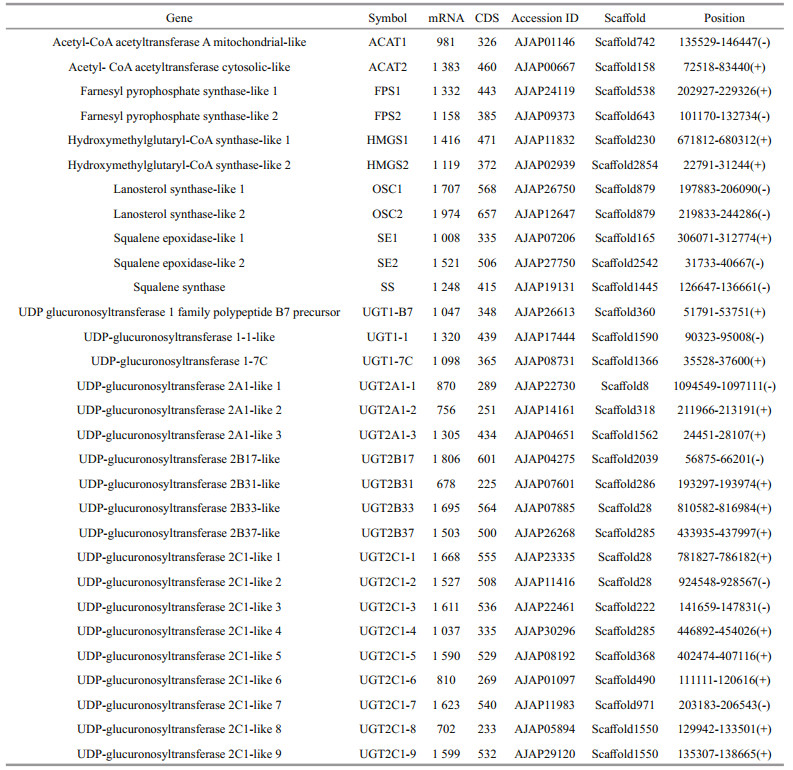
|
Upstream enzymes [2 genes for AACTs (ACAT1 and ACAT2), 2 genes for HMGS (HMGS1 and HMGS2), and 2 genes for FPSs (FPS1 and FPS2)].
Midstream enzymes [2 genes for OSCs (OSC1 and OSC2), 1 gene for SS, 2 genes for SEs (SE1 and SE2)].
Downstream enzyme [19 genes for UGTs, 3 enzymes for UGT1 subfamily, 3 enzymes for UGT2A subfamily, 4 for UGT2B subfamily, 9 for UGT2C subfamily].
In the overall phylogenetic analysis (Figs. 1 & 2), all of the saponin biosynthesis enzymes (i.e., AACTs, FPSs, HMGSs, OSCs, SS, SEs, and UGTs) in A. japonicus were first clustered with their counterparts in other echinoderm species, namely sea star and sea urchin. These genes were then grouped with the orthologous genes in ginseng. Two types of AACT enzymes were identified in A. japonicus and sea star, i.e., cytosolic and mitochondrial-like biocatalysts. Detailed phylogenetic analysis revealed that these two types of AACT enzymes were clustered into two separate clades. In A. japonicus, most UGTs were grouped with homologous copies in the genome and clustered with the corresponding sea urchin genes.
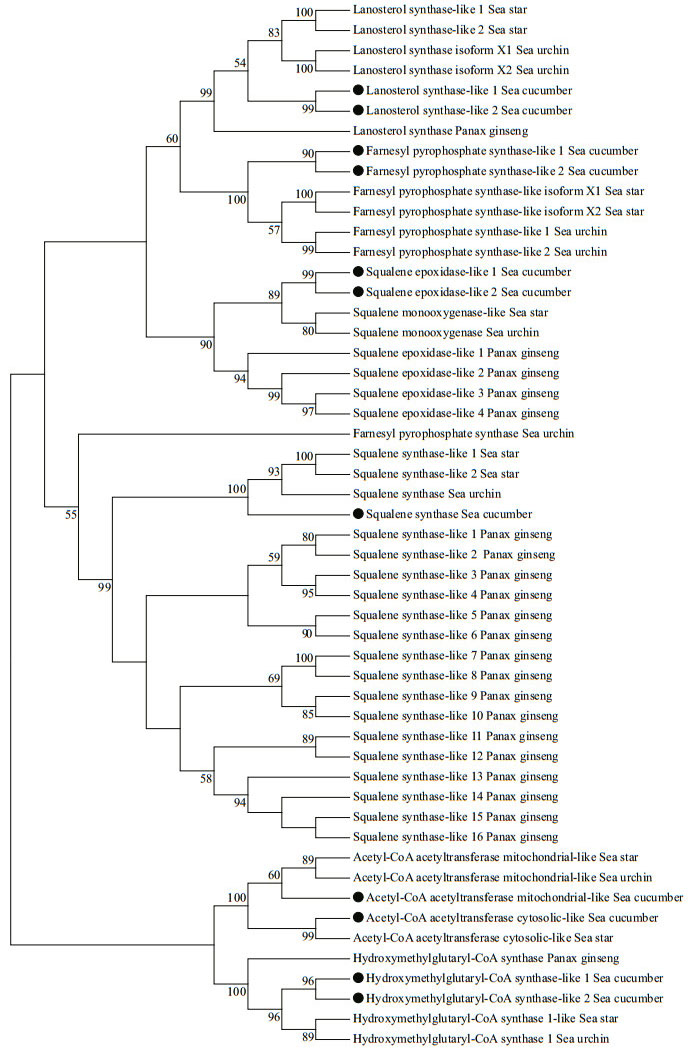
|
| Fig.1 Phylogenetic analysis of enzymes responsible for saponin biosynthesis using sequences from A. japonicus, sea star, sea urchin, and ginseng The black dots indicate saponin biosynthesis enzymes from A. japonicus. Bootstrap (1 000 replications) support values appear on the branches. |

|
| Fig.2 Phylogenetic analysis of UDP-glucuronosyltransferase using sequences from A. japonicus, sea star, sea urchin, and ginseng The black dots indicate saponin biosynthesis enzymes from A. japonicus. Bootstrap (1 000 replications) support values appear on the branches. |
The two types of AACTs, ACAT1 and ACAT2, both included a conserved domain of the Thiolase_N superfamily (Fig. 3). Furthermore, FPS1, FPS2, and SS contained a conserved domain of the Isoprenoid_ Biosyn_C1 superfamily. A conserved domain of the HMG_CoA_synt_C superfamily was identified in HMGS1 and HMGS2. In addition, OSC1 and OSC2 exhibited a conserved domain of the ISOPREN_C2_ like superfamily, while SE1 and SE2 contained a conserved domain of squalene monooxygenase. Lastly, UGTs shared a conserved domain of the UDP-glucuronosyltransferase (UDPGT) superfamily.
