Institute of Oceanology, Chinese Academy of Sciences
Article Information
- ZHANG Tongwei, XU Huangtao, LIU Jia, PAN Yongxin, CAO Changqian
- Determination of the heating efficiency of magnetotactic bacteria in alternating magnetic field
- Journal of Oceanology and Limnology, 39(6): 2116-2126
- http://dx.doi.org/10.1007/s00343-021-1071-4
Article History
- Received Mar. 1, 2021
- accepted in principle Mar. 23, 2021
- accepted for publication Apr. 30, 2021
2 Innovation Academy for Earth Science, Chinese Academy of Sciences, Beijing 100029, China;
3 College of Earth and Planetary Sciences, University of Chinese Academy of Sciences, Beijing 100049, China;
4 France-China Joint Laboratory for Evolution and Development of Magnetotactic Multicellular Organisms, Chinese Academy of Sciences, Beijing 100029, China
Magnetic hyperthermia is an important treatment strategy of diseases based on raising the temperature of local-regional tissues or whole body above physiological value by converting the magnetic energy into thermal energy. It has gone into clinic testing for therapy of malignant tumors due to its intrinsic high tissue penetration and non-invasion (Falk and Issels, 2001; Rosensweig, 2002; Johannsen et al., 2007; Périgo et al., 2015; Blanco-Andujar et al., 2018). Tumor tissues are more susceptible to heat than healthy tissue. When temperatures reach the 41–46 ℃ range (therapeutic window), tumor tissue tends to suffer irreversible damage, while healthy tissue is usually unharmed(Pankhurst et al., 2003; Ortega and Pankhurst, 2013). In clinical trials, it has been proved that hyperthermia combined with conventional treatment modalities (e.g., radiotherapy, chemotherapy) can improve response and survival outcome of tumor patients (Thiesen and Jordan, 2008; Maier-Hauff et al., 2011).
During the magnetic hyperthermia treatment process, magnetic nanoparticles (MNPs) with various sizes, ranging from superparamagnetism to magnetic single-domain (roughly 10 up to 100 nm), can be employed to complete the energy conversion through hysteresis loss or Néel/Brownian relaxation process in an alternating magnetic field (AMF) (Rosensweig, 2002; Hergt et al., 2006; Dadfar et al., 2019; Xu and Pan, 2019). Usually, the energy conversion efficiency is expressed by specific absorption rate (SAR), which is defined as the absorbed energy per unit of nanoparticle mass (Mason et al., 2000; Ortega and Pankhurst, 2013). The SAR is directly related to the initial slope of the temperature rising curves, and calculated using the next Eq.1 (Andreu and Natividad, 2013),
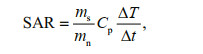 (1)
(1)where ms is the mass of solvent, mn is the mass of MNPs, Cp is the heat capacity of solvent, and ΔT/Δt is the initial slope of the temperature rising curve: fitting the curve with Box-Lucas equation [T(t)=a(1–e-bt)] to calculate the ΔT/Δt at t=0 (Kallumadil et al., 2009). Experimental results show that single-domain particles with narrow size distribution may theoretically provide the most desired heat generation efficiency (Hergt et al., 2008). High SAR ensures enhancement of specific heating power and reduction of dosage applied to the tumor.
MTBs are a kind of aquatic microorganisms that can live in oxic‐anoxic interface (OAI) environment and swim along the Earth's magnetic field lines (Blakemore, 1975; Frankel et al., 1997). A unique feature of this type of bacteria is that it has nanometer-sized (30–120 nm) magnetosomes (Fe3O4/Fe3S4) arranged in chains, which show characteristic single-domain magnetic properties (Bazylinski and Frankel, 2004; Ding et al., 2010; Zhang and Pan, 2018). Magnetosome is a kind of nanomaterial with wide application potential, which has been developed into excellent nanocarriers, such as anti-cancer drug carriers (Sun et al., 2007), transgenic carriers (Yang et al., 2016), and antibody carriers (Li et al., 2010). In addition, MTB Whole cells have been developed into nanobots for antimicrobial benefiting from the unique advantage of self-propulsion capability provided by their flagella and the guidance capabilities ensured by their magnetosome chain (Chen et al., 2019). Magnetosome chains are usually destroyed during extraction; however, the magnetosome crystals coated by membranes still have high heat efficiency due to well-developed crystallinity and particle uniformity (Muela et al., 2016), which are regulated strictly by a series of genes (Uebe and Schüler, 2016). Hergt et al. (2005) reported a maximum SAR value of 960 W/g (calculated with magnetosome mass) for magnetosomes with a mean diameter of 30 nm, under 10 kA/m field amplitude and 410 kHz frequency. Muela et al. (2016) determined a SAR of 2 394 W/g (calculated with magnetosome mass) for magnetosomes of 45 nm exposed to an AMF of 28 kA/m and a frequency of 532 kHz. In addition, each MTB intact cell with magnetosomes arranged in chains can also be used as magnetic hyperthermia agent, and the SAR of the intact cells is usually much higher than that of the isolated magnetosomes because of the low magnetostatic interactions (Fdez-Gubieda et al., 2020). Alphandéry et al. (2011) obtained the SAR value of 860 W/g (calculated with Fe mass) at 88 mT and 108 kHz for MTB intact cells using Magnetospirillum magneticum AMB-1 strain. Gandia et al. (2019) directly proved that MTB intact cells had higher SAR than the extracted magnetosomes under the same conditions using Magnetospirillum gryphiswaldense MSR-1 strain, and then, the MTB intact cells significantly inhibited the proliferation of cancer cells in AMF. Therefore, MTB whole cells are promising agent for targeted therapy of tumors.