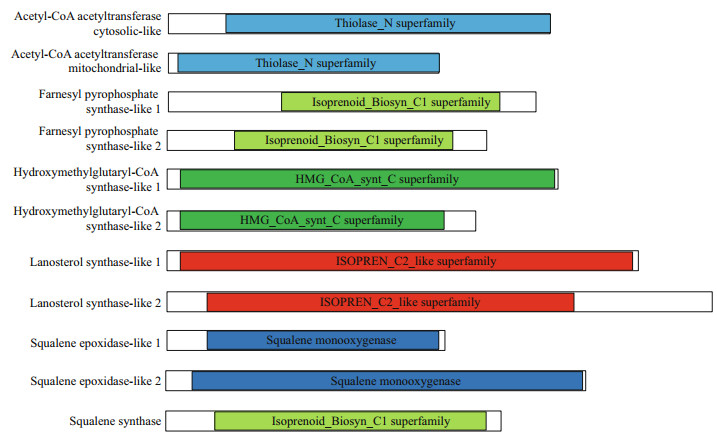
|
| Fig.3 Conserved domain analysis of enzymes responsible for saponin biosynthesis in A. japonicus |
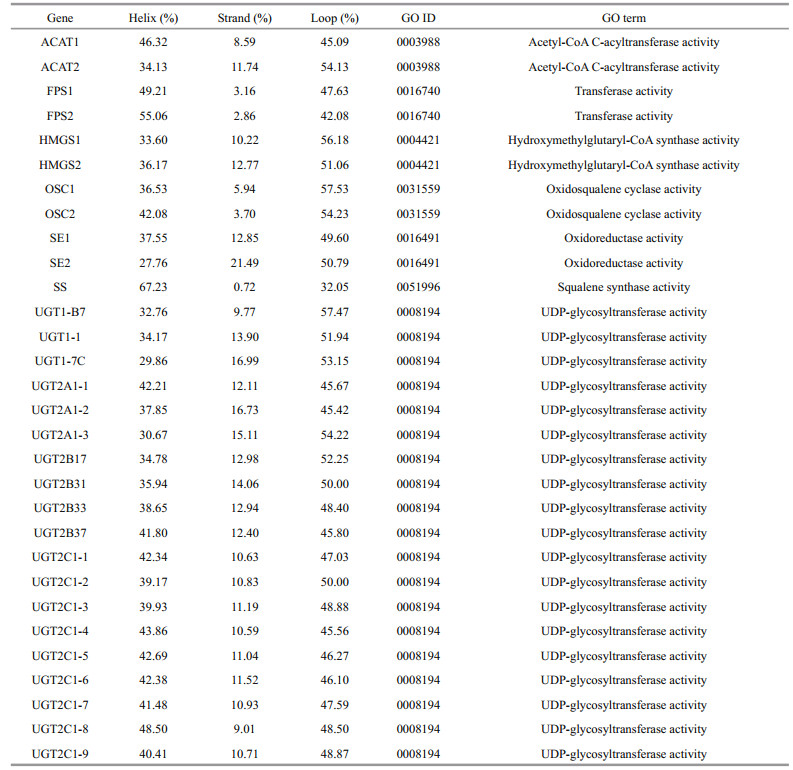
Analysis of the saponin biosynthesis enzymes revealed conserved tertiary structures comparable to those found in plants. The secondary structures of AACTs, HMGSs, SEs, and UGTs were composed of both α-helices and β-sheets. Notably, the secondary structures of FPSs, OSCs, and SSs displayed a relatively high level of α-helices and a low percentage of β-sheets (Fig. 4 & Table 2). Specifically, the secondary structure composition of ACAT2 was 34.13% helices and 11.74% strands. Moreover, the secondary structure of ACAT1 was made up of 46.32% helices and 8.59% strands. The FPSs were composed of 49.21%–55.06% helices and 2.86%– 3.16% strands, whereas the secondary structures of the HMGSs were comprised of 33.60%–36.17% helices and 10.22%–12.77% strands. Additionally, the OSCs consisted of 36.53%–42.08% helices and 3.70%–5.94% strands. The SEs were made up of 27.76%–37.55% helices and 12.85%–21.49% strands. The SS enzyme was composed of 67.23% helices and 0.72% strands. The UGTs were comprised of 29.86%– 48.50% helices and 9.01%–16.99% strands.
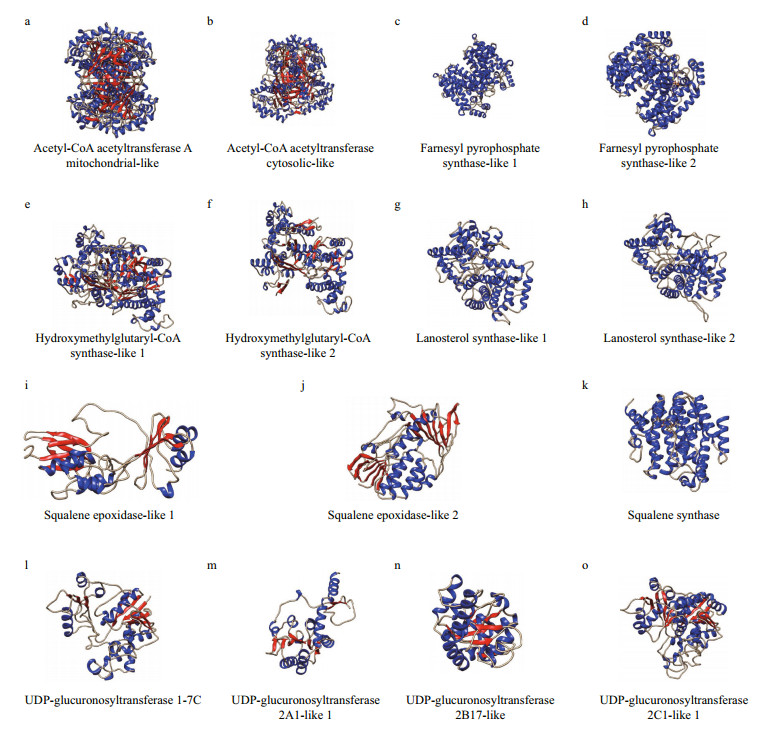
|
| Fig.4 Tertiary structures of saponin biosynthesis enzymes in A. japonicus α-helices in blue, whereas β-sheets in red. |
|
Most of the enzymes responsible for the saponin biosynthesis were categorized into the corresponding molecular function ontology via homology to the annotated proteins (Table 2). ACAT1 and ACAT2 belonged to the acetyl-CoA C-acyltransferase activity (GO: 0003988). Furthermore, HMGSs, OSCs, SS, and UGTs were categorized into the hydroxymethylglutaryl-CoA synthase (GO: 0004421), oxidosqualene cyclase (GO: 0031559), squalene synthase (GO: 0051996), and UDP-glycosyltransferase (GO: 0008194) activity, respectively. Lastly, FPSs and SEs were classified into the transferase (GO: 0016740) and oxidoreductase (GO: 0016491) activity, correspondingly.
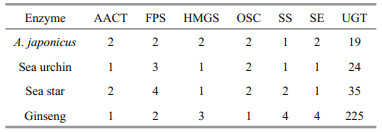
3.2 Copy numbers of saponin biosynthesis enzymesOverall, the copy numbers of the enzymes responsible for the saponin biosynthesis significantly varied between organisms, particularly those of UGTs (Table 3). It is noteworthy that the copy numbers of SS in ginseng were higher than those in the echinoderm species. Some duplicated saponin biosynthesis enzymes in the genome of A. japonicus might be a result of independent tandem duplication events. Two copies of the lanosterol synthase genes (FPS1 and FPS2) were determined as tandemly arrayed genes on scaffold879. Moreover, multiple copies of UGT2C1 were identified in the genome A. japonicus. Two copies (UGT2A1-1 and UGT2A1-2) and two additional paralogous copies (UGT2C1-8 and UGT2C1-9) were tandemly arrayed genes on scaffold28 and scaffold1550, respectively. Lastly, UGT2B37 and UGT2C1-4 were tandemly arrayed genes on scaffold285.
|
In A. japonicus, ACAT2 exhibited 57.55% sequence identity with the corresponding gene in the sea star. Notably, ACAT1 shared a 45.67% and 44.06% sequence identity with the homologous genes in the sea star and sea urchin. The HMGSs of A. japonicus shared amino acid sequence similarities of 44.32%–64.35% and 43.16%–64.55% with the sea star and sea urchin. Additionally, the OSCs in A. japonicus displayed 29.40%–36.30% and 29.62%– 36.30% sequence identity with the corresponding genes in the sea star and sea urchin. The SS enzyme in A. japonicus showed sequence identity of 57.51% and 53.43% with the relevant genes in the sea star and sea urchin. The SEs in A. japonicus shared an amino acid identity of 26.83%–39.22% with the corresponding protein in the sea star. However, the SEs in A. japonicus shared a relatively low amino acid sequence similarity of approximately 1.36%–15.62% with the corresponding genes in the sea urchin. The UGTs in A. japonicus exhibited low amino acid sequence similarities of approximately 9.69% and 11.36% with the sea star and sea urchin, respectively. Thus, the obtained results suggested that overall, the SEs and UGTs in A. japonicus shared low amino acid sequence similarities to the corresponding genes in other echinoderms.
Similar to A. japonicus, we also determined seven types of enzymes responsible for saponin biosynthesis in ginseng, i.e., AACTs, FPSs, HMGSs, OSCs, SS, SEs, and UGTs. The ginseng ACAT2 shared a sequence similarity of 43.10% with the relevant gene in A. japonicus. Moreover, FPSs shared 48.23%– 48.58% sequence similarity with the corresponding genes in A. japonicus. The ginseng HMGSs shared an amino acid similarity of 39.54%–52.06% with the homologous genes in A. japonicus. The OSCs in ginseng exhibited 33.33%–35.80% identities with the related genes in A. japonicus. The ginseng SEs shared a sequence identity of 37.28%–44.00% with the homologous genes in A. japonicus. Furthermore, the ginseng SS showed a 44.56%–48.89% amino acid sequence identity with the corresponding proteins in A. japonicus. It is recognized that sequence identities of most of the saponin biosynthesis enzymes have been evolutionarily conserved across the ginseng and marine organisms. Nonetheless, 19 and 225 UGTs were identified in A. japonicus and ginseng, respectively. Intriguingly, it was one of the largest gene families in ginseng (Xu et al., 2017); however, not in other plant species. The sequence similarities of UGTs between A. japonicus and ginseng ranged from 5.76% to 23.50%.