However, the detailed magnetic hyperthermia properties and optimum heat production conditions of MTB cells are not clear. Because the AMF conditions including magnetic field amplitude and frequency providing for testing magnetic hyperthermia treatment are usually limited due to the lack of commercial standard systems. In addition, the SAR value of MTB cells is often measured by home-made equipment under individual conditions. The inconsistency in accuracy and background caloric value produced from magnetic field coil may lead to incomparable results between different laboratories. Hence, benefiting from commercial standard system D5 series (NanoScale Biomagnetics, Zaragoza, Spain) providing AMF with wide range of frequency and amplitude, we implemented a comprehensive study of the hyperthermic response of Magnetospirillum gryphiswaldense MSR-1 strain under different magnetic conditions: frequency of 144–764 kHz, and field amplitude of 10–45 kA/m. The heating efficiency was measured using calorimetric method by dual channel fiber optic sensors. We also compared the heating rates of the samples with different magnetic parameters harvested from different medium and commercial magnetic nanoparticles to determine the hyperthermia mechanisms of MTB cells.
2 MATERIAL AND METHOD 2.1 Preparation of sampleThree samples used in this work, including two MSR-1 samples and a kind of commercial magnetic particle (Fe3O4 nanoparticles) with mean size about 30 nm bought from Sangon Biotech (Shanghai), are named MTBLA-1, MTBLA-2, and commercial magnetic particle (Scmp), respectively. MTBLA-1 was cultured using medium LA-1 and method reported by Sun et al. (2008). MTBLA-2 was harvested from medium LA-2 with sodium nitrate instead of ammonium chloride as sources of nitrogen referred to LA-1 and literature of Heyen and Schüler (2003).
Cell growth and magnetism were assessed by OD565 (optical density at 565 nm) and Cmag as described by literatures (Schüler et al., 1995). The iron concentrations of the samples were determined by the spectrophotometric ferrozine assay based on the standard curve (0, 0.5, 1, 2, 4, 6, 8, and 10 μg/mL) (Stookey, 1970; Han et al., 2020).
Fresh whole cells were collected by centrifugation at 8 000 r/min for 10 min at 4 ℃ after culturing for 24 h in medium LA-2, and then suspended in water with needed cell concentrations. Samples S1, S2, and S 3 were prepared from cells with concentration of OD565=8 through ultrasonic treatment for 0, 10, and 50 min in an ice-water mixture, respectively.
2.2 Transmission electronic microscopy analysisMorphological characteristics of samples involved in this work were examined by transmission electron microscopy (TEM, JEOL JEM-2100, Tokyo) operating at 200 kV at the Institute of Geology and Geophysics, Chinese Academy of Sciences (Beijing). The sizes of magnetosome were analyzed using standard analytical software. The major and minor axes of magnetosomes were used as the length (L) and width (W) of the crystal, respectively, and the grain size was defined as (L+W)/2.
2.3 Rock magnetic measurementsThe measurement of room-temperature hysteresis loops, firstorder reversal curves (FORCs) and saturation isothermal remanent magnetization (SIRM) were performed on a VSM3900 magnetometer (Princeton Measurements Corporation, USA, sensitivity 5.0×10-10 Am2). Hysteresis loops were measured with field increments of 8 mT, ranging between maximum fields of ±1.00 T with an average time of 500 ms. SIRM acquisition curves were obtained with logarithmic increments to a maximum field of 1.00 T. A total of 180 curves for each sample were measured in FORCs using an increasing field step of 1.2 mT with an average time of 400 ms in the range from -50 to 50 mT for Bu and from 0 to 100 mT for Bc. Bu and Bc are two parameters in the FORCs test programs, representing magnetostatic interactions and coercivity of samples. The FORC diagrams were processed using FORCinel version 3.06 software with a smooth factor of 3.
2.4 Magnetic hyperthermia analysisMagnetic hyperthermia measurements of all samples had been performed using a commercial system D5 series (NanoScale Biomagnetics, Zaragoza, Spain) at the Institute of Geology and Geophysics, Chinese Academy of Sciences (Beijing, China). Samples suspended in water (1.0 mL) in a 2-mL glass chromatography vial were set in the middle of the coil. The temperatures during measurement were recorded by dual channel optic fiber temperature probes with response temperature of 0.1 ℃. One probe was placed in the center of samples, and the other probe was in the gap between glass and coil. Every magnetic field condition was first tested by 1.0-mL water, and the two probes would stabilize to the same temperature, which was the initial temperature of the corresponding testing condition.
The effect of AMF parameters on the heat production efficiency of samples were investigated. Sample MTBLA-2 with iron concentration of 48.4 μg/ mL was tested at 339 kHz with varying AMF amplitudes (10, 15, 20, 25, 30, 35, 40, and 45 kA/m), and at 30 kA/m with different frequencies (144, 298, 339, 377, 485, 621, and 764 kHz), respectively. All the other tests were performed under the magnetic field condition of 764 kHz and 30 k/Am.
The SAR values were calculated using the Eq.1 as mentioned above, where the solvent was 1-mLwater with the heat capacity of 4.185 J/(g·K).
3 RESULT 3.1 Structural characterizations of MTB cells and magnetosomes 3.1.1 Culture results of MSR-1After culturing for 24 h, the cells of MTBLA-1 and MTBLA-2 reached the states of OD565=0.9±0.1, Cmag=0.6±0.1, and OD565=0.9±0.1, Cmag=1.8±0.1, respectively. The high OD values imply that both LA-1 with ammonium chloride and LA-2 with sodium nitrate are suitable for the growth of MSR-1 under the culture conditions. Usually, the Cmag is well correlated with the average number of magnetosomes per cell and can be used for semi-quantitative assessment of magnetosome formation (Schüler et al., 1995). Thus, the larger Cmag value of MTBLA-2 indicates that it may contain more magnetosomes, and the medium LA-2 is more suitable for magnetosomes synthesis.