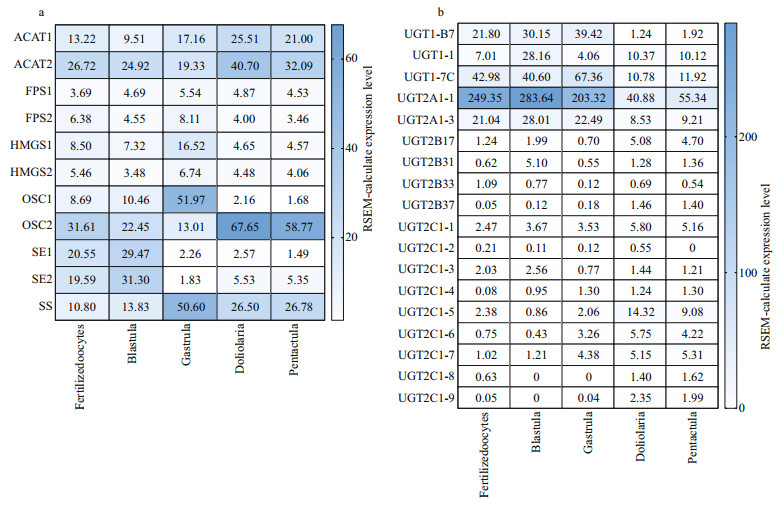
3.3 Expression of saponin biosynthesis enzymes during developmental stagesAACTs were expressed at the highest levels at the doliolaria stage (Fig. 5a). Moreover, ACAT2 was expressed at a higher level than ACAT1 at all five developmental stages. The expression levels of FPSs only marginally changed during the five developmental stages. It is noteworthy that the expression of HMGS1 was the highest at the gastrula stage. Similarly, OSC1 also showed the highest expression at the gastrula stage, whereas OSC2 was highly expressed at the remaining four stages (fertilized oocytes, blastula, doliolaria, and penractula). SE1 and SE2 were highly expressed at the two early stages (fertilized oocytes and blastula). SS exhibited higher expression levels at the gastrula, doliolaria, and penractula stages. In contrast, UGT1-B7, UGT1-1, UGT1-7C, UGT2A1-1, and UGT2A1-3 showed relatively high expression levels at the fertilized oocytes, blastula, and gastrula stages (Fig. 5b). The expression levels of UGTs were relatively low at all developmental stages. Among them, UGT2A1-1 showed the highest expression levels, while UGT2A1-2 was not detected in the analyzed RNA-seq datasets.
|
| Fig.5 RSEM-calculate expression levels of saponin biosynthesis enzymes in A. japonicus during developmental stages |
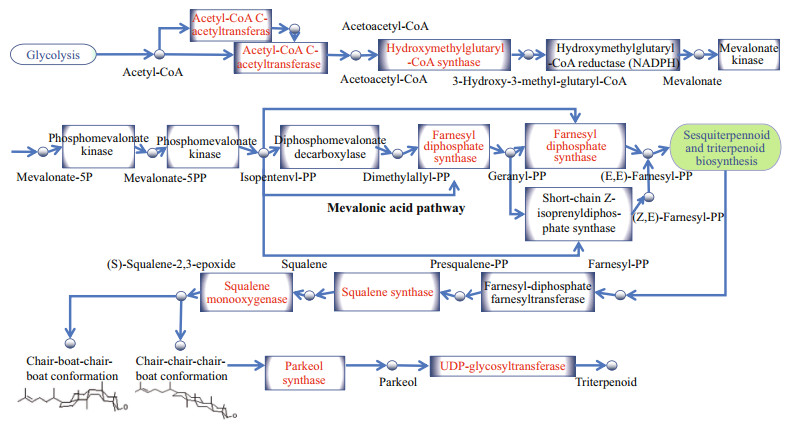
In the present study, the putative mechanisms of saponin biosynthesis in A. japonicus were predicted based on previous studies concerning triterpenoid saponins biosynthesis. As demonstrated in Fig. 6, the saponin biosynthesis involves several important biological reactions. Initially, AACTs generate acetoacetyl-CoA from acetyl-CoA (Haapalainen et al., 2007). HMGSs subsequently catalyze the reaction between acetyl-CoA and acetoacetyl-CoA to generate HMG-CoA (Rokosz et al., 1994). HMG-CoA reductase then facilitates the synthesis of mevalonate from HMG-CoA (Luskey and Stevens, 1985). Furthermore, mevalonate-5-diphosphate is biosynthesized from mevalonate by the action of mevalonate kinase and phosphomevalonate kinase (Miziorko, 2011). Subsequently, isopentenyl-PP and dimethylallyl-PP are generated via the mevalonic acid (MVA) pathway (Chang et al., 2013). FPSs catalyze the reaction of dimethylallyl pyrophosphate to form farnesyl-PP (Qian et al., 2017), while SS facilitates the conversion of farnesyl-PP to squalene (Kim et al., 2011). SEs then catalyze the transformation of squalene to (S)-2, 3-oxidosqualene (Zhao et al., 2010). In A. japonicus, OSCs exhibiting a plant-like motif produces parkeol instead of lanosterol (Li et al., 2018). Lastly, UGTs modify and produce diverse triterpenoid saponins (Seki et al., 2015).
|
| Fig.6 Putative triterpene saponins biosynthetic pathway in A. japonicus Green textboxes represent metabolic pathways. Red texts represent saponin biosynthesis enzymes in A. japonicus. Small circles represent metabolic intermediates. A. japonicus produces triterpenoid saponins through parkeol other than lanosterol. |
Saponins are a large family of amphiphilic glycosides of steroids and triterpenes (Yang et al., 2014). Although saponins have been widely identified and isolated from plants, their determination in aquaculture species has been limited. Many pharmacological activity studies have focused on the sea cucumber saponins (Chen et al., 2018; Ding et al., 2018; Kwon et al., 2018; Meng et al., 2018; Han et al., 2019). It has been found that enzymes, such as OSCs, P450s, UGTs, and several specialized transferases, are essential in the saponin biosynthesis (Adzhubei et al., 2013; Moses et al., 2014). A recent study on the evolution of OSC genes in A. japonicus (Li et al., 2018) as well as other work on the body wall transcriptome of H. scabra (Mitu et al., 2017) indicated that sea cucumber exhibited a plant-like saponin biosynthesis pathway.
In this work, we identified and characterized 30 enzymes responsible for the saponin biosynthesis by analyzing the genome of A. japonicus. The key saponin biosynthesis enzymes were classified into seven types, i.e., two AACTs, two FPSs, two HMGSs, two OSCs, one SS, two SEs, and 19 UGTs. The annotation of the enzymes responsible for saponin biosynthesis was based on the phylogenetic and sequence analyses. Sea cucumber, also called marine ginseng, displays similar appearance and medicinal properties to the plant ginseng (Bordbar et al., 2011). Both sea cucumber and ginseng contain a high abundance of potential anticancer compounds, including triterpene glycosides (Wang et al., 2007; Aminin et al., 2015). We found that the sequences of the upstream triterpenoid saponin biosynthesis genes in echinoderms and ginseng exhibited high sequence similarities. The comparison of animal and plant OSCs revealed that the OSCs in A. japonicus contained plant-like motifs lacking in sea urchin and starfish (Li et al., 2018). In addition, UGTs in A. japonicus shared relatively low amino acid sequence similarities with other echinoderms (9.69% and 11.36%), while those between A. japonicus and ginseng were 5.76%–23.50%. It is recognized that UGT enzymes participate in the generation of structurally diverse triterpenoid saponins via glycosylation reactions using a UDP-based sugar donor (Seki et al., 2015). It was previously reported that the tandem array of UGTs in Barbarea vulgaris led to higher order bisdesmosidic glycosylation and the formation of various saponins (Erthmann et al., 2018). It was hypothesized that in A. japonicus, the low homology of UGTs with the corresponding genes in other echinoderms might result in the production of structurally and functionally diverse saponins. The conserved domains (Fig. 3) as well as secondary structures of the key enzymes in the saponin biosynthesis pathway were evaluated based on their predicted protein sequences. AACTs, HMGSs, SEs, and UGTs were composed of both α-helices and β-sheets, whereas the secondary structures of FPSs, OSCs, and SS contained a relatively high level of α-helices and a low percentage of β-sheets.
Saponin biosynthesis is an important biological process occurring during the growth and development of numerous plants (Haralampidis et al., 2002). Nevertheless, research concerning the biosynthesis of saponins during the developmental stages of sea cucumber is limited. In this work, we determined that the enzymes involved in the saponin biosynthesis pathway exhibited dynamic expression patterns during the five critical stages of development of A. japonicus (fertilized oocytes, blastula, gastrula, doliolaria, and penractula). Mitochondrial ACAT1 and cytosolic ACAT2 showed differential expression in various tissues of non-human primates (Lee et al., 2000). It was proposed that the two enzymes had different functions, as ACAT1 was ubiquitous, while ACAT2 was specifically expressed in the liver and intestine (Lee et al., 2000). In A. japonicus, ACAT2 was expressed at a higher level than ACAT1 at all five developmental stages. OSC1 showed higher expression at the gastrula stage, whereas OSC2 was highly expressed at the remaining four stages (i.e., fertilized oocytes, blastula, doliolaria, and penractula). Conversely, in Stichopus horrens, during the triterpenoid biosynthesis, OSC1 and OSC2 were highly expressed in the intestine (Liu et al., 2018). Both in A. japonicus (Li et al., 2018) and Holothuria arenicola (Cordeiro et al., 1988), OSC1 and OSC2 were modified to synthesize parkeol instead of lanosterol. Furthermore, in A. japonicus, most UGTs were expressed at low levels during the critical development period, with the exception of the UGT1 family and UGT2A subfamily. UGT2A1-1 showed the highest expression levels. It is known that UGTs play essential roles in the structural diversity of saponins (Seki et al., 2015). Hence, higher expression levels of the UGT1 and UGT2A subfamilies might contribute to the formation of various saponins during the early development of A. japonicus.
The MVA and non-mevalonate pathway (MEP) pathways are critical in saponin biosynthesis (Zhao et al., 2014). The MEP pathway is a plant-specialized process, while the MVA pathway plays an essential role in the saponin biosynthesis as well as the production of saponin precursors in A. japonicus. In A. japonicus, parkeol is biosynthesized by OSCs (Li et al., 2018), and then modified by various UGTs to produce diverse structural triterpene saponins. In the present study, we presented putative mechanisms for the biosynthesis of saponin in A. japonicus (Fig. 6); however, further verification of the proposed mechanisms is required.
5 CONCLUSIONThirty genes responsible for biosynthesis of saponin were identified in A. japonicus. These included upstream (AACTs, FPSs, HMGSs), midstream (OSCs, SEs, SS), and downstream (UGTs) enzymes. The genomic organization and evolution analysis of the entire set of saponin biosynthesis enzymes in A. japonicus provided further understanding of crucial saponin biosynthesis components. It was determined that UGTs shared lower amino acid sequence similarity with the corresponding genes in other echinoderms than upstream and midstream enzymes, which could contribute to the production of more diverse types of saponins. The dynamic expression patterns of the saponin biosynthesis enzymes during developmental stages are important for understanding the role of saponin biosynthesis in the growth and development of sea cucumber. The putative mechanisms proposed herein provide a complete view of the saponin biosynthesis in A. japonicus and will be valuable for the evaluation of saponin biosynthesis in other echinoderms.
6 DATA AVAILABILITY STATEMENTThe datasets analyzed during the current study are available at NCBI under the accession number PRJNA354676.
Adzhubei I, Jordan D M, Sunyaev S R. 2013. Predicting functional effect of human missense mutations using PolyPhen-2. Current Protocols in Human Genetics, 76(1): 7.20.
DOI:10.1002/0471142905.hg0720s76 |
Aminin D L, Menchinskaya E S, Pisliagin E A, Silchenko A S, Avilov S A, Kalinin V I. 2015. Anticancer activity of sea cucumber triterpene glycosides. Marine Drugs, 13(3): 1202-1223.
DOI:10.3390/md13031202 |
Bahrami Y, Franco C M M. 2015. Structure elucidation of new acetylated saponins, Lessoniosides A, B, C, D, and E, and non-acetylated saponins, Lessoniosides F and G, from the viscera of the sea cucumber Holothuria lessoni. Marine Drugs, 13(1): 597-617.
DOI:10.3390/md13010597 |
Bahrami Y, Zhang W, Franco C. 2014. Discovery of novel saponins from the viscera of the sea cucumber Holothuria lessoni. Marine Drugs, 12(5): 2633-2667.