3.1.2 TEM characterization of MTB cells, magnetosomes, and commercial magnetic beadsFigure 1 shows the images of MTBLA-1, MTBLA-2 and commercial magnetic particle Scmp. It is obvious that the magnetosomes in MTBLA-1 and MTBLA-2 are arranged in chains, whereas the Scmp nanoparticles are clustered together (Fig. 1a–f). Size analysis reveals that one MTBLA-1 cell contains an average of 7.8±3.9 magnetosomes with the average particle size of (27.9±7.9) nm (Fig. 1a–b), and the cell of MTBLA-2 possess an average of 17.7±5.6 magnetosomes with the average particle size of (35.7±7.7) nm (Fig. 1c–d). Compared with MTBLA-2, the MTBLA-1 have fewer magnetosomes per cell and bimodal size distributions with a main peak at 32 nm and a secondary peak at 16 nm on the particle size distribution histogram, indicating more immature magnetosomes (Fig. 1b), while the MTBLA-2 cells have more magnetosomes with larger average particle size, consistent with the results of Cmag determination. The true average size of S cmp nanoparticles is (21.2±6.3) nm, which is smaller than the size described in the product specification, possibly due to the statistical errors caused by sample aggregation (Fig. 1e–f).
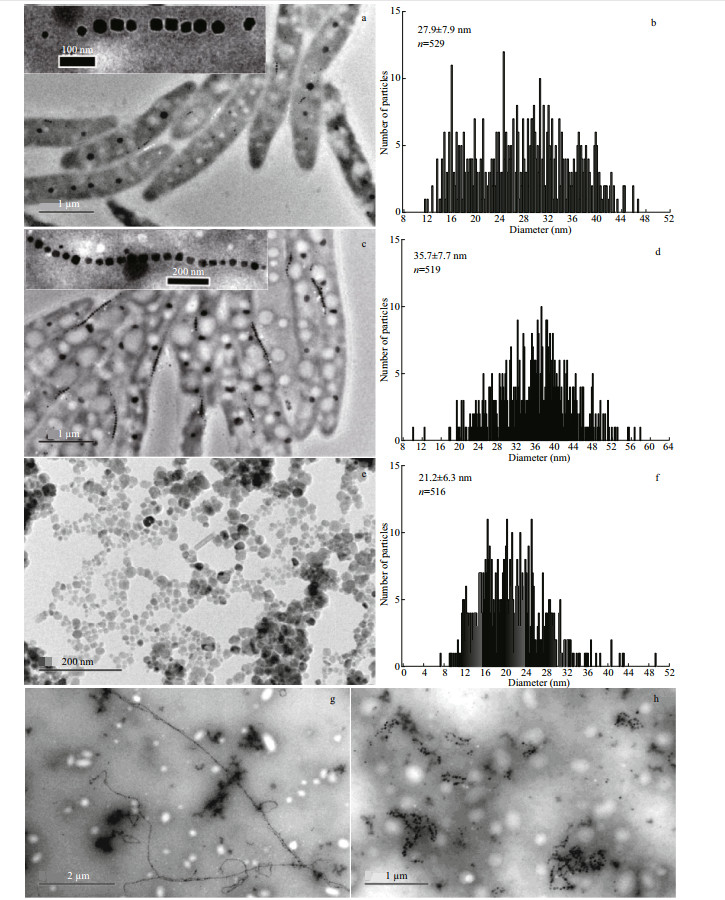
|
| Fig.1 TEM images (a, c, e, g, h) and statistics data of particle size (b, d, f) of MTB samples and commercial magnetic beads The data inserted in the figure b, d, f represent the average particle size and the number of statistical particles. a–b. MTBLA-1; c–d. MTBLA-2; e–f. Scmp; g. S2; h. S3. |
As displayed in Fig. 1g, the intact cell structures of the sample S2 are lost due to ultrasonic treatment for 10 min. With ultrasonic treatment for 50 min, both of the cell structure and magnetosome chain of sample S3 are completely destroyed, and the magnetosomes stick together (Fig. 1h). The sample S1 is not subjected to any ultrasonic treatment. Therefore, the S1 remains its original state with complete cell morphology and magnetosome chains.
3.2 Rock magnetic propertiesFORC diagrams show very weak magnetostatic interactions (Bu, 1/2=1.0 mT) among magnetite particles of MTB cells because of separation by magnetosome membranes and cell structure (Fig. 2a–b), which is consistent with our previous reports (Zhang and Pan, 2018). The coercivity value of MTBLA-2 (Bc, FORC= 20.4 mT) is larger than that of MTBLA-1 (Bc, FORC=17.4 mT), which is in agreement with the TEM results that the MTBLA-2 owns larger magnetosomes and longer magnetosome chains (Fig. 1a–d). On the contrary, the magnetostatic interactions among magnetite particles in Scmp are significantly stronger (Bu, 1/2=9.6 mT) (Fig. 2c), and the center of FORC diagram is much closer to the Y-axis than that of MTBLA-1 and MTBLA-2. This means that the coercivity of Scmp is smaller than MTB cells.