DOI:10.3390/md12052633 |
Bordbar S, Anwar F, Saari N. 2011. High-value components and bioactives from sea cucumbers for functional foods—a review. Marine Drugs, 9(10): 1761-1805.
DOI:10.3390/md9101761 |
Caulier G, Van Dyck S, Gerbaux P, Eeckhaut I, Flammang P. 2011. Review of saponin diversity in sea cucumbers belonging to the family Holothuriidae. SPC Beche-de-mer Information Bulletin, 31(1): 48-54.
|
Chang W C, Song H, Liu H W, Liu P H. 2013. Current development in isoprenoid precursor biosynthesis and regulation. Current Opinion in Chemical Biology, 17(4): 571-579.
DOI:10.1016/j.cbpa.2013.06.020 |
Chen C, Han X Q, Dong P, Li Z J, Yanagita T, Xue C H, Zhang T T, Wang Y M. 2018. Sea cucumber saponin liposomes ameliorate obesity-induced inflammation and insulin resistance in high-fat-diet-fed mice. Food & Function, 9(2): 861-870.
DOI:10.1039/C7FO01599B |
Claereboudt E J S, Caulier G, Decroo C, Colson E, Gerbaux P, Claereboudt M R, Schaller H, Flammang P, Deleu M, Eeckhaut I. 2019. Triterpenoids in echinoderms: fundamental differences in diversity and biosynthetic pathways. Marine Drugs, 17(6): 352.
DOI:10.3390/md17060352 |
Cordeiro N L, Kerr R G, Djerassi C. 1988. Biosynthetic studies of marine lipids 15. Conversion of parkeol (lanosta-9(11), 24-dien-3β-ol) to 14α-methylcholest-9(11)-en-3β-ol in the sea cucumber Holothuria arenicola. Tetrahedron Letters, 29(18): 2159-2162.
DOI:10.1016/S0040-4039(00)86698-8 |
Darriba D, Taboada G L, Doallo R, Posada D. 2011. ProtTest 3: fast selection of best-fit models of protein evolution. Bioinformatics, 27(8): 1164-1165.
DOI:10.1093/bioinformatics/btr088 |
Ding L, Zhang T T, Che H X, Zhang L Y, Xue C H, Chang Y G, Wang Y M. 2018. Saponins of sea cucumber attenuate atherosclerosis in ApoE−/− mice via lipid-lowering and anti-inflammatory properties. Journal of Functional Foods, 48: 490-497.
DOI:10.1016/j.jff.2018.07.046 |
Edgar R C. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research, 32(5): 1792-1797.
DOI:10.1093/nar/gkh340 |
Erthmann P Ø, Agerbirk N, Bak S. 2018. A tandem array of UDP-glycosyltransferases from the UGT73C subfamily glycosylate sapogenins, forming a spectrum of mono-and bisdesmosidic saponins. Plant Molecular Biology, 97(1-2): 37-55.
DOI:10.1007/s11103-018-0723-z |
Forester J, Hartmut T. 2006. MetaCyc Pathway: saponin biosynthesis I. Advances in Experimental Medicine and Biology, 405: 377-385.
|
Francis G, Kerem Z, Makkar H P S, Becker K. 2002. The biological action of saponins in animal systems: a review. British Journal of Nutrition, 88(6): 587-605.
DOI:10.1079/BJN2002725 |
Guex N, Peitsch M C. 1997. SWISS-MODEL and the Swiss-Pdb Viewer: an environment for comparative protein modeling. Electrophoresis, 18(15): 2714-2723.
DOI:10.1002/elps.1150181505 |
Haapalainen A M, Meriläinen G, Pirilä P L, Kondo N, Fukao T, Wierenga R K. 2007. Crystallographic and kinetic studies of human mitochondrial acetoacetyl-CoA thiolase: the importance of potassium and chloride ions for its structure and function. Biochemistry, 46(14): 4305-4321.
DOI:10.1021/bi6026192 |
Han X Q, Zhang L Y, Ding L, Shi H H, Xue C H, Zhang T T, Wang Y M. 2019. Synergistic effect of sea cucumber saponins and EPA-enriched phospholipids on insulin resistance in high-fat diet-induced obese mice. Food & Function, 10(7): 3955-3964.
DOI:10.1039/C9FO01147A |
Haralampidis K, Trojanowska M, Osbourn A E. 2002. Biosynthesis of triterpenoid saponins in plants. In: Dutta N N, Hammar F, Haralampidis K, Karanth N G, König A, Krishna S H, Kunze G, Nagy E, Orlich B, Osbourn A E, Raghavarao K S M S, Riedel K, Sahoo G C, Schomäcker R, Srinivas N D, Trojanowska M eds. History and Trends in Bioprocessing and Biotransformation. Springer, Berlin. p. 31-49, https://doi.org/10.1007/3-540-44604-4_2.
|
Itkin M, Heinig U, Tzfadia O, Bhide A J, Shinde B, Cardenas P D, Bocobza S E, Unger T, Malitsky S, Finkers R, Tikunov Y, Bovy A, Chikate Y, Singh P, Rogachev I, Beekwilder J, Giri A P, Aharoni A. 2013. Biosynthesis of antinutritional alkaloids in solanaceous crops is mediated by clustered genes. Science, 341(6142): 175-179.
DOI:10.1126/science.1240230 |
Kensil C R. 1996. Saponins as vaccine adjuvants. Critical Reviews in Therapeutic Drug Carrier Systems, 13(1-2): 1-55.
|
Kim T D, Han J Y, Huh G H, Choi Y E. 2011. Expression and functional characterization of three squalene synthase genes associated with saponin biosynthesis in Panax ginseng. Plant and Cell Physiology, 52(1): 125-137.
DOI:10.1093/pcp/pcq179 |
Krokida A, Delis C, Geisler K, Garagounis C, Tsikou D, Peña-Rodríguez L M, Katsarou D, Field B, Osbourn A E, Papadopoulou K K. 2013. A metabolic gene cluster in Lotus japonicus discloses novel enzyme functions and products in triterpene biosynthesis. New Phytologist, 200(3): 675-690.
DOI:10.1111/nph.12414 |
Kwon T R, Oh C T, Bak D H, Kim J H, Seok J, Lee J H, Lim S H, Yoo K H, Kim B J, Kim H. 2018. Effects on skin of Stichopus japonicus viscera extracts detected with saponin including Holothurin A: down-regulation of melanin synthesis and up-regulation of neocollagenesis mediated by ERK signaling pathway. Journal of Ethnopharmacology, 226: 73-81.
DOI:10.1016/j.jep.2018.08.007 |
Lee R G, Willingham M C, Davis M A, Skinner K A, Rudel L L. 2000. Differential expression of ACAT1 and ACAT2 among cells within liver, intestine, kidney, and adrenal of nonhuman primates. Journal of Lipid Research, 41(12): 1991-2001.
DOI:10.1016/S0022-2275(20)32360-9 |
Li B, Dewey C N. 2011. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics, 12(1): 323.
DOI:10.1186/1471-2105-12-323 |
Li Y L, Wang R J, Xun X G, Wang J, Bao L S, Thimmappa R, Ding J, Jiang J W, Zhang L H, Li T Q, Lv J, Mu C, Hu X L, Zhang L L, Liu J, Li Y Q, Yao L J, Jiao W Q, Wang Y F, Lian S S, Zhao Z L, Zhan Y Y, Huang X T, Liao H, Wang J, Sun H Z, Mi X, Xia Y, Xing Q, Lu W, Osbourn A, Zhou Z C, Chang Y Q, Bao Z M, Wang S. 2018. Sea cucumber genome provides insights into saponin biosynthesis and aestivation regulation. Cell Discovery, 4(1): 29.
DOI:10.1038/s41421-018-0030-5 |
Liu H L, Kong X, Chen J W, Zhang H B. 2018. De novo sequencing and transcriptome analysis of Stichopus horrens to reveal genes related to biosynthesis of triterpenoids. Aquaculture, 491: 358-367.
DOI:10.1016/j.aquaculture.2018.01.012 |
Luskey K L, Stevens B. 1985. Human 3-hydroxy-3-methylglutaryl coenzyme A reductase. Conserved domains responsible for catalytic activity and sterol-regulated degradation. Journal of Biological Chemistry, 260(18): 10271-10277.
DOI:10.1016/S0021-9258(17)39242-6 |
Maier M S, Roccatagliata A J, Kuriss A, Chludil H, Seldes A M, Pujol C A, Damonte E B. 2001. Two new cytotoxic and virucidal trisulfated triterpene glycosides from the Antarctic sea cucumber Staurocucumis liouvillei. Journal of Natural Products, 64(6): 732-736.
DOI:10.1021/np000584i |
Makarieva T N, Stonik V A, Kapustina I I, Boguslavsky V M, Dmitrenoik A S, Kalinin V I, Cordeiro M L, Djerassi C. 1993. Biosynthetic studies of marine lipids. 42. Biosynthesis of steroid and triterpenoid metabolites in the sea cucumber Eupentacta fraudatrix. Steroids, 58(11): 508-517.
DOI:10.1016/0039-128X(93)90026-J |
Marchler-Bauer A, Lu S, Anderson J B, Chitsaz F, Derbyshire M K, DeWeese-Scott C, Fong J H, Geer L Y, Geer R C, Gonzales N R, Gwadz M, Hurwitz D I, Jackson J D, Ke Z X, Lanczycki C J, Lu F, Marchler G H, Mullokandov M, Omelchenko M V, Robertson C L, Song J S, Thanki N, Yamashita R A, Zhang D C, Zhang N G, Zheng C J, Bryant S H. 2010. CDD: a Conserved Domain Database for the functional annotation of proteins. Nucleic Acids Research, 39(S1): D225-D229.
DOI:10.1093/nar/gkq1189 |
Meng J, Hu X Q, Zhang T T, Dong P, Li Z J, Xue C H, Chang Y G, Wang Y M. 2018. Saponin from sea cucumber exhibited more significant effects than ginsenoside on ameliorating high fat diet-induced obesity in C57BL/6 mice. MedChemComm, 9(4): 725-734.
DOI:10.1039/C7MD00653E |
Mitu S A, Bose U, Suwansa-Ard S, Turner L H, Zhao M, Elizur A, Ogbourne S M, Shaw P N, Cummins S F. 2017. Evidence for a saponin biosynthesis pathway in the body wall of the commercially significant sea cucumber Holothuria scabra. Marine Drugs, 15(11): 349.
DOI:10.3390/md15110349 |
Miziorko H M. 2011. Enzymes of the mevalonate pathway of isoprenoid biosynthesis. Archives of Biochemistry and Biophysics, 505(2): 131-143.
DOI:10.1016/j.abb.2010.09.028 |
Moghimipour E, Handali S. 2015. Saponin: properties, methods of evaluation and applications. Annual Research & Review in Biology, 5(3): 207-220.
DOI:10.9734/ARRB/2015/11674 |
Moses T, Papadopoulou K K, Osbourn A. 2014. Metabolic and functional diversity of saponins, biosynthetic intermediates and semi-synthetic derivatives. Critical Reviews in Biochemistry and Molecular Biology, 49(6): 439-462.
DOI:10.3109/10409238.2014.953628 |
Mugford S T, Louveau T, Melton R, Qi X Q, Bakht S, Hill L, Tsurushima T, Honkanen S, Rosser S J, Lomonossoff G P, Osbourn A. 2013. Modularity of plant metabolic gene clusters: a trio of linked genes that are collectively required for acylation of triterpenes in oat. The Plant Cell, 25(3): 1078-1092.
DOI:10.1105/tpc.113.110551 |
Mugford S T, Osbourn A. 2012. Saponin synthesis and function. In: Concepts N, Approaches E eds. Isoprenoid Synthesis in Plants and Microorganisms. Springer, New York. p. 405-424, https://doi.org/10.1007/978-1-4614-4063-5_28.
|
Pettersen E F, Goddard T D, Huang C C, Couch G S, Greenblatt D M, Meng E C, Ferrin T E. 2004. UCSF Chimera—a visualization system for exploratory research and analysis. Journal of Computational Chemistry, 25(13): 1605-1612.