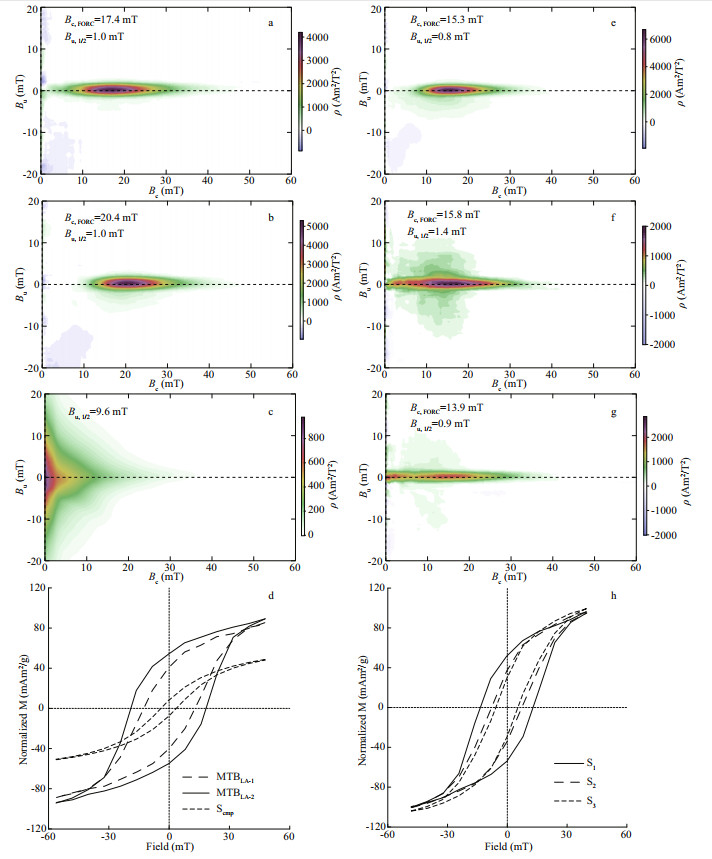
|
| Fig.2 Rock magnetic characterization of samples a–c. FORC diagrams of MTBLA-1, MTBLA-2, and Scmp; d. room-temperature hysteresis loops of MTBLA-1, MTBLA-2, and Scmp; e–g. FORC diagrams of S1, S2, and S3; h. room-temperature hysteresis loops of S1, S2, and S3. The magnetization was normalized by the mass of Fe. |
The coercivity (Bc), saturation magnetization (Ms), remanent coercivity (Bcr), and saturation remanent magnetization (Mrs) of MTBLA-1, MTBLA-2, and Scmp are determined by hysteresis loops and SIRM curves, and the results are shown in Fig. 2d and Table 1 (Ms is normalized by iron mass). It is noted that these samples have similar potbelly shape loop, which is characteristic of cubic type of magnetic anisotropy. The MTBLA-1, MTBLA-2, and Scmp samples have Bc values of 12.7, 18.7, and 3.8 mT; Ms values of 105.1, 116.8, and 69.5 mAm2/g; Bcr values of 18.0, 21.6, and 9.8 mT; and Mrs/Ms values of 0.37, 0.48, and 0.11, respectively. As a result, the area surrounded by hysteresis loop, in descending order, are MTBLA-2, MTBLA-1, and Scmp, respectively.
The changes in magnetosome chain structure can significantly affect the rock magnetism of MTB cells. The FORCs diagrams of the two ultrasound-treated samples, S2 and S3, are clearly different from that of intact cells, S1, (Fig. 2e–g). In particular, there are two hot center spots in FORC diagrams of sample S3. This might have been caused by chain-structure destruction. As the results of hysteresis loops and SIRMs shown in Table 1; Bc, Bcr, and Mrs/Ms values of S1, S2, and S3 are reduced in turn, and the corresponding areas of hysteresis loops also decreased in the same order (Fig. 2h).
3.3 Heating efficiency of MTB in AMFAs shown in Fig. 3a, the temperature rising curves of MTBLA-2 under AMF (30 kA/m, 764 kHz) show that the same kind of MTB cells under the same cell concentration (OD=8 or 20) have the same rate of temperature rise in the overlapped curves. This means that the heat production stability of MTB in AMF is stable and repeatable in aqueous medium. The change in temperature (ΔT) values increase linearly with the concentration of MTB cells (Fig. 3b). At the same iron concentration, the ΔT of MTBLA-2 at 10 min is higher than that of MTBLA-1 (Fig. 3b insert), indicating that the magnetosomes of MTBLA-2 have stronger heat conversion ability. This is consistent with the results of TEM (Fig. 1a–d) and magnetic characterization (Fig. 2a–b); therefore, the MTBLA-2 cells with more and larger magnetosomes have superior magnetic properties and heat conversion efficiency.
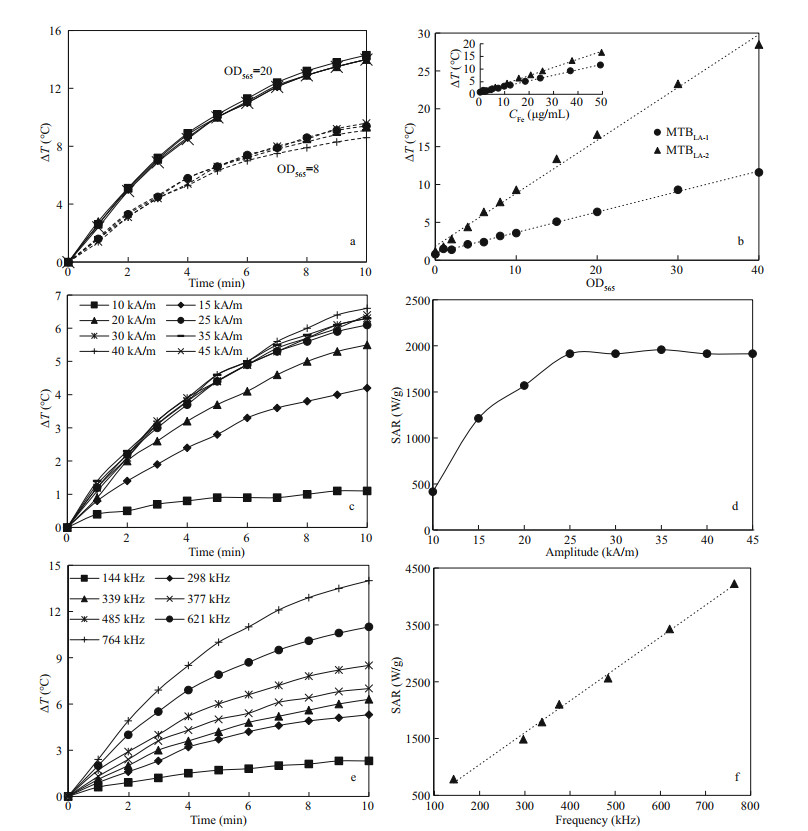
|
| Fig.3 Heating efficiency of MTB cells in AMF a. repeatability tests of temperature rising curves of MTB in aqueous under AMF with frequency of 764 kHz and amplitude of 30 kA/m, the solid and dashed lines represent MTBLA-2 cell concentrations of OD565=20 and OD565=8, respectively; b. ΔT of MTBLA-1 and MTBLA-2 at 10 min versus cell and iron concentrations; c, d. temperature rising and SAR curves of MTBLA-2 with varying amplitude under frequency of 339 kHz; e, f. temperature rising and SAR curves of MTBLA-2 with different frequency under amplitude of 30 kA/m. |
In order to evaluate the effect of magnetic field amplitude on the heat production efficiency of MTB, we tested the temperature rising curves of MTBLA-2 with iron concentration of 48.4 μg/mL using AMF frequency of 339 kHz at different amplitudes of 10, 15, 20, 25, 30, 35, 40, 45 kA/m. As AMF amplitude increased from 10 to 25 kA/m, the ΔT rises from 1.1 to 6.1 ℃ in 10 min, and the SARs of MTBLA-2 are increased from 415.7 to 1 913.8 W/g (Fig. 3c–d).However, the ΔT and SARs stop increasing with the amplitude when the amplitude exceeded 25 kA/m.