DOI:10.1002/jcc.20084 |
Qian J Y, Liu Y, Chao N X, Ma C T, Chen Q C, Sun J, Wu Y S. 2017. Positive selection and functional divergence of farnesyl pyrophosphate synthase genes in plants. BMC Molecular Biology, 18(1): 3.
DOI:10.1186/s12867-017-0081-4 |
Rokosz L L, Boulton D A, Butkiewicz E A, Sanyal G, Cueto M A, Lachance P A, Hermes J D. 1994. Human cytoplasmic 3-hydroxy-3-methylglutaryl coenzyme A synthase: expression, purification, and characterization of recombinant wild-type and Cys129 mutant enzymes. Archives of Biochemistry and Biophysics, 312(1): 1-13.
DOI:10.1006/abbi.1994.1273 |
Rost B, Yachdav G, Liu J F. 2004. The PredictProtein server. Nucleic Acids Research, 32(S2): W321-W326.
DOI:10.1093/nar/gkh377 |
Schultz J, Copley R R, Doerks T, Ponting C P, Bork P. 2000. SMART: a web-based tool for the study of genetically mobile domains. Nucleic Acids Research, 28(1): 231-234.
DOI:10.1093/nar/28.1.231 |
Seki H, Tamura K, Muranaka T. 2015. P450s and UGTs: key players in the structural diversity of triterpenoid saponins. Plant and Cell Physiology, 56(8): 1463-1471.
DOI:10.1093/pcp/pcv062 |
Silchenko A S, Kalinovsky A I, Avilov S A, Andryjashchenko P V, Dmitrenok P S, Kalinin V I, Stonik V A. 2012. 3β-O-Glycosylated 16β-acetoxy-9β-H-lanosta-7, 24-diene-3β, 18, 20β-triol, an intermediate metabolite from the sea cucumber Eupentacta fraudatrix and its biosynthetic significance. Biochemical Systematics and Ecology, 44: 53-60.
DOI:10.1016/j.bse.2012.04.008 |
Skropeta D. 2008. Deep-sea natural products. Natural Product Reports, 25(6): 1131-1166.
DOI:10.1039/b808743a |
Stonik V A. 2001. Marine polar steroids. Russian Chemical Reviews, 70(8): 673-715.
DOI:10.1070/RC2001v070n08ABEH000679 |
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution, 30(12): 2725-2729.
DOI:10.1093/molbev/mst197 |
Van Dyck S, Gerbaux P, Flammang P. 2010. Qualitative and quantitative saponin contents in five sea cucumbers from the Indian Ocean. Marine Drugs, 8(1): 173-189.
DOI:10.3390/md8010173 |
Vincken J P, Heng L, De Groot A, Gruppen H. 2007. Saponins, classification and occurrence in the plant kingdom. Phytochemistry, 68(3): 275-297.
DOI:10.1016/j.phytochem.2006.10.008 |
Wang W, Zhao Y Q, Rayburn E R, Hill D L, Wang H, Zhang R W. 2007. In vitro anti-cancer activity and structure– activity relationships of natural products isolated from fruits of Panax ginseng. Cancer Chemotherapy and Pharmacology, 59(5): 589-601.
DOI:10.1007/s00280-006-0300-z |
Xiao G Z, Shao X F, Zhu D P, Yu B. 2019. Chemical synthesis of marine saponins. Natural Product Reports, 36(5): 769-787.
DOI:10.1039/C8NP00087E |
Xu J, Chu Y, Liao B S, Xiao S M, Yin Q G, Bai R, Su H, Dong L L, Li X W, Qian J, Zhang J J, Zhang Y J, Zhang X Y, Wu M L, Zhang J, Li G Z, Zhang L, Chang Z Z, Zhang Y B, Jia Z W, Liu Z X, Daniel A, Ruth N, Zhang L J, Cheng R Y, Zhu Y J, Zhu G W, Rao W, Zhou C, Qiao L R, Huang Z H, Cheng Y C, Chen S L. 2017. Panax ginseng genome examination for ginsenoside biosynthesis. GigaScience, 6(11): 1-15.
DOI:10.1093/gigascience/gix093 |
Yang Y, Laval S, Yu B. 2014. Chemical synthesis of saponins. Advances in Carbohydrate Chemistry and Biochemistry, 71: 137-226.
DOI:10.1016/B978-0-12-800128-8.00002-9 |
Zhang X J, Sun L N, Yuan J B, Sun Y M, Gao Y, Zhang L B, Li S H, Dai H, Hamel J F, Liu C Z, Yu Y, Liu S L, Lin W C, Guo K M, Jin S J, Xu P, Storey K B, Huan P, Zhang T, Zhou Y, Zhang J Q, Lin C G, Li X N, Xing L L, Huo D, Sun M Z, Wang L, Mercier A, Li F H, Yang H S, Xiang J H, Tyler-Smith C. 2017. The sea cucumber genome provides insights into morphological evolution and visceral regeneration. PLoS Biology, 15(10): e2003790.
DOI:10.1371/journal.pbio.2003790 |
Zhao C L, Cui X M, Chen Y P, Liang Q. 2010. Key enzymes of triterpenoid saponin biosynthesis and the induction of their activities and gene expressions in plants. Natural Product Communications, 5(7): 1147-1157.
|
Zhao S J, Wang L, Liu L, Liang Y L, Sun Y, Wu J J. 2014. Both the mevalonate and the non-mevalonate pathways are involved in ginsenoside biosynthesis. Plant Cell Reports, 33(3): 393-400.
DOI:10.1007/s00299-013-1538-7 |
Zhao Y C, Xue C H, Zhang T T, Wang Y M. 2018. Saponins from sea cucumber and their biological activities. Journal of Agricultural and Food Chemistry, 66(28): 7222-7237.
DOI:10.1021/acs.jafc.8b01770 |
 2021, Vol. 39
2021, Vol. 39