To understand the effect of frequency on heat efficiency of MTB cells, we also perform heating test using the sample MTBLA-2 with iron concentration of 48.4 μg/mL as AMF frequency increased from 144 to 764 kHz under the constant amplitude of 30 kA/m. With the frequency increased, the ΔT grows from 2.4 to 14.0 ℃ at 10 min (Fig. 3e), and SARs grow linearly from 788.0 to 4 226.0 W/g (Fig. 3f).
3.4 Comparison of heat production efficiency between MTB and ScmpThe temperature rising curves of MTBLA-1, MTBLA-2, and Scmp with same iron concentration of 49.2 μg/mL are tested under amplitude of 30 kA/m and frequency of 764 kHz. The ΔT values at 10 min are 11.6℃ for MTBLA-1, 16.6 ℃ for MTBLA-2, and 2.9 ℃ for S cmp, respectively (Fig. 4a), and the corresponding SAR values are 3 440.2 W/g for MTBLA-1, 4 925.6 W/g for MTBLA-2, and 657.3 W/g for Scmp, respectively. As expected, sample MTBLA-2 with more and larger magnetosomes possesses stronger ability of heat generation than sample MTBLA-1. Both of the two MTB samples produce more heat than commercial magnetic beads under the same conditions because of the advantages in magnetic properties, such as Bc, Ms, Mrs/Ms.
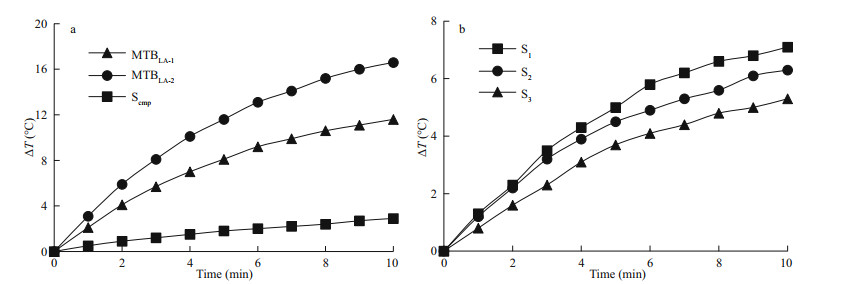
|
| Fig.4 Temperature rising curves under amplitude of 30 kA/m and frequency of 764 kHz a. MTBLA-1, MTBLA-2, and Scmp; b. S1, S2, and S3. |
To evaluate the effect of magnetosome chain integrity on heat production, the temperature rising curves of sample S1, S2, and S3 with same iron concentration of 22.3 μg/mL are tested under amplitude of 30 kA/m and frequency of 764 kHz, and the corresponding SAR values were calculated according to the temperature rising curves. It is obvious that the sample S1 owing to intact cell structure and magnetosome chain exhibit the strongest ability of heat production with ΔT value of 7.1 ℃ at 10 min (Fig. 4b). By contrast, the ΔT values of S2 and S 3 at 10 min are only 6.3 ℃ and 5.3 ℃, respectively. Likewise, the SAR of S1 is 4 643.6 W/g, which is larger than that of S2 (4 209.4 W/g) and S3 (3 114.6 W/g). This highlights the important role of the intact magnetosome chain for heat generation during the magnetic hyperthermia process.
4 DISCUSSIONThere are three physical principles involved in the heat transfer mechanisms involved in magnetic hyperthermia, namely: (i) resistance heating due to eddy currents, (ii) magnetic heating due to hysteresis loss, and (iii) magnetic heating due to Néel and Brownian relaxation processes (Ortega and Pankhurst, 2013). In the case of single-domain particles, hysteresis losses are higher than any of the other losses (Hergt et al., 1998). When the magnetic field vary with time, the relationship between the area under AC hysteresis loop and the amount of heat generated per cycle (PFM) follows the next equation:
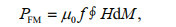 (2)
(2)where f is the frequency of the applied field, H is the field amplitude and M is the magnetization. It is well known that magnetosomes belong to single-domain magnetic particles (Ding et al., 2010; Zhang and Pan, 2018), and MTB shows typical rock magnetic characteristics of single-domain (Fig. 2a–d), thus the magnetic hyperthermia mechanism of MTB mainly follows the principle of hysteresis loss. The experimental results accord with the prediction of the Eq.2: (i) ΔT and SARs linearly increase with field frequency; (ii) ΔT and SARs increase with field amplitude only within a certain range. When the magnetization of MTB is saturated and the area under the AC hysteresis loop reaches maximum, ΔT and SARs will no longer increase with the amplitude. In a word, the three main parameters of AC hysteresis loop, Ms, Mrs, and Bc, determine the area under AC hysteresis loop, and thus determine the heat production efficiency of single domain particles at the same frequency. Unfortunately, due to the limitation of equipment, we only test the low-frequency hysteresis loop, which could not be directly used in the calculation of heat production efficiency, but could be used to evaluate the capacity of heat generation of the samples.
It is obvious that the MTB whole cells have huge advantages in heat production compared with commercial magnetic particles under the conditions of nearly similar particle size, same iron concentration and magnetic field. Under the experimental conditions described above, the ΔT values of MTBLA-1 and MTBLA-2 at 10 min are 3 times and 4.7 times more than that of sample Scmp, respectively (Fig. 4a). As the values of Bc, Ms, and Mrs/Ms of MTB are significantly greater than those of sample Scmp, it means the lager value of AC hysteresis loop area and higher capacity of heat generation. Comparing with the sample Scmp, the magnetic parameters and heat production efficiency of MTB whole cells greatly benefit from the inherent chain-like arrangement of magnetosomes.
Combined the rock magnetic and hyperthermia measurements, it indicates that the chain alignment of magnetosomes plays important roles in the magnetic parameters and heating efficiency of MTB intact cells. The destruction of the magnetosome chain is directly proportional to the decrease in magnetic parameters, such as Bc, Bcr, and Mrs/Ms (Li et al., 2012). Magnetic hyperthermia also follows the same law (Fig. 4b). The MTB intact cell organizes magnetosomes like necklaces through magnetosome membrane and cytoskeletal proteins, which reduces the interaction between magnetic particles and increases magnetic anisotropy and coercivity. The excellent magnetic properties make MTB whole cells have great heat production efficiency and promising medical application prospects.
The MTB whole cells used in this study shows excellent property in heat production; however, there are still some limitations. For safety and patient tolerance reasons, the applied magnetic field is limited in clinical magnetic hyperthermia therapy by "Brezovich criterion" (Brezovich, 1988). In this case, achieving the "therapeutic window" of tumor would requires 109 magnitude of bacteria, equivalent to a dose of approximately 1-mL OD565=20 bacteria cells with iron concentration of about 49.2 μg/mL, because one single MSR-1 cell contains only limited number of magnetosomes, averaging of 17.7 per cell in our study. Large number of foreign bacteria entering the body is bound to increase the safety risk. One strategy for reducing the dose of MTB is to continue to improve the heat production efficiency of unit MTB cells. Our study and previous literatures show that using sodium nitrate instead of ammonium chloride as medium nitrogen source could significantly improve the parameters of magnetosome (Heyen and Schüler, 2003), and then improve the heat production efficiency. Li et al. (2016) proves that cobalt-containing magnetosomes with higher coercivity could be synthesized by adding cobalt to the culture medium. It is well known that MSR-1 cultured in fermenter have higher Cmag value than that cultured in shaking table (Zhang et al., 2011), indicating higher coercivity and greater efficiency in heat generation. All these indicate that the MSR-1 cells with higher heat efficiency can be obtained by optimizing medium and culture mode. In addition, as more and more species of MTB have been discovered, we are pleasantly surprised to find a kind of giant rod‐shaped MTB containing hundreds of magnetosomes per cell (Lin et al., 2009; Li et al., 2020), which means huge heat efficiency and potential for clinical application. However, the vast majority of MTB, including giant rod‐shaped MTB, have not achieved pure culture at present. Therefore, it is very significant to study the culture of MTB in future.
5 CONCLUSIONMTB whole cells with single-domain magnetosome exhibit excellent magnetic hyperthermia ability, and the heat generation principle mainly follows the hysteresis loss mechanism. So the heat production efficiency of MTB cells is closely related to the parameters of hysteresis loop, such as Bc, Ms, and Mrs. Benefitting from the chain structures of magnetosomes, MTB cells have more superior magnetic properties than commercial magnetic beads with similar size, and thus have higher efficiency in heat production. As expected, MTB cells with more and larger magnetosomes possess greater efficiency in heat production. Therefore, improving the magnetic properties of MTB cells by optimizing the medium and culture method may further improve the heat conversion efficiency.
6 DATA AVAILABILITY STATEMENTThe data that support the findings of the current study are available on reasonable request from the corresponding author.
7 ACKNOWLEDGMENTWe thank Tang XU from the Institute of Geology and Geophysics, CAS for his help in TEM analysis.
Alphandéry E, Faure S, Raison L, Duguet E, Howse P A, Bazylinski D A. 2011. Heat production by bacterial magnetosomes exposed to an oscillating magnetic field. The Journal of Physical Chemistry C, 115(1): 18-22.
DOI:10.1021/jp104580t |
Andreu I, Natividad E. 2013. Accuracy of available methods for quantifying the heat power generation of nanoparticles for magnetic hyperthermia. International Journal of Hyperthermia, 29(8): 739-751.
DOI:10.3109/02656736.2013.826825 |
Bazylinski D A, Frankel R B. 2004. Magnetosome formation in prokaryotes. Nature Reviews Microbiology, 2(3): 217-230.
DOI:10.1038/nrmicro842 |
Blakemore R. 1975. Magnetotactic bacteria. Science, 190(4212): 377-379.
DOI:10.1126/science.170679 |
Blanco-Andujar C, Teran F J, Ortega D. 2018. Chapter 8: Current Outlook and Perspectives on Nanoparticle-Mediated Magnetic Hyperthermia. Iron Oxide Nanoparticles for Biomedical Applications: Synthesis, Functionalization and Application. Elsevier. p. 197-245.
|
Brezovich I A. 1988. Low frequency hyperthermia: capacitive and ferromagnetic thermoseed methods. Medical Physics Monograph, 16: 82-111.
|
Chen C Y, Chen L J, Wang P P, Wu L F, Song T. 2019. Steering of magnetotactic bacterial microrobots by focusing magnetic field for targeted pathogen killing. Journal of Magnetism and Magnetic Materials, 479: 74-83.
DOI:10.1016/j.jmmm.2019.02.004 |
Dadfar S M, Roemhild K, Drude N I, Von Stillfried S, Knüchel R, Kiessling F, Lammers T. 2019. Iron oxide nanoparticles: diagnostic, therapeutic and theranostic applications. Advanced Drug Delivery Reviews, 138: 302-325.
DOI:10.1016/j.addr.2019.01.005 |
Ding Y, Li J H, Liu J N, Yang J, Jiang W, Tian J S, Li T, Pan Y X, Li J L. 2010. Deletion of the ftsZ-like gene results in the production of superparamagnetic magnetite magnetosomes in Magnetospirillum gryphiswaldense. Journal of Bacteriology, 192(4): 1097-1105.
DOI:10.1128/JB.01292-09 |
Falk M H, Issels R D. 2001. Hyperthermia in oncology. International Journal of Hyperthermia, 17(1): 1-18.
DOI:10.1080/02656730118511 |
Fdez-Gubieda M L, Alonso J, García-Prieto A, García-Arribas A, Barquín L F, Muela A. 2020. Magnetotactic bacteria for cancer therapy. Journal of Applied Physics, 128(7): 070902.
DOI:10.1063/5.0018036 |
Frankel R B, Bazylinski D A, Johnson M S, Taylor B L. 1997. Magneto-aerotaxis in marine coccoid bacteria. Biophysical Journal, 73(2): 994-1000.
DOI:10.1016/S0006-3495(97)78132-3 |
Gandia D, Gandarias L, Rodrigo I, Robles-García J, Das R, Garaio E, García J Á, Phan M H, Srikanth H, Orue I, Alonso J, Muela A, Gubieda M L F. 2019. Unlocking the potential of magnetotactic bacteria as magnetic hyperthermia agents. Small, 15(41): 1902626.
DOI:10.1002/smll.201902626 |
Han X H, Tomaszewski E J, Sorwat J, Pan Y X, Kappler A, Byrne J M. 2020. Effect of microbial biomass and humic acids on abiotic and biotic magnetite formation. Environmental Science & Technology, 54(7): 4121-4130.
DOI:10.1021/acs.est.9b07095 |
Hergt R, Andra W, d'Ambly C G, Hilger I, Kaiser W A, Richter U, Schmidt H G. 1998. Physical limits of hyperthermia using magnetite fine particles. IEEE Transactions on Magnetics, 34(5): 3745-3754.
DOI:10.1109/20.718537 |
Hergt R, Dutz S, Müller R, Zeisberger M. 2006. Magnetic particle hyperthermia: nanoparticle magnetism and materials development for cancer therapy. Journal of Physics: Condensed Matter, 18(38): S2919.
DOI:10.1088/0953-8984/18/38/S26 |
Hergt R, Dutz S, Röder M. 2008. Effects of size distribution on hysteresis losses of magnetic nanoparticles for hyperthermia. Journal of Physics: Condensed Matter, 20(38): 385214.
DOI:10.1088/0953-8984/20/38/385214 |
Hergt R, Hiergeist R, Zeisberger M, Schüler D, Heyen U, Hilger I, Kaiser W A. 2005. Magnetic properties of bacterial magnetosomes as potential diagnostic and therapeutic tools. Journal of Magnetism and Magnetic Materials, 293(1): 80-86.
DOI:10.1016/j.jmmm.2005.01.047 |
Heyen U, Schüler D. 2003. Growth and magnetosome formation by microaerophilic Magnetospirillum strains in an oxygen-controlled fermentor. Applied Microbiology and Biotechnology, 61(5): 536-544.
DOI:10.1007/s00253-002-1219-x |
Johannsen M, Gneveckow U, Thiesen B, Taymoorian K, Cho C H, Waldöfner N, Scholz R, Jordan A, Loening S A, Wust P. 2007. Thermotherapy of prostate cancer using magnetic nanoparticles: feasibility, imaging, and threedimensional temperature distribution. European Urology, 52(6): 1653-1662.
DOI:10.1016/j.eururo.2006.11.023 |
Kallumadil M, Tada M, Nakagawa T, Abe M, Southern P, Pankhurst Q A. 2009. Suitability of commercial colloids for magnetic hyperthermia. Journal of Magnetism and Magnetic Materials, 321(10): 1509-1513.
DOI:10.1016/j.jmmm.2009.02.075 |
Li A H, Tang T, Zhang H Y, Wang Q, Tian J S, Li Y. 2010. Modification of Bacterial magnetosomes and application of magnetosome-antibody complex in pathogen detection. Acta Biophysica Sinica, 26(8): 680-690.
(in Chinese with English abstract) |
Li J H, Liu P Y, Wang J, Roberts A P, Pan Y X. 2020. Magnetotaxis as an adaptation to enable bacterial shuttling of microbial sulfur and sulfur cycling across aquatic oxicanoxic interfaces. Journal of Geophysical Research: Biogeosciences, 125(12): e2020JG006012.
DOI:10.1029/2020JG006012 |
Li J H, Menguy N, Arrio M A, Sainctavit P, Juhin A, Wang Y Z, Chen H T, Bunau O, Otero E, Ohresser P, Pan Y X. 2016. Controlled cobalt doping in the spinel structure of magnetosome magnetite: new evidences from elementand site-specific X-ray magnetic circular dichroism analyses. Journal of the Royal Society Interface, 13(121): 20160355.
DOI:10.1098/rsif.2016.0355 |
Li J H, Wu W F, Liu Q S, Pan Y X. 2012. Magnetic anisotropy, magnetostatic interactions and identification of magnetofossils. Geochemistry, Geophysics, Geosystems, 13(12): Q10Z51.
DOI:10.1029/2012GC004384 |
Lin W, Li J H, Schüler D, Jogler C, Pan Y X. 2009. Diversity analysis of magnetotactic bacteria in Lake Miyun, northern China, by restriction fragment length polymorphism. Systematic and Applied Microbiology, 32(5): 342-350.
DOI:10.1016/j.syapm.2008.10.005 |
Maier-Hauff K, Ulrich F, Nestler D, Niehoff H, Wust P, Thiesen B, Orawa H, Budach V, Jordan A. 2011. Efficacy and safety of intratumoral thermotherapy using magnetic ironoxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. Journal of Neuro-Oncology, 103(2): 317-324.
DOI:10.1007/s11060-010-0389-0 |
Mason P A, Hurt W D, Walters T J, D'Andrea J A, Ryan K L, Nelson D A, Smith K I, Ziriax J M. 2000. Effects of frequency, permittivity, and voxel size on predicted specific absorption rate values in biological tissue during electromagnetic-field exposure. IEEE Transactions on Microwave Theory and Techniques, 48(11): 2050-2058.
DOI:10.1109/22.884194 |
Muela A, Muñ oz D, Martin-Rodriguez R, Orue I, Garaio E, de Cerio A A D, Alonso J, García J Á, Fdez-Gubieda M L. 2016. Optimal parameters for hyperthermia treatment using biomineralized magnetite nanoparticles: theoretical and experimental approach. The Journal of Physical Chemistry C, 120(42): 24437-24448.
DOI:10.1021/acs.jpcc.6b07321 |
Ortega D, Pankhurst Q A. 2013. Magnetic hyperthermia. In: P. O'Brien. Nanoscience: Volume 1: Nanostructures through Chemistry. Royal Society of Chemistry: Cambridge. p. 60-88, https://doi.org/10.1039/9781849734844-00060.
|
Pankhurst Q A, Connolly J, Jones S K, Dobson J. 2003. Applications of magnetic nanoparticles in biomedicine. Journal of Physics D: Applied Physics, 36(13): R167.
DOI:10.1088/0022-3727/36/13/201 |
Périgo E A, Hemery G, Sandre O, Ortega D, Garaio E, Plazaola F, Teran F J. 2015. Fundamentals and advances in magnetic hyperthermia. Applied Physics Reviews, 2(4): 041302.
DOI:10.1063/1.4935688 |
Rosensweig R E. 2002. Heating magnetic fluid with alternating magnetic field. Journal of Magnetism and Magnetic Materials, 252: 370-374.
DOI:10.1016/S0304-8853(02)00706-0 |
Schüler D, Uhl R, Bäuerlein E. 1995. A simple light scattering method to assay magnetism in Magnetospirillum gryphiswaldense. FEMS Microbiology Letters, 132(1-2): 139-145.
DOI:10.1111/j.1574-6968.1995.tb07823.x |
Stookey L L. 1970. Ferrozine-a new spectrophotometric reagent for iron. Analytical Chemistry, 42(7): 779-781.
DOI:10.1021/ac60289a016 |
Sun J B, Duan J H, Dai S L, Ren J, Zhang Y D, Tian J S, Li Y. 2007. In vitro and in vivo antitumor effects of doxorubicin loaded with bacterial magnetosomes (DBMs) on H22 cells: the magnetic bio-nanoparticles as drug carriers. Cancer Letters, 258(1): 109-117.
DOI:10.1016/j.canlet.2007.08.018 |
Sun J B, Zhao F, Tang T, Jiang W, Tian J S, Li Y, Li J L. 2008. High-yield growth and magnetosome formation by Magnetospirillum gryphiswaldense MSR-1 in an oxygencontrolled fermentor supplied solely with air. Applied Microbiology and Biotechnology, 79(3): 389-397.
DOI:10.1007/s00253-008-1453-y |
Thiesen B, Jordan A. 2008. Clinical applications of magnetic nanoparticles for hyperthermia. International Journal of Hyperthermia, 24(6): 467-474.
DOI:10.1080/02656730802104757 |
Uebe R, Schüler D. 2016. Magnetosome biogenesis in magnetotactic bacteria. Nature Reviews Microbiology, 14(10): 621-637.
DOI:10.1038/nrmicro.2016.99 |
Xu H T, Pan Y X. 2019. Experimental evaluation on the heating efficiency of magnetoferritin nanoparticles in an alternating magnetic field. Nanomaterials, 9(10): 1457.
DOI:10.3390/nano9101457 |
Yang W J, Bai Y, Wang X, Dong X X, Li Y, Fang M Y. 2016. Attaching biosynthesized bacterial magnetic particles to polyethylenimine enhances gene delivery into mammalian cells. Journal of Biomedical Nanotechnology, 12(4): 789-799.
DOI:10.1166/jbn.2016.2213 |
Zhang T W, Pan Y X. 2018. Constraining the magnetic properties of ultrafine-and fine-grained biogenic magnetite. Earth, Planets and Space, 70(1): 206.
DOI:10.1186/s40623-018-0978-2 |
Zhang Y, Zhang X J, Jiang W, Li Y, Li J L. 2011. Semicontinuous culture of Magnetospirillum gryphiswaldense MSR-1 cells in an autofermentor by nutrient-balanced and isosmotic feeding strategies. Applied and Environmental Microbiology, 77(17): 5851-5856.
DOI:10.1128/AEM.05962-11 |
 2021, Vol. 39
2021, Vol. 39